Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV2) and associated COVID-19 infection continue to impact patients globally. Patients with underlying health conditions are at heightened risk of adverse outcomes from COVID-19; however, research involving patients with rare health conditions remains scarce. The amyloidoses are a rare grouping of protein deposition diseases. Light-chain and transthyretin amyloidosis are the most common disease forms, often present with systemic involvement of vital organs including the heart, nerves, kidneys, and GI tracts of affected individuals. The Amyloidosis Program of Calgary examined 152 ATTR patients and 103 AL patients analyzing rates of vaccination, COVID-19 testing, infection outcomes, influence referrals, and excess deaths. Results showed 15 total PCR-confirmed COVID-19 infections in the tested population of amyloid patients, with a higher frequency of infections among patient with AL compared to the ATTR cohort (26.2% vs 5.1%). Four patients (26.6%) required hospital admission for COVID-19 infection, 2 ATTR, and 2 AL patients. Of the confirmed cases, 1 (0.07%) unvaccinated ATTR patient died of a COVID-19 infection. An excess of deaths was found in both the ATTR and AL cohorts when comparing pre-pandemic years 2018 and 2019 to the pandemic years of 2020 and 2021. The finding suggests that amyloidosis patients are likely at a high risk for severe COVID-19 infection and mortality, especially those of advanced age, those on an active treatment with chemotherapy, and those with concomitant B-cell or plasma cell disorder. The impact of virtual healthcare visits and pandemic measures on the excess of deaths observed requires further research.
Keywords: Amyloid, Light-chain amyloidosis, Transthyretin amyloidosis, COVID-19, SARS CoV2
Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV2) is a highly contagious microorganism causing coronavirus disease 2019 (COVID-19) [1]. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic [1]. Globally, there have been 500,186,525 confirmed cases of COVID-19, including 6,190,349 deaths, reported to the WHO in 223 countries around the world (as of April 14, 2022)[2]. In Alberta, Canada, 552,403 patients have had confirmed COVID-19 positive tests and 222,273 of which reside in Calgary (as of April 11, 2022)[3].
A correlation between the severity of COVID-19 and preexisting comorbidities, including malignancy and cardiovascular disease, has been largely described [4, 5]. Amyloidosis represents a group of diseases that are characterized by extracellular deposition of amyloid fibrils in organs and tissues [6]. These amyloid fibrils are composed of misfolded protein aggregates [6]. To date, nearly 40 amyloid precursor proteins have been identified and transthyretin (ATTR) and light-chain (AL) remain the most common [7]. Patients with amyloidosis are suspected to be at a higher risk for COVID-19 complications and mortality due to age, organ dysfunction, and immunosuppression secondary to plasma cell clone proliferation or the effects of immunosuppressive therapies [8]. Since December 2019, when COVID-19 emerged in Wuhan City and rapidly spread throughout China and around the world, data has been needed to better understand its impact on patients with chronic conditions and cancer patients, in particular, those with plasma cell disorders [9, 10].
Recently, the Amyloidosis Program of Calgary (APC) was established. This program provides specialized and comprehensive multidisciplinary care for patients with ATTR and AL amyloidosis and is predominantly organized out of the Tom Baker Cancer Centre (TBCC) hematology clinics and cardiac amyloidosis clinics at the South Health Campus in Calgary. Based on the above mentioned, we aimed to assess the impacts of COVID-19 in the management and outcomes of patients with AL and ATTR treated or seen via the APC.
Methods
Patients included in the ATTR and AL amyloidosis databases of the APC were evaluated. Briefly, this project included a cohort of patients over the age of 18 with a diagnosis AL or ATTR amyloidosis from January 2004 to the present day. This cohort was analyzed for frequency of vaccinations, COVID-19 testing, outcomes of COVID-19 infections, and methods of assessment.
A separate cohort of consecutive symptomatic newly diagnosed ATTR and AL patients from January 2019 to December 2021 was evaluated aiming to assess patterns of referrals and diagnosis. Approval for the review of these records was obtained from the Tom Baker Cancer Centre (TBCC) Institutional Review Board and Informed consent was obtained.
