Abstract
Supplemental oxygen is an essential medication in critical care. The optimal oxygen dose delivery system remains unclear, however. The “dose” and “delivery” of oxygen carry significant importance for resource-limited settings, such as low- and middle-income countries (LMICs). Regrettably, LMICS often experience significant inequities in oxygen supply and demand, with major impacts on preventable mortality. These inequities have become particularly prominent during the global COVID-19 pandemic, highlighting the need for additional investment and research into the best methods to utilize supplemental oxygen and ensure stable access to medical oxygen.
Keywords: Oxygen, Critical illness, Global health, COVID-19
Key points
-
•
Supplemental oxygen is an essential medication for critically ill patients with acute hypoxic respiratory failure and is necessary to maintain normal cellular metabolism.
-
•
The optimal oxygen dose and delivery system remain unknown, and the risks and benefits may differ among disease processes seen in the intensive care unit, with clinical studies demonstrating mixed findings.
-
•
Low- and middle-income countries experience significant disparities in both the global burden of acute hypoxic respiratory failure and oxygen supply. Clinical studies to understand optimal oxygen delivery in these areas are ongoing and are vital to optimal care for patients in resource-limited settings.
-
•
The COVID-19 pandemic has greatly exacerbated health care disparities prevalent in low- and middle-income countries and has accelerated the global response to address shortfalls in oxygen supply.
Introduction
The administration of supplemental oxygen as a medical treatment is among the most common therapies provided to acutely ill patients. This is particularly true in critical care medicine, where up to half of the patients in intensive care units (ICUs) require supplemental oxygen.1 Oxygen is currently on the World Health Organization’s (WHO’s) List of Essential Medicines for acutely ill individuals and for people with chronic diseases resulting in hypoxemia.2 Medical oxygen’s status as an essential medicine has become even more prominent given the global COVID-19 pandemic, with the virus’ most common and marked clinical manifestation being hypoxemia.
Adequate oxygen availability at the cellular level is vital for normal metabolism in humans, traversing a path from “mouth to mitochondria.”3 Diseases that affect oxygen transfer anywhere along this path, from the pulmonary alveoli to end-organ mitochondria, can cause negative downstream effects and lead to a common array of symptoms. Supplemental oxygen at a concentration greater than atmospheric partial pressure is commonly administered to overcome pathophysiologic barriers in oxygen uptake and delivery. However, oxygen can also be toxic despite its essential nature in maintaining normal aerobic respiration. Excess oxygen can damage proteins, nucleic acids, and other pieces of cellular machinery through increased oxidative stress.4 Concerns regarding oxygen toxicity in critical care medicine have been noted as far back as the 1960s with descriptions of “respirator lung syndrome” seen in mechanically ventilated patients exposed to high oxygen concentrations.5 Despite the long-standing evidence of a therapeutic window for supplemental oxygen therapy, the optimal dose and delivery method of oxygen remain a significant scientific gap with varying recommendations from international critical care societies.6, 7, 8
A significant number of critically ill patients are exposed to high levels of oxygen support in excess of their needs in many ICUs.9 Yet, undertreatment of hypoxemia also remains a major problem, especially in resource-limited settings. Under-recognition and undertreatment, frequently because of a lack of oxygen supply and monitoring equipment, are associated with a substantial increase in mortality even among patients not admitted to an ICU.10 Among low- and middle-income countries (LMIC), almost one-quarter of hospitals do not have supplemental oxygen available.11 The COVID-19 pandemic has exacerbated and highlighted these health care inequities, as multiple LMICs have experienced oxygen shortages during surges of viral transmission, leading to increased preventable mortality and impacting patients with and without COVID-19.12 These events acted as catalysts for various international efforts to increase oxygen supply.13 Additionally, unanswered questions remain as to the best approaches to delivering oxygen in LMIC and reduced-resource settings.14
In this review, we explore the history of oxygen therapy in medicine and briefly review the important physiology. We subsequently outline the clinical relevance of determining the optimal delivery method and “dose” of supplemental oxygen among critically ill patients. Finally, we explore the impact of oxygen therapy in a global context, discussing the questions and considerations of oxygen therapy in LMICs and highlighting the patient-level and systemic challenges of oxygen supply and a global respiratory pandemic.
