Abstract
Background
Respiratory syncytial virus (RSV) is a significant cause of severe lower respiratory tract disease in children and older adults, but has no approved vaccine. This study assessed the potential of Ad26.RSV.preF to protect against RSV infection and disease in an RSV human challenge model.
Methods
In this double-blind, placebo-controlled study, healthy adults aged 18–50 years were randomized 1:1 to receive 1 × 1011 vp Ad26.RSV.preF or placebo intramuscularly. Twenty-eight days postimmunization, volunteers were challenged intranasally with RSV-A (Memphis 37b). Assessments included viral load (VL), RSV infections, clinical symptom score (CSS), safety, and immunogenicity.
Results
Postchallenge, VL, RSV infections, and disease severity were lower in Ad26.RSV.preF (n = 27) vs placebo (n = 26) recipients: median VL area under the curve (AUC) quantitative real-time polymerase chain reaction: 0.0 vs 236.0 (P = .012; predefined primary endpoint); median VL-AUC quantitative culture: 0.0 vs 109; RSV infections 11 (40.7%) vs 17 (65.4%); median RSV AUC-CSS 35 vs 167, respectively. From baseline to 28 days postimmunization, geometric mean fold increases in RSV A2 neutralizing antibody titers of 5.8 and 0.9 were observed in Ad26.RSV.preF and placebo, respectively. Ad26.RSV.preF was well tolerated.
Conclusions
Ad26.RSV.preF demonstrated protection from RSV infection through immunization in a human challenge model, and therefore could potentially protect against natural RSV infection and disease.
Clinical Trials Registration
NCT03334695; CR108398, 2017-003194-33 (EudraCT); VAC18193RSV2002.
Keywords: respiratory syncytial virus, vaccine, challenge study, adenoviral vectors, RSV fusion protein, Pre-F protein, adults
Ad26.RSV.preF, an investigational vaccine against Respiratory Syncytial Virus (RSV) was evaluated in an RSV human challenge model. Vaccinated individuals showed a reduction in infections, viral load and disease severity compared to placebo. Ad26.RSV.preF warrants further investigation in field efficacy studies.
Globally, respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infection and is a major cause of hospitalizations, morbidity, and mortality in children ≤5 years of age and older adults, particularly those aged ≥60 years [1–4]. Annually, RSV infection develops in 3%–7% of older adults, where RSV-associated hospitalizations are often more severe than those associated with influenza [5, 6].
RSV glycoprotein (RSV-G), which initiates viral attachment to the host cell, and RSV fusion protein (RSV-F), which in its prefusion (pre-F) conformation mediates viral cell membrane fusion, play a major role in the RSV viral life cycle [7]. Unlike influenza, RSV is antigenically stable, with only the RSV-G protein undergoing significant changes by season [8]. Therefore, the RSV-F protein is a favorable target for an effective vaccine, as it is highly conserved season-to-season across RSV strains [7, 8]. Epitopes specific to the pre-F protein appear to be more potent than those on the postfusion conformation (post-F) of RSV-F [7, 8]. However, wild-type pre-F is highly unstable and rapidly converts to a post-F conformation [9]. RSV vaccine candidates based on the post-F conformation so far failed to protect against RSV-mediated lower respiratory tract disease (LRTD) [10, 11]. However, a recent study showed that the RSV-F protein stabilized in the pre-F conformation induced [12].
Ad26.RSV.preF is a recombinant adenovirus serotype 26 vector (Ad26) that encodes for a full-length RSV-F protein stabilized in the pre-F protein conformation [13]. Ad26-based vectors investigated to date have elicited both humoral and cell-mediated immune responses without requiring an adjuvant, and have a good safety profile [14, 15]. Ad26.RSV.preF has demonstrated immunogenicity and induced robust RSV-specific humoral and cell-mediated immune responses that were durable for at least 2 years postimmunization in a first-in-human phase 1 study in older healthy adults aged ≥60 years [16]. A trend for a higher humoral immune response was observed with the higher vaccine dose (1 × 1011 viral particles [vp]) compared with the lower dose (5 × 1010 vp), with no safety concerns [16].
This study aimed to demonstrate the potential of Ad26.RSV.preF to protect against RSV infection and disease in healthy adult volunteers, utilizing the established human viral challenge (HVC) model for the first time to evaluate an RSV vaccine candidate.
METHODS
Study Design
This randomized, placebo-controlled, double-blind phase 2a HVC study, conducted at a single quarantine unit (hVIVO, United Kingdom) comprised screening, immunization, viral challenge, and outpatient phases (Supplementary Figure 1). The screening phase was conducted between days –84 and –31. Historical prescreening data collected through the Ethics Committee–approved hVIVO screening protocol within 56 days to 3 days prior to vaccination (90 days for viral serology) could be used for screening procedures. Historical prescreening data obtained prior to this window could be reassessed any time from 40 days to 3 days prior to immunization. In the immunization phase, volunteers were randomized 1:1 to receive either 1 × 1011 vp Ad26.RSV.preF or placebo (0.9% saline) intramuscularly at day –28 and were followed up 28 days postimmunization. In the viral challenge phase, volunteers entered the quarantine unit 1–2 days prior to challenge. Volunteers were inoculated intranasally with RSV-A (4 log10 plaque-forming units [PFU]/mL Memphis 37b) on day 0 (generally within 25–33 days postimmunization with allowance of up to 90 days) and followed up for 12 days. Volunteers were discharged from the unit on day 12 if RSV was not detected in the nasopharyngeal sample collected before discharge. If symptoms were present but RSV was not detected, the volunteer was discharged at the discretion of the investigator. If appropriate, volunteers were able to reside in quarantine for an additional night or longer before discharge. In the outpatient phase, volunteers were followed up for 6 months postimmunization.
Participants
Healthy adult volunteers aged 18–50 years were prescreened (to enrich the population for increased susceptibility to RSV infection) before inclusion in the study. Volunteers with levels of RSV neutralizing antibodies compatible with susceptibility to RSV infection were included. Susceptibility was determined within 90 days of immunization and on entry to the quarantine unit (ie, 1–2 days prior to challenge with RSV-A Memphis 37b virus, for post hoc confirmation of susceptibility). The cutoff was based on the bottom 25th percentile of the previous 12 months of screening results (half-maximal inhibitory concentration [IC50] of 810). Full inclusion and exclusion criteria are detailed in the Supplementary Appendix. All volunteers provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (1996 version), the International Conference on Harmonization Good Clinical Practice guidelines, applicable regional and local regulations, and the study protocol.
