Graphical abstract
Vaccines play an important role in the control and prevention of human infectious diseases such as the ongoing COVID-19 pandemic. The successful vaccination needs to effectively deliver antigenic substances to induce a strong protective or therapeutic immune response. In this review, the lymph node structure, the administration routes and the most popular vaccine delivery systems together with their applications are carefully summarized with a special focus on the designing of the vaccine delivery strategies to overcome the barriers and strengthen the immune response against infectious diseases.
Keywords: Vaccine, Lymph nodes, Delivery barriers, Targeted delivery, Lymphocytes, Immune response
Abstract
Strategies of improving vaccine targeting ability toward lymph nodes have been attracting considerable interest in recent years, though there are remaining delivery barriers based on the inherent properties of lymphatic systems and limited administration routes of vaccination. Recently, emerging vaccine delivery systems using various materials as carriers are widely developed to achieve efficient lymph node targeting and improve vaccine-triggered adaptive immune response. In this review, to further optimize the vaccine targeting ability for future research, the design principles of lymph node targeting vaccine delivery based on the anatomy of lymph nodes and vaccine administration routes are first summarized. Then different designs of lymph node targeting vaccine delivery systems, including vaccine delivery systems in clinical applications, are carefully surveyed. Also, the challenges and opportunities of current delivery systems for vaccines are concluded in the end.
1. Introduction
Vaccines have received considerable attention in recent years when considering urgent needs due to their outstanding therapeutic and prophylactic efficacy against emerging intractable diseases. Especially nowadays, COVID-19 vaccine is considered as the hope for ending the worsening coronavirus pandemic [1]. Functionalizing as triggers to stimulate human immune systems fighting against foreign pathogens, the efficacy of vaccines depends upon the potent delivery performance towards immune-related organs to optimize the vaccine availability and retention time at the lymphatic organs. Hence, as a critical part of vaccine development, vaccine delivery strategies utilizing agent delivery platforms are fundamental to the targeting delivery towards human immune-related systems or the controllable release of vaccine agents at targeted sites. The improved specificity of vaccine delivery is also beneficial to eliminate immune-related adverse effects like antibody-mediated hypersensitivity [2].
Since the vaccinal antigens primarily activate the adaptive immune system that memorizes and clears antigens as foreign invaders, the potency of the vaccine relies on the extent of the vaccine-boosted adaptive immune response in human bodies [3]. The adaptive immune response is mainly initiated in lymph nodes, which are the crucial residence of adaptive immunity-related lymphocytes, including CD4+ helper T cells, CD8+ T cells, B cells, and antigen presenting cells (APCs). The intact adaptive immune response is initially based on the antigen presenting behavior of APCs, including macrophages, dendritic cells (DCs) like follicular dendritic cells, and B cells [4]. Antigen uptake mechanisms of APCs vary among different cell types. As for macrophages and the majority of DCs, phagocytosis plays a vital role in antigen uptake through engulfing exogenous vaccinal antigens into APCs, following by the progress of processing and presenting the antigens as major histocompatibility complex (MHC) class I and class II proteins on the surface of APCs [5]. Subsequently, those APCs carrying with MHC I or MHC II complexes would migrate from antigen-exposed tissue sites to near-by draining lymph nodes (dLNs) and present MHC complexes to T lymphocytes and B lymphocytes. Therefore, activated T cells and B cells within lymph nodes will respond to the vaccination, regulate adaptive immune response against foreign antigens, and form potential long-term immune responses. Follicular dendritic cells, however, have a totally distinct pathway to trigger the adaptive immune response. As the DCs are originated in primary and secondary lymph follicles of lymphoid tissues, the surface receptors of follicular dendritic cells could recognize and capture antigen–antibody complexes, also named immune complexes, which circulate systematically, and retain them in the lymph nodes to maintain long-lasting immunity [6]. It is now well established from a variety of studies that the potency of T cell-associated immune response activated by vaccination correlates with the transportation efficiency of vaccine-activated APCs towards lymph node immunization site [7], [8], [9]. Among these, professional DCs with antigen cross-presentation ability have markedly more promising T cell activation effectiveness compared with macrophages and B cells [10]. According to the above, for enhancing the vaccination-triggered adaptive response, how to achieve the targeted transport of either vaccine antigens into lymph nodes for APCs inside or activated APCs into lymph nodes for priming T cells and B cells has been instrumental in the research field of vaccine delivery.
Despite the hopeful therapeutic efficacy of lymph node-targeted vaccination, the barriers for lymph node-targeted vaccine transportation tremendously hinder the achievement of significant vaccination efficacy, which requires robust vaccine delivery platform designs. Lymph node-targeting vaccine delivery barriers hinge mainly on the constraints between the following key elements: the administration route of the vaccine, lymph node physiological structures and microenvironment, and intrinsic characteristics of the vaccine in vivo [11]. Therefore, to accomplish compelling lymph node-targeted vaccine delivery, the development of a vaccine delivery platform overcoming existing barriers is of great importance. So far, with a better understanding of the entry pathways of either vaccine or vaccine-activated APCs into lymph nodes and how adaptive immune response takes place inside draining lymph nodes, rational design of vaccine carriers has achieved significant progress. Various biomaterials-based vaccine delivery systems, including liposome-based vaccine delivery systems, cell-based vaccine delivery systems, nucleic acid-based vaccine delivery systems, and peptide-based vaccine delivery systems, have shown great promises in advancing delivery efficiency by overcoming the transport challenges.
Therefore, this review article aims to highlight the lymph node's physiological architecture and the administration routes of vaccines, and how they impede the targeting delivery of vaccines towards lymph nodes. The corresponding design principles of vaccine towards lymph nodes are reviewed in detail. The clinical trials of diverse lymph node-targeted vaccine delivery systems, as well as preclinical research, are summarized. Lastly, the challenges and future directions of lymph node-targeted vaccine delivery systems for further development are discussed and outlooked.
2. Administration routes of vaccine towards lymph nodes
The systematic analysis of vaccine administration routes is imperative and helpful to better comprehend and achieve the immune activation by lymph node-targeted vaccine. Commercialized vaccines currently available are primarily administrated to patients in two different routes: oral route and interstitial injection, which includes subcutaneous, intramuscular, intradermal injection. Considering the most desirable and patient-compliant administration route, oral vaccines are valued in developing countries as potential strategies to improve vaccine distribution and efficacy by reducing the administering costs and increasing patient adherence to vaccine [12]. However, the oral route is not the desired route for lymph node targeting due to the transportation barriers in the human GI tract, including a highly acidic environment, complicated ingredients of digestive enzymes, and more importantly, the multiple epithelial barriers created by tight cellular junctions [13]. The harsh gastrointestinal environment and self-downregulated immune response in the GI tract significantly impede vaccinal antigens from getting through intestinal barriers and being internalized by APCs, resulting in dissatisfied lymph node targeting effectiveness. Therefore, for evading the depletion and metabolism of foreign vaccine antigens during circulation, recently there has been increasing interest in direct intranodal injection of vaccine, especially for the dendritic cell-based vaccines [14], [15]. Studies have shown that by intranodally delivering vaccines for lymph node targeting, better accumulation of vaccines at lymph nodes enables a relatively lower dose of vaccines to achieve enhanced immune response. However, the clinical implementation of intranodal vaccination is hindered mainly by technical challenges in locating lymph nodes even with the help of ultrasound or the nontoxic tracer dye [16], and also the highly delicate structure of lymph nodes and cytokine gradients inside might be irreparably damaged by direct injection. Taken together, traditional interstitial vaccination still could not be substituted so far due to the immature development of intranodal vaccination.
Interstitial vaccination, in contrast to intranodal vaccination, could not avoid the long circulation pathway through lymphatic vessels before reaching lymph nodes to initiate the adaptive immune response, which primarily affects the therapeutic efficacy of vaccines. Currently, for a better understanding of the barriers for vaccine delivery towards lymph nodes, two possible transport pathways of vaccines from peripheral tissues into lymph nodes are recognized. For either subcutaneous, intramuscular, or intradermal vaccination, injected antigens could be captured by APCs like macrophages and DCs that reside in peripheral tissues, following with being digested and presented as MHC. Afterward, activated MHC-expressed APCs will move across the lymphatic endothelial cells from the interstitium and enter lymphatic vessels with interstitial fluid. For example, mature DCs migrate towards lymphatic vessels due to the upregulated signaling of CCR7 [17]. Traveling along with lymphatic fluid from initial lymphatic vessels, through collecting lymphatic vessels, APCs transport into the draining lymph nodes with the help of chemokine gradients and luminal valves inside the collecting lymphatic vessels [18]. Consequently, APCs could encounter and present antigens to naïve lymphocytes in the lymph nodes, known as the reservoir of T cells and B cells, triggering the corresponding adaptive immunity. For antigens that are not processed by APCs in the periphery, instead of transporting with APCs, antigens could only passively drain into the lymphatic vessels and be unidirectionally transported to lymph nodes. However, the lymph node targeting capabilities of non-captured vaccine antigens are relatively poor without the digestion and presentation by APCs because the majority of vaccine particles still remain in the peripheral injection sites owing to the complex delivery barriers generated by the dynamic physiological environment within the interstitium [19]. The clearance of vaccines along with blood capillaries and possible endocytosis of vaccines by other cell types would also reduce the delivery efficacy of vaccines towards lymph nodes, leading to a higher dosage of antigens for activating sufficient immune response [20]. What is more, the remaining antigens in the periphery cannot trigger enough adaptive immune response compared with inside lymph nodes since the low amount of peripheral immature DCs could hardly prime adequate amounts of T or B cells [21]. Therefore, how to overcome the transport challenges of interstitially injected free antigens towards lymph nodes would be the critical point for enhancing the immune-stimulating efficacy. To get better lymph node targeting results, various vaccine delivery carriers have been designed to increase the vaccine delivery efficiency for better priming vaccination efficacy.
3. Lymph nodes physiological architecture and antigen transportation inside lymph nodes
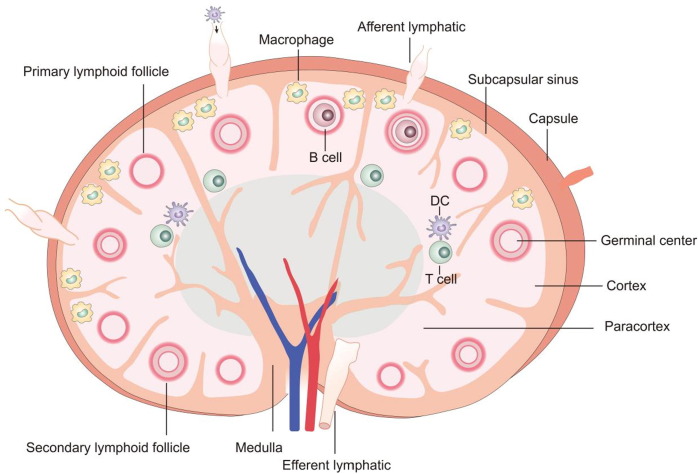
Once reaching the lymph nodes with lymphatic fluid through the afferent lymphatic vessels, antigens or antigen-presented APCs originated from vaccines and antigen injection sites will start to provoke a robust immunostimulatory response with lymphocytes clustered inside lymph nodes. As the smallest compartment of lymph nodes, lymphoid lobules provide the place for immune response activation (Fig. 1 ). Surrounded by complex lymphatic sinuses, lymphoid lobules are divided into two major compartments: the innermost medulla and the cortex formed of paracortex and outer cortex [22]. The medulla composing medullary cords separated by lymph-draining sinuses is the compartment containing plasma cells, macrophages, and some memory T cells [23]. Furthermore, in the cortex beneath the subcapsular sinus, abundant T lymphocytes and B lymphocytes are located within the paracortex and lymphoid follicle zone, respectively [24]. When enriching T lymphocytes and B lymphocytes are activated to mature into effector cells or memory cells in response to antigens and leave the lymph nodes via lymphatic vessels by expressing different chemokine receptors, adaptive immune responses are triggered, providing specific and long-lasting protection by clearance of corresponding pathogens and antigens [25].
Fig. 1.
Lymph node's physiological architecture and the distribution of immune-related cells inside lymph nodes.
Lymphatic fluids flowed from collecting lymphatic vessels, firstly pass through the subcapsular sinuses wrapped by capsules from outside, following with cortical sinus. The succedent routes of migration largely depend on the size of immune-modulatory particles/cells [26], which present totally different movement behaviors of antigens or migrating APCs. As for antigen-carrying APCs, especially DCs, lymphatic endothelial cells between subcapsular sinuses and T cell inhabited cortex zone are the main barriers for cell migration inside lymph nodes. Due to the large size of cells, APCs could not permeate from sinuses through single endothelial cell layers without the help of chemokine gradients across the sinus floor [27]. For example, CCRL1 chemokine receptors expressed on the lymph node periphery are the driving force of the formation of CCL19 and CCL21 functional gradients across the sinus floors, which initiates the migration of DCs across the single endothelial cell layers to the paracortex for the antigen presentation to resided T cells [28]. Unlike antigen carried APCs, the processing procedure of free vaccine antigens inside lymph nodes, which transport from peripheral tissues, involves the participation of resided immature antigen presenting cells in the lymph nodes [29]. It is worth noting that depending on the size of antigens or antigen-loaded delivery vehicles, whether they could enter the conduits significantly influences the transportation time of reaching either B cells or macrophages. Small antigens with less than 70 KDa molecular weight are suitable for transiting from subcapsular sinus through conduit system of lymph nodes, and subsequently be recognized by APCs in lymph nodes, for example, B cell in follicle zone and DCs within the T cell area [30]. However, for large antigens with molecular weight more than 70 KDa, or hydrodynamic radius larger than 4 nm, the size exclusion function of the conduit system due to small-sized tubules originating between the sinus-lining cells become the biggest challenge for antigen transportation [19], [31], [32]. Macrophages inside the subcapsular sinus, as the frontline immune cells embracing lymph-borne pathogens, take the responsibility of the capture and digestion of those trapped large antigens to assist antigen transportation to B cells in the underlying follicles triggering adaptive immune response [18]. Afterward, matured lymphocytes like T cells and B cells and antibodies produced in lymph nodes are concentrated in the inner medulla and drain into the efferent lymphatic vessels from medullary sinuses entering the systematic circulation. Consequently, as the main target sites of vaccination, lymph nodes stimulate robust immune responses corresponding to the vaccine injection.
As a particular type of normal lymph node, tumor-draining lymph node is the primary compartment for tumor antigen presentation to the naive immune system, which shows immunological significance in either anti-tumor immune activation or immune tolerance, favoring itself as an ideal target for cancer vaccines. The microenvironment of tumor-draining lymph node changes under the influence of the upstream cancer condition, resulting in pro-tumoral lymph node structures and cell composition, including suppressing resident dendritic cells during the tumor progression [33]. Tumor-derived biological substances, for example, IL-6, TGF-β, and VEGF, could stimulate the lymphangiogenesis, which facilitates cancer metastasis with the increase of lymph flow [34], [35] and could also disrupt the cross-presentation of tumor antigens by suppressing resident dendritic cells and effector T cells while activating tumor-specific regulatory T cells [36], [37]. Furthermore, the overexpression of chemokine receptors like CXCR4 and CCR7 also promotes the migration of tumor cells through the lymphatic capillaries [38]. Therefore, by targeting tumor-draining lymph nodes to modulate suppressive anti-tumor immune responses in the lymph nodes, the efficacy of the cancer vaccine will be enhanced with induced prophylactic immunity and restrained pro-tumor immune tolerance.
4. Design principles and delivery barriers of vaccine towards lymph nodes
Studies of the lymph node targeting ability of vaccines have shown the importance of size-dependent behaviors of vaccine agents. A huge difference in the sizes of existing vaccine antigens, which vary from smaller than 10 nm to larger than 10 µm, determines their innate targeting capability towards lymph nodes [26]. The optimal size of vaccine systems is largely decided by the antigen transportation process from the periphery, in order to efficiently move through the interstitium and passively drain into lymph nodes through lymphatic capillaries. Moving through the water channels with size limitations, vaccine antigens that are larger than 100 nm would be easily excluded by the dense extracellular matrix and are incapable of diffusing and converting through channels [39]. What is more, antigen particles with even larger size (above 200–500 nm) would be detained in the matrix and would also be refused to enter the initial lymphatic vessels through cell junctions, which could be only facilitated by APCs active transportation towards lymph nodes [40]. However, for the best lymph node targeting performance, the case is not always the smaller the vaccine agents are, the better accumulation in lymph nodes can achieve. The circulation through cardiovascular systems could attract antigens with a size of less than 5 nm (16–20 k Da for proteins) into vascular capillaries and induce the accelerated clearance effects, leading to poor vaccination efficacy and potential side effects [41], [42], [43]. Studies also show that compared to larger particles (diameter greater than 1 μm), antigen particles with size of around 300 nm are more favorable for dendritic cells uptake [44], implying superior immune activation. Therefore, besides taking advantage of the transition functions of APCs for larger antigens, designing a vaccine delivery system with the size of around 10–100 nm would be essential for the achievement of better lymph node targeting and improved vaccine-triggered immune responses [45].