History of oxygen therapy in critical care medicine
The history of oxygen reaches back centuries, with theories regarding molecular oxygen and gas exchange being promulgated as early as the thirteenth century.3 Yet, the eighteenth century saw an acceleration of scientific knowledge regarding oxygen that laid the framework for its future use as a medical therapy.3 By the late nineteenth century and early twentieth century, pioneering work by two prominent physicians, Sir William Osler and Jonathan Campbell Meakins, elucidated the role of oxygen therapy in treating hypoxemia caused by pneumonia.15 Through this work, they established supplemental oxygen therapy as the standard of care for pneumonia and, ultimately, other diseases of hypoxemia in the 1920s.15
ICUs were initially developed in Europe in response to the epidemic of respiratory failure secondary to the polio epidemic with the development of negative-pressure ventilators, including the commonly known “iron lung.”16 In the 1960s and 1970s, with the advent of critical care medicine as a specialty and the development of positive-pressure mechanical ventilators, the ability to use supplemental oxygen concentrations above atmospheric levels grew substantially. Positive-pressure ventilators combine the ability to provide ventilation invasively through an endotracheal tube or a tracheostomy tube with the ability to entrain supranormal concentrations of oxygen. Early on, patients routinely received high tidal volumes and high oxygen concentrations in an effort to aggressively normalize patients’ physiology.16 Over the next several decades, however, it was increasingly recognized that high volumes, in conjunction with high oxygen concentrations, were harmful to many patients.5 , 17 Efforts to normalize physiology were exchanged for “lung protective ventilation” with lower tidal volumes and increased positive end-expiratory pressures, or PEEP, to avoid many of the untoward effects of high oxygen concentrations.17 , 18
At the same time that invasive mechanical ventilation and oxygen delivery were developing, noninvasive forms of respiratory support were growing in use as well. The use of positive-pressure ventilation through a mask apparatus was known as far back as the 1940s and 1950s; however, it was predominantly limited to patients requiring long-term ventilatory support and oxygen therapy.19 These devices combine the ability to entrain higher concentrations of oxygen with ventilatory support without the need for an endotracheal tube or tracheostomy, and their use has grown exponentially in the ICU. In the late 1980s and early 1990s, however, multiple studies demonstrated the benefit of noninvasive mechanical ventilation in patients with chronic obstructive pulmonary disease (COPD) and congestive heart failure.20 , 21 In the last decade, another noninvasive oxygen delivery method called high-flow nasal cannula (HFNC) has changed practice in treating acute hypoxemic respiratory failure (AHRF) by providing high oxygen flow rates through a nasal cannula apparatus. These noninvasive methods of providing high-level oxygen support have transformed critical care medicine, providing vital tools in the treatment of hypoxemic respiratory failure. Despite revolutionizing critical care medicine and oxygen therapy more broadly, the physiologic and clinical importance of these oxygen delivery systems, and how they may differ in settings with varying resources, is an ongoing area of active research.
Physiologic and clinical relevance of oxygen therapy
Physiology of Oxygen Therapy
Diatomic oxygen (O2) is one of the foundational elements on our planet, forming the backbone of bioenergetic function in aerobic organisms. On Earth, the overall oxygen concentration in the atmosphere is approximately 21%, with an inspired partial pressure of roughly 150 mmHg at sea level.22 At the level of the pulmonary alveoli, the partial pressure of oxygen is approximately 100 mm Hg after accounting for the partial pressure of carbon dioxide and the respiratory exchange ratio. Most of the oxygen that is inspired and traverses the pulmonary capillaries binds to hemoglobin, and a small amount is dissolved in the plasma. The overall concentration of oxygen in the blood can be quantified as follows: Cao 2 (mL O2/100 mL blood) = (1.36 × Hgb × Sao 2/100) × 0.003 × Pao 2, where Cao 2 is the concentration of oxygen in arterial blood, 1.36 is the carrying capacity of hemoglobin (1.36 mL of O2 for 1 g of hemoglobin), Hgb is hemoglobin level, Sao 2 is the arterial oxygen–hemoglobin saturation percentage, and Pao2 is the dissolved partial pressure of oxygen in arterial blood.22 From the aforementioned equation, it can be discerned that oxygen bound to hemoglobin provides the majority of oxygen available to be delivered to tissues, whereas dissolved oxygen (Pao2) provides a smaller contribution. The percentage of arterial oxygen bound to hemoglobin at rest in healthy individuals near sea level who are breathing an ambient oxygen concentration of 21% is approximately 98%. By increasing the fraction of inspired oxygen (Fio2) in patients with impaired oxygen exchange, a greater proportion of available hemoglobin is bound to oxygen. It can then be delivered to tissue, assuming cardiac output and oxygen extraction at the tissue level are normal, with modest increases in the dissolved oxygen concentration. Additionally, increasing the mean pressure in pulmonary alveoli above that of sea-level atmospheric pressure while also increasing the Fio2 (ie, “hyperbaric” oxygen) provides an even greater increase in available oxygen to tissues, predominantly by increasing the partial pressure of dissolved oxygen.3 Each of these approaches (increasing Fio2 and increasing pressure at the pulmonary alveoli) has important clinical uses in increasing the delivery of oxygen.
Clinical Relevance
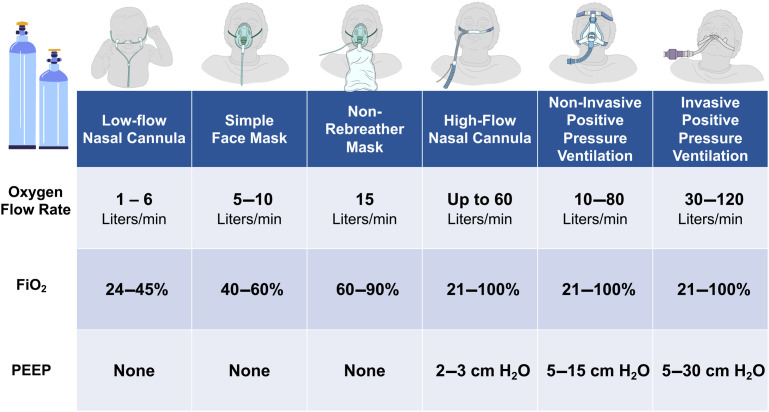
Oxygen therapy, both in the ICU and outside of the ICU, is a fundamental necessity for the care of acutely ill patients, and there are several different systems available to deliver oxygen. The choice of an oxygen delivery system is multifaceted (Fig. 1 ), impacted by the degree of illness, the necessary “dose” of oxygen, underlying conditions, and patient safety. Each of these factors needs to be carefully considered in the use of oxygen therapy, particularly when resources or settings may also dictate additional limitations on delivery methods.