Procedures
From day 2 postchallenge, nasal wash samples were collected (twice daily from day 2 to day 11, once daily on day 12) and symptom score cards were completed thrice daily by volunteers at entry to the quarantine unit (day 1) until day 11 after challenge, and once at discharge from the quarantine unit on day 12, and reviewed by the attending physician. Tissue counts and mucus weights were accumulated every 24 hours. Blood samples for humoral immunity were collected preimmunization, 1–2 days prior to viral challenge, and at 28 days postchallenge. Isolated peripheral blood mononuclear cells were collected to measure cell-mediated immunity (CMI). Serious adverse events (SAEs) were reported by volunteers from immunization until 6 months postchallenge. Solicited adverse events (AEs) were reported by volunteers for 7 days postimmunization. Unsolicited AEs were reported by volunteers 28 days postimmunization and 28 days postchallenge.
RSV infection was defined as either asymptomatic and symptomatic (≥2 quantifiable real-time polymerase chain reaction [rt-PCR] measurements above the lower limit of quantification [LLOQ]), liberal (≥2 quantifiable rt-PCR measurements above the LLOQ plus any clinical symptom of any severity), or conservative (≥2 quantifiable rt-PCR measurements above the LLOQ and symptoms from 2 different categories [upper respiratory, lower respiratory, systemic] from the symptom score card or any grade 2 symptom from any category). Peak viral load (VL) was defined as the maximum VL of nasal wash samples determined by quantitative rt-PCR assay observed over the quarantine period.
Outcomes
The prespecified primary endpoint was the area under the VL-time curve of RSV (RSV VL-AUC) from challenge to discharge determined by quantitative rt-PCR assay of nasal wash samples. Other efficacy endpoints included RSV VL-AUC determined by quantitative culture, volunteers with RSV infection and peak VL, AUC of the total clinical symptom score (CSS; a composite of 13 self-reported symptoms, detailed in the Supplementary Appendix), total mucus weight, and tissue count (number of tissues used for nasal secretions). Immunogenicity endpoints included RSV A2 neutralizing antibody titers (virus neutralization assay), pre-F and post-F RSV-F protein binding serum antibody (immunoglobulin G [IgG]) titers (enzyme-linked immunosorbent assay [ELISA]), intranasal pre-F immunoglobulin A (IgA) antibody titers (ELISA), and Ad26 neutralizing antibody titers (adenovirus neutralization assay). Safety endpoints included solicited local and systemic AEs, unsolicited AEs, and SAEs. Post hoc, the vaccine efficacy (VE) based on RSV-infection definitions was calculated. Post hoc analysis of the CSS and VL as determined by rt-PCR and by quantitative culture was conducted on volunteers with breakthrough infections (≥2 timepoints with rt-PCR VL values above the LLOQ). Post hoc analyses were also conducted on the time to first of 2 positive rt-PCR results and first positive viral culture, and the relationship between infection and the prechallenge titers of neutralizing antibodies to the RSV A2 strain in Ad26.RSV.preF recipients.
Statistical Analysis
The study was only powered for the primary efficacy endpoint with details of power calculations discussed in the Supplementary Appendix. All volunteers who were randomized and received Ad26.RSV.preF or placebo, regardless of the occurrence of protocol deviations, were included in the safety population (full analysis set). Randomized volunteers who received Ad26.RSV.preF or placebo and viral challenge, were in the primary analysis population for efficacy evaluation (intent-to-treat challenge population). All randomized volunteers who received Ad26.RSV.preF or placebo and had immunogenicity data available (excluding volunteers with a major protocol deviation impacting the immunogenicity outcomes) were included in the per-protocol immunogenicity population, upon which immunogenicity analysis was conducted. For the primary endpoint, an exact Wilcoxon rank-sum test was performed, and the 1-sided P value was interpreted at the 5% and 20% significance level. The same test was used to assess all other continuous secondary endpoints. VE was evaluated using a Farrington–Manning score method. A generalized linear regression was used to model the probability of being infected in the Ad26.RSV.preF group using the log-transformed prechallenge titers of neutralizing antibodies to the RSV A2 strain as an independent variable.
RESULTS
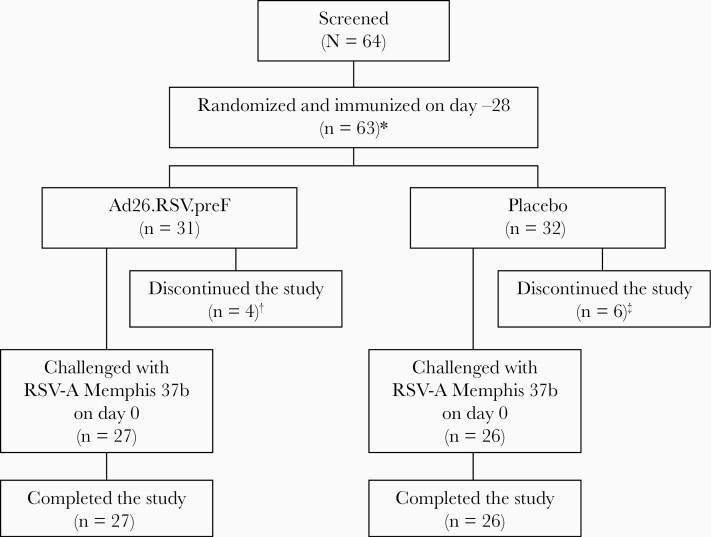
Overall, 63 volunteers were randomized and immunized with Ad26.RSV.preF (n = 31) or placebo (n = 32), with the study conducted from 2 August 2017 through 27 November 2018. Ten volunteers receiving Ad26.RSV.preF (n = 4) or placebo (n = 6) discontinued before receiving viral challenge; 6 were lost to follow-up (Ad26.RSV.preF, n = 3; placebo, n = 3), 3 discontinued as per the investigator’s decision (Ad26.RSV.preF, n = 1; placebo, n = 2), and 1 discontinued in the placebo group due to a positive cotinine test. Fifty-three patients were subsequently challenged with RSV (Ad26.RSV.preF, n = 27; placebo, n = 26) 25–33 days following immunization, with the exception of 1 volunteer in the placebo group who was challenged 73 days postimmunization (Figure 1). Baseline characteristics were similar between groups (Table 1).
