To the Editor:
Immunocompromised patients who develop COVID-19 infection have worse outcomes [1, 2]. Patients with hematological malignancies (HM) who receive hematopoietic cell transplantation (HCT) or chimeric antigen receptor T-cell (CAR-T) therapy are at a higher risk for infections due to lymphodepletion chemotherapy, B cell aplasia, and cytopenias. COVID-19-attributable mortality rates have been shown to be as high as 41% among CAR-T recipents [3, 4].
Though current data are limited to small studies, vaccine responses are blunted among HCT and CAR-T recipients [5–7]. In our previous review, we reported a pooled humoral response to primary vaccination series of 31% among 40 CAR-T recipients in 5 studies [5]. We also observed that BCMA-directed CAR-T patients might mount superior vaccine responses compared to recipients of CD19-directed CAR-T therapy.
The novel finding that target antigen and CAR-T-related factors can influence vaccine responses prompted us to reexamine these phenomena in a larger cohort as well as the impact of booster vaccine doses. Overall, we aimed to characterize better the determinants of vaccine responses for optimal intervals, vaccine types, number of doses, and the potential impact of CAR-T target antigen on seroconversion.
We conducted a comprehensive electronic search of Medline (Ovid), Scopus, Web of Science, Cochrane databases, and preprint servers starting the date when the first SARS-CoV-2 vaccine received emergency use authorization (EUA) on 11 December 2020 to 1 March 2022. We also manually searched studies reporting SARS-CoV-2 vaccine responses in CAR-T recipients. Further study methodology, including the full search strategy, PRISMA flowchart, study selection, outcomes definition, risk-of-bias assessment, and statistical analyses, are provided in Supplementary Materials S1-3.
The search overview, humoral response assessments, quality of studies, and risk of bias are detailed in Supplementary Materials S4–6. Eighteen studies reported humoral vaccine responses in 236 CAR-T recipients (Table 1). Fifteen of these studies included 181 fully vaccinated patients who completed the primary vaccination series. A primary vaccination regimen included either 2 doses of the BNT162b2, mRNA1273, or ChAdOx1 nCoV-19 vaccines, or a single dose of the JNJ-78436735 vaccine. Four studies included humoral responses to a third or fourth dose of either the BNT162b2 or mRNA1273 vaccines in 55 patients (Supplementary Materials S10) [5]. The humoral response assessment platforms and antibody assays are outlined in Table 1.
Table 1.
Studies examining SARS-CoV-2 vaccine responses of primary doses among CAR-T recipients.
| Study | Study design | Underlying disease | CAR-T construct | Vaccine type | Number of patients | Median age, years (range) | Median time from CAR-T to vaccine, months (range) | Humoral immune response | Antibody assay | Duration between last dose and response assessment, (range) | Predictors of immune response | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bergman et al. | Prospective | NR | CD19 | BNT162b2 | 3a | NR | NR | CD19: 0% (0/2) | Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay | NR | Solid organ transplant treated with MMF (−); CLL treated with ibrutinib (−) | One patient had a fatal suspected vaccine-related pulmonary infection |
| Dahiya et al. | Prospective | LBCL, FL, MCL | CD19 | BNT162b2; mRNA1273 | 18b | 50.5 (24–87) | NR | CD19: 7% (1/14) | Unspecified ELISA for S1 and RBD antigen | 4 weeks after 2nd dose (n = 14); 4 weeks after 1st dose (n = 4) | NR | NR |
| Dhakal et al. | Retrospective | NR | CD19/20 (n = 6); CD19 (n = 4); BCMA (n = 3); Allogeneic CD19 (n = 1)d,e | BNT162b2; mRNA1273; JNJ-78436735 | 14 | 66 (34–80)c | 24 (8–31) | CD19/CD20: 33% (2/6); CD19: 0% (0/4); BCMA: 0% (0/3) Allogeneic CD19: 0% (0/1)d,e | EUROIMMUN ELISA | NR | ↑IgG levels (+); corticosteroid use (−) in alloHCT cohort | NR |
| Fox et al. | Prospective | NR | NR | BNT162b2; ChAdOx1 nCoV-19 | 9 | 60 (27–82)f | NR | CAR T: 22% (2/9) | Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay | NR | ↑Total blood lymphocyte, CD19, CD4, CD56 counts (+); vaccinated >6 months after B-cell-malignancy therapy (+) | NR |