Statistics
Patient demographic variables have been summarized by appropriate central measures of tendency and dispersion. Two-sided Fisher exact test was used to test for differences between categorical variables. A P value of < 0.05 was considered significant. Excess deaths were estimated by comparing the raw number of patients who died in pre-pandemic years 2018 and 2019 compared to the pandemic years of 2020 and 2021. A measure that is more comparable across countries is the P-score, which calculates excess mortality as the percentage difference between the number of deaths in 2020 and 2021 and the average number of deaths in the same period in 2018 and 2019. Survival curves were constructed according to the Kaplan–Meier method and compared using the log-rank test. All statistical analyses were performed by using the SPSS 24.0 software.
Results
A total of 152 ATTR and 103 AL amyloid patients were evaluated. Clinical characteristics are summarized in Table 1. Patients in the ATTR group were generally older age males with a trend towards higher levels of troponin T, calcium, albumin, and creatinine. Most patients in the ATTR group were wild-type and had a higher prevalence of cardiac and nerve involvement when compared to AL amyloidosis patients (cardiac involvement 96.1% vs 59.2%; nerve involvement 34.9% vs 13.5; p < 0.001). In contrast, patients with AL amyloidosis were generally younger (median age of 66) and the majority had evidence of systemic AL involvement (84.4%). The AL patients exhibited a higher degree of kidney and GI involvement when compared to the ATTR group (kidney involvement 61.1% vs 0.0%; and GI involvement 17.4% vs 2.6%; p < 0.001).
Table 1.
Clinical characteristics of ATTR patients seen at the Amyloid Program of Calgary from 2014 to 2021
| Characteristic | ALL patients n = 255 |
ATTR patients n = 152 |
AL amyloidosis patients n = 103 |
Pvalue | |
|---|---|---|---|---|---|
| Median age (years, range) | 77 (40–97) | 82 (57–97) | 66 (40–82) | < 0.001 | |
| Gender, n (%) | < 0.001 | ||||
| Female | 64 (25.1%) | 19 (12.5%) | 45 (43.6%) | ||
| Male | 191 (74.9%) | 133 (87.5%) | 58 (56.3%) | ||
| 142 (93.4%) | Localized AL | ||||
| 16 (15.5%) | |||||
| 10 (6.6%) | Systemic AL | ||||
| 87 (84.4%) | |||||
| Median hemoglobin, range (g/L) | 133 (76–173) | 137 (76–172) | 126 (85–173) | 0.3 | |
| Median calcium, mmol/L | 2.34 (1.9–3.5) | 2.35 (1.9–3) | 2.32 (1.96–3.5) | 0.03 | |
| Median creatinine, μmol/L Median | 99 (48–671) | 100 (48–364) | 81 (48–671) | 0.04 | |
| Median eGFR (mL/min/1.73m2) | 59.5 (6–117) | 58 (12–100) | 64 (6–117) | 0.2 | |
| Median albumin, g/L | 33 (7–46) | 35 (7–46) | 29 (8–45) | < 0.001 | |
| Median LDH (U/L) | 221 (48–770) | 223 (60–549) | 205 (48–770) | 0.2 | |
| Median NTproBNP (ng/L) (0–300) | 2462 (40–62,951) | 2960 (40–40,650) | 1185 (50–62,951) | 0.3 | |
| Median hsTroponin T (0–13 ng/L) | 44 (13–357) | 49 (9–357) | 43 (13–337) | 0.04 | |
| Organ Involvement | |||||
| Heart | 207 (81.1%) | 146 (96.1%) | 61/103 (59.2%) | < 0.001 | |
| Kidney | 63 (24.7%) | 0 |
61/87 (70.1%) for Systemic AL only |
< 0.001 | |
| Nerve | 67 (26.2%) | 53 (34.9%) | 63/103 (61.1%) | < 0.001 | |
| Bladder | 4 (1.5%) | 2 (1.3%) | 0.3 | ||
| Gastrointestinal | 22 (8.6%) | 4 (2.6%) | 14/103 (13.5%) | < 0.001 | |
| 2 (1.9%) | |||||
| 18/103 (17.4%) | |||||
Abbreviations: eGFR, estimated glomerular filtration rate = hemoglobin; BMPC, bone marrow plasma cells; NTproBNP, N-terminal pro-b type natriuretic peptide
A total of 17 newly diagnosed cases of AL amyloidosis were seen in 2019, compared to 11 in 2020, and 15 in 2021. This equated to a 36% decrease of new AL cases seen in 2020 via the APC when compared to 2019 (representing new cases in Calgary not the province of Alberta). Within the ATTR cohort, similar numbers of new diagnoses were seen pre-pandemic and during the pandemic. A total of 33 ATTR patients were diagnosed in 2019, compared to 38 in 2020, and 27 in 2021 (representing new cases in Calgary not the province of Alberta).
