Abstract
The Escherichia coli umuD and umuC genes comprise an operon and encode proteins that are involved in the mutagenic bypass of normally replication-inhibiting DNA lesions. UmuD is, however, unable to function in this process until it undergoes a RecA-mediated cleavage reaction to generate UmuD′. Many homologs of umuDC have now been identified. Most are located on bacterial chromosomes or on broad-host-range R plasmids. One such putative homolog, humD (homolog of umuD) is, however, found on the bacteriophage P1 genome. Interestingly, humD differs from other umuD homologs in that it encodes a protein similar in size to the posttranslationally generated UmuD′ protein and not UmuD, nor is it in an operon with a cognate umuC partner. To determine if HumD is, in fact, a bona fide homolog of the prokaryotic UmuD′-like mutagenesis proteins, we have analyzed the ability of HumD to complement UmuD′ functions in vivo as well as examined HumD’s physical properties in vitro. When expressed from a high-copy-number plasmid, HumD restored cellular mutagenesis and increased UV survival to normally nonmutable recA430 lexA(Def) and UV-sensitive ΔumuDC recA718 lexA(Def) strains, respectively. Complementing activity was reduced when HumD was expressed from a low-copy-number plasmid, but this observation is explained by immunoanalysis which indicates that HumD is normally poorly expressed in vivo. In vitro analysis revealed that like UmuD′, HumD forms a stable dimer in solution and is able to interact with E. coli UmuC and RecA nucleoprotein filaments. We conclude, therefore, that bacteriophage P1 HumD is a functional homolog of the UmuD′-like proteins, and we speculate as to the reasons why P1 might require the activity of such a protein in vivo.
In a wild-type Escherichia coli cell, most cellular mutagenesis is dependent on the UmuD′2C complex, which together with RecA allows DNA polymerase III to traverse otherwise replication-blocking lesions (33, 43, 44). Both UmuD′ and UmuC are induced as part of the global SOS response to DNA damage (13, 21). The activity of the Umu proteins is normally kept to a minimum by a variety of transcriptional and posttranslational mechanisms (reviewed in reference 48). An essential step in this process is the generation of UmuD′: the protein is synthesized as a larger mutagenically inactive precursor, UmuD, and achieves its mutagenically active form only after a RecA-mediated self-processing reaction that is believed to be mechanistically similar to that by which signal peptidases act on their substrates (4, 26, 27, 30, 31, 37).
Many homologs of the E. coli umuDC genes have now been identified, and a recent search of GenBank (release 110.0) identified what appear to be 11 bona fide homologs. In such cases, these homologs are arranged in an operon and are likely to be negatively regulated at the transcriptional level by LexA. Perhaps not too surprisingly, certain proteins with structural similarities are identified as homologs, but they lack the characteristic operon arrangement or do not appear to be regulated by LexA. As a consequence, in the absence of functional data, it is difficult to determine whether they are indeed functional homologs.
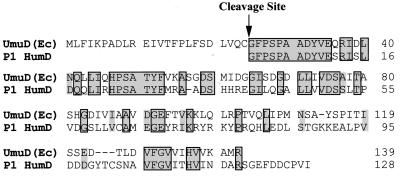
One such case is the bacteriophage P1 humD gene. This putative homolog was identified by Lewis et al., in 1994 (25), in a search for genes that might be regulated by LexA. Although a potential LexA-binding site was identified upstream of humD, the downstream region lacked an associated P1 umuC homolog. Perhaps the most striking observation was the fact that the translational start signals predict a protein with identity to the posttranslationally generated or recombinant UmuD′ protein (Fig. 1); therefore, although negatively regulated by LexA, once expressed, HumD might be able to functionally substitute for UmuD′ without the need to undergo any posttranslational modification.
FIG. 1.
Alignment of E. coli UmuD and bacteriophage P1 HumD proteins. The E. coli UmuD and bacteriophage P1 HumD proteins were aligned by using the program Geneworks 2.51 (Oxford Molecular, Campbell, Calif.). Residues that are identical are shaded grey and boxed, while highly conserved residues are shaded in grey. Interestingly, the start of the HumD corresponds exactly to the mutagenically active posttranslational cleavage product of UmuD, UmuD′. Overall, E. coli UmuD and P1 HumD are approximately 33% identical to each other, but as can be seen, most identity is in the N-terminal tail, in which 24 of the first 29 residues are identical. HumD has an extended C-terminal tail, which may be important for dimerization of the protein.
Since the mutagenically active Umu complex consists of a heterotrimer of UmuD′2C (43, 44), one would have to postulate that if HumD is functionally similar to UmuD′, it should be a dimer in solution as well as physically interact with UmuC to form a HumD2-UmuC complex in such a way as to promote error-prone translesion DNA synthesis. To test this hypothesis, we have subcloned a ∼1-kb fragment of the P1 genome containing humD and have characterized HumD both in vivo and in vitro. All of our experimental results support the conclusion that HumD is, in fact, a functional homolog of UmuD′ and substitutes for UmuD′ in damage-induced mutagenesis.
During the course of this work, the entire genome (46,375 bp) of the lambdoid bacteriophage N15 was sequenced (GenBank accession no. AF64539). Sequence analysis revealed that like P1, N15 carries a gene predicted to encode a HumD protein related to the UmuD′ family of proteins. Presumably, humD (umuD′-like) genes are maintained on the relatively small genomes of N15 (∼46 kb) and P1 (∼100 kb) because they provide some function that is evolutionarily advantageous to both phages. Here, we hypothesize as to what these functions might be.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli strains used in this study are listed in Table 1. Strains RW260, RW262, RW264, and RW406 were made by P1 transduction (29) of the Δ(umuDC)595::cat allele from RW82 (47) into EWRP1, JS431, EW181 (42), and WP2uvrA (17), respectively, by selecting for chloramphenicol resistance and screening for loss of damage-induced mutagenesis.
TABLE 1.
E. coli strains used in this study
| Strain | Type, relevant genotype | Reference |
|---|---|---|
| RW82 | K-12, Δ(umuDC)595::cat uvrA6 | 47 |
| EC10 | K-12, recA+ lexA51(Def) Δ(umuDC)596::ermGT | 10 |
| JS431 | B, recA430 lexA71(Def)::Tn5 umuDC+ uvrA155 trpE65(oc) | 42 |
| EW181 | B, recA718 lexA71(Def)::Tn5 umuDC+ uvrA155 trpE65(oc) | 42 |
| WP2uvrA | B, recA+ lexA+ umuDC+ uvrA155 trpE65(oc) | 17 |
| RW260 | B, recA+ lexA51(Def) Δ(umuDC)595::cat uvrA155 trpE65(oc) | This study |
| RW262 | B, recA430 lexA71(Def)::Tn5 Δ(umuDC)595::cat uvrA155 trpE65(oc) | This study |
| RW264 | B, recA718 lexA71(Def)::Tn5 Δ(umuDC)595::cat uvrA155 trpE65(oc) | This study |
| RW406 | B, recA+ lexA+ Δ(umuDC)595::cat uvrA155 trpE65(oc) | This study |
Plasmids used in this study (Table 2) were constructed by using standard methods of recombinant DNA technology (34). pOS27, a low-copy-number plasmid expressing humD, was constructed as follows. Plasmid pAW711 (24) was digested with XmnI and BssHII, and the 1,035-bp humD-containing fragment was gel purified. The BssHII ends were blunt ended with DNA polymerase I (Klenow fragment), and EcoRI linkers (New England Biolabs, Beverly, Mass.) were added. The 1,055-bp EcoRI fragment was subsequently cloned into the unique EcoRI site of pGB2 (5).
