Abstract
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can affect multiple organs. Reports of persistent or newly emergent symptoms, including those related to the nervous system, have increased over the course of the pandemic, leading to the introduction of post-COVID-19 syndrome. However, this novel syndrome is still ill-defined and structured objectification of complaints is scarce. Therefore, we performed a prospective observational cohort study to better define and validate subjective neurological disturbances in patients with post-COVID-19 syndrome.
Methods
A total of 171 patients fulfilling the post-COVID-19 WHO Delphi consensus criteria underwent a comprehensive neurological diagnostic work-up including neurovascular, electrophysiological, and blood analysis. In addition, magnetic resonance imaging (MRI) and lumbar puncture were conducted in subgroups of patients. Furthermore, patients underwent neuropsychological, psychosomatic, and fatigue assessment.
Results
Patients were predominantly female, middle-aged, and had incurred mostly mild-to-moderate acute COVID-19. The most frequent post-COVID-19 complaints included fatigue, difficulties in concentration, and memory deficits. In most patients (85.8%), in-depth neurological assessment yielded no pathological findings. In 97.7% of the cases, either no diagnosis other than post COVID-19 syndrome, or no diagnosis likely related to preceding acute COVID-19 could be established. Sensory or motor complaints were more often associated with a neurological diagnosis other than post-COVID-19 syndrome. Previous psychiatric conditions were identified as a risk factor for developing post-COVID-19 syndrome. We found high somatization scores in our patient group that correlated with cognitive deficits and the extent of fatigue.
Conclusions
Albeit frequently reported by patients, objectifiable affection of the nervous system is rare in post-COVID-19 syndrome. Instead, elevated levels of somatization point towards a pathogenesis potentially involving psychosomatic factors. However, thorough neurological assessment is important in this patient group in order to not miss neurological diseases other than post-COVID-19.
Keywords: Post-COVID, Long COVID, COVID-19, Neurological deficits, Neuropsychological assessment, Somatization, Fatigue
Key Summary Points
| Why carry out this study? |
| To validate subjective neurological complaints in patients with post-COVID-19 by applying a comprehensive neuro-psychiatric diagnostic workup. |
| What was learned from the study? |
| The nervous system is rarely affected in patients with post-COVID-19 syndrome. |
| Psychosomatic factors probably contribute to the pathogenesis of post-COVID-19 syndrome. |
| Patients presenting with post-COVID-19 should be thoroughly assessed in order to not miss other diagnoses. |
Introduction
Since the first description of the coronavirus disease 2019 (COVID-19), more than 500,000,000 infections with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been verified as of May 2022 [1]. Initially thought to mainly present as respiratory tract infection, COVID-19 has been identified as a multiorgan disease, among others, affecting the kidneys, the heart, and the nervous system in many patients [2].
Following the acute infection, symptoms in some individuals persist while others develop new symptoms, a phenomenon that the World Health Organization (WHO) had recently named post-COVID-19 condition or ‘long’ COVID-19 [3]. Even though varying definitions exist for these two terms, they are used equivalently in clinical practice. Reported frequency of post-COVID-19 syndrome ranges between 10 and 35% [4, 5]. However, these numbers mainly have been collected during the pre-vaccination era and at a time when the alpha and delta variants of SARS-CoV-2 were still dominant. Whether they also apply to the omicron variant of the virus or vaccinated individuals remains unknown. Recent studies suggest that post-COVID-19 syndrome can also occur following infection with omicron [6] or even as a result of the vaccination against the corona virus [7]. Interestingly, even asymptomatic individuals or those with only mild COVID-19 symptoms seem to be at risk of developing post-COVID-19 syndrome. Of note, even children can develop post-COVID-19 syndrome, although the risk appears to be lower compared to adults. However, with the increased transmissibility of novel SARS-CoV-2 variants, pediatric post-COVID-19 might occur more frequently [2, 8].
According to previous descriptions, disturbances related to nearly every organ system can occur in individuals with post-COVID-19 syndrome, making the classification of this symptom complex and a challenging task in clinical practice [5, 9, 10]. However, neurological and/or neuropsychological deficits such as memory loss, “brain fog”, fatigue, dizziness, headaches, or generalized pain [5] are frequent complaints, thus placing the nervous system at the center of interest when studying this condition.
The pathophysiological mechanisms triggering post-COVID-19 are still under debate. Several groups have suggested virus persistence, long-lasting overactivation of the immune system including autoimmune phenomena or ongoing thrombus formation in the microvasculature as potential factors [11]. With respect to the nervous system, distinct patterns of brain atrophy have recently been reported in post-COVID-19 patients suffering from impaired cognition [12] and cerebral spinal fluid (CSF) analyses suggested dysfunctional inflammatory processes as the underlying cause of the disease [13].
However, the clinical significance of these often selective findings-in relation to the broad and heterogeneous symptomatology—remains unclear, and so does the validity of the diagnostic tests performed, many of which can be considered rather experimental or not established in routine practice [14, 15]. Therefore, an increasing number of research groups have recently stressed the potential importance of psychosomatic factors in the emergence and perpetuation of post-COVID-19 syndrome [16, 17]. This idea is further supported by similarities between post-COVID-19 syndrome and the presentation of posttraumatic distress syndrome, depression, anxiety disorder, or poorly defined disease entities such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [18, 19]. Coherently, supportive psychotherapy appears to be effective in alleviating the symptoms of post-COVID-19 [20].
In an attempt to further put into context the manifold complaints brought forward by post-COVID-19 patients, we performed a prospective observational cohort study encompassing a comprehensive neurological diagnostic work-up.
Methods
Study Cohort
The study was conducted in accordance with the Declaration of Helsinki. A total of 171 participants fulfilling the WHO Delphi consensus criteria for post-COVID-19 syndrome [4] were included in this prospective observational cohort study. Individuals were recruited from the post-COVID-19 outpatient center at the Department of Neurology, University Medicine Essen, Germany between January 2021 and February 2022.
The cohort represents a typical “real-world” cohort in a German tertiary COVID-19 and neurology center. A broad range of patients with post-COVID-19 syndrome including those with previous cerebrovascular disease has been included into our study. Patients with comorbidities, in particular cerebrovascular disease, did not present any severe physical or mental deficits at the time of study entry and all patients were able to undergo the full set of diagnostic tests including neurocognitive assessment.
All patients were of legal age and gave written informed consent prior to study inclusion. The study was approved by the local ethics committee of the University Duisburg-Essen, Germany (reference numbers 20-9284-BO, 20-9307-BO).
Diagnostics
Information on demographics, previous medical history, the severity of the preceding COVID-19 infection, and the profession were collected via structured interviews. All patients underwent full neurological and physical examination according to standards recommended by the European Academy of Neurology [21]. If clinically indicated or if more specific complaints were brought forward by the patient, additional diagnostics such as MRI or lumbar puncture were performed to substantiate or exclude diagnoses.
In 171 patients, extensive nerve conduction studies were performed (tibial nerve, sural nerve, median and ulnar nerve), recording motor distal latency, motor conduction velocity, compound muscle action potential amplitude, F-waves, temporal dispersion and compound muscle action potential duration in motor nerves and sensory nerve action potential amplitude, and conduction velocity in sensory nerves. Furthermore, sensory and motor evoked potentials (SEP, MEP), blink reflex, and masseter reflex were conducted. Electrophysiological studies were expanded at the physicians’ discretion (n = 12 patients). Ultrasound examination of the extra- and intracranial head and neck arteries was performed in 76 patients. In 41 patients, clinical findings justified brain MRI and nine patients underwent lumbar puncture. In addition, all patients received pulmonary function tests (vital capacity, forced 1-s capacity) using a handheld spirometer (Micro Spirometer, Vitalograph), and peripheral oxygen saturation was measured using a finger clip (Pulox, PO-200). Blood was collected from all patients and analyzed for routine parameters, full blood count, and analysis of inflammatory parameters.
