Abstract
The characteristics of the respiratory system of Acetobacter diazotrophicus PAL5 were investigated. Increasing aeration (from 0.5 to 4.0 liters of air min−1 liter of medium−1) had a strong positive effect on growth and on the diazotrophic activity of cultures. Cells obtained from well-aerated and diazotrophically active cultures possessed a highly active, membrane-bound electron transport system with dehydrogenases for NADH, glucose, and acetaldehyde as the main electron donors. Ethanol, succinate, and gluconate were also oxidized but to only a minor extent. Terminal cytochrome c oxidase-type activity was poor as measured by reduced N,N,N,N′-tetramethyl-p-phenylenediamine, but quinol oxidase-type activity, as measured by 2,3,5,6-tetrachloro-1,4-benzenediol, was high. Spectral and high-pressure liquid chromatography analysis of membranes revealed the presence of cytochrome ba as a putative oxidase in cells obtained from diazotrophically active cultures. Cells were also rich in c-type cytochromes; four bands of high molecular mass (i.e., 67, 56, 52, and 45 kDa) were revealed by a peroxidase activity stain in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. KCN inhibition curves of respiratory oxidase activities were biphasic, with a highly resistant component. Treatment of membranes with 0.2% Triton X-100 solubilized c-type cytochromes and resulted in a preparation that was significantly more sensitive to cyanide. Repression of diazotrophic activity in well-aerated cultures by 40 mM (NH4)2SO4 caused a significant decrease of the respiratory activities. It is noteworthy that the levels of glucose dehydrogenase and putative oxidase ba decreased 6.8- and 10-fold, respectively. In these cells, a bd-type cytochrome seems to be the major terminal oxidase. Thus, it would seem that glucose dehydrogenase and cytochrome ba are key components of the respiratory system of A. diazotrophicus during aerobic diazotrophy.
Acetobacter diazotrophicus is an obligatory aerobe that fixes nitrogen (1, 5, 7, 16, 39). All nitrogen-fixing bacteria have the ability to utilize atmospheric nitrogen gas as their source of nitrogen for metabolic biosynthesis (5). Otherwise, they represent species from rather different taxonomic groups with different life-styles. These microorganisms must use some mechanism to protect the nitrogenase components from oxygen. In fact, all the nitrogenases from anaerobic, facultatively aerobic, strictly aerobic, symbiotically associated, or even photosynthetic bacteria (22) that have been purified are irreversibly inactivated by oxygen. More than 25 years ago, Drozd and Postgate postulated the existence of a mechanism for the protection of nitrogenase from oxygen inactivation in nitrogen-fixing cells of Azotobacter vinelandii (11). “Respiratory protection” was suggested as a mechanism whereby the extremely high respiratory rates of the cells maintain an intracellular oxygen concentration at levels low enough to not affect the nitrogenase components.
Among the nitrogen-fixing bacteria, A. diazotrophicus is interesting because it carries out nitrogen fixation under aerobic growth conditions. It appears to be a plant endophyte (10) that is capable of excreting almost half of the fixed nitrogen in a form that is potentially available to plants (8). However, its respiratory system and mechanism of protection of nitrogenase under aerobic conditions have not been explored. Hence, the aim of this work is to gain insight into the components of its respiratory system and its relationship to nitrogen fixation metabolism.
MATERIALS AND METHODS
Strain, growth conditions, preparation of membranes, and culture methods.
A. diazotrophicus PAL5 ATCC49037, kindly provided by G. Martínez-Drets (1), was grown under conditions described by Reis et al. (37) with LGIP medium supplemented with 1.0 or 40 mM (NH4)2SO4. Preparative cultures were grown aerobically at 30°C in a 20-liter-working-volume fermentor stirred at 250 rpm and sparged with 32 liters of air min−1 to give an O2 transfer coefficient (KLa) value of 160 (see below).
Active inocula (1.0 liter) were obtained after 24 h of growth in 2.8-liter Ferenbach flasks stirred at 250 rpm. Cells were harvested at the end of the exponential growth phase (36 h) and washed twice with cold 50 mM Tris-HCl (pH 7.4) containing 5 mM CaCl2 and 5 mM MgCl2 (TCM buffer). The cell suspension was supplemented with phenylmethylsulfonyl fluoride (15 μg ml−1) and disrupted in a Dyno-mill (WAB Maschinen-Fabrik, Basel, Switzerland) as previously described (13). Unbroken cells and debris were eliminated by centrifugation at 8,000 × g for 10 min. Membranes were prepared by centrifugation at 144,000 × g for 30 min and thereafter washed twice with TCM buffer. The membranes were used immediately for assay of enzymatic activities or stored in liquid nitrogen.
Analytical cultures were performed in an Applikon laboratory minifermentor with a working volume of 1.0 liters at 30°C and stirred at 320 rpm. Aeration was varied from 0.5 to 4.0 liters of air min−1. At selected times, samples were withdrawn to determine growth (measured as optical density at 560 nm [OD560]) and medium pH. Ammonium concentration and the amount of O2 dissolved in culture medium were measured amperometrically by using an Orion 95-12 ammonia electrode and a Clark-type oxygen electrode. In these samples, metabolism was instantaneously arrested by adding HgCl2 to a final concentration of 1.0 mM.
The culture O2 demand was measured in a model 53YSI oxygen meter by using a 10-fold dilution of culture samples in fresh media. Nitrogenase activity in whole cells was determined by the acetylene reduction assay (37, 39). Samples (2 ml) removed from the fermentor were placed into 10-ml sealed vials, and acetylene was injected to give a 15% concentration in the gas phase; the vials were incubated with stirring at 250 rpm for 30 min at 30°C. The ethylene produced was determined in 10-μl aliquots on a Poropak N column by using a Variant 3400 gas chromatography system fitted with a flame ionization detector.
The oxygen transfer coefficient, KLa was estimated for the 1.0-liter fermentor system by the static method of gassing out as described by Stanbury and Whitaker (38). That is, the oxygen concentration of a fresh culture medium (agitated at 320 rpm) was lowered by gassing the liquid with nitrogen gas, the deoxygenated medium was then aerated, and the increase in dissolved-oxygen concentration was monitored continuously with a fermentor-installed Clark oxygen electrode. KLa values under different aeration conditions (0.5 to 4.0 liters of air min−1) were calculated as described by the same authors (38).
Spectral analysis of cytochromes.