COVID-19 vaccination
Of the 118 ATTR patients evaluable since the pandemic declaration, 11 (10%) were not vaccinated at the time of analysis. Of the 107 (90.6%) vaccinated ATTR patients, 103 (96.3%) received Cominarty (Pfizer) and 4 (3.7%) Spikevax (Moderna). Of the 60 AL patients evaluable since the pandemic declaration, 11 (18.3%) were not vaccinated. Of those 49 (81.6%) vaccinated AL patients, 40 (81.6%) received Cominarty (Pfizer) and 9 (18.4%) Spikevax (Moderna). At the time of analysis, 8 AL amyloid patients had received 2 vaccine doses; 37 patients had received 3 doses; 4 patients received 4 doses.
COVID-19 testing in ATTR and AL amyloidosis patients seen at APC
COVID-19 testing was performed for amyloidosis patients according to the Alberta Health Services (AHS) guidelines. The province of Alberta has one of the highest testing rates in the world. There have been 6, 825,883 PCR tests completed for 2,723,069 people of a total population of approximately 4.37 million [3]. Our analysis was based on PCR confirmed tests and did not include rapid antigen tests.
Of the 60 patients with active AL amyloid assessed since the time of pandemic declaration, 42 patients were tested for COVID-19 with a median of 2 COVID-19 swabs (range 1–8). Of the AL patients tested, 11 of 42 (26.2%) cases were positive for SARS CoV2. Of the 131 ATTR patients assessed during the pandemic, 78 patients were tested for SARS CoV2, and of those, 4 patients tested positive (5.1%). Of the 15 total amyloid patients who tested positive for COVID-19, 6 were unvaccinated; 1 patient had only one vaccine dose; 3 patients had 2 vaccine doses; 5 patients had received 3 doses of mRNA vaccine at the time of COVID-19 infection.
COVID-19 infections in ATTR and AL amyloidosis patients seen at APC
Clinical characteristics and outcomes of positive COVID-19 cases are presented in Table 2. Briefly, 1 of the 4 cases (25%) with ATTR died as a direct result of a COVID-19 infection. The patient was unvaccinated, had cardiac involvement, and was of advanced age. Two additional deaths were noted among patients in our analysis who tested positive for COVID-19; both deaths occurred 3 and 6 months after their COVID-19 infections, and the cause of death was secondary to decompensated heart failure. One male ATTR patient of advanced age who was partially vaccinated with 1 dose of vaccine. The second patient was a young woman with advanced-stage cardiac AL amyloid who was fully vaccinated. Of the 15 amyloid patients with confirmed COVID-19 infections, 6 patients did not receive any supportive treatment, 3 received Sotrovimab, 2 Dexamethasone, 1 Prednisone, and 3 patients were treated with combined Dexamethasone and Remdesivir therapy.
Table 2.
COVID-19 and amyloidosis
| Patient | Age/Gender AL type | Current treatment | Amyloid status at COVID- 19 infection | Time from diagnosis (Months) | Prior lines of thera py | Immunopa resis and ALC* at COVID-19 | Summary Status (alive/dead) Infection severity (Inf) Treatment (Trx) Vaccine status (Vax) |
|---|---|---|---|---|---|---|---|
| 1 | 65/MAL amyloidosis, Stage IIIA | CyBorD | PR | 6 | 0 | Yes (IgG and IgM low) ALC = 1.3 | Status: Alive |
| Inf: Mild | |||||||
| Trx: Sotrovimab | |||||||
| Vax:Postvaccinex3 | |||||||
| 2 | 46/FAL amyloidosis, Stage II | CyBorD | NR | 5 | 0 | Yes (IgG and IgA low) ALC = 2.6 | Status: Alive |
| Inf: Moderate | |||||||
| Trx: No treatment | |||||||
| Vax: Unvaccinated | |||||||