Neuropsychological Assessment
Patients (n = 146) underwent structured neuropsychological testing by trained personnel. Patients were asked for self-perceived current cognitive status and subjective cognitive decline after COVID-19. A standardized cognitive assessment was performed using the following neuropsychological tests: word list of ten words to assess immediate and delayed verbal memory [22], d2 test to assess selective attention [23], verbal fluency (words with the letter “S” at the beginning) as executive function [24], Trail Making Test A to assess processing speed and Trail Making Test B to assess cognitive flexibility as executive function [25], digit span forward to assess verbal short-term memory and digit span backwards to assess verbal working-memory [26] and symbol digit modalities test to assess attention, processing speed, visual scanning and memory [27].
Results were transferred to z-scores using the age-appropriate mean and standard deviation (SD) of the corresponding test. If the performance was more than 1 SD below the mean, it was considered impaired. Results were depicted as a radar plot to visualize the proportion of individuals with impaired results.
Assessment of Fatigue and Somatization
In addition, study participants completed the German adaptation of the Fatigue Inventory Scale (FIS, n = 128) to evaluate fatigue [28, 29]. FIS scores 40 items, ranging from 0 (no problem) to 4 (severe problem), adding up to a maximum score of 160. In addition, subdimensions for cognitive, physical, and psychosocial domains were calculated.
The severity of individual somatic symptoms was evaluated using the Patient Health Questionnaire-15 (PHQ-15, n = 128). The survey is a valid and reliable screening tool for detecting patients at risk for somatoform disorders [21] and requests 15 symptoms, accountable for more than 90% symptoms reported in somatoform disorders. PHQ-15 also records severity of somatization [30], scoring from 0 (“not bothered at all”) to 2 (“bothered a lot”) [31].
Statistical Analysis
All statistical analyses were performed using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. IBM Corp. Armonk, NY, USA).
The two-step clustering method was performed to identify underlying clusters of complaints using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. IBM Corp. Armonk, NY, USA). Clusters were identified using log-likelihood distance measure and Schwarz’s Bayesian clustering criterion.
Odds ratios (OR) for the complaints were calculated and Cramer's V test for statistical significance was performed. We used logistic regression analyses to examine the association of risk factors (“no” as reference) with patients’ symptoms resulting in odds ratios (OR) and 95% confidence interval (CI). Comparison of means was performed using Mann–Whitney U test, and analysis of multiple groups was performed using Kruskal–Wallis ANOVA with Dunn’s post hoc test after testing for parametric distribution with Shapiro–Wilk test. Influencing factors on somatization (PHQ-15) and cognitive dimensions were identified with linear regression calculation with the method enter. Correlations were analyzed by bivariate correlation and spearman’s rho. The level of significance was set at p < 0.05.
Results
Cohort Demographics
The demographics of the patient cohort are summarized in Table 1. The cohort comprised 171 patients with a mean age of 45.2 ± 12.7 years; 66.7% of the study participants were of female sex; 75.3% of the patients visited our department within 7 months after the diagnosis of acute COVID-19. The average time between diagnosis of acute COVID-19 and study entry was 4.0 months ± 1.5 months; 32.5% of participants were employed in the sector of public education, thereby representing the largest group; 29.9% were working in administration and service, while 18.8% held positions within the healthcare system. Skilled trade workers or individuals employed in the field of science and research were less frequently represented in our cohort (2.6%); 48.5% of the study participants had an academic degree or A level educational degree (Table 1).
Table 1.
Demographics, severity of COVID-19, and medical history of patients
| Demographics | |
| Participants | 171 |
| Age, years | 45.2 ± 12.7 (18–74) |
| Female | 66.7% |
| Severity COVID | % |
| Mild | 34.5 |
| Moderate | 64.9 |
| Severe | 0.6 |
| Treatment | % |
| No professional medical care | 94.1 |
| Hospitalized without ventilation | 5.3 |
| Required mechanical ventilation | 0.6 |
| Medical history | % |
| Previous cardiovascular condition (arterial hypertension (86%), myocardial infarction (4%), other heart diseases (10%)) | 28.3 |
| Previous neurological conditions (Migraine (44%), stroke (16.3%), MS (4.1%), PNP (8.2%), CPS (8.2%), epilepsy (4.1%), post-infectious fatigue (2%), others (12.2%)) | 29.0 |
| Previous psychiatric preconditions (Depression (66.7%), anxiety disorder (20.1%), post-traumatic stress disorder (3.3%), somatic disorder (3.3), adjustment disorder (3.3%), borderline disorder (3.3%)) | 19.0 |
| Allergies | 5.0 |
| Diabetes | 3.0 |
| Previous oncological conditions | 3.1 |
| Professions | % |
| Nursery/teaching | 32.5 |
| Administration/Professional service | 29.9 |
| Healthcare | 18.8 |
| Craft/Construction | 9.4 |
| Student/further education | 6.8 |
| Academic sector | 2.6 |
| Educational level | % |
| Academic degree | 22.8 |
| A-level degree | 25.7 |
| Secondary school certificate | 17.0 |
| Vocational school certificate | 7.0 |
| Others | 1.8 |
| Missing | 25.7 |
MS multiple sclerosis, PNP polyneuropathy, CPS carpal tunnel syndrome
The severity of the preceding acute SARS-CoV-2 infection was rated according to the COVID-19 severity classification of Buonseno et al. [32]; 34.5% of the participants had suffered from mild, 64.9% from moderate, and 0.6% from severe COVID-19; 94.1% required no professional medical care and no specific antiviral or anti-inflammatory treatment over the course of COVID-19; 5.3% were hospitalized without requiring ventilation, and only one study participant (0.6%) was mechanically ventilated; 29.0% of the participants suffered from neurological comorbidities such as migraine, stroke, or epilepsy. One patient had experienced cerebral ischemia during acute COVID-19 and one patient had a history of fatigue following Epstein–Barr virus (EBV) infection. However, the cohort did not comprise patients with previous post-infectious disorders such as Guillain–Barré syndrome (GBS) or myelitis, which could have been reactivated after SARS-CoV-2 infection; 28.3% had preceding cardiovascular conditions (e.g., hypertension, history of heart attack), and 19.0% had psychiatric comorbidities including depression, anxiety disorder, post-traumatic stress disorder, or somatic disorder (Table 1). Importantly, participants with comorbidities did not present any severe physical or mental deficits at the time of study entry and all participants were able to undergo the full set of diagnostic tests including neurocognitive assessment.
Timeline of SARS-CoV-2 Infections in the Cohort
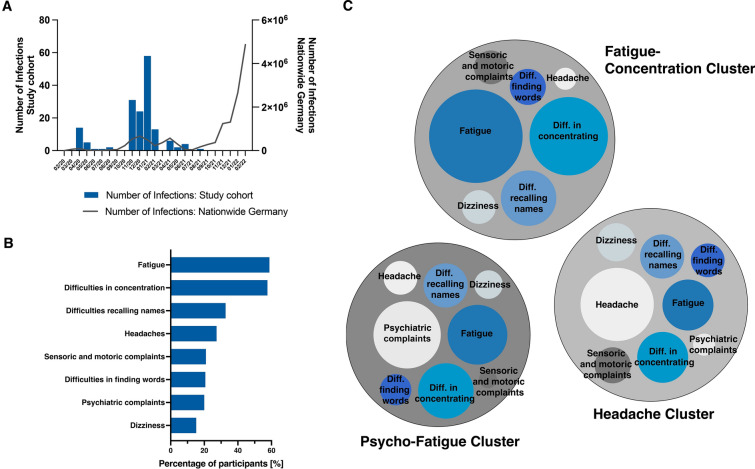
First of all, we compared the SARS-CoV-2 infection kinetics of our study participants with the overall infection kinetics in Germany (Fig. 1A) [33]. We found that all participants had been infected during the first two infection waves in Germany between March 2020 and August 2021. This indicates that study patients had been contaminated with the alpha to delta variants of SARS-CoV-2 since the omicron variant did not emerge in Germany before November 2021 [34]. At the time most of the participants were recruited to our study, routine sequencing of the viral genome in order to determine the specific variant had not yet been routinely established in Germany. Moreover, due to the prioritization strategy followed by German healthcare authorities, vaccination against SARS-CoV-2 was not available in Germany before January 2021 [35]. To the broader public, i.e., younger individuals without active comorbidities, similar to the participants from our cohort, vaccination was not available before summer 2021 and we did not routinely assess the vaccination status of our patients.