Membranes were suspended in TCM buffer containing 50% (vol/vol) glycerol and analyzed in an SLM-Aminco DW 2000 spectrophotometer. Difference spectra at 77 K (liquid nitrogen) were recorded in cuvettes with a 2-mm light path. Samples were reduced with a few grains of sodium dithionite in the absence or presence of 1.0 mM KCN; the reference samples were oxidized with a few grains of ammonium persulfate. Reduced-plus-CO minus reduced difference spectra were also recorded at 77 K. The concentrations of cytochromes in membranes and derived preparations were calculated from the difference spectra (dithionite-reduced minus persulfate-oxidized or dithionite-reduced plus CO minus dithionite-reduced) at room temperature by using the following wavelength pairs and absorption coefficients: cytochrome c, extinction coefficient at 550 to 540 nm (E550–540) = 19.1 mM−1 cm−1; cytochrome b, E562–575 = 22 mM−1 cm−1; cytochrome a1-CO, E427–440 = 60 mM−1 cm−1; cytochrome d-CO, E622–642 = 18 mM−1 cm−1 (13, 20, 28, 29).
Extraction and analysis of hemes and cytochromes c.
Hemes were extracted with 0.01 N HCl in acetone as described by Goodhew et al. (17). The membrane residues obtained were used to determine cytochrome c without the spectral interference of b-type cytochromes.
Cytochromes c were solubilized by resuspending membrane pellets (10 mg of protein) with 1.0 ml of 0.2% Triton X-100 in 50 mM potassium phosphate (pH 6.0). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in 16- by 14-cm plates with 10% polyacrylamide and a 5% stacking gel by the method of Goodhew et al. (17). Cytochrome c bands were revealed by detection of the peroxidase activity. Protein blotting and heme peroxidase detection were performed with the Amersham enhanced chemiluminescence Western blotting detection reagents, as reported by Miranda-Ríos et al. (33). SDS treatment removes noncovalently bound hemes; therefore, a peroxidase stain on SDS-gels specifically reveals c-type cytochromes.
Heme composition was determined on a Waters chromatography system equipped with a Waters model 996 photodiode array detector and Waters Delta-Pak HPIC18 300 Å (2 by 150 mm) reverse-phase high-pressure liquid chromatography (HPLC) column. The data was analyzed with Millennium 2000 software. Hemes extracted and purified from membranes (40 mg of protein) as described by Puustinen and Wikström (36) were dissolved in 0.5% trifluoroacetic acid–acetonitrite solution and applied to a column previously equilibrated with 0.5% trifluoroacetic acid–25% acetonitrite in water. The hemes were eluted by an acetonitrile gradient as described previously (25). The following standards were used: hemes B and O extracted from membranes of Escherichia coli, hemes B and A extracted from bovine mitochondria particles, and protoheme IX obtained from Sigma.
Respiratory activities.
Oxidase activities were determined with either of the following substrates (final concentrations are given): 3 mM NADH, 10 mM glucose, 50 mM succinate, 10 mM gluconate; 10 mM ethanol, 10 mM acetaldehyde, 10 mM ascorbate plus 2 mM TMPD (N,N,N′,N′-tetramethyl-p-phenylenediamine), or 10 mM ascorbate plus 1.5 mM THQ (2,3,5,6-tetrachloro-1,4-benzenediol). The reactions were initiated with 0.1 mg of membrane protein and measured polarographically with a Clark oxygen electrode in 2 ml of 50 mM potassium phosphate buffer (pH 7.4 or 6.0) at 30°C.
Dehydrogenase activities were measured spectrophotometrically with potassium ferricyanide as the electron acceptor. The assay mixture contained 0.1 M potassium phosphate buffer (pH 7.4 or 6.0), 1.0 mM test substrate, 1 mM potassium ferricyanide, and 0.03 mg of membrane protein. The reaction was started by the addition of substrate, and the reduction of ferricyanide was monitored by measuring the OD660 (2, 3). One unit of activity is defined as that causing the reduction of 1 μmol of ferricyanide per min under these conditions. THQ was dissolved in dimethyl sulfoxide. The solvent alone had no significant effect on the respiratory activities tested. Protein concentrations were determined by a modification (12) of the Lowry method.
RESULTS
A. diazotrophicus has been recognized as an aerotolerant diazotroph (39) in which oxygen is instrumental for the generation of the large quantities of ATP required for nitrogen fixation. Hence, experiments were performed to explore the effect of increasing aeration on the growth properties and nitrogen fixation activity of A. diazotrophicus in batch culture at 30°C (Fig. 1).
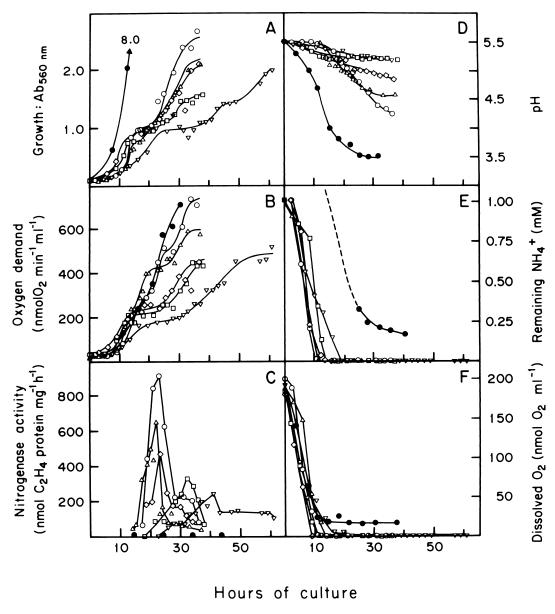
FIG. 1.
Growth properties and diazotrophic activity of A. diazotrophicus on LGIP medium with increased aereation (liters of air min−1 liters of medium−1): ▿, 0.5; □, 1.0; ◊, 1.5; ▵, 2.5; ○, 4.0. The medium contained 1.0 mM (NH4)2SO4 as the nitrogen starting dose; cultures were performed at 30°C in a fermentor with a working volume of 1.0 liter, agitated to 320 rpm. Cultures were initiated with 20 ml of inoculum from a 24-h shaker culture (200 rpm). At the times noted, samples were withdrawn to determine culture growth at 560 nm (A), culture oxygen demand (B), nitrogenase activity as measured by the acetylene reduction assay (C), medium pH (D), concentration of ammonium in the medium (E), and dissolved-oxygen concentration in the medium (F). For comparison, growth profiles obtained in LPGIP medium containing 40 mM (NH4)2SO4 and with an air flow of 4.0 liters of air min−1 are displayed (●) in each of the sets. Details of culture and assay methods are described in Materials and Methods.
