Abstract
The temperate phage TPW22, induced from Lactococcus lactis subsp. cremoris W22, and the evolutionarily interesting integrase of this phage were characterized. Phage TPW22 was propagated lytically on L. lactis subsp. cremoris 3107, which could also be lysogenized by site-specific integration. The attachment site (attP), 5′-TAAGGCGACGGTCG-3′, of phage TPW22 was present on a 7.5-kb EcoRI fragment, a 3.4-kb EcoRI-HindIII fragment of which was sequenced. Sequence information revealed the presence of an integrase gene (int). The deduced amino acid sequence showed 42 and 28% identity with integrases of streptococcal and lactococcal phages, respectively. The identities with these integrase-encoding genes were 52 and 45%, respectively, at the nucleotide level. This could indicate horizontal gene transfer. A stable integration vector containing attP and int was constructed, and integration in L. lactis subsp. cremoris MG1363 was obtained. The existence of an exchangeable lactococcal phage integration module was suggested. The proposed module covers the phage attachment site, the integrase gene, and surrounding factor-independent terminator structures. The phages φLC3, TP901-1, and TPW22 all have different versions of this module. Phylogenetically, the TPW22 Int links the φLC3 lactococcal integrase with known Streptococcus thermophilus integrases.
The interactions of lactococcal phages with bacterial hosts have been subjected to studies not only because of the ability of bacteriophages to disturb fermentation of industrial products but also because of the potential use of phage genetic elements in the study and genetic modification of lactococcal bacteria.
Much of this work has been inspired by the findings and knowledge achieved from work with bacteriophage λ and its host, Escherichia coli. A model of the insertion of the bacteriophage λ genome into the chromosome of the host bacterium through site-specific recombination between the phage attachment site (attP) and the bacterial attachment site (attB) has been suggested (19). It has been shown that the insertion is mediated by an integrase protein (Int) encoded by bacteriophage λ (29, 67) as well as an integration host factor encoded by the host bacterium (41, 51). The mechanism of this insertion, as well as the excision of bacteriophage λ, is complicated but well characterized (for a review, see reference 45).
Both the integrase-encoding genes (int) and the phage attachment sites have been identified and sequenced in five separate temperate lactococcal phages. The sequence information on the int gene of the φLC3 phage was the first published and revealed relationship with the Int family of site-specific recombinases (5, 47). Later, the int genes of Tuc2009, r1t, and BK5-T were also reported (11, 63, 64). The integrase proteins deduced from these sequences are found to be almost identical with φLC3 Int and could be called the φLC3 type of integrases. In addition, the cores of the attachment sites in these phages are identical. The Int protein of the lactococcal phage TP901-1 shows features relating to the resolvase-integrase family of site-specific recombinases (23, 58).
Botstein (10) has proposed that the evolution of lambdoid phages in particular happens by exchange of genes organized in functional modules with homologous areas interspersed between the genes as recombination sites. Examples supporting this kind of recombination in lambdoid phages have been summarized by Campbell (20). In temperate lactococcal phages, the application of this model has been validated by the observation of different codon usage in lysin and holin genes of the phages φLC3 and Tuc2009 (4, 9); by the presence of moderate homology among genes of temperate Lactococcus lactis, Streptococcus thermophilus, and Lactobacillus phages (17, 24, 26, 42, 43, 52, 60); and by the proposal of a conserved integration cassette of the phages BK5-T, φLC3, Tuc2009, and r1t (11, 12, 64). Additional examples supporting the Botstein evolutionary theory have not been presented for temperate lactococcal phages, although it is well established that lactococcal phages are able to evolve by acquisition of host chromosomal DNA (31, 50).
In this paper we report on the characterization of a temperate phage, TPW22, from a bacterial isolate, Lactococcus lactis subsp. cremoris W22, isolated from the mixed starter culture TK5 (39). The integrase of this phage is identified and characterized.
MATERIALS AND METHODS
Bacteria, bacteriophages, and plasmids.
The L. lactis subsp. cremoris strains, the E. coli strain, plasmids, and bacteriophages used in this study are listed in Table 1. Lactobacillus strains were grown at 30°C in M17 medium (Oxoid Ltd.) with 0.5% glucose. When the phages were propagated, 5 mM CaCl2 and 20 mM MgCl2 were added to the medium. The E. coli transformants with plasmids containing the 7.5-kb EcoRI fragment were grown at 27°C, and other E. coli transformants were grown at 37°C in Luria-Bertani broth (Difco Laboratories) as described by Sambrook et al. (56).
TABLE 1.
Bacterial strains, plasmids, and bacteriophages
| Strain, plasmid, or phage | Relevant characteristicsc | Reference or source |
|---|---|---|
| Strains | ||
| L. lactis subsp. cremoris | ||
| 3107 | Indicator strain for the phages TPW22 and TP901-1; lysogenic for phage TP3107 | This study, 13 |
| AM1 | Indicator strain for phage r1t | 49 |
| W22, | Strain from the TK5 starter culture | 38 |
| 901-1 | Lysogenic for phage TP901-1 | 13 |
| 3107L1-9 | 3107::TPW22 | This study |
| MG1363 | Plasmid-free strain | 28 |
| MG1363I1.6 | MG1363::pAP2 erm | This study |
| E. coli XL1-Blue MRF′ | Δ(mcrA)183 Δ(mcrCB-hsdSMA-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tetr)] | Stratagene, La Jolla, Calif. |
| Plasmids | ||
| pGEM-3Zf(−) | lacZ bla | Promega, Madison, Wis. |
| pG7f5 | pGEM-7Zf(+)::1.8-kb EcoRI TP901-1 bla | 22 |
| pSJ1327 | A pUC19 derivative containing cata | S. T. Jørgensen, Novo Nordisk, Denmark |
| pAG23 | pSKII-cm1::pJW563 2.3-kb EcoRI cat | 31 |
| pSMC | pGEM-3Zf(−)::1.1-kb cat from pSJ1327 in the NdeI site bla cat | This study |
| pSMAC | pSMC::pAG23 2,010-bp EcoRI-SacI in the HincII site bla cat | This study |
| pAP1 | pSMC::7.5-kb EcoRI TPW22 bla cat | This study |
| pAB201 | pGEM-3Zf(−)::attP-int TP901-1 bla | 15 |
| pLEB22 | pUC6S::1.2-kb ClaI-PdeI with ermLb from pERY1 in the EcoRV site bla erm | 55 |
| pG3E | pGEM-3Zf(−)::1.2-kb NotI-BamHI with ermLb from pLEB22 in the NdeI site bla erm | This study |
| pAP2 | pG3E::1.6-kb attP-int TPW22 bla erm | This study |
| pAP3 | pAP2 ΔPstI attP bla erm | This study |
| pAP4 | pSMC::2.1-kb EcoRI-PstI TPW22 bla erm | This study |
| Bacteriophages | ||
| TPW22 | L. lactis subsp. cremoris 3107 | This study |
| r1t | L. lactis subsp. cremoris AM1 | 49 |
| φLC3 | L. lactis subsp. cremoris IMN-C18 | 48 |
| TP901-1 | L. lactis subsp. cremoris 3107 | 13 |
| P335 | L. lactis subsp. lactis biovar. diacetylactis F7/2 | 13 |
The cat resistance gene of pSJ1327 originates from pC194 (33).
ermL sequence information was obtained from Morten Skaugen, Biotechnology Department, Agricultural University of Norway, Ås, Norway.
