Abstract
Background
Direct-acting antiviral (DAA) treatment has revolutionized hepatitis C virus (HCV) care. We aimed to evaluate the risk for the development of hepatocellular carcinoma (HCC) in patients aged 75–84 years with chronic hepatitis C after HCV elimination.
Methods
This multicenter cohort study included 2405 consecutive patients with chronic hepatitis C without a history of HCC who achieved HCV elimination by DAAs. Patients in whom HCC developed within 1 year of DAA initiation were excluded. Propensity score matching analysis was used to evaluate differences in HCC risk between patients aged 75–84 versus 60–74 years.
Results
The median observational period was 3.5 years. Among patients aged 75–84 years with a high Fibrosis-4 (FIB-4) index (≥3.25 at baseline), there was no significant difference in the annual incidence of HCCs between groups with an FIB-4 index ≥3.25 (2.75 per 100 person-years [PY]) versus <3.25 (2.16 per 100 PY) at 12 weeks after the end of treatment, unlike the results in those aged 60–74 years (3.61 and 1.51 per 100 PY, respectively) (adjusted hazard ratio, 2.20; P = .04). In 495 pairs matched by propensity score matching, in patients without cirrhosis, the cumulative HCC incidence was significantly higher in the 75–84-year than in the 60–74-year age group (P = .04).
Conclusions
Older patients aged 75–84 years remained at high risk for the development of HCC, even after HCV elimination and the improvement of the FIB-4 index to <3.25.
Keywords: hepatitis C virus, hepatocellular carcinoma, direct-acting antivirals, elderly person
This study was performed to elucidate the risk of hepatocellular carcinoma (HCC) after elimination of hepatitis C virus in patients aged ≥75–84 years. Fibrosis biomarker improvement had little positive effect on HCC risk for patients in this age group.
Chronic hepatitis C (CHC) affects approximately 80 million people globally [1] and is the leading risk factor for hepatocellular carcinoma (HCC) [2]. With the advent of highly effective and well-tolerated direct-acting antivirals (DAAs) in 2013, the vast majority of treated patients are now able to achieve hepatitis C virus (HCV) eradication [3, 4]. In the era of interferon-based regimens, older patients were often ineligible for treatment owing to the increased prevalence of multiple comorbid conditions associated with an increased risk of adverse effects [5]. All-oral DAAs made it possible for these previously difficult-to-treat older patients to achieve sustained viral response (SVR).
In real-world cohorts, almost all older patients, even the oldest generations aged >75 years, were able to obtain an excellent virological outcome by DAA treatment, as is seen with younger patients [6, 7]. The age of Japanese patients with CHC has progressively increased, ahead of other countries [8, 9], with a similar trend predicted for various countries in the near future; for example, 70% of patients with CHC in the United States were born between 1945 and 1965 [10]. Age has consistently been found to be a major factor influencing the progression of fibrosis in patients with CHC, which could lead to the development of liver-related complications, such as cirrhosis, HCC, and portal hypertension, all of which have an effect on life expectancy [11, 12].
HCV elimination by DAA treatment is associated with improved clinical outcomes, including a reduced risk of HCC and prolonged life expectancy [13–15]. In almost all cohorts studied outside Japan, however, the mean age was about 60 years. Thus, much remains to be clarified regarding much older cohorts, such as patients aged ≥75 years, for the development of HCC after SVR. The definition of “old age” in Japan has been changed from ≥65 years to ≥75 years because of improved physical and psychological functioning, compared with a few decades ago [16], and this trend is spreading globally. Posttreatment data for the older generations of patients would be useful for designing HCC surveillance strategies.
The purpose of this study was to assess the risk for the development of HCC in patients aged 75–84 years with CHC and without a history of HCC after achievement of SVR by DAA treatment. In addition, we used propensity score matched (PSM) analysis to evaluate differences in that risk between patients aged 75–84 and those aged 60–74 years.
PATIENTS AND METHODS
Study Cohort
The Kyushu University Liver Disease Study (KULDS) Group consists of hepatologists from Kyushu University Hospital and its affiliated hospitals located in the northern Kyushu area of Japan. This large-scale, multicenter cohort study analyzed the data of 3128 consecutive Japanese patients who were enrolled from September 2014 through December 2019 for treatment with interferon-free DAA regimens for chronic HCV infection. Exclusion criteria for our original cohort were (1) age <18 years at the initiation of treatment, (2) decompensated cirrhosis (Child-Pugh B or C), (3) concomitant human immunodeficiency virus or hepatitis B virus infection, (4) excessive active alcohol consumption, and (5) history of organ transplantation. Moreover, to focus this study, we excluded patients (1) aged ≥85 years at the initiation of treatment, (2) with a history of HCC, (3) with DAA treatment failure, (4) with unknown DAA treatment outcome, (5) with HCC developing within 1 year of DAA initiation, or (6) with a follow-up of <1 year.
This study was approved by the Ethics Committees of Kyushu University Hospital, and each study site is registered as a clinical study on the University Hospital Medical Information Network (identification no. 000027342). Data were acquired from patients’ medical records from a prospectively maintained database of all patients who have been treated with DAAs.