Fig. 1.
Major oxygen delivery systems. FiO2, fraction of inspired oxygen (percentage); PEEP, positive end-expiratory pressure.
(Images courtesy of the OpenCriticalCare.org Project.)
Low-flow oxygen therapy is perhaps the most frequent delivery method used in hospitals. Low-flow oxygen, at rates from 1 L to 6 L per minute in adults, is commonly supplied through nasal prongs or by facemask. Because of the lower flow rates, open system design, and patient-level factors, such as respiratory rate, room air (with an oxygen concentration of 21%) mixes with the supplied oxygen, reducing the overall Fio2 delivered to the alveoli to a range of 25% to 40%.23 Higher Fio2 can be delivered through the use of partial and nonrebreathing masks, which utilize oxygen reservoirs and/or valve systems to limit the entrainment of room air.24 Over the last 2 decades, the use of HFNC systems that provide heated and humidified flows up to 60 L per minute and supply oxygen concentrations greater than 90% has grown exponentially.25 Because of the increased flow rates intrinsic to these delivery systems, there is a reduced entrainment of room air, a washout of carbon dioxide with high oxygen concentrations, and small increases in mean airway pressure, providing an effective method of increasing arterial oxygen concentration.26 , 27 Providing supplemental oxygen through the use of HFNC devices has been shown to reduce the risk of intubation and death in patients with AHRF,28 as well as reduce the risk of reintubation in patients who were liberated from invasive mechanical ventilation when compared with conventional oxygen therapy.29 Lastly, the other predominant form of oxygen delivery system is noninvasive and invasive mechanical ventilation provided through positive pressure ventilation devices. Oxygen can be supplied through these devices across a range of concentrations up to 100%. These devices also provide various methods to increase mean alveolar pressure through the use of positive PEEP or longer durations at peak inspiratory pressures while also providing ventilatory support to facilitate carbon dioxide removal.16 Although, perhaps, the most efficient method for providing accurate and titratable oxygen concentrations, the use of positive-pressure ventilation systems is resource-intensive and expensive; this can substantially limit their use in certain settings. Noninvasive systems without masks, including tent or hood enclosure systems, exist. Yet, their use is largely limited to a few regions globally, and their effectiveness is actively being studied.30
Choosing the right “dose” of oxygen
Regardless of the delivery system, substantial knowledge gaps persist regarding the “dose” of oxygen needed for a variety of conditions treated in critical care medicine. A prime example is the use of supplemental oxygen in patients experiencing acute cardiac ischemia. The previous rationale for using supplemental oxygen, even in patients without hypoxemia, during a myocardial infarction was that it could potentially limit the overall area of infarcted myocardium.31 However, a large randomized controlled trial (RCT) of continuous supplemental oxygen therapy versus no oxygen in patients with acute myocardial infarction without hypoxemia showed no difference in mortality or rehospitalization.32 Of note, a systematic review of several RCTs of oxygen therapy found that in patients with cardiac ischemia and no hypoxemia, oxygen therapy was associated with significantly increased mortality.33 Similarly, in a large clinical trial in patients with acute cerebrovascular accidents, continuous and nocturnal oxygen therapy did not improve outcomes when compared with no oxygen therapy.34 Changes in the most recent guidelines now reflect the results of these trials in recommending against supplemental oxygen in patients with cardiac or cerebrovascular ischemia who are not hypoxemic.35
Several recent RCTs have also shed light on the role of oxygen therapy in general ICU patients. In the OXYGEN-ICU trial, 434 patients at a single hospital in Italy were randomized to conservative oxygen therapy with a target oxygen saturation of 94% to 98% (Pao2 of 70–100 mm Hg) or “standard” oxygen therapy with a target oxygen saturation of 97% to 100% (Pao2 up to 150 mm Hg) with a primary outcome of ICU mortality. The mortality rate was significantly lower in the conservative oxygen arm (11.6% vs. 20.2%). Notably, the study enrolled patients on mechanical ventilation as well as those who were not. The study was also significantly limited by the fact that it was stopped early and did not reach the predetermined sample size. In the more recent, and larger, ICU-ROX trial, mechanically ventilated general ICU patients randomized to a conservative oxygen target of 91% to 96% versus a “usual care” (higher) target had no difference in ventilator-free days nor any difference in 90-day or 180-day mortality.36 Subgroup analyses in this trial suggested that patients with hypoxic ischemic encephalopathy had better outcomes with conservative oxygen therapy, however. Additionally, patients in the usual care (higher oxygen target) group reported increased difficulties in mobility and self-care. In contrast, in a study of liberal versus conservative oxygen targets in mechanically ventilated patients with acute respiratory distress syndrome (ARDS), there was no difference between the liberal or conservative oxygen therapy arms in terms of survival at 28 days. However, the study was stopped for safety concerns due to five mesenteric ischemia events in the conservative oxygen target group, though this finding of intestinal ischemia was not seen in a subsequent larger follow-up study.37 , 38 These studies highlight the ongoing need for further clarity regarding optimal oxygen dosage in critically ill patients. Observational data indicates that well over half of patients treated in ICUs are exposed to excess oxygen concentrations,39 yet they may also experience clinically significant hypoxemia.40 These findings have immediate implications for critical illness outcomes, such as patients with COPD,41 but they also highlight important considerations for oxygen supply, appropriate utilization, and the best approaches to oxygen therapy in resource-limited settings. Given that many hospitals in LMICs lack adequate oxygen supplies and that inadvertent hypoxemia impacts mortality in resource-limited settings,10 , 11 appropriate dosing and delivery of oxygen in critically ill patients that maximizes benefit, minimizes harm, and increases availability is of vital importance for global health.