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant characteristics | Reference |
|---|---|---|
| pAW711 | Ampr, medium-copy-number, pBR322-based plasmid that carries ∼5 kb of the P1 genome | 24 |
| pOS27 | Spcr, low-copy-number, pGB2-based plasmid expressing HumD | This study |
| pRW66 | Spcr, low-copy-number, pGB2-based plasmid expressing UmuD′ | 49 |
| pRW124 | Ampr, medium-copy-number, pBR322-based plasmid that expresses E. coli UmuC | 49 |
| pOS30 | Ampr, high-copy-number, pBluescriptKS+-based plasmid that expresses HumD in the same orientation as LacZ | This study |
| pOS31 | Ampr, high-copy-number, pBluescriptKS+-based plasmid that expresses HumD in the opposite orientation to LacZ | This study |
| pOS32 | Ampr, high-copy-number, pBluescriptKS+-based plasmid that expresses UmuD′ in the same orientation as LacZ | This study |
| pOS33 | Ampr, high-copy-number, pBluescriptKS+-based plasmid that expresses RumA′ in the same orientation as LacZ | This study |
| pOS34 | Ampr, high-copy-number, pBluescriptKS+-based plasmid that expresses MucA′ in the same orientation as LacZ | This study |
| pRW274 | Spcr, low-copy-number, pGB2-based plasmid expressing UmuC | 14 |
| pOS35 | Spcr, low-copy-number, pGB2-based plasmid expressing RumB | This study |
| pOS37 | Spcr, low-copy-number, pGB2-based plasmid expressing MucB | This study |
| pOS36 | Ampr, medium-copy-number, pBR322-based plasmid that expresses HumD from an IPTG-inducible T7 RNA polymerase promoter | This study |
High-copy-number vectors expressing UmuD′-like proteins were constructed by cloning the various genes into the polylinker of pBluescript KS+ (Stratagene, La Jolla, Calif.) as follows: pOS30 and pOS31 were generated by cloning the EcoRI humD fragment from pOS27 into the unique EcoRI site of pBluescript KS+. HumD is translated in the same orientation as LacZ′ in pOS30 and in the opposite direction in pOS31. pOS32 was generated by cloning a 980-bp BglII E. coli umuD′-containing fragment from pGW2123 (30) into the BamHI site of pBluescript KS+. pOS33 was similarly constructed by cloning a 1,491-bp HindIII rumA′ fragment from pRW320 (23) into HindIII digested pBluescript KS+. pOS34 was constructed by cloning a 489-bp mucA′-containing BglII-HindIII fragment from pRW294 (23) into BamHI-HindIII-digested pBluescript KS+.
Low-copy-number vectors expressing UmuC-like proteins were constructed by introducing frameshift mutations in their cognate UmuD′-like partner as follows: pRW274 (umuC+) by digesting pRW134 (7) with NcoI, filling the ends, and subsequent religation (14); pOS35 (rumB+) by digesting pRW320 (23) with TthIII, filling the ends, and subsequent religation; and pOS37 (mucB+) by partial digestion of pRW294 (23) with BspMI, followed by end filling and religation.
A HumD-overproducing plasmid was constructed by PCR amplification of the humD gene from pOS30, using the 22-mer T7 sequencing primer (Stratagene) and a 39-mer, 5′-GAGGTGAAAACGCCATGGGCTTCCCTTCTCCTGCGGCGG-3′, which is identical to the start of the humD gene except that it contains a T→C transition at the −1 position relative to the ATG codon. This change introduces an NcoI restriction enzyme site (underlined) at the start of the humD gene. The PCR product was subsequently digested with NcoI and EcoRI and subcloned into a similarly digested T7 expression vector, pEC46 (12). The humD gene in this new plasmid, pOS36, was sequenced (Lark Technologies, Houston, Tex.) to confirm that no mutations were inadvertently generated during PCR. pOS36 therefore expresses HumD from an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible T7 RNA polymerase promoter.
UV survival assays.
To test the ability of HumD to complement UmuD′ functions and restore UV resistance to normally UV-sensitive recA718 umuDC cells (46), cultures were grown in Luria-Bertani (LB) medium until they reached a cell density of 108/ml, at which point they were harvested and resuspended in SM buffer (50 mM Tris-HCl [pH 7.5], 8 mM MgSO4, 100 mM NaCl, 0.01% gelatin) (29). Cells were exposed to UV light at a fluence of 0.25 J/m2/s, and appropriate dilutions were plated on LB agar plates. Surviving colonies were scored after overnight incubation at 37°C.
Qualitative spontaneous mutagenesis plate assay.
Assays were performed essentially as described elsewhere (49). Aliquots (1 ml) from a fresh overnight culture were centrifuged and resuspended in an equal volume of SM buffer (29). The ability of particular plasmid-bearing strains to promote Umu-dependent SOS mutator activity in the absence of exogenous DNA damage was judged by plating 100-μl aliquots on Davis-Mingioli minimal agar plates supplemented with a trace amount of tryptophan (0.75 μg/ml) (3). Umu-dependent chemically induced mutagenesis was determined as described above except that 1 μl of methyl methanesulfonate (MMS; Sigma, St. Louis, Mo.) was applied to a small sterile disk in the center of the plate. Both spontaneously arising and MMS-induced Trp+ mutants were scored after 3 days of incubation at 37°C.
Purification of the bacteriophage P1 HumD protein.
HumD protein was purified from a 3-liter culture of E. coli BL21(λDE3)/pOS36 that had been induced with 1.0 mM IPTG for 3 h prior to harvesting. The purification strategy was based upon that successfully used to isolate the structurally homologous UmuD′ and MucA′ proteins (12) and includes selective precipitation with ammonium sulfate followed by DEAE-Sephacel (Pharmacia, Piscataway, N.J.) and hydroxyapatite (Bio-Rad, Hercules, Calif.) ion-exchange chromatography and AcA54 (BioSepra, Cergy St. Christopher, France) gel filtration.
Polyclonal antisera to the highly purified HumD protein were raised in rabbits, using standard methods, by Covance (Vienna, Va.). The serum was subsequently affinity purified by standard methods (34).
Steady-state levels of HumD in vivo.
The steady-state level of HumD expressed from pOS30 in various genetic backgrounds was determined as described previously (11). Briefly, cells were grown in LB broth at 37°C to early exponential phase, at which time 1.5-ml aliquots were harvested and resuspended in sample buffer (50 mM Tris-HCl [pH 6.8], 10% glycerol, 2.0% sodium dodecyl sulfate [SDS], 0.1% bromophenol blue, 10 mM dithiothreitol). Aliquots representing equal cell numbers were electrophoresed on SDS–17% polyacrylamide gels. Proteins were then transferred to an Immobilon P membrane (Millipore, Bedford, Mass.) and subsequently probed with a 1:10,000 dilution of the affinity-purified polyclonal antibodies raised against HumD. The transferred proteins were subsequently visualized using the CSPD-Star chemiluminescence assay (Tropix, Bedford, Mass.). Membranes were exposed to Kodak Bio-Max MR film for periods of 1 to 10 min.
Stability of HumD and UmuC in vivo.