In confirmation of the results of Stephan et al. (39), A. diazotrophicus did not grow in N-free LGIP medium at aeration levels of 0.5 to 4.0 liters air min−1 in a 1.0-liter-working-volume minifermentor agitated at 320 rpm (data not shown). Therefore, the medium was supplemented with 1.0 mM (NH4)2SO4 as the nitrogen starting dose. Under these conditions, high aeration (as above) had a strong positive effect on the growth properties of A. diazotrophicus; growth was faster and higher optical densities were obtained (Fig. 1A). Under each of the aeration conditions tested, the curves for growth (Fig. 1A) and oxygen demand (Fig. 1B) of the culture ran in parallel and showed biphasic kinetics. The first phase of growth seems to rely on the initial dose of NH4+, as suggested by the concomitant removal of this ion from the medium (Fig. 1E). After a few hours of adaptation, growth was resumed; this stage was accompanied by an expression of nitrogenase activity (Fig. 1C). The highest specific activity of nitrogenase was registered in the best-aerated (i.e., 4.0 liters of air min−1) and fastest-growing culture. Therefore, this suggested that the second phase of growth depends on the diazotrophic activity of cultures.
Sucrose utilization by A. diazotrophicus leads to the acidification of media due to the accumulation of gluconic acids (5, 9, 39). Accordingly, increasing the aeration of cultures accelerated and increased the acidification of media during the second phase of growth (Fig. 1D), suggesting that during this time an intense oxidation of glucose to gluconic acid by glucose dehydrogenase provided appropriate metabolic conditions to generate enough ATP for growth and continuously remove O2 from the medium (Fig. 1F) so as to protect the diazotrophic activity.
We found that at all levels of aeration, nitrogenase activity appeared after the initial dose of ammonium had been exhausted (Fig. 1E) and the dissolved O2 concentration had dropped to nondetectable concentrations (Fig. 1F).
For a comparison, growth profiles obtained in LGIP medium containing 40 mM (NH4)2SO4, with aeration of 4 liters of air min−1, are displayed in each of the panels of Fig. 1. Ammonium had a large impact on the growth properties of A. diazotrophicus; fast growth producing high optical densities (i.e., OD560 = 8.0 after 34 h) was observed. Rapid growth was accompanied by a fast and deep acidification of the medium (final pH = 3.5) and a quantitative removal of NH4+ from the medium. The profile for oxygen demand did not reach the levels expected for such high cell densities achieved by growth. Dissolved O2 in the medium decreased to a constant low level (i.e., 20 nmol ml−1) after 10 h of growth. As expected, nitrogenase activity was not detected at any time during culture.
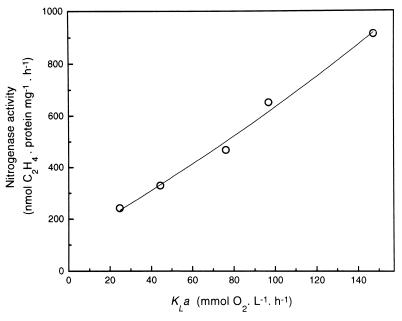
To gain further insight into the impact of increasing aeration on the expression levels of nitrogenase activity, oxygen transfer coefficients (i.e., KLa) were estimated at the aeration levels used in the experiment in Fig. 1. A plot of KLa values against the top registered values of nitrogenase activity (Fig. 2) showed that the specific activity of nitrogenase increased linearly within the KLa range tested (i.e., 25 to 145 mmol of O2 liter−1 h−1). This implies that the O2 supply to the medium was the rate-limiting step for N2-dependent growth.
FIG. 2.
Nitrogenase activities of A. diazotrophicus in a fermentor (1.0-liter working volume) at increased values of oxygen transfer coefficient (KLa). Values for KLa (see Materials and Methods) were determined under each of the aeration conditions shown in Fig. 1 and plotted against the recorded maximal values of nitrogenase activity (as shown in Fig. 1C).
Cytochromes.
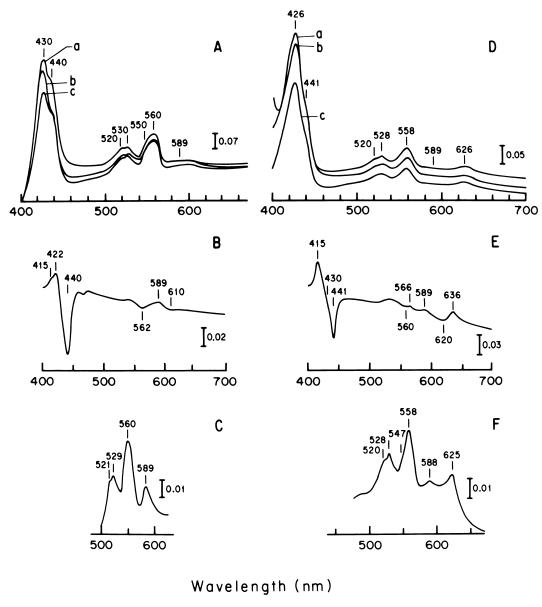
The respiratory system of A. diazotrophicus was characterized in membranes obtained from cells grown aerobically in LGIP medium supplemented with 1.0 mM (NH4)2SO4 and was compared to that of cells grown under same conditions with 40 mM (NH4)2SO4. The spectroscopic analysis of the cytochrome composition of the two membrane preparations showed significant differences (Fig. 3). Reduced-minus-oxidized spectra (77 K) of cells grown on low ammonium (Fig. 3A) showed b-type cytochromes (peaks at 430, 530, and 560 nm). c-type and a-type cytochromes were respectively suggested by shoulders at 520 and 550 nm and by a shoulder at 440 nm plus a weak signal around 600 nm. Difference spectra (77 K) produced by carbon monoxide (Fig. 3B) and cyanide (Fig. 3C) of the reduced preparation revealed the presence of an a-type cytochrome; however, the reduced cytochrome-CO compound produced signals at 422 and 440 nm, i.e., a shift of a few nanometers toward the violet, relative to the typical aa3-CO complex (29). On the other hand, the reaction of CN− with the reduced preparation was accompanied by a large enhancement of the signal at 589 nm. This hyperchromic effect of cyanide on the reduced spectrum has been considered a reliable criterion for the identification of cytochrome ba oxidase (29). It is relevant that cytochrome ba oxidase (also named cytochrome a1) has been identified in Acetobacter aceti (28, 29).
FIG. 3.
Low-temperature (77 K) spectra of membranes of A. diazotrophicus PAL5 grown aerobically in LGIP medium supplemented with 1.0 mM (A to C) or 40 mM (D to E) (NH4)2SO4. (A and D) reduced-minus-oxidized spectra. Difference spectra were generated by adding sodium dithionite (spectra a), NADH (spectra b), and glucose (spectra c) to sample cuvettes and ammonium persulfate to reference cuvettes. (B and E) Reduced plus CO minus reduced difference spectra. Membranes in both cuvettes were reduced by dithionite and CO gas bubbled through sample cuvettes. (C and F) Reduced plus 1.0 mM KCN minus oxidized spectra. Sample cuvettes were reduced with dithionite in the presence of 1.0 mM KCN. Reference cuvettes were oxidized with ammonium persulfate. All samples contained 2.5 mg of membrane protein ml−1. Cells were collected at early stationary phase once they reached OD560 = 2.5 (low NH+4) and 6 to 8 (high NH+4).