Global and public health impact
Epidemiology of Acute Hypoxemic Respiratory Failure and Resource Constraints in Low- and Middle-Income Countries
LMICs account for the majority of the world’s population, and the majority of the global burden of disease, including respiratory infections, which are among the most common causes of mortality.42 Additionally, LMICs experience a proportionally higher rate of critical illness when compared with high-income countries (HICs), and they also experience greater mortality rates in disease processes that would potentially benefit from critical care.43 AHRF is one of the most common diagnoses in LMICs. Hypoxemia leads to excess mortality in infectious diseases prevalent in some LMICs such as malaria and tuberculosis, as well as noncommunicable diseases, including heart failure and chronic lung disease.44, 45, 46 In one study of 7300 adults presenting to an emergency department in a Ugandan hospital, 4.5% of patients presented with AHRF, most commonly due to pneumonia.47 Despite being relatively young (median age of 38), the in-hospital mortality for this cohort was 77%. The authors note that only 6% of these patients received mechanical ventilation due to resource limitations, including ICU bed availability. In a similar cross-sectional point prevalence study of adult inpatients admitted to two hospitals in Malawi, 4% of patients had hypoxemia, yet 89% of them were not receiving oxygen therapy.48 ARDS, an inflammatory form of AHRF, is also highly prevalent in LMICs. In a study of inpatient admissions to one hospital in Rwanda over a 6 week period, 4% of adults met criteria for ARDS using the Kigali modification of the Berlin criteria.46 Only 31% of patients were admitted to an ICU, however, and in-hospital mortality was 50% for all patients with ARDS.
Despite the burden of critical illness in LMICs, hospitals in these areas are often undersupplied. Caregiving for critically ill patients is a resource-intensive endeavor, fraught with challenges related to the cost of care, staffing, and equipment needs. Supplemental oxygen and pulse oximeters for monitoring oxygen saturation are foundational resources that often have limited availability in LMICs.11 In a survey of over 200 health care institutions in 12 African countries, less than half had consistent, uninterrupted sources of oxygen, and less than one-quarter had an oxygen concentrator.49 Whereas the availability of supplemental oxygen, and particularly pulse oximetry, has been shown to improve mortality,50 , 51 logistical challenges related to maintenance, electricity, and supply significantly limit oxygen availability in LMICs.52 In LMICs where the availability of pulse oximetry is limited, inadvertent and undiagnosed hypoxemia is frequent and impacts clinical outcomes and care.53 Evans and colleagues, in a cross-sectional study of all adult patients admitted to a large teaching hospital in Malawi, found that 14 out of 144 patients needed oxygen therapy due to desaturations lower than 90% during a 24 hour period, but only 4 were receiving oxygen because of the lack of functional oxygen concentrators.54 A related study found that over half of patients at one Rwandan hospital with hypoxemia received no or limited supplemental oxygen on one or more days of their inpatient admission.10 This lack of oxygen supply has a direct impact on mortality. Duke and colleagues found that after the introduction of pulse oximeters, oxygen concentrators, and a protocol for detecting and treating hypoxemia in children admitted to 5 hospitals in Papua New Guinea, mortality due to pneumonia fell by 35%.50
In contrast, inappropriate oxygen use may also occur frequently and impact oxygen supply downstream for other patients. For example, a retrospective study of pulse oximeter use in pediatric patients at 18 Kenyan hospitals found that 8.6% of patients had oxygen prescribed, but 87% of patients either did not have pulse oximetry performed or did not have oxygen saturations lower than 90%.55 In situations where oxygen supply is limited, liberal and nontitrated dosing of supplemental oxygen may lead to or exacerbate supply shortages. In a study of patients in the adult emergency department of a Rwandan teaching hospital, Sutherland and colleagues found that 12% of patients were hypoxemic, but on over 80% of days, patients were either over or undertreated with oxygen to achieve a target oxygen saturation of 90% to 95%.56 Following a targeted educational intervention on appropriate oxygen targets and monitoring, however, the number of oxygen tanks used daily in the emergency department decreased significantly, with concomitant increases in oxygen tank reserves. These studies demonstrate the importance of optimal dosing of oxygen therapy for clinical outcomes and managing supplies of supplemental oxygen in resource-limited settings.