| 3 | 86/MAL amyloidosis, Stage II | Lenalidomide and dexamethasone | VGPR | 96 | 1 | Yes (IgG and IgM low) ALC = 0.9 | Status: Alive |
| Inf: Mild-Moderate | |||||||
| Trx: Sotrovimab | |||||||
| Vax: Postvaccinex3 | |||||||
| 4 | 64/FAL amyloidosis, Stage II | Daratumumab | VGPR | 36 | 1 | Yes (IgG and IgM low) ALC = 2.3 | Status: Alive |
| Inf: Mild | |||||||
| Trx: No treatment | |||||||
| Vax: Unvaccinated | |||||||
| 5 | 61/FAL amyloidosis, Stage II | None | VGPR | 36 | 1 | No ALC = 1.6 | Status: Alive |
| Inf: Mild | |||||||
| Trx: No treatment | |||||||
| Vax: Post vaccinex3 | |||||||
| 6 | 56/MAL amyloidosis, Stage II | Lenalidomide and dexamethasone | VGPR | 40 | 1 | No ALC = 1.6 | Status: Alive |
| Inf: Mild | |||||||
| Trx: No treatment | |||||||
| Vax: Unvaccinated | |||||||
| 7 | 76/MAL amyloidosis, Stage II and co-diagnosis of CLL | CyBorMe | VGPR | 12 | 0 | No ALC = 11 | Status: Alive |
| Inf: Severe. Pneumonia required hospital admission | |||||||
| Trx: Dexamethasoneand Remdesivir | |||||||
| Vax: Postvaccinex2 | |||||||
| 8 | 52/MAL amyloidosis, Stage II | CyBorD | NA | 1 | 0 | Yes (IgG low) ALC = 1.9 | Status: Alive |
| Inf: Moderate. Pneumonia. No hospitalization | |||||||
| Trx: Dexamethasone | |||||||
| Vax: Unvaccinated | |||||||
| 9 | 67/MAL amyloidosis, Stage IIIA | None | Untreated | < 1 | 0 | Yes (IgA and IgM low) ALC = 2.3 | Status: Alive |
| Inf: Moderate. Pneumonia required hospital admission | |||||||
| Trx: Sotrovimab | |||||||
| Vax: Postvaccinex2 | |||||||
| 10 | 62/FAL amyloidosis, Stage IIIB | None | Untreated | < 1 | 0 | Yes (IgA and IgM low) ALC = 1.3 | Status: Died 3 months post Covid-19 infection |
| Inf: Mild | |||||||
| Trx: Untreated | |||||||
| Vax: Postvaccinex2 | |||||||
| 11 | 79/F Localized AL amyloid (Lung) | None | Untreated | 0 | 0 | No ALC = 2.0 | Status: Alive |
| Inf: Mild-Moderate | |||||||
| Trx: Dexamethasone | |||||||
| Vax: Postvaccinex3 | |||||||
| 12 | 80/MATTR with cardiac and nerve involvement | Tafamidis | NA | 36 | 0 | Unknown ALC = 1 | Status: Alive |
| Inf: Moderate. Pneumonia required hospital admission | |||||||
| Trx: Dexamethasone and Remdesivir | |||||||
| Vax: Unvaccinated | |||||||
| 13 | 76/MATTR with cardiac involvement | Supportive Care | NA | 12 | 0 | Unknown ALC = 0.3 | Status: Died |
| Inf: Severe. Pneumonia required hospital admission | |||||||
| Trx: Dexamethasose and Remdesivir | |||||||
| Vax: Unvaccinated | |||||||
| 14 | 87/MATTR with cardiac involvement | Tafamidis | NA | 24 | 0 | No ALC = 1.4 | Status: Alive |
| Inf: Mild | |||||||
| Trx: No treatment | |||||||
| Vax: Postvaccinex3 | |||||||
| 15 | 89/MATTR with cardiac involvement | Supportive Care | NA | 12 | 0 | Unknown ALC = 2.3 | Status: Died 6 months after Covid-19 infection |
| Inf: Moderate. Pneumonia. No hospitalization | |||||||
| Trx: Prednisone | |||||||
| Vax: Postvaccinex1 |
*Absolute lymphocyte count (ALC) measured in 10E9/L
Of the 15 total patients who tested positive, 4 required hospital admission for COVID-19 infection; 2 patients with AL amyloid and 2 with ATTR. The AL amyloid patients who required hospital admission had both received 2 doses of a COVID-19 mRNA vaccine and both the ATTR patients who required admission were unvaccinated. Of the hospitalized AL patients, 1 was not on active treatment and the second was receiving chemotherapy with CyBorMe and had a concomitant diagnosis of chronic lymphocytic leukemia. Of the hospitalized ATTR patients, both had cardiac involvement and 1 was actively being treated with Tafamidis.