The cytochrome analysis of cells grown on high ammonium (i.e., 40 mM) showed a different picture. In the reduced-minus-oxidized spectra (Fig. 3D), maxima at 426, 528, and 558 nm indicated b-type cytochromes; signals for c-type cytochromes at 418, 520, and 550 nm were hardly discernible. However, the most dramatic changes were those related to putative oxidases. Signals for reduced cytochrome ba at 440 and 589 nm were significantly low, but a peak at 626 nm (Fig. 3D) was clearly apparent, suggesting the presence of a cytochrome bd. That conclusion was reinforced by the CO difference spectrum (Fig. 3E); it showed a weak signal at 589 nm for the cytochrome a-CO compound and conspicuous signals at 620 (through) and 636 nm (peak); these data would be consistent with the presence of a cytochrome d-CO-type compound.
Taken together, the results indicate that when culture requirements for nitrogen are satisfied by an excess of ammonium, nitrogenase is not expressed, and that cytochrome bd seems to replace cytochrome ba as the main putative oxidase in A. diazotrophicus. Moreover, when a culture grown for 24 h (KLa = 150, OD560 = 1.2) in 1.0 mM NH4+ was switched to NH4+-dependent growth by increasing the (NH4)2SO4 concentration to 40 mM (results not shown), there was a change in the composition of terminal oxidases; i.e., cytochrome bd was spectrocopically detectable after 2.5 h in high NH4+ (OD560 = 1.5) and became the dominant oxidase 2.5 h later (OD560 = 3.2). On the other hand, the high levels of cytochrome ba at the time of (NH4)2SO4 addition decreased steadily during NH4+-dependent growth. Indeed, 5 h after the addition of (NH4)2SO4, the cytochrome pattern registered in whole cells was very similar to that registered in membranes of cells obtained from cultures in high ammonium (Fig. 2E to F). On the other hand, it is noted that cytochrome bd was at no time detected in cells removed from a control culture grown for 72 h in 1.0 mM (NH4)2SO4 medium (results not shown). These results contrast with previous reports of experiments with Azotobacter vinelandii that indicate that a cytochrome bd accounts for respiratory protection of nitrogenase (18, 19, 34).
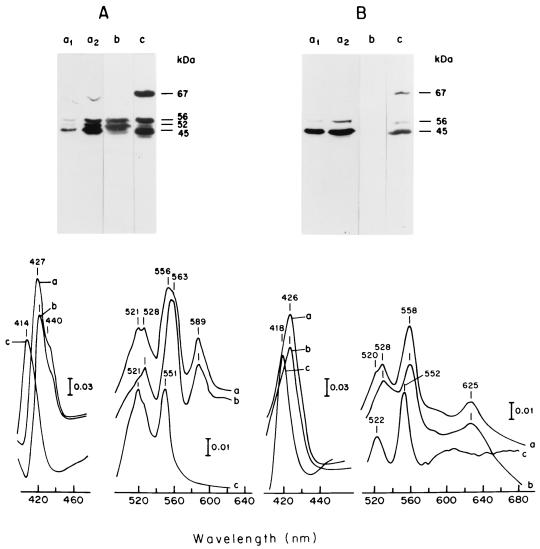
The c-type cytochromes were further characterized in cells grown on limited ammonium (Fig. 4A) and on excess ammonium (Fig. 4B). Treatment of membranes with 0.2% Triton X-100 caused selective solubilization of c-type cytochromes (spectra c in Fig. 4), while b-, a-, and d-type cytochromes remained attached to membrane residues (spectra b), compared to spectra of whole membranes (spectra a). SDS-PAGE analysis of membranes and 0.2% Triton X-100 fractions showed that cells grown on limited ammonium (Fig. 4A) are rich in c-type cytochromes; four bands (67, 56, 52, and 45 kDa [lanes a1 and a2]) positive for peroxidase (33) were apparent. The bands of 67, 56, and 45 kDa were released by 0.2% Triton X-100 (lane b), but the 52-kDa band remained membrane bound (lane c). Membranes of cells grown in high ammonium (Fig. 4B) contained decreased levels of c-type cytochromes, and only the 45-kDa band was present at significant levels. In this respect, it is noted that alcohol dehydrogenases (28, 31) and aldehyde dehydrogenases (3) of acetic acid bacteria contain subunits bearing cytochrome c, with molecular masses ranging from 45 to 82 kDa. In consonance with these observations, our 0.2% Triton X-100 supernatant of cells grown on limited ammonium was rich in dehydrogenase activities for ethanol and acetaldehyde (data not shown) and the membrane residues, depleted of cytochromes c, retained full capacity for glucose and NADH oxidation (data not shown).
FIG. 4.
c-type cytochromes associated with membranes of A. diazotrophicus PAL5 grown aerobically in LGIP medium supplemented with 1.0 mM (A) or 40 mM (B) (NH4)2SO4. Membranes (50 mg of protein) were extracted with 5.0 ml of 0.2% Triton X-100 in 50 mM potassium phosphate (pH 6.0) for 2 h at 4°C. Membrane residues were sedimented at 140,000 × g for 1 h. SDS-PAGE and peroxidase stain of heme C-containing proteins of whole membranes (lanes a1 and a2), membrane residues after 0.2% Triton X-100 treatment (lanes b), and supernatant after 0.2% Triton X-100 treatment (lanes c). Dithionite-reduced minus persulfate-oxidized spectra at 77 K were obtained from the same samples with spectra a to c as above. The protein contents for spectra a, b, and c were 5.0, 4.0, and 1.0 mg ml−1, respectively. The protein contents in SDS-PAGE of samples in panel A were 80, 160, 940, and 1,000 μg for samples a1, a2, b, and c, respectively; those in panel B were: 500 μg for sample a1 and 1,000 μg for samples a2, b, and c. SDS treatment removes noncovalently bound hemes. Hence, a peroxidase stain on SDS-gels specifically reveals c-type cytochromes.
The concentration of each type of cytochromes was calculated from spectra recorded at room temperature; the results (Table 1) showed that the concentration of b-type cytochromes was similar in membranes of both types of cells. On the other hand, in cells grown in low ammonium, the concentration of c-type and a-type cytochromes was 2- and 10-fold higher, respectively than in cells grown in high ammonium. Cytochrome d was detected at high concentrations only in the latter type of cells.
TABLE 1.