The relative stability of the HumD and UmuC proteins was measured essentially as described above except that when the cells reached mid-log phase (time zero), chloramphenicol (100 μg/ml) was added to the medium to inhibit further protein synthesis. Aliquots of 1.5 ml were removed at various time points thereafter, and samples were processed as described above. The apparent half-life of HumD (or UmuC) was determined by simple analysis of the relative steady-state level of the protein at each time point (11).
Glutaraldehyde cross-linking studies.
To determine if HumD is capable of forming a dimer with itself, ∼250 ng of HumD was incubated at 37°C for 30 min in 20 mM Tris (pH 7.5)–1 mM EDTA–10% glycerol–50 mM NaCl–1 mM dithiothreitol, after which time protein complexes were chemically cross-linked by adding glutaraldehyde to a final concentration of 0.05%. After 15 min of further incubation at room temperature, complexes were separated in an SDS–15% polyacrylamide gel and electrotransferred to an Immobilon P membrane. The monomeric/multimeric state of HumD was determined after probing the membrane with polyclonal antibodies to HumD and subsequently visualized with the CSPD-Star chemiluminescence kit as described above.
Ability of HumD to bind to a RecA nucleoprotein filament.
The ability of HumD to bind to a RecA nucleoprotein filament was determined essentially as previously described for UmuD′ (12). Reaction mixtures (10 μl) contained 40 ng of φX174 DNA (New England Biolabs), 20 mM HEPES buffer (pH 7.5, 20 mM NaCl, 1 mM EDTA, 1 mM ATPγS, 1.5 μg of RecA (added where indicated), 1 mM dithiothreitol, and 50 μg of bovine serum albumin. To initiate nucleoprotein formation, MgCl2 was added to a final concentration of 10 mM and the mixture incubated at 37°C for 20 min, after which time 300 ng of UmuD′ or HumD was added to the reaction mixture and incubated for an additional 20 min at 37°C. Nucleoprotein complexes were chemically cross-linked by adding glutaraldehyde (final concentration of 0.05%) at room temperature for 10 min. These complexes were separated by electrophoresis in a nondenaturing 0.95% agarose gel, and the relative mobility of the nucleoprotein complex was determined after chemiluminescence immunoanalysis as described above.
RESULTS
Restoration of cellular mutagenesis by P1 HumD to normally nonmutable lexA(Def) recA430 strains.
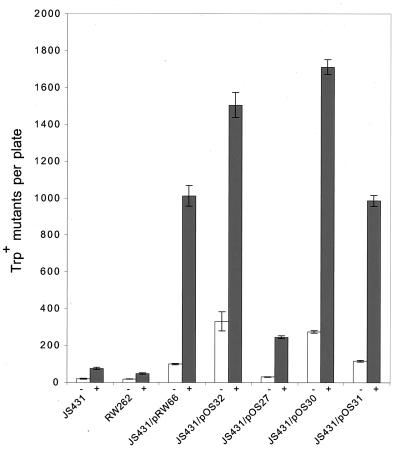
To test the hypothesis that HumD might encode a protein with a function similar to that of the UmuD′-like mutagenesis proteins, we subcloned a ∼1-kb fragment of the P1 genome, containing humD, into a low-copy-number plasmid and introduced the plasmid, pOS27, into the lexA(Def) recA430 strain, JS431 (42). Although this strain expresses derepressed levels of the chromosomally encoded UmuDC proteins, it does not exhibit any cellular mutagenesis because RecA430 is unable to mediate cleavage of UmuD (4, 37). Both spontaneous and damage-induced mutagenesis can, however, be restored, if UmuD′ is provided in trans from a high copy-number plasmid (30, 42) and to a lesser extent by a low-copy-number plasmid (7; Fig. 2). Unlike the low-copy-number E. coli plasmid, pRW66, which resulted in an 5-fold increase in spontaneous mutagenesis and 13-fold increase in MMS-induced mutagenesis over background, the low-copy-number HumD plasmid, pOS27, resulted in no discernible increase in spontaneous mutagenesis and only a 3-fold increase (from 74 to 245 Trp+ mutants) in MMS mutagenesis (Fig. 2). Although these effects are clearly smaller than those observed for the low-copy-number UmuD′ plasmid, we were nevertheless encouraged by these results since the qualitative plate assay is a sensitive and accurate reflection of the ability of cells to promote damage-induced mutagenesis (15).
FIG. 2.
Restoration of mutagenesis functions to a normally nonmutable lexA(Def) recA430 strain by P1 HumD. The ability of E. coli UmuD′ and P1 HumD to restore mutagenesis functions to JS431, a recA430 lexA(Def) strain, was monitored in a qualitative plate assay by following reversion of the trpE65(oc) allele in the absence of exogenous DNA damage (−) or after exposing the cells to MMS (+). recA430 lexA(Def) strains are nonmutable because they are unable to posttranslationally mediate the cleavage of UmuD to UmuD′ and therefore give rise to essentially the same number of Trp+ revertants as the isogenic ΔumuDC strain, RW262. Mutagenesis can, however, be restored to JS431 by providing a recombinant UmuD′ in trans. As can be seen, the extent of restoration is dependent on the copy number of the plasmid; pRW66 is a low-copy-number UmuD′ plasmid, and pOS32 is a high-copy-number UmuD′ plasmid. Similarly, HumD also restores mutagenesis to JS431, with the extent of mutagenesis related to the copy number/expression of the relevant HumD plasmid: pOS31, low-copy-number HumD; pOS30, high-copy-number HumD translated in the same direction as LacZ′ (expressed from the vector polylinker); pOS31, high-copy-number HumD translated in the opposite direction to LacZ′. The data presented are the means from three independent isolates and three plates per isolate. The error bars represent the standard error of the mean for each experiment.
As noted above, the extent to which UmuD′ restores mutagenesis to recA430 cells depends on the copy-number plasmid from which it is expressed (7) (Fig. 2). We were interested in determining if the same is true for HumD. As a consequence, we subcloned the humD gene from pOS27 into the high-copy-number plasmid pBluescript KS+. Plasmids were obtained with the humD gene transcribed in the same and opposite directions as lacZ′, and one plasmid from each group was chosen for further study. Introduction of these plasmids (pOS30 and pOS31) resulted in a dramatic increase in the extent of both spontaneous and MMS-induced Trp+ reversion (Fig. 2). The greatest increase was observed with pOS30, in which HumD is translated in the same direction as LacZ′. Indeed, the amount of mutagenesis promoted by pOS30 was comparable to that seen with the high-copy-number E. coli UmuD′ plasmid, pOS32. By comparison, pOS31, in which HumD is translated in the opposite direction to LacZ′, yielded twofold fewer Trp+ revertants, but this was still significantly higher than that seen with the low-copy-number plasmid, pOS27 (Fig. 2). Based on these observations, we conclude that HumD can substitute for E. coli UmuD′ in cellular mutagenesis. The fact that very little mutagenesis is seen with a low-copy-number plasmid, and the most is found when translation is in the same orientation and distal to the lac promoter from a high-copy-number plasmid, suggests, however, that production of high levels of HumD-dependent mutagenesis requires maximal expression of HumD.
Specificity of HumD’s ability to restore SOS mutagenesis.