Study Assessments
Clinical parameters were measured by standard laboratory techniques at a commercial laboratory at baseline within the 3 months before DAA initiation, every 4 weeks during DAA treatment, then every 12–24 weeks after achievement SVR, which was defined as undetectable HCV RNA (target not detected) at 12 weeks after the end of treatment (pw12). HCV RNA was measured using a real-time reverse-transcriptase polymerase chain reaction assay (COBAS TaqMan HCV assay, version 2.0) (Roche Molecular Diagnostics) with a lower limit of quantitation of 15 IU/mL. Cirrhosis was determined by transient elastography (FibroScan; Echosens) or by the presence of clinical, histological, radiologic, or endoscopic evidence of cirrhosis and/or portal hypertension (nodular contour on imaging, splenomegaly, presence of varices). For each patient, we calculated the Fibrosis-4 (FIB-4) index using the following formula: Age (years) × aspartate aminotransferase (U/L)/[platelet count (109/L) × (alanine aminotransferase [ALT] [U/L])½], at least at baseline and at the time of SVR evaluation (pw12). We categorized FIB-4 indexes as <1.45 (low), 1.45 to <3.25 (moderate), or ≥3.25 (high) [17].
The primary end point was de novo HCC incidence after achievement of SVR with DAA treatment for patients aged 75–84 years. The follow-up period reflects the time between the start date of DAA treatment and the date of the last imaging assessment. All patients were examined for HCC by means of abdominal ultrasonography, dynamic computed tomography, and/or magnetic resonance imaging at baseline and every 3–6 months after the initiation of treatment. Moreover, deaths that occurred during follow-up, even after development of HCC and whether or not they were liver related, were obtained from the medical records of each hospital.
Statistical Analysis
Standard descriptive and comparative statistical analyses were performed for all demographic and clinical variables. Categorical variables are described as proportions (percentages), and continuous variables as medians (with interquartile ranges). We calculated the HCC incidence rate with 95% confidence intervals (CIs) as the number of HCCs divided by total person-years (PY) of follow-up. The Kaplan-Meier method was used to estimate the cumulative risks of HCC and death. The log-rank test was used to compare the difference in HCC incidence and death rates between the groups.
For comparison of the patient groups aged 60–74 and 75–84 years, we chose to use PSM to balance them. Variables used in the PSM model included sex, diabetes mellitus, serum albumin and ALT levels, platelet count, HCV genotype, baseline cirrhosis status, and treatment experience. Caliper matching of the propensity scores was done, and pairs were matched to within a range of 0.2 standard deviations of the logit of the propensity scores [18]. We assessed the adequacy of the propensity score specification by comparing the standardized difference in baseline covariates after stratification. A standardized mean difference of <0.1 was used to indicate no significant difference between baseline covariates. Cox proportional hazards regression was used to estimate the hazard ratio (HR) and 95% CI for the risk of HCC incidence during the follow-up period.
An additional predefined sensitivity analysis included patients with the censoring criteria of HCC incidence within 1 year of DAA initiation and those who were followed up for <1 year. We calculated the HCC incidence rate and used the log-rank test to compare differences in HCC incidence according to the FIB-4 transition score. Moreover, PSM analysis was performed in the same way as an original analysis. All statistical analyses were conducted using SPSS Statistics software, version 25.0 (IBM SPSS). Differences were considered statistically significant at P < .05 (2 tailed).
RESULTS
Demographics
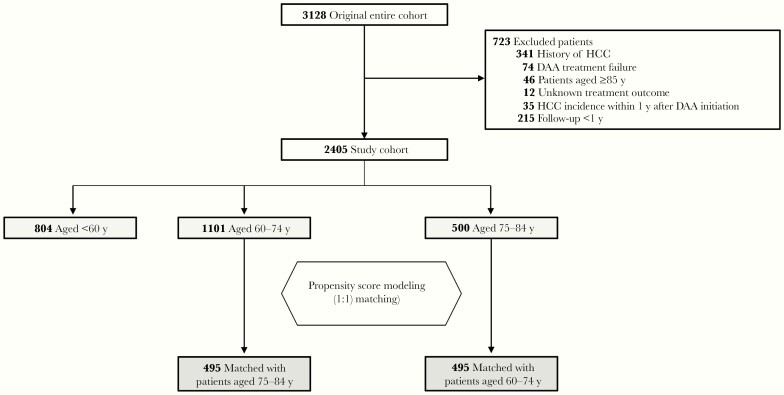
We identified 3128 patients who received DAA treatment and excluded 723 (23.1%) in accordance with the criteria, leaving the data of 2405 patients available for analysis (Figure 1). The median observational period was 3.5 years (range, 1.0–5.2 years). Of the patients, 500 (20.8%) were aged 75–84 and 1101 (45.8%) aged 60–74 years at the time of DAA initiation (Table 1). In the older group, 34.8% were men, 27.2% had a diagnosis of cirrhosis, and 71.6% were treatment naive. Approximately two-thirds had an FIB-4 index ≥3.25 at baseline. Compared with patients aged 60–74, those aged 75–84 years had higher FIB-4 indexes and lower serum albumin levels and were more likely to have experienced HCV treatment.
Figure 1.
Study flowchart. Abbreviations: DAA, direct-acting antiviral; HCC, hepatocellular carcinoma.
Table 1.