Fig. 1.
Timeline of SARS-CoV-2 infections and self-reported complaints. A Number of SARS-CoV-2 infections per month of the study participants in comparison to the different SARS-CoV-2 infection waves in Germany. All study participants had been infected during the first two infection waves in Germany between March 2020 and August 2021. B Frequency of complaints reported by patients with fatigue and difficulties in concentration as most prevalent complaints. C Clusters of complaints. Complaints brought forward by the participants were clustered with a two-step clustering method. Three distinct clusters could be identified. Diameter indicates the number of patients reporting the respective complaint. Cluster 1: Headache (n = 46) is defined by the complaint of headaches and fatigue. Cluster 2: Psycho-Fatigue (n = 34) is predominated by psychiatric complaints and fatigue. In Cluster 3: Fatigue-Concentration (n = 60), fatigue and difficulties in concentration constitute most frequent complaints
Type and Frequency of post-COVID-19 Symptoms
The most frequent complaints reported were fatigue (58.2%) and deficits in concentration (58.2%) (Fig. 1B). Difficulties in recalling names (32.7%), headaches (27.3%), and sensory or motor disturbances (21.0%) were also frequently mentioned. Word finding difficulties affected 20.6% of patients. Psychiatric complaints (20.0% in total) encompassed depressive mood (41.0%), the feeling of being stressed (22.5%), anxiety (22.5%), listlessness (19.3%), irritability/aggression (6.4%), the feeling of being restless (9.6%), and 3.2% other complaints. Moreover, 15.2% of study participants complained of dizziness.
Despite the broad variety of complaints brought forward by the patients, we were able to identify three distinct clusters of symptoms by using a two-step clustering method (Fig. 1C): a cluster with the predominant complaint of headaches (n = 46); a cluster (n = 34) predominately including psychiatric symptoms, fatigue, and difficulties in concentration designated as “Psycho-Fatigue”; and finally the cluster “Fatigue-Concentration” (n = 60) including patients that mainly reported fatigue, difficulties in concentration and recalling names. The complaints expressed by the remaining patients did not allow for specific clustering. The “Fatigue-Concentration cluster” included a higher proportion of females (73.3%) as compared to the “Headache” (56.5%) and “Psycho-Fatigue clusters” (61.8%). Moreover, symptoms of cognitive impairment were significantly associated with the presence of fatigue (number of abnormal tests to fatigue levels, r = 0.283, p < 0.01).
We further analyzed whether the pre-existence of certain disease states was associated with distinct post-COVID-19 symptoms. Patients with a previous history of psychiatric conditions were more likely to report psychiatric symptoms (OR 3.5, CI 1.47–8.32, p < 0.01), fatigue (OR 2.43, CI 1.11–5.82, p < 0.05), or difficulties in concentration (OR 2.58, CI 1.01–6.19, p < 0.05) (Fig. 2A). A previous neurological condition was associated with a higher probability of complaining about deficits in sensory or motor functions (OR 2.49, CI 1.10–5.62, p < 0.05) and dizziness (OR 2.13, CI 1.02–4.42, p < 0.05) (Fig. 2B). A history of migraine or other types of headaches increased the risk of developing headaches after COVID-19 (OR 3.52, CI 3.52, 1.34–9.36, p < 0.01) (Fig. 2C).
Fig. 2.
Evaluation of risk factors by calculation of odds ratios (OR) and 95% confidence interval (CI) for presenting a particular complaint. A Patients with a history of psychiatric conditions were more likely to complain of fatigue (OR 2.43, CI 1.11–5.82, p < 0.05), difficulties in concentration (OR 2.58, CI 1.01–6.19, p < 0.05), and to report psychiatric complaints (OR 4.50, CI 1.47–8.32, p < 0.01). B Patients with a history of neurological conditions more frequently reported sensor and motor disturbances (OR 2.49, CI 1.10–5.62, p < 0.05) as well as dizziness (OR 2.13, CI 1.02–4.42, p < 0.05). C History of headaches was a risk factor for reporting headache as part of post-COVID-19 syndrome (OR 3.52, CI 1.34–9.36, p < 0.01). OR are given with respective 95% confidence intervals (95% CI), x-axis is depicted in logarithmic scale. Only OR that reached a level of significance p < 0.05 are reported *p < 0.05, **p < 0.01, ***p < 0.001
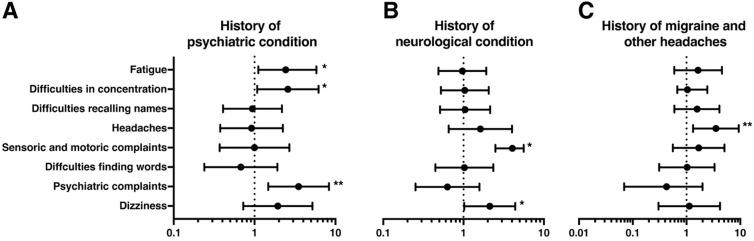
We next quantified the extent of neuropsychiatric and neuropsychological symptoms in our patient cohort by using standardized questionnaires and neuropsychological assessment. Analysis of FIS (n = 128) revealed a considerable high level of fatigue in post-COVID-19 patients (86.1 ± 37.12). Females had significantly higher fatigue sum scores than males (95 ± 34.0 vs. 67.0 ± 36.5, p < 0.001, data not shown). For the cluster of complaints, patients in the cluster “Fatigue” presented the highest fatigue levels, whereas those in cluster “Headache” had the lowest sum scores (62.3 ± 42.1 vs. 95.8 ± 33.2, p < 0.001). A subgroup analysis of patients with previous psychiatric conditions (n = 33) demonstrated significantly elevated total fatigue scores (110.2 ± 27.4 vs. 79.8 ± 38.0, p < 0.01) compared to patients without a history of psychiatric condition. In contrast, neurological comorbidities were not associated with elevated fatigue scores (Fig. 3A). Since patients with former stroke and multiple sclerosis are prone to developing fatigue, we in addition performed a subgroup analysis in these patients (n = 10). Indeed, we found a significant association between fatigue levels and structural brain damage due to previous stroke or multiple sclerosis (beta 0.274, p < 0.05; data not shown).
Fig. 3.
Fatigue Impact Scale (FIS), neuropsychological assessment and Patient Health Questionnaire 15 (PHQ15) for clusters of complaints and previous neurological and psychiatric conditions. A Highest levels of fatigue (95.7 ± 33.2) were found in the “Fatigue-Concentration cluster” while lowest levels were found in the “Headache cluster” (62.3 ± 42.0, p < 0.001). Range of reference encompassed fatigue levels of MS patients (high fatigue levels, 93.2) and patients with high blood pressure (HBP; low fatigue levels, 31.2) [28]. The presence of a previous psychiatric condition was associated with markedly higher FIS (110 ± 27.4 vs. 79.8 ± 38.0, p < 0.01) while previous neurological conditions did not influence levels of fatigue. B The proportion of patients having abnormal neuropsychological test results is depicted as radar plot. For the total cohort, assessment revealed deficits in all tested qualities of neurocognition. “Fatigue-concentration cluster” and patients with previous psychiatric conditions displayed the highest frequency of abnormal test results. C Assessment of PHQ15 revealed the strongest tendency towards somatization in the “Fatigue-concentration cluster” as compared to “Headache cluster” (p < 0.01; PHQ15 > 15, dotted line). Patients with a previous psychiatric condition had significantly higher PHQ15 scores, whereas those with previous neurological conditions showed the lowest levels of somatization (Dotted line: mean score of the total cohort (PHQ15: 14.0)). *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant
To validate the self-reported cognitive deficits, a neuropsychological assessment was performed by trained personnel (n = 141) (Fig. 3B). In the total cohort, 79.6% of individuals had deficits in one or more subdomains tested, i.e., immediate verbal memory, processing speed, or cognitive flexibility. In the “Fatigue-Concentration cluster”, almost half of the patients (48.0%) presented with deficits in three or more test dimensions, whereas this was the case in only 20% of patients in the “Headache cluster”. Of patients presenting with psychiatric comorbidities, 57.8% were found to have deficits in three or more test dimensions. In contrast, pre-existing neurological conditions had no specific impact on post-COVID-19 neurocognition. Of note, structural brain damage due to previous stroke or multiple sclerosis (n = 10) did not negatively influence the performance in standardized cognitive testing (p > 0.05, not shown). In addition, the level of education could be ruled out as a potential confounder on cognitive performance (p > 0.05, not shown). Linear regression analyses showed that only “complex scanning and visual tracking” as cognitive dimension was significantly influenced by the level of education. Further eight dimensions were not influenced by education, therefore arguing against academic degree being a major confounding factor during cognitive assessment. Notably, despite a considerable high frequency of participants showing poor performance in neuropsychological tests in our cohort across all different clusters, we were not able to identify a distinct pattern of neuropsychological deficits suggestive of any specific neurodegenerative disease, e.g., dementia or a predominantly affected cognitive domain.