The influence of hydrophilicity in lymph node targeting vaccine delivery also needs to be interpreted with caution due to its dual-edged characteristics in the lymph node targeting process and APC uptake process. To achieve lymph node targeting, how to overcome the hindrance of the interstitial extracellular matrix is a crucial issue for vaccine agent delivery. Vaccine delivery systems transport through the interstitium via mainly water channels, which prefers the hydrophilic surface properties of delivery vehicles [45]. Studies have emphasized the benefits of hydrophilicity of drug delivery carriers in lymphatic uptake and lymph node retention, taking advantage of the hydrophilic environment of the interstitial matrix [46]. Polyethylene glycol (PEG), as a hydrophilic molecule, is widely used as an agent for improving vaccine delivery efficacy through vehicle surface hydrophilic modification, which is also well known as particle size controller when synthesizing nanoparticle-based vaccine delivery systems [47]. In the meantime, PEG decoration provides additional protection of vaccine agents from enzyme degradation and renal clearance. Therefore, the stabilization and targeting capability of vaccine agents during the systemic circulation through PEGylation show great potentials in lymph node targeting vaccines, with enhanced immune response activation [48]. Other materials like hydrophilic functional groups like silanol groups (Si-OH) are also applied to boost lymph node targeting transportation [49]. Nonetheless, PEGylation is not preferred by the APC uptake procedure, opposing its functions in lymph node targeting delivery. The interaction between cellular membranes of phagocytic cells and particle surfaces is facilitated by the hydrophobicity property of vaccine particles, while PEGylation leads to the hydrophilic modification of the vaccine delivery system surfaces [50]. Even though PEGylation could enhance the vaccine delivery toward lymph nodes, adaptive immune response triggered by vaccination is still unsatisfactory because of the weak antigen presentation by DCs or macrophages either in the periphery or inside lymph nodes. Interestingly, there has been growing interest in zwitterionic materials as an emerging material for vaccine delivery, especially for DNA vaccine delivery. Zwitterionic materials, including hydrolytic cationic polycarboxy-betaine esters and the mannosylated zwitterionic-based cationic liposome, have shown great capability of protecting DNA vaccine from enzyme degradation and prolonging the retention of DNA antigens in dLNs [51], [52]. The amphiphile adjuvant with protein vaccine also shows great lymph node accumulation properties when treating SARS-CoV-2 [53]. Surface charge, another vital property of vaccine delivery systems, also shows adverse effects in the lymph node targeting process and APC antigen uptake process. Owing to the composition of the interstitium, which contains mostly negatively charged glycosaminoglycans, positively charged carrier systems could be trapped in the interstitium, and transportation of vaccines toward lymphatic capillaries will be largely hindered [33]. Hence, negatively charged materials as the vaccine delivery carriers are more favorable for better interstitial movement for a better delivery performance of lymphatic uptake and lymph node retention [45]. Studies have shown that vaccines modified by anionic materials could increase the accumulation of vaccines towards lymphatics [54]. Surface charge has been proved to be one of the main influencing factors of their internalization efficiency by APCs and also cytotoxicity. Anionic surfaces become the delivery barriers when reaching APCs for antigen capture and presentation, While the uptake of vaccine particles could be significantly enhanced by decorating the vaccine carriers with positively charged materials like polypeptides poly-l-lysine, due to the electrostatically binding with negatively charged surface groups on the antigen presenting cell membranes and enhanced cellular internalization [55], [56]. Overall, charged materials have higher cytotoxicity than neutral materials, where positively charged materials show increased toxicity against nonphagocytic cells in comparison with particles with negative charge [57]. Mechanistically, the high cytotoxicity of positively charged materials is primarily correlated with the disruption of the negatively charged cell membranes during the cell membrane penetration process of materials, which induces cell death [58]. Thus, the development of charge-reversal materials, which can maintain the neutral or negative surface charge in the physiological environment while been transformed to positive charge upon interacting with APCs, will be of great significance in promoting antigen uptake and ameliorating cytotoxicity [59], [60]. Hence, both hydrophilicity-hydrophobicity balance and surface charge balance are required for careful consideration when developing vaccine delivery systems (Fig. 2 A).
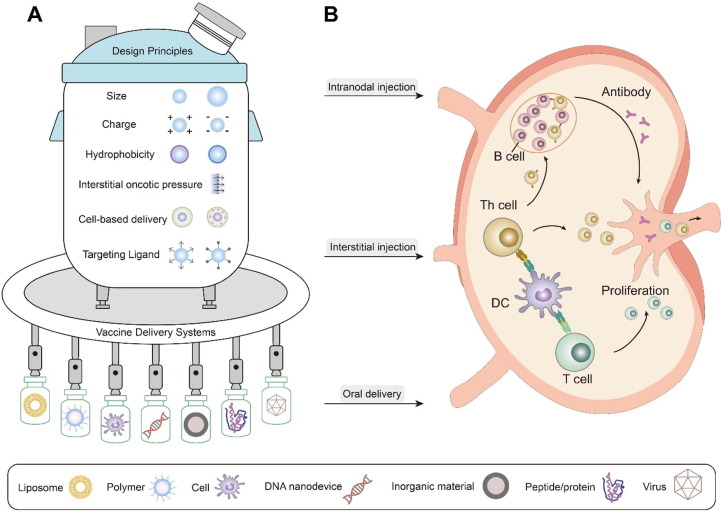
Fig. 2.
Vaccine delivery towards lymph nodes. (A) Basic design principles for vaccine delivery systems and representative vaccine delivery systems, including lipid-based vaccine delivery systems, polymer-based vaccine delivery systems, cell-based vaccine delivery systems, DNA nanodevice-based vaccine delivery systems, inorganic materials-based vaccine delivery systems, peptide/protein-based vaccine delivery systems, virus-based vaccine delivery systems. (B) Administration routes of vaccine delivery towards lymph nodes, including intranodal injection, interstitial injection, oral delivery.
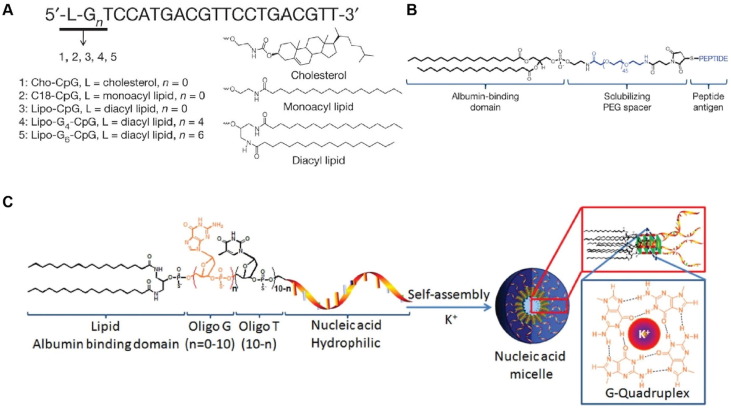
Another factor that largely influences the efficacy of vaccine delivery towards lymph nodes is the interstitial oncotic pressure. Since the driving force of the antigen movement along lymphatic systems is the difference of interstitial oncotic pressure between the peripheral interstitium and draining lymph nodes, compared to the low pressure inside lymph nodes, creating a higher interstitial oncotic pressure in the injection site has great potential in targeting delivery and accelerating flow rate to achieve a better immune response. Lymphatic uptake could be significantly improved with a combination of a larger amount of vaccine antigens reaching the draining lymph node through lymphatics and decrease of the loss of antigens during the systematic circulation, and immune activation could be consequently stimulated more quickly [43]. The interstitial injection as the administration route of vaccines shows markedly distinction of lymphatic transportation efficacy among all different injection sites. Because in the skin, the oncotic pressure is higher than that in other interstitial sites, the intradermal injection could increase the flow toward lymph nodes comparing with subcutaneous or intramuscular injection [43], [61]. To further strengthen the targeting ability of the vaccine to lymph nodes, vaccine adjuvants are introduced to vaccine delivery systems, among which albumin is one of the most widely utilized in vaccine design. Albumin is a non-toxic intrinsic protein enriching in blood vessels, which displays excellent lymph node targeting ability as vaccine carriers for lipophilic vaccine molecules [62]. Meanwhile, since the albumin concentration is relatively high in the blood capillaries, the “albumin hitchhiking” approach could prevent vaccine agents from entering blood circulation through blood capillaries and mostly drain into lymphatics. By modifying the antigens or adjuvants with a lipophilic albumin-binding domain (Fig. 3 ), this efficient lymph node-targeted delivery approach has been proved its boosting vaccine presentation by DCs and targeting ability to lymph nodes [63], [64]. Consequently, adaptive immune response activated by vaccines could be enhanced by the increased interstitial oncotic pressure.
Fig. 3.
Design of the lymph nodes-targeted molecular adjuvant, amphiphiles-peptides, and CpG-DNA amphiphiles-vaccines. (A) Structure of amphiphiles-CpGs. (B) Structure of amphiphiles -peptides. (C) Structure of G-quadruplex-stabilized CpG adjuvants and its three-dimensional spherical micelle structure in aqueous solutions. Reproduced with permission from Ref. [64].
Recently, expect focusing on developing vaccine carriers based on their physical properties, more attention is paid to how to take advantage of the intrinsic biological characteristics of human bodies in lymphatics targeting, especially APC-mediated vaccine trafficking and ligand decoration to target specific receptors on APCs or lymphatic endothelial cells [33]. APCs play an essential role in immune-related activities, which have their unique strengths as vaccine carriers, such as long-lasting half-life, superior ability to cross lipid membrane barriers, migration ability between tissues, chemotaxis, and tumor homing ability. Selecting cell-based delivery systems based on various disease mechanisms and pathological characteristics is expected to achieve long circulation, specific targeting, and penetration into diseased tissues. DCs, known as the most potent APCs so far, can process antigens efficiently and present them to T and B lymphocytes to activate the specific immunity [65]. Given that activated DCs have natural lymph node targeting ability, dendritic cell-based vaccine delivery systems can promote the accurate and efficient delivery of antigens to lymph nodes. Besides, DCs could secrete cytokines and regulate chemokine gradients, facilitating DC vaccines to exert systemic immunity [66]. Furthermore, considering the contradiction in vaccine delivery system design, DC-based vaccine carriers formed in vitro cleverly evade the conundrum of how to balance the properties of vaccine carriers, like size and surface charge, to conducing to either APC uptake or lymph node targeting. In addition to APC-based vaccine delivery, another attractive biomimetic vaccine delivery strategy is making use of the receptors on the surface of cells surrounding lymph nodes, including endothelial cells and lymphocytes. Instead of passively draining into the lymph nodes, the ligand-receptor interaction could actively drive the vaccine agents to the lymph node targeting site, activating the potent immune response. Using DCs as examples, 33D1 and CD205 receptors are served as targets for antibody chaperones in vaccine delivery [67], [68], [69]. These receptors are highly expressed on the surface of DCs located in lymph nodes, and their corresponding antigens could direct vaccine agents towards lymph nodes and further enhance the antigen uptake ability of DCs to promote the immune response (Fig. 2B). Also, for the high endothelial venules in lymph nodes, peptides like MECA79 antibody, could guide vaccine agents towards the draining lymph nodes [70]. Therefore, the strategy of using receptors as targeting ligands for lymph node homing vaccine shows high potential and feasibility in the design of effective vaccine delivery systems.
5. Preclinical vaccine delivery systems
5.1. Liposome-based vaccine delivery systems
Liposomes are bilayer membrane-based nanovesicles composed of phospholipids and cholesterol, which have attracted much attention in the field of drug delivery because of superior biocompatibility and encapsulation properties for a variety of cargoes [71]. With the long-circulating characteristics, the liposome-based vaccine delivery systems, including targeted liposomes, environmentally responsive liposomes, and immunoliposomes, can efficiently deliver antigens in various forms, including polypeptides, proteins, and genes, providing a universal platform for vaccination [72], [73], [74]. The encapsulation in the vesicle structure of liposomes could prevent antigens from the degradation by various biological enzymes in vivo, thus ensuring the desired effectiveness of the vaccines. It is also advantageous to deliver antigens from liposomes to APC cells through membrane fusion, which improves the internalization efficiency of antigens to enhance the immune activation [75]. Therefore, with reasonable particle size control, liposome-based vaccines can efficiently and stably deliver antigens to lymph nodes, potentially addressing the challenges of weak targeting and low immune efficacy of free antigens [76].
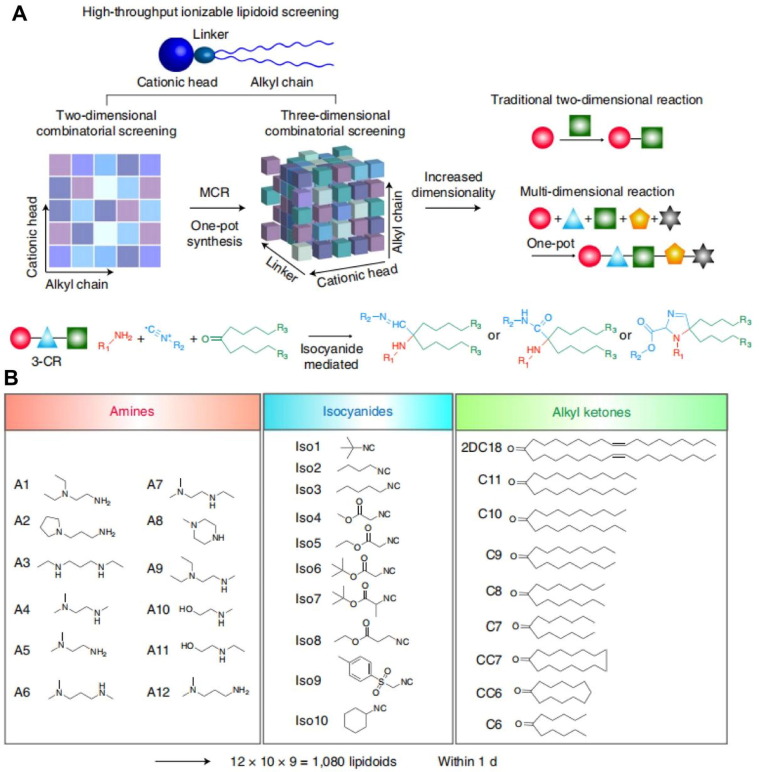
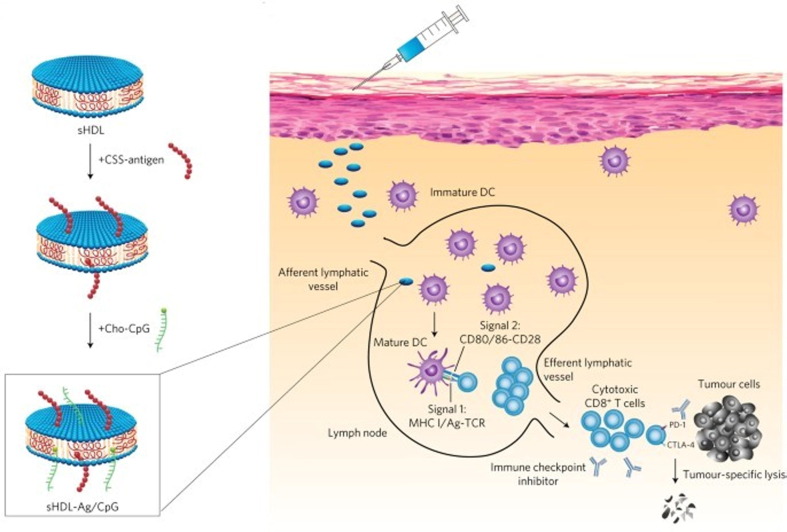
For liposome-based vaccine delivery, it is worth noting how liposomes entrap antigens are essential for the immune activation process. Lovell and co-workers mixed a recombinant malaria antigen (Pfs25) with a polyhistidine tag (His-tag) and liposomes containing cobalt porphyrin-phospholipid (CoPoP) to prepare spontaneous nanoliposome antigen particleization (SNAP) [77]. The His-tag was inserted in the CoPoP bilayer through metal coordination so that the antigen could be stably presented on the liposome surface. This antigen-binding method ensured the serum stability of SNAP during transport from the injection site to the lymph nodes and further increased the antigen uptake by APC cells. Compared with other commonly used adjuvants, SNAP performed greatly in inducing a safe and durable immune response. Two key points in liposome-based vaccine delivery system design are that the way antigens are attached to liposomes has a particular impact on the vaccine immune efficacy, and also the phospholipid structure of the liposomes has been demonstrated to affect the immune activation pathway. Anderson and co-workers established a three-dimensional multi-component combinatorial library of lipid molecules and screened the optimal liposome structure for intracellular delivery of mRNA antigens, including unsaturated lipid tails, dihydroimidazole linkers, and positive cyclic amine head [78]. The mRNA antigen was bound to lipid carrier through electrostatic action, and the cyclic amine structure promoted the vaccine to induce the maturation of the APCs through the stimulator of interferon genes (STING) pathway, showing a robust anti-tumor immune effect (Fig. 4 A-B). Besides traditional liposome-structured vaccine delivery carriers, synthetic high-density lipoprotein (sHDL) nanodiscs composed of phospholipids and apolipoprotein A1-mimetic peptides, which have similar chemical components with liposomes, could also serve as nanoplatforms for the co-delivery of antigen peptides and adjuvants towards draining lymph nodes (Fig. 5 ) [79]. Surprisingly, sHDL-assisted antigen and adjuvant delivery could increase the accumulation of antigens inside lymph nodes and further enhance the antigen presentation on dendritic cells.
