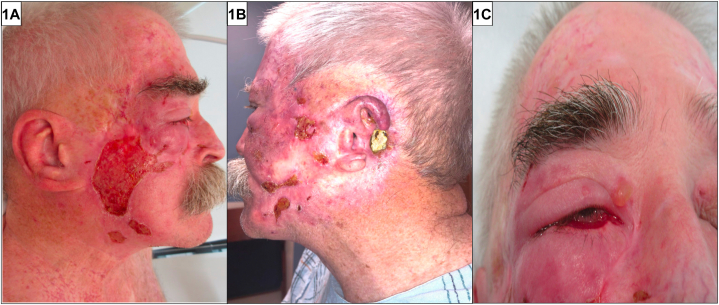
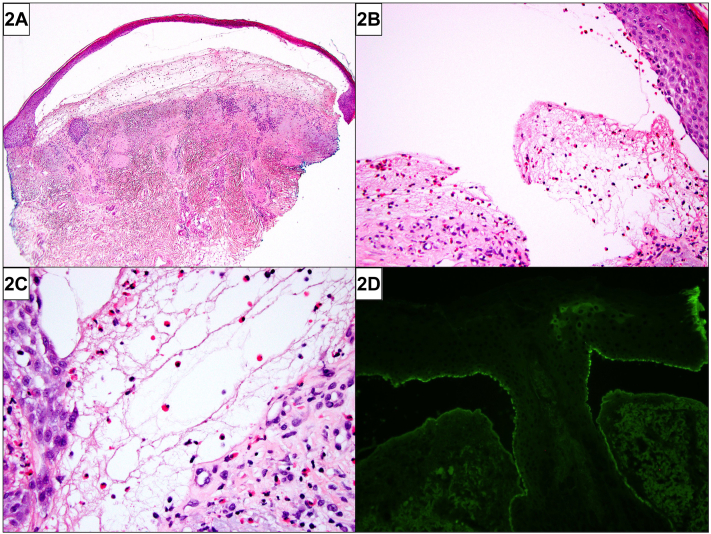
A 72-year-old man with multiple prior head and neck skin cancers presented with 8 months of facial erosions. He had received excision, radiation therapy, and carboplatin/paclitaxel for advanced facial squamous cell carcinomas and completed pembrolizumab for stage III melanoma of the chest 1 year prior to presentation. Examination revealed an afebrile man with geometric bilateral cheek erosions and a bulla above the right medial canthus (Fig 1). Histopathology demonstrated a subepidermal blister with eosinophil-rich infiltrate along the dermoepidermal junction (Fig 2). Direct immunofluorescence (DIF) for complement component 3 (C3) (Fig 2) revealed a hemidesmosomal pattern of reactivity along the basal layer of the blister roof.
Fig 1.
Fig 2.
Question 1: What is the most likely diagnosis?
-
A.
Pemphigus vulgaris (PV)
-
B.
Linear IgA bullous dermatosis
-
C.
Porphyria cutanea tarda
-
D.
Immune checkpoint inhibitor-induced bullous pemphigoid (ICI-BP)
-
E.
Bullous cellulitis
Answer:
-
A.
PV – Incorrect. PV is an autoimmune bullous disease with predominant mucosal involvement. PV is characterized by flaccid bullae and oral lesions. DIF demonstrates intercellular IgG and C3 deposition between keratinocytes.
-
B.
Linear IgA bullous dermatosis – Incorrect. Linear IgA dermatosis is an immune-mediated disease commonly associated with widespread vesicular and bullous involvement with mucosal erosions. DIF shows linear deposition of IgA along the dermoepidermal junction.
-
C.
Porphyria cutanea tarda – Incorrect. Porphyria cutanea tarda is a disease of defective heme biosynthesis characterized by photosensitivity, vesicles, bullae, and erosions in photodistributed areas. This patient does not have typical risk factors associated with porphyria cutanea tarda, such as excess iron, alcohol or tobacco use, hepatitis or HIV infection. Furthermore, positive C3/IgG in a hemidesmosomal pattern on DIF is not expected.
-
D.
ICI-BP – Correct. ICI-BP is a rare variant of drug-induced BP. Like idiopathic BP, it is characterized by tense bullae, pruritus, and IgG/C3 deposition at the dermoepidermal junction on DIF. Unlike idiopathic BP, ICI-BP is characterized by a longer pruritic prodrome, delayed diagnosis, and increased likelihood of requiring systemic immunosuppression for disease remission.1 This patient’s ICI-BP is also likely associated with radiation therapy given localized disease onset limited to previously irradiated areas.
-
E.
Bullous cellulitis – Incorrect. Cellulitis is caused by bacterial infection of dermal and subcutaneous tissue. It is characterized by erythema, pain, and swelling along with constitutional symptoms. Although vesicles and bullae may be present in severe cases, it is unlikely to be the diagnosis in this patient with longstanding pruritus and facial erosions.
Question 2: Which of the following is a risk factor for development of this condition in this patient?
-
A.