Figure 1.
Volunteer disposition. *One volunteer was screened and randomized, but not immunized. †Three volunteers were lost to follow-up, and 1 volunteer was discontinued per the investigator’s decision (not due to an adverse event [AE]). ‡Three volunteers were lost to follow-up, 2 volunteers were discontinued per the investigator’s decision (not due to an AE), and 1 volunteer was discontinued due to a positive cotinine test, an exclusion criterion. Abbreviation: RSV-A, respiratory syncytial virus subtype A.
Table 1.
Baseline Characteristics of Immunized Volunteers
| Characteristic | Ad26.RSV.preF | Placebo |
|---|---|---|
| Intent-to-treat challenge analysis set | ||
| No. | 27 | 26 |
| Female sex | 12 (44.4) | 6 (23.1) |
| Median age, y, range (min, max) | 24 (18, 45) | 25 (18, 41) |
| Racea | ||
| Asian | 2 (7.7) | 1 (4) |
| Black or African American | 1 (3.8) | 0 |
| White | 22 (84.6) | 24 (96) |
| Multiracial | 1 (3.8) | 0 |
| Median weight, kg (min, max) | 71.7 (50.7, 94.5) | 74.35 (55.8, 103.5) |
| Median BMI, kg/m2 (min, max) | 24 (17.6, 28.4) | 23.15 (20.7, 33.3) |
| Full analysis set | ||
| No. | 31 | 32 |
| Female sex | 12 (38.7) | 6 (18.8) |
| Median age, y, range (min, max) | 24.0 (18, 45) | 25.0 (18, 41) |
| Racea | ||
| Asian | 3 (10.3) | 1 (3.4) |
| Black or African American | 1 (3.4) | 0 |
| White | 24 (82.8) | 28 (96.6) |
| Multiracial | 1 (3.4) | 0 |
| Median weight, kg (min, max) | 75.10 (50.7, 112.8) | 74.35 (54.7, 107.5) |
| Median BMI, kg/m2 (min, max) | 24.20 (17.6, 32.7) | 23.15 (18.4, 36.2) |
| Baseline immunogenicity (per-protocol immunogenicity set) | ||
| No. | 30 | 31 |
| Titers of neutralizing antibodies to RSV A2, IC50, (95% CI) | 267 (222–320) | 283 (248–322) |
| Pre-F IgG serum antibody response, ELISA units/L (95% CI) | 109 (85–141) | 112 (91–139) |
| Post-F IgG serum antibody response, ELISA units/L (95% CI) | 101 (76–135) | 97 (78–121) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; IC50, half-maximal inhibitory concentration; IgG, immunoglobulin G; RSV, respiratory syncytial virus.
aTwo volunteers did not report race.
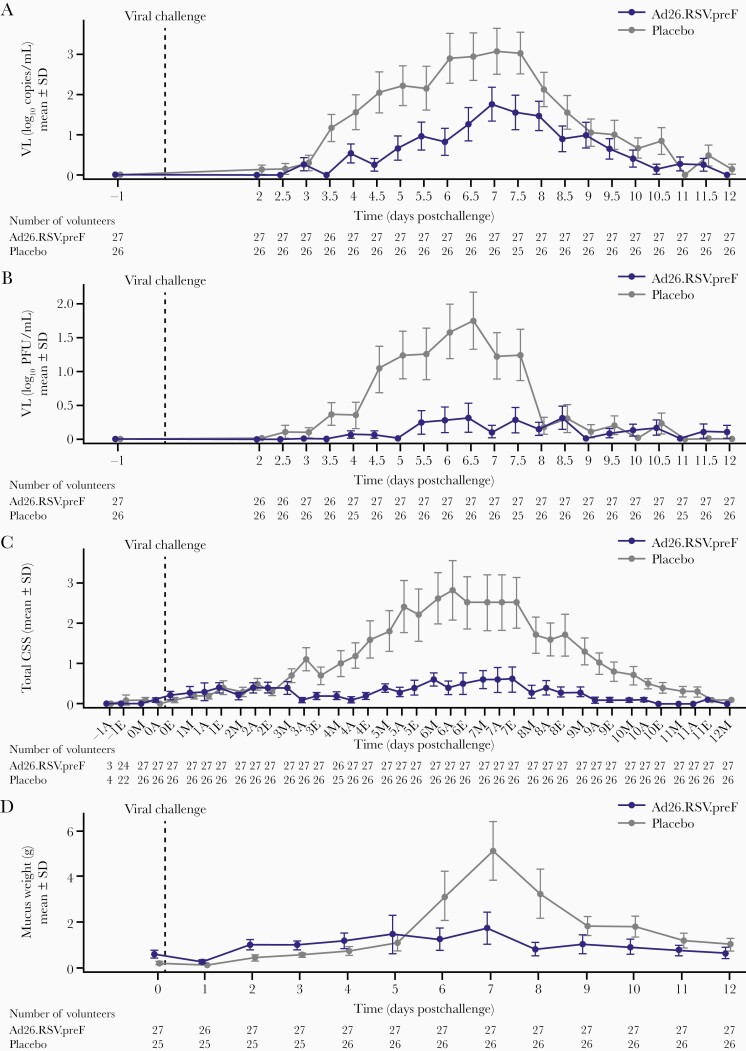
Postchallenge with RSV, the infection rate was lower in recipients of Ad26.RSV.preF than placebo. The VL remained below the LLOQ postchallenge in 14 of 27 (51.9%) volunteers receiving Ad26.RSV.preF and in 6 of 26 (23.1%) volunteers receiving placebo. The primary endpoint (rt-PCR–determined RSV VL-AUC) was significantly lower in volunteers who received Ad26.RSV.preF than placebo (median, 0.0 vs 236 log10 copies × hour/mL) (P = .012) (Table 2). In both groups, mean VL peaked after approximately 7 days postchallenge, with the mean VL being considerably lower in Ad26.RSV.preF than placebo recipients at most timepoints (Figure 2A). Similarly, quantitative culture–determined RSV VL-AUC and peak VL (rt-PCR) were both substantially lower in those who received Ad26.RSV.preF compared with placebo (Table 2). In the placebo group, mean VL (as determined by quantitative culture) began to increase 3 days postchallenge, peaking 6.5 days postchallenge and decreasing substantially 7.5 days postchallenge. Mean VL in the Ad26.RSV.preF group was <0.5 log10 PFU/mL throughout the study (Figure 2B). Post hoc analysis of volunteers with breakthrough infections yielded similar results (Supplementary Figure 2).