Baseline Characteristics of Patients Before and After Propensity Score Matching
| Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Aged <60 y (n = 804) | Aged 60–74 (n = 1101) | Aged 75–84 y (n = 500) | Standardized Mean Differencea | Aged 60–74 y (n = 495) | Aged 75–84 y (n = 495) | Standardized Mean Differencea |
| Age, median (IQR), y | 51 (43–55) | 67 (65–71) | 78 (76–81) | … | 68 (65–71) | 78 (76–81) | … |
| Male sex, no. (%) | 457 (56.8) | 426 (38.7) | 174 (34.8) | 0.07 | 173 (34.9) | 172 (34.7) | 0.004 |
| BMI, median (IQR)b | 23.0 (20.8–25.8) | 22.6 (20.6–24.8) | 22.2 (19.9–24.5) | 0.13 | 22.4 (20.1–24.7) | 22.2 (19.9–24.5) | 0.08 |
| Cirrhosis, no. (%) | 113 (14.1) | 252 (22.9) | 136 (27.2) | 0.09 | 141 (28.5) | 135 (27.3) | 0.03 |
| Diabetes mellitus, no. (%) | 104 (13.0) | 224 (20.3) | 90 (18.0) | 0.05 | 98 (19.8) | 87 (17.6) | 0.06 |
| FIB-4 index, median (IQR) | 1.57 (1.02–2.64) | 2.98 (2.16–4.37) | 3.95 (2.85–5.74) | 0.33 | 3.12 (2.28–4.78) | 3.94 (2.85–5.73) | 0.26 |
| FIB-4 index group, no. (%) | |||||||
| <1.45 | 364 (45.4) | 59 (5.3) | 5 (1.0) | 21 (4.2) | 5 (1.0) | ||
| 1.45 to <3.25 | 304 (38.0) | 567 (51.7) | 165 (33.0) | 242 (48.9) | 163 (32.9) | ||
| ≥3.25 | 133 (16.6) | 471 (42.9) | 330 (66.0) | 232 (46.9) | 327 (66.1) | ||
| ND | 3 | 4 | 0 | 0 | 0 | ||
| Prior treatment, no. (%) | |||||||
| Treatment naive | 649 (80.7) | 727 (66.0) | 358 (71.6) | 0.11 | 355 (71.7) | 354 (71.5) | 0.004 |
| Treatment experienced | 155 (19.3) | 374 (34.0) | 142 (28.4) | 140 (28.3) | 141 (28.5) | ||
| Interferon based | 151 | 373 | 139 | 139 | 139 | ||
| All-oral DAAs | 4 | 1 | 3 | 1 | 2 | ||
| Laboratory values, median (IQR) | |||||||
| Total bilirubin, mg/dL | 0.7 (0.5–0.9) | 0.7 (0.6–0.9) | 0.7 (0.6–0.9) | 0.13 | 0.7 (0.6–1.0) | 0.7 (0.6–0.9) | 0.14 |
| Albumin, g/dL | 4.2 (4.0–4.5) | 4.1 (3.8–4.3) | 3.9 (3.7–4.2) | 0.24 | 4.0 (3.6–4.2) | 3.9 (3.7–4.2) | 0.03 |
| AST, U/L | 40 (27–69) | 41 (29–61) | 41 (31–56) | 0.04 | 39 (28–54) | 41 (30–56) | 0.09 |
| ALT, U/L | 49 (29–89) | 38 (25–63) | 32 (24–52) | 0.22 | 34 (23–52) | 33 (24–52) | 0.02 |
| γ-GTP, U/L | 43 (23–89) | 31 (20–54) | 27 (19–42) | 0.23 | 29 (19–46) | 27 (19–42) | 0.13 |
| eGFR, mL/min/1.73 m2 | 85 (74–97) | 73 (63–83) | 65 (56–75) | 0.34 | 72 (61–82) | 65 (56–75) | 0.27 |
| Platelet count, 103/µL | 189 (142–230) | 152 (117–192) | 143 (112–178) | 0.09 | 147 (111–185) | 143 (112–178) | 0.04 |
| α-Fetoprotein, ng/mL | 4.0 (2.6–6.6) | 4.4 (2.9–8.0) | 4.4 (3.0–8.9) | 0.05 | 4.5 (2.9–8.3) | 4.4 (3.0–8.7) | 0.05 |
| HCV RNA, log IU/mL | 6.2 (5.4–6.6) | 6.1 (5.6–6.5) | 6.1 (5.6–6.4) | 0.08 | 6.1 (5.6–6.5) | 6.1 (5.6–6.4) | 0.05 |
| HCV genotype | |||||||
| 1 | 383 (47.6) | 821 (74.6) | 393 (78.6) | 0.09 | 396 (80.0) | 388 (78.4) | 0.04 |
| 2 | 420 (52.2) | 280 (25.4) | 107 (21.4) | 99 (20.0) | 107 (21.6) | ||
| Other | 1 (0.1) | 0 | 0 | 0 | 0 | ||
| DAA regimen | |||||||
| LDV/SOF | 213 (26.5) | 449 (40.8) | 222 (44.4) | 0.07 | 220 (44.4) | 220 (44.4) | … |
| SOF + RBV | 336 (41.8) | 240 (21.8) | 83 (16.6) | 0.12 | 80 (16.2) | 83 (16.8) | 0.02 |
| 2D | 25 (3.1) | 37 (3.4) | 13 (2.6) | 0.04 | 12 (2.4) | 13 (2.6) | 0.01 |
| ASV + DCV | 48 (6.0) | 190 (17.3) | 81 (16.2) | 0.003 | 100 (20.2) | 78 (15.8) | 0.11 |
| EBR + GZR | 63 (7.8) | 111 (10.1) | 63 (12.6) | 0.07 | 52 (10.5) | 63 (12.7) | 0.07 |
| GLE/PIB | 119 (14.8) | 74 (6.7) | 38 (7.6) | 0.03 | 31 (6.3) | 38 (7.7) | 0.05 |
Abbreviations: 2D, ombitasvir-paritaprevir-ritonavir; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASV, asunaprevir; BMI, body mass index; DAA, direct-acting antiviral; DCV, daclatasvir; EBR, elbasvir; eGFR, estimated glomerular filtration rate; FIB-4, Fibrosis-4; GLE, glecaprevir; γ-GTP, γ-glutamyl transpeptidase; GZR, grazoprevir; HCV, hepatitis C virus; IQR, interquartile range; LDV, ledipasvir; ND, not determined; PIB, pibrentasvir; PSM, propensity score matching; RBV, ribavirin; SOF, sofosbuvir.