For bacteriophages, strain propagated on.
Induction of bacteriophage TPW22 was achieved by mixing 1 volume of L. lactis subsp. cremoris W22 culture at an optical density of 0.3 at 600 nm with 2 volumes of M17 medium containing 0.5% glucose and mitomycin C to a final concentration of 3 μg per ml and incubation at 30°C overnight. Phages liberated from L. lactis subsp. cremoris W22 were propagated lytically on the host strain L. lactis subsp. cremoris 3107. UV induction of phage TP3107 and phage TP901-1 from their respective hosts was performed as described for phage TP901-1 (22). Propagation of phage φLC3 was performed as suggested by Lillehaug et al. (48). Phages from culture supernatants were precipitated by treatment with 1 M NaCl and 10% (wt/vol) polyethylene glycol 6000 and further purified by two subsequent CsCl step gradients as described for bacteriophage λ (56). Strains of L. lactis subsp. cremoris 3107 lysogenized with TPW22 were obtained as described for the isolation of TP901-1 lysogenes (22). Phage titers were determined as described by Terzaghi and Sandine (61). Agar-agar (Merck KGaA, Darmstadt, Germany) was used at 1.5% (wt/vol) in solid media and 0.5% (wt/vol) in GM17 top layer.
Integration stability was tested by growing integrants for more than 100 generations without erythromycin, and 100 single colonies from each vector integrant were restreaked on plates with and without erythromycin.
Electron microscopy.
Twenty microliters of a TPW22 phage stock containing 1010 PFU/ml was placed on a carbon-coated grid. After 5 min the grid was negatively stained in 2% (wt/vol) uranyl acetate and examined in a Philips CM 100 transmission electron microscope.
SDS-PAGE.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed on dialyzed stocks of the phages TPW22 and φLC3, which were boiled for 10 min in SDS-PAGE sample buffer (44) before electrophoresis in a Protean II xi vertical electrophoresis cell (Bio-Rad Laboratories, Richmond, Calif.). Phage samples of 109 to 1010 PFU were loaded in each slot. Gels containing 12.5% (wt/vol) polyacrylamide (National Diagnostics) were silver stained as described by Sambrook et al. (56).
DNA preparation.
Phage DNA was extracted from purified phages as described for bacteriophage λ (56). Plasmid DNA from E. coli was purified according to the instructions of Qiagen Ltd. (Hilden, Germany) with or without use of the column. Chromosomal DNA, including plasmid DNA, was extracted from Lactobacillus strains as described by Johansen and Kibenich (36) by using 10 or 200 ml of culture at an optical density of approximately 1 at 600 nm and collected by centrifugation at 5,000 × g for 15 min. DNA preparations were stored at 5°C.
Recombinant DNA techniques.
DNA restriction fragments for cloning and hybridization were isolated from 0.7% low-melting-point SeaKem GTG agarose (FMC, Rockland, Ohio) by extraction with phenol and chloroform as described by Sambrook et al. (56). PCR products for sequencing and cloning were purified with the QIAquick PCR purification kit (Qiagen Ltd.). Restriction endonuclease enzymes (New England Biolabs, Beverly, Mass.), T4 DNA ligase (U.S. Biochemical Corp., Cleveland, Ohio), shrimp alkaline phosphatase (Amersham Pharmacia Biotech, Uppsala, Sweden), and Klenow DNA polymerase (Boehringer Mannheim GmbH, Mannheim, Germany) were used as recommended by the suppliers.
Southern hybridization.
Endonuclease-digested DNA was separated on a 0.6% agarose gel and transferred to Hybond N+ membranes (Amersham Pharmacia Biotech) by vacuum blotting as described by the supplier of the vacuum blotter (Amersham Pharmacia Biotech). DNA probes were labeled with the ECL direct nucleic acid labeling and detection system (Amersham Pharmacia Biotech). Hybridization and detection were performed as suggested by the supplier, corresponding to an estimated signal identity of 95 and 77% at 42°C (high stringency) or 25°C (low stringency) for hybridization and for washing with the primary wash buffer containing 6 M urea and 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The formulas for calculating melting temperature and stringency are given by Sambrook et al. (56), and the information from the supplier of the ECL kit (Boehringer Mannheim), that addition of 6 M urea is equivalent to 50% formamide, was used for the calculation.
A phage TP901-1 integrase-specific probe was produced from pAB201 plasmid DNA obtained from Anne Breüner, Department of Microbiology, Technical University of Denmark, Lyngby, Denmark (15). The insert of this plasmid spanning from bp 1219 to 2702 of the published sequence (23) was purified after digestion with BamHI and SalI. A fragment of the integrase-encoding region of phage φLC3 covering bp 469 to 951 of the published sequence was PCR amplified with the primers G-210387 and C-262804 (48). The primers were kindly supplied by Dag Lillehaug, Department of Biotechnology Sciences, Agricultural University of Norway, Ås, Norway.
Transformation and selection.
E. coli XLI-Blue MRF′ was made electrocompetent and transformed with a Gene Pulser apparatus as recommended by the supplier (Bio-Rad Laboratories) (8). This equipment was also used for electrotransformation of L. lactis subsp. cremoris as described by Holo and Nes (32). However, only 0.2 M sucrose was used for osmotic stabilization of L. lactis subsp. cremoris 3107. E. coli transformants were selected on Luria-Bertani plates containing 100 μg of ampicillin per ml or 10 μg of chloramphenicol per ml, 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml, and 1 mM isopropyl-β-d-thiogalactopyranoside. When introducing the ermL cassette, selection in E. coli was performed with 250 μg of erythromycin per ml. However, only 150 μg of erythromycin per ml was used for selection of integration vectors. Lactococcal integrants were selected on 2 μg of erythromycin per ml. L. lactis subsp. cremoris 3107 transformants with pSMAC and pSMC and their derivatives were selected with 4 μg of chloramphenicol per ml.
Construction of vectors.
The 7.5-kb EcoRI and the 2.1-kb EcoRI-PstI fragments from digested phage TPW22 DNA were cloned in the pSMC vector to construct the plasmids pAP1 and pAP4. The plasmid pSMC is a pGEM-3Zf(−) vector with a cat gene introduced at the NdeI site and functional lacZ screening ability (Table 1). The plasmid pSMAC was obtained by introducing the 2.0-kb EcoRI-SacI fragment from pAG23 containing the lactococcal replicon repA563 (30) into Klenow DNA polymerase-treated HincII-digested pSMC.
In addition, vectors with the ermL gene from pLEB22 (55) were constructed. A 1.2-kb NotI-BamHI fragment carrying ermL was introduced into the Klenow DNA polymerase-treated NdeI site of pGEM-3Zf(−) to create pG3E. The ermL gene originates from Lactobacillus reuteri and is carried by the plasmid pERY1 (6).
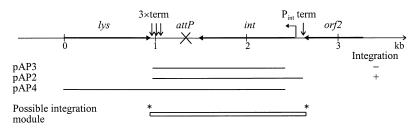
In order to define the region containing the functional integrase, a 1.6-kb fragment was PCR amplified with Vent DNA polymerase (New England Biolabs). The PCR product, 1.6-kb attP-int (Table 2), extending from the second base pair in the stop codon of the putative lysin gene to 73 bp upstream from the putative int gene of phage TPW22, was purified, digested with BamHI and KpnI, and repurified from an agarose gel. The fragment was ligated into the pG3E vector digested with the same enzymes, and the construct obtained was named pAP2. In order to examine whether the presence of attP alone could mediate integration, a 206-bp deletion of the Int coding region was made in pAP2. The PstI site in the multiple cloning linker and the PstI site located 119 bp upstream from the start codon of the integrase gene (int) were joined by religation in the construction named pAP3.