Table 2.
Summary of Efficacy Outcomes Following Intranasal Viral Challenge With Respiratory Syncytial Virus Subtype A After Receiving Intramuscular Immunization With Either Ad26.RSV.preF or Placebo (Intent-to-Treat Challenge Population)
| Endpoint | Ad26.RSV.preF (n = 27) | Placebo (n = 26) | Difference Between Ad26.RSV.preF and Placebo |
|---|---|---|---|
| Primary endpoint, median (Q1, Q3) | |||
| RSV VL-AUC (rt-PCR) (log10 copies × h/mL) | 0.0 (0.0, 268.8) | 236.0 (20.3, 605.8) | P = .012a |
| Secondary endpoints, median (Q1, Q3) | |||
| RSV VL-AUC (quantitative culture) (log10 PFU × h/mL) | 0.0 (0.0, 20.3) | 109.0 (0.0, 227.5) | P = .002a,b |
| Peak VL (rt-PCR) (log10 copies/mL) | 0.0 (0.0, 4.5) | 5.4 (3.0, 6.7) | P = .004a,b |
| AUC of mucus weight (g × h) | 102.0 (10.4, 380.2) | 333.0 (46.4, 669.0) | P = .056a,b |
| AUC of total clinical symptom score (TSS × h) | 35.0 (0.0, 91.2) | 167 (38.7, 428.5) | P = .002a,b |
| Secondary endpoints, No. (%) | VE, % (95% CI)c | ||
| % RSV-infected volunteers (asymptomatic and symptomatic)d | 11 (40.7) | 17 (65.4) | 37.7 (–5.7 to 69.2) |
| RSV-infected volunteers (liberal)e | 9 (33.3) | 16 (61.5) | 45.8 (–1.0 to 73.8) |
| RSV-infected volunteers (conservative)f | 6 (22.2) | 12 (46.2) | 51.9 (–7.4 to 83.2) |
Abbreviations: AUC, area under the curve; CI, confidence interval; PFU, plaque-forming units; Q1, first quartile; Q3, third quartile; RSV, respiratory syncytial virus; rt-PCR, real-time polymerase chain reaction; TSS, total symptom score; VE, vaccine effectiveness; VL, viral load.
aWilcoxon rank-sum test, 1-sided P value.
bAs this was an exploratory study, hypothesis testing was only planned for the primary endpoint. No testing strategy (and corresponding α-level adjustment) was specified for any other analysis; therefore, P values should be interpreted with caution.
cFarrington–Manning score method.
dAsymptomatic and symptomatic: ≥2 quantifiable rt-PCR measurements.
eLiberal: ≥2 quantifiable rt-PCR measurements and any clinical symptom (regardless grade or class).
fConservative: ≥2 quantifiable rt-PCR measurements and clinical symptoms of 2 different categories or any grade 2.
Figure 2.
Efficacy as determined by viral load as determined by real-time polymerase chain reaction (rt-PCR) and quantitative culture, and disease severity as determined by total clinical symptom score (CSS) and mucus weight over time following intranasal viral challenge with respiratory syncytial virus subtype A (RSV-A) after receiving immunization with either Ad26.RSV.preF or placebo intramuscularly (intent-to-treat challenge population). A, Viral load as determined by rt-PCR. B, Viral load as determined by quantitative culture over time. C, Total CSS over time. On the x-axis, A indicates afternoon; E, evening; M, morning. D, Total mucus weight over time. Viral load as determined by rt-PCR, viral load as determined by quantitative culture, CSS, and mucus weight are shown from viral challenge at day 0 until 12 days postchallenge in the intent-to-treat challenge set. From days 2 to 12 postchallenge, nasal wash samples were collected (twice daily from days 2 to 11, once daily on day 12), and symptom score cards were completed by volunteers (thrice daily from days 2 to 11, once daily on day 12) and reviewed by the attending physician. Mucus weights were determined every 24 hours. Placebo and Ad26.RSV.preF were measured at the same timepoints, jitter was applied to ensure both Ad26.RSV.preF and placebo means, and confidence intervals are visible even if close to each other. Abbreviations: CSS, clinical symptom score; SD, standard deviation; VL, viral load.
The AUC of the total CSS was markedly lower in those who received Ad26.RSV.preF compared with placebo (35.0 vs 167.0; Table 2). Volunteers who received Ad26.RSV.preF had fewer symptoms and lower severity of disease than placebo (Supplementary Figure 3). Disease severity remained low across timepoints in recipients of Ad26.RSV.preF, whereas severity started to increase 3 days postchallenge for placebo, peaking 6 days postchallenge and then decreasing 7 days postchallenge (Figure 2C). Objective markers of severity, the AUC of excreted mucus weight and the number of tissues used, were markedly lower in those who received Ad26.RSV.preF compared with placebo (Table 2; Supplementary Figure 4). The mean weight of mucus produced peaked in the placebo group 7 days postchallenge, while it remained consistently low in the Ad26.RSV.preF group throughout the study (Figure 2D).