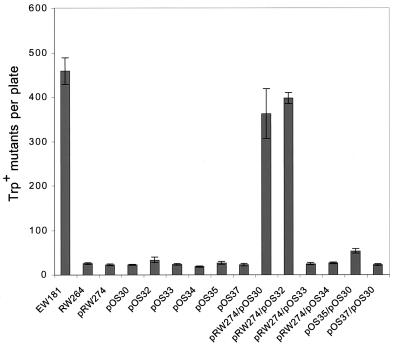
HumD clearly has the ability to restore mutagenesis functions to a lexA(Def) recA430 strain, and we were interested in determining if the effect might be greater in other recA mutant strains. An excellent background to assay for Umu-like activity is in a lexA(Def) recA718 strain (18, 42). When fully derepressed, the RecA718 protein exhibits a modest coprotease activity that results in a spontaneous mutator activity (18, 42) (Fig. 3). This activity is clearly Umu dependent, as strains carrying a ΔumuDC mutation do not exhibit the spontaneous mutator phenotype (18, 42) (Fig. 3). Based on genetic studies, it has been hypothesized that the mutator activity reflects the ability of the Umu-like proteins to promote extension from mispaired bases generated during normal replication (9).
FIG. 3.
Specificity of HumD’s ability to restore mutagenesis functions in a ΔumuDC recA718 lexA(Def) strain. The specificity of HumD’s ability to restore mutagenesis functions to RW264 was assayed by introducing compatible plasmids expressing various combinations of E. coli UmuD′ and/or UmuC, pKM101 MucA′ and/or MucB, and R391 RumA′ and/or RumB together with high-copy-number HumD. Mutagenesis was monitored as described in the legend to Fig. 2 by following spontaneous reversion of the trpE65(oc) allele. Strain EW181 demonstrates the level of spontaneous mutagenesis promoted by the chromosomally encoded UmuD(D′) proteins, and RW264 is the isogenic ΔumuDC strain. As can be seen, background levels of spontaneous mutagenesis were observed with RW264 harboring pRW274 (low-copy-number E. coli UmuC), pOS30 (high-copy-number HumD), pOS32 (high-copy-number E. coli UmuD′), pOS33 (high-copy-number R391 RumA′), pOS34 (high-copy-number pKM101 MucA′), pOS35 (low-copy-number R391 RumB), pOS37 (low-copy-number pKM101 MucB) alone or with pRW274/pOS33 (low-copy-number E. coli UmuC and high-copy-number R391 RumA′), pRW274/pOS34 (low-copy-number E. coli UmuC and high-copy-number pKM101 MucA′), and pOS37/pOS30 (low-copy-number pKM101 and high-copy-number HumD). A twofold increase over background was observed with pOS35/pOS30 (low-copy-number R391 RumB and high-copy-number HumD). In dramatic contrast, significant levels of spontaneous mutagenesis, comparable to that seen in the umu+ strain, were observed with pRW274/pOS32 (low-copy-number E. coli UmuC and high-copy number E. coli UmuD′) and pRW274/pOS30 (low-copy-number E. coli UmuC and high-copy-number HumD). The data presented are the means from three independent isolates and three plates per isolate. The error bars represent the standard error of the mean for each experiment.
Many UmuD′C-like homologs have now been cloned, and we were interested in determining if bacteriophage P1 HumD could interact with these homologs in addition to E. coli UmuC. The Salmonella typhimurium Umu proteins are closely related to their E. coli counterparts (38, 45), and one might intuitively think that they would be the best candidates to determine if HumD has a broad specificity in promoting mutagenesis. Unfortunately, S. typhimurium is poorly mutable because it appears to have acquired mutations in its umuC gene that render the protein less active (20, 36). As a consequence, we decided to look at the ability of HumD to promote mutagenesis in combination with the more diverged but normally very active MucB and RumB proteins (22). Lack of complementation between Umu homologs has previously been demonstrated and is understandable given their evolutionary divergence (36). Indeed, no complementation is seen with a high-copy-number HumD plasmid and a low-copy-number MucB plasmid, and only a very slight increase in MMS mutagenesis is seen with a high-copy-number HumD plasmid and a low-copy-number RumB plasmid (Fig. 3). Likewise, no mutagenesis is seen with a low-copy-number E. coli UmuC plasmid and a compatible MucA′ or RumA′ plasmid (Fig. 3). In fact, the only combination of plasmids that resulted in significant levels of spontaneous mutagenesis in the ΔumuDC background was the high-copy-number HumD plasmid or high-copy-number UmuD′ plasmid in combination with the low-copy-number UmuC plasmid (Fig. 3).
HumD shares only 39% identity with E. coli UmuD′ (Fig. 1) and slightly less with MucA′ (35%) and RumA′ (30%), suggesting that it is not the overall degree of identity that is important for the HumD-UmuC interaction, but rather that the residues conserved between the HumD and UmuD′ proteins presumably play a critical role in their ability to interact with UmuC.
Ability of HumD to restore UV resistance to UV-sensitive recA718 ΔumuDC bacteria.
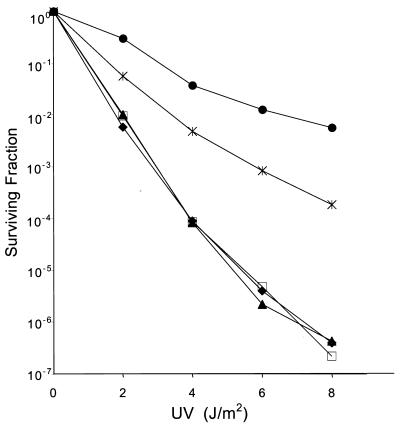
Several years ago, Witkin and colleagues demonstrated that a recA718 umuDC uvrA155 strain was very sensitive to the killing effects of UV light because the strain was unable to resume replication after sustaining DNA damage (46). Similar to the mutator phenotype described above, this UV-sensitive phenotype is dependent on the Umu-like proteins, as resistance to UV light is restored in the presence of the chromosomally encoded Umu proteins (46) or by Umu-complementing plasmids (18). As can be seen in Fig. 4, the recA718 ΔumuDC lexA(Def) uvrA155 strain, RW264, is very sensitive to UV light, with survival dropping 6 orders of magnitude after a dose of approximately 6 J/m2. Transformation with either the low-copy-number UmuC plasmid, pRW274, or the high-copy-number HumD plasmid, pOS30, alone has no obvious effect on UV survival. If, however, the strain is cotransformed with both plasmids, UV resistance increases dramatically (103-fold at 6 Jm−2), but is roughly 10-fold less than that seen with pRW274 and the compatible high-copy-number UmuD′ plasmid, pOS32 (Fig. 4). These findings are consistent with the data from the mutagenesis assays, described above, by suggesting that HumD is able to interact with UmuC.
FIG. 4.
Restoration of UV resistance to a normally UV-sensitive ΔumuDC lexA(Def) recA718 strain by HumD-UmuC. The ability of various plasmids to restore UV resistance to RW264 [ΔumuDC lexA(Def) recA718 uvrA155] was assayed by exposing plasmid containing cultures to UV light and plating serial dilutions on LB agar plates. Surviving colonies were scored after 24 h of incubation at 37°C. The data presented are the means from three independent isolates and three plates per UV dose. Strains analyzed were RW264 alone (⧫), RW264/pOS30 (high-copy-number HumD) (□), RW264/pRW274 (low-copy-number E. coli UmuC) (▴), RW264/pRW274/pOS30 (low-copy-number E. coli UmuC with high-copy-number HumD) (X), and RW264/pRW274/pOS32 (low-copy-number E. coli UmuC with high-copy-number E. coli UmuD′) (●).