TABLE 2.
Nucleotide sequences of PCR primers
| Set of primers | Name | Sequence | Name | Sequence | Amplified region |
|---|---|---|---|---|---|
| 1 | G-210387 | 5′-AATGGAAATGATAAAGCCG-3′ | C-2628045 | 5′-GTAAGTGGAGTAGAAATC-3′ | 473 bp of φLC3 int |
| 2 | APattLFP | 5′-CGTTAACTCACGATAAACGTTG-3′ | APattRRP | 5′-CTCACCAGCTAAAAGAGATGG-3′ | attP |
| 3 | APattLFP | 5′-CGTTAACTCACGATAAACGTTG-3′ | APattBLYP | 5′-TCCTCTACAGACGGCTCATC-3′ | attL |
| 4 | APattBRYP | 5′-TTTATGTTGTCAGCAGGGAC-3′ | APattRRP | 5′-CTCACCAGCTAAAAGAGATGG-3′ | attR |
| 5 | APattBLYP | 5′-TCCTCTACAGACGGCTCATC-3′ | APattBRYP | 5′-TTTATGTTGTCAGCAGGGAC-3′ | attB |
| 6 | AP3Kpn | 5′-TGGGGTACCAATAATCATGCCTGGCTTCG-3′ | AP1Bam | 5′-TCGGGATCCGGTAAAAATTGACAAAACAGTCAAGTC-3′ | attP-int |
DNA sequencing.
The nucleotide sequence of the integrase region was determined by sequencing on the insert of plasmid pAP1 and its derivatives. All sequence reactions were conducted with the cycle-sequencing dideoxynucleotide chain termination method (kit RPN2436; Amersham Pharmacia Biotech) with 0.5 to 2 μg of DNA and 5 pmol of indodicarbocyanine amidite-labeled primers deduced from known sequences. The labeled-cycle PCR products were separated in a gel containing 6% (wt/vol) Long Ranger acrylamide (FMC), 7 M urea (ICN Biochemicals Inc., Aurora, Ohio), and 1.2× TBE (108 mM Tris-borate, 2.4 mM EDTA). The gel was run on an ALFexpress DNA sequencer (Amersham Pharmacia Biotech) with 0.6× TBE as a running buffer.
Sequence assembly and further analysis of the sequences were performed with the Wisconsin Package versions 8.1 and 9.1 (Genetics Computer Group, Inc., Madison, Wis.). Putative prokaryotic factor-independent RNA polymerase terminator sequences were predicted with the program Terminator (14). A helix-turn-helix motif was found according to the method of Dodd and Egan (25) with the program HelixTurnHelix. Database searches were performed by using the FASTA (54) and Gapped BLAST (2) programs. The sequence data was matched against the following databases: SWISSPROT (release 37.0), GenBank (release 111.0), and EMBL (release 58.0). The Gapped BLAST searches were performed at the National Center for Biotechnology Information with the BLAST network service. The phenogram was created with the CLUSTAL W Multiple Sequence Alignment Program (62) and the Phylip Phylogeny Inference Package version 3.5C by Joseph Felsenstein. The tree was estimated by the unweighted pair group method with arithmetic averages (59) with the programs PROTDIST and NEIGHBOR of the Phylip package, and the program DRAWGRAM was used to draw the phenogram.
Isolation of attL, attR, and attB DNA templates.
Inverse PCR was used to obtain sequence templates containing the left and right attachment sites (attL and attR, respectively) of the L. lactis subsp. cremoris 3107 lysogenized with TPW22. Fragments of PstI-digested L. lactis subsp. cremoris 3107 TPW22-lysogenized chromosomal DNA ranging in size from 2.3 to 2.7 and from 3.7 to 4.2 kb were extracted from an agarose gel after electrophoresis. The fragments were self-ligated and used for inverse PCR with primers resulting in an 1.5-kb fragment containing attL and a 3.0-kb fragment containing attR (Table 2). Primers for amplification of attB were designed from the sequence information obtained from attL and attR inverse-PCR products. The inverse PCR and other PCRs were carried out with the Expand High Fidelity PCR system (Boehringer Mannheim GmbH) or the GeneAmp PCR reagent kit with AmpliTaq DNA polymerase (Perkin-Elmer Cetus), respectively, as recommended by the suppliers. All custom-made primers were delivered from DNA Technology Aps (Århus, Denmark).
Nucleotide sequence accession numbers.
The nucleotide sequence data of 3.4 kb of the phage TPW22 genome and the attB sequence of L. lactis subsp. cremoris 3107 have been deposited in GenBank under accession no. AF066865 and AF065985, respectively.
RESULTS
Characterization of phage TPW22.
Induction experiments showed that a temperate phage, TPW22, could be induced from the bacterial isolate L. lactis subsp. cremoris W22, isolated from the mixed cheddar starter culture TK5 (38). Induction of phage TPW22 was tested by treatment with UV light, heat, and mitomycin C addition. The amount of TPW22 spontaneously released from L. lactis subsp. cremoris W22 was 5 · 104 PFU/ml. A maximum titer of 5 · 106 PFU/ml was reached with the addition of mitomycin C. By further propagation on L. lactis subsp. cremoris 3107, a titer of 1010 PFU/ml could be obtained.
After induction and lytic propagation on L. lactis subsp. cremoris 3107, TPW22 could still lysogenize the indicator strain, L. lactis subsp. cremoris 3107. Isolation of TPW22-lysogenic L. lactis subsp. cremoris 3107 strains confirmed that phage TPW22 has a lysogenic life cycle. The lysogens showed immunity to infection with both the lytically propagated TPW22 phages and the mitomycin C-induced TPW22 phages (data not shown). The spontaneous release was found to be 105 to 106 PFU/ml in an overnight culture.
Electron microscopy showed that phage TPW22 is a small isometric-headed phage with a head diameter of 54 nm and a tail length of 128 nm (data not shown).
SDS-PAGE analysis revealed that eight of the proteins of phage TPW22 migrated to positions similar to those of proteins of phage φLC3. The molecular masses of these proteins were estimated to be 60, 50, 34.5, 26, 16.5, 16, 13.5 (48), and 15 kDa. Only small migration differences were seen between the phage φLC3 proteins estimated to have molecular masses of 82.5 and 42.5 kDa and the corresponding phage TPW22 proteins estimated to have molecular masses of 72 and 44 kDa (data not shown).
When AccI-digested phage TPW22 genomic DNA was heat treated at 65°C for 5 min, a 12-kb fragment was dissociated into two new fragments of 4.8 and 6.8 kb, respectively (data not shown). These fragments could reanneal upon slow cooling. Digestion with other restriction enzymes confirmed that phage TPW22 has a cohesive site (cos) (data not shown). By adding up the fragment sizes of five different endonuclease digestions of the phage TPW22 genomic DNA, the molecular size of the double-stranded phage genome was found to be approximately 35 kb (data not shown).