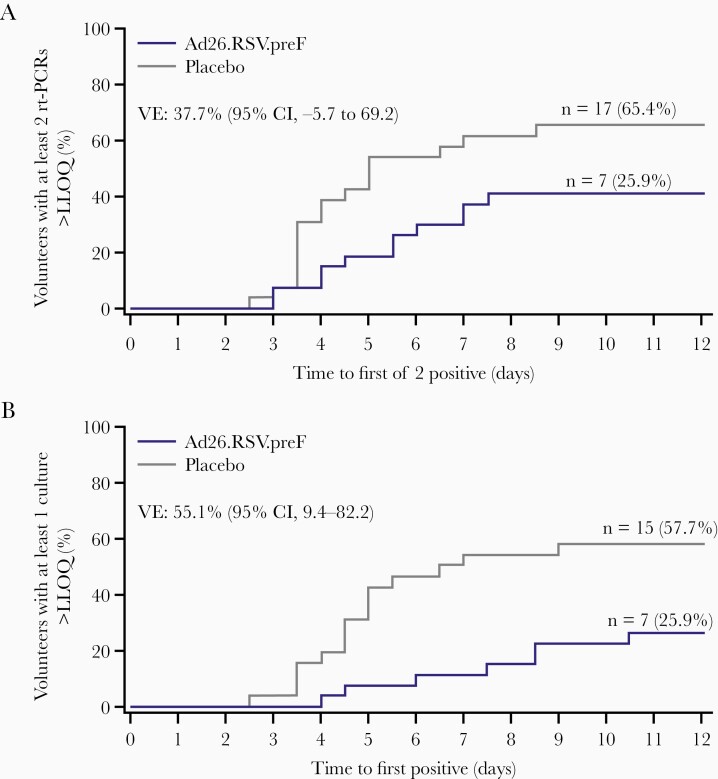
The asymptomatic and symptomatic, liberal, and conservative definitions of RSV infection resulted in VE of 37.7%, 45.8%, and 51.9%, respectively (Table 2). The time to the first of 2 positive rt-PCR results was longer in the Ad26.RSV.preF group than placebo with more volunteers having 2 positive rt-PCR results in the placebo group (65.4%) than the Ad26.RSV.preF group (40.7%) by day 12 (VE, 37.7%). This pattern was more pronounced in the time to first positive culture, with a greater difference between the groups at day 12 (Ad26.RSV.preF, 18.5%; placebo, 53.8%; VE, 55.1%) (Figure 3).
Figure 3.
Kaplan-Meier curves of time to the first of 2 (A) respiratory syncytial virus (RSV) real-time polymerase chain reaction–positive (B) viral cultures, following intranasal viral challenge with RSV subtype A after receiving immunization with Ad26.RSV.preF or placebo intramuscularly (intent-to-treat challenge population). From days 2 to 12 postchallenge, nasal wash samples were collected (twice daily from day 2 to day 11, once daily on day 12). Positive was defined as above the lower limit of quantification (LLOQ). Results below the LLOQ were allocated 0. Abbreviations: CI, confidence interval; LLOQ, lower limit of quantification; n, number of volunteers; rt-PCR, real-time polymerase chain reaction; VE, vaccine efficacy.
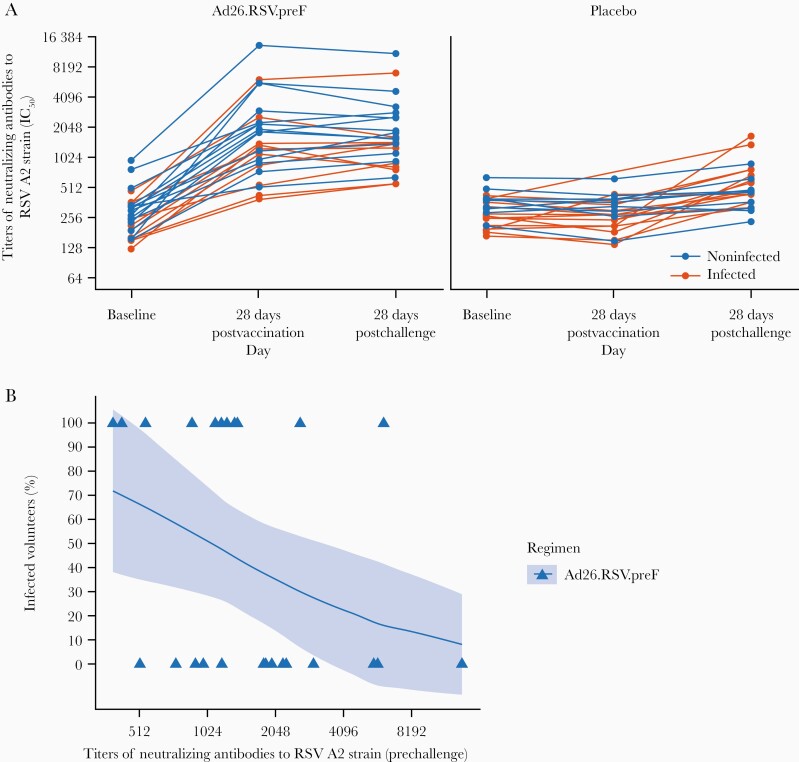
Baseline geometric mean titers (GMTs) of RSV A2 neutralizing antibodies were similar in the Ad26.RSV.preF (267 IC50 [95% confidence interval {CI}, 222–320]) and placebo groups (283 IC50 [95% CI, 248–322]). From baseline to 28 days postimmunization, there was a 5.8-fold (95% CI, 4.4–7.8) increase in GMTs of RSV A2 neutralizing antibodies to 1589 IC50 (95% CI, 1136–2224) in the Ad26.RSV.preF group, whereas RSV A2 neutralizing antibody GMTs remained similar in placebo (268 IC50 [95% CI, 229–313]) (Figure 4A). There was a further GMT fold increase from prechallenge to 28 days postchallenge of 6.1 (95% CI, 4.8–7.9) to 1617 (95% CI, 1214–2152) in the Ad26.RSV.preF group and of 1.7 (95% CI, 1.4–2.1) to 538 IC50 (95% CI, 436–664) in placebo. Post hoc analysis indicated that higher RSV A2 neutralizing antibody titers present at the time of the RSV challenge were associated with a reduction of the probability of being infected with RSV-A Memphis 37b (Figure 4B).
Figure 4.
Titers of neutralizing antibodies to respiratory syncytial virus (RSV) A2 strain over time, and proportion of infected volunteers vs prechallenge titers of neutralizing antibodies to RSV A2 strain following immunization with either Ad26.RSV.preF or placebo intramuscularly (per-protocol immunogenicity set). A, Titers of neutralizing antibodies to RSV A2 strain are shown from baseline to 28 days postchallenge in the per-protocol immunogenicity set. Geometric mean titers with 95% confidence intervals (CIs) are shown. The majority of the volunteers had their 28-day postimmunization sample taken between 22 and 33 days after immunization. One volunteer had 2 prechallenge samples taken at day 45 and day 70; these 2 samples are not part of the analysis. Infection was defined based on the symptomatic and asymptomatic definition: if a volunteer had ≥2 quantifiable real-time polymerase chain reaction (rt-PCR) measurements above the lower limit of quantification (LLOQ). B, Infected and noninfected volunteers receiving Ad26.RSV.preF were plotted vs their prechallenge titers of neutralizing antibodies to RSV A2 strain. Generalized linear regression was used to model the probability of those in the Ad26.RSV.preF group being infected using the log-transformed prechallenge titers of neutralizing antibodies to RSV A2 strain as an independent variable. The solid lines represent the estimated probability of being infected and the shaded area around it represents the 95% CI. Infection was defined based on the symptomatic and asymptomatic definition: if a volunteer had ≥2 quantifiable rt-PCR measurements above the LLOQ. Abbreviations: IC50, half-maximal inhibitory concentration; RSV, respiratory syncytial virus.