Purification of HumD.
The genetic data strongly suggest that HumD can functionally substitute for E. coli UmuD′. To better characterize the HumD protein and its interactions with other components of the mutasome, we overproduced bacteriophage P1 HumD by placing the humD gene under the control of an IPTG-inducible T7 RNA polymerase promoter (41). Addition of IPTG to exponentially growing cells resulted in the induction of a protein with the expected molecular mass of HumD (Fig. 5). A large proportion of the overproduced protein was found in the soluble fraction of cell extracts, thereby allowing us to follow purification by simple visualization with Coomassie brilliant blue. HumD was purified to greater than 95% homogeneity (Fig. 5) by using the same protocol as used for other UmuD′-like proteins (12). N-terminal sequence analysis of the highly purified protein confirmed its identity as HumD and revealed that like UmuD′ (12), the formylmethionyl had been removed by amino-terminal peptidase (1) (data not shown). Like other homologs, HumD eluted from the gel filtration at a position consistent with it being a dimer in solution.
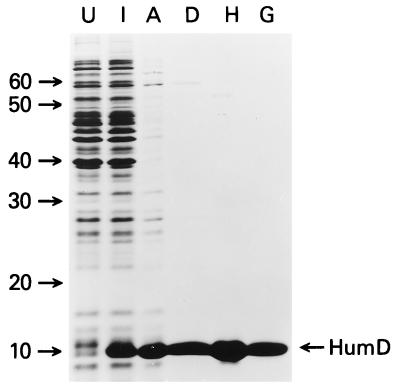
FIG. 5.
Overproduction and purification of HumD. Bacteriophage P1 HumD was purified from BL21(λDE3) cells carrying the HumD-overproducing plasmid pOS36. Proteins were separated in SDS–15% polyacrylamide gels, and proteins were visualized after staining with Coomassie blue. Lanes U and I are whole-cell extracts from uninduced and IPTG-induced cells, respectively; lanes A, D, H, and G are fractions obtained after selective ammonium sulfate precipitation and DEAE-Sephacel, hydroxyapatite, and AcA54 gel filtration chromatography, respectively. The position of monomeric HumD is marked on the right, and the molecular masses of marker proteins are indicated (in kilodaltons) on the left.
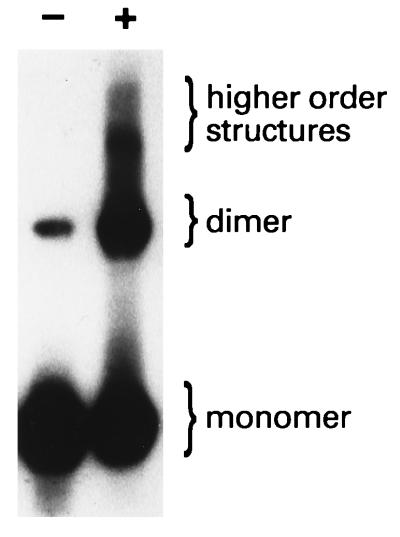
We further analyzed the ability of HumD to form dimers by chemically cross-linking HumD (Fig. 6). Most of the uncross-linked HumD migrated at a position consistent with it being a monomer (under denaturing conditions). However, a significant quantity of HumD migrated at a position of dimeric HumD (even under apparently denaturing conditions). The intensity of this dimeric HumD band increased dramatically upon cross-linking, as did the appearance of higher-order structures (Fig. 6) that we have previously seen with UmuD′ under similar conditions (32). We conclude, therefore, that like UmuD′, HumD is a dimer in solution.
FIG. 6.
HumD is a dimer in solution. To determine if HumD is capable of forming a dimer with itself, HumD was chemically cross-linked with glutaraldehyde as described in Materials and Methods. Untreated (−) and cross-linked (+) samples were separated in an SDS–15% polyacrylamide gel and transferred to an Immobilon P membrane. The monomeric/multimeric state of HumD was determined by probing the membrane with polyclonal antibodies to HumD and subsequent visualization with the CSPD-Star chemiluminescent substrate. The positions of HumD monomers, dimers, and higher-order structures are indicated on the right.
Expression of HumD in vivo.
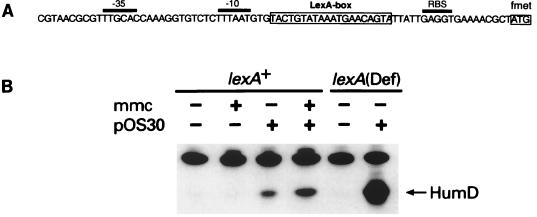
The location of a LexA-binding site immediately upstream of HumD (25) (Fig. 7) strongly suggests that under normal conditions, HumD is negatively regulated by LexA and is damage inducible. To confirm these suggestions, we analyzed the in vivo level of HumD expressed from pOS30 in a wild-type lexA+ cell, with and without exposing the cells to the DNA-damaging agent MMC (Fig. 7). Like the umu operon, the humD gene has a potential LexA-binding site that has a very good match with the consensus binding site (25). In the case of HumD, it deviates from this consensus by only three nucleotides and as a consequence would be expected to be tightly regulated by LexA. Surprisingly, the basal level of expression was higher than expected, and probably arose from the inability of the chromosomally expressed LexA to bind to all of the humD operator sites on the multicopy plasmid pOS30 (>100 copies per cell). Exposure of the wild-type cells to MMC resulted in only an ∼1.7-fold induction of HumD and presumably reflects incomplete derepression and/or tight binding by LexA to the operator sequence, as the level of HumD expressed in a lexA(Def) strain was 10- to 20-fold greater than in the induced lexA+ background. These observations therefore support the hypothesis that HumD is part of the LexA regulon and is induced as a consequence of cellular DNA damage.
FIG. 7.
Damage-inducible expression of HumD. (A) Nucleotide sequence of the humD promoter/operator region. The initiator codon (formylmethionyl [fmet]) of HumD is at the far right and boxed; positions of the −35 and −10 promoter elements and of the ribosome binding site (RBS) are overlined. The proposed LexA-binding site is located between the −10 promoter element and the RBS and is also boxed. It should be noted that this LexA-binding site deviates from the consensus at only three positions. (B) Steady-state levels of HumD expressed from pOS30 in an undamaged or MMC-treated lexA+ strain (RW406) or in an undamaged lexA51(Def) strain (RW260). The position of HumD is indicated on the right. As can be seen, the HumD antiserum recognizes another cellular protein in addition to HumD. The identity of this protein is unknown, but it serves as a useful internal control, ensuring that equal amounts of protein extract have been applied to the gel.
Perhaps the most surprising aspect of these studies was the actual amount of HumD protein expressed in the cell. As noted above, pOS30 is a high-copy-number plasmid, and so we expected that HumD would be significantly overproduced. In fact, even when fully derepressed, the amount of HumD expressed from pOS30 was roughly similar to that of UmuD′ from a low-copy-number plasmid (11) (data not shown). The poor expression of HumD can potentially be explained by weak promoter activity, especially as the sequence of the −35 box deviates from the consensus (19) and similar substitutions in the −35 box of the umu operon resulted in reduced expression of UmuD′ (28). The limited expression of HumD from its native promoter might also explain why complementation of mutagenesis functions is greatly enhanced in the presence of the high-copy-number plasmid: there is simply not enough HumD expressed from the low-copy-number plasmid for functional complementation.
Stability of HumD in vivo.
