Hypercholesterolemia is a significant risk factor for cardiovascular disease (CVD), and the presence of chronic kidney disease (CKD) represents an independent risk factor for CVD. Therefore, developing safe and effective treatment strategies to control hyperlipidemia in patients with and without CKD is of high importance, even more so when considering that the prevalence of both CVD and CKD is increasing in the global population. Although hypercholesterolemia does not represent an independent risk factor for the development of CKD, the concept of how the accumulation of perirenal fat, renal sinus fat, and intraparenchymal fat may contribute to CKD progression has gained a lot of attention. The fatty transformation of the kidney in the setting of kidney diseases was first reported in the British Medical Journal in 1883. What was not known at the time was if and how intrarenal fat may contribute to the development and progression of CKD. A large body of literature now suggests that altered renal lipid metabolism may cause proteinuria and CKD progression. However, what is not yet known are the pathways responsible for this association between altered lipid metabolism and CKD progression. In fish-eye disease, the association between deficiency of lecithin cholesterol acyltransferase and the development of nephrotic syndrome leading to ESKD indeed suggests a causative link between cholesterol efflux and kidney disease development. Interestingly, however, in familiar hyperlipidemia, which is mainly associated with mutation of the LDL receptor (LDLR) or other proteins such as proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9), the development of CKD is not often reported, suggesting that altered intrinsic renal lipid metabolism rather than the renal deposit of circulating lipids may contribute to disease progression. In fact, the biology of PCSK9 is quite complex because circulating PCSK9 may have a different mechanism of action than cell-derived PCSK9 and may modulate pathways other than LDLR.
PCSK9 is a protein involved in cholesterol homeostasis through its activity of reducing LDLR levels on the plasma membrane of hepatocytes. When PCSK9 is active, the reduction in LDLR on plasma membranes results in halted uptake and internal metabolism of LDL particles, leading to hypercholesterolemia. This protein has gained recent attention because PCSK9 inhibitors (PCSK9i), a new class of lipid-lowering drugs, have recently revolutionized the treatment of dyslipidemia by decreasing LDL by blocking the recycling of the LDLR in hepatocytes. The overwhelming conclusion from recent clinical trials of PCSK9i (DESCARTES and ODYSSEY) is that these treatments are safe and effective (1,2), even when administered to patients with established CKD. However, little is known about the potential renoprotective effects of PCSK9i. In addition, the LDLR-dependent and -independent signaling pathways involving PCSK9 remain partially unknown.
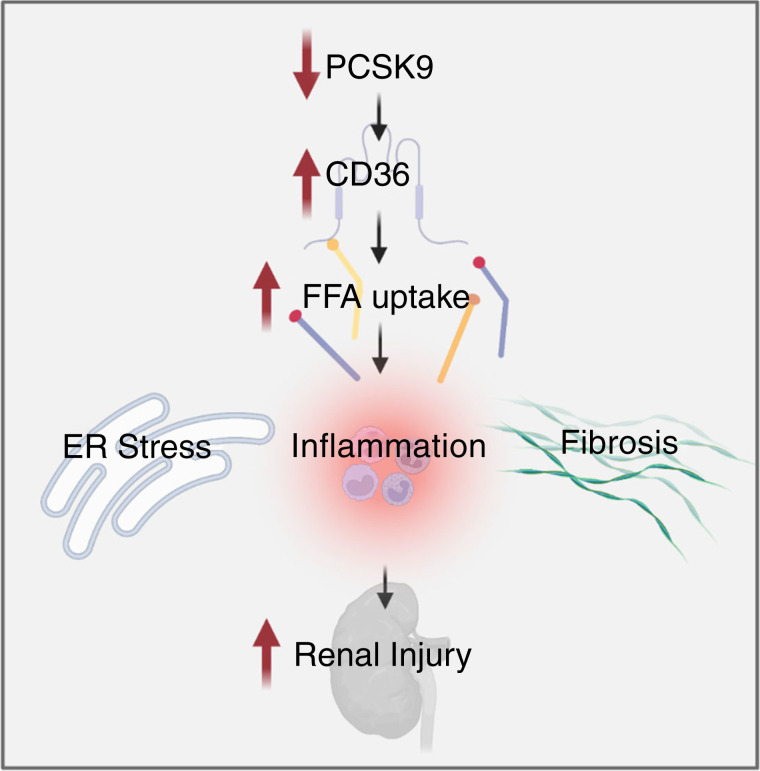
Cellular lipid overload, or lipotoxicity, leads to chronic cellular dysfunction and injury in multiple disease states, including CVD and CKD. Lipotoxicity is primarily driven by excessive free fatty acid (FFA) uptake and impaired fatty acid oxidation. Therapeutics aimed at decreasing lipotoxicity in various cell types will continue to hold promise in treating diseases encompassed by the term “metabolic syndrome.” In this issue of Kidney 360, Byun et al. observed that circulating PCSK9 suppresses CD36-dependent FFA uptake in the kidney. The authors describe that high-fat diet–induced renal injury is associated with decreased circulating PCSK9, promoting CD-36-dependent renal lipid accumulation and subsequent renal injury in both in vivo and in vitro models (Figure 1). Although administration of PCSK9i to patients leads to beneficial effects in CVD, this study shed new light on the apparent contradiction between the beneficial effect of circulating PCSK9 on the kidney and the current success of PCSK9i in treating hyperlipidemia and CVD.