| Greenberger et al. | Prospective | DLBCL, CLL, FL, MM | CD19 (n = 7); BCMA- or CD138 (n = 5) | BNT162b2; mRNA1273 | 12 | 68 (16–110)g | NR | BCMA or CD138: 80% (4/5); CD19: 14% (1/7) | Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay | mRNA-1273: 41 days BNT162b2: 42 days | mRNA-1273 > BNT162b2; BCMA- or CD138-CAR T > CD19+ CAR-T | NR |
| Gastinne et al. | Retrospective | NHL (n = 20); ALL (n = 3) | CD19 (n = 22); Allogeneic CD19 (n = 1) | BNT162b2 | 23h | 62 (21–79) | 13 (range, 4–27) | CD19: 30% (6/20) | Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay | 52 days (21–99) | NR | Lesser pain in patients compared to controls |
| Haggenburg et al. | Prospective | NR | CD19 (n = 44) | mRNA1273 | 53i | Mean 60 (SD: 11)j | 30 weeks (1–346) | CD19: 11% (5/44) | Bead-based multiplex assay | 28 days | Lymphoma, ruxolitinib, hypomethylating therapy, CLL on ibrutinib (−); serum IgG4, absolute B and NK cell count (+) | NR |
| Healy et al. | Prospective | NR | NR | BNT162b2 | 2 | 60 (51–67)k | NR | CAR T: 0% (0/2) | Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay | 35 days | NR | NR |
| Lim et al. | Prospective | B-NHL | NR | BNT162b2; ChAdOx1 nCoV-19 | 4l | NR | NR (11–23) | CAR T: 50% (1/2) | Meso Scale Discovery electrochemiluminescence assay | 2-4 weeks | Vaccinated while receiving systemic anti-lymphoma therapy (−) | NR |
| Parvathaneni et al. | Prospective | B-ALL (n = 6); DLBCL (n = 1); NHL (n = 1); MCL (n = 1); CLL (n = 3) | CD19 (n = 7); CD19/22 (n = 5) | BNT162b2 (n = 6); mRNA1273 (n = 6) | 12 | 53 (16–74) | 22 (3–126) | CD19 or CD19/22: 42% (5/12) | Unspecified ELISA for RBD-IgG | NR | Circulating B cell count (+) | NR |
| Ram et al. (2021) | Prospective | NR | CD19 | BNT162b2 | 14 | 65 (23–83)m | 9 (3–17) | CD19: 36% (5/14) | Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay | 7–14 days | B-cell aplasia (−); ↑ cells and vaccine interval (+); females (+); ↑ cell dose (+) | Grade 3 or 4 cytopenia in ~5% of the entire cohort |
| Shapiro et al. | Cross-sectional | NR | CD19 (n = 6); BCMA (n = 1) | BNT162b2; mRNA1273; JNJ-7846735 | 7 | 70.5 (27–91)n | 17.5 (NR) | CD19: 0% (0/6); BCMA: 100% (1/1) | AdviseDx SARS-CoV-2 IgG II assay | NR | NHL (−); cytotoxic therapy, IVIG, CAR T, CD20 mAb (−); PCN (+); immunomodulatory agents, proteasome inh (+); prior COVID-19 infection (+) | Mild sore arm, muscle aches, fatigue, and fever most observed; No life threatening AE observed |
| Tamari et al. | Prospective | NR | NR | BNT162b2; mRNA1273 | 7 | 66.4 (25.8–84.1)o | 218 days (66–825) | CAR T: 29% (2/7) | AdviseDx SARS-CoV449 2 IgG II assay | ~2 months | >1 year after cellular therapy (+); ↑ CD4 count, CD19 count, mitogen proliferation response, IgG level (+) | NR |
| Thakkar et al. | Prospective (n = 213); retrospective (n = 29)p | NR | NR | BNT162b2; mRNA1273; JNJ-7846735 | 3 | 67 (27–90)q | NR | CAR T: 0% (0/3) | AdviseDx SARS-CoV-2 IgG II assay | 7–14 days | Anti-CD20 therapy, SCT (−); immune checkpoint inh; hormonal therapy (+); prior COVID-19 infection (+); mRNA-based > adenovirus-based vaccine | Sore arm and muscle aches most observed |
| Van Oekelen et al. | Prospective (n = 112) and retrospective (n = 208) | MM | BCMA | BNT162b2; mRNA1273r | 23s | 68 (38–93)t | NR | BCMA: 79% (15/19) | COVID-SeroKlir Kantaro SARS-CoV-2 IgG assay | 51 (11–118) | Prior COVID-19 infection (+); Neutropenia (−); anti-CD38 mAb, BCMA-targeted therapy (−); no active MM treatment (+) | NR |
CAR-T chimeric antigen receptor T-cells, MMF mycophenolate mofetil, CLL chronic lymphocytic leukemia, LBCL large B-cell lymphoma, FL follicular lymphoma, MCL mantle cell lymphoma, AlloHCT allogeneic hematopoietic cell therapy, AutoHCT autologous hematopoietic cell therapy, RBD R# binding domain, DLBCL diffuse large B-cell lymphoma, MM multiple myeloma, NHL Non-Hodgkin’s lymphoma, MM multiple myeloma, NK natural killer, BCMA B-cell maturation antigen, IVIG intravenous immunoglobulin, mAb monoclonal antibody, inh inhibitor, SCT stem cell therapy, SD standard deviation, AE adverse effect, N/A not applicable, NR not reported.