aValues <0.1 indicate adequate balance between the groups aged 60–74 and 75–84 years.
bBMI is calculated as weight in kilograms divided by height in meters squared.
Predictors of HCC Development for the Overall Cohort
Overall, 64 patients (2.7%) had HCC develop during the follow-up period, starting 1 year after treatment initiation. The Kaplan-Meier curves were used to determine the cumulative HCC incidence by age (<60, 60–74, or 75–84 years), as in Supplementary Figure 1. In multivariable analysis adjusted for background characteristics and parameters at pw12, the following variables were extracted as giving a higher risk of HCC: age 60–74 years (HR, 4.95; 95% CI, 1.57–15.6; P = .006), age 75–84 years (6.93; 2.00–24.0; P = .002), male sex (2.29; 1.25–4.18; P = .007), cirrhosis (2.41; 1.40–4.15; P = .002), pw12 serum albumin level (in grams per deciliiter) (0.28; .14–.58; P < .001), pw12 ALT level (in units per liter) (1.01; 1.00–1.02; P = .04), and pw12 α-fetoprotein (AFP) level (in nanograms per milliliter) (1.12; 1.08–1.18; P < .001) (Supplementary Table 1).
Incidence Rates for HCC Development in Patients Aged 75–84 Years
There were 24 cases of HCC after achievement of SVR during 1170.7 PY of follow-up from 1 year after the initiation of DAAs. We analyzed the incidence of HCCs according to the transition in the FIB-4 index from baseline to pw12. The FIB-4 index was decreased in most patients (447 of 500 [89.4%]). For patients in the group with high FIB-4 indexes (≥3.25 at baseline), there was no significant difference in the annual incidence of HCCs between the pw12 FIB-4 index ≥3.25 and <3.25 groups (incidence rate, 2.75 and 2.16 per 100 PY, respectively; P = .69) (Table 2, upper panel). Of the patients in the groups with moderate or low FIB-4 indexes (<3.25 at baseline), HCC developed in only 1 (2.4%) patient with a pw12 FIB-4 index <1.45 (Table 2, lower panel). In multivariable analysis for evaluating the predictors of HCC development, pw12 serum albumin and pw12 AFP levels were extracted as giving a higher risk of HCC (Supplementary Table 2, right panel).
Table 2.
Incidence Rate of Hepatocellular Carcinoma, Stratified by Age and the Fibrosis-4 Index 1 Year After Direct-Acting Antiviral Initiation
| FIB-4 Index | Patients, No. | HCC, No. | PY of Follow-upa | HCC Incidence Rate per 100 PY (95% CI) | Crude HR (95% CI) | P Value | Adjusted HRb (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Baseline ≥3.25 | ||||||||
| Patients aged 75–84 y | ||||||||
| <3.25 at pw12 | 161 | 8 | 370.1 | 2.16 (.53–3.79) | 1 | .69 | 1 | .67 |
| ≥3.25 at pw12 | 161 | 11 | 400.6 | 2.75 (1.03–4.46) | 1.20 (.48–3.03) | 1.31 (.22–4.90) | ||
| Patients aged 60–74 y | ||||||||
| <3.25 at pw12 | 244 | 9 | 594.7 | 1.51 (.44–2.59) | 1 | .02 | 1 | .04 |
| ≥3.25 at pw12 | 218 | 20 | 554.7 | 3.61 (2.00–5.22) | 2.49 (1.13–5.46) | 2.20 (1.02–4.89) | ||
| Baseline <3.25 | ||||||||
| Patients aged 75–84 y | ||||||||
| <1.45 at pw12 | 42 | 1 | 87.2 | 1.15 (0–4.77) | 1 | .88 | 1 | .60 |
| 1.45 to <3.25 at pw12 | 128 | 4 | 292.6 | 1.37 (0–2.96) | 1.19 (.13–10.6) | 1.96 (.16–24.3) | ||
| Patients aged 60–74 y | ||||||||
| <1.45 at pw12 | 171 | 1 | 368.1 | 0.27 (0–1.17) | 1 | .49 | 1 | .57 |
| 1.45 to <3.25 at pw12 | 441 | 6 | 1068.5 | 0.56 (.05–1.07) | 2.09 (.25–17.4) | 1.85 (.22–15.5) |
Abbreviations: CI, confidence interval; FIB-4, Fibrosis-4; HCC, hepatocellular carcinoma; HR, hazard ratio; pw12, 12 weeks after the end of direct-acting antiviral treatment; PY, person-years.
aPY of follow-up were calculated from 1 year after initiation of direct-acting antivirals.
bAdjusted for sex, body mass index, diabetes mellitus, estimated glomerular filtration rate, hepatitis C virus (HCV) genotype, HCV RNA level, serum albumin level at pw12, and treatment experience.