Figure 1.
Proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) modulation of lipid uptake in the kidney. PCSK9 deficiency leads to increased surface expression of CD36 in HK-2 cells. Activation of CD36 in renal tubular cells and podocytes leads to increased endoplasmic reticulum stress, inflammation, and fibrosis, resulting in increased renal injury. Created with BioRender.com.
Byun et al. clearly demonstrate that circulating PCSK9 acts to degrade CD36 and may protect against CD36-induced renal damage, even though studies by others have suggested that genetic loss of CD36 did not alter fatty acid uptake or triglyceride accumulation, adding complexity to the interpretation of these new findings. In particular, the authors demonstrate that circulating PCSK9 protects against diet-induced renal injury in both cultured cells and mice by enhancing the degradation of renal CD36. Applying genetic and pharmacologic inhibition of PCSK9, the authors observed heightened CD36 expression in the renal parenchyma, associated with increased long-chain saturated FFA-induced endoplasmic reticulum stress in tubular cells and renal tissue.
Increased CD36 expression was associated with increased FFA uptake in cultured renal tubular cells and podocytes, consistent with findings by others. These findings are highly significant because inhibition or deficiency of CD36 can prevent fibrosis and proteinuria (3), and CD36 is upregulated in podocytes during proteinuric injury (4), as after exposure to nicotine and fasting. Blockade of CD36 in podocytes leads to reduced apoptosis and oxidative stress levels (5); therefore, blockade of CD36-dependent pathways, including degradation of CD36 by PCSK9, may provide a novel therapeutic strategy for a variety of kidney diseases.
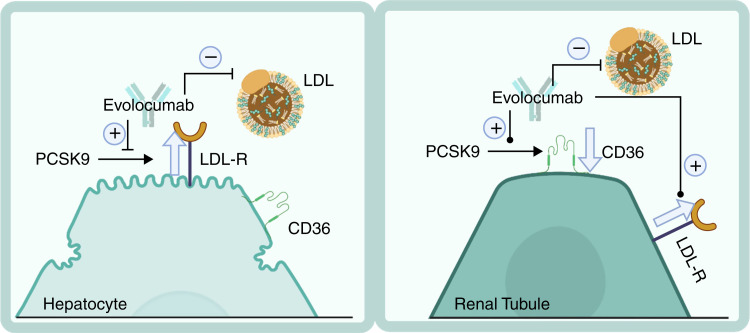
CD36 activation in the kidney results in renal injury by promoting inflammation and fibrosis. Previous studies demonstrate that CD36 activation promotes intrarenal oxidative and endoplasmic reticulum stress by increasing the uptake of modified lipoproteins and FFAs (6). PCSK9 has been identified to decrease the expression of CD36 in adipocytes and murine livers (5,7), and a similar mechanism in the kidney could reduce renal lipotoxicity. Therefore, these findings suggest that the beneficial effect of PCSK9i in treating hyperlipidemia may be accompanied by worsening renal injury in the setting of CKD. On the contrary, this study demonstrates that although the PCSK9i evolocumab blocks the interaction of PCSK9 with LDLR and prevents PCKS9-dependent hepatic production of LDL, it does not block the interaction between PCSK9 and CD36, thus allowing for the PCSK9-induced protection from lipotoxic stress by degradation of CD36 by PCSK9 in renal tissue (Figure 2). This differential action on renal tissue is even more critical when considering that increased levels of circulating PCSK9 were described with renal disease, where they may represent a defense mechanism from renal lipotoxicity, and after clinical (8) and experimental (9) treatment with evolocumab. Therefore, evolocumab may be an even more valuable tool for treating CVD, particularly CVD presenting with comorbidities such as CKD. These findings also challenge the current development of therapeutics to reduce circulating PCSK9 levels, such as incisiran, a PCSK9 siRNA in phase 3 clinical trials. It may be essential to determine the safety of this therapy in patients with CKD.