Effect of ammonium concentration in LGIP medium on the composition of cytochromes associated with membranes of A. diazotrophicus PAL5 grown aerobicallya
| Cytochrome | Amt of cytochrome (nmol/mg of protein in cells grown in:
|
|
|---|---|---|
| Low ammonium | High ammonium | |
| c | 0.12 | 0.06 |
| b | 0.25 | 0.17 |
| a1-CO | 0.16 | 0.01 |
| d-CO | NDb | 0.16 |
Cultures were performed at 30°C in LGIP medium supplemented with 1.0 mM (low) or 40 mM (high) (NH4)2SO4, in a 20-liter-working-volume fermentor sparged with 32 liters of air min−1 and stirred at 250 rpm (KLA = 165). Cells were collected at early stationary phase: OD560 = 2.5 (low ammonium) and 6 to 8 (high ammonium).
ND, not detected.
Hemes.
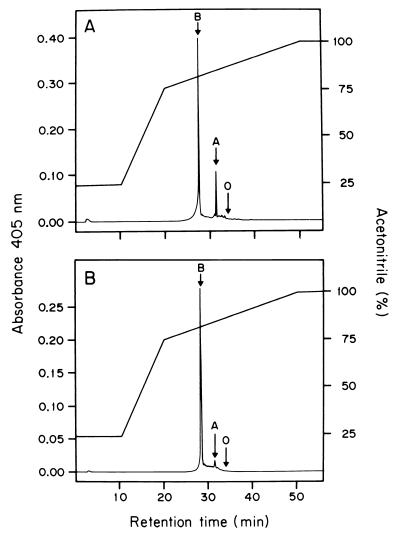
Hemes were extracted, purified from membranes, and analyzed by reverse-phase HPLC (Fig. 5); the column was calibrated with hemes A, B, and O. Samples purified from A. diazotrophicus grown on limited ammonium (Fig. 5A) showed two main peaks with retention times of 28.5 and 31.4 min, corresponding to hemes B and A, respectively. A different heme composition was observed in cells grown in high ammonium (Fig. 5B). The expected peak for heme B and a small peak for heme A were observed. Heme D was not detected by the HPLC procedure we used (40), but its presence in cells grown in high ammonium was confirmed by preparation of its pyridine hemochrome derivative (results not shown). We also found that heme O (retention time = 34 min) was not detected in either of the two types of cells.
FIG. 5.
Reverse-phase HPLC chromatograms of the membrane-bound hemes from A. diazotrophicus PAL5 grown aerobically in LGIP medium supplemented with 1.0 mM (A) or 40 mM (B) (NH4)2SO4. The system was calibrated with the following standards: hemes B and O extracted from membranes of E. coli, hemes B and A extracted from submitochondrial particles from bovine heart, and photoheme IX obtained from Sigma Chemical Co. The retention times for the standards and hemes of A. diazotrophicus were as follows: heme B, 28.5 min; heme A, 31.4 min; heme O, 34 min. The relative amounts of hemes B and A were estimated to be 1.0:0.26 (A) and 1.0:0.042 (B).
Respiratory activities.
Membrane particles of A. diazotrophicus grown in medium with low ammonium had respiratory specific activities higher than those of cells grown at high ammonium concentrations (Table 2). In decreasing order, NADH, glucose, and acetaldehyde were the best physiological substrates for both types of cells. Oxidase activities with NADH, acetaldehyde, and glucose were 2.2-, 2.9-, and 5.5-fold higher, respectively in membrane preparations obtained from cultures in low ammonium than in those from cultures in high ammonium. The outstanding increase observed for glucose oxidase was due to a 6.8-fold increment in the glucose dehydrogenase activity. Gluconate, ethanol, and succinate were significantly less efficient as electron donors in the two cell preparations.
TABLE 2.
Effect of ammonium concentration in LGIP medium on the respiratory activities associated with membranes of A. diazotrophicus PAL5 grown aerobicallya
| Substrate | Low ammoniumb
|
High ammoniumb
|
Low/high ratio for oxidase | ||
|---|---|---|---|---|---|
| Oxidase activity (ng-atoms of O2) | Dehydrogenase activity (nmol of ferricyanide) | Oxidase activity (ng-atoms of O2) | Dehydrogenase activity (nmol of ferricyanide) | ||
| NADH | 2,824 | 2,563 | 1,293 | 1,693 | 2.2 |
| Glucose | 2,085 | 2,180 | 379 | 319 | 5.5 |
| Acetaldehyde | 1,082 | 1,633 | 364 | 130 | 2.9 |
| THQ-ascorbate | 750 | 363 | 2.0 | ||
| Gluconate | 314 | 127 | 91 | 34 | 3.4 |
| Ethanol | 235 | 826 | 141 | 84 | 1.6 |
| Succinate | 235 | 680 | 172 | 209 | 1.3 |
| TMPD-ascorbate | 141 | 108 | 1.3 | ||
Cultures were performed on LGIP medium supplemented with 1.0 mM (low ammonium) or 40 mM (high ammonium) (NH4)2SO4, as described in Table 1.
Respiration rates were measured polarographically with a Clark-type electrode, while dehydrogenase activities were determined spectrophotometrically with potassium ferricyanide as the electron acceptor. In both cases, the specific activities per milligram of membrane protein per minute are displayed. Each value is the mean of values obtained from three individual cultures. The standard deviations were less than 15% of the means given. Activities shown are those obtained at the optimal pH: glucose, acetaldehyde, ethanol, and gluconate at pH 6.0; NADH and succinate at pH 7.4. Activities with THQ-ascorbate and TMPD-ascorbate were measured at pH 6.0 to minimize the chemical oxidation of these cocktails.
It is noteworthy that the quinol TQH was oxidized at high rates by both types of cell membranes whereas the TMPD-ascorbate mixture was the poorest electron donor, suggesting that terminal oxidases in both types of membranes belong to the group of quinol oxidases (15).
Oxidase and dehydrogenase activities with glucose, acetaldehyde, ethanol, and gluconate determined at pH 6.0 (Table 2) were 3-, 1.5-, 1.3-, and 1.2-fold higher respectively, than at pH 7.4 (results not shown). For NADH and succinate, we found that activities at pH 7.4 (Table 2) were 1.3- to 1.5-fold higher than at pH 6.0 (results not shown).
Cyanide inhibition.