The Kaplan-Meier curves for HCC incidence classified according to the FIB-4 transition score (from ≥3.25 to ≥3.25 [ie, ≥3.25 at both time points], from ≥3.25 to <3.25, from <3.25 to a range from 1.45 to <3.25, and from <3.25 to <1.45) are shown in Figure 2. The 5-year cumulative rates of de novo HCC were 11.4%, 6.0%, and 4.2% for the following FIB-4 transitions, respectively: from ≥3.25 to ≥3.25, from ≥3.25 to <3.25, and from <3.25 to a range from 1.45 to <3.25. There was no significant difference in HCC incidence between the ≥3.25-to-≥3.25 and ≥3.25-to-<3.25 groups (P = .80; log-rank test). None of the patients whose FIB-4 index transitioned from <3.25 to <1.45 had HCCs develop during follow-up.
Figure 2.
Cumulative development of hepatocellular carcinoma (HCC) after the initiation of direct-acting antiviral treatment for patients aged 75–84 years, stratified by Fibrosis-4 (FIB-4) index transition. (Note: 1.45–<3.25 represents range from 1.45 to <3.25.)
Incidence Rates for HCC Development in Patients Aged 60–74 Years
There were 36 cases of HCC after achievement of SVR during 2670.3 PY of follow-up from 1 year after DAA initiation. The FIB-4 index was decreased in most patients (933 of 1101 [84.7%]). For patients in the group with high FIB-4 indexes (≥3.25 at baseline), HCC developed in 20 patients with a pw12 FIB-4 index ≥3.25, an annual incidence of 3.61 per 100 PY (95% CI, 2.00–5.22), significantly higher than the 1.51 per 100 PY (.44–2.59) incidence rate among patients with a pw12 FIB-4 index <3.25 (adjusted HR, 2.20; 95% CI, 1.02–4.89; P = .04) (Table 2, upper panel). For patients with a moderate or low FIB-4 index (<3.25 at baseline), the annual incidences of HCC were low in patients with a pw12 FIB-4 index of 1.45 to <3.25 or <1.45 (incidence rate, 0.56 and 0.27 per 100 PY, respectively; P = .49) (Table 2, lower panel). In multivariable analysis performed to evaluate predictors of HCC development, male sex, cirrhosis, and pw12 AFP level were extracted as giving a higher risk of developing HCC (Supplementary Table 2, left panel).
The Kaplan-Meier curves for HCC incidence classified according to the FIB-4 transition score are shown in Figure 3. Unlike for patients aged 75–84 years, this score, especially in those with an FIB-4 index of ≥3.25 at both time points, had a significant impact on the development of de novo HCC.
Figure 3.
Cumulative development of hepatocellular carcinoma (HCC) after the initiation of direct-acting antiviral treatment for patients aged 60–74 years, stratified by Fibrosis-4 (FIB-4) index transition. (Note: 1.45–<3.25 represents range from 1.45 to <3.25.)
Crude Survival Rate and Causes of Death
During the time frame including after the development of HCC, 33 (2.1%) of the patients aged ≥60 years died. Twelve (36.4%) died of liver-related complications due mainly to HCC (Supplementary Table 3). Although the crude overall survival for those with an FIB-4 index of ≥3.25 at both time points was lower than in the other FIB-4 transition groups in the 75–84-year age group, the difference was not significant (P = .48) (Supplementary Figure 2). In the 60–74-year age group, those with an FIB-4 index of ≥3.25 at both time points had a crude overall survival similar to that in the other FIB-4 transition groups (Supplementary Figure 3).
Predictors for HCC Development in the PSM Cohort
After PSM, there were 495 patients in both age groups (75–84 and 60–74 years), with no significant demographic differences except for FIB-4 index and estimated glomerular filtration rate, which were directly affected by age (Table 1). Sex, body mass index, prior treatment experience, hepatic reserve, and DAA regimen were very similar between the groups. The significant variables predicting HCC incidence were male sex (adjusted HR, 2.62; 95% CI, 1.39–4.93; P = .003), cirrhosis (2.22; 1.03–4.75; P = .04), and pw12 AFP level (>7 ng/mL) (2.80; 1.33–5.91; P = .007) (Table 3).
Table 3.