a1 patient died after first dose of vaccine.
bOnly 14 patients had response assessed.
cControl group for entire cohort.
dData provided by authors.
eAllogeneic CAR-T was CD19-directed.
fEntire cohort of 55 patients.
gEntire cohort of 1445 patients.
h20 patients had response assessed.
IOnly 44 patients had humoral response assessed.
jExpanded cohort of 53 CAR-T recipients.
kExpanded cohort of 90 HSCT/CAR-T recipients.
l2 patients had response assessed after second vaccine dose.
mEntire cohort of 80 patients.
nEntire cohort of 116 patients.
oEntire cohort of 217 patients.
pTotal of 242 patients total assessed for inclusion.
qFrom vaccine efficacy analysis performed in a cohort of 200 patients.
r3.8% of cohort received an unknown vaccine type.
s19 patients had response assessed.
tEntire cohort of 320 patients.
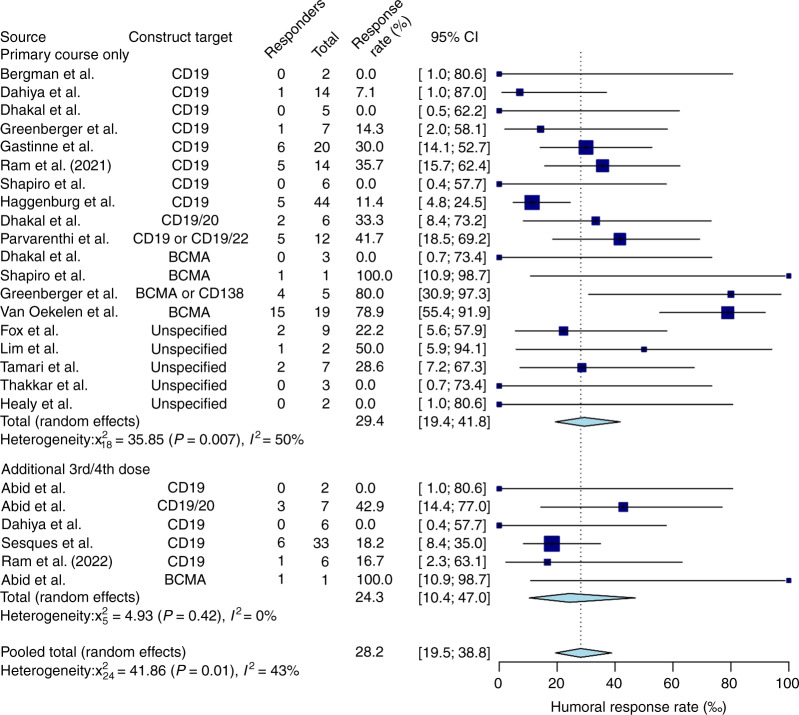
The pooled humoral response rate was 28.2% (61/236) (95% CI, 19.5–38.8%, I2 = 43%) among all 18 studies (Fig. 1). The vaccine response rate among patients who only completed their primary vaccine series, without a third or a fourth dose, was 29.4% (50/181) (95% CI, 19.4–41.8%, I2 = 50%) across 15 studies. The pooled seroconversion rate after receiving a third or fourth vaccine dose was 24.3% (11/55) (95% CI, 10.4–47%, I2 = 0%), reported in 4 studies (Supplementary Materials S7). Subgroup analysis comparing the recipients of a primary series of SARS-CoV-2 vaccines compared to third or fourth doses was not suggestive of a difference in humoral response rate with additional vaccine doses (p = 0.453) (Fig. 1).