Oxygen Delivery Systems in Low- and Middle-Income Countries
Studies regarding the optimal dose and delivery of oxygen therapy have predominantly been limited to HICs, with inadequate studies regarding oxygen therapy in the care of critically ill patients in LMICs. The use of nasal bubble continuous positive airway pressure (bCPAP) ventilation versus standard low-flow oxygen therapy was trialed in 644 hypoxemic pediatric patients with pneumonia in one hospital in Malawi.57 There was no difference in in-hospital mortality between the bCPAP group and the nasal low-flow oxygen groups, though the study was stopped early before enrolling its targeted sample size due to futility. Of note, these therapies were provided in a non-ICU setting, and there were more adverse events, including deaths, in the bCPAP group, raising questions regarding the feasibility of providing noninvasive ventilatory support outside of the ICU. A similar multicenter randomized clinical trial named the Children’s Oxygen Administration Strategies, or COAST, trial investigated HFNC versus low-flow nasal cannula versus permissive hypoxemia. Maitland and colleagues enrolled 1852 pediatric patients with pneumonia in 4 Ugandan and 2 Kenyan hospitals, stratifying them according to severity of hypoxemia, with severe hypoxemia considered to be oxygen saturations of less than 80%.58 Patients with severe hypoxemia were randomly assigned to either HFNC or low-flow oxygen therapy, whereas those with less hypoxemia (>80% to 91%) were randomly assigned to either HFNC or low-flow oxygen therapy or were allowed to have permissive hypoxemia down to 80%. The trial was ultimately stopped early by the Trial Steering Committee because of ethical concerns raised regarding randomization to the permissive hypoxemia arm. Although definitive conclusions regarding the efficacy of these approaches to oxygen dosing cannot be drawn from this trial due to its early termination, there are suggestions of potential benefit that require further study. First, at 48 hours, there was a 4% lower mortality in the HFNC arm when compared with low-flow oxygen in the stratum with severe hypoxemia, though this did not quite reach predetermined significance (P = .076). Second, in both strata, HFNCs were associated with significantly lower volumes of oxygen used. The finding of oxygen conservation is important and encouraging, as it may suggest that higher flow oxygen delivery may actually reduce overall oxygen requirements. This is likely because of the fact that many children achieved adequate oxygenation from high flows of room air without requiring any oxygen. Whether oxygen conservation may occur through the small amounts of intrinsic PEEP (and thus higher mean alveolar pressure) or reduction of physiologic dead space remains to be proven given the preliminary nature of these findings.
Whether there are clinical benefits to certain oxygen delivery systems for critically ill adults in lower resource settings is unknown. It is also unclear whether delivery systems, such as HFNC oxygen therapy, which have substantially higher flows, can reduce oxygen supply utilization in adults similar to children in the COAST trial given that flow rates in adults can be relatively higher (up to 60 L/min). A handful of upcoming studies may help answer these questions. The Adult Respiratory Failure Intervention Study – Africa (ARISE-Africa), a stepped wedge cluster randomized trial (Clinicaltrials.gov ID: NCT04693403), will study 470 critically ill adults in Uganda with AHRF randomized to HFNC oxygen, continuous positive airway pressure plus oxygen, or standard low-flow oxygen therapy and compare 28-day mortality. Another upcoming trial called the Building Respiratory support in East Africa Through High flow versus low flow oxygen Evaluation (BREATHE), funded by the Wellcome Trust, will evaluate the use of HFNC oxygen therapy versus low-flow oxygen therapy in five hospitals from three East African countries—Rwanda, Kenya, and Malawi. This study will utilize a type 1 effectiveness-implementation hybrid trial design with the primary aim of determining the effectiveness of HFNC oxygen therapy versus low-flow oxygen therapy and a secondary aim of determining the feasibility and utility of protocolized oxygen use with monitoring and titration. Although protocolized low-flow oxygen therapy has been shown to reduce mortality in pediatric patients with pneumonia,50 it has not been studied in adults and not in comparison with high-flow nasal oxygen therapy, which may have unique benefits due to high flow rates but also impact oxygen utilization and supply. The Circumvent Project in Nigeria is examining helmet noninvasive ventilation using an implementation science framework.59 These studies, and others, are crucial to improving our understanding of how to best deliver supplemental oxygen in an efficient manner while accounting for the unique context of health care institutions in LMICs.
Oxygen therapy during the COVID-19 pandemic
The arrival of a novel respiratory coronavirus, called severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), in 2019 has fundamentally changed critical care medicine as a specialty throughout the world and highlighted existing inequities in ICU resources between countries. In its severe form, coronavirus disease 2019, or COVID-19 as it is commonly named, manifests as severe respiratory distress and AHRF in a substantial percentage of patients infected with SARS-CoV-2 and has strained ICU resources globally. Given the already limited resources present in many LMICs, the pandemic has particularly impacted critical care in LMICs. Medical oxygen was initially the only known therapy for the disease, and remains one of the most important therapies in the treatment of COVID-19 worldwide; limited supply has threatened the lives of thousands of patients in LMICs, both with COVID-19 and those with other diseases that require supplemental oxygen. The WHO continues to highlight oxygen supply in LMIC as a global health emergency.12 , 60 A recently developed tool for estimating country and global oxygen needs demonstrates that over 3.6 million oxygen cylinders per day are needed in LMICs for COVID-19 patients alone.61
Although inequities in oxygen supply existed long before the arrival of COVID-19, the pandemic has accelerated the global response to oxygen supply. Early on, much of the global focus was on the provision of mechanical ventilators and diagnostic therapies. The WHO’s Access to COVID-19 Tools Accelerator (ACT-A) initiative, launched in April of 2020, was designed to coordinate a global effort in the development of tools to fight COVID-19 and ensure access to such resources in LMICs.62 Although not originally part of the initial pillars of the ACT-A initiative, following several high-profile reports of desperate oxygen shortages and associated mortality in several LMICs, the ACT-A Oxygen Emergency Taskforce was launched in February of 2021. This task force represents an effort by over 20 global health agencies to tackle and prevent medical oxygen shortages that were exacerbated by the pandemic. The task force was able to generate more than $700 million US dollars of funding in an attempt to mitigate the damage of oxygen shortages. This funding, provided by various entities, including The Global Fund, United Nations Children’s Fund, Wellcome Trust, and Unitaid, was used for the purchases of oxygen supplies and associated equipment, such as pulse oximeters and monitors as well as providing funding directly to governments in LMIC for the purchase of supplies and in their use and maintenance. In late 2021, the updated ACT-A strategic plan called for an additional 1.4 billion dollars of funding to be obtained to continue efforts in supplying oxygen and supplies to LMICs in 2022.13 Additionally, the United States Agency for International Development has provided support for the development and delivery of oxygen plants in some countries, in addition to other necessary oxygen supplies.63 Given the ongoing difficulties in consistent oxygen supply as well as logistical insults to health care infrastructure in LMICs during the COVID-19 pandemic, increased and ongoing support in providing oxygen delivery systems and associated equipment will be vital to saving lives in the pandemic. Additionally, there is a substantial need to train end users (eg, clinicians) on the titration of oxygen based on best practice and for logistical and engineering expertise in the maintenance of oxygen supply, monitoring systems, and pulse oximetry to guarantee the sustainability of oxygen systems in LMICs.