Predictors of Hepatocellular Carcinoma Development in the Propensity Score–Matched Cohort
| Univariable Analysis | Multivariable Analysisa | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
| Age group | ||||
| 60–74 y | 1 (Referent) | .83 | 1 (Referent) | .42 |
| 75–84 y | 1.07 (.59–1.94) | 1.31 (.68–2.50) | ||
| Sex | ||||
| Female | 1 (Referent) | .008 | 1 (Referent) | .003 |
| Male | 2.26 (1.24–4.12) | 2.62 (1.39–4.93) | ||
| Body mass indexb | ||||
| <25 | 1 (Referent) | .44 | 1 (Referent) | .51 |
| ≥25 | 1.31 (.66–2.63) | 1.28 (.61–2.68) | ||
| Baseline fibrosis status | ||||
| No cirrhosis | 1 (Referent) | <.001 | 1 (Referent) | .04 |
| Cirrhosis | 3.66 (1.98–6.77) | 2.22 (1.03–4.75) | ||
| FIB-4 index | ||||
| Baseline ≥3.25 | 3.26 (1.51–7.04) | .003 | … | … |
| pw12 ≥3.25 | 2.37 (1.30–4.32) | .005 | 1.07 (.50–2.29) | .86 |
| Diabetes mellitus | 1.04 (.48–2.24) | .92 | 0.67 (.28–1.59) | .36 |
| Serum albumin | ||||
| Baseline ≤3.5 g/dL | 3.69 (2.03–6.72) | <.001 | … | … |
| pw12 ≤3.5 g/dL | 2.66 (1.18–5.98) | .018 | 1.99 (.78–5.10) | .15 |
| ALT | ||||
| Baseline ≥30 U/L | 1.96 (.99–3.90) | .054 | … | … |
| pw12 ≥30 U/L | 1.93 (.86–4.34) | .11 | 1.58 (.68–3.68) | .29 |
| α-Fetoprotein | ||||
| Baseline >7 ng/mL | 3.85 (2.05–7.24) | <.001 | … | … |
| pw12 >7 ng/mL | 4.18 (2.23–7.85) | <.001 | 2.80 (1.33–5.91) | .007 |
| Genotype | ||||
| 2 | 1 (Referent) | .24 | 1 (Referent) | .20 |
| 1 | 1.75 (.69–4.47) | 1.97 (.69–5.61) | ||
| History of HCV treatment | ||||
| Treatment naive | 1 (Referent) | .38 | 1 (Referent) | .93 |
| Treatment experienced | 1.32 (.71–2.45) | 0.97 (.49–1.90) | ||
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; FIB-4, Fibrosis-4; HCV, hepatitis C virus; HR, hazard ratio; pw12, 12 weeks after the end of treatment.
aMultivariable analysis included background characteristics plus the FIB-4 index and serum albumin, ALT, and α-fetoprotein levels at pw12.
bBody mass index is calculated as weight in kilograms divided by height in meters squared.
The Kaplan-Meier curves for HCC incidence classified by age and cirrhosis status for the PSM cohort are shown in Figure 4. Among patients without cirrhosis, the 5-year cumulative incidence of HCC was significantly higher in the 75–84-year than in the 60–74-year age group (5.2% vs 1.3%, respectively; P = .048).
Figure 4.
Cumulative development of hepatocellular carcinoma (HCC) after initiation of direct-acting antiviral treatment in a propensity score–matched cohort with patients aged 75–84 or 60–74 years. Abbreviations: LC, liver cirrhosis; Non-LC, non-liver cirrhosis.
Sensitivity analysis was performed, including patients in whom HCC developed or who discontinued follow-up within 1 year after DAA initiation. Of the 1760 patients with data available for analysis, 563 (32.0%) were aged 75–84 and 1197 (68.0%) aged 60–74 years at the time of DAA initiation (Supplementary Table 3). In total, 86 cases of HCC (34 in patients aged 75–84 and 52 in those aged 60–74 years) which occurred over the course of the study follow-up.
Kaplan-Meier curves for HCC incidence, classified according to FIB-4 transition score for participants aged 75–84 or 60–74 years, are shown in Supplementary Figures 4 and 5, respectively. Similar to findings in the original cohort, the improvement in FIB-4 index to <3.25 contributed to the decrease in HCC incidence for those aged 60–74 years but not for those aged 75–84 years. After PSM, the data from 559 pairs of patients were available for analysis. The Kaplan-Meier curves for HCC incidence classified by age and cirrhosis status for the PSM cohort are shown in Supplementary Figure 6. There was no significant difference in cumulative HCC incidence rate between the two age groups among patients with cirrhosis (P = .48). In contrast, among patients without cirrhosis, the cumulative HCC incidence rate was significantly higher in the 75–84-year than in the 60–74-year age group (P = .03).
DISCUSSION
We believe that the current study provides further insight into the importance of treating the older generation of patients in whom HCV is eliminated by DAA. Our study was focused on elderly persons aged ≥75 years, a population that has been underrepresented in clinical trials. Our main findings revealed that even though there was improvement in the FIB-4 index to <3.25 or no cirrhosis at baseline, most patients aged 75–84 years remained at high risk for the development of HCC.
The FIB-4 index is a useful noninvasive tool for evaluating advanced fibrosis that can identify groups at high risk for the development of HCC [16]. When using the FIB-4 index in older patients, however, these values have the potential to overestimate fibrosis because age is included in the calculating formula [19]. In fact, most patients aged 75-84 years have difficulty achieving an FIB-4 index of <1.45 even after HCV elimination, owing to their age. Nevertheless, the FIB-4 index plays an important role in determining the risk for HCC. In the assessment of patients aged 60–74 years, a reduction in FIB-4 index from ≥3.25 to <3.25 was associated with a decrease in the risk of HCC, consistent with a previous report [20].