Baseline GMTs of pre-F IgG binding antibodies were similar in the Ad26.RSV.preF (109 ELISA units/L [95% CI, 85–141]) and placebo groups (115 ELISA units/L [95% CI, 96–138]). There was a 6.8 (95% CI, 5.1–9.1) fold increase in pre-F IgG binding antibody GMTs to 743 ELISA units/L (95% CI, 587–941) from baseline to 28 days postimmunization in the Ad26.RSV.preF group, while remaining similar in placebo (112 ELISA units/L [95% CI, 91–139]) (Supplementary Figure 6). There was a further GMT fold increase from prechallenge to 28 days postchallenge of 8.2 (95% CI, 6.3–10.6) to 855 ELISA units/L (95% CI, 704–1038) in the Ad26.RSV.preF group vs a 2.1 GMT fold increase (95% CI, 1.7–2.6) to 281 ELISA units/L (95% CI, 227–348) in placebo. Further details of post-F IgG binding antibodies and neutralizing antibodies directed against the Ad26 vector are discussed in the Supplementary Appendix.
Safety
The most frequently reported solicited local AEs were pain/tenderness and swelling/induration at the injection site, and all solicited local AEs were grade 1 or 2 (Table 3). The most frequently reported solicited systemic AEs were myalgia, fatigue, and headache. Four volunteers reported at least 1 grade 3 solicited systemic AE postimmunization (Ad26.RSV.preF, n = 3; placebo, n = 1); the grade 3 solicited systemic AEs reported in volunteers receiving Ad26.RSV.preF were chills (n = 2), fatigue (n = 2), myalgia (n = 2), headache (n = 2), arthralgia (n = 1), and nausea (n = 1). The grade 3 solicited systemic AE reported in the volunteer receiving placebo was fatigue, and all reported grade 3 solicited systemic AEs were considered to be related to the vaccine by the investigator. All other solicited systemic AEs were grade 1 or 2. All unsolicited AEs were grade 1 or 2, with headache the most common unsolicited AE postimmunization (3.2% Ad26.RSV.preF; 12.5% placebo). The only unsolicited AEs considered related to study vaccine by the investigator in the Ad26.RSV.preF group were increased aspartate aminotransferase and increased alanine aminotransferase (ALT), occurring in 2 cases each (6.5%). Postchallenge, increased ALT, lymphadenopathy, and pharyngitis were the most common AEs, and both increased ALT and lymphadenopathy were more frequent in the Ad26.RSV.preF group (25.9% ALT; 22.2% lymphadenopathy) than in the placebo group (11.5% ALT; 3.8% lymphadenopathy). One volunteer in the Ad26.RSV.preF group had an SAE (right ovarian cyst) that occurred 8 weeks postchallenge. This was considered to be unrelated to Ad26.RSV.preF or challenge virus by the investigator.
Table 3.
Adverse Events Following Immunization With Either Ad26.RSV.preF or Placebo Intramuscularly (Full Analysis Set)a
| Event or Abnormality | Ad26.RSV.preF (n = 31) | Placebo (n = 32) |
|---|---|---|
| Solicited local AEs | ||
| Any solicited local AE | 31 (100.0) | 6 (18.8) |
| Pain/tenderness | 31 (100.0) | 6 (18.8) |
| Swelling/induration | 9 (29.0) | 1 (3.1) |
| Erythema | 1 (3.2) | 0 (0.0) |
| Any solicited local AE of grade 3/4 | 0 (0.0) | 0 (0.0) |
| Solicited systemic AEs | ||
| Any solicited systemic AE | 31 (100.0) | 16 (50.0) |
| Myalgia | 28 (90.3) | 4 (12.5) |
| Fatigue | 26 (83.9) | 12 (37.5) |
| Headache | 26 (83.9) | 8 (25.0) |
| Chills | 17 (54.8) | 2 (6.3) |
| Arthralgia | 14 (45.2) | 2 (6.3) |
| Nausea | 11 (35.5) | 2 (6.3) |
| Fever | 4 (12.9) | 1 (3.1) |
| Any solicited systemic AE of grade 3 | 3 (9.7) | 1 (3.1) |
| Any solicited systemic AE of grade 4 | 0 (0.0) | 0 (0.0) |
| Unsolicited AEs | ||
| Postimmunization | ||
| Any unsolicited AE | 11 (35.5) | 15 (46.9) |
| Headache | 1 (3.2) | 4 (12.5) |
| Postchallengea | ||
| Any unsolicited AE | 20 (74.1) | 18 (69.2) |
| Increased ALT | 7 (25.9) | 3 (11.5) |
| Dry skin | 3 (11.1) | 2 (7.7) |
| Epistaxis | 0 (0.0) | 3 (11.5) |
| Lymphadenopathy | 6 (22.2) | 1 (3.8) |
| Pharyngitis | 3 (11.1) | 4 (15.4) |
| Rhinorrhea | 1 (3.7) | 4 (15.4) |
Data are presented as No. (%).
Abbreviations: AE, adverse event; ALT, alanine aminotransferase.
aUnsolicited AEs postchallenge were collected from the intent-to-treat challenge population (Ad26.RSV.preF, n = 27; placebo, n = 26). Solicited AEs were defined (local and systemic) events that volunteers were specifically asked about and which were noted by volunteers in a diary in the evening after immunization and then daily for the next 7 days, which was reviewed by the investigator. Solicited AEs are presented in order of decreasing incidence in the Ad26.RSV.preF group and were based on the full analysis set (volunteers who were randomized and received at least 1 dose of the study vaccine). The severity of solicited AEs were graded in the diary by the volunteer based on the severity assessment provided in the diary and then verified by the investigator. For unsolicited AEs, only events occurring in at least 10% of volunteers in 1 arm per period are shown.