To quantify the degree of somatization in our study cohort, we used the PHQ15 questionnaire (n = 128, Fig. 3C). In general, females had significantly higher somatization scores than males (13.3. ± 5.5 vs. 8.6. ± 4.4, p < 0.001, data not shown). The lowest PHQ15 score (8.6 ± 5.4) was found in the “Headache cluster”, whereas patients from the “Fatigue-Concentration cluster” reported a sum score of 13.6 ± 5.5 (p < 0.01), indicative of a medium to a high level of somatization. Moreover, patients with psychiatric comorbidities were at a higher risk for somatization compared to patients with no history of psychiatric disorder (14.0 ± 5.5 vs. 11.3 ± 5.5, p < 0.05). Interestingly, patients with pre-existing neurological diseases reported significantly lower PHQ15 scores than those not suffering from neurological comorbidities (12.0 ± 5.5 vs. 8.9 ± 4.5, p < 0.001). We observed a correlation between the extent of somatization (PHQ15) and the extent of fatigue (FIS, r = 0.656, p < 0.001, data not shown). Furthermore, patients with higher somatization scores and higher levels of fatigue more frequently exhibited abnormal findings in neurocognition assessment as compared to individuals with lower somatization scores (r = 0.257, p < 0.01) and lower fatigue levels (r = 0.262, p < 0.01, data not shown).
Diagnostic Work-up
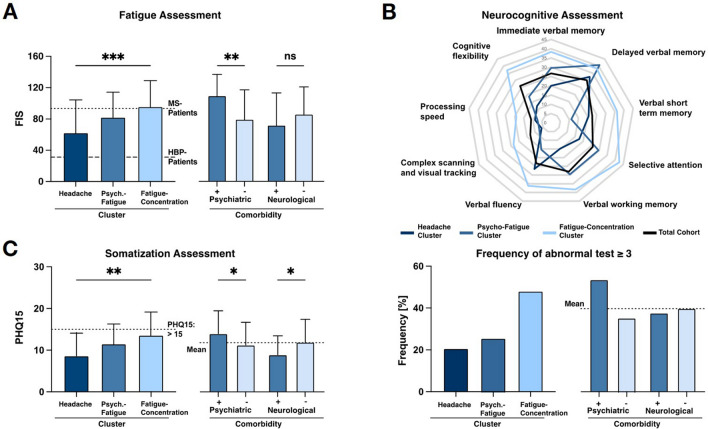
An extensive diagnostic workup-up was performed to align the different post-COVID-19 symptoms to any specific neuroanatomical or neurophysiological pathology (Table 2 and Fig. 4A–D).
Table 2.
Results of neurological assessment
| Explaining at least one complaint | ||
|---|---|---|
| Neurological examination (n = 171) | n (%) | n (%) |
| No pathological findings | 147 (85.8) | |
| Sensomotoric deficits | 21 (12.4) | 16/21 (76.2) |
| Tremor | 2 (1.2) | 1/2 (50) |
| Ataxia | 1 (0.6) | 1/1 (100) |
| Electrophysiological assessment (n = 171) | n (%) | |
| No pathological findings | 153 (89.2) | |
| Axonal/demyelinating damage | 18 (10.8) | 10/18 (55.0) |
| Imaging (n = 41) | n (%) | |
| No pathological finding | 35 (85.4) | |
| Microangiopathy | 3 (7.3) | 0/3 (0.0) |
| MS lesions | 3 (7.3) | 2/3 (66.6) |
| Neurovascular assessment (n = 76) | n (%) | |
| No pathological finding | 75 (98.3) | |
| Sub-intimal bulging | 1(1.7) | 0/1 (0.0) |
| CSF diagnostics (n = 11) | n (%) | |
| No pathological findings | 9 (81.8) | |
| Oligoclonal bands (MS) | 1 (6.1) | 1/1 (100) |
| Elevated cell count | 1 (6.1) | 1/1 (100) |
| Laboratory assessment (n = 167) | Mean ± SD | |
| CRP | 0.50 ± 0.33 mg/dl | |
| Above cut-off (< 0.5 mg/dl) | 12.0% | 0/20 (0.0) |
| Leukocytes | 6.68 ± 2.04 /nl | |
| Above cut-off (> 11/nl) | 6.0% | 0/10 (0.0) |
| Respiratory assessment (n = 171) | Mean ± SD | |
| VC [l] | 3.65 ± 0.88 | 0/171 (0.0) |
| FEV1, [%] | 94.07 ± 20.58 | 0/171 (0.0) |
| SpO2, [%] | 97.48 ± 1.23 | 0/171 (0.0) |
Results are given in absolute numbers with relative percentage in parenthesis
All findings were evaluated as to whether the complaints can be explained by the diagnostic finding
VC vital capacity, FEV1 forced expiratory volume in 1 s, SpO2 peripheral oxygen saturation, MS multiple sclerosis, CRP C-reactive protein, CSF cerebrospinal fluid
Fig. 4.
Results of neurological diagnostics. Findings evaluated as to whether they were able to explain at least one reported complaint. A In 85.8%, the neurological examination (n = 171) revealed no abnormalities. Sensory and motor deficits (11.8%), tremor (1.2%) and ataxia (1.2%) added up to 14.2% of cases with focal neurological deficits; 10.0% of these findings could be attributed to one complaint brought forward by the patient. B Electrophysiological assessment in 89.2% yielded normal and in 10.8% pathological findings; 6.3% of these findings explained a reported complaint, in 4.5% the electrophysiological finding was incidental and not related to any complaints. C 41 MRI scans were performed, revealing no pathological findings in 85.0% of the cases. Of 15.0% noticeable findings, 5.0% (stable MS lesions) were considered explanatory for the complaints of the patients. D In 81.9% of patients, cerebrospinal fluid analysis (n = 11) yielded no pathological findings; in 18.1% of cases either elevated cell count or oligoclonal bands (new MS diagnosis) were found
In most of the patients (85.8%), neurological examination did not yield any abnormal findings. Sensory and motor deficits (11.8%), tremor (1.2%), and ataxia (1.2%) added up to 14.2% of cases with focal neurological deficits. In 10.0% of the cases, the finding from the neurological examination matched with at least one of the subjective complaints (Fig. 4A).
Electrophysiological assessment (n = 171) including extensive nerve conduction studies of sensory and motor nerves, sensory and motor evoked potentials and brainstem reflexes revealed no pathological findings in 89.2% of study participants (Fig. 4B). In 10.8%, axonal or demyelinating peripheral nerve damage with prolonged nerve conduction velocity or reduced amplitude was detected. The electrophysiological findings covered the complaints brought forward by the patient in 6.3% (e.g., symptoms of sensory alterations in the lower extremities and electrophysiological findings of axonal sensory nerve damage diagnosed as diabetogenic polyneuropathy). In the remaining 4.5%, electrophysiological pathological findings were incidental and not related to any complaints.
Cranial MRI (n = 41) was normal in 85% of examined patients (Fig. 4C). Three patients (7.3%) were found with relevant cerebral microangiopathy. Three additional patients (7.3%) had MRI lesions typical for multiple sclerosis. In two of these cases, a diagnosis of multiple sclerosis had already been established before COVID-19, while the remaining case who presented with vertigo and dizziness was newly diagnosed for multiple sclerosis in our post-COVID-19 outpatient center.