It is important to note that the rapid decrease in the FIB-4 index after DAA treatment mainly represents improvement in hepatic inflammation with the reduction in aspartate aminotransferase and ALT levels more than it represent fibrosis regression. In the current study, many patients whose FIB-4 index decreased from ≥3.25 to <3.25 potentially did not have cirrhosis at baseline (73.5% in the 60–74-year age group); therefore, the reduction in FIB-4 index would explain their better prognosis. In contrast, a reduction in FIB-4 index was not associated with HCC incidence for patients aged 75–84 years with a high FIB-4 index at baseline. The annual rate for the development of HCC remained relatively high (2.16%) for patients aged 75–84 years with FIB-4 index transitioning from ≥3.25 to <3.25, despite the fact that 78.1% did not have cirrhosis at baseline. Going forward, longer-term study will be warranted to elucidate the development of HCC among patients with improvement in the FIB-4 index.
Many recent reports have associated DAA therapy with a significant reduction in the risk of HCC and death [13–15, 20]. Fibrosis status at baseline and some serum biomarkers, including posttreatment AFP and Mac-2 binding protein level, could be useful for predicting the development of HCC [21, 22]. According to a cost-effectiveness study [23], surveillance is likely cost-effective for patients with cirrhosis but not for those with advanced fibrosis. However, almost none of the cohorts studied have included patients aged ≥75 years.
One strength of this study that makes it unique is that it includes 500 patients aged 75–84 years. For patients with cirrhosis, the difference between age groups (75–84 vs 60–74 years) in the development of HCC was borderline significant (P = .08). However, the gap was reduced in the sensitivity analysis (P = .48) because in 7 cirrhotic patients in the 75–84-year age group (4.7%) HCC developed within 1 year after DAA initiation. In contrast, we demonstrated that patients without cirrhosis, considered at low risk for HCC, had differing HCC risks when divided into groups aged 75–84 or 60–74 years. Approximately 65% of the studied patients aged 75–84 years in whom de novo HCC developed were able to have curative treatment, such as resection or radiofrequency ablation; moreover, none of them have died of liver-related complications during the follow-up period. According to data on the cancer survival rate, HCC had the second lowest 5-year relative survival for cases diagnosed in 2006–2012, at 18.1%, with only pancreatic cancer having a lower survival rate, at 8.5% [24]. Taken together, the findings indicate that we need to establish better HCC surveillance programs, especially for patients aged ≥75 years, irrespective of fibrosis stage. In addition, cost-effectiveness studies should be conducted to assess the relative costs of treating HCV based on age.
There are some limitations to this study. First, it was retrospective and had no untreated controls; therefore, in could not show conclusively but only indicated that DAAs reduce HCC incidence among older patients. However, we enrolled consecutive patients treated with DAA in each hospital, reviewed every medical record individually in detail, and used objective criteria, which included laboratory results, radiology reports, biopsy results when available, and physician notes, to determine cirrhosis and HCC status. Second, our study included only Japanese patients. Japan has among the world’s longest healthy life expectancies, both for men and women [25]; moreover, this longevity trend is expected to continue owing to well-organized comprehensive community health promotion and social security services. Therefore, we were able to include a large sample size of elderly patients. Further studies of other ethnic groups and countries are needed to generalize our findings.
Third, the mortality rate that we have shown as a subanalysis might be underestimated, because some patients with terminal illness were transferred to another hospital after discontinuing follow-up. Finally, no analyses of FIB-4 index elevation to ≥3.25 after HCV elimination were done in this study. Actually, there were only 31 patients (1.9%) whose FIB-4 index increased from <3.25 to ≥3.25. The causes of the progression of liver fibrosis after HCV elimination could include a broad range of possibilities, including alcoholic or nonalcoholic steatohepatitis. We hope other studies will be conducted regarding patients with worsening liver fibrosis after HCV elimination by DAA.
In summary, DAA treatment in patients with CHD aged 75–84 years helped reduce the FIB-4 index; however, this had little positive effect on persistent HCC risk in patients with an FIB-4 index ≥3.25 at baseline. In addition, the risk of HCC development in noncirrhotic patients aged 75–84 years was higher than in those aged 60–74 years. Our findings suggest that, to avoid problems that accompany advancing age, early antiviral treatment is needed in patients with CHC and patients aged ≥75 years should continue careful surveillance for HCC, even after HCV elimination.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Note
Potential conflicts of interest. N. F. has received grants from Merck Sharp & Dohme and Gilead Sciences. All other authors report no potential conflicts.
Contributor Information
Eiichi Ogawa, Department of General Internal Medicine, Kyushu University Hospital, Fukuoka, Japan.
Hideyuki Nomura, The Center for Liver Disease, Shin-Kokura Hospital, Kitakyushu, Japan; Department of Internal Medicine, Haradoi Hospital, Fukuoka, Japan.
Makoto Nakamuta, Department of Gastroenterology, Kyushu Medical Center, National Hospital Organization, Fukuoka, Japan.