DISCUSSION
This study assessed the potential of Ad26.RSV.preF to protect against RSV infection and disease in healthy adult volunteers in the RSV HVC model. Such models have been used to successfully demonstrate efficacy in RSV antiviral studies [17, 18], but this is the first such study to assess the proof of concept for vaccine-mediated protection against RSV infection. The primary endpoint was met; RSV VL-AUC (rt-PCR) postchallenge was significantly lower in volunteers who received Ad26.RSV.preF vs placebo (P = .012). We successfully demonstrated protection from RSV infection through active immunization with Ad26.RSV.preF in humans experimentally infected with RSV.
The majority of potent neutralizing antibodies in the serum of RSV-positive individuals are directed against pre-F specific sites [8, 9, 12]. Ad26.RSV.preF led to an increase in pre-F and post-F IgG serum antibody response and RSV A2 neutralizing antibody titers compared with placebo. In a previous challenge study, preexisting RSV-antibody serum concentrations correlated with protection against RSV infection, indicating that an effective RSV vaccine should likely elicit the production of serum neutralizing antibodies [19]. This is consistent with the protective effect of the increase in RSV A2 neutralizing antibody titers observed following immunization with Ad26.RSV.preF. In our study, a post hoc analysis demonstrated there was a correlation between the proportion of volunteers infected and their postimmunization RSV A2 neutralizing antibody titers, suggesting that Ad26.RSV.preF-induced titers are indeed contributing to protection.
Other immune functions induced by Ad26.RSV.preF likely also play a role in protection. Ad26-expressed proteins elicit stronger protective immunity than directly delivered soluble proteins [20]. The level of intranasal pre-F IgA antibodies was increased after immunization with Ad26.RSV.preF compared with the placebo group prechallenge, but did not correlate with protection. This is in contrast with a previous study reporting an association between the level of preexisting RSV-F specific IgA antibodies and susceptibility to infection after challenge of nonimmunized healthy adults [19]. These differences could be due to variability between studies and the limited number of volunteers included in our trial, or reflect a different mechanism of protection between natural and vaccine-induced immunity. CMI was not measured as the quality of the samples was insufficient to perform analysis, but Ad26.RSV.preF has been shown to induce durable RSV-specific CMI previously [16]. Overall, Ad26.RSV.preF has demonstrated its ability to elicit a functional immune response, which induces protection from RSV infection in the adult population, partly mediated by neutralizing antibodies. The preexisting Ad26 neutralizing antibodies measured at baseline did not impact the vaccine-induced immune responses.
VL has been shown to drive disease progression in RSV infection, and high RSV VL in respiratory secretions is associated with increased clinical disease severity in natural infection [21, 22]. Furthermore, reducing infectious virus quantity within respiratory secretions likely interrupts human-to-human RSV transmission [23]. Mathematical modeling predicts that an effective RSV vaccine would reduce viral transmission, thus indirectly reducing the burden of RSV [23]. Ad26.RSV.preF significantly reduced VL and reduced infectious virus quantity in the upper respiratory tract and therefore has potential for inducing an additional herd immunity effect.
The HVC model mimics natural infection as closely as possible and RSV Memphis 37b viral challenge models have shown parallels with natural infection, indicating that Ad26.RSV.preF may be efficacious against natural RSV infection [21, 24]. However, in this challenge model, adults infected with RSV usually experience mild, cold-like symptoms limited to upper respiratory tract infections (URTIs). Few URTI symptoms were noted in those who received Ad26.RSV.preF and were of reduced severity compared with placebo recipients, suggesting that Ad26.RSV.preF may induce protection from URTI symptoms that lead to upper respiratory tract disease (URTD), which has not been demonstrated with previous vaccine candidates [10, 11]. Severe RSV disease is characterized by an infection of the lower respiratory tract and results from the progression of RSV from the upper airways to the lower airways; therefore, protection from URTI symptoms that lead to URTD suggest that Ad26.RSV.preF could also provide protection from lower respiratory tract infection symptoms that lead to LRTD. Such a hypothesis has been consistently corroborated by data for influenza, which is another respiratory virus with similar disease progression. More specifically, many influenza vaccines have demonstrated clinically significant levels of efficacy against URTI symptoms that lead to URTD in the influenza challenge model similar to those observed with Ad26.RSV.preF against RSV in our study [25, 26]. These influenza vaccines were ultimately shown in larger pre- and postmarketing studies to demonstrate even more significant reductions in rates of influenza-associated pneumonia, hospitalization, and death among older adults and/or persons with high-risk underlying medical conditions [27–29]. Increased levels of efficacy have been observed in parallel with increased severity of case definition [30], which was consistent with our findings.
However, as volunteers in this study were healthy adults who were not at high risk of developing LRTD, and as previous RSV therapeutics that have demonstrated efficacy in adult RSV challenge models have not yet translated into efficacy in trials against natural RSV infection [17, 18], further studies are needed in high-risk populations to determine the degree of protection afforded by Ad26.RSV.preF from RSV LRTD.
A limitation of this study is that volunteers were inoculated with a clinical strain of RSV-A, so protection from a currently circulating strain or RSV-B was not demonstrated here. Additionally, the time from immunization to RSV challenge was relatively short. This study only demonstrated durability of immune responses 25–33 days postimmunization and 28 days postchallenge against RSV-A. However, durability of humoral and cellular immune responses induced by Ad26.RSV.preF for at least 2 years and breadth of immune responses against both RSV-A and RSV-B in older adults (aged ≥60 years) have been previously demonstrated [16].
In conclusion, VL, RSV infection, and URTD severity following challenge with RSV-A were consistently lower in volunteers immunized with Ad26.RSV.preF vs placebo. Ad26.RSV.preF demonstrated immunogenicity and was well tolerated and has the potential to decrease viral transmission in those with breakthrough infection. These findings support further evaluation of Ad26.RSV.preF, the first RSV vaccine candidate to be tested in an HVC model, in field trials for efficacy against natural RSV infection in RSV-experienced populations at risk of severe RSV disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: IDWeek, Washington, District of Columbia, October 2019; RSVVW’19, Accra, Ghana, 12–14 November 2019 (Abstract 902).
Author contributions. J. S., E. D. P., J. D.-V., J. M., B. M., A. R. B., W. H., N. N., K. E., A. G., R. L.-W., H. S., and B. C. were involved in the in the conception and design of the study. J. S., B. M., A. R. B., W. H., N. N., and B. C. were responsible for the data collection and acquisition. All authors were involved in the interpretation of the data and the drafting of the manuscript. The authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the study to the protocol.