In 11 patients, symptoms justified sampling of CSF (Fig. 4D). CSF analysis was normal in 81.9% of the cases. In one patient presenting with headache, fatigue, and difficulties in concentration, CSF examination finally yielded EBV meningitis while in another patient findings from CSF were in line with the diagnosis of multiple sclerosis.
Neurovascular ultrasound (n = 76) yielded pathological findings in only 1.7% of the patients, whereas 98.3% of the examinations were normal (Table 2). Finally, routine laboratory parameters (n = 171), including markers of inflammation, were unremarkable in the vast majority of patients (Table 2), as was the assessment of respiratory function (vital capacity, forced expiratory volume in 1 s, peripheral oxygen saturation).
Diagnostic Attribution
First, we assessed the type and frequency of confirmed neurological diagnoses in our cohort of post-COVID-19 patients. If symptoms and findings from diagnostic work-up matched the criteria for a particular disease entity according to clinical standards, they were attributed to the respective condition other than post-COVID-19. In 20.5% of the patients, at least one of the reported symptoms was attributed to an ICD-10 coded neurological diagnosis other than post-COVID-19 syndrome (Fig. 5A). These included polyneuropathy, carpal tunnel syndrome, and restless legs syndrome. In only 2.3% of the patients with a neurological diagnosis, a causative link to preceding COVID-19 appeared plausible. These cases comprised one patient with myelitis, one patient with cerebellar ataxia, and two cases of GBS. Of note, our cohort did not comprise individuals with previous post-infectious disorders such as GBS or myelitis, which could have been reactivated after SARS-CoV-2 infection. In the remaining 18.2%, a potential association of the neurological diagnosis with the previous COVID-19 did not appear likely (Fig. 5A).
Fig. 5.
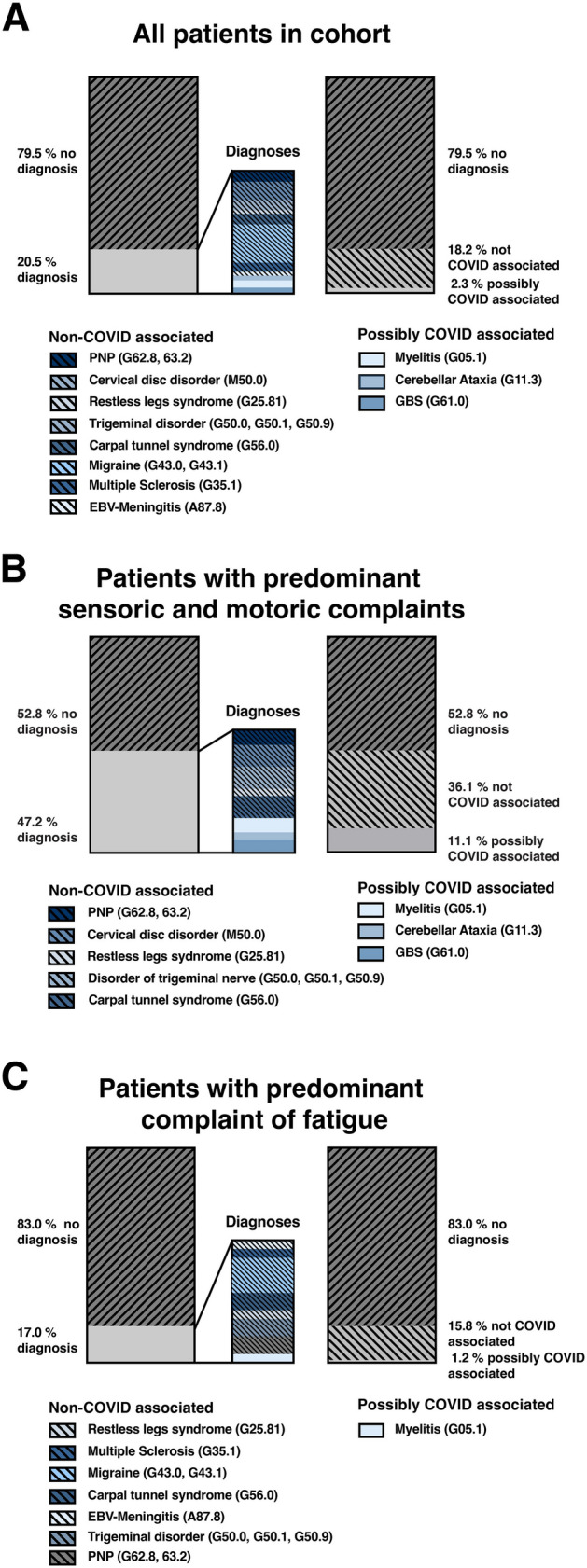
Neurological diagnoses explaining at least one subjective complaint and the possible association with COVID-19. A For the total cohort, 20.5% of participants received a neurological diagnosis apart from post-COVID-19, of which 18.2% were not associated with COVID-19. B 47.2% of individuals with sensory or motor dysfunction were finally found with a neurological diagnosis and in 36.1% this diagnosis was not associated with COVID-19. C In 17.0% of individuals complaining of fatigue, a neurological diagnosis was found explaining at least one subjective complaint; 1.2% of these were related to COVID-19
In post-COVID-19 patients presenting with sensory or motor deficits as main symptoms, the proportion of neurological diagnoses explaining the reported complaints, e.g., diabetic polyneuropathy, was considerably high (47.2%) (Fig. 5B). A plausible link to previous COVID-19 could be made in 11.1% of cases in this group.
In contrast, only 17.0% of patients primarily complaining of fatigue were finally found with a neurological diagnosis, and only 1.2% of these diagnoses were probably related to COVID-19 in this group (Fig. 5C). Patients reporting sensory or motor deficits without an established neurological diagnosis had significantly higher somatization scores (PHQ15) compared to patients with any neurological diagnosis (mean 13.6 ± 4.8 vs. 9.1 ± 6.6, p = 0.05, Fig. 5C).
Discussion
Our study showed that patients with post-COVID-19 syndrome suffer from a great variety of neuropsychiatric syndromes. However, damage of the central or peripheral nervous system could only be rarely objectified or, if present, was attributable to a distinct neurological disease rather than post-COVID-19 syndrome. Moreover, we found evidence of significant psychiatric comorbidities and high levels of somatization, pointing towards the possibility that psychosomatic mechanisms like somato-sensoric amplification might play a substantial role in the pathogenesis of post-COVID-19 syndrome.
The majority of our patients were female and reported multiple complaints of which fatigue, difficulties in concentration, and headache were most frequently expressed. This observation underlines that neurological and neuropsychiatric symptoms represent key features of post-COVID-19 syndrome and is in accordance with several previous reports on the symptomatology and female predominance in post-COVID-19 syndrome [10, 36, 37]. Most of the patients included in our study had only a mild-to-moderate COVID-19 course, with only 5% requiring hospitalization. This underrepresentation of severe COVID-19 cases might have biased the findings in post-COVID-19 syndrome afterwards. On the other hand, it is well established that there is no clear correlation between the severity of acute COVID-19 and the risk of developing post-COVID-19 syndrome, since only mildly affected COVID-19 patients or even asymptomatic SARS-CoV-2-positive individuals can develop persisting symptoms [38–40].
Interestingly, stratification according to different occupational groups revealed that professions requiring academic qualifications or from the administrative and teaching field were more frequently represented in our cohort in comparison to professions going along with physical activity. We are not aware of any other study on post-COVID-19 that has captured the occupational status of the study participants and the reasons for this specific distribution are unknown. However, different educational levels of the study participants leading to a higher interest in health issues in academics, in particular women [41], or disparities in social security and the job situation between the different professions might play a role.