Survival outcomes
At the time of analysis, 57 out of 87 systemic and 16 out of 16 localized AL amyloidosis patients were alive (57.5% and 100%). Of the ATTR cohort, 106 of the 152 (69.7%) patients were alive. Out of the 15 patients with COVID-19 infection, 1 died of COVID-19–related complications and 2 succumbed a few months after the diagnosis of COVID-19 due to cardiovascular complications related to cardiac amyloidosis.
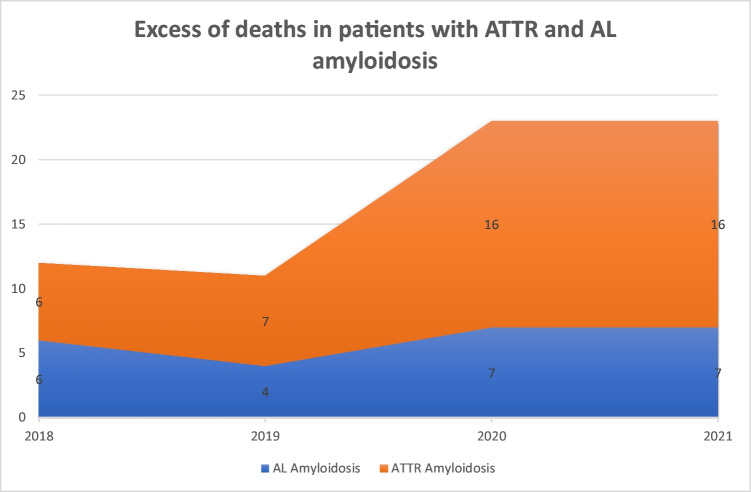
With regard to excess of deaths (Fig. 1), 6 ATTR patients died in 2018, 7 in 2019, 16 in 2020, and 16 in 2021. None of the 16 patients that died in 2020 were vaccinated, whereas 11 of the 16 that died in 2021 had received a mRNA vaccine. Of those that died in 2021, 5 received only 1 vaccine dose, 3 received 2 doses, and 3 received 3 doses of an mRNA COVID-19 vaccine.
Fig. 1.
Excess mortality for patients with AL and ATTR amyloidosis
This represents an excess of deaths of 128% (P-score) among the ATTR cohort when comparing pre-pandemic years 2018 and 2019 to the pandemic years of 2020 and 2021. In the AL cohort, 6 patients died in 2018, 4 in 2019, 7 in 2020, and 7 in 2021. None of the patients that died on 2020 were vaccinated, whereas 5 of the 7 that died in 2021 had received a mRNA vaccine. Of those that died in 2021, 3 received 1 dose of mRNA vaccine, 1 received 2 doses, and 1 received 3 doses before they died. This represents an excess of deaths of 75% in 2020 and 2021 when compared to 2019, and a 16% excess of deaths when compared to 2018 (P-score).
Method of patient assessment and evaluation
After March 11, 2020, when WHO declared the pandemic [1], the TBCC initiated a telemedicine program. All AL patients seen via the hematology clinics at TBCC from January to February of 2020 were assessed as an in-person clinic visit. In March 2020, 24% of multiple myeloma and AL patients were assessed virtually (92 out of 382 visits), increasing to 70% in April of 2020 (377 out of 536 visits). All virtually assessed patients were contacted by phone and laboratory testing was restricted to local laboratory facilities where the immunocompromised patients were offered reserved spots by the Alberta Health Services. As the first wave improved, in-person appointments increased; however, with the advent of the second COVID-19 wave in Alberta, up to 60% of cases were again assessed virtually (380 out of 626 visits in December of 2020). All patients not on active treatment were evaluated with phone consults and laboratory testing was delayed at the peak of the outbreak.
Discussion
SARS-CoV2 is known to put individuals with chronic conditions at higher risk of adverse health outcomes [4, 5, 12]; although minimal data currently exists on the risks and impacts SARS-CoV2 has had on patients with rarer conditions, such as amyloidosis [8]. Disease manifestations and impacts on immune function can vary widely between different types of amyloid, adding to the challenge of managing these complex patients within the constraints of the pandemic. Additionally, with attempts to mitigate risks of exposure, many health agencies around the globe have utilized telemedicine and virtual approaches to care, of which, the long-term influences have not been fully understood [8, 11]. Our analysis of AL and ATTR amyloid patients seen via the APC provides much-needed data and insight on the impact that COVID-19 vaccination, testing, and infections have had on amyloidosis patients and the delivery of care throughout the pandemic.