Hybridization experiments with EcoRI-digested phage TPW22 DNA as a probe resulted in extensive signals with the EcoRI-digested DNA of the phages P335, φLC3, and r1t under high-stringency conditions (data not shown). Phage TPW22 DNA gave signals with all EcoRI fragments of phage φLC3 DNA and phage r1t DNA. However, three of the EcoRI fragments of phage r1t (13,378, 1,740, and 1,074 bp) gave weak signals with the probe. The 13,378-bp fragment encodes the holin, the lysin, the integrase, the repressor, and putative gene products involved in DNA replication. The 1,740-bp fragment encodes a putative dUTPase, and the 1,074-bp fragment encompasses a part of orf42. The fragments with high homology contain genes encoding structural proteins (64).
Hybridization studies with DNA probes covering the integrase of phage TP901-1 and a part of the integrase of phage φLC3 to TPW22 DNA revealed no DNA homology with these phage integrases when conditions corresponding to an estimated 77% identity were used. This indicated the presence of an integrase of phage TPW22 with low or no homology to the previously identified lactococcal phage integrases belonging to two different families of site-specific recombinases.
Series of hybridization studies localized attP on a 7.5-kb EcoRI fragment. This fragment was cloned into pSMC to generate pAP1.
Sequence analysis.
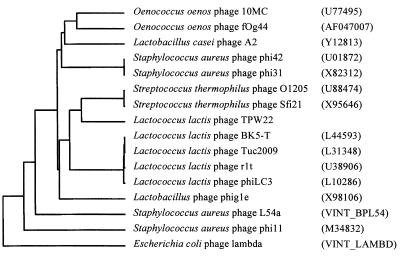
The 3.4-kb HindIII-EcoRI insert of plasmid pAP1 was sequenced. Analysis of the sequence revealed a putative integrase gene, int, an open reading frame (ORF), orf2, and part of a putative lysin gene, lys (Fig. 1). A putative promoter sequence and a putative factor-independent terminator structure were positioned between lys and int. A DNA sequence alignment of the int genes of the phages TPW22, φO1205, and r1t showed a DNA identity of 51% between int of phage TPW22 and int of the S. thermophilus phage φO1205 and 44% identity between int of phage TPW22 and int of the lactococcal phage r1t (data not shown). The deduced protein of int showed 41% identity with the three almost-identical integrases of the S. thermophilus phages TP-J34, φSfi21, and φO1205 (17, 52, 60) and 28% identity with the integrases of the L. lactis phages of the φLC3 type (11, 48, 63, 64) but also 25 to 28% identity with other phage integrases of gram-positive bacteria, such as the integrases of the Lactobacillus phages φg1e and A2 and of the Staphylococcus aureus phages φ42 and φ11 (3, 21, 40, 66). An alignment of TPW22 Int and these integrases revealed a high degree of identity in boxes 1 and 2, which are conserved regions among the integrases of the Int family (1, 5, 53) (data not shown). The conserved amino acids Arg-212 (1), His-308, Arg-311, and Tyr-342 (5) of the site-specific integrase family (the numbers refer to λ Int) were present in the phage TPW22 integrase along with a weak helix-turn-helix motif located between residues 67 and 88. A phylogenetic-tree analysis with site-specific integrases of closely related phages of gram-positive bacteria and of bacteriophage λ indicated that TPW22 Int belongs to the same branch of integrases as those of S. thermophilus phages instead of lactococcal phage integrases (Fig. 2). Thus, the TPW22 integrase was more closely related to the three streptococcal phage integrases than to any of the known lactococcal integrases of the Int family of site-specific integrases.
FIG. 1.
Overview of the 3.4-kb sequenced region stretching from the EcoRI site to the HindIII site positioned 3.4 kb downstream. The map includes the position of attP, putative genes drawn as arrows, four putative factor-independent terminator structures marked “term,” and a putative promoter called Pint. The clones used in integration experiments are indicated as lines. +, ability to integrate into L. lactis subsp. cremoris MG1363; −, lack of ability to integrate. The extension of the proposed integration module is indicated by a box, with the transition points shown as asterisks.
FIG. 2.
Phenogram of the TPW22 integrase, integrases of closely related bacteriophages of gram-positive bacteria, and the integrase of bacteriophage λ. The GenBank accession numbers of the sequences are given in parentheses.
The 65 C-terminal residues of the gene product of orf2 of TPW22 showed 69% identity to the orf2 gene product of S. thermophilus phage φO1205 (60). The deduced protein sequence of lys showed 95% identity to the 314 C-terminally positioned amino acids of the lysin genes of the lactococcal phages Tuc2009 and φLC3 (4, 9).
Site-specific phage integration.
Hybridization with the insert of the plasmid pAP3 (shown in Fig. 1) as a probe was performed in order to identify fragments containing attL and attR sequences in EcoRI-digested chromosomal DNA of TPW22-lysogenic L. lactis subsp. cremoris 3107 (Fig. 3, lanes 1 to 5). When the phage genome was integrated on the bacterial chromosome of L. lactis subsp. cremoris 3107, a 7.5-kb EcoRI fragment was divided at the attP site into two fragments associated with chromosomal fragments. Two junction fragments of 18 and 3.2 kb were detected after digestion with EcoRI, as both fragments gave a signal with labeled TPW22 DNA, suggesting that these two fragments contain the left and right attachment sites (attL and attR). A weak hybridization signal corresponding to the 7.5-kb EcoRI phage genome fragment was also present in the chromosomal DNA preparation (Fig. 3, lanes 2 and 3). The signal from TPW22-lysogenic L. lactis subsp. cremoris 3107 was weaker than the signal from L. lactis subsp. cremoris W22, and at the same time, L. lactis subsp. cremoris W22 has a less efficient spontaneous phage release (100 times lower) than TPW22-lysogenic L. lactis subsp. cremoris 3107. Therefore, it is believed that this signal is caused mainly by the presence of intracellular TPW22 DNA existing either as unpackaged phage DNA or as phages rather than extracellular TPW22 DNA.
FIG. 3.
Identification of the TPW22 phage attachment site (attP) by Southern blot hybridization. Lane 1, TPW22 phage genomic DNA digested with EcoRI. Lanes 2 to 4, EcoRI-digested chromosomal DNA of L. lactis subsp. cremoris W22, L. lactis subsp. cremoris 3107L8, and L. lactis subsp. cremoris 3107, respectively. Lane 5, TP3107 phage DNA digested with PstI. Lane 6, TPW22 phage genomic DNA digested with PstI. Lanes 7 to 8, PstI-digested chromosomal DNA of L. lactis subsp. cremoris W22 and L. lactis subsp. cremoris 3107L8. The DNA was hybridized with the labeled pAP3 insert containing attP of phage TPW22 as a probe. The migration positions and molecular sizes in kilobases of the fragments containing the left attachment site (attL), the right attachment site (attR), and the phage attachment site (attP) are marked. The autoradiograph was scanned with Hewlett-Packard DeskScan II, and labels were added in Adobe Photoshop 4.0.
PstI-digested chromosomal DNA from nine independently isolated TPW22-lysogenic L. lactis subsp. cremoris 3107 strains, 3107L1 to -9, showed identical hybridization signals when probed with the insert of pAP3. The probe gave a signal with a 3.5-kb PstI fragment from TPW22 phage DNA and with 2.5 and 4.0-kb PstI fragments of phage TPW22-lysogenic L. lactis subsp. cremoris 3107, which was expected to contain attL and attR, respectively (Fig. 3, lanes 6 and 8). The result from one of nine identical integration analyses is presented in Fig. 3, lane 8. A signal was also obtained with a 3.5-kb fragment from PstI-digested phage DNA in the lysogenic hosts (Fig. 3, lane 7).