Fig. 1. Pooled humoral response rates to SARS-CoV-2 primary vaccination series and additional doses in CAR-T recipients.
The Forest plot shows that the pooled humoral response rate was 28.2% (95%CI, 19.5-38.8%, I2=43%) among all 18 studies. The vaccine response rate among patients who only completed their primary vaccine series, without a third or a fourth dose, was 29.4% (95%CI, 19.4-41.8%, I2=50%) across 15 studies. After receiving a third or fourth vaccine dose, the pooled seroconversion rate was 24.3% (95%CI, 10.4-47%, I2=0%), reported in 4 studies (S7).
When stratified by CAR-T target antigen, recipients of any CD19-directed CAR-T, including dual-targeting CAR-T products, had an overall humoral response rate of 22.8% (25/130) (95% CI, 15.7–32%, I2 = 19%) to primary series across 9 studies (Supplementary Materials S8 and S10). On subgroup analysis restricted to CAR-T products that exclusively target CD19, the pooled humoral response rate was 19.3% (18/112) (95%CI, 12.6–28.4%, I2 = 10%) based on data from 8 studies (Supplementary Materials S9).
There was a significant difference in humoral response rates between recipients of CD19-directed CAR-T and BCMA- or CD138-directed CAR-T therapy recipients (p = 0.003). Multiple myeloma (BCMA- or CD138-directed) CAR-T recipients had a pooled vaccine response rate of 73.2% (20/28) (95% CI, 52.1-87.2%, I2 = 29%) across 4 studies (Supplementary Materials S8). When subgrouping only patients who received BCMA-targeting CAR-T therapy exclusively, the response rate was 71.9% (16/23) (95% CI, 48.9–87.3%, I2 = 51%) from 3 studies (Supplementary Materials S9).
This systematic review and meta-analysis estimate diminished humoral response rate among CAR-T therapy recipients. Irrespective of CAR-T construct, number of vaccine doses, or underlying disease, the pooled humoral response rate was 28.2% across 18 studies. Stratifying seroconversion rates by CAR-T target antigen demonstrated significantly blunted vaccine response rates in patients who received CD19-directed therapy (19.3%) compared to recipients of BCMA-directed products (71.9%). Though data are limited, booster doses of SARS-CoV-2 vaccines likely have a similar seroconversion rate in CAR-T patients [8]. Notably, most of these patients initiated their vaccinations 6 months after CAR-T (Table 1).
Despite the low seroconversion rates of additional vaccine doses reported in Dahiya et al., Abid et al., and Ram et al. (2022), our results suggest that boosters can be successful in CAR-T recipients in a targeted manner. As proposed by Tamari et al., who associated lower rates of seroconversion with a shorter interval of cellular therapy to vaccination, it is plausible that initial non-responders, who seroconverted after an additional vaccine dose, partly responded due to enhanced immune reconstitution with additional elapsed time from cellular therapy [9]. While we could not examine the impact of the CAR-T therapy and vaccination due to lack of granular data, Ram et al. (2021) reported that increased duration after allogeneic HCT to vaccination was a positive predictor of seroconversion to SARS-CoV-2 vaccination [7]. Currently, the American Society of Hematology (ASH) and the American Society of Transplantation and Cellular Therapy (ASTCT) also recommend that patients with HM, including HCT and CAR-T recipients, wait 3 or more months after cellular therapy before receiving a SARS-CoV-2 vaccine.
Interestingly, these results diverge from our initial hypothesis that BCMA+ CAR-T recipients are less likely to seroconvert in response to SARS-CoV-2 vaccines compared to recipients of CD19+ CAR-T cells [4]. Though both therapies eliminate antibody-producing B-cells, preservation of CD19+ populations may be critical in mounting humoral immune responses to SARS-CoV-2 vaccines, as suggested by the lower seropositivity rates among CD19-directed CAR-T recipients. Interestingly, CD19+ naïve B-cells and plasmablasts are essential to SARS-CoV-2 vaccine immunogenicity in immunocompromised hosts [10]. Conversely, BCMA-targeting therapies eliminate mature plasma cells responsible for long-term immunity [11]. While targeting BCMA eliminates preexisting immunity from vaccinations before CAR-T therapy, it may be less disruptive to post-treatment immunity than CD19-directed treatment.