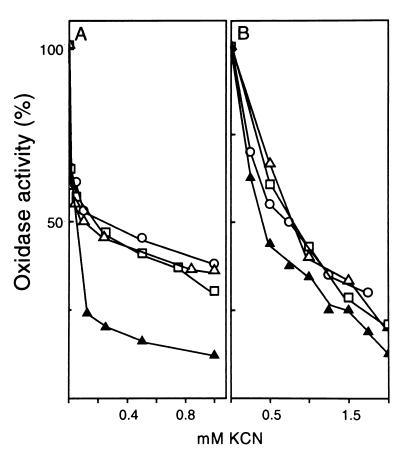
The oxidase activities with NADH, glucose, and acetaldehyde were titrated with KCN in membranes of cells grown in low ammonium (Fig. 6A). KCN inhibition at the terminal oxidase step with all substrates tested was clearly biphasic, and about 50% of the respiratory activity was abolished by 100 μM KCN. The second kinetic component was relatively resistant to the inhibitor. The residual membranes obtained after treatment with 0.2% Triton X-100 (Fig. 4) exhibited a fully active glucose oxidase which was significantly more sensitive to the inhibitor; i.e., 75% of the activity was inhibited by 100 μM KCN. Triton X-100 treatment removed most of the c-type cytochromes from the membrane (Fig. 4). It is thus possible that the cyanide-resistant respiration in A. diazotrophicus involves c-type cytochromes, as shown for Gluconobacter suboxydans (31).
FIG. 6.
Cyanide inhibition of oxidase respiratory activities of A. diazotrophicus PAL5 grown aerobically in LGIP medium supplemented with 1.0 mM (A) or 40 mM (B) (NH4)2SO4. Oxidase activities for NADH (○), glucose (▵), and acetaldehyde (□) in membranes were titrated with KCN. Alternatively, the KCN titration was performed on (▴) glucose oxidase of membrane residues obtained after treatment with 0.2% Triton X-100 (see Results and Fig. 4). The membrane protein used, assay conditions, and oxidase specific activities were similar to those shown in Table 2.
The cyanide titration curves for the same oxidase activities in membranes of cells grown on high ammonium presented a single cyanide-resistant component; i.e., the three oxidases tested retained more than 80% of their activity with 100 μM KCN and about 45% survived treatment with 1.0 mM KCN. As noted above, these types of membranes contain low levels of c-type cytochromes and significant levels of cytochrome bd; this oxidase has been described as typically resistant to cyanide (20, 35). Treatment of these membranes with 0.2% Triton X-100 did not modify the cyanide titration curve significantly (Fig. 6B).
DISCUSSION
A. diazotrophicus belongs to the selected group of bacterial species endowed with the capacity for nitrogen fixation; it is remarkable that this ability can be demonstrated in culture and increased by aerobic conditions (reference 39 and this work). This peculiar life-style requires an efficient mechanism for protection of nitrogenase activity from deleterious oxygen (21–23). Therefore, it is relevant that we found that A. diazotrophicus PAL5 grown in well-aerated media possesses a respiratory system with the following remarkable features.
(i) It had an amazingly high respiratory capacity. The respiratory rates with NADH and glucose determined here (Table 2) are among the highest ever reported for aerobic bacteria (see examples in references 6, 13, 21, 29, and 30).
(ii) The O2 demand of A. diazotrophicus during N2-dependent growth was sufficient to remove continuously dissolved O2 in well-aerated cultures, thus producing an adequate intracellular environment for nitrogen fixation. In fact, within the KLa range tested (i.e., 24 to 145 mmol of O2 liter−1 h−1), the O2 supply to the medium was the limiting step rate for N2-dependent growth.
(iii) Respiration of glucose deserves special consideration. Galar and Boiardi (14) showed that glucose dehydrogenase activity increased when A. diazotrophicus was grown under nitrogen-fixing conditions. Along these lines, Alvarez and Martínez-Drets (1) suggested that the catalytic site of this enzyme faces the periplasmic space, thus enabling oxidation without permeation. As expected for an enzyme whose catalytic site is oriented to the outer acidic medium, we found that the glucose oxidation rate at pH 6.0 was threefold higher than at pH 7.4. Likewise, we found that the dehydrogenase activities for acetaldehyde, ethanol, and gluconate were higher at pH 6.0 than at pH 7.4. The ample number of dehydrogenases feeding electrons to the respiratory system without mediation of NAD (reference 1 and this study) would seem to provide a varied menu of direct electron donors that ensure sufficient electron flux for ATP synthesis and oxygen consumption.
(iv) Cytochrome a1 (cytochrome ba) seems to be the major oxidase expressed during aerobic N2-dependent growth. This enzyme, rare among bacteria, was identified by its CO difference spectrum and by the intensification of its α-band at 589 nm when cyanide reacted with the reduced form. Its characteristics were similar to those of the well-established cytochrome a1 of A. aceti (29). The presence of heme A in membranes of cells grown aerobically at low NH+4 concentrations was confirmed by HPLC analysis.
(v) Interestingly, a bd-type cytochrome is absent in cells displaying nitrogen-fixing ability during aerobic growth in limiting NH+4. However, the spectral features of a bd-type cytochrome were conspicuous in membranes of aerobic cells grown in excess NH+4. These results contrast with previous reports of studies with Azotobacter vinelandii, where a bd-type cytochrome plays the major role in the respiratory protection of nitrogenase while a bo-type oxidase seems to be involved in a coupled step in the generation of ATP (21, 24, 32). The presence of a bo-type cytochrome in A. diazotrophicus could not be confirmed in cells grown in low-NH4+ cultures, suggesting that the putative oxidase ba might play the role of a highly coupled site.
Membranes of A. diazotrophicus grown in low NH+4 were rich in c-type cytochromes with high molecular masses. By analogy to the respiratory chain of G. suboxydans (2, 4), A. methanolicus (30), and A. aceti (31), these c-type cytochromes could function as electron carriers in the segments preceding ubiquinone, i.e., those associated with primary dehydrogenases. It is known that alcohol and aldehyde dehydrogenases from A. aceti (31), A. methanolicus (30), and G. suboxydans (3, 4) contain subunits bearing c-type cytochrome with molecular masses that range from 40 to 80 kDa. The values are within the range of those found in this work (i.e., 67, 56, 52, and 45 kDa [Fig. 4]). The TMPD oxidase activity registered here (Table 2) was negligible, thus discounting a role for cytochrome c in the high-potential side of the respiratory system. Moreover, ubiquinol cytochrome c reductase activity could not be detected in membranes of A. diazotrophicus with NADH as an electron donor and horse cytochromes c as an electron acceptor in the presence of 2 mM KCN (results not shown).
Oxidase ba could be identified as the highly sensitive target for KCN in cells obtained from low-NH+4 cultures. The high-molecular-mass c-type cytochromes might be components of the KCN-resistant branch, since its selective release from membranes by Triton X-100 results in a membrane preparation that was more sensitive to the inhibitor.
Here we presented persuasive evidence suggesting that the ammonium concentration in the culture plays a determinant role in the expression of components of the respiratory system of A. diazotrophicus. This could be a unique property among acetic acid bacteria. It is noteworthy that the levels of glucose dehydrogenase, c-type cytochromes, and alternative oxidases ba and bd are strongly affected by ammonium concentration in media and that all this seems to be related to the unique life-style of A. diazotrophicus as a facultative diazotroph among acetic acid bacteria.