Characterization of attachment sites.
The 440-bp stretch between the stop codon of the integrase gene, int, and the stop codon of the putative lysin gene, lys, was predicted to be noncoding with an A+T content of 64.6%, which corresponds to the frequency normally reported for L. lactis (57). This region was expected to contain attP, as indicated by hybridization experiments. Also, this region contained several direct and inverted repeats (data not shown). Amplification products of 1.5 and 3.0 kb were obtained by inverse PCR with a 2.5-kb PstI fragment containing attL and a 4.0-kb PstI fragment containing attR, respectively. The PstI fragments were isolated from the chromosome of TPW22-lysogenic L. lactis subsp. cremoris 3107L8. The sequence information obtained from the inverse-PCR products, was used for the design of primers for amplification of attL, attR, and attB (Table 2). Amplification of attL, attR, and attB resulted in PCR products of 479, 500, and 535 bp, respectively. When sequence information from the amplification products was aligned, a 14-bp core region common to the chromosomal and phage genomic sequences was found to be 5′-TAAGGCGACGGTCG-3′ (Fig. 4B). In this common core, DNA strand exchange is expected to occur during phage genome integration into the bacterial chromosome. Identical attB, attL, and attR sequences were found in TPW22-lysogenic L. lactis subsp. cremoris 3107 and in L. lactis subsp. cremoris W22, indicating that the integration takes place in the same major attachment site in the two strains. Sequences of attL and attB indicated that the core was located 133 bp downstream from the start codon of a putative gene (Fig. 4A). The deduced amino acid sequence showed a high degree of homology with several polydeoxyribonucleotide synthetases (DNA ligases). The highest degree of homology (52% identity in a stretch of 40 amino acids) was obtained with the N terminus of the Haemophilus influenzae DNA ligase (EC 6.5.1.2) (27). An inverted repeat of 16 bp was located between positions 216 and 231 and positions 261 and 246 in the sequence of the attB region (Fig. 4A).
FIG. 4.
(A) Schematic representation of the bacterial chromosomal attachment site (attB) of L. lactis subsp. cremoris 3107 containing part of a putative gene. The deduced gene product has homology to DNA ligases. Inverted repeats are drawn as thin arrows, and the common core is indicated as a boxed region. The nucleotide positions of the sequenced part of the ORF and of the common core are indicated as numbers. (B) Aligned nucleotide sequences of the regions containing attP, attL, attR, and attB. Phage TPW22 sequences are underlined, while the L. lactis subsp. cremoris 3107 chromosomal sequences are double underlined.
Site-specific vector integrations.
The vectors pAP3 and pAP2 (Fig. 1), with the ermL gene from pLEB22 (55), were constructed and used in integration experiments in order to study the ability of attP and the int gene to mediate integration. These vectors possess an erythromycin resistance selection marker but lack gram-positive replication ability. The vector pAP2, containing attP and int, integrated in L. lactis subsp. cremoris MG1363. However, the frequency was only 102 erythromycin-resistant transformants per μg of plasmid DNA. No integrants could be obtained with vector pAP3, a construction with a 206-bp deletion in the int gene. By transforming with pSMAC, which is able to replicate in L. lactis subsp. cremoris MG1363, a frequency of 107 transformants per μg of DNA was reached.
Sequence analysis on amplified PCR products from vector integrants confirmed that the site of vector integration in L. lactis subsp. cremoris MG1363 was identical to that of phage genomic integration in L. lactis subsp. cremoris 3107. In addition, site-specific vector integration in L. lactis subsp. cremoris MG1363 was confirmed in a hybridization experiment with EcoRI-digested chromosomal DNA from 20 individual integrants (data not shown).
Stabilities of the integrants.
The stabilities of the integrants obtained with pAP2 were tested, and comparable colony numbers were obtained with and without erythromycin after 100 generations. All 100 investigated colonies of each integrant were still erythromycin resistant upon restreaking.
Identification of putative transition.
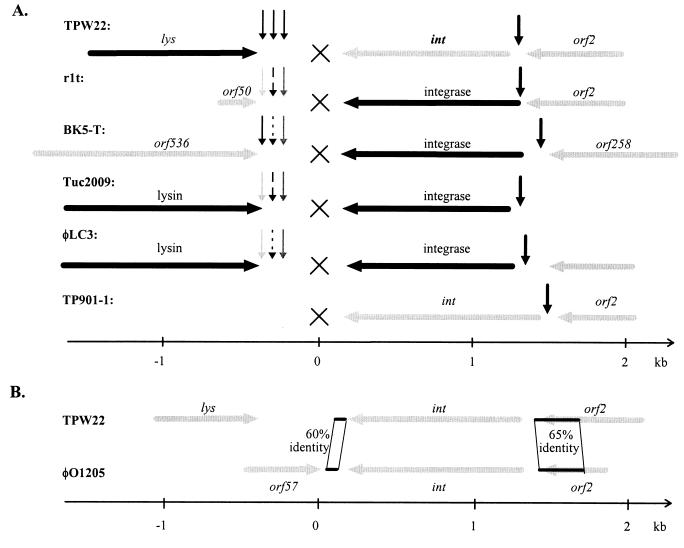
A nucleotide alignment of the phage attachment region including 50 bp of the lysin genes and 50 bp of the int genes of the lactococcal phages TPW22, BK5-T, φLC3, r1t, and Tuc2009 was performed, and an overview of the identity in that region is presented in Fig. 5A. A transition point between heterologous and homologous DNA was found at the 3′ end of the lysin gene of phage TPW22 and the lysin genes of the phages Tuc2009 and φLC3. Although these two phages have nearly identical lysin, holin, and int genes, the sequences between the first and the second putative terminator structures located downstream from the lysin genes are different.
FIG. 5.
(A) An overview of the genome organization in the region surrounding int of the temperate lactococcal phages TPW22, r1t (GenBank accession no. U38906) (64), BK5-T (L44593) (11), Tuc2009 (L31348) (63), φLC3 (U04309 and L10286) (9, 47), and TP901-1 (Y14232) (23). ORFs or parts of ORFs with gene products showing more than 95% identity are indicated as solid horizontal arrows, and the conserved gene products are stated above. Other ORFs are presented as shaded horizontal arrows with the name stated. Vertical arrows represent conserved putative terminator structures. Arrows of the same type (except the vertical arrows of phage TPW22) indicate a high degree of homology between the putative terminator structures. The first base pair in the attachment core is 1 and is indicated as a cross. (B) Overview of homologies in the region around int of the phages TPW22 and φO1205. ORFs are indicated as shaded arrows. Homologous areas upstream and downstream of int are drawn as black lines. Homologous areas are connected with thin lines, and the identity is shown.
Nearly identical sequences of the phages BK5-T, φLC3, r1t, and Tuc2009 start in the third putative terminator structure located between the lysin gene and the common core. The identity continues through the int gene and comes to an end in a putative terminator structure located between int and orf2. This putative terminator structure is nearly conserved in all the temperate lactococcal phages BK5-T, φLC3, r1t, and Tuc2009 and even in the phages TPW22, TP901-1, and φO1205 (Fig. 6).
FIG. 6.