Given patients with underlying chronic health conditions are at high risk of adverse outcomes from SARS-CoV2 infection [4, 5, 12], the COVID-19 vaccination was recommended for all of our amyloid patients [13]. Vaccine uptake in the analyzed cohort of amyloid patients was similar to that of the vaccinated population in Alberta, of which 86% of the population above 12 years of age has had 2 or more vaccine doses [3]. On subgroup analysis, the AL amyloid cohort had a higher percentage of unvaccinated patients when compared to the ATTR cohort and the general Alberta population (Al = 18.3%; ATTR = 10%; AB pop = 13.3%). Consequently, patients in the AL cohort had a higher incidence of positive COVID-19 infections when compared to the ATTR cohort (26.2% versus 5.1%). Given the difference in pathogenesis and formation of amyloid fibrils between AL and ATTR amyloid [6, 14], vaccination rates alone likely do not reflect the difference in infection rates seen between these groups. On further analysis, only 4 of the 11 AL amyloid patients with confirmed COVID-19 infections were unvaccinated. As AL amyloidosis is precipitated by the proliferation of monoclonal immunoglobulin light-chain fibrils arising in the bone marrow [14], likely, plasma cell dysfunction could also increase AL amyloid patients’ risk of infection and dampened vaccine responses [13, 15].
Sixty-four percent of the AL amyloid patients in our cohort who tested positive for COVID-19 were on active treatment with chemotherapy. Mixed vaccine immune responses have been noted in patients receiving cancer treatments with chemo-immunotherapy, and patients with a hematologic malignancy in particular may not mount a protective vaccine response [13, 15, 16]. Although there are currently no published data looking at vaccine response in larger cohorts of amyloid patients, Salton and colleagues analyzed a cohort of myeloma (n = 178) and AL amyloid patients (n = 8) concerning vaccine responses [15]. Salton et al. noted dampened humoral immune responses after 2nd dose of mRNA vaccine from patients who were older, on active therapy (especially an anti-CD38 agent), or were heavily pre-treated [15]. Given the increased frequency of AL amyloid patients being treated with chemo-immunotherapy, it remains likely that despite vaccination, AL amyloid patients may not mount a protective immune response predisposing them to a higher risk of adverse outcomes from COVID-19 infection [8, 15].
With attention to the severity of infection, equal numbers of ATTR and AL amyloid patients required hospital admission. All of the patients who required hospital admission had cardiac amyloid involvement. One older patient with ATTR amyloid, a suppressed lymphocyte count, and unvaccinated status, died as a direct result of COVID-19 infection. The two other patients in our analysis died months after COVID-19 infections from decompensated heart failure, both having had preexisting cardiovascular amyloid involvement. Although COVID-19 infection was not the direct cause of death in these cases, it could have been a precipitating factor. Notably, SARS-CoV2 has a high affinity for angiotensin converting enzyme 2 (ACE2) and is thought to affect patients’ microvascular function by damaging endothelial cells [17]. Lasting cardiovascular complications including dysrhythmias, ischemia, and heart failure, have been described up to 12 months post COVID-19 infection [18]. Our data on the severity of infection adds to current evidence that patients with cardiovascular disease, including cardiac amyloidosis, are at higher risk for adverse outcomes and more severe COVID-19 infections [5, 17, 18].
During the initial phase of the pandemic, recommendations were made by Alberta Health Services to adopt a virtual approach to health care where appropriate. Within the province of Alberta, similarly to other countries, there was a widespread shift to telemedicine. During the infection peaks, the majority of patients at the cancer center were assessed virtually. The main goal of switching to virtual visits was to decrease infection spread in hopes of protecting both the compromised patients and the front-line healthcare staff during the height of the pandemic. Additionally, during the peak of infections in Calgary, the province of Alberta had initiated measures to reduce the spread including suspending non-urgent surgeries, limiting socialization to a person’s immediate household, recommendations to avoid non-essential travel, limiting support person and visitors to hospitals and long-term care facilities, and triaging access to diagnostics for a narrower cohort. The full impact of this shift to virtual healthcare is still being uncovered; however, these pandemic measures have undoubtedly played a role in access to healthcare services, as noted in multiple other cancer care studies [8, 19, 20].