Fig. 4.
Liposome based vaccine delivery. (A) Schematic illustration of the screening and reaction of the lipid-based material. (B) Chemical Structures of the lipidoid in the synthesis library. Reproduced with permission from Ref. [78].
Fig. 5.
Schematic illustration of the structure of sHDL nanodiscs and the lymph node targeting pathway of antigens and adjuvants-loaded sHDL nanodiscs, resulting in robust anti-tumor therapeutic efficacy with the elicitation of robust antigen-specific CD8α + cytotoxic T-lymphocyte responses. Reproduced with permission from Ref. [79].
As mentioned above, the vaccine delivery from the injection site to the lymph nodes includes transportation through the lymphatic vessels and the blood vessels, which can be further classified into direct transportation and hitchhiking transportation by APCs [76]. The particle size, charge, and specific ligands of the carrier have certain effects on maintaining the circulation stability of the vaccine, avoiding the clearance by the endothelial reticulum system, and enhancing the targeting ability toward lymph nodes. Owing to the diversity of synthetic lipid structures, liposomes with different properties and functions can be simply obtained by changing the lipid structure components or physically/chemically combining different lipids. This detachable carrier structure is conducive to the study of the structure–activity relationship of liposome vaccines, and the flexible adjustment of the liposome vaccine formula is expected to meet the individual needs of clinical vaccination. Meanwhile, the manufacturing process of liposomes is relatively easy and quality-controllable, and the development of microfluidic technology has created possibilities for obtaining liposome vaccines with a uniform particle size that promote lymph node targeting [80]. However, the relatively slow release rate of the cargos from liposomes highlights the need for the design of lymph node environment-triggered drug release systems for antigen presentation and improve overall immune efficiency.
5.2. Polymer-based vaccine delivery systems
Polymeric delivery systems consist of nano-solid particles, nanogels, micelles, polymer vesicles, and core–shell nanoparticles based on natural or synthetic polymeric materials [81], [82], [83], [84], [85], [86]. The surface of polymeric nanoparticles is rich in modifiable chemical groups, which could be used to imitate the biochemical characteristics of pathogens to trigger a potent immune response. Polymeric nanoparticles have been applied to mimic pathogens for the prevention and treatment of infectious diseases [87]. A lot of polymer materials present good biocompatibility and negligible toxic side effects in long-term use [88]. Notably, changing the molecular weight, chemical or physical cross-linking degree, hydrophilicity or hydrophobicity, and other polymers' properties can easily optimize its lymph node targeting ability and the dynamic features of the loading and release of vaccines. Therefore, polymer-based vaccine delivery systems have great potential to activate immunity effectively.
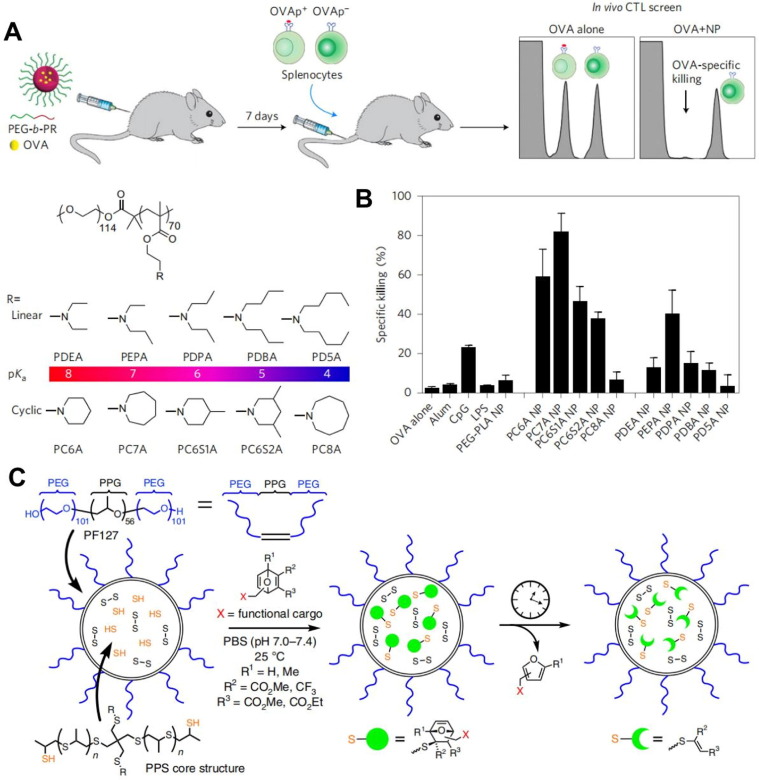
The key processes for vaccines to exert efficacy include the enrichment of antigens to lymph nodes, internalization by APCs, and cross-presentation. So far, almost all existing commercialized vaccines that can effectively activate the immune response need assistance from vaccine adjuvants, which unavoidably complicates the composition of vaccines. To simply the vaccine delivery system, Luo et al. constructed a polyethylene glycol-polymethacrylate-based polymeric nanoparticle vaccine system (PC7A) (Fig. 6 A-B) [89]. Its 29 nm size was conducive to lymph node accumulation, and the nitrogen-containing six-membered ring in its polymeric structure promoted the cytoplasmic delivery of antigens through the proton sponge effect and initiated the stimulator of interferon genes (STING) pathway for immune cell activation, which significantly suppressed the growth of B16-F10 melanoma and MC38 colon tumors. Collectively, incorporation of multifunctional polymers and simplification of vaccine delivery system could significantly improve the vaccination efficacy and holds great promise in clinical translation. Besides the vaccine delivery issues that hinder the vaccination efficacy, the penetration of the vaccines accumulated in the lymph nodes is another determining factor for potent immune response. To facilitate the vaccine penetration, Schudel et al. proposed a two-stage delivery platform (OND-NP) based on thiolated poly (propylene sulfide) (PPS) nanoparticles and oxaboranediene (OND) linkers (Fig. 6C) [90]. The optimized size of the vaccine delivery system allowed it to accumulate inside lymph nodes after intradermal injection and then spread to the deep lymph nodes after the degradation of OND. A small library was established by incorporated OND-NPs with various OND likers that could degrade at different timepoints, and the authors screened the nanoparticle structure to optimize the delivery efficacy and penetration capability of vaccines toward the lymph nodes and the desired immune cells. Studies have also shown that OND-NP with a programmable drug release rate can promote the delivery and accumulation of CpG adjuvants. After intradermal injection, it promoted the proliferation and activation of T cells, B cells, and DCs in the draining lymph nodes and effectively inhibited the development and metastasis of mouse EL4 lymphoma. The polymeric nanoparticles with the capability of overcoming the lymphatic and intra-lymph node transport barriers hold great promise for improving the immune effect of the therapeutic vaccine.
Fig. 6.
Polymer-based vaccine delivery systems. (A) Schematic illustration of the chemical structure of the polymer and the delivery strategy of the polymer-based OVA vaccine. (B) Specific CTL killing after activated by the polymer-based vaccine. Reproduced with permission from Ref. [89]. (C) Schematic illustration of the NP-OND preparation and controlled release of furan-tagged cargos. Reproduced with permission from Ref. [90].
Another efficient polymer-based vaccine delivery system is transdermal microneedle that can provide minimally invasive administration of therapeutic agents [91], [92]. Percutaneous microneedles-based vaccination is usually effective because the skin contains a more abundant network of antigen-presenting cells, which are critical and straightforward for initiating the immune response [93]. Due to the advantages of easy preparation, painless injection, effective transdermal delivery, superior biocompatibility and degradability, polymer microneedles-based vaccination has become an important and popular approach for transdermal vaccine delivery [94], [95].
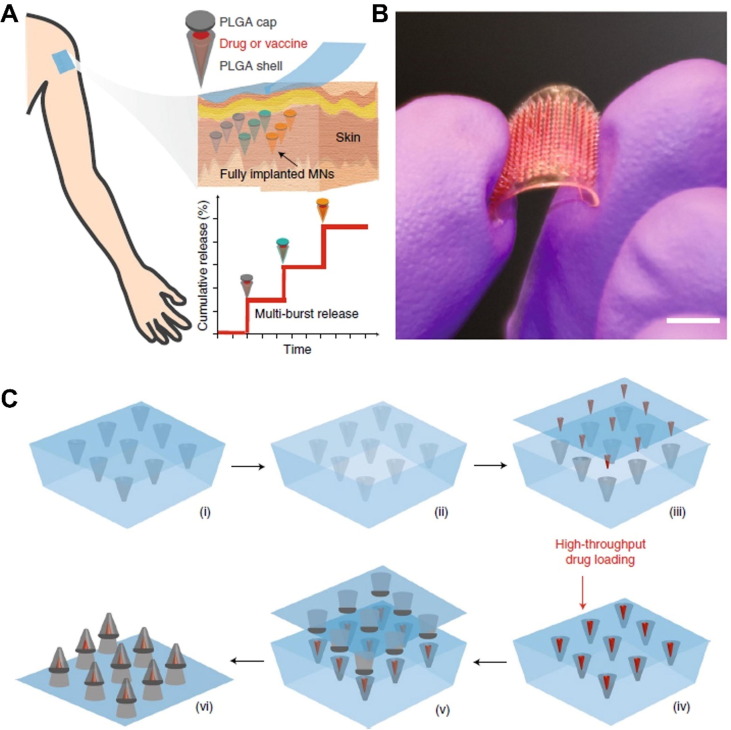
Sullivan et al. reported a dissolving polymer microneedles patch for vaccination to prevent influenza [96]. The microneedles were fabricated by polymerization of a vinyl pyrrolidone monomer within a microneedle mold, and the inactivated influenza virus was loaded into the microneedles for vaccination. Robust humoral and cellular immune responses were generated after the vaccination, as evidenced by the increased IgG titers and IL-4 and IFN-γ production. Recently, Tran et al. developed transdermal microneedles to achieve programmable release of the vaccine payloads for a single-dose vaccination [94]. The PLGA polymer-based microneedles with vaccine cargos were inserted into the skin with multiple burst releases profile over a long period of time (Fig. 7 A). The microneedle showed a core–shell structure, and vaccine agents were encapsulated in the cap and base layers (Fig. 7B-C). To verify the immunogenicity of the microneedle-based vaccine delivery strategy, the microneedle patches were loaded with OVA and inserted into the skin of rats. After 44 days, a strong anti-OVA immune response was observed that was comparable to the conventional multiple bolus vaccine injections. Moreover, after being loaded with Prevnar-13, a clinically approved vaccine for the prevention of Streptococcus pneumoniae related pneumonia, the microneedle-based vaccine delivery strategy induced a similar immune response compared to multiple subcutaneous bolus vaccination and protected the rats against lethal bacterial infection.
Fig. 7.
Microneedles based vaccine delivery. (A) Schematic illustration of the working mechanism of the microneedles-based vaccine delivery. (B) Optical image of the microneedle patch. (C) Schematic illustration of the preparation of the vaccine-loaded microneedles. Reproduced with permission from Ref. [94].
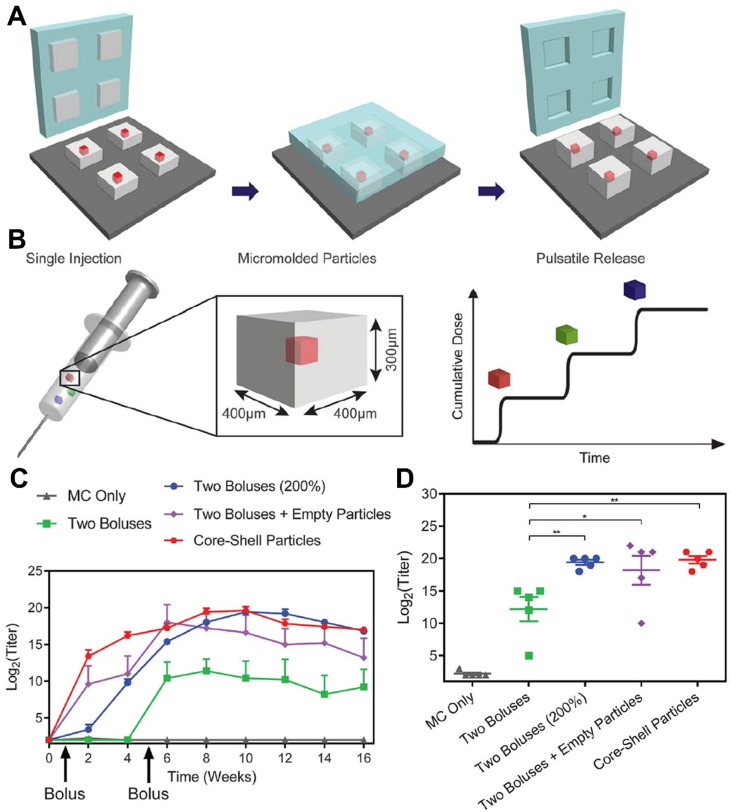
Many vaccines require multiple doses to complete the full immunization, which may cause economic burden and low patient compliance due to injection-related pain, relatively high cost, and complex injection plan, which is more significant in economically disadvantaged areas with insufficient medical resources [97]. In order to simplify the vaccination, McHugh et al. developed a “3D weaving” technology that used PLGA polymers to make a “core-shell” microparticle, which can encapsulate a large number of vaccines [98]. The PLGA microparticles were formed through a microfabrication method where the microparticle base was prepared and subsequently capped after loading with vaccines through a dispensing technique (Fig. 8 A). After one-time injection into the body, multiple release of vaccines can be achieved by precisely controlling the degradation time of PLGA, which is equivalent to the effect of multiple bolus vaccination (Fig. 8B). Specifically, the core–shell particle-based vaccine delivery strategy can achieve comparable immune activation with two boluses (200%) treatment as evidenced by the longitudinal and peak antibody titers (Fig. 8C-D).
Fig. 8.
Single injection vaccination strategy. (A) Schematic illustration of the preparation of the vaccine-loaded microparticles. (B) Schematic illustration of the single injection of the microparticles and the pulsatile release profile of the antigens. (C) Antibody titers over 16 weeks and (D) peak antibody titers after vaccination. Reproduced with permission from Ref. [98].
The vaccine delivery system based on polymeric nanoparticles shows unique advantages. First, the diversity of polymer structures provides a basis for the realization of co-delivery of multiple immune molecules [99]. The incorporation of environmentally responsive components is expected to build a smart vaccine delivery system based on the pathological characteristics of different diseases [100]. Second, certain polymers can function as immune adjuvants due to their immune modulation properties [89]. Moreover, the flexibility of designing polymeric nanoparticles through adjusting size, rigidity, charge, and chemical composition pave a facile way to optimize the effective vaccine delivery platform [89], [101]. Additionally, polymer-based microneedle vaccine delivery systems as a minimally invasive, painless, and effective vaccination strategy will bring convenient vaccination experience to patients [94], [102]. Despite the great advances of polymeric nanoparticle-based vaccine delivery systems, there are still several bottlenecks limiting the clinical translation. For example, factors such as organic solvents, temperature, or mechanical force in the polymer preparation process may cause the degradation or denaturation of sensitive peptide/protein-based antigens [103]. Also, the synthesis of polymeric materials is often complicated, which limits the scale-up production, maintaining batch-to-batch repeatability, and ensuring the clinical efficacy of polymeric systems based vaccination [104]. Moreover, whether the biological metabolites of various polymers in the body can induce non-specific immune responses still remains to be further verified. In addition, for the polymer-based microneedle vaccine delivery systems, the batch consistency, stability of the loaded vaccine, precisely controlled vaccine release, and sterilization of the delivery system need to be further improved to achieve wider application in the clinic.
5.3. Cell-based vaccine delivery systems
The essential roles of APCs, especially professional DCs, in vaccine-triggered immune system activation have been thoroughly investigated from the emergence of vaccination, while an emerging trend of applying DCs as vaccine delivery vehicles towards lymph nodes to bypass the vaccine delivery and internalization by DCs have attracted considerable interest [105]. Early studies have shown that applying DC vaccines labeled with autologous tumor antigens to patients with lymphoma can induce anti-tumor immune responses and promote tumor regression [106]. At present, a variety of DC vaccines such as HybriCell and CreaVax-RCC have been approved for clinical application, and a large amount of DC tumor vaccine products have entered clinical trials. The preparation of tumor vaccines based on DCs involves the acquisition of DCs and the introduction of different forms of tumor-associated antigens. The source of DCs includes the direct extraction or induced differentiation of monocytes, hematopoietic stem cells isolated from peripheral blood [107]. Notably, reprogramming of somatic cells is an emerging method of obtaining DC-like cells in recent years, so-called induced DC technology. Fibroblasts have been successfully reprogrammed into DC-like cells by inducing the expression of key transcription factors in the development of DCs [108]. Besides, the immune activation capability of the DC vaccine is limited by the absence of suitable cytokines to induce the maturation of DCs [109], which requires the assistance of bioengineering technologies such as RNA interference (RNAi), gene editing, and virus transduction to create conditions for enhancing the antigen presentation ability and the overall immune mobilization ability of DC vaccines [110], [111], [112]. For example, the introduction of specific cytokine-related genes coding for Toll-like receptors (TLR) and CD40L is of great significance to promote the maturation of DCs [113]. Furthermore, the knockout of the YTHDF1 gene, which is related to intracellular antigen degradation, has been shown to improve the efficiency of antigen cross-presentation and effectively suppress the growth of B16 melanoma and MC38 colon tumors [114]. The down-regulation of the immune checkpoint PD-L1 ligand expression on the surface of DCs also helps DC vaccines to efficiently prime T cells, while the upregulation of the expression of CXCL9 and CCL21 is conducive to the recruitment of a variety of immune cells [115], [116], [117]. It is worth noting that the DCs-mediated vaccination immune response varies with the source of DCs, highlighting the significance of designing the personalized DC vaccines [118].