The conserved putative factor-independent terminator structure between int and orf2 of the lactococcal phages φLC3 (GenBank accession no. L24560) (47), r1t (U38906) (64), Tuc2009 (L31348) (63), TP901-1 (X85213) (23), BK5-T (L44593) (11), and TPW22 and of the S. thermophilus phage φO1205 (U88974) (60). Residues are shaded if four or more are identical, and the consensus sequence is shown at the bottom.
Southern hybridization experiments indicated a high degree of homology between the phages TP901-1 and φLC3 in the lysis region (46). In order to find putative transition sites between homologous and heterologous DNAs of TPW22 and TP901-1, we investigated whether the C-terminal end of the putative lysin gene of phage TPW22 has a high degree of homology with TP901-1 phage DNA. A probe containing the 2.3-kb EcoRI-PstI fragment from pAP4 (Fig. 1) was hybridized to EcoRI-digested chromosomal DNA of phage TP901-1 in a Southern hybridization experiment. A signal was obtained, but not when DNA of pAP3 was used as a probe (data not shown). The 2.3-kb EcoRI-PstI fragment contains 945 bp of the 3′ end of lys, which is not present on the insert of pAP3 (Fig. 1). The phage attachment sites of TP901-1 and TPW22 were found to be different (22), so transition from homologous to heterologous DNA in phage TP901-1 and TPW22 could be at a point in the lysin gene or just downstream from the lysin gene.
FASTA searches with the nucleotide sequences flanking TPW22 int revealed a sequence of 280 bp with 65% identity between the phages TPW22 and φO1205. In both phages this homologous sequence starts approximately 100 bp upstream from int in the putative terminator structure and extends about 260 bp into the following ORF (Fig. 5B and 6). Twenty-five base pairs downstream from TPW22 int a sequence of 78 bp shows 60% identity with a sequence 85 bp downstream from int of phage φO1205 (Fig. 5B).
DISCUSSION
We have identified the integrase of the lactococcal temperate phage TPW22 and found it to be distinct from the other lactococcal integrases of the Int family.
Extensive hybridization signals from TPW22 DNA were obtained with probes of the lytic-type phage P335 and those of the phages φLC3 and r1t, both lysogenic members of the P335 phage species (13, 35, 46) (data not shown). The head and tail structures of phage TPW22 (data not shown) are the same size as those reported for phage r1t (34) and slightly smaller than the corresponding structures of phage φLC3 (48). The presence of similar-molecular-size proteins in the virion structures of the phages φLC3, r1t (37, 48, 64), and TPW22, the extensive hybridization signals between the DNAs of these phages, and the similar morphologies strongly suggest that the structural elements encoded by the TPW22 genome are highly homologous with those of P335 species members and that phage TPW22 belongs to the P335 phage species.
The L. lactis subsp. cremoris 3107 and the L. lactis subsp. cremoris MG1363 bacterial site of integration and the corresponding TPW22 genomic site were revealed as a common core of 14 bp with no homology to other attB sequences in GenBank.
Although a relatively A+T-rich attachment site region is usually found, as in phage λ (45) and in temperate lactococcal phages (11, 22, 48, 63, 64), the A+T percentage of the attachment site of phage TPW22 is not significantly higher than that of the lactococcal host (57). This suggests that the attachment site of phage TPW22 is more closely related to those of phages with a lower A+T percentage, like S. thermophilus phage φO1205 (60), rather than to those of other temperate lactococcal phages (11, 22, 48, 63, 64).
The site-specific integration of phage TPW22 into L. lactis subsp. cremoris 3107 at a specific attachment site was demonstrated. Both the position next to the core sequence and the deduced protein homology point to Int as a site-specific integrase. The integration experiments prove that Int must be functional to obtain integrants and that attP alone cannot mediate integration.
Although the structural proteins of phage TPW22 are closely related to those of the lactococcal phages r1t and φLC3, and the integrases of TPW22, r1t, φLC3, Tuc2009, and BK5-T all belong to the Int family of site-specific recombinases, the TPW22 integrase is clearly different from these integrases of the φLC3 type. It is more closely related to the nearly identical integrases of the S. thermophilus bacteriophages φO1205, φSfi21, and TP-J34 and belongs to the same branch, although the identity is only 42% (Fig. 2) (11, 17, 18, 48, 52, 60, 63, 64). In this way, TPW22 Int links the S. thermophilus and lactococcal phage integrases of the Int family. The TPW22 integrase shows no homology with the resolvase-like integrase of the lactococcal phage TP901-1 (23).
The integrative elements of phage TPW22 are likely to represent a lactococcal integration module other than the one proposed for the phages r1t, BK5-T, φLC3, and Tuc2009 (11). The TPW22 integration module may have evolved as an interchangeable module from a common ancestor which has been introduced into a lactococcal phage in accordance with the theory of Botstein (10) (Fig. 1). Putative recombination sites could be located at the 5′ end of the putative lysin gene of TPW22. Both this region and the putative factor-independent terminator structure between int and orf2 (Fig. 2), which could have been involved in acquisition of the resolvase-related integrase of TP901-1 (23), are conserved among the phages TP901-1, Tuc2009, φLC3, and TPW22 (Fig. 5A). Therefore, it may be hypothesized that at least three different exchangeable integration modules of lactococcal phages exist containing a φLC3 type, a TP901-1 type, or a TPW22 type of Int, attP, and surrounding putative terminator structures.
It could also be speculated that the conserved structures positioned 100 to 125 bp upstream from the start codons and downstream from the stop codons of the int genes of TPW22 and φO1205 (Fig. 5B) could be putative transition points responsible for DNA exchange of attP and int. In the noncoding regions flanking int, point mutations could be allowed, giving rise to these nonhomologous regions of the two phages.
Also, the higher nucleotide identity than amino acid identity of TPW22 int with int of the S. thermophilus phages φO1205, φSfi21, and TP-J34 could indicate horizontal gene transfer of an integrative module between an S. thermophilus phage and a lactococcal phage. Such a gene transfer could have happened, as both S. thermophilus and Lactobacillus spp. are present in milk and the dairy environment (16).
Also, in the lambdoid phages λ, 434, and HK022 recombination events may have occurred in the lysogenic region. Comparisons of sequences from these phages reveal that recombination has taken place within the int gene (7, 20). Among the related lactococcal, staphylococcal, and S. thermophilus phage integrases presented in Fig. 2, sequence comparisons point to the presence of a complete integration cassette. A common mechanism of exchange of integration cassettes seems possible, as a conserved region upstream from int is found in the four staphylococcal phages φ13, φ42, φ11, and φL54a (21, 65, 66).
ACKNOWLEDGMENTS
This work was supported by a Ph.D. scholarship granted by the Royal Veterinary and Agricultural University to A. Petersen. We thank the Department of Dairy and Food Science for grant payment for the supervision of M. Johnsen.
J. Bresciani is thanked for helping with the electron micrographs of phage TPW22. We thank D. Lillehaug for phage φLC3 and two primers, S. T. Jørgensen for the pSJ1327 plasmid, M. Skaugen for providing the ermL-containing fragment and sequence information, A. Breüner for pAB201 DNA, and E. W. Nielsen for L. lactis subsp. cremoris W22. We also thank F. K. Vogensen and K. Hammer for helpful discussions.