Summary
Medical oxygen therapy is a fundamental cornerstone of critical care medicine. Molecular oxygen is a necessary substrate in normal aerobic metabolism in humans, and it has a vital role in maintaining organ function in both health and disease. As medicine has continued to develop, numerous methods to supply oxygen have been devised, ranging from low flows of concentrated oxygen through nasal cannulas to invasive mechanical ventilation with supplementation of 100% oxygen. Yet, many questions remain regarding the right “dose” and delivery method of oxygen in a number of diseases that lead to critical illness, especially in LMICs. Additionally, the logistics of providing supplemental oxygen in different delivery systems vary and have substantial impacts on LMICs, who bear the greatest burden of disease-related hypoxemia and mortality. LMICs often have inconsistent oxygen supplies, leading to preventable excess mortality. Future studies will help answer questions regarding optimal oxygen delivery approaches and their impact on supply. The COVID-19 pandemic has unearthed and accelerated the inequities in oxygen supply and delivery, increasing international attention to the need but also necessitating further investment and research into the supply and best use of this essential medication.
Clinics care points
-
•
Supplemental oxygen therapy is a vital component in the management of critically ill patients. However, the optimal dose and delivery method of oxygen across the range of critical illness remains unclear.
-
•
Low- and middle-income countries (LMICs) suffer from significant inequities related to oxygen availability and supply, a fact only exacerbated by the COVID-19 pandemic, further confounding decisions regarding optimal approaches to oxygen therapy in these under-resourced settings.
-
•
Further studies addressing the optimal dose and delivery method of supplemental oxygen in LMICs, along with analyses of cost and supply barriers, will guide future public health interventions to reduce impact of acute hypoxemic respiratory failure and oxygen shortages.
Acknowledgments
Disclosure
Department of Veterans Affairs Tennessee Valley Health Care System Geriatric Research, Education and Clinical Center (GRECC) and VA-MERIT, the National Institutes of Health under awards (R01AG027472, R01AG035117, R01HL111111, R01GM120484, and R01AG058639).
Footnotes
The authors do not plan to order reprints. Role of the Funder/Sponsor: This material is based upon work supported by the U.S. Department of Veterans Affairs (VA) Office of Academic Affiliations, VA National Quality Scholars Program, and with resources and use of facilities at VA Tennessee Valley Healthcare System in Nashville, Tennessee.
References
- 1.Vincent J.L., Akça S., De Mendonça A., et al. The epidemiology of acute respiratory failure in critically ill patients(∗) Chest. 2002;121(5):1602–1609. doi: 10.1378/chest.121.5.1602. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva, Switzerland: 2017. The selection and use of essential medicines: report of the WHO Expert Committee, 2017 (including the 20th WHO model list of essential medicines and the 6th WHO Model list of essential medicines for children) [Google Scholar]
- 3.Brugniaux J.V., Coombs G.B., Barak O.F., et al. Highs and lows of hyperoxia: physiological, performance, and clinical aspects. Am J Physiol Regul Integr Comp Physiol. 2018;315(1):R1–R27. doi: 10.1152/ajpregu.00165.2017. [DOI] [PubMed] [Google Scholar]
- 4.Davies K.J.A. In: Rice-Evans C., Halliwell B., Lunt G.G., et al., editors. vol. 61. Portland Press; London, United Kingdom: 1995. Oxidative stress: the paradox of aerobic life; pp. 1–31. (Biochemical Society Symposia). [DOI] [PubMed] [Google Scholar]
- 5.Nash G., Blennerhassett J.B., Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artifical ventilation. N Engl J Med. 1967;276(7):368–374. doi: 10.1056/NEJM196702162760702. [DOI] [PubMed] [Google Scholar]
- 6.O’Driscoll B.R., Howard L.S., Earis J., et al. British Thoracic Society mergency oxygen guideline group, BTS emergency oxygen guideline development group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1–ii90. doi: 10.1136/thoraxjnl-2016-209729. [DOI] [PubMed] [Google Scholar]
- 7.Beasley R., Chien J., Douglas J., et al. T horacic S ociety of A ustralia and N ew Z ealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags. ’ Respirology. 2015;20(8):1182–1191. doi: 10.1111/resp.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan E., Del Sorbo L., Goligher E.C., et al. An official American thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S., Eastwood G.M., Peck L., et al. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. 2013;28(5):647–654. doi: 10.1016/j.jcrc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland T., Musafiri S., Twagirumugabe T., et al. Oxygen as an essential medicine: under- and over-treatment of hypoxemia in low- and high-income nations. Crit Care Med. 2016;44(10):e1015–e1016. doi: 10.1097/CCM.0000000000001912. [DOI] [PubMed] [Google Scholar]
- 11.Meara J.G., Leather A.J.M., Hagander L., et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386(9993):569–624. doi: 10.1016/S0140-6736(15)60160-X. [DOI] [PubMed] [Google Scholar]
- 12.Usher A.D. Medical oxygen crisis: a belated COVID-19 response. Lancet. 2021;397(10277):868–869. doi: 10.1016/S0140-6736(21)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Every Breath Counts Coalition One year after the launch of the access to COVID-19 tools accelerator (ACT-A) oxygen emergency taskforce, what has been achieved? https://stoppneumonia.org/wp-content/uploads/2022/02/ACTAOxygenTaskforceAnniversaryStatement23February2022.pdf Available at: Accessed March 2, 2022.