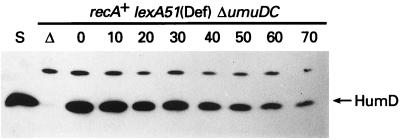
The intracellular levels of E. coli UmuD and UmuD′ proteins are exquisitely regulated by targeted proteolysis (10). UmuD is relatively labile in vivo and is targeted for degradation by the Lon protease (10, 14). Homodimeric UmuD′ is, in contrast, relatively insensitive to Lon, and it is stable in vivo until it forms a heterodimer with UmuD and is rapidly degraded by ClpXP (10). Analysis of the stability of HumD (Fig. 8) revealed that like homodimeric UmuD′, it is very stable in vivo, with a half-life estimated to be greater than 50 min. Similar results were obtained when HumD was coexpressed with UmuD, suggesting that HumD and UmuD heterodimers are insensitive to proteolysis or that HumD and UmuD are unable to form heterodimers (data not shown).
FIG. 8.
In vivo stability of HumD. Plasmid pOS30 was introduced into the Δ(umuDC)596::ermGT recA+ lexA51(Def) strain EC10, and the relative stability of HumD was measured after protein synthesis was inhibited by the addition of chloramphenicol (100 μg/ml) at time zero. Additional aliquots were removed at 10-min intervals. Whole-cell extracts were separated in an SDS–15% polyacrylamide gel, and proteins were visualized using HumD antibodies and the CSPD-Star chemiluminescence assay. Track S is ∼20 ng of highly purified HumD protein; track Δ is an extract of the strain lacking pOS30. As shown in Fig. 7, the HumD antiserum recognizes another cellular protein in addition to HumD that serves as a useful internal control, ensuring that equal amounts of protein extract have been applied to the gel. The position of HumD is indicated on the right.
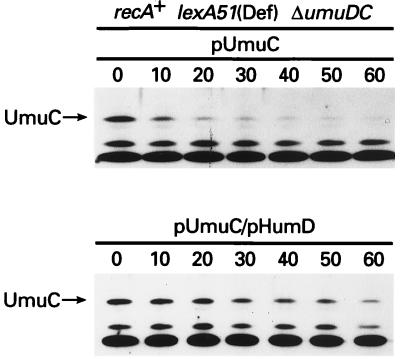
In vivo interaction of HumD and UmuC.
Our observation that HumD is able to restore mutagenesis functions specifically in combination with E. coli UmuC strongly suggests that the proteins physically interact with each other. Like UmuD, UmuC is labile in vivo because it is rapidly degraded by Lon (10) (Fig. 9). However, in the presence of UmuD′, the stability of UmuC increases dramatically (11), presumably as a result of UmuD′ protecting UmuC from Lon degradation (10). Similarly, when HumD and UmuC are coexpressed, UmuC exhibits a dramatic increase in its observed half-life (Fig. 9). Thus, although not direct evidence of a protein-protein interaction, taken together with the genetic complementation studies, these observations suggest that HumD and UmuC are able to physically interact.
FIG. 9.
Stabilization of E. coli UmuC by HumD. A plasmid expressing UmuC alone (pRW274) or coexpressing HumD (pOS30) was introduced into the Δ(umuDC)596::ermGT recA+ lexA51(Def) strain, EC10. The relative stability of UmuC was assayed after protein synthesis was inhibited by the addition of chloramphenicol (100 μg/ml) at time zero. Whole-cell extracts were separated in an SDS–15% polyacrylamide gel, and proteins were visualized by using UmuC antibodies and the CSPD-Star chemiluminescence assay. The position of UmuC is indicated by an arrow at the left.
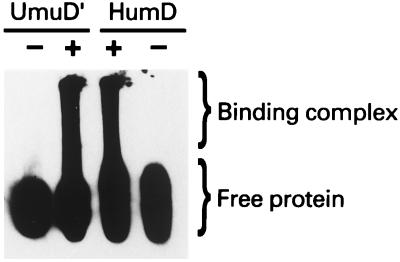
Ability of HumD to interact with a RecA nucleoprotein filament.
As noted in the introduction, SOS mutagenesis requires the formation of a multiprotein “mutasome” (6). A key step in the formation of the mutasome is an interaction between UmuD′2C and RecA that most likely positions the Umu complex at the site of a DNA lesion (2, 39, 40). HumD, Like UmuD′, is an acidic protein with a predicted pI of ∼5.1, and it does not bind directly to DNA (Fig. 10). If the DNA is, however, coated with RecA, HumD migrates at a position similar to that of RecA-DNA-UmuD′ complexes, suggesting that it too binds to a RecA nucleoprotein filament (Fig. 10). We hypothesize, therefore, that like UmuD′, HumD interacts with a RecA nucleoprotein filament in such a way as to target the limited number of HumD molecules to sites where they may promote translesion DNA synthesis.
FIG. 10.
Interaction of HumD with a RecA-nucleoprotein filament. Nucleoprotein complexes were formed as described in Materials and Methods. After electrophoretic separation in 0.9% agarose, nucleoprotein complexes were transferred to a support membrane. The membrane was cut, and the blots were probed with UmuD′ and HumD antibodies, as indicated. Both blots were subsequently visualized using the CSPD-star chemiluminescence immunoassay. Tracks labeled − lack RecA protein, whereas those labeled + contain RecA and are therefore able to form RecA nucleoprotein filaments. Because of their large size, these nucleoprotein complexes have limited mobility in the agarose gel and are therefore retained at the top of the gel. In contrast, the free protein migrates much more quickly. These gels are run under native conditions, and the smear observed in the presence of the nucleoprotein filaments presumably arises from the dissociation of some of the un-cross-linked UmuD′ and HumD from the filament during electrophoresis. The positions of free UmuD′ and HumD as well as the binding complex are indicated at the right.
DISCUSSION
Bacteriophage P1 HumD is a functional homolog of UmuD′.
Based on sequence comparisons, Lewis et al. (25) suggested that the bacteriophage P1 humD gene might encode a protein similar in function to UmuD′-like proteins. We have tested this hypothesis directly and find that HumD can in fact functionally substitute for E. coli UmuD′ in vivo. Complementation between Umu-like orthologs is rare (16, 36), and it could be argued that since HumD does not have a natural cognate partner, it may have a more relaxed specificity for the necessary protein-protein interactions required for mutasome formation. However, we observed no restoration of mutagenesis functions with HumD and MucB and only very slight complementation with HumD and RumB, suggesting that the HumD-UmuC-RecA interaction is quite specific. Clues as to the sites of these protein-protein interactions come from comparison of HumD’s predicted structure to that of UmuD′ (32). Although overall homology is quite low (∼39%) between UmuD′ and HumD, when one aligns the primary amino acid sequences of both proteins (Fig. 1), it is clear that most homology is in the N-terminal tail, where 24 of 29 residues (∼83%) are identical. This region has been implicated as a RecA binding site as deletion of the first 20 amino acids of UmuD′ results in a protein that has a reduced capacity to interact with a RecA-nucleoprotein filament (32). In addition, the longer N-terminal tail, along with the shorter C-terminal tail, is important for UmuD′ dimerization (8, 32). In particular, the short C-terminal tails of the two UmuD′ protomers form a β-sheet that helps dimerization. While there is limited identity between HumD and UmuD′ in their C termini, the C terminus of HumD is 10 amino acids longer than that of UmuD′, suggesting that it may be able to form a more extensive β sheet with a cognate protomer, thereby explaining the appearance of a HumD dimer, even under apparently denaturing conditions (Fig. 5).