The DC vaccine holds great promise to bypass several limiting factors in antigen internalization and presentation, such as in vivo cellular uptake of antigens, lysosomal escape, and translation of mRNA antigens. However, the preparation process of the DC vaccine involves multiple biotechnology approaches with certain technical bottlenecks, which is more time-consuming and expensive than traditional vaccines. More regrettably, the clinical efficacy of the DC vaccine is not satisfactory with an objective response rate (ORR) less than 15% [119]. A potential factor leading to the low efficacy of the DC vaccine may be the compromised viability of the modified DC cells. The application of biomaterials as scaffolds for adoptive cells may address this issue [107]. For example, DC vaccines coated by the fibrin gel could maintain good viability, where a single injection showed equivalent efficacy to multiple injections of free DC vaccine in inhibiting TC-1 tumors [120]. Moreover, studies have shown that only 5% of intradermal DC vaccines actually migrated to lymph nodes [121]. Yang et al. accordingly further improved the lymph node targeting ability of DC vaccine by up-regulating the lymph node homing factor CCR7 of DC vaccine [122]. Furthermore, combining DC vaccines with traditional therapy modalities such as chemotherapy and radiotherapy or other immunotherapies, including immune checkpoint suppression, could achieve better anti-tumor effects.
5.4. DNA nanodevice-based vaccine delivery systems
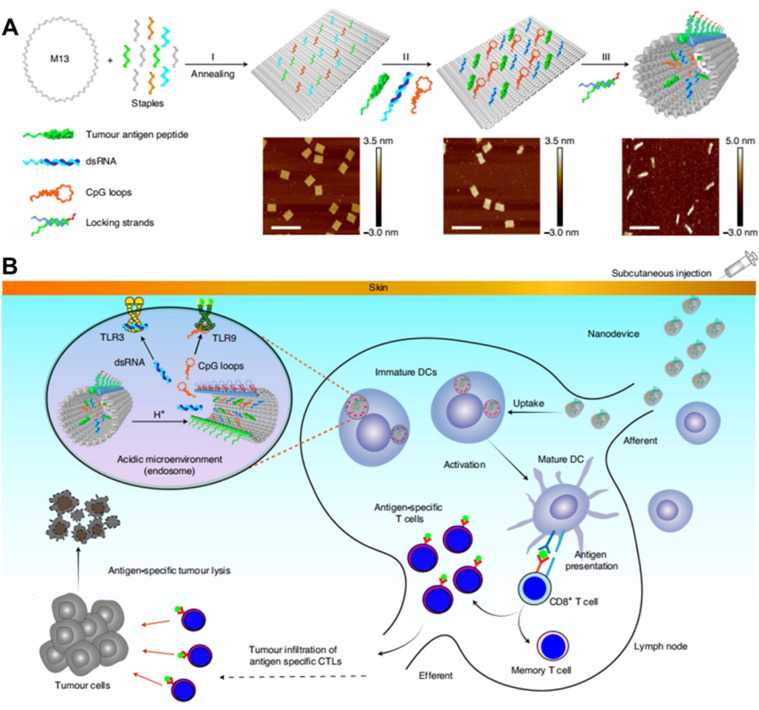
In 1982, Nadrian Seeman proposed the concept of DNA nanotechnology, aiming to use the strong coding structural characteristics of DNA to construct novel nanomaterials [123]. Later in 2006, Rothemund et al. invented DNA origami, which has shown application prospects in the fields of nano-optoelectronic devices and biomedicine [124]. DNA origami can be used to construct a smart DNA-based drug delivery system that specifically recognizes and responds to the nucleolin on the vascular endothelium to release carried thrombin [82]. DNA nanomaterials based on molecular self-assembly possess the characteristics of controllable size, facile drug loading, cost-effective preparation, and biodegradability, presenting excellent application potential in the targeted delivery of vaccines to lymph nodes [125], [126]. For example, Liu et al. used nucleic acid molecular hybridization to co-load antigen peptides and nucleic acid immune adjuvants (TLR3 agonist dsRNA and TLR9 agonist CpG) into hollow DNA nanotubes to develop a tumor vaccine (Fig. 9 A-B) [127]. The loading ratio of the different components is highly consistent with the grafting ratio of their nucleic acid complementary strands on the DNA carrier. Moreover, the vaccine can be efficiently enriched in the lymph nodes due to its optimized size. Afterwards, the slightly acidic environment in DCs opened the nucleic acid molecular lock responsively and released the functional components, thereby inducing an anti-tumor immune response and effectively suppressing MC38 colon cancer growth.
Fig. 9.
Construction and working mechanism of the DNA nanodevice based vaccine. (A) Preparation of the DNA nanodevice vaccine constructed by M13mp18 DNA, tumor antigen peptide, dsRNA, CpG loops, and locking strands. (B) Schematic illustration of the lymph node targeting, acidic microenvironment responsive antigen release, and specific immune activation of the DNA nanodevice vaccine. Reproduced with permission from Ref. [127].
The DNA-based vaccine delivery system can co-deliver various immune molecules, including antigens, adjuvants, and agonistic/antagonistic antibodies, to achieve anti-tumor immunotherapy by regulating the immune environment and promoting the interaction of immune cells [128], [129], [130]. More importantly, the loading of various immune molecules can be located and quantified, which is conducive to the coordinated and on-demand release of antigen and adjuvant spatiotemporally for enhanced vaccination efficacy [67]. Besides, DNA origami development has enabled DNA nanomaterials to expand from flat structures to frames, curved surfaces, and even other complex and ordered structures [131], providing a basis for further exploration of the optimal geometry in lymph node targeting. However, there are still substantial limitations for DNA-based vaccine delivery systems. For example, the stimuli that promote the unwinding of the DNA double-strand are limited [127], making the responsive release mode of the vaccine relatively less intelligent. Certain chemical modifications to DNA molecules may further expand the development of responsive vaccine delivery systems based on DNA nanomaterials [132]. Besides, forming hollow DNA nanotubes from a DNA flat plate does not have precise winding directionality, which may expose the antigen/adjuvant on the outer surface of the hollow tube, resulting in the degradation of antigen/adjuvant by multiple enzymes in human bodies before reaching the lymph nodes. Therefore, combining other physical or chemical methods (such as increasing the difference in flexibility between the inner and outer surfaces, etc.) to further control DNA origami's spatial directionality will help to better achieve the safety and effectiveness of vaccine delivery. Finally, whether the introduction of exogenous DNA sequences will induce unfavorable gene recombination and whether the biocompatibility of DNA nanocomposites will change if mixed with other materials, remain to be further elucidated to promote the clinical translation of DNA nanodevice-based vaccine delivery systems [125].
5.5. Inorganic materials-based vaccine delivery systems
Inorganic nanoparticles, including gold nanoparticles, quantum dots, carbon nanotubes, iron oxide nanoparticles, and silica nanoparticles, are widely used in catalysis, optoelectronics, and medical fields because of their excellent surface selective adsorption, good conductivity, special quantum size effect, and macroscopic quantum tunnel effect [133], [134]. Moreover, investigation of the underlying mechanism of innate immune regulatory properties of inorganic nanoparticles has become one of the new research hotspots in the field of immunity [135], [136]. Studies have shown that inorganic nanoparticles with large specific surface area and high specific surface energy can stably adsorb biomacromolecules such as antigens, thus showing great application prospects in vaccine delivery [137].
Inorganic nanoparticle-based vaccine delivery systems can enhance immune activation by promoting drainage of antigens to the lymph node, uptake of antigens by dendritic cells, maturation of dendritic cells, and antigen presentation (DUMP cascade) [10], [49]. Among them, mesoporous silicon nanoparticles (MSNs) have become a class of excellent vaccine delivery vectors due to their large surface area, easy modification, negative charge, and hydrophilicity [138]. Hong et al. explored the effect of mesoporous silica nanoparticles with different pore sizes (7.8, 10.3, and 12.9 nm) on the immune response of the vaccines [49]. The results showed that the increase of mesoporous pore size promoted the efficiency of MHC class I antigen presentation in the DUMP cascade process and enhanced the potency of cellular immune response. It is worth noting that silica nanoparticles with large pore size could efficiently encapsulate complex antigens consisting of cell lysate and cell membrane at the same time and induced a strong cellular immune response against B16 melanoma without the assistance of other adjuvants. Besides, the degradation rate of mesoporous silica nanoparticles with large pore size was relatively faster, which could promote the release of antigens in lymph nodes. Besides the application in cellular immune response, vaccine delivery systems based on inorganic materials are also used in humoral immune research mediated by antibody secretion. Similar to cellular immunity, effective humoral immune stimulation requires prolonging the retention time of antigens in dendritic cells to present them to B cells in lymph node follicles [139]. Zhang et al. used gold nanoparticles (AuNPs) to deliver the model antigen OVA into lymph follicles and explored the influence of gold nanoparticles size on the fate of antigen in lymph nodes [140]. Due to the long-term retention effect in lymph nodes, the antigen delivery of large-sized (50–100 nm) gold nanoparticles was 175 times higher than that of small-sized (less than 15 nm) gold nanoparticles, and the production of specific antibodies was also increased by five times.
The wide range of sizes, various shapes and structures, and controllable surface charge of inorganic nanoparticles are favorable for their lymph node vaccine delivery. The easy modification of its surface can support the co-delivery of multiple immune therapeutics, and its considerable surface area can realize the efficient exposure of antigens and other immune-related molecules [141]. Meanwhile, the resistance of inorganic materials to general chemical degradation can prevent the denaturation of biomacromolecules such as antigens attached or encapsulated and prolong their half-life in vivo, which can promote the efficiency of antigen presentation [137]. Moreover, inorganic nanoparticles also have unique properties, including optical imaging functions and thermal and magnetic properties [142], [143], which provides a basis for the combination of immunotherapy with photothermal therapy, magnetic resonance imaging, and other diagnostic and therapeutic treatment modalities. However, only 10% of the nanoparticles approved for clinical use nowadays belong to the category of inorganic nanoparticles [144]. Compared with other materials, inorganic nanoparticles are usually slowly biodegradable, imposing significant concerns on their long-term in vivo application. Furthermore, some recent studies have pointed out that commonly used inorganic nanoparticles such as titanium dioxide, silicon dioxide, and gold nanoparticles can accelerate the infiltration and extravasation of breast cancer cells after intravenous injection, increase the degree of existing cancer metastasis and promote the emergence of new tumor metastases [145].
5.6. Peptide/protein-based vaccine delivery systems
Due to good biocompatibility and relative accessibility, peptide/protein based biological nanomaterials has been widely used in nanoreactor, drug delivery, disease diagnosis, and vaccine development [146], [147], [148]. The structure of peptide/protein biomacromolecules could be easily adjusted to control the loading and release of vaccine and further be subtly designed for a better vaccine delivery system with the help of computation technology [149], [150]. Currently, viral-like particles (VLPs) and non-viral caged protein nanoparticles (CPs) are investigated as protein-based carriers for vaccine delivery [151]. VLPs retain the structure and conformation of the virus but lack viral genetic materials, so that they do not have the safety concerns like self-replication and strong pathogenicity [152]. The proteins used for VLPs construction mainly come from the capsid proteins of icosahedral plant virus (such as cowpea mosaic virus CPMV) [153], baculovirus (such as tobacco mosaic virus TMV) [154], and bacteriophage Qβ [155]. Similar to VLPs, CPs are highly ordered and symmetrical, which is composed of proteins from mainly heat-shock proteins [156], bacterial E2 proteins [157], and ferritin [158]. Generally, the sizes of these protein nanoparticles' diameters are between 20 and 200 nm, which are suitable for efficient lymph node targeting.
The vaccine delivery systems based on the self-assembled protein carrier have emerged as an effective and safe vaccine strategy. Wang et al. reported a hepatitis B virus (HBV) vaccine with both preventive and therapeutic effects based on fireball ferritin carrier (NP-PreS1) [159]. PreS1, as one type of HBV capsid protein, was decorated on protein nanoparticles' surface by using the Spytag-Spycatcher protein assembly system. The NP-PreS1 was enriched in lymph nodes after subcutaneous inoculation, and PreS1 was delivered to specific bone marrow cells (SIGNR1+ dendritic cells and SIGNR1+ macrophages) responsible for the recognition of various virus particles, further activating T follicular helper cells and B lymphocytes. The vaccine could induce a high level and long-lasting anti-PreS1 immune response and achieve sufficient virus clearance and serological changes in the chronic HBV mouse model. Escolano et al. developed an RC1 chimeric VLPs vaccine (RC1-4fill VLPs) based on Spytag-Spycatcher protein assembly system, which effectively activated B lymphocytes to express broad-spectrum neutralizing antibodies precursor molecules in polyclonal repertoires in mice, rabbits, and rhesus monkeys [160]. The antibody produced by RC1-4fill VLPs could recognize the V3-glycan patch on the envelope protein of HIV-1. RC1-4fill VLPs could mask competitive non-targeted epitopes through the polymerization of RC1 on VLPs, thus minimizing competition into germinal centers and promoting the production of specific antibodies against the V3-glycan patch.
Protein-based vaccine vectors have optimizable lymphatic targeting functionalities, providing a new platform for vaccine delivery. Employing gene fusion or covalent coupling, multiple epitopes of antigens can be integrated into the protein carrier, and then their immunogenicity and the immune responses can be regulated. The fusion of some functional protein fragments with antigenic peptides can further enhance their immune activation effect. For example, conjugation of bacteria-derived proteins with antigenic peptides can induce a strong local immune response [161]. The coupling of the transmembrane peptide with antigenic peptide can increase the cellular entry of antigen and improve its delivery efficiency [162]. Inspired by the geometry and functionality of virus, protein-based nanoparticles mimicking the virus after incorporation of virus-related biological components may increase APCs' antigen uptake, leading to enhanced immunogenicity [151]. Although immunogenicity is one of the critical properties of vaccines, antibody reaction based on the immune recognition of protein vector itself may cause neutralization of vaccines. For example, CPMV proteins induce specific IgG antibodies in vivo, leading to a rapid clearance of the protein-based vaccines [163]. Modification of the carrier with PEG or special membrane proteins such as CD47 may avoid the non-specific clearance mediated by phagocytes and increase the plasma circulation time [164], [165].
5.7. Virus based vaccine delivery systems
Over the decades, various viral vectors, including lentivirus, retrovirus, adenovirus (AV), and adeno-associated virus (AAV), have been used to transfer functional nucleic acids to cells and tissues, among which lentivirus and retrovirus show the outstanding capability to integrate their genes into the genome to achieve long-term stable foreign genes expression. AAV is one of the most powerful tools to study gene function in vivo because of its safety, low immunogenicity, and long-term expression capability [166]. The combination of AAV vector with transposon and CRISPR system can achieve efficient gene editing of CD8 T lymphocytes and screen several new targets for tumor immunotherapy [167]. AAV vector can also be utilized to transfer CRISPR activation molecules to activate several specific target gene expression in vivo and improve the effectiveness of immune recognition and immune clearance [168]. However, the capacity of AAV to carry foreign genes is limited [169], which can be well compensated by AV. In addition, AV has been widely used in gene transduction, gene therapy, vaccination, and other fields due to its wide infection range, high infection efficiency, easy operation, and no-host genome integration [170], [171]. More importantly, the AV-based vaccine requires a shorter development time than traditional inactivated and attenuated vaccines [172], which is conducive to the rapid containment of infectious diseases, including the COVID-19 pandemic.