- 14.Calligaro G.L., Lalla U., Audley G., et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine. 2020;28:100570. doi: 10.1016/j.eclinm.2020.100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren C.P.W. The introduction of oxygen for pneumonia as seen through the writings of two McGill University professors, William Osler and Jonathan Meakins. Can Respir J. 2005;12(2):81–85. doi: 10.1155/2005/146951. [DOI] [PubMed] [Google Scholar]
- 16.MacIntyre N., Rackley C., Khusid F. Fifty years of mechanical ventilation-1970s to 2020. Crit Care Med. 2021;49(4):558–574. doi: 10.1097/CCM.0000000000004894. [DOI] [PubMed] [Google Scholar]
- 17.Acute Respiratory Distress Syndrome Network, Brower R.G., Matthay M.A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 18.Brower R.G., Lanken P.N., MacIntyre N., et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 19.Pierson D.J. History and epidemiology of noninvasive ventilation in the acute-care setting. Respir Care. 2009;54(1):40–52. [PubMed] [Google Scholar]
- 20.Meduri G.U., Conoscenti C.C., Menashe P., et al. Noninvasive face mask ventilation in patients with acute respiratory failure. Chest. 1989;95(4):865–870. doi: 10.1378/chest.95.4.865. [DOI] [PubMed] [Google Scholar]
- 21.Brochard L., Isabey D., Piquet J., et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990;323(22):1523–1530. doi: 10.1056/NEJM199011293232204. [DOI] [PubMed] [Google Scholar]
- 22.West J.B., Luks A.M. Lippincott Williams & Wilkins; Philadelpha, Pennsylvania: 2020. West’s respiratory physiology. [Google Scholar]
- 23.Bazuaye E.A., Stone T.N., Corris P.A., et al. Variability of inspired oxygen concentration with nasal cannulas. Thorax. 1992;47(8):609–611. doi: 10.1136/thx.47.8.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bateman N.T., Leach R.M. ABC of oxygen. Acute oxygen therapy. BMJ. 1998;317(7161):798–801. doi: 10.1136/bmj.317.7161.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helviz Y., Einav S. A systematic review of the high-flow nasal cannula for adult patients. Crit Care. 2018;22(1):71. doi: 10.1186/s13054-018-1990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rochwerg B., Granton D., Wang D.X., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 27.Levy S.D., Alladina J.W., Hibbert K.A., et al. High-flow oxygen therapy and other inhaled therapies in intensive care units. Lancet. 2016;387(10030):1867–1878. doi: 10.1016/S0140-6736(16)30245-8. [DOI] [PubMed] [Google Scholar]
- 28.Frat J.P., Thille A.W., Mercat A., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 29.Hernández G., Vaquero C., Colinas L., et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 30.Ferreyro B.L., Angriman F., Munshi L., et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maroko P.R., Radvany P., Braunwald E., et al. Reduction of infarct size by oxygen inhalation following acute coronary occlusion. Circulation. 1975;52(3):360–368. doi: 10.1161/01.cir.52.3.360. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann R., James S.K., Jernberg T., et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377(13):1240–1249. doi: 10.1056/NEJMoa1706222. [DOI] [PubMed] [Google Scholar]
- 33.Chu D.K., Kim L.H.Y., Young P.J., et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 34.Roffe C., Nevatte T., Sim J., et al. Effect of routine low-dose oxygen supplementation on death and disability in adults with acute stroke: the stroke oxygen study randomized clinical trial. JAMA. 2017;318(12):1125–1135. doi: 10.1001/jama.2017.11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siemieniuk R.A.C., Chu D.K., Kim L.H.Y., et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. doi: 10.1136/bmj.k4169. [DOI] [PubMed] [Google Scholar]
- 36.ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Mackle D., Bellomo R., et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382(11):989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 37.Barrot L., Asfar P., Mauny F., et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 38.Schjørring O.L., Klitgaard T.L., Perner A., et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384(14):1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 39.Helmerhorst H.J., Schultz M.J., van der Voort P.H., et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23. doi: 10.1186/s13613-014-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trial Group S.R.L.F. Hypoxemia in the ICU: prevalence, treatment, and outcome. Ann Intensive Care. 2018;8(1):82. doi: 10.1186/s13613-018-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Echevarria C., Steer J., Wason J., et al. Oxygen therapy and inpatient mortality in COPD exacerbation. Emerg Med J. 2021;38(3):170–177. doi: 10.1136/emermed-2019-209257. [DOI] [PubMed] [Google Scholar]
- 42.Rudd K.E., Johnson S.C., Agesa K.M., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murthy S., Adhikari N.K. Global health care of the critically ill in low-resource settings. Ann Am Thorac Soc. 2013;10(5):509–513. doi: 10.1513/AnnalsATS.201307-246OT. [DOI] [PubMed] [Google Scholar]