Norihiro Furusyo, Department of General Internal Medicine, Kyushu University Hospital, Fukuoka, Japan; General Internal Medicine, Taihaku Avenue Clinic, Fukuoka, Japan.
Eiji Kajiwara, Kajiwara Clinic, Kitakyushu, Japan.
Kazufumi Dohmen, Department of Internal Medicine, Chihaya Hospital, Fukuoka, Japan.
Akira Kawano, Department of Medicine, Kitakyushu Municipal Medical Center, Kitakyushu, Japan.
Aritsune Ooho, Department of Hepatology, Steel Memorial Yawata Hospital, Kitakyushu, Japan.
Koichi Azuma, Department of Medicine, Kyushu Central Hospital, Fukuoka, Japan.
Kazuhiro Takahashi, Department of Medicine, Hamanomachi Hospital, Fukuoka, Japan.
Takeaki Satoh, Center for Liver Disease, National Hospital Organization Kokura Medical Center, Kitakyushu, Japan.
Toshimasa Koyanagi, Department of Medicine, Fukuoka City Hospital, Fukuoka, Japan.
Yasunori Ichiki, Department of Internal Medicine, JCHO Kyushu Hospital, Kitakyushu, Japan.
Masami Kuniyoshi, Department of Gastroenterology, Kyushu Rosai Hospital, Kitakyushu, Japan.
Kimihiko Yanagita, Department of Internal Medicine, Saiseikai Karatsu Hospital, Karatsu, Japan.
Hiromasa Amagase, Amagase Clinic, Kitakyushu, Japan.
Chie Morita, Department of Internal Medicine, Kyushu Railway Memorial Hospital, Kitakyushu, Japan.
Rie Sugimoto, Department of Gastroenterology, Kyushu Cancer Center, Fukuoka, Japan.
Masaki Kato, Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Shinji Shimoda, Department of Medicine and Biosystemic Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Jun Hayashi, Kyushu General Internal Medicine Center, Haradoi Hospital, Fukuoka, Japan.
References
- 1. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61:S45–57. [DOI] [PubMed] [Google Scholar]
- 2. Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017; 3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoratti MJ, Siddiqua A, Morassut RE, et al. Pangenotypic direct acting antivirals for the treatment of chronic hepatitis C virus infection: a systematic literature review and meta-analysis. EClinicalMedicine 2020; 18:100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogawa E, Furusyo N, Kajiwara E, et al. ; Kyushu University Liver Disease Study [KULDS] Group . Evaluation of the adverse effect of premature discontinuation of pegylated interferon α-2b and ribavirin treatment for chronic hepatitis C virus infection: results from Kyushu University Liver Disease Study. J Gastroenterol Hepatol 2012; 27:1233–40. [DOI] [PubMed] [Google Scholar]
- 6. Toyoda H, Kumada T, Tada T, et al. Efficacy and tolerability of an IFN-free regimen with DCV/ASV for elderly patients infected with HCV genotype 1B. J Hepatol 2017; 66:521–7. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa E, Furusyo N, Azuma K, et al. ; Kyushu University Liver Disease Study (KULDS) Group . Elbasvir plus grazoprevir for patients with chronic hepatitis C genotype 1: a multicenter, real-world cohort study focusing on chronic kidney disease. Antiviral Res 2018; 159:143–52. [DOI] [PubMed] [Google Scholar]
- 8. Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol 2017; 14:122–32. [DOI] [PubMed] [Google Scholar]
- 9. Toyoda H, Kumada T, Takaguchi K, Shimada N, Tanaka J. Changes in hepatitis C virus genotype distribution in Japan. Epidemiol Infect 2014; 142:2624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med 2012; 157:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C: the OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–32. [DOI] [PubMed] [Google Scholar]
- 12. Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 2002; 36:S47–56. [DOI] [PubMed] [Google Scholar]
- 13. Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol 2018; 69:1088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singal AG, Rich NE, Mehta N, et al. Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology 2019; 157:1253–63.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2020; 71:44–55. [DOI] [PubMed] [Google Scholar]
- 16. Ouchi Y, Rakugi H, Arai H, et al. ; Joint Committee of Japan Gerontological Society (JGLS) and Japan Geriatrics Society (JGS) on the definition and classification of the elderly . Redefining the elderly as aged 75 years and older: proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int 2017; 17:1045–7. [DOI] [PubMed] [Google Scholar]
- 17. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 2013; 158:807–20. [DOI] [PubMed] [Google Scholar]
- 18. Rosenbaum PR, Rubin DB. Constructing a control-group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985; 39:33–8. [Google Scholar]
- 19. McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017; 112:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ioannou GN, Beste LA, Green PK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology 2019; 157:1264–78.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogawa E, Furusyo N, Nomura H, et al. ; Kyushu University Liver Disease Study (KULDS) Group . Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther 2018; 47:104–13. [DOI] [PubMed] [Google Scholar]
- 22. Hsu YC, Jun T, Huang YT, et al. Serum M2BPGi level and risk of hepatocellular carcinoma after oral anti-viral therapy in patients with chronic hepatitis B. Aliment Pharmacol Ther 2018; 48:1128–37. [DOI] [PubMed] [Google Scholar]
- 23. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med 2015; 162:407–19. [DOI] [PubMed] [Google Scholar]
- 24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst 2017; 109:djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1859–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.