The sites on UmuD′ required for an interaction with UmuC are unknown, but it is clear from our functional studies that they are conserved in HumD. All of the available evidence is therefore consistent with the hypothesis that HumD is a bona fide homolog of the UmuD′ mutagenesis protein.
At first glance, one might consider whether the existence of humD on P1 is a peculiarity of nature. Very recently, however, a gene (GenBank accession no. AF064539) that is approximately 70% identical to P1 HumD has been identified on the lambdoid phage N15 (not shown), and it seems likely that other bacteriophage orthologs will be identified. One obvious question is, why do P1 and N15 carry such orthologs on their small chromosomes? Like all organisms, both phage are subject to evolutionary pressures, and so humD presumably confers some advantage over phage that have lost the gene.
Our in vivo complementation studies revealed that HumD’s mutagenesis promoting activity is manifested, albeit at low levels, when expressed from a low-copy-number plasmid. Both P1 and N15 are normally maintained at one to two copies in a lysogenic state, and so by analogy to the phenotype observed with the low-copy-number plasmid, it is possible that HumD’s function is to allow the host to survive the potentially fatal consequences of replication-inhibiting DNA lesions, thereby obviating the need for the phage to enter a lytic cycle. Such an activity seems hardly warranted in E. coli, which already possesses active UmuD′2C proteins, but what about additional hosts of P1, N15, and other humD-bearing phage? Many enteric bacteria possess Umu homologs which, based upon their cross-reactivity to E. coli Umu antibodies (35), are clearly closely related to their E. coli counterparts. Moreover, unlike the common E. coli laboratory strains with which most of us work, many naturally occurring E. coli strains are poorly mutable (35), suggesting ineffective Umu systems. Furthermore, some bacteria, such as Klebsiella aerogenes and Citrobacter intermedius, are apparently rendered nonmutable because they are unable to posttranslationally process UmuD to mutagenically active UmuD′ (35) and are therefore analogous to the E. coli recA430 lexA(Def) strain used for Fig. 2. Under such conditions, any activity provided by HumD may, in fact, provide a significant evolutionary advantage to both the host and the phage.
Of course, the other possibility is that the function of HumD is to protect the phage itself, rather than its host. After all, once the phage has entered the lytic cycle, the cell is doomed anyway. Under lytic conditions, the copy number of these temperate phage increases dramatically (over 100-fold) before eventual lysis, and this could be considered analogous to the conditions wherein we observed the considerable mutagenesis-promoting activity of HumD when expressed from a high-copy-number plasmid (Fig. 2 and 3). Presumably, if the phage has entered the lytic cycle because of host DNA damage, it may have acquired damage itself, and the role of HumD is to protect the phage genome by allowing translesion DNA synthesis across otherwise unrepairable lesions.
Another formal possibility is that the retention of humD on phage genomes is completely unrelated to HumD’s translesion-promoting activity. For example, we have recently demonstrated that E. coli UmuD′ is an enzyme that possesses the ability to cleave intact UmuD in vitro and in vivo (26, 27). Given the structural similarities between UmuD′ and HumD (32), including the active-site residues necessary for cleavage, it is likely that HumD possesses similar enzymatic activities and that it is this property which provides the selective pressure for its retention on certain phage genomes.
ACKNOWLEDGMENTS
Mary P. McLenigan and Olga I. Kulaeva contributed equally to this work.
We thank Arun Alagappan for technical help with the bacterial mutagenesis assays shown in Fig. 2 and 3, Michael Yarmolinsky for plasmid pAW711 and helpful insights into P1 biology and Martín Gonzalez for stimulating discussions during the course of this work.
REFERENCES
- 1.Ben-Bassat A, Bauer K, Chang S Y, Myambo K, Boosman A, Chang S. Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol. 1987;169:751–757. doi: 10.1128/jb.169.2.751-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudsocq F, Campbell M, Devoret R, Bailone A. Quantitation of the inhibition of Hfr × F− recombination by the mutagenesis complex UmuD′C. J Mol Biol. 1997;270:201–211. doi: 10.1006/jmbi.1997.1098. [DOI] [PubMed] [Google Scholar]
- 3.Bridges B A, Woodgate R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc Natl Acad Sci USA. 1985;82:4193–4197. doi: 10.1073/pnas.82.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burckhardt S E, Woodgate R, Scheuermann R H, Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification and cleavage by RecA. Proc Natl Acad Sci USA. 1988;85:1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 6.Echols H, Goodman M F. Mutation induced by DNA damage: a many protein affair. Mutat Res. 1990;236:301–311. doi: 10.1016/0921-8777(90)90013-u. [DOI] [PubMed] [Google Scholar]
- 7.Ennis D G, Levine A S, Koch W H, Woodgate R. Analysis of recA mutants with altered SOS functions. Mutat Res. 1995;336:39–48. doi: 10.1016/0921-8777(94)00045-8. [DOI] [PubMed] [Google Scholar]
- 8.Ferentz A E, Opperman T, Walker G C, Wagner G. Dimerization of the UmuD′ protein in solution and its implications for regulation of SOS mutagenesis. Nat Struct Biol. 1997;4:979–983. doi: 10.1038/nsb1297-979. [DOI] [PubMed] [Google Scholar]
- 9.Fijalkowska I J, Dunn R L, Schaaper R M. Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J Bacteriol. 1997;179:7435–7445. doi: 10.1128/jb.179.23.7435-7445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank E G, Ennis D G, Gonzalez M, Levine A S, Woodgate R. Regulation of SOS mutagenesis by proteolysis. Proc Natl Acad Sci USA. 1996;93:10291–10296. doi: 10.1073/pnas.93.19.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank E G, Gonzalez M, Ennis D G, Levine A S, Woodgate R. In vivo stability of the Umu mutagenesis proteins: a major role for RecA. J Bacteriol. 1996;178:3550–3556. doi: 10.1128/jb.178.12.3550-3556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank E G, Hauser J, Levine A S, Woodgate R. Targeting of the UmuD, UmuD′ and MucA′ mutagenesis proteins to DNA by RecA protein. Proc Natl Acad Sci USA. 1993;90:8169–8173. doi: 10.1073/pnas.90.17.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 14.Gonzalez M, Frank E G, Levine A S, Woodgate R. Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green M H L, Muriel W J. Mutagen testing using TRP+ reversion in Escherichia coli. Mutat Res. 1976;38:3–32. doi: 10.1016/0165-1161(76)90076-5. [DOI] [PubMed] [Google Scholar]
- 16.Gruz P, Matsui K, Sofuni T, Nohmi T. Construction of a new system for separate expression of mutagenesis proteins: the abilities to promote UV mutagenesis and interchangeability of MucA′, MucB, SamA′ and SamB proteins in Salmonella typhimurium. Mutat Res. 1996;354:157–170. doi: 10.1016/0027-5107(96)00006-1. [DOI] [PubMed] [Google Scholar]
- 17.Hill R F. Location of genes controlling excision repair of UV damage and mutator activity in Escherichia coli WP2. Mutat Res. 1970;9:341–344. doi: 10.1016/0027-5107(70)90135-1. [DOI] [PubMed] [Google Scholar]