REFERENCES
- 1.Abremski K E, Hoess R H. Evidence for a second conserved arginine residue in the integrase family of recombination proteins. Protein Eng. 1992;5:87–91. doi: 10.1093/protein/5.1.87. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez M A, Herrero M, Suárez J E. The site-specific recombination system of the Lactobacillus species bacteriophage A2 integrates in Gram-positive and Gram-negative bacteria. Virology. 1998;250:185–193. doi: 10.1006/viro.1998.9353. [DOI] [PubMed] [Google Scholar]
- 4.Arendt E K, Daly C, Fitzgerald G F, van de Guchte M. Molecular characterization of lactococcal bacteriophage Tuc2009 and identification and analysis of genes encoding lysin, a putative holin, and two structural proteins. Appl Environ Microbiol. 1994;60:1875–1883. doi: 10.1128/aem.60.6.1875-1883.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argos P, Landy A, Abremski K, Egan J B, Haggard-Ljungquist E, Hoess R H, Kahn M L, Kalionis B, Narayana S V L, Pierson III L S, Sternberg N, Leong J M. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986;5:433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axelsson L T, Ahrné S E I, Andersson M C, Ståhl S R. Identification and cloning of a plasmid-encoded erythromycin resistance determinant from Lactobacillus reuteri. Plasmid. 1988;20:171–174. doi: 10.1016/0147-619x(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 7.Baker J, Limberger R, Schneider S J, Campbell A. Recombination and modular exchange in the genesis of new lambdoid phages. New Biol. 1991;3:297–308. [PubMed] [Google Scholar]
- 8.Bio-Rad Laboratories. Pulse controller, operating instructions and applications guide, accessory for bacterial and fungal electro-transformation. Catalog number 165-2098. Hercules, Calif: Bio-Rad Laboratories; 1992. [Google Scholar]
- 9.Birkeland N K. Cloning, molecular characterization, and expression of the genes encoding the lytic functions of lactococcal bacteriophage φLC3: a dual lysis system of modular design. Can J Microbiol. 1994;40:658–665. doi: 10.1139/m94-104. [DOI] [PubMed] [Google Scholar]
- 10.Botstein D. A theory of modular evolution for bacteriophages. Ann N Y Acad Sci. 1980;354:484–491. doi: 10.1111/j.1749-6632.1980.tb27987.x. [DOI] [PubMed] [Google Scholar]
- 11.Boyce J D, Davidson B E, Hillier A J. Spontaneous deletion mutants of the Lactococcus lactis temperate bacteriophage BK5-T and localization of the BK5-T attP site. Appl Environ Microbiol. 1995;61:4105–4109. doi: 10.1128/aem.61.11.4105-4109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce J D, Davidson B E, Hillier A J. Identification of prophage genes expressed in lysogens of the Lactococcus lactis bacteriophage BK5-T. Appl Environ Microbiol. 1995;61:4099–4104. doi: 10.1128/aem.61.11.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun V, Jr, Hertwig S, Neve H, Geis A, Teuber M. Taxonomic differentiation of bacteriophages of Lactococcus lactis by electron microscopy, DNA-DNA hybridization, and protein profiles. J Gen Microbiol. 1989;135:2551–2560. [Google Scholar]
- 14.Brendel V, Trifonov E N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984;12:4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breüner A G. Factors involved in site-specific recombination in the temperate lactococcal bacteriophage TP901-1. Ph.D. thesis. Lyngby, Denmark: Technical University of Denmark; 1998. [Google Scholar]
- 16.Brüssow H, Bruttin A, Desiere F, Lucchini S, Foley S. Molecular ecology and evolution of Streptococcus thermophilus bacteriophages—a review. Virus Genes. 1998;16:95–109. doi: 10.1023/a:1007957911848. [DOI] [PubMed] [Google Scholar]
- 17.Bruttin A, Desiere F, Lucchini S, Foley S, Brüssow H. Characterization of the lysogeny DNA module from the temperate Streptococcus thermophilus bacteriophage φSfi21. Virology. 1997;233:136–148. doi: 10.1006/viro.1997.8603. [DOI] [PubMed] [Google Scholar]
- 18.Bruttin A, Foley S, Brüssow H. The site-specific integration system of the temperate Streptococcus thermophilus bacteriophage φSfi21. Virology. 1997;237:148–158. doi: 10.1006/viro.1997.8769. [DOI] [PubMed] [Google Scholar]
- 19.Campbell A. Episomes. Adv Genet. 1962;11:101–145. [Google Scholar]
- 20.Campbell A. Comparative molecular biology of lambdoid phages. Annu Rev Microbiol. 1994;48:193–222. doi: 10.1146/annurev.mi.48.100194.001205. [DOI] [PubMed] [Google Scholar]
- 21.Carroll D, Kehoe M A, Cavanagh D, Coleman D C. Novel organization of the site-specific integration and excision recombination functions of the Staphylococcus aureus serotype F virulence-converting phages φ13 and φ42. Mol Microbiol. 1995;16:877–893. doi: 10.1111/j.1365-2958.1995.tb02315.x. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen B, Johnsen M G, Stenby E, Vogensen F K, Hammer K. Characterization of the lactococcal temperate phage TP901-1 and its site-specific integration. J Bacteriol. 1994;176:1069–1076. doi: 10.1128/jb.176.4.1069-1076.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christiansen B, Brøndsted L, Vogensen F K, Hammer K. A resolvase-like protein is required for the site-specific integration of the temperate lactococcal bacteriophage TP901-1. J Bacteriol. 1996;178:5164–5173. doi: 10.1128/jb.178.17.5164-5173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desiere F, Lucchini S, Bruttin A, Zwahlen M C, Brüssow H. A highly conserved DNA replication module from Streptococcus thermophilus phages is similar in sequence and topology to a module from Lactococcus lactis phages. Virology. 1997;234:372–382. doi: 10.1006/viro.1997.8643. [DOI] [PubMed] [Google Scholar]
- 25.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engel G, Altermann E, Klein J R, Henrich B. Structure of a genome region of the Lactobacillus gasseri temperate phage φadh covering a repressor gene and cognate promoters. Gene. 1998;210:61–70. doi: 10.1016/s0378-1119(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Goeghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 28.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingery R, Echols H. Mutants of bacteriophage λ unable to integrate into the host genome. Proc Natl Acad Sci USA. 1967;58:1507–1514. doi: 10.1073/pnas.58.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gravesen A, Josephsen J, von Wright A, Vogensen F K. Characterization of the replicon from the lactococcal theta-replicating plasmid pJW563. Plasmid. 1995;34:105–118. doi: 10.1006/plas.1995.9996. [DOI] [PubMed] [Google Scholar]
- 31.Hill C, Miller L A, Klaenhammer T R. In vivo genetic exchange of a functional domain from a type II A methylase between lactococcal plasmid pTR2030 and a virulent bacteriophage. J Bacteriol. 1991;173:4363–4370. doi: 10.1128/jb.173.14.4363-4370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iordänescu S. Three distinct plasmids originating in the same Staphylococcus aureus strain. Arch Roum Path Exp Microbiol. 1976;35:111–118. [PubMed] [Google Scholar]
- 34.Jarvis A W. The serological differentiation of lactic acid streptococcal bacteriophages. N Z J Dairy Sci Technol. 1977;12:176–181. [Google Scholar]