Acknowledgments. Medical writing support was provided by Jack Wigham (Zoetic Science, an Ashfield company, part of UDG Healthcare plc, Macclesfield, United Kingdom) and funded by Janssen Vaccines & Prevention.
Financial support. The study was sponsored by Janssen Vaccines & Prevention and was designed by the sponsor, hVIVO, and J. D. V. The study was managed by the sponsor.
Potential conflicts of interest. J. S., E. D. P., E. G., J. M., A. R. B., A. V., W. H., C. C., E. H., H. S., and B. C. are employed by Janssen Pharmaceuticals, a Johnson & Johnson company, and may be Johnson & Johnson stockholders. J. D.-V. has received RSV-related research contracts (through the University of Tennessee) and consultancy fees from Janssen, ReViral, Pulmocide, ADMA Biologics, Ark Pharma, Pfizer, and MedImmune/AstraZeneca, and has received consultancy fees from VIR Biotechnology, Alveo, and Enanta, all related to RSV antivirals, RSV preventions, and vaccines. B. M., N. N., K. E., A. G., and R. L.-W. were employees/contractors of hVIVO during the conduct of this study.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Jerald Sadoff, Janssen Vaccines and Prevention BV, Leiden, The Netherlands.
Els De Paepe, Janssen Infectious Diseases, Beerse, Belgium.
John DeVincenzo, University of Tennessee School of Medicine, Memphis, Tennessee, USA.
Efi Gymnopoulou, Janssen Infectious Diseases, Beerse, Belgium.
Joris Menten, Janssen Infectious Diseases, Beerse, Belgium.
Bryan Murray, hVIVO, London, United Kingdom.
Arangassery Rosemary Bastian, Janssen Vaccines and Prevention BV, Leiden, The Netherlands.
An Vandebosch, Janssen Infectious Diseases, Beerse, Belgium.
Wouter Haazen, Janssen Infectious Diseases, Beerse, Belgium.
Nicolas Noulin, hVIVO, London, United Kingdom.
Christy Comeaux, Janssen Vaccines and Prevention BV, Leiden, The Netherlands.
Esther Heijnen, Janssen Vaccines and Prevention BV, Leiden, The Netherlands.
Kingsley Eze, hVIVO, London, United Kingdom.
Anthony Gilbert, hVIVO, London, United Kingdom.
Rob Lambkin-Williams, hVIVO, London, United Kingdom.
Hanneke Schuitemaker, Janssen Vaccines and Prevention BV, Leiden, The Netherlands.
Benoit Callendret, Janssen Vaccines and Prevention BV, Leiden, The Netherlands.
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 3. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222:S577–83. [DOI] [PubMed] [Google Scholar]
- 4. Fleming DM, Elliot AJ, Cross KW. Morbidity profiles of patients consulting during influenza and respiratory syncytial virus active periods. Epidemiol Infect 2007; 135:1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pastula ST, Hackett J, Coalson J, et al. Hospitalizations for respiratory syncytial virus among adults in the United States, 1997–2012. Open Forum Infect Dis 2017; 4:ofw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ackerson B, Tseng HF, Sy LS, et al. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis 2019; 69:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol 2013; 372:83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ngwuta JO, Chen M, Modjarrad K, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7:309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gillman MS, Castellanos CA, Chen M, et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol 2016; 1:eaaj1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falloon J, Yu J, Esser MT, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novovax Press Release . Topline RSV F vaccine data from two clinical trials in older adults. https://ir.novavax.com/news-releases/news-release-details/novavax-announces-topline-rsv-f-vaccine-data-two-clinical-trials. Accessed 16 April 2020.
- 12. Crank MC, Ruckwardt TJ, Chen M, et al. ; VRC 317 Study Team . A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019; 365:505–9. [DOI] [PubMed] [Google Scholar]
- 13. Krarup A, Truan D, Furmanova-Hollenstein P, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun 2015; 6:8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geisbert TW, Bailey M, Hensley L, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol 2011; 85:4222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barouch DH, Liu J, Peter L, et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). J Infect Dis 2013; 207:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams K, Bastian AR, Feldman RA, et al. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥60 years. J Infect Dis 2020; 222:979–88. [DOI] [PubMed] [Google Scholar]
- 17. DeVincenzo JP, Whitley RJ, Mackman RL, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 2014; 371:711–22. [DOI] [PubMed] [Google Scholar]
- 18. DeVincenzo JP, McClure MW, Symons JA, et al. Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med 2015; 373:2048–58. [DOI] [PubMed] [Google Scholar]
- 19. Bagga B, Cehelsky JE, Vaishnaw A, et al. Effect of preexisting serum and mucosal antibody on experimental respiratory syncytial virus (RSV) challenge and infection of adults. J Infect Dis 2015; 212:1719–25. [DOI] [PubMed] [Google Scholar]
- 20. Barouch DH, Alter G, Broge T, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 2015; 349:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeVincenzo JP, Wilkinson T, Vaishnaw A, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 2010; 182:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee N, Chan MC, Lui GC, et al. High viral load and respiratory failure in adults hospitalized for respiratory syncytial virus infections. J Infect Dis 2015; 212:1237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamin D, Jones FK, DeVincenzo JP, et al. Vaccination strategies against respiratory syncytial virus. Proc Natl Acad Sci U S A 2016; 113:13239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim YI, DeVincenzo JP, Jones BG, et al. Respiratory syncytial virus human experimental infection model: provenance, production, and sequence of low-passaged Memphis-37 challenge virus. PLoS One 2014; 9:e113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999; 18:899–906. [DOI] [PubMed] [Google Scholar]
- 26. Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008; 167:775–85. [DOI] [PubMed] [Google Scholar]
- 27. Beck CR, McKenzie BC, Hashim AB, Harris RC, Nguyen-Van-Tam JS; University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group . Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis 2012; 206:1250–9. [DOI] [PubMed] [Google Scholar]
- 28. Poudel S, Shehadeh F, Zacharioudakis IM, et al. The effect of influenza vaccination on mortality and risk of hospitalization in patients with heart failure: a systematic review and meta-analysis. Open Forum Infect Dis 2019; 6:ofz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US medicare beneficiaries in preventing post-influenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215:510–17. [DOI] [PubMed] [Google Scholar]
- 30. Jain VK, Rivera L, Zaman K, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369:2481–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.