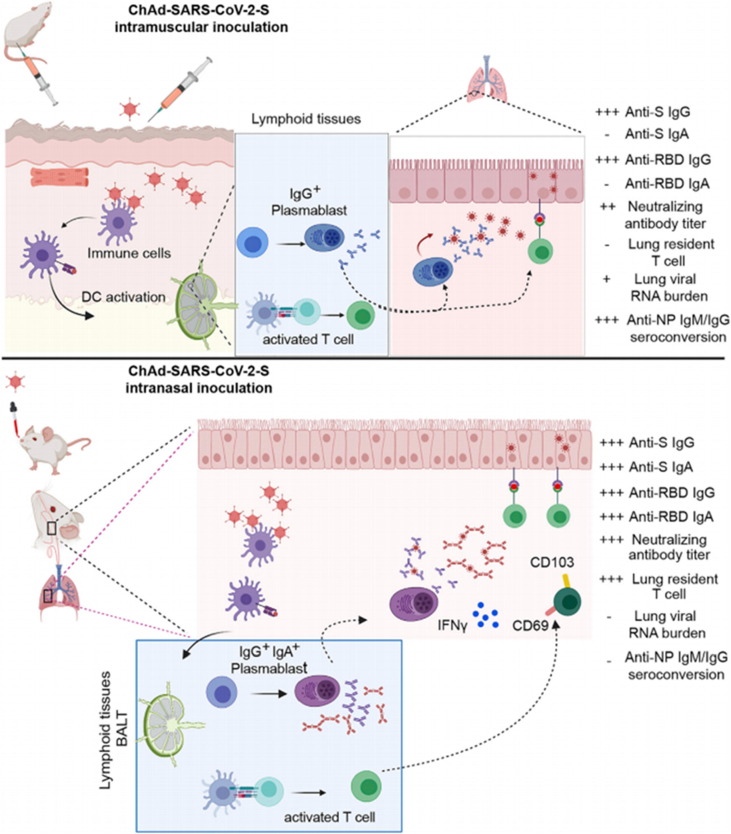
The AV-based vaccine takes AVs as the carrier and recombines the protective antigen gene into the genome of AVs, presenting the antigenicity but no toxicity against the virus, to trigger specific immune response. At present, a new type of AV-based coronal vaccine (Ad5-nCoV) has been authorized for use in China, Mexico, Pakistan [171]. The vaccine integrates the spike protein (S) gene of SARS-CoV-2 into the genome of replication-deficient human AVs type 5 (Ad5) to prevent SARS-CoV-2 infection. It is well known that SARS-CoV-2 usually infects the body from the nasopharynx [173]. Although intramuscular injection can effectively activate systemic immunity, it fails to eliminate local infection effectively. Therefore, intranasal inoculation can provide immunity for the cells in the nasal cavity and pharynx (Fig. 10 ) [174]. Similarly, Feng et al. reported a codon-optimized AVs vaccine (Ad5-S-nb2), which achieved a one-month-long immune effect in mice and rhesus macaques after a single vaccination [175]. Intramuscular inoculation can induce systemic humoral and cellular immunity, while intranasal inoculation has a weak effect on cellular immunity [174]. Wu et al. further evaluated the immune effect of intranasal injection of Ad5-nCoV [176]. Studies have shown that single intranasal inoculation can effectively protect the upper and lower respiratory tract from SARS-CoV-2 infection in mice.
Fig. 10.
Schematic illustration of the working mechanism of the adenovirus-vector based vaccine for SARS-CoV-2 by intramuscular delivery and intranasal delivery. Reproduced with permission from Ref. [174].
Compared with inactivated or attenuated virus vaccines, nucleic acid vaccines have unique vaccine development advantages, such as the simple preparation process and short manufacture time [177]. Viral vector-based vaccine delivery system can protect nucleic acid antigens in vivo, overcome the biological barrier of the multilayer cell membrane, and promote its transport to antigen-presenting cells [178]. Also, viral vectors can be obtained by cell culture in vitro with low production cost and easy industrialization. What is more exciting is that with advanced molecular biology technology, viral vectors can be used as a general vaccine platform for efficient delivery of antigen molecules to produce a wide range of protective and strong immunity against a variety of infectious diseases. However, there are still some deficiencies in virus vectors. For example, most viral vectors are weak in disease site targeting, which may cause adverse reactions in normal tissues and cells. Besides, some viral proteins can stimulate the immune response, which will inevitably affect the first and subsequent immunization [179].
6. Clinical vaccine delivery systems
The ongoing novel coronavirus COVID-19 pandemic brings considerable challenges to human health [180]. Correspondingly, the vaccine research for the COVID-19 is also in full swing. In terms of scale and speed, the global vaccine research and development for the COVID-19 pandemic are unprecedented, bringing vaccine research to a new climax [181]. The most representative one is the BNT162b2 mRNA COVID-19 vaccine jointly developed by BioNTech and Pfizer. On December 11, 2020, the US FDA authorized Pfizer’s BioNTech COVID-19 vaccine to be used for active immunization under an emergency use authorization to prevent the COVID-19 pandemic caused by the SARS-CoV-2 virus (Table 1 ) [182]. Besides, the mRNA-1273 vaccine, jointly developed by Moderna and the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health, is as effective as 94.1% in preventing COVID-19 infection according to the results of phase III clinical trial [183]. Both vaccines utilize lipid materials as the vaccine delivery systems, demonstrating the huge application prospects of liposome delivery systems in vaccine development [184], [185]. Lipid-based vaccine delivery systems have also shown broad application prospects in treating tumors and other infectious diseases [186], [187]. For example, the MPER-656 liposome vaccine developed by NIAID to treat HIV infection is in phase I clinical research, and the GLA-LSQ liposome vaccine developed by NIAID to treat Plasmodium Falciparum Infections is also in phase I clinical trial. Moreover, in the treatment of lung cancer, glioma, ovarian cancer, and cervical cancer, lipid-based vaccine delivery systems are in accelerating clinical research and development [188], [189].
Table 1.
Clinical trials & FDA approved product of lymph node-targeted vaccine delivery systems.
| Catalog | Name | Company/Sponsor | Phase | Disease |
|---|---|---|---|---|
| Liposome | BNT162b2 | Pfizer&BioNTech | Approved 2020 | COVID-19 |
| CVnCoV | CureVac AG | Phase 3 | COVID-19 | |
| mRNA-1273 | ModernaTX, Inc. | Phase 3 | COVID-19 | |
| MPER-656 | NIAID | Phase 1 | HIV Infections | |
| BLP25 | ECOG-ACRIN Cancer Research Group | Phase 2 | Lung Cancer | |
| RNA-LP | University of Florida | Phase 1 | Adult Glioblastoma | |
| W_ova1 | BioNTech | Phase 1 | Ovarian Cancer | |
| GLA-LSQ | NIAID | Phase 1 | Plasmodium Falciparum Infections | |
| PDS0101 | M.D. Anderson Cancer Center | Phase 2 | Cervical Cancer | |
| Polymer | Grippol® Plus | NPO Petrovax | Phase 3 | Influenza Infections |
| DC cell | PROVENGE® | Dendreon | Approved 2010 | Prostatic Cancer |
| DC/AML vaccine | Dana-Farber Cancer Institute | Phase 2 | Acute Myelogenous Leukemia | |
| DC/MM fusion vaccine | NHLBI | Phase 2 | Multiple Myeloma | |
| Ad.p53-DC vaccines | H. Lee Moffitt Cancer Center and Research Institute | Phase 2 | Small Cell Lung Cancer | |
| PEP-DC vaccine | Centre Hospitalier Universitaire Vaudois | Phase 1 | Pancreatic Adenocarcinoma | |
| HER-2 pulsed DC vaccine | H. Lee Moffitt Cancer Center and Research Institute | Phase 1 | Breast Cancer | |
| MIDRIX4-LUNG DC vaccine | University Hospital, Ghent | Phase 1 | Non-small Cell Lung Cancer | |
| DC1 vaccine | Roswell Park Cancer Institute | Phase 2 | Cancer of Prostate | |
| MART-1 peptide DC vaccine | Jonsson Comprehensive Cancer Center | Phase 2 | Melanoma | |
| nDC vaccine | Radboud University | Phase 3 | Melanoma | |
| DC vaccine | Oslo University Hospital | Phase 3 | Glioblastoma | |
| Peptide/protein | EBV gp350-Ferritin vaccine | NIAID | Phase 1 | Epstein-Barr Virus Infections |
| Virus | ERVEBO® | Merck | Approved 2019 | Ebola Infections |
| Ad5-nCoV | CanSino Biologics Inc. | Approved 2021 | COVID-19 | |
| AZD1222 | AstraZeneca | Approved 2020 | COVID-19 | |
| Gam-COVID-Vac | Gamaleya Research Institute of Epidemiology and Microbiology | Approved 2020 | COVID-19 | |
| Ad26.COV2.S | Janssen Vaccines & Prevention B.V. | Approved 2020 | COVID-19 | |
| Ad26.ZEBOV | Janssen Vaccines & Prevention B.V. | Phase 3 | Ebola Infections | |
| Ad26.Mos4.HIV | Janssen Vaccines & Prevention B.V. | Phase 3 | HIV Infections | |
| ChAdOx1-HBV | Vaccitech Limited | Phase 1 | Hepatitis B Infections | |
| Ad26.RSV.preF | Janssen Vaccines & Prevention B.V. | Phase 2 | Respiratory syncytial virus Infections | |
| Ad5Ag85A | McMaster University | Phase 1 | Tuberculosis | |
| ChAdOx1-MVA 5 T4 | University of Oxford | Phase 2 | Prostate Cancer | |
| Ad-CEA | NCI | Phase 2 | Colorectal Tumors |
Though there are existing side effects caused by most viruses to human health, including SARS-COV-2, scientists gradually use the virus to develop vaccine delivery systems [190]. Encouragingly, in December 2019, the U.S. FDA announced the approval of Merck’s Ervebo vaccine to be used to prevent Ebola virus disease caused by Zaire ebolavirus infection in people over 18 years of age. Ervebo is a genetically engineered live attenuated vaccine consisting of a modified vesicular stomatitis virus (VSV) as a carrier and an important glycoprotein on the Ebola virus's surface [191], [192]. Meanwhile, another promising virus-based vaccine for Ebola infections developed by Janssen Vaccines & Prevention B.V., Ad26.ZEBOV, is in the Phase III clinical trial [193]. Several adenovirus-based vaccines for COVID-19 have been approved for clinical application, including Ad5-nCoV by CanSino Biologics Inc., AZD1222 by AstraZeneca, Gam-COVID-Vac by Gamaleya Research Institute of Epidemiology and Microbiology and Ad26.COV2.S by Johnson & Johnson, demonstrating the tremendous clinical application potential of adenovirus vectors in the field of vaccine delivery [171], [194], [195], [196]. Besides, virus-based vaccine delivery systems for HIV infections, hepatitis B infections, respiratory syncytial virus infections, tuberculosis, prostate cancer, and colorectal tumors are also in development for clinical application [197], [198], [199], [200].
As the key initiator of the immune response, DCs have also been developed into vaccine delivery systems in clinical research [107]. In April 2010, Dendreon's autoimmune cell therapy PROVENGE® also known as Sipuleucel-T, was approved by FDA for the treatment of castration-resistant (hormone-refractory) prostate cancer (CRPC) as the first therapeutic tumor vaccine approved by FDA [201]. Inspired by the success of PROVENGE®, many DC-based vaccine delivery systems have entered the clinical trials especially for cancer treatment, such as Ad.p53-DC vaccines vaccine by H. Lee Moffitt Cancer Center and Research Institute for small cell lung cancer, PEP-DC vaccine by Centre Hospitalier Universitaire Vaudois for pancreatic adenocarcinoma, DC/MM fusion vaccine by National Heart, Lung, and Blood Institute for multiple myeloma, etc [202], [203], [204].
Furthermore, Grippol® Plus assisted by polymer-based vaccine delivery systems, is in Phase III clinical trial to treat influenza infections [205]. For peptide/protein-based vaccine delivery systems, EBV gp350-Ferritin vaccine developed by NIAID is in Phase I clinical trial for the treatment of Epstein-Barr Virus Infections. Other delivery systems like inorganic materials-based vaccine delivery systems and emerging DNA nanodevice-based vaccine delivery systems are still under development towards clinical progress. Collectively, for clinical studies of vaccines, despite the antigen forms (protein, peptide, mRNA, or DNA), appropriate delivery systems are essential for the stability, safety, and lymph node delivery efficiency of vaccines [206], [207], [208]. The key to the future development of vaccine delivery is to take advantage of the delivery system to help the vaccine reach the lymph nodes and play a higher efficacy at a same or lower dose to reduce the systemic toxic and side effects. Personalized vaccine delivery systems based on gene technology, virology, and cell biology will play a critical role in disease treatment and prevention towards precision medicine, including cancer and infectious diseases [209], [210].
7. Conclusion and outlook
The discovery and successful development of vaccines is a milestone in human history to activate the body's immune system fighting against various types of human diseases. Notably, as the main immune regulating tissues, the lymph node is widely distributed in the human body and plays an essential role in the immune system [211], [212]. Therefore, the delivery of antigens towards lymph nodes is critical for the achievement of better vaccination efficacy. However, free vaccine administration is suffering from the low targeting efficiency of antigens towards lymph nodes, resulting in a higher dose needed to take desired effect, which potentially causes systemic or local side effects [213]. Thus, the lymph node targeting ability of vaccine delivery systems urgently needs to be further developed and improved.
The vaccine delivery systems currently developed in preclinical and clinical studies include various vaccine systems based on liposomes, polymers, cells, inorganic materials, DNA, peptides/proteins, and virus-based vaccine delivery systems (Table 2 ) [49], [78], [89], [114], [152], [174], [215]. Compared with free antigen-based vaccination, the design of the vaccine delivery systems can effectively improve the targeting ability and delivery efficiency of antigens towards lymph nodes, and increase the controllability and biosafety of the vaccines. Different delivery systems often use selected administration routes and exhibit different lymph node targeting capabilities based on the unique designing and properties of the carriers. For example, liposome-based vaccine delivery systems can be injected intramuscularly or subcutaneously [77]. While the polymer-based vaccine delivery systems can usually be injected subcutaneously, or it can be made into microneedles for delivery through non-invasive microneedle insertion [89], [94]. As for inorganic materials-based vaccine delivery system, protein/peptide-based vaccine delivery system and DNA nanodevice materials-based vaccine delivery system can also be delivered by subcutaneous injection [127]. For different delivery systems or even unified delivery systems, the administration route depends on the demand of disease treatment, delivered antigens and the specific structure of the carriers. For example, to develop a single dose vaccine for patient compliance improvement, microneedle and micro-fabricated particles have been developed with a pulsatile release profile after subcutaneous injection [96], [98]. Furthermore, for virus-based delivery systems in the treatment of lung diseases such as COVID-19, compared with intramuscular injection, a single dose of intranasal delivery achieved high levels of immune activation and superior COVID-19 protection [174].
Table 2.
Summary of the vaccine delivery systems towards lymph nodes. LN: lymph node; OVA: ovalbumin; CPG: 5′-C-phosphate-G-3′.
| Vaccine delivery systems | Antigen type | Administration routes | Delivery efficiency | Disease type |
|---|---|---|---|---|
| Liposome | Recombinant Pfs25 | Intramuscularly | Enhanced several-fold | Malaria [77] |
| Liposome | mRNA | Subcutaneously | Induced protein expression | Tumor [78] |
| Lipoprotein | Antigens and CpG | Subcutaneously | Increased LN accumulation | Tumor [79] |
| Polymer | OVA | Subcutaneously | Efficient LN accumulation | Tumor [89] |
| Polymer | Prevnar-13 | Microneedle insertion | Controlled antigen release | Pneumonia [94] |
| Polymer | Inactivated influenza virus | Microneedle insertion | Efficient LN immune activation | Influenza [96] |
| Cell | Hybrid cells | Intradermal Immunization |
Immune function recovery | Tumor [214] |
| DNA nanodevice | Tumor antigen peptide/CpG loop/dsRNA | Subcutaneously | Enhanced antigen-fluorescence signals in LN | Tumor [127] |
| Inorganic materials | OVA | Subcutaneously | Much greater extent in LN | Tumor [49] |
| Peptide/protein | preS1 | Subcutaneously | Mainly captured by SIGNR1+ DCs and macrophages in LN | Chronic hepatitis B [159] |
| Virus | Prefusion stabilized spike protein | Intramuscularly/ Intranasally | Protecting upper and lower respiratory tracts | SARS-CoV-2 [174] |
It is worth noting that the full protective immunity usually requires multiple vaccine injections, which will bring increasing difficulties for full vaccination and decrease the compliance of patients due to increased injection pain. Particularly, in some developing countries, the limited medical resources and patient access significantly hampered the patients from getting necessary protective immunity against infectious diseases, which is more vital under the context of COVID now [97], [216]. Thus, replacing a multiple-dosing vaccine with a single injection vaccine with comparable or better vaccination efficacy will be of great significance in fighting infectious diseases globally, which is more impactful in developing countries with resource-poor setting by increasing vaccine coverage. To accomplish single injection vaccine, controlled vaccine release systems have been developed to achieve long-term vaccine release, while the mechanistic study revealed that sustained and slow vaccine release induced rapid elution of antigens from adjuvants in vivo [217], [218], diminishing the immune response and subsequent protective immunity. Recently, vaccine delivery system with pulsatile-release profile, which could release loaded vaccines at pre-set time points mimicking the multiple bolus vaccine dosing plan, has been developed, showing comparable vaccination efficacy with multiple bolus injections. The micro-fabricated particles and microneedle patch, where the release time of loaded vaccine could be precisely controlled by degradation of polymers, have been designed for single injection vaccination [94], [96], [98]. However, there are still challenges in applying this polymer-based single vaccine delivery systems. The gradual degradation of polymers will generate unfavorable environment for encapsulated vaccine, potentially deactivating the vaccine. For instance, the activity of pH-sensitive vaccine encapsulated in PLGA-microparticles will be impacted during the degradation of PLGA generating acidic environment [219]. Also, the majority of vaccine in the single vaccine delivery system displays a burst release as proposed, while there is still basal release of vaccine during the degradation of delivery system, which potentially causes diminished immune response. In addition, the intervals between vaccine dosing regimens are usually set for few weeks to few months, which put forward significant challenges in protecting vaccine activity in vivo [220]. Collectively, vaccine-friendly polymers with better biocompatibility and vaccine protection against environment, and more precise control on pulsatile-release fashion will be key for the development of single injection vaccine.