Fatigue was the most prominent complaint in our cohort, which is in line with previous observations [5, 9, 10, 42, 43]. The degree of post-COVID-19 fatigue observed here was much higher than in healthy individuals and similar to the extent of fatigue in patients with multiple sclerosis [28, 44], further underlining that fatigue is one of the most disturbing symptoms in post-COVID-19 syndrome, although we cannot completely rule out from our findings that some neurological comorbidities present in our study participants, in particular former stroke and multiple sclerosis amplified fatigue levels. The mechanisms causing extensive fatigue in post-COVID-19 syndrome are still not fully understood. Albeit post-infectious fatigue is a common and sometimes long-lasting clinical phenomenon, e.g., following EBV infections or tick-borne encephalitis [45, 46], the high frequency, the strong manifestation, and the persistence of fatigue in patients with post-COVID-19 argue in favor of distinct mechanisms. In addition, significant tissue hypoxemia as another potential cause of fatigue [47] appeared unlikely in our cohort since the assessment of blood oxygenation was normal in all patients. Also, cranial MRI did not reveal any structural abnormalities, e.g., strategic lesions that could have accounted for the occurrence of fatigue. In the absence of any specific measurements in our study and evidence from the literature, we cannot exclude that continuously elevated levels of certain proinflammatory cytokines (e.g., IL-6) or the re-composition of different immune cell subsets in the CSF are involved in the occurrence of post-COVID-19 fatigue, as it has also been suggested for acute COVID-19 [48, 49]. Finally, we did not routinely assess iron metabolism (e.g., ferritin and transferrin levels) in our study participants, which has been described to affect the frequency and severity of fatigue [50]. However, hemoglobin levels, which are linked to iron metabolism [51], were normal in 97.7% of patients, therefore arguing against a severe iron deficit in our cohort.
Interestingly, patients with pre-existing psychiatric conditions most often reported fatigue in our study. It is well known that the perception of fatigue also depends on emotional factors such as mood and motivation [52, 53], and it can sometimes be challenging in clinical practice to strictly distinguish fatigue from depression [54].
Some groups suggested that patients having recovered from COVID-19 are at a higher risk of developing dementia afterwards. For instance, neurofilament and amyloid-beta serum levels were increased in elderly and severely affected COVID-19 patients, as was the extent of brain atrophy [55, 56]. In line with these findings, the feeling of suffering from cognitive deficits, e.g., difficulties in recalling names or word finding difficulties, was also frequently reported by our study participants. Indeed, the structured neuropsychological assessment revealed low performance in cognition in these patients. However, the deficits did not follow any specific pattern, but were distributed across all measured cognitive domains, resembling neuropsychological patterns in individuals with mood disorders such as depression [57–59], conditions which are well known for their reduced impetus to perform exhausting tests [60]. Also, MRI assessment in our study did not reveal remarkable brain atrophy, albeit we might have missed subtle changes in brain volumes since we did not perform volumetric brain measurements and the cross-sectional study design did not allow for collecting longitudinal MRI data. Patients were examined according to common clinical standards, which in Germany do not include brain volumetry. According to the best possible clinical judgement also from our neuroradiologists we did not observe any ‘obvious’ brain atrophy in our study patients. In addition, we were not able to detect SARS-CoV-2 RNA in the CSF of any of our study participants, which is in accordance with our larger CSF studies on COVID-19 [49, 61], arguing against an excessive neurotropism of SARS-CoV-2 or virus persistence in the CNS. Moreover, most patients with post-COVID-19 syndrome spontaneously recover after several months [42, 62, 63], a phenomenon that explicitly excludes the diagnosis of dementia [64, 65]. In addition, elevated neurofilament serum levels have also been reported in hospitalized COVID-19 patients without CNS involvement, and in non-hospitalized COVID-19 patients, biomarker levels of neurodegeneration were found to be normal [55]. Interestingly, study participants comorbid of stroke and multiple sclerosis did not perform worse in neurocognitive testing in comparison to the other participants. Nevertheless, we cannot fully exclude the possibility that in some of our post-COVID-19 patients pre-existing neuropsychiatric diseases might have negatively impacted cognitive results.
Despite the multitude of diagnostic tests and examinations applied, findings were normal in most of our patients. Although we did not perform some of the diagnostic tests that have recently been claimed to be specifically or causally linked to post-COVID-19 syndrome, such as autoantibodies against g-protein-coupled receptors [15] or Xenon CT of the chest [14], the diagnostic armamentarium used in our study well represents the current standard in clinical neurology. Apart from that, most current experimental tests and diagnostics offered to patients with post-COVID-19 syndrome have not been validated independently or in larger groups of patients, and their actual pathophysiological relevance is unknown. Of note, some of the reported abnormal findings, for instance related to the autonomic nervous system, might even be triggered through conditioning mechanisms (fear conditioning) rather than structural organ changes [66]. Moreover, from a clinical perspective, it is questionable that any single abnormal finding can explain the large heterogeneity of post-COVID-19 symptoms. Accordingly, specific neurological diagnoses plausibly linked to preceding COVID-19 could be established only in single patients in our study. GBS, transverse myelitis, and cerebellitis have been described in temporal proximity to the infection with SARS-CoV-2 [67–70].
Considering the absence of any specific abnormalities in the CNS and PNS, evidence of high somatization scores, and a higher frequency of neuropsychiatric symptoms in individuals with pre-existing psychiatric disorder in our cohort, one should consider that in some individuals post-COVID-19 syndrome might be a rather psychosomatic provenance. Indeed, symptoms of post-COVID-19 syndrome even occurred in patients with retrospectively falsified diagnosis of SARS-CoV-2 infection [71]. In contrast, patients aware of a previous infection reported higher rates of post-COVID-19 syndrome than those not being aware. Furthermore, the predisposition of developing symptoms of post-COVID-19 in patients with comorbid psychiatric disorders has already been reported by other groups [72, 73] and psychiatric medications such as antidepressants have been described to relieve symptoms of post-COVID-19 [74]. Finally, the observed gender and age distribution in post-COVID-19 is typical for patient populations suffering from psychosomatic disorders [75], and there seems to be considerable overlap in terms of symptomatology and postulated pathophysiological concepts with similarly enigmatic disease entities such as chronic fatigue syndrome or ME/CFS [19, 76].
Nevertheless, we cannot exclude from our findings that post-COVID-19 syndrome represents a spectrum of different entities and that subgroups of post-COVID-19 patients exist with more specific organotypic abnormalities that could be of causal relevance. In any case, it is crucial to take the complaints of patients with alleged post-COVID-19 syndrome seriously, since in 18.2% of our study participants we were finally able to secure a neurological diagnosis unrelated to post-COVID-19 including multiple sclerosis, migraine, and encephalitis. In particular, individuals presenting with sensory or motor symptoms should be thoroughly examined since they were at a higher risk of suffering from a neurological disease rather than from post-COVID-19, although we cannot fully exclude self-reporting of symptoms related to previous disease states (e.g., stroke) or re-activation of neurological symptoms as a consequence of SARS-CoV-2 infection.
Our study has several limitations. First, we did not collect longitudinal data. Therefore, we cannot make any firm statement regarding the natural course of post-COVID-19 or the possible development of brain atrophy over time in our cohort. Nevertheless, previous studies have shown that symptoms in populations with post-COVID-19 significantly improve or even completely resolve in the majority of patients after 6 months [42, 62, 63]. Furthermore, due to the monocentric and observational study design, we cannot fully rule out selection bias or unidentified confounders. Moreover, infection of our study participants happened during a time of the pandemic when the alpha and delta variants of SARS-CoV-2 were still dominant, and vaccination was not broadly available. Hence, our data do not allow for conclusions as to whether symptoms of post-COVID-19 are different according to virus variants or vaccination status. For ethical reasons, we were not able to apply all diagnostics, e.g., lumbar puncture, to each study participant, and our population was probably biased towards ‘Neuro post-COVID-19’ rather than pulmonary or cardiac disturbances, for instance. Hence, we might have missed specific findings in some individuals. However, our approach reflects common clinical practice and diagnostic decisions were strictly based on the results from patient interviews and neurological examination. Also, most of the patients suffering from post-COVID-19 report neuropsychiatric symptoms [5, 9, 10, 77]. Another limitation of our study is that psychosomatic assessment was solely based on the PHQ15 score. Yet, PHQ15 is a well-established test in psychosomatic medicine and can detect psychosomatic disorders with a high level of sensitivity and specificity [78, 79]. Nevertheless, further investigations are warranted to better characterize how psychosomatic factors contribute to the pathophysiology of post-COVID-19 syndrome.
Conclusions
In conclusion, this study shows that objectifiable affection of the nervous system is rare in patients with post-COVID-19 syndrome. However, this does not release physicians from the responsibility to thoroughly examine these patients to discern alternative disease entities apart from post-COVID-19. Future research should not exclusively focus on potential organic causes of post-COVID-19 syndrome, but rather should also take into account psychosomatic factors as possible triggers.
Acknowledgements
We thank the participants of the study. We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Mark Stettner, Christoph Kleinschnitz, and Martin Teufel concepted the study. Data collection was performed by Michael Fleischer, Muriel Tovar, Hannah Dinse, Klaas Herchert, Daniel Jokisch, and Julia Stögbauer. Analysis and figure preparation was performed by Michael Fleischer, Mark Stettner, Adam Schweda, Daniel Jokisch, Martha Jokisch, and Hannah Dinse. The first draft of the manuscript was written by Mark Stettner, Christoph Kleinschnitz, Michael Fleischer, and Fabian Szepanowski. All authors commented on previous versions of the manuscript. Supervision and critical revision were done by Martin Köhrmann, Martha Jokisch, Anne K. Mausberg, Eva-Maria Skoda, Cornelius Deuschl, Dagny Holle-Lee, Martin Teufel, Christoph Kleinschnitz, and Mark Stettner. All authors read and approved the final manuscript.
Disclosures
Michael Fleischer, Fabian Szepanowski, Muriel Tovar, Klaas Herchert, Hannah Dinse, Adam Schweda, Anne K. Mausberg, Dagny Holle-Lee, Martin Köhrmann, Julia Stögbauer, Daniel Jokisch, Martha Jokisch, Cornelius Deuschl, Eva-Maria Skoda, Martin Teufel, Mark Stettner, and Christoph Kleinschnitz declare that they have no competing interest in connection with the submitted material.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki. All patients were of legal age and gave written informed consent prior to study inclusion. The study was approved by the local ethics committee of the University Duisburg-Essen, Germany (reference numbers 20-9284-BO, 20-9307-BO).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Mark Stettner and Christoph Kleinschnitz contributed equally to this work.
References
- 1.World Health Organization. WHO Coronavirus (COVID19) dashboard. 2022. https://covid19.who.int
- 2.Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6(9):e005427. doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . A clinical case definition of post COVID-19 condition by a Delphi consensus. World Health Organization; 2021. [Google Scholar]
- 4.Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52(6):575–581. doi: 10.1016/j.arcmed.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayoubkhani D, Bosworthor M. Self-reported long COVID after infection with Omicron variant in the UK. Office of National Statistics: Office for National Statistics; 2022.
- 7.Chen Y, Xu Z, Wang P, Li X, Shuai Z, Ye D, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 8.Thallapureddy K, Thallapureddy K, Zerda E, Suresh N, Kamat D, Rajasekaran K, et al. Long-term complications of COVID-19 infection in adolescents and children. Curr Pediatr Rep. 2022;10(1):11–17. doi: 10.1007/s40124-021-00260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClin Med. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John S, Hussain SI, Piechowski-Jozwiak B, Dibu J, Kesav P, Bayrlee A, et al. Clinical characteristics and admission patterns of stroke patients during the COVID 19 pandemic: a single center retrospective, observational study from the Abu Dhabi. United Arab Emirates Clin Neurol Neurosurg. 2020;199:106227. doi: 10.1016/j.clineuro.2020.106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grist JT, Collier GJ, Walters H, Kim M, Chen M, Abu Eid G, et al. Lung abnormalities depicted with hyperpolarized xenon MRI in patients with long COVID. Radiology. 2022;24:220069. doi: 10.1148/radiol.220069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallukat G, Hohberger B, Wenzel K, Fürst J, Schulze-Rothe S, Wallukat A, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisenius S, Kersting A. Psychosomatische Aspekte von long-Covid. MMW Fortschritte Med. 2022;164(1):40–41. doi: 10.1007/s15006-021-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stengel A, Malek N, Zipfel S, Goepel S. Long haulers—What is the evidence for post-COVID fatigue? Front Psychiatry. 2021;21(12):677934. doi: 10.3389/fpsyt.2021.677934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forte G, Favieri F, Tambelli R, Casagrande M. COVID-19 Pandemic in the Italian population: validation of a post-traumatic stress disorder questionnaire and prevalence of PTSD symptomatology. Int J Environ Res Public Health. 2020;17(11):4151. doi: 10.3390/ijerph17114151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systemic review and comparison of clinical presentation and symptomatology. Medicina (Mex) 2021;57(5):418. doi: 10.3390/medicina57050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi Ferrario S, Panzeri A, Cerutti P, Sacco D. The psychological experience and intervention in post-acute COVID-19 inpatients. Neuropsychiatr Dis Treat. 2021;17:413–422. doi: 10.2147/NDT.S283558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Oertzen TJ, Macerollo A, Leone MA, Beghi E, Crean M, Oztuk S, et al. EAN consensus statement for management of patients with neurological diseases during the COVID-19 pandemic. Eur J Neurol. 2021;28(1):7–14. doi: 10.1111/ene.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–609. doi: 10.1212/WNL.44.4.609. [DOI] [PubMed] [Google Scholar]
- 23.Brähler E, Holling H, Leutner D, Petermann F, editors. Brickenkamp-Handbuch psychologischer und pädagogischer Tests. 1. 3., vollst. überarb. u. erw. Aufl. Göttingen Bern: Hogrefe Verl. für Psychologie; 2002. 726 p.
- 24.Aschenbrenner O, Lange K. Regensburger Wortflüssigkeits-Test. Goettingen, Bern: Hogrefe Verl. für Psychologie; 2002.
- 25.Tombaugh T. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 26.Härting C, Neufeld H, Calabrese P. Wechsler Gedächtnistest- Revidierte Fassung. Bern: Hans Huber; 2000. [Google Scholar]
- 27.Smith A. Symbol digits modalities test: manual. Western Psychological Services; 2007.
- 28.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(1):S79–S83. doi: 10.1093/clinids/18.Supplement_1.S79. [DOI] [PubMed] [Google Scholar]
- 29.Hauser W, Almouhtasseb R. Validierung der deutschen version der fatigue impact scale FIS-D. Z Für Gastroenterol. 2003;41(10):973–982. doi: 10.1055/s-2003-42927. [DOI] [PubMed] [Google Scholar]
- 30.Interian A, Allen LA, Gara MA, Escobar JI, Díaz-Martínez AM. Somatic complaints in primary care: further examining the validity of the patient health questionnaire (PHQ-15) Psychosomatics. 2006;47(5):392–398. doi: 10.1176/appi.psy.47.5.392. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Buonsenso D, Parri N, De Rose C, Valentini P. Toward a clinically based classification of disease severity for paediatric COVID-19. Lancet Infect Dis. 2021;21(1):22. doi: 10.1016/S1473-3099(20)30396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert-Koch-Institut. COVID19: Fallzahlen in Deutschland und weltweit. 2022. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html
- 34.RKI. Anzahl und Anteil von VOC und VOI in Deutschland. RKI; 2022. https://giftbot.toolforge.org/deref.fcgi?url=https%3A%2F%2Fwww.rki.de%2FDE%2FContent%2FInfAZ%2FN%2FNeuartiges_Coronavirus%2FDaten%2FVOC_VOI_Tabelle.xlsx%3F__blob%3DpublicationFile
- 35.Wichmann O, Scholz S, Waize M, Schmid-Küpke N, Hamouda O, Wieler LH, et al. Welche Impfquote ist notwendig, um COVID-19 zu kontrollieren? 2021 Jul 5. https://edoc.rki.de/handle/176904/8483
- 36.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torjesen I. COVID-19: Middle-aged women face greater risk of debilitating long-term symptoms. BMJ. 2021;25:n829. doi: 10.1136/bmj.n829. [DOI] [PubMed] [Google Scholar]
- 38.Malkova A, Kudryavtsev I, Starshinova A, Kudlay D, Zinchenko Y, Glushkova A, et al. Post COVID-19 syndrome in patients with asymptomatic/mild form. Pathogens. 2021;10(11):1408. doi: 10.3390/pathogens10111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y, Pinto MD, Borelli JL, Mehrabadi MA, Abrihim H, Dutt N, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: looking for clarity in the haze of the pandemic. Infectious diseases (except HIV/AIDS); 2021. 10.1101/2021.03.03.21252086
- 40.Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’—The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research. 2021;9:1349. doi: 10.12688/f1000research.27287.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Heide I, Wang J, Droomers M, Spreeuwenberg P, Rademakers J, Uiters E. The relationship between health, education, and health literacy: results from the Dutch adult literacy and life skills survey. J Health Commun. 2013;18(sup1):172–184. doi: 10.1080/10810730.2013.825668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schou TM, Joca S, Wegener G, Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19—a systematic review. Brain Behav Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (Long COVID): a scoping review. Front Med. 2021;1(8):750378. doi: 10.3389/fmed.2021.750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroencke DC, Lynch SG, Denney DR. Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler J. 2000;6(2):131–136. doi: 10.1177/135245850000600213. [DOI] [PubMed] [Google Scholar]
- 45.Chiffi G, Grandgirard D, Sendi P, Dietmann A, Bassetti CLA, Leib SL. Sleep-wake and circadian disorders after tick-borne encephalitis. Microorganisms. 2022;10(2):304. doi: 10.3390/microorganisms10020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen M, Asprusten TT, Godang K, Leegaard TM, Osnes LT, Skovlund E, et al. Fatigue in Epstein–Barr virus infected adolescents and healthy controls: a prospective multifactorial association study. J Psychosom Res. 2019;121:46–59. doi: 10.1016/j.jpsychores.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Fan JL, Kayser B. Fatigue and exhaustion in hypoxia: the role of cerebral oxygenation. High Alt Med Biol. 2016;17(2):72–84. doi: 10.1089/ham.2016.0034. [DOI] [PubMed] [Google Scholar]
- 48.Heming M, Li X, Räuber S, Mausberg AK, Börsch AL, Hartlehnert M, et al. Neurological manifestations of COVID-19 feature T cell exhaustion and dedifferentiated monocytes in cerebrospinal fluid. Immunity. 2021;54(1):164–175.e6. doi: 10.1016/j.immuni.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarius S, Pache F, Körtvelyessy P, Jelčić I, Stettner M, Franciotta D, et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflammation. 2022;19(1):19. doi: 10.1186/s12974-021-02339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Share JB. Review of drug treatment for Down’s syndrome persons. Am J Ment Defic. 1976;80(4):388–393. [PubMed] [Google Scholar]
- 51.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 52.St Clair Gibson A, Baden DA, Lambert MI, Lambert EV, Harley YXR, Hampson D, et al. The conscious perception of the sensation of Fatigue. Sports Med. 2003;33(3):167–176. doi: 10.2165/00007256-200333030-00001. [DOI] [PubMed] [Google Scholar]
- 53.Zadra JR, Clore GL. Emotion and perception: the role of affective information: emotion and perception. Wiley Interdiscip Rev Cogn Sci. 2011;2(6):676–685. doi: 10.1002/wcs.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corfield EC, Martin NG, Nyholt DR. Co-occurrence and symptomatology of fatigue and depression. Compr Psychiatry. 2016;71:1–10. doi: 10.1016/j.comppsych.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Frontera JA, Boutajangout A, Masurkar AV, Betensky RA, Ge Y, Vedvyas A, et al. Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or Alzheimer’s dementia. Alzheimers Dement. 2022;18(5):899–910. doi: 10.1002/alz.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu X, Marks AR. Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement. 2022;18(5):955–965. doi: 10.1002/alz.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Godard J, Grondin S, Baruch P, Lafleur MF. Psychosocial and neurocognitive profiles in depressed patients with major depressive disorder and bipolar disorder. Psychiatry Res. 2011;190(2–3):244–252. doi: 10.1016/j.psychres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 58.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24(1):9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- 59.Schwert C, Stohrer M, Aschenbrenner S, Weisbrod M, Schröder A. Neurocognitive profile of outpatients with unipolar depressive disorders. J Clin Exp Neuropsychol. 2019;41(9):913–924. doi: 10.1080/13803395.2019.1634180. [DOI] [PubMed] [Google Scholar]
- 60.Mohn C, Rund BR. Neurocognitive profile in major depressive disorders: relationship to symptom level and subjective memory complaints. BMC Psychiatry. 2016;16(1):108. doi: 10.1186/s12888-016-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleischer M, Köhrmann M, Dolff S, Szepanowski F, Schmidt K, Herbstreit F, et al. Observational cohort study of neurological involvement among patients with SARS-CoV-2 infection. Ther Adv Neurol Disord. 2021;14:175628642199370. doi: 10.1177/1756286421993701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: What is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Helvoort MA, Pop-Purceleanu M, Tendolkar I, Everaerd DS. Neuropsychiatric recovery after COVID-19—an observational cohort-study. Tijdschr Voor Psychiatr. 2021;63(7):514–521. [PubMed] [Google Scholar]
- 64.Gale SA, Acar D, Daffner KR. Dementia. Am J Med. 2018;131(10):1161–1169. doi: 10.1016/j.amjmed.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 65.Fink G. Die interdisziplinäre S 3-Leitlinie Demenzen: Fortschritte der Neurologie Psychiatrie. Fortschritte Neurol Psychiatr. 2010;78(09):503–504. doi: 10.1055/s-0029-1245689. [DOI] [PubMed] [Google Scholar]
- 66.Norcliffe-Kaufmann L, Palma JA, Martinez J, Camargo C, Kaufmann H. Fear conditioning as a pathogenic mechanism in the postural tachycardia syndrome. Brain J Neurol. 2022 doi: 10.1093/brain/awac249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute Transverse Myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 Vaccine (AZD1222) Front Immunol. 2021;26(12):653786. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan JL, Murphy KA, Sarna JR. Myoclonus and cerebellar ataxia associated with COVID-19: a case report and systematic review. J Neurol. 2021;268(10):3517–3548. doi: 10.1007/s00415-021-10458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matta J, Wiernik E, Robineau O, Carrat F, Touvier M, Severi G, et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182(1):19. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skoda EM, Bäuerle A, Schweda A, Dörrie N, Musche V, Hetkamp M, et al. Severely increased generalized anxiety, but not COVID-19-related fear in individuals with mental illnesses: a population-based cross-sectional study in Germany. Int J Soc Psychiatry. 2021;67(5):550–558. doi: 10.1177/0020764020960773. [DOI] [PubMed] [Google Scholar]
- 73.Thye AYK, Law JWF, Tan LTH, Pusparajah P, Ser HL, Thurairajasingam S, et al. Psychological symptoms in COVID-19 patients: insights into pathophysiology and risk factors of long COVID-19. Biology. 2022;11(1):61. doi: 10.3390/biology11010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazza MG, Zanardi R, Palladini M, Rovere-Querini P, Benedetti F. Rapid response to selective serotonin reuptake inhibitors in post-COVID depression. Eur Neuropsychopharmacol. 2022;54:1–6. doi: 10.1016/j.euroneuro.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hulme K, Hudson JL, Rojczyk P, Little P, Moss-Morris R. Biopsychosocial risk factors of persistent fatigue after acute infection: a systematic review to inform interventions. J Psychosom Res. 2017;99:120–129. doi: 10.1016/j.jpsychores.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 76.Lim EJ, Son CG. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2020;18(1):289. doi: 10.1186/s12967-020-02455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kocalevent RD, Hinz A, Brähler E. Standardization of a screening instrument (PHQ-15) for somatization syndromes in the general population. BMC Psychiatry. 2013;20(13):91. doi: 10.1186/1471-244X-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Ravesteijn H, Wittkampf K, Lucassen P, van de Lisdonk E, van den Hoogen H, van Weert H, et al. Detecting somatoform disorders in primary care with the PHQ-15. Ann Fam Med. 2009;7(3):232–238. doi: 10.1370/afm.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.