- 44.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 45.Stein F., Perry M., Banda G., et al. Oxygen provision to fight COVID-19 in sub-Saharan Africa. BMJ Glob Health. 2020;5(6) doi: 10.1136/bmjgh-2020-002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riviello E.D., Kiviri W., Twagirumugabe T., et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the kigali modification of the berlin definition. Am J Respir Crit Care Med. 2016;193(1):52–59. doi: 10.1164/rccm.201503-0584OC. [DOI] [PubMed] [Google Scholar]
- 47.Kwizera A., Nakibuuka J., Nakiyingi L., et al. Acute hypoxaemic respiratory failure in a low-income country: a prospective observational study of hospital prevalence and mortality. BMJ Open Respir Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kayambankadzanja R.K., Schell C.O., Mbingwani I., et al. Unmet need of essential treatments for critical illness in Malawi. PLoS One. 2021;16(9):e0256361. doi: 10.1371/journal.pone.0256361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belle J., Cohen H., Shindo N., et al. Influenza preparedness in low-resource settings: a look at oxygen delivery in 12 African countries. J Infect Dev Ctries. 2010;4(7):419–424. doi: 10.3855/jidc.859. [DOI] [PubMed] [Google Scholar]
- 50.Duke T., Wandi F., Jonathan M., et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. The Lancet. 2008;372(9646):1328–1333. doi: 10.1016/S0140-6736(08)61164-2. [DOI] [PubMed] [Google Scholar]
- 51.Graham H.R., Bakare A.A., Ayede A.I., et al. Oxygen systems to improve clinical care and outcomes for children and neonates: a stepped-wedge cluster-randomised trial in Nigeria. Plos Med. 2019;16(11):e1002951. doi: 10.1371/journal.pmed.1002951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duke T., Graham S.M., Cherian M.N., et al. Oxygen is an essential medicine: a call for international action. Int J Tuberc Lung Dis. 2010;14(11):1362–1368. [PMC free article] [PubMed] [Google Scholar]
- 53.Foran M., Ahn R., Novik J., et al. Prevalence of undiagnosed hypoxemia in adults and children in an under-resourced district hospital in Zambia. Int J Emerg Med. 2010;3(4):351–356. doi: 10.1007/s12245-010-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans H.G.T., Mahmood N., Fullerton D.G., et al. Oxygen saturations of medical inpatients in a Malawian hospital: cross-sectional study of oxygen supply and demand. Pneumonia (Nathan) 2012;1:3–6. doi: 10.15172/pneu.2012.1/208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuti T., Aluvaala J., Akech S., et al. Pulse oximetry adoption and oxygen orders at paediatric admission over 7 years in Kenya: a multihospital retrospective cohort study. BMJ Open. 2021;11(9):e050995. doi: 10.1136/bmjopen-2021-050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sutherland T., Moriau V., Niyonzima J.M., et al. The “Just right” amount of oxygen. Improving oxygen Use in a Rwandan emergency department. Ann Am Thorac Soc. 2019;16(9):1138–1142. doi: 10.1513/AnnalsATS.201811-763QI. [DOI] [PubMed] [Google Scholar]
- 57.McCollum E.D., Mvalo T., Eckerle M., et al. Bubble continuous positive airway pressure for children with high-risk conditions and severe pneumonia in Malawi: an open label, randomised, controlled trial. Lancet Respir Med. 2019;7(11):964–974. doi: 10.1016/S2213-2600(19)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maitland K., Kiguli S., Olupot-Olupot P., et al. Randomised controlled trial of oxygen therapy and high-flow nasal therapy in African children with pneumonia. Intensive Care Med. 2021;47(5):566–576. doi: 10.1007/s00134-021-06385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahonkhai A.A., Musa A.Z., Fenton A.A., et al. The CircumVent Project: a CPAP/O2 helmet solution for non-invasive ventilation using an implementation research framework. Implement Sci Commun. 2021;2(1):93. doi: 10.1186/s43058-021-00193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skrip L., Derra K., Kaboré M., et al. Clinical management and mortality among COVID-19 cases in sub-Saharan Africa: a retrospective study from Burkina Faso and simulated case analysis. Int J Infect Dis. 2020;101:194–200. doi: 10.1016/j.ijid.2020.09.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.PATH COVID-19 oxygen needs tracker. https://www.path.org/programs/market-dynamics/covid-19-oxygen-needs-tracker/ Available at: Accessed March 8, 2022.
- 62.World Health Organization The access to COVID-19 tools (ACT) accelerator. https://www.who.int/initiatives/act-accelerator Available at: Accessed March 4, 2022.
- 63.United States Agency for International Development (USAID) Usaid ASSISTANCE to Tajikistan for the COVID-19 CRISIS. https://www.usaid.gov/sites/default/files/documents/2021_10_USAID_Assistance_to_Tajikistan_for_the_COVID-19_Crisis_Fact_Sheet.pdf Available at: Accessed March 6, 2022.