Although outstanding achievement has been made so far in the field of engineered lymph node targeting delivery systems for vaccine treatments by adjusting the particle size, surface charge, hydrophobicity, and targeting ligand conjugation, currently, there are still challenges that need to be addressed further to improve the delivery efficacy vaccine agents: 1) Liposome materials have shown good application prospects in pre-clinical and clinical vaccine development. However, the rapid leaking of antigens from liposome led by liposome-lipoprotein interaction during the blood circulation largely limits the intravenous administration of liposomes [221]. 2) The biosafety and immunomodulation capability of biomaterials themselves should be thoroughly investigated. 3) Although the new DNA nanodevice has demonstrated vaccine delivery efficacy and disease control outcomes, The stability of the delivered antigens and consistency of treatment efficacy should be studied particularly for potential clinical application. 4) Inorganic nano-vaccine delivery system has shown strong loading efficacy in preclinical research, but its biodegradability brings significant challenges to the on-demand release of encapsulated antigens and raises long-term safety concerns. 5) The immunogenicity of the protein carrier itself will reduce the specificity of the immune response activated by the antigens, thus limiting the in vivo retention and persistence of vaccine delivered by protein carriers. 6) The targeting ability and quality control of viral vectors need to be further improved to avoid systemic side effects on normal cells. Besides vaccine systems mentioned above, although other innovative vaccine delivery systems like T cell-based vaccine delivery are attracting significant attention, the quality of T cells after vaccination and difficulties of manufacturing hinder its clinical application [222], [223]. What is more, the emerging new vaccine agents including mRNA vaccines and virus-based vaccines, put forward higher requirements for the applicability of current existing delivery systems to maintain equivalent lymph node targeting efficacy. Therefore, based on the current state of lymph node targeting vaccine delivery systems, several improvements could be made to overcome existing limitations. First, it is crucial to deeply understand unknown physiological mechanisms of vaccine delivery in the human body in order to design a better delivery system with enhanced targeting efficiency on account of the biological features of lymphatic systems. Also, a more solid study of how the properties of immune systems change in different disease models is necessary, which could potentially influence targeting vaccine delivery through the lymphatic system and its retention time in lymph nodes. Moreover, innovative bio-inspired vaccine delivery systems, for example, dendritic cell-based cancer vaccines, show a great potency of better therapeutic efficacy, which might serve as good biocompatible delivery platforms to achieve lymph node targeting [224].
Overall, the improvement and innovation of lymph node targeting vaccine delivery systems are critical for preventing and treating human diseases. Though the existence of difficulties faced by the design of vaccine delivery systems, the adjustability and design flexibility in the various vaccine carriers make it possible to promote the targeting ability of vaccination towards lymph nodes. With the development of genetic engineering, bioengineering, and chemical engineering, more new inventions of lymph node targeting vaccine delivery systems would occur with the integration of contributions from various disciplines, and the vaccine delivery systems will be further optimized and become the leading means of personalized treatment and preventive approach in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the start-up package from University of Wisconsin-Madison.
References
- 1.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone C.A., Jr, Rukasin C.R., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019;85:2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sompayrac L.M. John Wiley & Sons; 2019. How the Immune System Works. [Google Scholar]
- 4.Clem A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Diseas. 2011;3:73. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantegazza A.R., Magalhaes J.G., Amigorena S., Marks M.S. Presentation of phagocytosed antigens by MHC class I and II. Traffic. 2013;14:135–152. doi: 10.1111/tra.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heesters B.A., Myers R.C., Carroll M.C. Follicular dendritic cells: dynamic antigen libraries. Nat. Rev. Immunol. 2014;14:495–504. doi: 10.1038/nri3689. [DOI] [PubMed] [Google Scholar]
- 7.Gaudino S.J., Kumar P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 2019;10:360. doi: 10.3389/fimmu.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair D.A., Turner D.L., Bose T.O., Pham Q.-M., Bouchard K.R., Williams K.J., McAleer J.P., Cauley L.S., Vella A.T., Lefrançois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. J. Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L., Zhou J., Hu L., Han X., Zou X., Chen Q., Chen Y., Liu Z., Wang C. In situ formed fibrin scaffold with cyclophosphamide to synergize with immune checkpoint blockade for inhibition of cancer recurrence after surgery. Adv. Funct. Mater. 2020;30:1906922. [Google Scholar]
- 10.Chen Y., De Koker S., De Geest B.G. Engineering strategies for lymph node targeted immune activation. Acc. Chem. Res. 2020;53:2055–2067. doi: 10.1021/acs.accounts.0c00260. [DOI] [PubMed] [Google Scholar]
- 11.Schudel A., Francis D.M., Thomas S.N. Material design for lymph node drug delivery. Nat. Rev. Mater. 2019;4:415–428. doi: 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez J.E.V., Sharpe L.A., Peppas N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017;114:116–131. doi: 10.1016/j.addr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Smet R., Allais L., Cuvelier C.A. Recent advances in oral vaccine development: yeast-derived β-glucan particles. Human Vac. Immunotherapeut. 2014;10:1309–1318. doi: 10.4161/hv.28166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bol K.F., Figdor C.G., Aarntzen E.H., Welzen M.E., van Rossum M.M., Blokx W.A., van de Rakt M.W., Scharenborg N.M., de Boer A.J., Pots J.M. Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients. Oncoimmunology. 2015;4 doi: 10.1080/2162402X.2015.1019197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesterhuis W.J., de Vries I.J.M., Schreibelt G., Lambeck A.J., Aarntzen E.H., Jacobs J.F., Scharenborg N.M., van de Rakt M.W., de Boer A.J., Croockewit S. Route of administration modulates the induction of dendritic cell vaccine–induced antigen-specific T cells in advanced melanoma patients. Clin. Can. Res. 2011;17:5725–5735. doi: 10.1158/1078-0432.CCR-11-1261. [DOI] [PubMed] [Google Scholar]
- 16.Radomski M., Zeh H.J., Edington H.D., Pingpank J.F., Butterfield L.H., Whiteside T.L., Wieckowski E., Bartlett D.L., Kalinski P. Prolonged intralymphatic delivery of dendritic cells through implantable lymphatic ports in patients with advanced cancer. J. ImmunoTher. Cancer. 2016;4:24. doi: 10.1186/s40425-016-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randolph G.J., Angeli V., Swartz M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 18.Liao S., von der Weid P.-Y. Lymphatic system: an active pathway for immune protection, Seminars in cell & developmental biology. Elsevier. 2015:83–89. doi: 10.1016/j.semcdb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas S.N., Schudel A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Eng. 2015;7:65–74. doi: 10.1016/j.coche.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon Y.J., James E., Shastri N., Fréchet J.M. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc. Natl. Acad. Sci. 2005;102:18264–18268. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy S.T., Swartz M.A., Hubbell J.A. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27:573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 22.I. Bujoreanu, V. Gupta, Anatomy, Lymph Nodes, StatPearls [Internet], (2020). [PubMed]
- 23.von Andrian U.H., Mempel T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 24.Willard-Mack C.L. Normal structure, function, and histology of lymph nodes. Toxicol. Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 25.B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts, P. Walter, Lymphocytes and the cellular basis of adaptive immunity, Molecular Biology of the Cell. 4th edition, Garland Science2002.
- 26.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 27.Luther S.A., Bidgol A., Hargreaves D.C., Schmidt A., Xu Y., Paniyadi J., Matloubian M., Cyster J.G. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 28.Ulvmar M.H., Werth K., Braun A., Kelay P., Hub E., Eller K., Chan L., Lucas B., Novitzky-Basso I., Nakamura K. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014;15:623–630. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- 29.Wilson N.S., El-Sukkari D., Belz G.T., Smith C.M., Steptoe R.J., Heath W.R., Shortman K., Villadangos J.A. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 30.Sixt M., Kanazawa N., Selg M., Samson T., Roos G., Reinhardt D.P., Pabst R., Lutz M.B., Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Roozendaal R., Mebius R.E., Kraal G. The conduit system of the lymph node. Int. Immunol. 2008;20:1483–1487. doi: 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- 32.Rantakari P., Auvinen K., Jäppinen N., Kapraali M., Valtonen J., Karikoski M., Gerke H., Iftakhar-E-Khuda I., Keuschnigg J., Umemoto E. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 2015;16:386–396. doi: 10.1038/ni.3101. [DOI] [PubMed] [Google Scholar]
- 33.Du G., Sun X. Elsevier; Biomaterials for Cancer Therapeutics: 2020. Lymph node targeting for improved potency of cancer vaccine; pp. 527–548. [Google Scholar]
- 34.Rotman J., Koster B.D., Jordanova E.S., Heeren A.M., de Gruijl T.D. Unlocking the therapeutic potential of primary tumor-draining lymph nodes. Cancer Immunol. Immunother. 2019;68:1681–1688. doi: 10.1007/s00262-019-02330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duong T., Koopman P., Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J. Oncol. 2012;2012 doi: 10.1155/2012/204946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munn D.H., Mellor A.L. The tumor-draining lymph node as an immune-privileged site. Immunol. Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 37.van Pul K.M., Fransen M.F., van de Ven R., de Gruijl T.D. Immunotherapy goes local: the central role of lymph nodes in driving tumor infiltration and efficacy. Front. Immunol. 2021;12:518. doi: 10.3389/fimmu.2021.643291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandrasekaran S., King M.R. Microenvironment of tumor-draining lymph nodes: opportunities for liposome-based targeted therapy. Int. J. Mol. Sci. 2014;15:20209–20239. doi: 10.3390/ijms151120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard G.P., Verma G., Ke X., Thayer W.M., Hamerly T., Baxter V.K., Lee J.E., Dinglasan R.R., Mao H.-Q. Critical size limit of biodegradable nanoparticles for enhanced lymph node trafficking and paracortex penetration. Nano Res. 2019;12:837–844. doi: 10.1007/s12274-019-2301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manolova V., Flace A., Bauer M., Schwarz K., Saudan P., Bachmann M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 41.Ryan G.M., Kaminskas L.M., Porter C.J. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J. Control. Release. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 42.Kim S.-Y., Noh Y.-W., Kang T.H., Kim J.-E., Kim S., Um S.H., Oh D.-B., Park Y.-M., Lim Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56–66. doi: 10.1016/j.biomaterials.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 43.Trevaskis N.L., Kaminskas L.M., Porter C.J. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discovery. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 44.Brenner J.S., Pan D.C., Myerson J.W., Marcos-Contreras O.A., Villa C.H., Patel P., Hekierski H., Chatterjee S., Tao J.-Q., Parhiz H. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J. Control. Release. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Oussoren C., Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: III. Influence of surface modification with poly (ethyleneglycol) Pharm. Res. 1997;14:1479–1484. doi: 10.1023/a:1012145410859. [DOI] [PubMed] [Google Scholar]
- 47.Cai S., Yang Q., Bagby T.R., Forrest M.L. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2011;63:901–908. doi: 10.1016/j.addr.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moghimi S.M., Hawley A.E., Christy N.M., Gray T., Illum L., Davis S.S. Surface engineered nanospheres with enhanced drainage into lymphatics and uptake by macrophages of the regional lymph nodes. FEBS Lett. 1994;344:25–30. doi: 10.1016/0014-5793(94)00351-3. [DOI] [PubMed] [Google Scholar]
- 49.Hong X.Y., Zhong X.F., Du G.S., Hou Y.Y., Zhang Y.T., Zhang Z.R., Gong T., Zhang L., Sun X. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waku T., Nishigaki S., Kitagawa Y., Koeda S., Kawabata K., Kunugi S., Kobori A., Tanaka N. Effect of the hydrophilic-hydrophobic balance of antigen-loaded peptide nanofibers on their cellular uptake, cellular toxicity, and immune stimulatory properties. Int. J. Mol. Sci. 2019;20:3781. doi: 10.3390/ijms20153781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiao C., Liu J., Yang J., Li Y., Weng J., Shao Y., Zhang X. Enhanced non-inflammasome mediated immune responses by mannosylated zwitterionic-based cationic liposomes for HIV DNA vaccines. Biomaterials. 2016;85:1–17. doi: 10.1016/j.biomaterials.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 52.Jin Q., Chen Y., Wang Y., Ji J. Zwitterionic drug nanocarriers: a biomimetic strategy for drug delivery. Colloids Surf., B. 2014;124:80–86. doi: 10.1016/j.colsurfb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 53.Steinbuck M.P., Seenappa L.M., Jakubowski A., McNeil L.K., Haqq C.M., DeMuth P.C. A lymph node–targeted Amphiphile vaccine induces potent cellular and humoral immunity to SARS-CoV-2. Sci. Adv. 2021;7:eabe5819. doi: 10.1126/sciadv.abe5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao D.A., Forrest M.L., Alani A.W., Kwon G.S., Robinson J.R. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J. Pharm. Sci. 2010;99:2018–2031. doi: 10.1002/jps.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foged C., Brodin B., Frokjaer S., Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 56.Zheng M., Pan M., Zhang W., Lin H., Wu S., Lu C., Tang S., Liu D., Cai J. Poly (α-l-lysine)-based nanomaterials for versatile biomedical applications: Current advances and perspectives. Bioact. Mater. 2021;6:1878–1909. doi: 10.1016/j.bioactmat.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foroozandeh P., Aziz A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018;13:1–12. doi: 10.1186/s11671-018-2728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012;7:5577. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z., Shen Y., Tang J., Fan M., Van Kirk E.A., Murdoch W.J., Radosz M. Charge-reversal drug conjugate for targeted cancer cell nuclear drug delivery. Adv Funct Mater. 2009;19:3580–3589. [Google Scholar]
- 60.Shen Y., Zhou Z., Sui M., Tang J., Xu P., Kirk E.A.V., Murdoch W.J., Fan M., Radosz M. Charge-reversal polyamidoamine dendrimer for cascade nuclear drug delivery. Nanomedicine. 2010;5:1205–1217. doi: 10.2217/nnm.10.86. [DOI] [PubMed] [Google Scholar]
- 61.Nicolas J.-F., Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Exp. Rev. Vac. 2008;7:1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 62.Abdallah M., Mullertz O.O., Styles I.K., Mörsdorf A., Quinn J.F., Whittaker M.R., Trevaskis N.L. Lymphatic targeting by albumin-hitchhiking: Applications and optimisation. J. Control. Release. 2020 doi: 10.1016/j.jconrel.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 63.Wang P., Zhao P., Dong S., Xu T., He X., Chen M. An albumin-binding polypeptide both targets cytotoxic T lymphocyte vaccines to lymph nodes and boosts vaccine presentation by dendritic cells. Theranostics. 2018;8:223. doi: 10.7150/thno.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu H., Moynihan K.D., Zheng Y., Szeto G.L., Li A.V., Huang B., Van Egeren D.S., Park C., Irvine D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.R.M. Steinman, Decisions About Dendritic Cells: Past, Present, and Future, in: W.E. Paul (Ed.) Annual Review of Immunology, Vol 302012, pp. 1-22. [DOI] [PubMed]
- 66.Garg A.D., Coulie P.G., Van den Eynde B.J., Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Irvine D.J., Aung A., Silva M. Controlling timing and location in vaccines. Adv. Drug Deliv. Rev. 2020;158:91–115. doi: 10.1016/j.addr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reddy S.T., Van Der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neil C.P., Lee L.K., Swartz M.A., Hubbell J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 69.Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M.C., Steinman R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azzi J., Yin Q., Uehara M., Ohori S., Tang L., Cai K., Ichimura T., McGrath M., Maarouf O., Kefaloyianni E. Targeted delivery of immunomodulators to lymph nodes. Cell Rep. 2016;15:1202–1213. doi: 10.1016/j.celrep.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 72.Khadke S., Roces C.B., Cameron A., Devitt A., Perrie Y. Formulation and manufacturing of lymphatic targeting liposomes using microfluidics. J. Control. Release. 2019;307:211–220. doi: 10.1016/j.jconrel.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Liang R.J., Xie J., Li J., Wang K., Liu L.P., Gao Y.J., Hussain M., Shen G.X., Zhu J.T., Tao J. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. 2017;149:41–50. doi: 10.1016/j.biomaterials.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 74.Le Moignic A., Malard V., Benvegnu T., Lemiegre L., Berchel M., Jaffres P.A., Baillou C., Delost M., Macedo R., Rochefort J., Lescaille G., Pichon C., Lemoine F.M., Midoux P., Mateo V. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J. Control. Release. 2018;278:110–121. doi: 10.1016/j.jconrel.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 75.Benner B., Good L., Quiroga D., Schultz T.E., Kassem M., Carson W.E., Cherian M.A., Sardesai S., Wesolowski R. Pexidartinib, a novel small molecule CSF-1R inhibitor in use for tenosynovial giant cell tumor: a systematic review of pre-clinical and clinical development. Drug Des. Dev. Therapy. 2020;14:1693. doi: 10.2147/DDDT.S253232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura T., Harashima H. Dawn of lipid nanoparticles in lymph node targeting: potential in cancer immunotherapy. Adv. Drug Deliv. Rev. 2020;167:78–88. doi: 10.1016/j.addr.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Huang W.C., Deng B.B., Lin C.Y., Carter K.A., Geng J.M., Razi A., He X.D., Chitgupi U., Federizon J., Sun B.Y., Long C.A., Ortega J., Dutta S., King C.R., Miura K., Lee S.M., Lovell J.F. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 2018;13:1174-+. doi: 10.1038/s41565-018-0271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miao L., Li L.X., Huang Y.X., Delcassian D., Chahal J., Han J.S., Shi Y.H., Sadtler K., Gao W.T., Lin J.Q., Doloff J.C., Langer R., Anderson D.G. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019;37:1174-+. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 79.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maeki M., Kimura N., Sato Y., Harashima H., Tokeshi M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018;128:84–100. doi: 10.1016/j.addr.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 81.Wang X., Wilhelm J., Li W., Li S.X., Wang Z.H., Huang G., Wang J., Tang H.L., Khorsandi S., Sun Z.C., Evers B., Gao J.M. Polycarbonate-based ultra-pH sensitive nanoparticles improve therapeutic window. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavallaro G., Lazzara G., Milioto S., Parisi F., Evtugyn V., Rozhina E., Fakhrullin R. Nanohydrogel formation within the halloysite lumen for triggered and sustained release. ACS Appl. Mater. Interfaces. 2018;10:8265–8273. doi: 10.1021/acsami.7b19361. [DOI] [PubMed] [Google Scholar]
- 83.Chen H., Fan Y., Yu X., Semetey V., Trepout S., Li M.-H. Light-gated nano-porous capsules from stereoisomer-directed self-assemblies. ACS Nano. 2020 doi: 10.1021/acsnano.0c07400. [DOI] [PubMed] [Google Scholar]
- 84.Chen Q., Chen J.W., Yang Z.J., Xu J., Xu L.G., Liang C., Han X., Liu Z. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 2019;31 doi: 10.1002/adma.201802228. [DOI] [PubMed] [Google Scholar]
- 85.Li Z., Zhu L., Sun H., Shen Y., Hu D., Wu W., Wang Y., Qian C., Sun M. Fluorine assembly nanocluster breaks the shackles of immunosuppression to turn the cold tumor hot. Proc. Natl. Acad. Sci. USA. 2020;117:32962–32969. doi: 10.1073/pnas.2011297117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z., Wang Y., Shen Y., Qian C., Oupicky D., Sun M. Targeting pulmonary tumor microenvironment with CXCR4-inhibiting nanocomplex to enhance anti-PD-L1 immunotherapy. Sci. Adv. 2020;6:eaaz9240. doi: 10.1126/sciadv.aaz9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noh H.J., Noh Y.W., Heo M.B., Kim E.H., Park S.J., Kim Y.I., Choi Y.K., Lim Y.T. Injectable and pathogen-mimicking hydrogels for enhanced protective immunity against emerging and highly pathogenic influenza virus. Small. 2016;12:6279–6288. doi: 10.1002/smll.201602344. [DOI] [PubMed] [Google Scholar]
- 88.Griffin M., Castro N., Bas O., Saifzadeh S., Butler P., Hutmacher D.W. The current versatility of polyurethane three-dimensional printing for biomedical applications. Tissue Eng. B-Rev. 2020;26:272–283. doi: 10.1089/ten.TEB.2019.0224. [DOI] [PubMed] [Google Scholar]
- 89.Luo M., Wang H., Wang Z.H., Cai H.C., Lu Z.G., Li Y., Du M.J., Huang G., Wang C.S., Chen X., Porembka M.R., Lea J., Frankel A.E., Fu Y.X., Chen Z.J.J., Gao J.M. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;12:648-+. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schudel A., Chapman A.P., Yau M.K., Higginson C.J., Francis D.M., Manspeaker M.P., Avecilla A.R.C., Rohner N.A., Finn M.G., Thomas S.N. Programmable multistage drug delivery to lymph nodes. Nat. Nanotechnol. 2020;15:491-+. doi: 10.1038/s41565-020-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z., Wang J., Li H., Yu J., Chen G., Kahkoska A.R., Wu V., Zeng Y., Wen D., Miedema J.R., Buse J.B., Gu Z. Dual self-regulated delivery of insulin and glucagon by a hybrid patch. PNAS. 2020;117:29512–29517. doi: 10.1073/pnas.2011099117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen G., Chen Z., Wen D., Wang Z., Li H., Zeng Y., Dotti G., Wirz R.E., Gu Z. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. PNAS. 2020;117:3687–3692. doi: 10.1073/pnas.1917891117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.G. Erdos, S.C. Balmert, C.D. Carey, G.D. Falo, N.A. Patel, J. Zhang, A. Gambotto, E. Korkmaz, L.D. Falo, Jr., Improved Cutaneous Genetic Immunization by Microneedle Array Delivery of an Adjuvanted Adenovirus Vaccine, J Invest Dermatol, 140 (2020) 2528-2531 e2522. [DOI] [PMC free article] [PubMed]
- 94.Tran K.T.M., Gavitt T.D., Farrell N.J., Curry E.J., Mara A.B., Patel A., Brown L., Kilpatrick S., Piotrowska R., Mishra N., Szczepanek S.M., Nguyen T.D. Transdermal microneedles for the programmable burst release of multiple vaccine payloads. Nat Biomed Eng. 2020 doi: 10.1038/s41551-020-00650-4. [DOI] [PubMed] [Google Scholar]
- 95.del Pilar Martin M., Weldon W.C., Zarnitsyn V.G., Koutsonanos D.G., Akbari H., Skountzou I., Jacob J., Prausnitz M.R., Compans R.W. Local response to microneedle-based influenza immunization in the skin. mBio. 2012;3:e00012. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sullivan S.P., Koutsonanos D.G., Del Pilar Martin M., Lee J.W., Zarnitsyn V., Choi S.O., Murthy N., Compans R.W., Skountzou I., Prausnitz M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ochoa T.J., Zea-Vera A., Bautista R., Davila C., Salazar J.A., Bazan C., Lopez L., Ecker L. Vaccine schedule compliance among very low birth weight infants in Lima, Peru. Vaccine. 2015;33:354–358. doi: 10.1016/j.vaccine.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McHugh K.J., Nguyen T.D., Linehan A.R., Yang D., Behrens A.M., Rose S., Tochka Z.L., Tzeng S.Y., Norman J.J., Anselmo A.C., Xu X., Tomasic S., Taylor M.A., Lu J., Guarecuco R., Langer R., Jaklenec A. Fabrication of fillable microparticles and other complex 3D microstructures. Science. 2017;357:1138–1142. doi: 10.1126/science.aaf7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shae D., Baljon J.J., Wehbe M., Christov P.P., Becker K.W., Kumar A., Suryadevara N., Carson C.S., Palmer C.R., Knight F.C., Joyce S., Wilson J.T. Co-delivery of peptide neoantigens and stimulator of interferon genes agonists enhances response to cancer vaccines. ACS Nano. 2020;14:9904–9916. doi: 10.1021/acsnano.0c02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duong H.T.T., Yin Y., Thambi T., Nguyen T.L., Phan V.H.G., Lee M.S., Lee J.E., Kim J., Jeong J.H., Lee D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials. 2018;185:13–24. doi: 10.1016/j.biomaterials.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Blakney A.K., Zhu Y.Q., McKay P.F., Bouton C.R., Yeow J., Tang J.Q., Hu K., Samnuan K., Grigsby C.L., Shattock R.J., Stevens M.M. Big is beautiful: enhanced saRNA delivery and immunogenicity by a higher molecular weight bioreducible, cationic polymer. ACS Nano. 2020;14:5711–5727. doi: 10.1021/acsnano.0c00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu J., Wang J., Zhang Y., Chen G., Mao W., Ye Y., Kahkoska A.R., Buse J.B., Langer R., Gu Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed Eng. 2020;4:499–506. doi: 10.1038/s41551-019-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wibowo D., Jorritsma S.H.T., Gonzaga Z.J., Evert B., Chen S., Rehm B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials. 2020;268 doi: 10.1016/j.biomaterials.2020.120597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Du Y.Q., Xia Y.F., Zou Y.J., Hu Y.N., Fu J.Q., Wu J., Gao X.D., Ma G.H. Exploiting the lymph-node-amplifying effect for potent systemic and gastrointestinal immune responses via polymer/lipid nanoparticles. ACS Nano. 2019;13:13809–13817. doi: 10.1021/acsnano.9b04071. [DOI] [PubMed] [Google Scholar]
- 105.Li Z.T., Wang Y.X., Ding Y.Y., Repp L., Kwon G.S., Hu Q.Y. Cell-based delivery systems: emerging carriers for immunotherapy. Adv Funct Mater. 2021 [Google Scholar]
- 106.Hsu F.J., Benike C., Fagnoni F., Liles T.M., Czerwinski D., Taidi B., Engleman E.G., Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat. Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 107.Sabado R.L., Balan S., Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosa F.F., Pires C.F., Kurochkin I., Ferreira A.G., Gomes A.M., Palma L.G., Shaiv K., Solanas L., Azenha C., Papatsenko D., Schulz O., Sousa C.R.E., Pereira C.F. Direct reprogramming of fibroblasts into antigen-presenting dendritic cells. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aau4292. [DOI] [PubMed] [Google Scholar]
- 109.de Vries I.J.M., Lesterhuis W.J., Scharenborg N.M., Engelen L.P.H., Ruiter D.J., Gerritsen M.J.P., Croockewit S., Britten C.M., Torensma R., Adema G.J., Figdor C.G., Punt C.J.A. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin. Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 110.Laustsen A., Bak R.O., Krapp C., Kjaer L., Egedahl J.H., Petersen C.C., Pillai S., Tang H.Q., Uldbjerg N., Porteus M., Roan N.R., Nyegaard M., Denton P.W., Jakobsen M.R. Interferon priming is essential for human CD34+cell-derived plasmacytoid dendritic cell maturation and function. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-05816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee K., Kim T.S., Seo Y., Kim S.Y., Lee H. Combined hybrid structure of siRNA tailed IVT mRNA (ChriST mRNA) for enhancing DC maturation and subsequent anticancer T cell immunity. J. Control. Release. 2020;327:225–234. doi: 10.1016/j.jconrel.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 112.Rossi A., Dupaty L., Aillot L., Zhang L., Gallien C., Hallek M., Odenthal M., Adriouch S., Salvetti A., Buning H. Vector uncoating limits adenoassociated viral vector-mediated transduction of human dendritic cells and vector immunogenicity. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-40071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilgenhof S., Corthals J., Heirman C., van Baren N., Lucas S., Kvistborg P., Thielemans K., Neyns B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol. 2016;34:1330-+. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 114.Han D.L., Liu J., Chen C.Y., Dong L.H., Liu Y., Chang R.B., Huang X.N., Liu Y.Y., Wang J.Y., Dougherty U., Bissonnette M.B., Shen B., Weichselbaum R.R., Xu M.M., He C. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270-+. doi: 10.1038/s41586-019-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bergamaschi C., Pandit H., Nagy B.A., Stellas D., Jensen S.M., Bear J., Cam M., Valentin A., Fox B.A., Felber B.K., Pavlakis G.N. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-gamma, CXCL9 and CXCL10. J. ImmunoTher. Cancer. 2020;8 doi: 10.1136/jitc-2020-000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee J.M., Lee M.H., Garon E., Goldman J.W., Salehi-Rad R., Baratelli F.E., Schaue D., Wang G., Rosen F., Yanagawa J., Walser T.C., Lin Y., Park S.J., Adams S., Marincola F.M., Tumeh P.C., Abtin F., Suh R., Reckamp K.L., Lee G., Wallace W.D., Lee S., Zeng G., Elashoff D.A., Sharma S., Dubinett S.M. Phase I Trial of intratumoral injection of CCL21 gene-modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8(+) T-cell infiltration. Clin. Cancer Res. 2017;23:4556–4568. doi: 10.1158/1078-0432.CCR-16-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hassannia H., Chaleshtari M.G., Atyabi F., Nosouhian M., Masjedi A., Hojjat-Farsangi M., Namdar A., Azizi G., Mohammadi H., Ghalamfarsa G., Sabz G., Hasanzadeh S., Yousefi M., Jadidi-Niaragh F. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology. 2020;159:75–87. doi: 10.1111/imm.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Balan S., Ollion V., Colletti N., Chelbi R., Montanana-Sanchis F., Liu H., Manh T.P.V., Sanchez C., Savoret J., Perrot I., Doffin A.C., Fossum E., Bechlian D., Chabannon C., Bogen B., Asselin-Paturel C., Shaw M., Soos T., Caux C., Valladeau-Guilemond J., Dalod M. Human XCR1(+) dendritic cells derived in vitro from CD34(+) progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells. J. Immunol. 2014;193:1622–1635. doi: 10.4049/jimmunol.1401243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anguille S., Smits E.L., Lion E., van Tendeloo V.F., Berneman Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:E257–E267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 120.Verma V., Kim Y., Lee M.C., Lee J.T., Cho S., Park I.K., Min J.J., Lee J.J., Lee S.E., Rhee J.H. Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression. Oncotarget. 2016;7:39894–39906. doi: 10.18632/oncotarget.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anguille S., Smits E.L., Bryant C., Van Acker H.H., Goossens H., Lion E., Fromm P.D., Hart D.N., Van Tendeloo V.F., Berneman Z.N. Dendritic cells as pharmacological tools for cancer immunotherapy. Pharmacol. Rev. 2015;67:731–753. doi: 10.1124/pr.114.009456. [DOI] [PubMed] [Google Scholar]
- 122.Yang X.Q., Lian K.K., Meng T.T., Liu X., Miao J., Tan Y.N., Yuan H., Hu F.Q. Immune adjuvant targeting micelles allow efficient dendritic cell migration to lymph nodes for enhanced cellular immunity. ACS Appl. Mater. Interfaces. 2018;10:33532–33544. doi: 10.1021/acsami.8b10081. [DOI] [PubMed] [Google Scholar]
- 123.Seeman N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982;99:237–247. doi: 10.1016/0022-5193(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 124.Rothemund P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440:297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 125.Hu Q.Q., Li H., Wang L.H., Gu H.Z., Fan C.H. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019;119:6459–6506. doi: 10.1021/acs.chemrev.7b00663. [DOI] [PubMed] [Google Scholar]
- 126.Halley P.D., Patton R.A., Chowdhury A., Byrd J.C., Castro C.E. Low-cost, simple, and scalable self-assembly of DNA origami nanostructures. Nano Res. 2019;12:1207–1215. [Google Scholar]
- 127.S.L. Liu, Q. Jiang, X. Zhao, R.F. Zhao, Y.N. Wang, Y.M. Wang, J.B. Liu, Y.X. Shang, S. Zhao, T.T. Wu, Y.L. Zhang, G.J. Nie, B.Q. Ding, A DNA nanodevice-based vaccine for cancer immunotherapy, Nature Materials. [DOI] [PubMed]
- 128.Veneziano R., Moyer T.J., Stone M.B., Wamhoff E.C., Read B.J., Mukherjee S., Shepherd T.R., Das J., Schief W.R., Irvine D.J., Bathe M. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat. Nanotechnol. 2020;15:716-+. doi: 10.1038/s41565-020-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu X.W., Xu Y., Yu T., Clifford C., Liu Y., Yan H., Chang Y. A DNA nanostructure platform for directed assembly of synthetic vaccines. Nano Lett. 2012;12:4254–4259. doi: 10.1021/nl301877k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang P., Liu X.G., Liu P., Wang F., Ariyama H., Ando T., Lin J.P., Wang L.H., Hu J., Li B., Fan C.H. Capturing transient antibody conformations with DNA origami epitopes. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-16949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu X.G., Zhang F., Jing X.X., Pan M.C., Liu P., Li W., Zhu B.W., Li J., Chen H., Wang L.H., Lin J.P., Liu Y., Zhao D.Y., Yan H., Fan C.H. Complex silica composite nanomaterials templated with DNA origami. Nature. 2018;559:593–598. doi: 10.1038/s41586-018-0332-7. [DOI] [PubMed] [Google Scholar]
- 132.Z.R. Wang, L.L. Song, Q. Liu, R. Tian, Y.X. Shang, F.S. Liu, S.L. Liu, S. Zhao, Z.H. Han, J.S. Sun, Q. Jiang, B.Q. Ding, A Tubular DNA Nanodevice as a siRNA/Chemo-Drug Co-delivery Vehicle for Combined Cancer Therapy, Angewandte Chemie-International Edition. [DOI] [PubMed]
- 133.Li Z., Zhuang T., Dong J., Wang L., Xia J., Wang H., Cui X., Wang Z. Sonochemical fabrication of inorganic nanoparticles for applications in catalysis. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang C.Y., Jiao K., Yan J.F., Wan M.C., Wan Q.Q., Breschi L., Chen J.H., Tay F.R., Niu L.N. Biological and synthetic template-directed syntheses of mineralized hybrid and inorganic materials. Prog. Mater Sci. 2021;116 [Google Scholar]
- 135.Hu Z.L., Song B., Xu L., Zhong Y.L., Peng F., Ji X.Y., Zhu F., Yang C.K., Zhou J.Y., Su Y.Y., Chen S.N., He Y., He S.D. Aqueous synthesized quantum dots interfere with the NF-kappa B pathway and confer anti-tumor, anti-viral and anti-inflammatory effects. Biomaterials. 2016;108:187–196. doi: 10.1016/j.biomaterials.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 136.Kakizawa Y., Lee J.S., Bell B., Fahmy T.M. Precise manipulation of biophysical particle parameters enables control of proinflammatory cytokine production in presence of TLR 3 and 4 ligands. Acta Biomater. 2017;57:136–145. doi: 10.1016/j.actbio.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 137.Hess K.L., Medintz I.L., Jewell C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today. 2019;27:73–98. doi: 10.1016/j.nantod.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Teng Z.G., Li W., Tang Y.X., Elzatahry A., Lu G.M., Zhao D.Y. Mesoporous organosilica hollow nanoparticles: synthesis and applications. Adv. Mater. 2019;31 doi: 10.1002/adma.201707612. [DOI] [PubMed] [Google Scholar]
- 139.Cyster J.G., Allen C.D.C. B cell responses: cell interaction dynamics and decisions. Cell. 2019;177:524–540. doi: 10.1016/j.cell.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y.N., Lazarovits J., Poon W., Ouyang B., Nguyen L.N.M., Kingston B.R., Chan W.C.W. Nanoparticle size influences antigen retention and presentation in lymph node follicles for humoral immunity. Nano Lett. 2019;19:7226–7235. doi: 10.1021/acs.nanolett.9b02834. [DOI] [PubMed] [Google Scholar]
- 141.Xie J.J., Xu W.X., Wu Y.H., Niu B.N., Zhang X.K. Macroporous organosilicon nanocomposites co-deliver Bcl2-converting peptide and chemotherapeutic agent for synergistic treatment against multidrug resistant cancer. Cancer Lett. 2020;469:340–354. doi: 10.1016/j.canlet.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 142.Beik J., Khateri M., Khosravi Z., Kamrava S.K., Kooranifar S., Ghaznavi H., Shakeri-Zadeh A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019;387:299–324. [Google Scholar]
- 143.Anani T., Rahmati S., Sultana N., David A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics. 2021;11:579–601. doi: 10.7150/thno.48811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ventola C.L. Progress in nanomedicine: approved and investigational nanodrugs. P & T: A Peer-reviewed J. Formulary Manage. 2017;42:742–755. [PMC free article] [PubMed] [Google Scholar]
- 145.Peng F., Setyawati M.I., Tee J.K., Ding X.G., Wang J.P., Nga M.E., Ho H.K., Leong D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019;14:279-+. doi: 10.1038/s41565-018-0356-z. [DOI] [PubMed] [Google Scholar]
- 146.McCoy K., Uchida M., Lee B., Douglas T. Templated assembly of a functional ordered protein macromolecular framework from P22 virus-like particles. ACS Nano. 2018;12:3541–3550. doi: 10.1021/acsnano.8b00528. [DOI] [PubMed] [Google Scholar]
- 147.Maassen S.J., van der Schoot P., Cornelissen J. Experimental and theoretical determination of the pH inside the confinement of a virus-like particle. Small. 2018;14 doi: 10.1002/smll.201802081. [DOI] [PubMed] [Google Scholar]
- 148.Crone M.A., Priestman M., Ciechonska M., Jensen K., Sharp D.J., Anand A., Randell P., Storch M., Freemont P.S. A role for Biofoundries in rapid development and validation of automated SARS-CoV-2 clinical diagnostics. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Neek M., Kim T.I., Wang S.W. Protein-based nanoparticles in cancer vaccine development. Nanomed.-Nanotechnol. Biol. Med. 2019;15:164–174. doi: 10.1016/j.nano.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan Y.S., Lane D.P., Verma C.S. Stapled peptide design: principles and roles of computation. Drug Discov Today. 2016;21:1642–1653. doi: 10.1016/j.drudis.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 151.Manspeaker M.P., Thomas S.N. Lymphatic immunomodulation using engineered drug delivery systems for cancer immunotherapy. Adv. Drug Deliv. Rev. 2020;160:19–35. doi: 10.1016/j.addr.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mohsen M.O., Speiser D.E., Knuth A., Bachmann M.F. Virus-like particles for vaccination against cancer. Wiley Interdiscipl. Rev.-Nanomed. Nanobiotechnol. 2020;12 doi: 10.1002/wnan.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shukla S., Myers J.T., Woods S.E., Gong X.J., Czapar A.E., Commandeur U., Huang A.Y., Levine A.D., Steinmetz N.F. Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials. 2017;121:15–27. doi: 10.1016/j.biomaterials.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 154.Langowski M.D., Khan F.A., Bitzer A.A., Genito C.J., Schrader A.J., Martin M.L., Soto K., Zou X.Y., Hadiwidjojo S., Beck Z., Matyas G.R., Livingstone M.C., Batchelor A.H., Dutta S. Optimization of a Plasmodium falciparum circumsporozoite protein repeat vaccine using the tobacco mosaic virus platform. PNAS. 2020;117:3114–3122. doi: 10.1073/pnas.1911792117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maphis N.M., Peabody J., Crossey E., Jiang S.Y., Ahmad F.A.J., Alvarez M., Mansoor S.K., Yaney A., Yang Y.R., Sillerud L.O., Wilson C.M., Selwyn R., Brigman J.L., Cannon J.L., Peabody D.S., Chackerian B., Bhaskar K. Q beta Virus-like particle-based vaccine induces robust immunity and protects against tauopathy. NPJ Vaccines. 2019;4 doi: 10.1038/s41541-019-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Richert L.E., Servid A.E., Harmsen A.L., Rynda-Apple A., Han S., Wiley J.A., Douglas T., Harmsen A.G. A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine. 2012;30:3653–3665. doi: 10.1016/j.vaccine.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neek M., Tucker J.A., Kim T.I., Molino N.M., Nelson E.L., Wang S.W. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials. 2018;156:194–203. doi: 10.1016/j.biomaterials.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shaddeau A.W., Schneck N.A., Li Y.L., Ivleva V.B., Arnold F.J., Cooper J.W., Lei Q.P. Development of a new tandem ion exchange and size exclusion chromatography method to monitor vaccine particle titer in cell culture media. Anal. Chem. 2019;91:6430–6434. doi: 10.1021/acs.analchem.9b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang W.J., Zhou X.X., Bian Y.J., Wang S., Chai Q., Guo Z.Q., Wang Z.N., Zhu P., Peng H., Yan X.Y., Li W.H., Fu Y.X., Zhu M.Z. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020;15:406-+. doi: 10.1038/s41565-020-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Escolano A., Gristick H.B., Abernathy M.E., Merkenschlager J., Gautam R., Oliveira T.Y., Pai J., West A.P., Barnes C.O., Cohen A.A., Wang H.Q., Golijanin J., Yost D., Keeffe J.R., Wang Z.J., Zhao P., Yao K.H., Bauer J., Nogueira L., Gao H., Voll A.V., Montefiori D.C., Seaman M.S., Gazumyan A., Silva M., McGuire A.T., Stamatatos L., Irvine D.J., Wells L., Martin M.A., Bjorkman P.J., Nussenzweig M.C. Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques. Nature. 2019;570:468-+. doi: 10.1038/s41586-019-1250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sanchez J., Holmgren J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 2008;65:1347–1360. doi: 10.1007/s00018-008-7496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Piai A., Fu Q., Cai Y., Ghantous F., Xiao T., Shaik M.M., Peng H., Rits-Volloch S., Chen W., Seaman M.S., Chen B., Chou J.J. Structural basis of transmembrane coupling of the HIV-1 envelope glycoprotein. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-16165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Singh P., Prasuhn D., Yeh R.M., Destito G., Rae C.S., Osborn K., Finn M.G., Manchester M. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J. Control. Release. 2007;120:41–50. doi: 10.1016/j.jconrel.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee K.L., Shukla S., Wu M.Z., Ayat N.R., El Sanadi C.E., Wen A.M., Edelbrock J.F., Pokorski J.K., Commandeur U., Dubyak G.R., Steinmetz N.F. Stealth filaments: polymer chain length and conformation affect the in vivo fate of PEGylated potato virus X. Acta Biomater. 2015;19:166–179. doi: 10.1016/j.actbio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rodriguez P.L., Harada T., Christian D.A., Pantano D.A., Tsai R.K., Discher D.E. Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang D., Tai P.W.L., Gao G.P. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discovery. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ye L.P., Park J.J., Dong M.B., Yang Q.J., Chow R.D., Peng L., Du Y.Y., Guo J.J., Dai X.Y., Wang G.C., Errami Y., Chen S.D. In vivo CRISPR screening in CD8 T cells with AAV-Sleeping Beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat. Biotechnol. 2019;37:1302-+. doi: 10.1038/s41587-019-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang G.C., Chow R.D., Bai Z.G., Zhu L.Y., Errami Y., Dai X.Y., Dong M.B., Ye L.P., Zhang X.Y., Renauer P.A., Park J.J., Shen L., Ye H.H., Fuchs C.S., Chen S.D. Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nat. Immunol. 2019;20:1494-+. doi: 10.1038/s41590-019-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Levy J.M., Yeh W.H., Pendse N., Davis J.R., Hennessey E., Butcher R., Koblan L.W., Comander J., Liu Q., Liu D.R. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 2020;4:97–110. doi: 10.1038/s41551-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Boucher P., Cui X.X., Curiel D.T. Adenoviral vectors for in vivo delivery of CRISPR-Cas gene editors. J. Control. Release. 2020;327:788–800. doi: 10.1016/j.jconrel.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P., Wang Z., Wu X.H., Xu J.J., Zhang Z., Jia S.Y., Wang B.S., Hu Y., Liu J.J., Zhang J., Qian X.A., Li Q., Pan H.X., Jiang H.D.C., Deng P., Gou J.B., Wang X.W., Wang X.H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sayedahmed E.E., Elkashif A., Alhashimi M., Sambhara S., Mittal S.K. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines. 2020;8 doi: 10.3390/vaccines8040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yilmaz A., Marklund E., Andersson M., Nilsson S., Andersson L.-M., Lindh M., Gisslen M. Upper respiratory tract levels of severe acute respiratory syndrome coronavirus 2 RNA and duration of viral RNA shedding do not differ between patients with mild and severe/critical coronavirus disease 2019. J. Infect. Dis. 2021;223:15–18. doi: 10.1093/infdis/jiaa632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., Chen R.E., Winkler E.S., Wessel A.W., Case J.B., Kashentseva E., McCune B.T., Bailey A.L., Zhao H.Y., VanBlargan L.A., Dai Y.N., Ma M.S., Adams L.J., Shrihari S., Danis J.E., Gralinski L.E., Hou Y.J., Schafer A., Kim A.S., Keeler S.P., Weiskopf D., Baric R.S., Holtzman M.J., Fremont D.H., Curiel D.T., Diamond M.S. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169-+. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Feng L.Q., Wang Q., Shan C., Yang C.C., Feng Y., Wu J., Liu X.L., Zhou Y.W., Jiang R.D., Hu P.Y., Liu X.L., Zhang F., Li P.C., Niu X.F., Liu Y.C., Zheng X.H., Luo J., Sun J., Gu Y.Y., Liu B., Xu Y.C., Li C.F., Pan W.Q., Zhao J.C., Ke C.W., Chen X.W., Xu T., Zhong N.S., Guan S.H., Yuan Z.M., Chen L. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu S.P., Zhong G.X., Zhang J., Shuai L., Zhang Z., Wen Z.Y., Wang B.S., Zhao Z.H., Song X.H., Chen Y., Liu R.Q., Fu L., Zhang J.L., Guo Q., Wang C., Yang Y.L., Fang T., Lv P., Wang J.L., Xu J.J., Li J.M., Yu C.M., Hou L.H., Bu Z.G., Chen W. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Flemming A. mRNA vaccine shows promise in autoimmunity. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Munis A.M., Mattiuzzo G., Bentley E.M., Collins M.K., Eyles J.E., Takeuchi Y. Use of heterologous vesiculovirus G proteins circumvents the humoral anti-envelope immunity in lentivector-based in vivo gene delivery. Molecular Therapy-Nucleic Acids. 2019;17:126–137. doi: 10.1016/j.omtn.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 182.Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., McClung N., Campos-Outcalt D., Morgan R.L., Mbaeyi S., Romero J.R., Talbot H.K., Lee G.M., Bell B.P., Dooling K. The advisory committee on immunization practices' interim recommendation for use of pfizer-BioNTech COVID-19 vaccine - United States. Mmwr-Morbidity and Mortality Weekly Report. 2020;69(2020):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H., m R.N.A.S.G. An mRNA vaccine against SARS-CoV-2-preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.L.R. Baden, H.M. El Sahly, B. Essink, K. Kotloff, S. Frey, R. Novak, D. Diemert, S.A. Spector, N. Rouphael, C.B. Creech, J. McGettigan, S. Khetan, N. Segall, J. Solis, A. Brosz, C. Fierro, H. Schwartz, K. Neuzil, L. Corey, P. Gilbert, H. Janes, D. Follmann, M. Marovich, J. Mascola, L. Polakowski, J. Ledgerwood, B.S. Graham, H. Bennett, R. Pajon, C. Knightly, B. Leav, W. Deng, H. Zhou, S. Han, M. Ivarsson, J. Miller, T. Zaks, C.S. Group, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, The New England journal of medicine, 384 (2021) 403-416. [DOI] [PMC free article] [PubMed]
- 186.Guevara M.L., Persano F., Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.V. Bernasconi, K. Norling, I. Gribonika, L.C. Ong, S. Burazerovic, N. Parveen, K. Schon, A. Stensson, M. Bally, G. Larson, F. Hook, N. Lycke, A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection, Mucosal Immunology. [DOI] [PubMed]
- 188.Feeney O.M., Gracia G., Brundel D.H.S., Trevaskis N.L., Cao E., Kaminskas L.M., Porter C.J.H. Lymph-directed immunotherapy - Harnessing endogenous lymphatic distribution pathways for enhanced therapeutic outcomes in cancer. Adv. Drug Deliv. Rev. 2020;160:115–135. doi: 10.1016/j.addr.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 189.Zhang C., Gao F., Wu W., Qiu W.X., Zhang L., Li R., Zhuang Z.N., Yu W., Cheng H., Zhang X.Z. Enzyme-driven membrane-targeted chimeric peptide for enhanced tumor photodynamic immunotherapy. ACS Nano. 2019;13:11249–11262. doi: 10.1021/acsnano.9b04315. [DOI] [PubMed] [Google Scholar]
- 190.Sasso E., D'Alise A.M., Zambrano N., Scarselli E., Folgori A., Nicosia A. New viral vectors for infectious diseases and cancer. Semin. Immunol. 2020;50 doi: 10.1016/j.smim.2020.101430. [DOI] [PubMed] [Google Scholar]
- 191.Preston K.B., Monticello C.R., Wong T.A.S., To A., Donini O., Lehrer A.T., Randolph T.W. Preservation of quaternary structure in thermostable, lyophilized filovirus glycoprotein vaccines: a search for stability-indicating assays. J. Pharm. Sci. 2020;109:3716–3727. doi: 10.1016/j.xphs.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Choi M.J., Cossaboom C.M., Whitesell A.N., Dyal J.W., Joyce A., Morgan R.L., Campos-Outcalt D., Person M., Ervin E., Yu Y.C., Rollin P.E., Harcourt B.H., Atmar R.L., Bell B.P., Helfand R., Damon I.K., Frey S.E. Use of Ebola vaccine: recommendations of the advisory committee on immunization practices, United States. Mmwr Recommendat. Rep. 2020;70(2021):1–12. doi: 10.15585/mmwr.rr7001a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pollard A.J., Launay O., Lelievre J.-D., Lacabaratz C., Grande S., Goldstein N., Robinson C., Gaddah A., Bockstal V., Wiedemann A., Leyssen M., Luhn K., Richert L., Betard C., Gibani M.M., Clutterbuck E.A., Snape M.D., Levy Y., Douoguih M., Thiebaut R. E.E.s. group, Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial, The Lancet. Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30476-X. [DOI] [PubMed] [Google Scholar]
- 194.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Belij-Rammerstorfer S., Berry L., Bibi S., Bittaye M., Cathie K., Chappell H., Charlton S., Cicconi P., Clutterbuck E.A., Colin-Jones R., Dold C., Emary K.R.W., Fedosyuk S., Fuskova M., Gbesemete D., Green C., Hallis B., Hou M.M., Jenkin D., Joe C.C.D., Kelly E.J., Kerridge S., