Abstract
Background
Many long noncoding RNAs (lncRNAs) have important roles in biological processes. The lncRNA HULC was found to be upregulated in human hepatoma tissues. HULC is thought to be involved in multiple steps of hepatoma development and progression; however, the relationship between HULC and hepatitis C virus (HCV) infection, which is a leading cause of hepatoma, remains unclear.
Methods
We examined the effect of HCV replication on HULC expression and the underlying mechanism using cell culture systems. Subsequently, we tested the effect of HULC suppression and overexpression on HCV replication. Finally, we examined the impact of HCV eradication on HULC expression using human liver tissue and blood samples.
Results
HCV replication increased HULC expression in cell cultures. A promoter assay showed that an HCV nonstructural protein, NS5A, increased HULC transcription. HULC suppression inhibited HCV replication; conversely, its overexpression enhanced HCV replication. These effects on HCV replication seemed to occur by the modification of HCV translation. Measurements from human liver and blood samples showed that HCV eradication significantly reduced HULC levels in the liver and blood.
Conclusions
HCV infection increases HULC expression in vitro and in vivo. HULC modulates HCV replication through an HCV internal ribosome entry site–directed translation step.
Keywords: hepatitis C virus, long non-coding RNA, HULC
HULC is a long noncoding RNA identified from human liver tissue. We found that HULC was upregulated by hepatitis C virus (HCV) infection in vivo and in vitro and that HULC modulated HCV replication through an HCV translation step.
Hepatitis C virus (HCV) is a positive-stranded RNA virus that belongs to the Flaviviridae family. Persistent infection of the human liver with HCV can cause chronic hepatitis and cirrhosis, which are frequently followed by hepatocellular carcinoma (HCC) [1]. Therefore, antiviral treatments that eliminate HCV from an infected liver are expected to reduce the mortality of liver-related diseases due to HCV infection. Currently, treatment with highly effective direct-acting agents against HCV can result in a sustained virologic response (SVR) rate that is close to 100% [2]. Although HCV elimination by direct-acting agents reduces the risk of HCC, even after achieving an SVR, HCC can develop in an HCV-eliminated liver [3–6]. The mechanisms by which HCV promotes hepatocarcinogenesis remain poorly understood; in addition, a prediction of HCC occurrence after SVR is important for the effective surveillance of HCC in large numbers of HCV-eliminated patients.
Transcripts consist of 2 forms of RNA, namely, coding RNA and noncoding RNA; noncoding RNA is further divided into small noncoding RNA and long noncoding RNA (lncRNA). The functions of small noncoding RNAs, such as small interfering RNA (siRNA) or microRNA (miR), have been analyzed in more depth than those of lncRNAs. For example, the liver-specific miR-122 interacts directly with the 5′-untranslated region (UTR) of HCV and positively modulates HCV replication [7]. LncRNAs generally possess a cap structure at the 5′-end and poly A tail at the 3′-end and are >200 nucleotides in length. Recently, many lncRNAs have been identified and reported to have important roles in biological processes, such as differentiation, apoptosis, development, and immune responses [8–11]. Furthermore, roles for several lncRNAs, such as lnc-Lethe [12], CSR32/EGOT [13], lncR 8 [14], and lncRNA-32 [15], in HCV infection/replication have been reported and some of these lncRNAs work by modifying host antiviral responses.
HULC is an lncRNA that was identified from analysis of HCC in humans and was found to be upregulated in HCC tissues [16]. HULC is reported to be involved in multiple steps of hepatocarcinogenesis and HCC progression; accordingly, HULC levels in the blood and liver are reported to be useful for the diagnosis and detection of HCC or the prediction of prognosis after treatment [17, 18]. In addition to HCC, the importance of HULC in the progression or diagnosis of other cancers, such as pancreatic [19], colon [20], and stomach [21] cancer, has been suggested.
The relationship between HCV infection and HULC expression remains unclear. In this study, we clearly demonstrated that HULC is transcriptionally upregulated by HCV infection in an HCV cell culture model. In addition, we found that the increased expression of HULC positively modulates HCV replication by enhancing HCV translation. We also showed that HULC expression is significantly reduced in human liver and blood after the successful eradication of HCV.
MATERIALS AND METHODS
Human Samples
We extracted total RNA from liver biopsies of 19 patients. All patients were treated with pegylated interferon (IFN) α2a/b-containing regimens and achieved an SVR. The patients had undergone a liver biopsy prior to antiviral treatment and after viral elimination. Total RNA was extracted from the liver samples as described previously [22].
A total of 228 HCV genotype 1b HCV-infected patients were treated with asunaprevir and daclatasvir in Kanazawa University Hospital and its associated hospitals belonging to the Hokuriku Liver Study Group. Blood samples were taken and stored by using the PAX gene before antiviral treatment and at 24 weeks after finishing antiviral treatment. Total RNA was extracted with a PAXgene Blood RNA Kit (Qiagen, Hilden, Germany).
Informed consent was obtained from all patients, and ethics approval for the study was obtained from the Ethics Committee for Human Genome/Gene Analysis Research at Kanazawa University Graduate School of Medical Science.
Other methods used in this study are described in the Supplementary Data.
RESULTS
HULC Expression Is Upregulated by HCV Infection and Replication
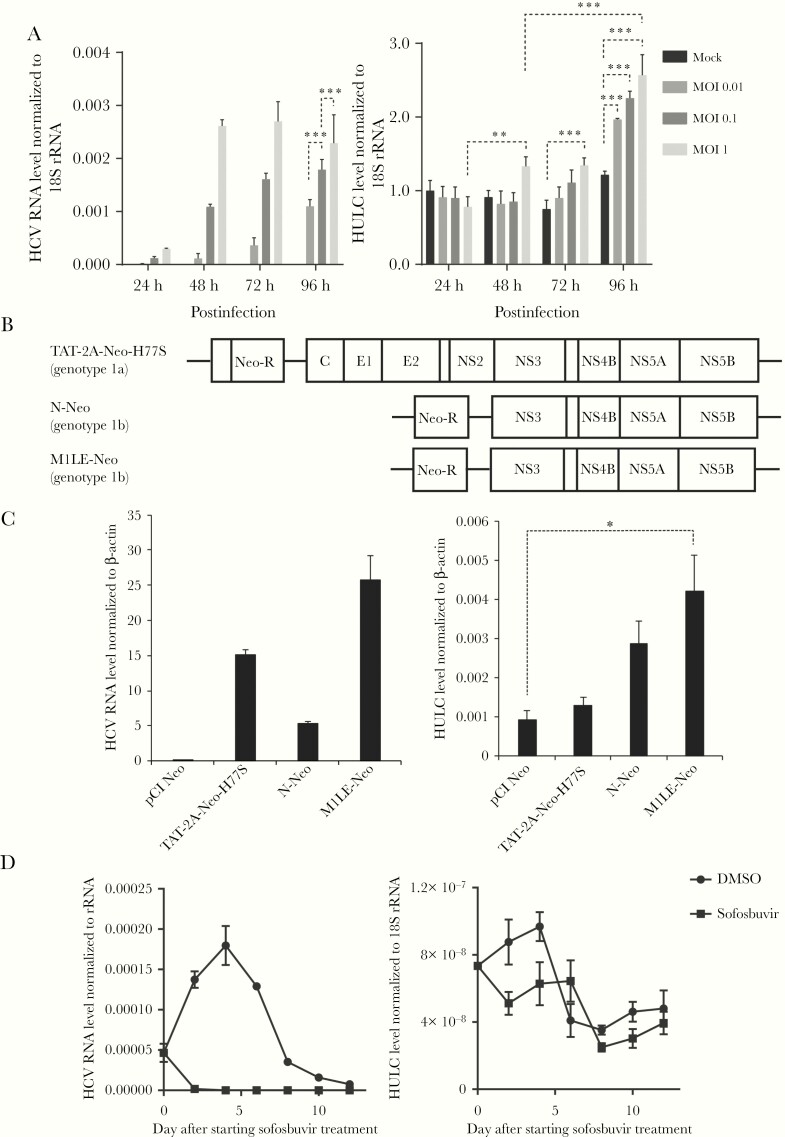
We examined whether HCV infection could increase HULC expression in an HCV cell culture system. For this purpose, Huh-7.5 cells were infected with cell culture–derived HCV from HJ3-5 [23], a chimera of genotype 1a H77S and genotype 2a JFH-1, at different multiplicities of infection (MOIs) of 0.01, 0.1, and 1. Total RNA was extracted every 24 hours from 24 to 96 hours postinfection, and HCV RNA and HULC levels were quantified by reverse-transcription quantitative polymerase chain reaction (RT-qPCR). HCV RNA levels increased from 24 hours postinfection in an MOI-dependent manner. While HULC expression was not altered at 24 hours postinfection, it started to increase from 48 hours postinfection in time- and MOI-dependent manners (Figure 1A). The increase of HULC levels by HCV infection was also observed in another Huh-7 subline, FT3-7 (Supplementary Figure 1). In addition, we observed a 20-fold difference in HULC levels depending on HCV infection in RNA-seq analyses (Supplementary Figure 2). We further investigated HULC expression in HCV-stably replicating Huh-7.5 cells by using HCV-replicon cells. Two subgenomic replicons derived from genotype 1b N [24] and M1LE [25, 26], and a full-genomic replicon from genotype 1a H77S [27, 28] were prepared by transfection of Huh 7.5 cells with the corresponding RNAs and subsequent selection of neomycin-resistant cells for 21 days (Figure 1B). As a control, neomycin-resistant cells were prepared by transfection with a neomycin-resistance gene-coding plasmid and subsequent treatment with neomycin. We quantified HCV RNA and HULC levels by RT-qPCR in these replicon cells. While HCV RNA was detected at different levels in these replicon-containing cells, HULC was also detected in these cells at a higher level than in the neomycin-resistant cells lacking an HCV replicon (Figure 1C). Furthermore, Huh-7.5 cells harboring the M1LE replicon were treated with 5 µM sofosbuvir, a nucleotide analogue NS5B polymerase inhibitor, to inhibit HCV replication or 0.5% dimethyl sulfoxide (DMSO) without neomycin. HCV RNA levels decreased sharply and became undetectable at day 6 after starting sofosbuvir treatment. HULC levels also quickly decreased at the same time with sofosbuvir treatment. In DMSO-treated cells, HCV RNA and HULC levels increased until day 4 and gradually decreased thereafter (Figure 1D). This result showed a good correlation between HULC and HCV RNA expression. These findings clearly show that HCV infection enhances HULC expression in vitro. The upregulation of HULC in HCV-infected liver was also observed in a human-liver chimeric mouse model (Supplementary Figure 3).
Figure 1.
Upregulation of HULC expression by hepatitis C virus (HCV) replication in cell culture. A, Huh-7.5 cells were infected with cell culture-derived HCV of HJ3-5 at multiplicities of infection (MOIs) of 0.01, 0.1, and 1 or mock. Total RNA was extracted at 24, 48, 72, and 96 hours postinfection. HCV RNA, HULC, and 18S ribosomal RNA (rRNA) levels were quantified by reverse-transcription quantitative polymerase chain reaction (RT-qPCR), and the levels of HCV RNA and HULC were normalized to those of 18S rRNA, and further normalized to the relative HULC level of mock at 24 hours, which was set to 1. Error bars show the standard deviation from 3 independent experiments, and the differences of means among each condition were analyzed by 2-way analysis of variance (ANOVA). B, Schematic representation of full-genomic and subgenomic replicons. Neo-R, neomycin resistance gene. C, Huh-7.5 cells harboring each replicon were established after selection with 0.5 mg/mL G418 for 21 days, and total RNA was extracted. The levels of HCV RNA, HULC, and β-actin were quantified by RT-qPCR, and HCV RNA and HULC levels were normalized to those of β-actin. Differences in the means were analyzed by one-way ANOVA. D, Huh-7.5 cells harboring the M1LE replicon were treated with 5 μM sofosbuvir or 0.5% dimethyl sulfoxide (DMSO) without neomycin. HCV RNA, HULC, and 18S rRNA levels were determined by RT-qPCR every other day, and HCV RNA and HULC levels were normalized to the level of 18S rRNA. *P < .05, **P < .01, ***P < .001.
Poly(I:C) Does Not Increase HULC Expression and HULC Is Not an Interferon-Stimulated Gene
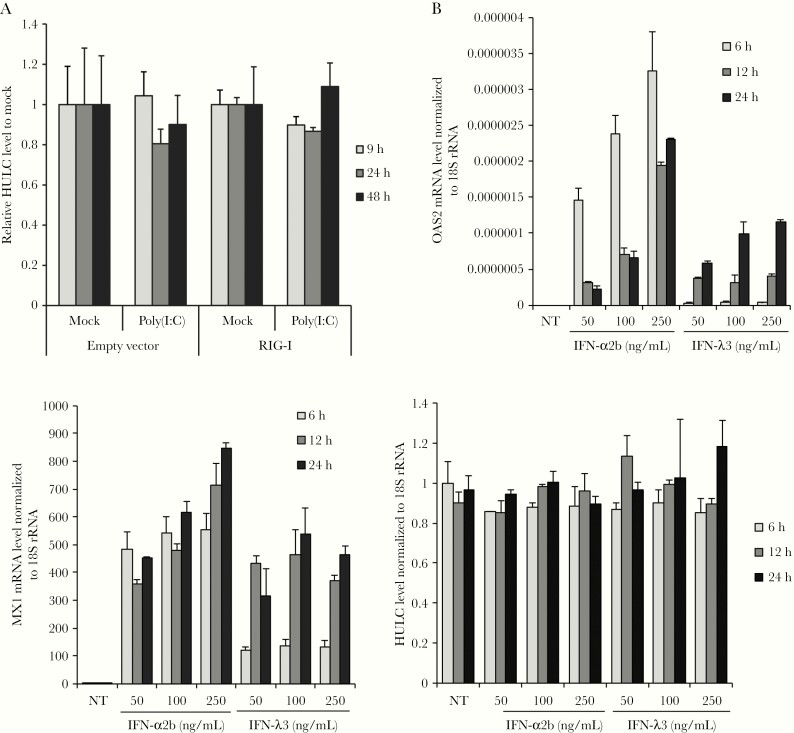
Next, we investigated the mechanism by which HCV infection or replication increases HULC expression. HCV replication is known to produce double-stranded RNA, which induces the expression of many IFN-stimulated genes (ISGs). We examined whether poly(I:C), a mimic of double-stranded RNA, could increase HULC expression by transfection of Huh-7.5 cells with poly(I:C). Because Huh-7.5 cells have a deficit in ISG induction upon poly(I:C) stimulation due to a mutation in RIG-I [29], we overexpressed wild-type RIG-I via plasmid transfection. The expression of exogenous RIG-I was confirmed by Western blotting (Supplementary Figure 4A), and the cells were transfected with poly(I:C). Poly(I:C) transfection induced ISG expression, including IFIT-1, OAS2, MX1, and IFN-β, in wild-type RIG-I–overexpressing cells, but not in empty vector-transfected cells (Supplementary Figure 4B); however, Poly(I:C) transfection did not affect HULC levels in empty vector-transfected or wild-type RIG-I–overexpressing cells. (Figure 2A). We also examined the possibility that HULC could be induced by IFN. For this purpose, the messenger RNA (mRNA) levels of 2 ISGs, MX1 and OAS2, and HULC were determined following IFN-α2b and IFN-λ3 treatment. While both ISGs were upregulated by IFN-α2b and IFN-λ3, HULC was not (Figure 2B). These results indicate that HULC is not an ISG.
Figure 2.
HULC is not an interferon (IFN)–stimulated gene. A, Huh-7.5 cells were transfected with a plasmid encoding N-terminal FLAG tagged RIG-I or the corresponding empty vector, and 24 hours later, they were transfected with 1 μg/mL poly(I:C) or mock. Total RNA was extracted 9, 24, and 48 hours after poly(I:C) transfection. HULC and β-actin messenger RNA (mRNA) levels were determined by reverse-transcription quantitative polymerase chain reaction (RT-qPCR). HULC expression was normalized to β-actin mRNA expression, and the normalized RNA levels from poly(I:C)-transfected cells were further normalized to those from mock-transfected cells at each time point and plasmid transfection. B, Huh-7.5 cells were treated with IFN-α2b or IFN-λ3 at the indicated concentrations and total RNA was extracted. The mRNA levels of MX1, OAS2, and 18S ribosomal RNA (rRNA) were quantified by RT-qPCR, and MX1 and OAS2 mRNA levels were normalized to those of 18S rRNA. NT indicates nontreatment.
NS5A Protein Increases HULC Transcription
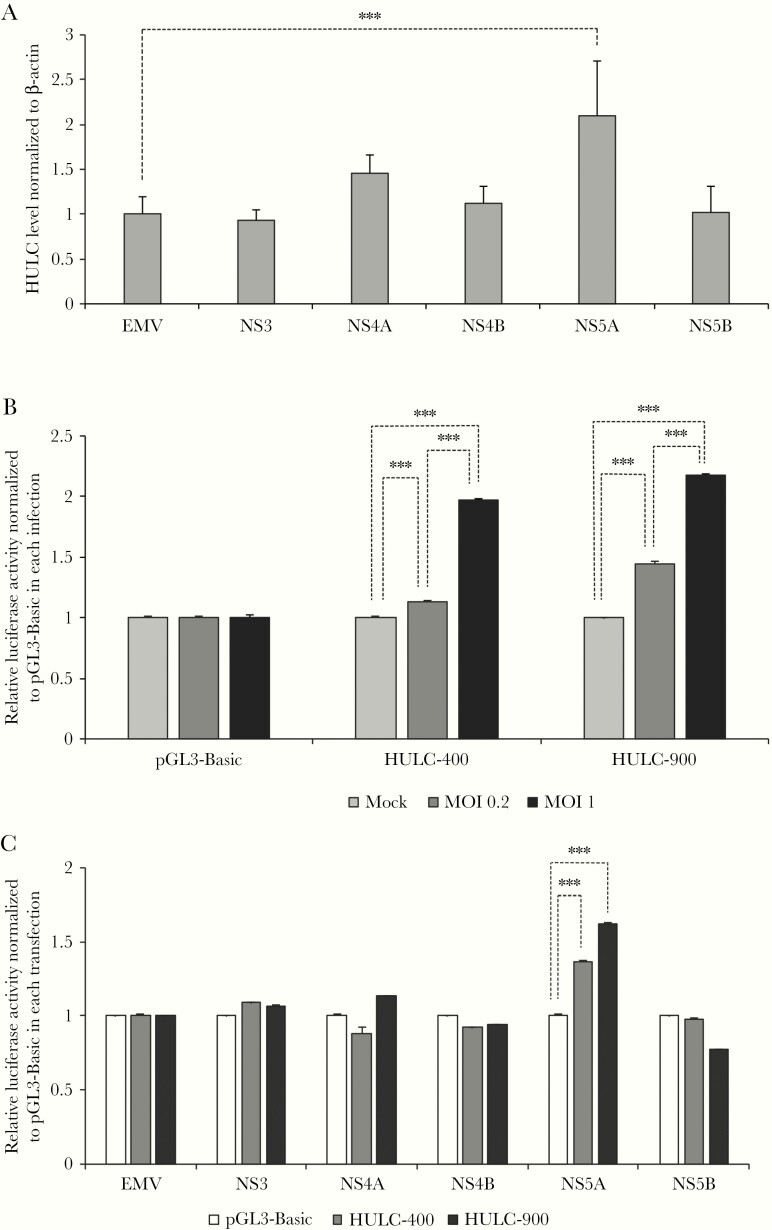
Subsequently, we examined whether HCV protein could increase HULC expression. Because HULC was upregulated by subgenomic and full-genomic replicons (Figure 1B and 1C), we speculated that HCV nonstructural proteins (NS3, NS4A, NS4B, NS5A, and NS5B) could increase HULC levels. To test this hypothesis, we overexpressed the nonstructural proteins individually in Huh-7.5 cells and confirmed their expression by Western blotting (Supplementary Figure 5). RT-qPCR analysis indicated that only NS5A overexpression significantly increased HULC expression (Figure 3A). We further examined the effect of HCV infection on HULC transcription. For this purpose, we introduced 2 HULC promoter regions, which are located approximately 400 and 900 nucleotides upstream from the transcription start site of HULC [30, 31], into the pGL3-Basic vector encoding a firefly luciferase gene whose expression is regulated by the introduced HULC promoter regions. By using these plasmids, we examined the effect of each viral protein on HULC promoter activity. When we infected Huh-7.5 cells, which had already been transfected with pGL3-Basic and pGL3 containing the 2 HULC promoter regions, with HCV at mock, MOI 0.2, and MOI 1, HCV infection significantly increased HULC promoter activity in an MOI-dependent manner for the 400 nucleotide- and 900 nucleotide-containing plasmids (Figure 3B). We further examined the ability of each nonstructural protein to increase HULC promoter activity. When we overexpressed the nonstructural proteins individually in Huh-7.5 cells, which had already been transfected with pGL3-Basic and pGL3 containing the 2 HULC promoter regions, NS5A overexpression increased HULC promoter activity (Figure 3C). These data suggest that NS5A can increase the transcription of HULC.
Figure 3.
HULC is transcriptionally upregulated by NS5A. A, Huh-7.5 cells were transfected with plasmids encoding NS3, NS4A, NS4B, NS5A, and NS5B from genotype 1a H77S.3 or empty vector (EMV). At 72 hours after transfection, total RNA was extracted, HULC and β-actin levels were quantified by reverse-transcription quantitative polymerase chain reaction, and HULC levels were normalized to those of β-actin. Furthermore, the relative HULC levels from plasmid-transfected cells were normalized to the relative HULC levels from EMV-transfected cells, which were set to 1. The differences of means between EMV-transfected cells and each NS protein-encoding plasmid were analyzed by one-way analysis of variance (ANOVA). B, The promoter regions of HULC, which are located approximately 400 and 900 nucleotides upstream from the transcription start site of HULC, were introduced into the pGL3-Basic vector encoding a firefly luciferase gene whose expression was regulated by the introduced HULC promoter regions, to create HULC-400 and HULC-900, respectively. Those plasmids were transfected into Huh-7.5 cells with a reporter plasmid containing a Renilla luminescent reporter gene. At 24 hours later, the cells were infected with cell culture–derived hepatitis C virus from HJ3-5 at multiplicities of infection (MOIs) 0.2 and 1 or mock. At 48 hours postinfection, firefly and Renilla luciferase activity was measured, and firefly luciferase activity was normalized to that of Renilla. Furthermore, activity was normalized to the activity of pGL3-Basic-transfected or mock-infected cells, which was set to 1. The differences of means at each condition were analyzed by 2-way ANOVA. C, Huh-7.5 cells were transfected with pGL3-Basic, HULC-400, or HULC-900 and then transfected with plasmids encoding NS3, NS4A, NS4B, NS5A, or NS5B from genotype 1a H77S.3 or EMV. At 48 hours after transfection, firefly and Renilla luciferase activity was measured, and firefly luciferase activity was normalized to that of Renilla. Furthermore, activity was normalized to that from pGL3-Basic-transfected cells for each nonstructural protein-coding plasmid, which was set to 1. The differences of means between pGL3-Basic and HULC-400 or HULC-900 at each NS protein overexpression were analyzed by 2-way ANOVA. ***P < .001.
HULC Knockdown Suppresses HCV Replication
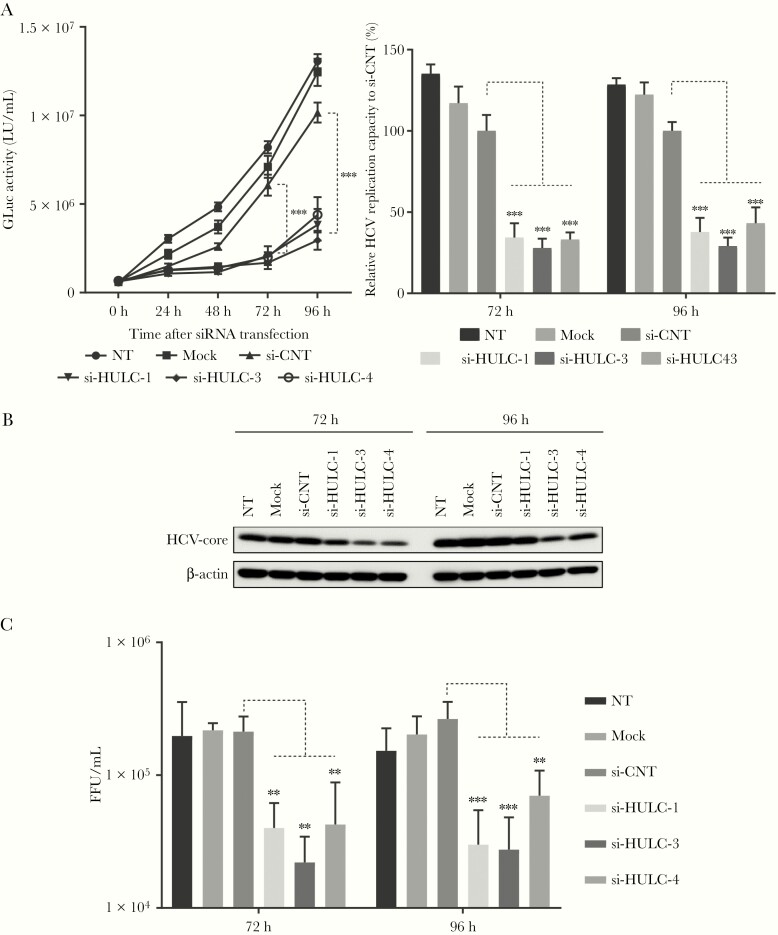
We examined the role of HULC, which is upregulated by HCV infection, in HCV replication. To suppress HULC expression, we designed 3 different siRNAs (si-HULC-1, -3, and -4) targeting HULC and confirmed the effective suppression of HULC by all 3 siRNAs in FT3-7 cells; the suppressive effect of si-HULC-3 was the most prominent among them (Supplementary Figure 6). To monitor HCV replication easily, we used HJ3-5/GLuc2A [32], in which the Gaussia luciferase (GLuc)–coding sequence, fused at its C terminus to the foot and mouth disease virus 2A autoprotease, was inserted between p7 and NS2 of HJ3-5. FT3-7 cells were initially transfected with HJ3-5/GLuc2A RNA, and at 48 hours later, they were transfected with si-HULC-1, -3, -4, or control siRNA (si-CNT). GLuc activity was then measured every 24 hours until 96 hours. From 48 hours after siRNA transfection, GLuc activity started to be lower in the cells transfected with siRNAs to HULC compared with those transfected with si-CNT or mock. At 72 and 96 hours after transfection, GLuc activity was 27%–43% lower in cells transfected with siRNAs to HULC compared with those transfected with si-CNT (Figure 4A). The suppression of HCV replication by siRNAs to HULC was also observed in Huh-7.5 cells (Supplementary Figure 7). The suppressive effect of si-HULC-3 on HCV replication was the most prominent among the siRNAs to HULC, which was consistent with the strong suppressive effect of si-HULC-3 on HULC expression. We also examined the effect of HULC suppression on cell proliferation by a WST-8 assay. HULC suppression by siRNA did not affect cell proliferation at 72 hours after siRNA transfection; however, a slight (∼10%) decrease was induced at 96 hours (Supplementary Figure 8). We also performed a similar experiment by using HJ3-5, which does not contain a GLuc-coding sequence. Following transfection of FT3-7 cells with HJ3-5 RNA, they were transfected with siRNAs to HULC or si-CNT, and then at 72 and 96 hours later, Western blot, RT-qPCR, and a focus-forming unit assay were performed. Western blot analysis showed that all siRNAs to HULC suppressed the expression of HCV core protein at 72 and 96 hours (Figure 4B). RT-qPCR showed that all siRNAs to HULC suppressed HCV replication by 18%–53% compared with si-CNT (Supplementary Figure 9). A focus-forming unit assay showed that HULC suppression reduced infectious virus production (Figure 4C). The Western blot, RT-qPCR, and focus-forming unit assay also showed that the suppressive effect of si-HULC-3 on HCV replication or infectious virus production was the most prominent among the 3 siRNAs to HULC. Si-HULC-3 inhibited HCV replication in a dose-dependent manner (Supplementary Figure 10), and this suppressive effect was also observed for genotypes 1a and 1b (Supplementary Figure 11).
Figure 4.
Inhibition of hepatitis C virus (HCV) replication by HULC suppression. A, FT3-7 cells were transfected with HJ3-5/GLuc2A RNA or HJ3-5 RNA and 72 hours later, the cells were transfected with 3 different small interfering RNAs (siRNAs) targeting HULC (si-HULC-1, si-HULC-3, and si-HULC-3) and control siRNA (si-CNT) at 20 nM. The medium was replaced at 24-hour intervals, and secreted GLuc activity was determined until 96 hours. The left panel shows the time course of GLuc activity after siRNA transfection and the right panel shows relative GLuc activity 72 and 96 hours after siRNA transfection to that from si-CNT–transfected cells, which was set to 100. The differences of means were analyzed by 2-way analysis of variance (ANOVA). B, HCV core protein and β-actin expression in HJ3-5 RNA-transfected cells was analyzed by Western blot analysis with appropriate antibodies 72 and 96 hours after siRNA transfection. C, Medium from HJ3-5 RNA-transfected cells was replaced at 24-hour intervals until 96 hours, and the media collected at 72 and 96 hours were used to infect Huh-7.5 cells to determine infectious virus yield using a conventional focus-forming unit (FFU) assay. Differences in the means at each time point were analyzed by one-way ANOVA. **P < .01, ***P < .001. NT indicates nontreatment.
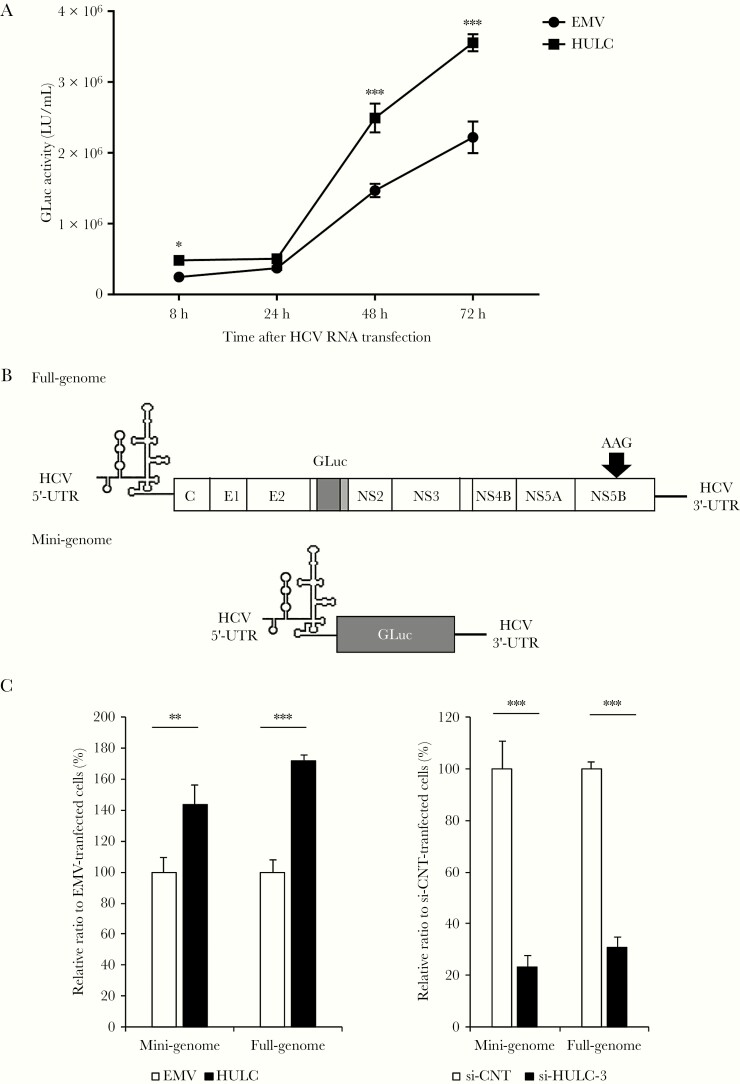
HULC Enhances Hepatitis C Virus Replication Through Its 5′ Region
We next examined the effect of HULC overexpression on HCV replication. To express HULC exogenously, we subcloned HULC complementary DNA into the vector pBapoCMV, in which HULC is expressed under the control of the cytomegalovirus promoter. We transfected Huh-7.5 cells with this HULC-coding vector or an empty vector, and at 48 hours later, the cells were transfected with HJ3-5/GLuc2A RNA. GLuc activity at 8 hours after transfection was significantly higher in the HULC-overexpressing cells than in the empty vector-transfected cells. At 48 hours and 72 hours, the HULC-overexpressing cells showed much higher GLuc activity than the empty vector-transfected cells (Figure 5A). A WST-8 assay showed that HULC overexpression did not alter cell proliferation (Supplementary Figure 12). These results indicate that HULC enhances HCV replication.
Figure 5.
Hepatitis C virus (HCV) replication is enhanced through HCV internal ribosome entry site (IRES)–directed translation. A, Huh-7.5 cells were transfected with a plasmid encoding HULC or an empty vector (EMV), and 24 hours later, they were transfected with HJ3-5/GLuc2A RNA. The medium was replaced at 24-hour intervals until 96 hours, and secreted GLuc activity was determined. Differences in the means of GLuc activity between EMV and HULC at each time point were analyzed with Student t test. B, Schematic representation of the nonreplicating RNAs used for the experiments for the effect of HULC on HCV IRES-directed translation. UTR indicates the untranslated region. C, Huh-7.5 cells were transfected with a plasmid encoding HULC or EMV, and 48 hours after transfection, the cells were transfected with full-genomic or mini-genomic RNA. Eight hours later, secreted GLuc activity was determined (left panel). Huh-7.5 cells were transfected with small interfering RNA (siRNA) targeting HULC or control siRNA (si-CNT) at 20 nM, and 48 hours after transfection, they were transfected with full-genomic or mini-genomic RNA. Eight hours later, secreted GLuc activity was determined (right panel). Differences in the means of relative ratios between CNT and HULC or si-CNT and si-HULC-3 were analyzed with Student t test. *P < .05, **P < .01, ***P < .001.
HULC Regulates HCV Translation
The enhancement of GLuc activity by HULC overexpression at 8 hours after transfection (Figure 5A) suggested that HULC could regulate the HCV internal ribosome entry site (IRES)–mediated translation step, because the transfected HCV RNA would not be amplified at this early time point; thus, GLuc activity at 8 hours reflects the levels of translated HCV proteins, rather than amplified HCV RNA. To examine the effect of HULC on translation directed by HCV IRES, we used 2 nonreplicating RNA genomes: one was a mini-genomic RNA sequentially containing HCV 5′-UTR, GLuc, and HCV 3′-UTR [33], and the other was an HCV full-genomic RNA containing the GLuc-coding sequence located between p7 and NS2 with the catalytic center GDD motif of NS5B mutated to AAG (Figure 5B) [32]. Huh-7.5 cells were transfected with the HULC-coding vector or an empty vector. At 48 hours later, they were transfected with mini-genomic or full-genomic HCV RNA, and GLuc activity was measured at 12 hours later. HULC overexpression enhanced GLuc activity in the mini-genomic and full-genomic HCV RNA-transfected cells compared with the empty vector-transfected cells (Figure 5C, left). We also examined the effect of HULC suppression by siRNA on HCV translation. Huh-7.5 cells were transfected with si-HULC-3 or si-CNT. At 48 hours later, they were transfected with mini-genomic or full-genomic HCV RNA, and GLuc activity was measured at 12 hours later. HULC suppression reduced GLuc activity in the mini-genomic and full-genomic HCV RNA–transfected cells compared with the si-CNT–transfected cells (Figure 5C, right). We examined the effect of HULC overexpression and knockdown on cap-dependent translation and encephalomyocarditis virus IRES-mediated translation with the same method as that used for HCV IRES-mediated translation. We found that neither HULC overexpression nor knockdown had an effect on these translations, except HCV (Supplementary Figure 13). These results suggest that HULC specifically regulates HCV IRES-mediated translation.
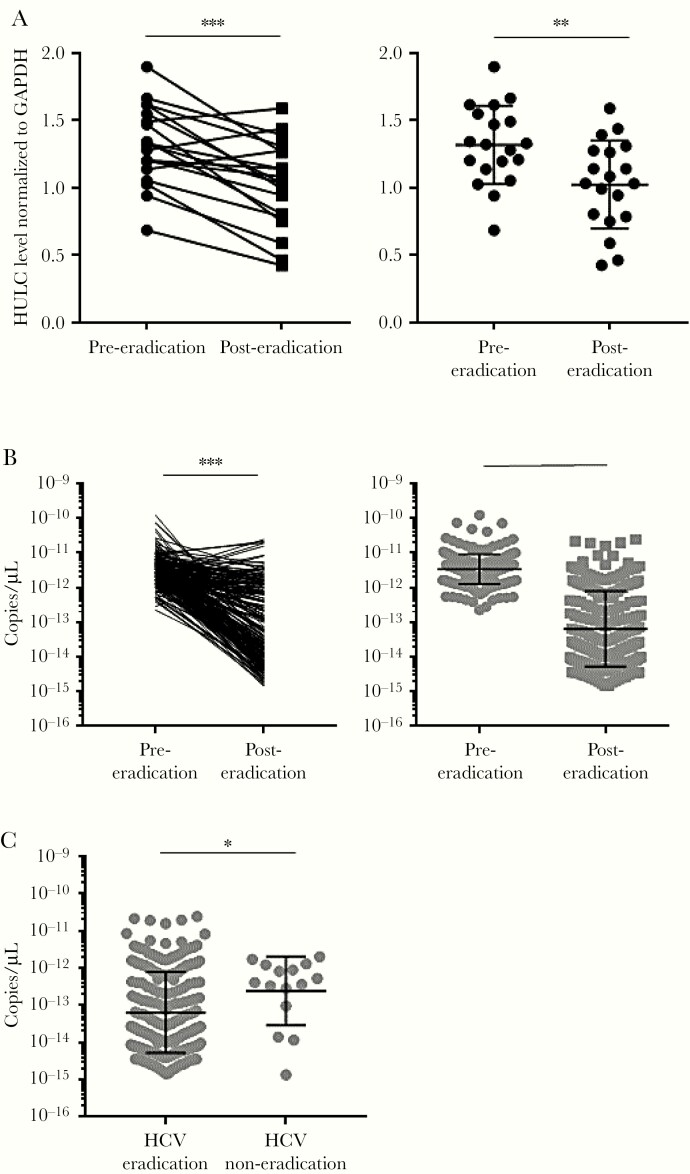
HULC in Human Liver and Blood
The data from Huh-7.5 cells, FT3-7 cells, and the human-liver chimeric mouse model clearly showed that HCV infection and replication increase HULC levels; however, it was unclear whether HCV infection could increase HULC expression in human liver. Therefore, we examined whether HCV eradication by antiviral treatment could reduce HULC expression in human liver. A total of 19 paired RNA samples from HCV-infected human liver were available from before antiviral treatment with pegylated IFN with or without ribavirin/telaprevir and after the eradication of HCV. The clinicopathological features of these patients are shown in Supplementary Table 1. When we compared HULC levels in human liver before and after HCV eradication, its expression was significantly decreased in 16 patients (Figure 6A). We also measured HULC levels in blood samples taken from HCV-infected patients. Because HULC levels in blood were expected to be very low, there is a possibility that HCV RNA could affect the quantitation of HULC in blood. Therefore, we confirmed that the presence of HULC and HCV RNA did not affect the PCR quantification of each by measuring serially diluted HCV- and HULC-encoding plasmids (Supplementary Figure 14). A total of 213 paired RNAs from HCV-infected human blood were available that were taken before antiviral treatment with the NS3/4A inhibitor asunaprevir and the NS5A inhibitor daclatasvir, and after the eradication of HCV. When we compared HULC levels in human blood before and after HCV eradication, its expression was significantly decreased in most cases (Figure 6B). We also had RNA samples isolated from blood samples of 15 patients in whom HCV was not eradicated by this treatment. We compared HULC expression in the blood after antiviral treatment between the 213 patients with successful HCV eradication and the 15 patients with unsuccessful HCV eradication. HULC expression was significantly higher in the blood of the HCV eradication–failed patients compared with the successfully treated patients (Figure 6C). These results show that HCV eradication can reduce HULC levels in human liver and blood and suggest that HCV infection increases HULC expression in humans.
Figure 6.
HULC levels in human liver and blood. A, Total RNA was isolated from liver samples of 19 patients at pre– and post–hepatitis C virus (HCV) eradication. HULC and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) RNA levels in human liver were quantified by reverse-transcription quantitative polymerase chain reaction (RT-qPCR). HULC levels were normalized to those of GAPDH RNA. Differences in HULC levels between pre- and posteradication were analyzed by a paired t test (left) and Mann–Whitney test (right). The left panel shows paired levels in individual patients and the right panel shows nonpaired levels for all patients. The bars show geometric mean with geometric standard deviation (SD). B, Total RNA was isolated from blood samples of 213 patients at pre- and post-HCV eradication. HULC levels in blood per microliter were quantified by RT-qPCR. The left panel shows paired levels in individual patients and the right panel shows nonpaired levels for all patients. The bars show geometric mean with geometric SD. Differences in HULC levels between pre- and post-eradication were analyzed by a paired t test (left) and Mann–Whitney test (right). C, Total RNA was isolated from blood samples of 15 patients in whom HCV eradication was unsuccessful and 213 patients with successful HCV eradication at postantiviral treatment. HULC levels in blood per microliter were quantified by RT-qPCR. The bars show geometric mean with geometric SD. Differences in HULC levels between HCV-eradicated and HCV-noneradicated patients were analyzed by Mann–Whitney test. *P < .05, **P < .01, ***P < .001.
DISCUSSION
In this study, we showed that HULC expression is upregulated by HCV infection in cell culture and human-liver chimeric mouse models. In addition, HULC expression in human liver and blood was significantly reduced by HCV eradication. These results suggest that HCV infection increases HULC levels. Furthermore, increased HULC expression augmented HCV RNA replication by enhancing HCV IRES-directed translation. Although HULC upregulation by HCV was reported by Sharma et al using a cell culture model [34], our study clearly showed that it is also the case in humans. Furthermore, this is the first report showing that HCV IRES-directed translation is regulated by an lncRNA.
In the mechanism of HULC upregulation by HCV infection, HCV core protein was shown to increase retinoid X receptor (RXR) α–mediated transcription, which induces HULC transcription [35]. In our study, HULC upregulation was observed in subgenomic replicon cells in which HCV core protein was absent, as well as in full-genomic replicon cells, suggesting that nonstructural proteins, as well as HCV core protein, could enhance HULC transcription. Furthermore, when we individually overexpressed nonstructural proteins, only NS5A overexpression increased HULC expression and HULC promoter activity, suggesting that NS5A increases the transcription of HULC. The mechanism of HULC upregulation has been examined in more depth for hepatitis B virus; HBx enhances the promoter activity of HULC through the transcription factor CREB [29]. Besides CREB, several transcription factors, such as RXR, Sp1, Sp3, and Sp4, are reported to upregulate HULC expression [35, 36]. In addition to transcriptional regulation, HULC is regulated by posttranscriptional destabilization via binding to IGF-2 mRNA-binding protein [37]. These studies suggest that HULC expression can be adjusted by various mechanisms. In a future study, we will clarify the mechanisms by which NS5A upregulates HULC promoter activity.
Sharma et al showed that HULC is upregulated during HCV infection and enhances HCV virus-particle release by increasing the number of lipid droplets (LDs) and promoting the association of HCV core protein with LDs [34]. LDs are important for infectious virus production, mainly through HCV core protein [38, 39]. Furthermore, NS5A interacts with the LD-associated protein tail-interacting protein 47, which coats LDs and is involved in their generation and turnover, and this interaction is important for HCV RNA replication [40]. Our results showed that HULC modulates HCV replication through an HCV IRES-directed translation step; however, in addition to this, the effects of HULC on HCV replication observed in this study could also be due to its effect on LDs. Taken together, these findings indicate that HULC modulates HCV infection through several steps including infectious virus production, HCV RNA amplification, and HCV IRES-directed translation.
Our results suggest that HULC regulates IRES-directed HCV translation. We speculated that HULC could interact directly with the 5′-UTR of HCV and modulate HCV IRES-directed translation. An in silico analysis predicted a potential interaction between nucleotides 319–324 of HCV 5′-UTR and nucleotides 163–168 of HULC (Supplementary Figure 15A). Although mutational analysis suggested they could interact via the predicted sites (Supplementary Figure 15B and 15C), it was difficult to prove that this interaction was crucial for the effect of HULC on HCV replication (Supplementary Figure 15D). Because HCV translation is regulated by many host factors, HULC could alter the expression of such HCV translation–related host proteins, resulting in the modulation of HCV translation. These possibilities will be examined in future studies.
Increased HULC expression in malignant tissues has been observed in pancreatic cancer [19], gastric cancer [21], osteosarcoma [35], and colorectal cancer that metastasizes to the liver [20]. Notably, HULC levels in blood are a useful noninvasive biomarker for the diagnosis and/or prognosis of HCC [18], and there is a strong correlation between the tissue and circulating levels of HULC [41]. We found that HULC expression in liver and blood was generally reduced by HCV eradication; however, in several patients, HULC expression was not reduced or even increased by HCV eradication. HULC exerts its oncogenic functions by promoting many steps, including cell survival, proliferation, colony formation, invasion, tumorgenicity, lipogenesis, epithelial-mesenchymal transition, and antigenicity, in several cancers, as summarized in a review article [42]. The multiple oncogenic features of HULC suggest the possibility that the patients whose HULC levels were not reduced after HCV eradication would have a higher risk of HCC occurrence. To test the hypothesis that HULC could be a useful predictive factor for HCC occurrence after HCV eradication, a prospective study is needed.
In this study, we clearly demonstrated that the oncogenic lncRNA HULC is upregulated by HCV infection in humans and in cultured cells. These results suggest crucial roles for HULC in hepatocarcinogenesis and cancer progression due to HCV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the following members of the Hokuriku Liver Study Group: Kanazawa University Hospital, Toyama Prefectural Central Hospital, Toyama City Hospital, Tonami General Hospital, Kurobe City Hospital, Kouseiren Namerikawa Hospital, Ogino Medical Office, National Hospital Organization Kanazawa Medical Center, Ishikawa Central Prefectural Hospital, Yawata Medical Center, Ushitsu General Hospital, Komatsu Sophia Hospital, Keiju Medical Center, Kanazawa Arimatsu Hospital, Kahoku Central Hospital, Hakui General Hospital, Noto General Hospital, Nomi Municipal Hospital, Wajima Municipal Hospital, Kanazawa Municipal Hospital, Kanazawa Red Cross Hospital, Kanazawa Seirei Hospital, Saiseikai Kanazawa Hospital, Public Central Hospital of Matto Ishikawa, Fukui-ken Saiseikai Hospital, Tsuruga Municipal Hospital, Fukui Prefectural Hospital, and Kumagai Clinic.
Disclaimer. The funding sources played no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit this article for publication.
Financial support. This work was supported by the following grants: Health and Labor Sciences Research Grant (grant number 12100730 to T. Shim.); the Promotion of Science (JSPS) KAKENHI (grant number JP15K08492, Grant-in-Aid for Scientific Research [C] to T. Shim.); the JSPS Core-to-Core Program, B, Asia-Africa Science Platforms (to S. K.); and the Japan Agency for Medical Research and Development (grant numbers JP19fk0210046 to T. Shir. and T. Shim. and JP20fk0210081 to K. M. and T. Shim.).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Juria Kitabayashi, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Takayoshi Shirasaki, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Tetsuro Shimakami, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Tomoaki Nishiyama, Advanced Science Research Center, Kanazawa University, Kanazawa, Japan.
Christoph Welsch, Department of Internal Medicine I, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany.
Masaya Funaki, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Kazuhisa Murai, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Ariunaa Sumiyadorj, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Hajime Takatori, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Kazuya Kitamura, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Kazunori Kawaguchi, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Kuniaki Arai, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Taro Yamashita, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Yoshio Sakai, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Tatsuya Yamashita, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Eishiro Mizukoshi, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Masao Honda, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
Shuichi Kaneko, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
The Hokuriku Liver Study Group, Department of Gastroenterology, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa, Japan.
References
- 1. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol 2014; 61:S58–68. [DOI] [PubMed] [Google Scholar]
- 2. Asselah T, Marcellin P, Schinazi RF. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int 2018; 38(Suppl 1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017; 153:996–1005e1. [DOI] [PubMed] [Google Scholar]
- 4. Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology 2019; 69:487–97. [DOI] [PubMed] [Google Scholar]
- 5. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma [manuscript published online ahead of print 5 September 2017]. J Hepatol 2017. doi:10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019; 393:1453–64. [DOI] [PubMed] [Google Scholar]
- 7. Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 2005; 309:1577–81. [DOI] [PubMed] [Google Scholar]
- 8. Wang P, Xue Y, Han Y, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014; 344:310–3. [DOI] [PubMed] [Google Scholar]
- 9. Huarte M, Guttman M, Feldser D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010; 142:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sauvageau M, Goff LA, Lodato S, et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife 2013; 2:e01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heward JA, Lindsay MA. Long non-coding RNAs in the regulation of the immune response. Trends Immunol 2014; 35:408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiong Y, Yuan J, Zhang C, et al. The STAT3-regulated long non-coding RNA Lethe promote the HCV replication. Biomed Pharmacother 2015; 72:165–71. [DOI] [PubMed] [Google Scholar]
- 13. Carnero E, Barriocanal M, Prior C, et al. Long noncoding RNA EGOT negatively affects the antiviral response and favors HCV replication. EMBO Rep 2016; 17:1013–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu P, Wilhelm J, Gerresheim GK, Shalamova LA, Niepmann M. Lnc-ITM2C-1 and GPR55 are proviral host factors for hepatitis C virus. Viruses 2019; 11:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishitsuji H, Ujino S, Yoshio S, et al. Long noncoding RNA #32 contributes to antiviral responses by controlling interferon-stimulated gene expression. Proc Natl Acad Sci U S A 2016; 113:10388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Panzitt K, Tschernatsch MM, Guelly C, et al. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology 2007; 132:330–42. [DOI] [PubMed] [Google Scholar]
- 17. Sonohara F, Inokawa Y, Hayashi M, et al. Prognostic value of long non-coding RNA HULC and MALAT1 following the curative resection of hepatocellular carcinoma. Sci Rep 2017; 7:16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie H, Ma H, Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int 2013; 2013:136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng W, Gao W, Feng J. Long noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancer. Med Oncol 2014; 31:346. [DOI] [PubMed] [Google Scholar]
- 20. Matouk IJ, Abbasi I, Hochberg A, Galun E, Dweik H, Akkawi M. Highly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasis. Eur J Gastroenterol Hepatol 2009; 21:688–92. [DOI] [PubMed] [Google Scholar]
- 21. Zhao Y, Guo Q, Chen J, Hu J, Wang S, Sun Y. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep 2014; 31:358–64. [DOI] [PubMed] [Google Scholar]
- 22. Shirasaki T, Honda M, Shimakami T, et al. Impaired interferon signaling in chronic hepatitis C patients with advanced fibrosis via the transforming growth factor beta signaling pathway. Hepatology 2014; 60:1519–30. [DOI] [PubMed] [Google Scholar]
- 23. Yi M, Ma Y, Yates J, Lemon SM. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol 2007; 81:629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ikeda M, Yi M, Li K, Lemon SM. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J Virol 2002; 76:2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kishine H, Sugiyama K, Hijikata M, et al. Subgenomic replicon derived from a cell line infected with the hepatitis C virus. Biochem Biophys Res Commun 2002; 293:993–9. [DOI] [PubMed] [Google Scholar]
- 26. Shimakami T, Hijikata M, Luo H, et al. Effect of interaction between hepatitis C virus NS5A and NS5B on hepatitis C virus RNA replication with the hepatitis C virus replicon. J Virol 2004; 78:2738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi M, Lemon SM. Adaptive mutations producing efficient replication of genotype 1a hepatitis C virus RNA in normal Huh7 cells. J Virol 2004; 78:7904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu F, Shimakami T, Murai K, et al. Efficient suppression of hepatitis C virus replication by combination treatment with miR-122 antagonism and direct-acting antivirals in cell culture systems. Sci Rep 2016; 6:30939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sumpter R Jr, Loo YM, Foy E, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 2005; 79:2689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du Y, Kong G, You X, et al. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J Biol Chem 2012; 287:26302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Liu X, Wu H, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res 2010; 38:5366–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shimakami T, Yamane D, Jangra RK, et al. Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc Natl Acad Sci U S A 2012; 109:941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimakami T, Honda M, Shirasaki T, et al. The acyclic retinoid peretinoin inhibits hepatitis C virus replication and infectious virus release in vitro. Sci Rep 2014; 4:4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma G, Tripathi SK, Das S. lncRNA HULC facilitates efficient loading of HCV-core protein onto lipid droplets and subsequent virus-particle release. Cell Microbiol 2019; 21:e13086. [DOI] [PubMed] [Google Scholar]
- 35. Cui M, Xiao Z, Wang Y, et al. Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res 2015; 75:846–57. [DOI] [PubMed] [Google Scholar]
- 36. Gandhy SU, Imanirad P, Jin UH, et al. Specificity protein (Sp) transcription factors and metformin regulate expression of the long non-coding RNA HULC. Oncotarget 2015; 6:26359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hämmerle M, Gutschner T, Uckelmann H, et al. Posttranscriptional destabilization of the liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding protein 1 (IGF2BP1). Hepatology 2013; 58:1703–12. [DOI] [PubMed] [Google Scholar]
- 38. Miyanari Y, Atsuzawa K, Usuda N, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 2007; 9:1089–97. [DOI] [PubMed] [Google Scholar]
- 39. Shavinskaya A, Boulant S, Penin F, McLauchlan J, Bartenschlager R. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J Biol Chem 2007; 282: 37158–69. [DOI] [PubMed] [Google Scholar]
- 40. Vogt DA, Camus G, Herker E, et al. Lipid droplet-binding protein TIP47 regulates hepatitis C virus RNA replication through interaction with the viral NS5A protein. PLoS Pathog 2013; 9:e1003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J, Wang X, Tang J, et al. HULC and Linc00152 Act as novel biomarkers in predicting diagnosis of hepatocellular carcinoma. Cell Physiol Biochem 2015; 37:687–96. [DOI] [PubMed] [Google Scholar]
- 42. Yu X, Zheng H, Chan MT, Wu WK. HULC: an oncogenic long non-coding RNA in human cancer. J Cell Mol Med 2017; 21:410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.