Age over 70
-
B.
Prior history of radiation therapy
-
C.
Prior history of melanoma
-
D.
Prior history of nonmelanoma skin cancer
-
E.
All of the above
Answer:
-
A.
Age over 70 – Incorrect. This is not the best answer given other choices available.
-
B.
Prior history of radiation therapy – Incorrect. This is not the best answer given other choices available.
-
C.
Prior history of melanoma – Incorrect. This is not the best answer given other choices available.
-
D.
Prior history of nonmelanoma skin cancer – Incorrect. This is not the best answer given other choices available.
-
E.
All of the above – Correct. All of the above listed answer choices are risk factors for BP. Based on a recent propensity score-matched case-control study, age greater than 70 years at the time of receiving first immune checkpoint inhibitor cycle, history of melanoma, and history of nonmelanoma skin cancers are all associated with increased risk of developing BP among a cohort of patients treated with ICIs.2 Radiotherapy is also an independent risk factor for BP.3 While the underlying pathophysiology is unknown, it has been hypothesized that radiotherapy causes tissue damage, leading to increased antigen exposure and increased autoantibody production.3 In addition to the risk factor of ICI usage, BP onset in this patient is likely associated with radiotherapy exposure given localized distribution of disease in previously irradiated areas on bilateral cheeks.
Question 3: Which of the following is true regarding this diagnosis?
-
A.
Future usage of biologics therapy is contraindicated in this patient
-
B.
This eruption will self-resolve with cessation of immune checkpoint inhibitor therapy
-
C.
This eruption may predict a positive tumor response to immunotherapy
-
D.
Vitamin E is an effective adjunctive therapy for this diagnosis
-
E.
Tetracycline is an ineffective treatment for this diagnosis
Answer:
-
A.
Future usage of biologics therapy is contraindicated in this patient – Incorrect. Biologics such as rituximab, a monoclonal antibody that binds CD20 antigen, and omalizumab, a monoclonal antibody that binds free immunoglobulin E, have been shown to be effective treatments of steroid-refractory BP.
-
B.
This eruption will self-resolve with cessation of immune checkpoint inhibitor therapy – Incorrect. While ICI-BP can be severe enough to warrant cessation of ICI treatment, it requires additional treatments such as topical and systemic steroids and is not expected to always self-resolve. Additionally, patients can develop ICI-BP after ICI regimen completion, such as in the case of our patient.
-
C.
This eruption may predict a positive tumor response to immunotherapy – Correct. Development of BP among patients has been associated with improved tumor objective response rates among patients receiving ICI.4 Although this has only been observed in case-control studies with small sample size, this is concordant with prior observation demonstrating positive associations between development of dermatological immune-related adverse events with improved tumor response and survival outcomes.4
-
D.
Vitamin E is an effective adjunctive therapy for this diagnosis – Incorrect. Nicotinamide (vitamin B3), not vitamin E, has been shown to be an effective adjunct to tetracycline in treating ICI-BP through modulation of inflammatory cytokines and its role as a poly-adenosine diphosphate ribose polymerase inhibitor.5
-
E.
Tetracycline is an ineffective treatment for this diagnosis – Incorrect. Tetracycline, a class of protein synthesis inhibiting antibiotics, is effective in treating BP. Its mechanism of action is not known but has been hypothesized to be secondary to its anti-inflammatory properties and inhibition of neutrophil and eosinophil chemotaxis.5
Conflicts of interest
None disclosed.
Footnotes
Funding sources: The article was supported in part by the Office of Scholarly Engagement, Harvard Medical School.
IRB approval status: Not applicable.
Patient consent: Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Molina G.E., Reynolds K.L., Chen S.T. Diagnostic and therapeutic differences between immune checkpoint inhibitor-induced and idiopathic bullous pemphigoid: a cross-sectional study. Br J Dermatol. 2020;183(6):1126–1128. doi: 10.1111/BJD.19313. [DOI] [PubMed] [Google Scholar]
- 2.Said J.T., Liu M., Talia J., et al. Risk factors for the development of bullous pemphigoid in US patients receiving immune checkpoint inhibitors. JAMA Dermatol. 2022;158(5):552–557. doi: 10.1001/JAMADERMATOL.2022.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen T., Kwan J.M., Ahmed A.R. Relationship between radiation therapy and bullous pemphigoid. Dermatology. 2014;229(2):88–96. doi: 10.1159/000362208. [DOI] [PubMed] [Google Scholar]
- 4.Nelson C.A., Singer S., Chen T., et al. Bullous pemphigoid after anti-PD-1 therapy: a retrospective case-control study evaluating impact on tumor response and survival outcomes. J Am Acad Dermatol. 2020 doi: 10.1016/J.JAAD.2019.12.068. [DOI] [PubMed] [Google Scholar]
- 5.Han A., Zeichner J.A. A practical approach to treating autoimmune bullous disorders with systemic medications. J Clin Aesthet Dermatol. 2009;2(5):19–28. [PMC free article] [PubMed] [Google Scholar]