Diagnosis of amyloidosis, especially AL amyloid, is often delayed given the wide range of clinical presentations and rarity of the condition [8, 21]. The incidence of AL amyloid has been described as 8–12 new cases per million person-years [22, 23] and when a diagnosis is established, patients often have systemic involvement or advanced disease, highlighting the importance of early diagnosis and prompt management [21]. Our analysis noted a 36% decrease in newly diagnosed AL amyloid patients in the year 2020 when compared to 2019. While there was no significant change in diagnosis of ATTR, this is in the context of increasing prevalence rates worldwide and therefore may represent a relative decrease [24]. It is challenging to pinpoint the cause of this decrease; however, it could be explained, in part, by the broad shift to telemedicine and the impact virtual visits could have on the delayed diagnosis of rarer conditions that require more detailed physical exams [8]. Additionally, a diagnosis of amyloid typically requires an extensive workup including advanced imaging, biopsies, and multiple organ function tests which could have been delayed or postponed during the height of the pandemic [6, 8, 11].
An excess of deaths was noted in both the ATTR cohort and the AL cohort when comparing pre-pandemic years to the pandemic years of 2020 and 2021. Excess of deaths was higher among the ATTR cohort, which, could be partially explained by the advanced age of the patients in this group. Vaccine status did not have an obvious impact on the excess of deaths observed. All patients who died in 2020 were unvaccinated; however, the majority of deceased AL and ATTR patients in 2021 received 1 or more vaccine doses. Overall, the excess of deaths observed is likely multifactorial and contributing factors could include virtual heath visits and pandemic measures to “flatten the curve.” It could be argued that virtual visits played a protective role, preventing further COVID-19 exposures, possible subsequent mortality, and further inflation of excess deaths noted. Alternatively, virtual visits and pandemic measures could have delayed amyloid patients from seeking healthcare services for fears of exposure, leading to either delayed diagnosis or disease progression [8] possibly contributing to the excess deaths noted. Additionally, the pandemic measures likely affected vulnerable elderly patients, of which our ATTR cohort mostly comprised, by limiting their access to support persons to help care for their health needs and from which to obtain collateral information. It is challenging to characterize completely the excess of deaths we noted during the pandemic and long-term research of pandemic measures on patient outcomes and mortality will be necessary.
Our study did have limitations. This analysis included patients seen via the APC, which is the first amyloid program in Canada. Given this, referral patterns and excess deaths may be under-representative of the true amyloid population in more rural settings and areas without a dedicated amyloid program. Additionally, vaccination rates, testing access, and pandemic measures differ widely between counties [2]. As such, the vaccination rates and COVID-19 outcomes we described may not be generalizable to other areas of the world due to lack of access. Future research will be necessary to analyze the long-term impacts of virtual healthcare and pandemic measures on patients, especially those with rarer conditions. Future data will be needed to assess vaccine efficacy and outcomes of infection in the advent of COVID-19 variants.
Conclusion
The pandemic has dramatically changed the landscape of healthcare and, 2 years post its declaration, the impacts of the SARS CoV2 virus are still being uncovered. COVID-19 data on patients with rare underlying conditions, such as amyloidosis, is limited. Given this, the APC sought to increase knowledge and understanding of the impacts COVID-19 has had on ATTR and AL amyloid patients. Our analysis notes that the majority of AL and ATTR patients have risk factors, which contribute to the increased severity of COVID-19 infection and mortality. AL and ATTR patients are often of advanced age, have underlying cardiovascular compromise, and have higher rates of concomitant plasma cell disorders. Additionally, virtual healthcare and pandemic measures to “flatten the curve” may have played a role in the decreased referrals for AL patients and the excess of deaths we observed. Future research analyzing the long-term influences of the pandemic and the outcome of COVID-19 infections in amyloidosis patients is necessary to fully appreciate the impact for individuals with this rare disorder.
Author contribution
EL and VJZ performed the research; collected, analyzed, and interpreted the data; performed the statistical analysis; and wrote the manuscript, and NF, RM, CH, SC, EM, SM, PN, PD, JT, and NB designed the research, analyzed and interpreted the data, and wrote the manuscript.