- 35.Jarvis A W, Fitzgerald G F, Mata M, Mercenier A, Neve H, Powell I B, Ronda C, Saxelin M, Teuber M. Species and type phages of lactococcal bacteriophages. Intervirology. 1991;32:2–9. doi: 10.1159/000150179. [DOI] [PubMed] [Google Scholar]
- 36.Johansen E, Kibenich A. Characterization of Leuconostoc isolates from commercial mixed strain mesophilic starter cultures. J Dairy Sci. 1991;75:1186–1191. [Google Scholar]
- 37.Johnsen M G, Neve H, Vogensen F K, Hammer K. Virion positions and relationships of lactococcal temperate bacteriophage TP901-1 proteins. Virology. 1995;212:595–606. doi: 10.1006/viro.1995.1517. [DOI] [PubMed] [Google Scholar]
- 38.Josephsen J, Nielsen E W. Plasmid profiles and bacteriophage sensitivity of bacteria of a Cheddar starter used for five years without rotation. Milchwissenschaft. 1988;43:219–223. [Google Scholar]
- 39.Josephsen J, Andersen N, Behrndt H, Brandsborg E, Christiansen G, Hansen M B, Hansen S, Nielsen E W, Vogensen F K. An ecological study of lytic bacteriophages of Lactococcus lactis subsp. cremoris isolated in a cheese plant over a five year period. Int Dairy J. 1994;4:123–140. [Google Scholar]
- 40.Kakikawa M, Oki M, Watanabe N, Yasukawa H, Masamune Y, Taketo A, Kodaira K I. Characterization of the genes encoding integrative and excisive functions of Lactobacillus phage φg1e: cloning, sequence analysis, and expression in Escherichia coli. Gene. 1997;185:119–125. doi: 10.1016/s0378-1119(96)00648-8. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi Y, Nash H A. The bacteriophage λ int gene product. A filter assay for genetic recombination, purification of Int, and specific binding to DNA. J Biol Chem. 1978;253:7149–7157. [PubMed] [Google Scholar]
- 42.Kodaira K I, Oki M, Kakikawa M, Watanabe N, Hirakawa M, Yamada K, Taketo A. Genome structure of the Lactobacillus temperate phage φg1e: the whole genome sequence and the putative promoter/repressor system. Gene. 1997;187:45–53. doi: 10.1016/s0378-1119(96)00687-7. [DOI] [PubMed] [Google Scholar]
- 43.Ladero V, García P, Bascarán V, Herrero M, Alvarez M A, Suárez J E. Identification of the repressor-encoding gene of the Lactobacillus bacteriophage A2. J Bacteriol. 1998;180:3474–3476. doi: 10.1128/jb.180.13.3474-3476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 45.Landy A. Dynamic, structural, and regulatory aspects of λ site-specific recombination. Annu Rev Biochem. 1989;58:913–949. doi: 10.1146/annurev.bi.58.070189.004405. [DOI] [PubMed] [Google Scholar]
- 46.Lillehaug D. The temperate bacteriophage φLC3: isolation, characterization and its use to develop an integration vector system for lactococci. Ph.D. thesis. Oslo, Norway: University of Oslo; 1994. [Google Scholar]
- 47.Lillehaug D, Birkeland N K. Characterization of genetic elements required for site-specific integration of the temperate lactococcal bacteriophage φLC3 and construction of integration-negative φLC3 mutants. J Bacteriol. 1993;175:1745–1755. doi: 10.1128/jb.175.6.1745-1755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lillehaug D, Lindqvist B H, Birkeland N K. Characterization of φLC3, a Lactococcus lactis subsp. cremoris temperate bacteriophage with cohesive single-stranded DNA ends. Appl Environ Microbiol. 1991;57:3206–3211. doi: 10.1128/aem.57.11.3206-3211.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowrie R J. Lysogenic strains of group N lactic streptococci. Appl Microbiol. 1974;27:210–217. doi: 10.1128/am.27.1.210-217.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moineau S, Pandian S, Klaenhammer T R. Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl Environ Microbiol. 1994;60:1832–1841. doi: 10.1128/aem.60.6.1832-1841.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nash H A, Robertson C A. Purification and properties of the Escherichia coli protein factor required for λ integrative recombination. J Biol Chem. 1981;256:9246–9253. [PubMed] [Google Scholar]
- 52.Neve H, Zenz K I, Desiere F, Koch A, Heller K J, Brüssow H. Comparison of the lysogeny modules from the temperate Streptococcus thermophilus bacteriophages TP-J34 and Sfi21: implications for the modular theory of phage evolution. Virology. 1998;241:61–72. doi: 10.1006/viro.1997.8960. [DOI] [PubMed] [Google Scholar]
- 53.Nunes-Düby S E, Kwon H J, Tirumalai R S, Ellenberger T, Landy A. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 1998;26:391–406. doi: 10.1093/nar/26.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiao M, Ye S, Koponen O, Ra R, Usabiaga M, Immonen T, Saris P E J. Regulation of the nisin operons in Lactococcus lactis N8. J Appl Bacteriol. 1996;80:626–634. doi: 10.1111/j.1365-2672.1996.tb03267.x. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Schleifer K H, Kraus J, Dvorak C, Kilpper-Bälz R, Collins M D, Fischer W. Transfer of Streptococcus lactis and related streptococci to the genus Lactobacillus gen. nov. Syst Appl Microbiol. 1985;6:183–195. [Google Scholar]
- 58.Sherratt D J. Site-specific recombination and the segregation of circular chromosomes. In: Eckstein F, Lilley D M J, editors. Nucleic acids and molecular biology. Vol. 7. Berlin, Germany: Springer-Verlag; 1993. pp. 202–216. [Google Scholar]
- 59.Sneath P H A, Sokal R R. Numerical taxonomy: the principles and practice of numerical classification. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 60.Stanley E, Fitzgerald G F, Le Marrec C, Fayard B, van Sinderen D. Sequence analysis and characterization of φO1205, a temperate bacteriophage infecting Streptococcus thermophilus CNRZ1205. Microbiology. 1997;143:3417–3429. doi: 10.1099/00221287-143-11-3417. [DOI] [PubMed] [Google Scholar]
- 61.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van de Guchte M, Daly C, Fitzgerald G F, Arendt E K. Identification of int and attP on the genome of lactococcal bacteriophage Tuc2009 and their use for site-specific plasmid integration in the chromosome of Tuc2009-resistant Lactococcus lactis MG1363. Appl Environ Microbiol. 1994;60:2324–2329. doi: 10.1128/aem.60.7.2324-2329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Sinderen D, Karsens H, Kok J, Terpstra P, Ruiters M H J, Venema G, Nauta A. Sequence analysis and molecular characterization of the temperate lactococcal bacteriophage r1t. Mol Microbiol. 1996;19:1343–1355. doi: 10.1111/j.1365-2958.1996.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 65.Ye Z H, Lee C Y. Nucleotide sequence and genetic characterization of staphylococcal bacteriophage L54a int and xis genes. J Bacteriol. 1989;171:4146–4153. doi: 10.1128/jb.171.8.4146-4153.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye Z H, Buranen S L, Lee C Y. Sequence analysis and comparison of int and xis genes from staphylococcal bacteriophages L54a and φ11. J Bacteriol. 1990;172:2568–2575. doi: 10.1128/jb.172.5.2568-2575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zissler J. Integration-negative (int) mutants of phage λ. Virology. 1967;31:189. doi: 10.1016/0042-6822(67)90030-x. [DOI] [PubMed] [Google Scholar]