Figure 2.
Evolovumab decreases circulating LDL by its actions on hepatocytes and enhances PCSK9 levels, reducing lipotoxic renal injury. Evolocumab decreases LDL by blocking LDL receptor (LDLR) recycling, leading to increased LDLRs on hepatocytes and decreased circulating LDL. In renal cells, evolocumab enhances the degradation of CD36 by PCSK9 while reducing LDL levels through hepatic mechanisms. Created with BioRender.com.
PCSK9 is also involved in non-LDL-related processes, including glucose metabolism and regulation of sodium reabsorption (10). Studies demonstrate a clear correlation, indicating that levels of circulating PCSK9 contribute to glucose metabolism and insulin release with PCSK9−/− mice having glucose intolerance (10). The mechanisms underlying this effect have not been fully described, and clinical studies investigating this relationship between PCSK9 and glucose metabolism yielded inconsistent results. Therefore, further studies of this effect in humans and model systems are needed to understand fully the effect of PCSK9 on glucose metabolism. PCSK9 also has a role in modulating Na+ channel (ENaC) expression in renal tubular epithelial cells, indicating that it may play a role in BP homeostasis (10). However, there seems to be a negligible effect of PCSK9 on BP regulation, with studies demonstrating that PCSK9 deficiency and PCSK9i do not cause significant alterations in BP (1,2,10). Additional studies are needed to determine the direct effect of PCSK9i on these processes and the potential off-target effects, which also contribute to CVD and CKD, of this new class of drugs. Consensus on the role of PCSK9 and the effect of PCSK9i on non-LDL-mediated signaling will provide valuable information for drug optimization and use in novel indications. Characterization of specific off-target effects may also allow for the identification of novel therapeutic targets.
Although this study provides clear evidence for the importance of a dual mechanism of action of PCSK9i in protecting renal tubular cells, future studies to address the effect of PCSK9i on CD36 expression in kidney cells, other than tubular cells, and in infiltrating immune-derived cells are warranted. Furthermore, it will be essential to investigate how PCSK9i affects ectopic fat deposition in other organs, and if and how it may protect from kidney diseases of nonmetabolic origin, where we and others have demonstrated an essential contribution of renal lipotoxicity to disease progression. Finally, the effect of PCSK9i on lipid metabolism is more extensive than merely controlling LDL or CD-36-dependent lipotoxicity. In the LAPLACE-2 study, the addition of evolocumab to statins in hypercholesterolemia patients improved apolipoprotein B, triglycerides, and lipoprotein (a) (11). As these lipids are also involved in renal disease, investigating the implications of PCSK9i on these other lipids of interest in the kidney is also warranted.
Disclosures
A. Fornoni reports consultancy for Dimerix, Horizon, Kaneka, and ONO; ownership interest in L&F Health LLC (CSO and Vice-President), River 3 Renal Corp. (shareholder), UpToDate (shareholder), and Zyversa Therapeutics (shareholder); research funding from Aurinia, Boheringer Ingelheim, and Kwoya Kirin; a patent on the use of cyclodextrin for the treatment of kidney diseases and a patent on the use of small molecule inducers of cholesterol efflux; an advisory or leadership role for Journal of Clinical Investigation and Kidney International; participation in a speakers’ bureau for APSA Physician Scientist Seminar Series, Drexer University, Dalian University, Duke University, ERA-EDTA, International Podocyte Meeting, MGH/Brigham combined Renal Grand Round, University of Heidelberg, UC Irvine, UCLA, USC, and WCN; and is the inventor on five pending US patents and one published patent. J. Pressly has nothing to disclose.