Declarations
Ethics approval and consent to participate
Approval for the review of these records was obtained from the TBCC Institutional Review Board and informed consent was also obtained. This study was performed in accordance with the ethical standards of TBCC research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from patients to participate in this project and our institutional database.
Conflict of interest
Dr. Miller has received research support and consulting fees from Pfizer. Dr. Hahn has received speaker’s fees from Alnylam and Sobi. The remaining authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coronavirus disease 2019 (COVID-19) situation report-51. World Health Organization. March 11, 2020. Accessed April 15, 2022. 20200311-sitrep-51-covid-19.pdf (who.int)
- 2.WHO Coronavirus (COVID-19) Dashboard. World Health Organization. Accessed April 15, 2022. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data
- 3.COVID-19 Alberta Statistics: interactive aggregate data on COVID-19 cases in Alberta. Government of Alberta. Updated April 4, 2022. Accessed April 15, 2022. COVID-19 Alberta statistics | alberta.ca
- 4.Beaumont AL, Vignes D, Sterpu R, et al. Factors associated with hospital admission and adverse outcome for COVID-19: role of social factors and medical care [published online ahead of print, 2022 Feb 13]. Infect Dis Now. 2022;S2666–9919(22)00032-X. 10.1016/j.idnow.2022.02.001 [DOI] [PMC free article] [PubMed]
- 5.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 7.Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2020;27(4):217–222. doi: 10.1080/13506129.2020.1835263. [DOI] [PubMed] [Google Scholar]
- 8.Kastritis E, Wechalekar A, Schönland S, Sanchorawala V, Merlini G, Palladini G, Minnema M, Roussel M, Jaccard A, Hegenbart U, Kumar S, Cibeira MT, Blade J, Dimopoulos MA. Challenges in the management of patients with systemic light chain (AL) amyloidosis during the COVID-19 pandemic. Br J Haematol. 2020;190:346–357. doi: 10.1111/bjh.16898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brannagan TH, 3rd, Auer-Grumbach M, Berk JL, et al. ATTR amyloidosis during the COVID-19 pandemic: insights from a global medical roundtable. Orphanet J Rare Dis. 2021;16(1):204. doi: 10.1186/s13023-021-01834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broom A, Kenny K, Page A, Cort N, Lipp ES, Tan AC, Ashley DM, Walsh KM, Khasraw M. The paradoxical effects of COVID-19 on cancer care: current context and potential lasting impacts. Clin Cancer Res. 2020;26(22):5809–5813. doi: 10.1158/1078-0432.CCR-20-2989. [DOI] [PubMed] [Google Scholar]
- 12.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buske C, Dreyling M, Alvarez-Larrán A, et al. Managing hematological cancer patients during the COVID-19 pandemic: an ESMO-EHA interdisciplinary expert consensus. ESMO Open. 2022;7(2):100403 . doi: 10.1016/j.esmoop.2022.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 15.Schiller Salton N, Szwarcwort M, Tzoran I, et al. Attenuated humoral immune response following anti-SARS-CoV-2 vaccine in heavily pretreated patients with multiple myeloma and AL amyloidosis. Am J Hematol. 2021;96(12):E475–E478. doi: 10.1002/ajh.26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths EA, Segal BH. Immune responses to COVID-19 vaccines in patients with cancer: promising results and a note of caution. Cancer Cell. 2021;39(8):1045–1047. doi: 10.1016/j.ccell.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Li X, Chen M, Feng Yi, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maringe Camille, Spicer James, Morris Melanie, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2021;21(8):1023–1034. doi: 10.1016/s1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCausland KL, White MK, Guthrie SD, et al. Light Chain (AL) Amyloidosis: the journey to diagnosis. Patient. 2018;11(2):207–216. doi: 10.1007/s40271-017-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79(7):1817–22. doi: 10.1182/blood.V79.7.1817.1817. [DOI] [PubMed] [Google Scholar]
- 23.Pinney JH, Smith CJ, Taube JB, Lachmann HJ, Venner CP, Gibbs SD, et al. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161(4):525–532. doi: 10.1111/bjh.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winburn I, Ishii T, Sumikawa T, Togo K, Yasunaga H. Estimating the prevalence of transthyretin amyloid cardiomyopathy in a large in-hospital database in Japan. Cardiol Ther. 2019;8(2):297–316. doi: 10.1007/s40119-019-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]