Although there is an underlying similarity in the organization of respiratory systems of acetic acid bacteria, there is still significant variation in its individual components, mainly at the level of the terminal oxidases. Previous descriptions of this subject (1, 4, 27–31) show that the distinct members of the group so far described can be distinguished by the possession of personalized sets of terminal oxidases. Hence, the presence of cytochromes ba and bd as terminal oxidases in A. diazotrophicus constitutes a distinctive set among acetic acid bacteria.
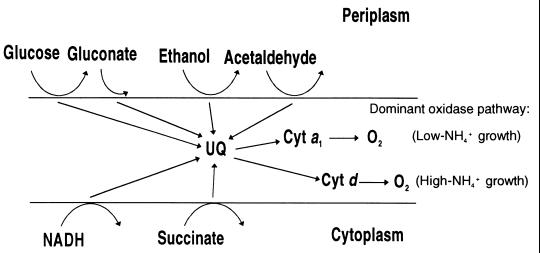
Figure 7 illustrates our proposal for the composition and organization of the respiratory system of A. diazotrophicus, as well as its variations according to the ammonium concentration in well-aerated cultures.
FIG. 7.
Postulated composition and organization of the aerobic respiratory system of A. diazotrophicus PAL5. Cytochrome a1 (also called ba) putative oxidase is preferentially expressed in N2-fixing cells, while cytochrome bd putative oxidase is conspicuous in cells grown with excess NH4+. Respiratory activities, notably glucose oxidase, are higher in cells displaying diazotrophic activity. Cyt, cytochrome.
The low-redox-potential side will be composed of several dehydrogenases directly coupled to the respiratory chain, including flavoprotein dehydrogenases for NADH and succinate with the catalytic site facing the cytoplasmic side of the membrane. The catalytic sites of quinoprotein glucose dehydrogenase and cytochrome c-containing dehydrogenases for ethanol and acetaldehyde are oriented facing the periplasmic space. A ubiquinone pool will collect reduced equivalents from all functional dehydrogenases, which in turn will transfer electrons to cytochrome a1 quinol-oxidase. Alternatively, cells grown in excess NH+4 and under aerobic conditions will contain cytochrome bd quinol oxidase and limited amounts of cytochrome a1; cyanide acts on the cytochrome a1 terminal oxidase. Membranes of A. diazotrophicus showed significant levels of cyanide-resistant respiration, which was selectively abolished with low concentrations of Triton X-100 (Fig. 6A). The nature of the components involved in this respiration remains to be explored, but in other work cytochrome c553 (subunit II of alcohol dehydrogenase) has been implicated as the main component of the cyanide-insensitive oxidase bypass of G. suboxydans (31).
Under nitrogen-fixing conditions, a rapid respiratory electron transport activity will be required to keep intracellular oxygen tension at very low levels. This could be accomplished through the high expenditure of ATP in nitrogen fixation and a physiological mechanism that carries out a high rate of uncoupled electron transport. An uncoupled respiratory pathway (cyanide resistant) and chemical uncoupling (acidification) have been proposed in G. suboxydans and in A. aceti respectively, (31). A. diazotrophicus PAL5 has one of the highest known rates of respiration and, very probably, the ability to adjust its oxygen consumption to match wide variations in its oxygen supply. Rapid respiration would be able to provide “respiratory protection” to the oxygen-labile nitrogenase during aerobic diazotrophy.
ACKNOWLEDGMENTS
This work was supported by grant DGAPA-UNAM IN-219397 and a CONACYT grant to J.E.E.
We express our deep appreciation to A. Gómez-Puyou, Mario Soberón, and Ann L. Lutterman for their generous help and criticism during the preparation of the manuscript. We are also indebted to Juan Méndez for his technical assistance and to Virginia Godínez for her secretarial assistance.
REFERENCES
- 1.Alvarez B, Martínez-Drets G. Metabolic characterization of Acetobacter diazotrophicus. Can J Microbiol. 1995;41:918–924. [Google Scholar]
- 2.Ameyama M, Adachi O. Alcohol dehydrogenase from acetic acid bacteria, membrane-bound. Methods Enzymol. 1982;89:450–457. [Google Scholar]
- 3.Ameyama M, Adachi O. Aldehyde dehydrogenase from acetic acid bacteria, membrane-bound. Methods Enzymol. 1982;89:491–497. [Google Scholar]
- 4.Ameyama M, Matsushita K, Shinagawa E, Adachi O. Sugar-oxidizing respiratory chain of Gluconobacter suboxydans. Evidence for a branched respiratory chain and characterization of respiratory chain-linked cytochromes. Agric Biol Chem. 1987;51:2943–2950. [Google Scholar]
- 5.Attwood M M, van Dijken J P, Pronk J T. Glucose metabolism and gluconic acid production by Acetobacter diazotrophicus. J Ferment Bioeng. 1991;72:101–105. [Google Scholar]
- 6.Barquera B, García-Horsman A, Escamilla J E. Cytochrome d expression and regulation pattern in free-living Rhizobium phaseoli. Arch Microbiol. 1991;155:114–119. [Google Scholar]
- 7.Cavalcante V A, Döbereiner J. A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil. 1988;108:23–31. [Google Scholar]
- 8.Cohjo E H, Reis V M, Schenberg A C, Döbereiner J. Interactions of Acetobacter diazotrophicus with an amylolytic yeast in nitrogen-free batch culture. FEMS Microbiol Lett. 1993;106:23–31. [Google Scholar]
- 9.Cozier G E, Anthony C. Structure of the quinoprotein glucose dehydrogenase of Escherichia coli modelled on that of methanol dehydrogenase from Methylobacterium extorquens. Biochem J. 1995;312:679–685. doi: 10.1042/bj3120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Döbereiner J, Reis V M, Paula M A, Olivares F L. Endophytes diazotrophs in sugar cane, cereals and tuber plants. Curr Plant Sci Biotechnol Agric. 1993;17:671–676. [Google Scholar]
- 11.Drozd J, Postgate J R. Effects of oxygen on acetylene reduction, cytochrome content and respiratory activity of Azotobacter chroococcum. J Gen Microbiol. 1970;63:63–73. doi: 10.1099/00221287-63-1-63. [DOI] [PubMed] [Google Scholar]
- 12.Dulley J R, Grieve P A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Biochem. 1975;64:136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- 13.Escamilla J E, Ramírez R, del Arenal I P, Zarzoza G, Linares V. Expression of cytochrome oxidases in Bacillus cereus: effects of oxygen tension and carbon source. J Gen Microbiol. 1987;133:3549–3555. [Google Scholar]