- 18.Ho C, Kulaeva O I, Levine A S, Woodgate R. A rapid method for cloning mutagenic DNA repair genes: isolation of umu-complementing genes from multidrug resistance plasmids R391, R446b, and R471a. J Bacteriol. 1993;175:5411–5419. doi: 10.1128/jb.175.17.5411-5419.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi M, Nagata K, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase: effect of base substitutions in the promoter −35 region on promoter strength. Nucleic Acids Res. 1990;18:7367–7372. doi: 10.1093/nar/18.24.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch W H, Kopsidas G, Meffle B, Levine A S, Woodgate R. Analysis of chimeric UmuC proteins: identification of regions in Salmonella typhimurium UmuC important for mutagenic activity. Mol Gen Genet. 1996;251:121–129. doi: 10.1007/BF02172909. [DOI] [PubMed] [Google Scholar]
- 21.Koch W H, Woodgate R. The SOS response. In: Nickoloff J A, Hoekstra M F, editors. DNA damage and repair: DNA repair in prokaryotes and lower eukaryotes. Totowa, N.J: Humana Press; 1998. pp. 107–134. [Google Scholar]
- 22.Kulaeva O I, Wootton J C, Levine A S, Woodgate R. Characterization of the umu-complementing operon from R391. J Bacteriol. 1995;177:2737–2743. doi: 10.1128/jb.177.10.2737-2743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence C W, Borden A, Woodgate R. Analysis of the mutagenic properties of the UmuDC, MucAB and RumAB proteins, using a site specific abasic lesion. Mol Gen Genet. 1996;251:493–498. doi: 10.1007/BF02172378. [DOI] [PubMed] [Google Scholar]
- 24.Lehnherr H, Guidolin A, Arber W. Bacteriophage P1 gene 10 encodes a trans-activating factor required for late gene expression. J Bacteriol. 1991;173:6438–6445. doi: 10.1128/jb.173.20.6438-6445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis L K, Harlow G R, Gregg-Jolly L A, Mount D W. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J Mol Biol. 1994;241:507–523. doi: 10.1006/jmbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- 26.McDonald J P, Frank E G, Levine A S, Woodgate R. Intermolecular cleavage of the UmuD-like mutagenesis proteins. Proc Natl Acad Sci USA. 1998;95:1478–1483. doi: 10.1073/pnas.95.4.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald J P, Peat T S, Levine A S, Woodgate R. Intermolecular cleavage by UmuD-like enzymes: identification of residues required for cleavage and substrate specificity. J Mol Biol. 1999;285:2199–2209. doi: 10.1006/jmbi.1998.2433. [DOI] [PubMed] [Google Scholar]
- 28.McLenigan M, Peat T S, Frank E G, McDonald J P, Gonzalez M, Levine A S, Hendrickson W A, Woodgate R. Novel Escherichia coli umuD′ mutants: structure function insights into SOS mutagenesis. J Bacteriol. 1998;180:4658–4666. doi: 10.1128/jb.180.17.4658-4666.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 30.Nohmi T, Battista J R, Dodson L A, Walker G C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci USA. 1988;85:1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paetzel M, Dalbey R E, Strynadka N C. Crystal structure of a bacterial signal peptidase in complex with a β-lactam inhibitor. Nature. 1998;396:186–190. doi: 10.1038/24196. [DOI] [PubMed] [Google Scholar]
- 32.Peat T S, Frank E G, McDonald J P, Levine A S, Woodgate R, Hendrickson W A. The UmuD′ protein filament and its potential role in damage induced mutagenesis. Structure. 1996;4:1401–1412. doi: 10.1016/s0969-2126(96)00148-7. [DOI] [PubMed] [Google Scholar]
- 33.Reuven N B, Tomer G, Livneh Z. The mutagenesis proteins UmuD′ and UmuC prevent lethal frameshifts while increasing base substitution mutations. Mol Cell. 1998;2:191–199. doi: 10.1016/s1097-2765(00)80129-x. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 35.Sedgwick S G, Ho C, Woodgate R. Mutagenic DNA repair in enterobacteria. J Bacteriol. 1991;173:5604–5611. doi: 10.1128/jb.173.18.5604-5611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedgwick S G, Lodwick D L, Doyle N, Crowne H M, Strike P. Functional complementation between chromosomal and plasmid mutagenic DNA repair genes. Mol Gen Genet. 1991;229:428–436. doi: 10.1007/BF00267466. [DOI] [PubMed] [Google Scholar]
- 37.Shinagawa H, Iwasaki H, Kato T, Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci USA. 1988;85:1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith C M, Koch W H, Franklin S B, Foster P L, Cebula T A, Eisenstadt E. Sequence analysis of the Salmonella typhimurium LT2 umuDC operon. J Bacteriol. 1990;172:4964–4978. doi: 10.1128/jb.172.9.4964-4978.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommer S, Bailone A, Devoret R. The appearance of the UmuD′C protein complex in Escherichia coli switches repair from homologous recombination to SOS mutagenesis. Mol Microbiol. 1993;10:963–971. doi: 10.1111/j.1365-2958.1993.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 40.Sommer S, Boudsocq F, Devoret R, Bailone A. Specific RecA amino acid changes affect RecA-UmuD′C interaction. Mol Microbiol. 1998;28:281–291. doi: 10.1046/j.1365-2958.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 41.Studier F W, Rosenberg A H, Dunn J J, Dubendorf J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 42.Sweasy J B, Witkin E M, Sinha N, Roegner-Maniscalco V. RecA protein of Escherichia coli has a third essential role in SOS mutator activity. J Bacteriol. 1990;172:3030–3036. doi: 10.1128/jb.172.6.3030-3036.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang M, Bruck I, Eritja R, Turner J, Frank E G, Woodgate R, O’Donnell M, Goodman M F. Biochemical basis of SOS-induced mutagenesis in Escherichia coli: reconstitution of in vitro lesion bypass dependent on the UmuD′2C mutagenic complex and RecA. Proc Natl Acad Sci USA. 1998;95:9755–9760. doi: 10.1073/pnas.95.17.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang M, Shen X, Frank E G, O’Donnell M, Woodgate R, Goodman M F. UmuD′2C is an error-prone DNA polymerase, Escherichia coli, DNA pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas S M, Crowne H M, Pidsley S C, Sedgwick S G. Structural characterization of the Salmonella typhimurium LT2 umu operon. J Bacteriol. 1990;172:4979–4987. doi: 10.1128/jb.172.9.4979-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witkin E M, Roegner-Maniscalco V, Sweasy J B, McCall J O. Recovery from ultraviolet light-inhibition of DNA synthesis requires umuDC gene products in recA718 mutant strains but not in recA+ strains of Escherichia coli. Proc Natl Acad Sci USA. 1987;84:6804–6809. doi: 10.1073/pnas.84.19.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodgate R. Construction of a umuDC operon substitution mutation in Escherichia coli. Mutat Res. 1992;281:221–225. doi: 10.1016/0165-7992(92)90012-7. [DOI] [PubMed] [Google Scholar]
- 48.Woodgate R, Levine A S. Damage inducible mutagenesis: recent insights into the activities of the Umu family of mutagenesis proteins. Cancer Surv. 1996;28:117–140. [PubMed] [Google Scholar]
- 49.Woodgate R, Singh M, Kulaeva O I, Frank E G, Levine A S, Koch W H. Isolation and characterization of novel plasmid-encoded umuC mutants. J Bacteriol. 1994;176:5011–5021. doi: 10.1128/jb.176.16.5011-5021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]