The consequences of prolonged corticosteroids (cumulative exposure) on SARS-CoV-2 vaccine responses have been reported in patients with HM and those undergoing HCT (Supplementary Materials S10) [4, 6]. Both corticosteroid and immunomodulator usage has consistently been demonstrated to increase the risk of infections while diminishing vaccine responses [12].
Although the results are hypothesis-generating for larger-scale prospective studies, our study has several limitations. The CAR-T studies have significant heterogeneity that can be attributed to differences in antibody assays, and variable definitions of seroconversion that make direct comparisons between studies challenging. Other notable differences included variability in vaccine type, underlying disease, timing of vaccination post-CAR-T, intervals between vaccine doses and response assessment, previous/current oncological therapy, and limited reported clinical data. Additionally, we were unable to examine the association of B cell aplasia, partial B cell recovery, immunoglobulin administration, or T cell responses due to a lack of data in the original studies.
Overall, we synthesized available data and provided working knowledge of SARS-CoV-2 vaccine responses among CAR-T recipients stratified by construct targets and booster doses. As CAR-T recipients maintain remission with prolonged cytopenias and hypogammaglobulinemias, novel strategies are needed to prevent severe infections among this profoundly immunocompromised group. Among CD19+ CAR-T recipients, additional vaccine doses may not render adequate protection; hence, monoclonal antibodies may be utilized earlier during illness. Despite the persistently low response rates following third and fourth vaccine doses, the fact that previous vaccine non-responders can successfully seroconvert supports their continued administration. In this regard, larger prospective vaccine studies are needed to determine optimal vaccine dosing strategies for CAR-T recipients.
Supplementary information
Author contributions
BU performed the statistical analysis and wrote the manuscript; MAA collected and analyzed the data and wrote the manuscript; ES performed the literature search and collected the data; MBA designed the study, collected, and analyzed the data, and wrote the manuscript. All authors wrote, reviewed, and approved the final version of the manuscript.
Data availability
Data are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-022-01795-3.
References
- 1.Wei-Jie G, Wen-Hua L, Yi Z, Heng-Rui L, Zi-Sheng C, Yi-Min L, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:640. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–8. doi: 10.1016/J.JACI.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spanjaart AM, Ljungman P, de La Camara R, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–8. doi: 10.1038/S41375-021-01466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meir J, Abid MA, Abid MB. State of the CAR-T: risk of infections with chimeric antigen receptor T-cell therapy and determinants of SARS-CoV-2 vaccine responses. Transpl Cell Ther. 2021;27:973–87. doi: 10.1016/J.JTCT.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abid MA, Abid MB. SARS-CoV-2 vaccine response in CAR T-cell therapy recipients: a systematic review and preliminary observations. Hematol Oncol. 2021. 10.1002/HON.2957. [DOI] [PubMed]
- 6.Abid MA, Nunley L, Abid MB. Could Coronavirus Disease 2019 (COVID-19) render natural immunity to re-infections? A spotlight on the therapeutic pipeline. Front Immunol. 2020;11. 10.3389/FIMMU.2020.01294. [DOI] [PMC free article] [PubMed]
- 7.Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single-center prospective cohort study. Transplant Cell Ther. 2021;27:788–94. doi: 10.1016/J.JTCT.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abid MB, Rubin M, Ledeboer N, Szabo A, Longo W, Mohan M, et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell. 2022. 10.1016/j.ccell.2022.02.010. [DOI] [PMC free article] [PubMed]
- 9.Tamari R, Politikos I, Knorr DA, Vardhana SA, Young JC, Marcello LT, et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov. 2021;2:577–85. doi: 10.1158/2643-3230.BCD-21-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz E, Hodl I, Forstner P, Hatzl S, Sareban N, Moritz M, et al. CD19+IgD+CD27- Naïve B cells as predictors of humoral response to COVID 19 mRNA vaccination in immunocompromised patients. Front Immunol. 2021;0:5245. doi: 10.3389/FIMMU.2021.803742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walti CS, Krantz EM, Maalouf J, Boonyaratanakornkit J, Keane-Candib J, Joncas-Schronce L, et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight. 2021;6. 10.1172/JCI.INSIGHT.146743. [DOI] [PMC free article] [PubMed]
- 12.Abid MB. Early immunomodulators with CAR T-cell immunotherapy in the COVID-19 era. Lancet Oncol. 2022;23:16–8. doi: 10.1016/S1470-2045(21)00695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the corresponding author.