Funding
JP is supported by the Ben J. Lipps Research Fellowship Program. AF is supported by grants from National Institutes of Health (R01DK104753 and R01CA227493], the Miami Clinical Translational Science Institute (UL1T R000460) and other network grants (U54DK083912, UM1DK100846,U01DK116101).
Acknowledgments
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Footnotes
See related article, “Inhibitory Antibodies against PCSK9 Reduce Surface CD36 and Mitigate Diet-Induced Renal Lipotoxicity,” on pages 1394–1410.
Author Contributions
J. Pressly wrote the original draft of the manuscript, and both authors reviewed and edited the manuscript.
References
- 1.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA; DESCARTES Investigators : A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 370: 1809–1819, 2014. 10.1056/NEJMoa1316222 [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM; ODYSSEY COMBO II Investigators : Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: The ODYSSEY COMBO II randomized controlled trial. Eur Heart J 36: 1186–1194, 2015. 10.1093/eurheartj/ehv028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YL, Lin SH, Chuang LY, Guh JY, Liao TN, Lee TC, Chang WT, Chang FR, Hung MY, Chiang TA, Hung CY: CD36 is a novel and potential anti‐fibrogenic target in albumin‐induced renal proximal tubule fibrosis. J Cell Biochem 101: 735–744, 2007. 10.1002/jcb.21236 [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Rui H-l, Yang M, Sun L-J, Dong H-R, Cheng H: CD36-mediated lipid accumulation and activation of NLRP3 inflammasome lead to podocyte injury in obesity-related glomerulopathy. Mediators Inflamm 2019: 3172647, 2019. 10.1155/2019/3172647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J-J, David JM, Wilbon SS, Santos JV, Patel DM, Ahmad A, Mitrofanova A, Liu X, Mallela SK, Ducasa GM, Ge M, Sloan AJ, Al-Ali H, Boulina M, Mendez AJ, Contreras GN, Prunotto M, Sohail A, Fridman R, Miner JH, Merscher S, Fornoni A: Discoidin domain receptor 1 activation links extracellular matrix to podocyte lipotoxicity in Alport syndrome. EBioMedicine 63: 103162, 2021. 10.1016/j.ebiom.2020.103162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamura DM, Pennathur S, Pasichnyk K, López-Guisa JM, Collins S, Febbraio M, Heinecke J, Eddy AA: CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol 20: 495–505, 2009. 10.1681/ASN.2008010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demers A, Samami S, Lauzier B, Des Rosiers C, Sock ETN, Ong H, Mayer GJA: PCSK9 induces CD36 degradation and affects long-chain fatty acid uptake and triglyceride metabolism in adipocytes and in mouse liver. Arterioscler Thromb Vasc Biol 35: 2517–2525, 2015. 10.1161/ATVBAHA.115.306032 [DOI] [PubMed] [Google Scholar]
- 8.Quiroga B, Ramos PM, Chiva VÁJN: Efficacy and safety of the PCSK9 inhibitors in the treatment of dyslipidemia in chronic kidney disease. Nefrologia (Engl Ed) 40: 499–505, 2020. 10.1016/j.nefro.2020.04.020 [DOI] [PubMed] [Google Scholar]
- 9.Byun JH, Lebeau P, Platko K, Carlisle R, Faiyaz M, Chen J, MacDonald M, Makda Y, Yousof T, Lynn EJK: Inhibitory antibodies against PCSK9 reduce surface CD36 and mitigate diet-induced renal lipotoxicity [published online ahead of print April 27, 2022]. Kidney360 10.34067/KID.0007022021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesaro A, Bianconi V, Gragnano F, Moscarella E, Fimiani F, Monda E, Scudiero O, Limongelli G, Pirro M, Calabrò PJB: Beyond cholesterol metabolism: the pleiotropic effects of proprotein convertase subtilisin/kexin type 9 (PCSK9). Genetics, mutations, expression, and perspective for long‐term inhibition. Biofactors 46: 367–380, 2020. 10.1002/biof.1619 [DOI] [PubMed] [Google Scholar]
- 11.Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott RJJ: Effect of evolocumab or ezetimibe added to moderate-or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA 311: 1870–1883, 2014. 10.1001/jama.2014.4030 [DOI] [PubMed] [Google Scholar]