- 14.Galar M L, Boiardi J L. Evidence for a membrane-bound pyrroloquinoline quinone-linked glucose dehydrogenase in Acetobacter diazotrophicus. Appl Microbiol Biotechnol. 1995;43:713–716. [Google Scholar]
- 15.García-Horsman J A, Barquera B, Rumbley J, Ma J, Gennis R B. The superfamily of heme-copper respiratory oxidases. J Bacteriol. 1994;176:5587–5600. doi: 10.1128/jb.176.18.5587-5600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillis M, Kersters K, Hoste B, Janssens D, Kroppenstedt R M, Stephan M P, Teixeira K R S, Döbereiner J, De Ley J. Acetobacter diazotrophicus sp. nov., a nitrogen-fixing acetic acid bacterium associated with sugarcane. Int J Syst Bacteriol. 1989;39:361–364. [Google Scholar]
- 17.Goodhew C F, Brown K R, Pettigrew G W. Heme staining in gels, as useful tool in the study of bacterial c-type cytochromes. Biochim Biophys Acta. 1986;852:288–294. [Google Scholar]
- 18.Hoffman P, Morgan T V, DerVartanian D V. Respiratory-chain characteristics of mutants of Azotobacter vinelandii negative to tetramethyl-p-phenylenediamine oxidase. Eur J Biochem. 1979;100:19–27. doi: 10.1111/j.1432-1033.1979.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones C W, Redfearn E R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966;113:467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- 20.Jünemann S. Cytochrome bd terminal oxidase. Biochim Biophys Acta. 1997;1321:107–127. doi: 10.1016/s0005-2728(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 21.Kelly M J S, Poole R K, Yates M G, Kennedy C. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. J Bacteriol. 1990;172:6010–6019. doi: 10.1128/jb.172.10.6010-6019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy I R, Tchan Y T. Biological nitrogen fixation in non-leguminous field crops: recent advances. Plant Soil. 1992;141:93–118. [Google Scholar]
- 23.Kim J, Rees D C. Nitrogenase and biological nitrogen fixation. Biochemistry. 1994;33:389–397. doi: 10.1021/bi00168a001. [DOI] [PubMed] [Google Scholar]
- 24.Leung D, van der Oost J, Kelly M J, Saraste M, Hill S, Poole R K. Mutagenesis of a gene encoding a cytochrome o-like terminal oxidase of Azotobacter vinelandii: a cytochrome o mutant is aero-tolerant during nitrogen fixation. FEMS Microbiol Lett. 1994;119:351–358. doi: 10.1111/j.1574-6968.1994.tb06912.x. [DOI] [PubMed] [Google Scholar]
- 25.Lübben M, Morand K. Novel prenylated hemes and cofactors of cytochrome oxidases. Archaea have modified hemes A and O. J Biol Chem. 1994;269:21473–21479. [PubMed] [Google Scholar]
- 26.Matsushita K, Ameyama M. d-Glucose dehydrogenase from Pseudomonas fluorescens, membrane bound. Methods Enzymol. 1982;89:149–154. doi: 10.1016/s0076-6879(82)89026-5. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita K, Shinawa E, Adachi O, Ameyama M. Reactivity with ubiquinone of quinoprotein d-glucose dehydrogenase from Gluconobacter suboxydans. J Biochem. 1989;105:633–637. doi: 10.1093/oxfordjournals.jbchem.a122716. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita K, Shinawa E, Adachi O, Ameyama M. Cytochrome a1 of Acetobacter aceti is a cytochrome ba functioning as ubiquinol oxidase. Proc Natl Acad Sci USA. 1990;87:9863–9867. doi: 10.1073/pnas.87.24.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita K, Ebisuya H, Ameyama M, Adachi O. Change of the terminal oxidase from cytochrome a1 in shaking cultures to cytochrome o in static cultures of Acetobacter aceti. J Bacteriol. 1992;174:122–129. doi: 10.1128/jb.174.1.122-129.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita K, Takahashi K, Takahashi M, Ameyama M, Adachi O. Methanol and ethanol oxidase respiratory chains of the methylotrophic acetic acid bacterium, Acetobacter methanolicus. J Biochem. 1992;111:739–747. doi: 10.1093/oxfordjournals.jbchem.a123829. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Toyama H, Adachi O. Respiratory chains and bioenergetics of acetic acid bacteria. Adv Microb Physiol. 1994;36:247–297. doi: 10.1016/s0065-2911(08)60181-2. [DOI] [PubMed] [Google Scholar]
- 32.McInerney M J, Holmes K S, Hoffman P, der Vartanian D V. Respiratory mutants of Azotobacter vinelandii with elevated levels of cytochrome d. Eur J Biochem. 1984;141:447–452. doi: 10.1111/j.1432-1033.1984.tb08212.x. [DOI] [PubMed] [Google Scholar]
- 33.Miranda-Ríos J, Morera C, Taboada H, Dávalos A, Encarnación S, Mora J, Soberón M. Expression of thiamin biosynthetic genes (thiCOGE) and production of symbiotic terminal oxidase cbb3 in Rhizobium etli. J Bacteriol. 1997;179:6887–6893. doi: 10.1128/jb.179.22.6887-6893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moshiri F, Kim J K, Changlin F, Maier R J. The FeSII protein of Azotobacter vinelandii is not essential for aerobic nitrogen fixation, but confers significant protection to oxygen-mediated inactivation of nitrogenase in vitro and in vivo. Mol Microbiol. 1994;14:101–114. doi: 10.1111/j.1365-2958.1994.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 35.Poole R K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983;726:205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- 36.Puustinen A, Wikström M. The heme groups of cytochrome o from Escherichia coli. Proc Natl Acad Sci USA. 1991;88:6122–6126. doi: 10.1073/pnas.88.14.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis V M, Olivares F L, Döbereiner J. Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J Microbiol Biotechnol. 1994;10:401–405. doi: 10.1007/BF00144460. [DOI] [PubMed] [Google Scholar]
- 38.Stanbury P F, Whitaker A. Principles of fermentation technology. New York, N.Y: Pergamon Press; 1984. pp. 169–191. [Google Scholar]
- 39.Stephan M P, Oliviera M, Teixeira K R S, Martínez-Drets G, Döbereiner J. Physiology and dinitrogen fixation of Acetobacter diazotrophicus. FEMS Microbiol Lett. 1991;77:67–72. [Google Scholar]
- 40.Svensson B, Lübben M, Hederstedt L. Bacillus subtilis ctaA and ctaB function in heme A biosynthesis. Mol Microbiol. 1993;10:193–201. doi: 10.1111/j.1365-2958.1993.tb00915.x. [DOI] [PubMed] [Google Scholar]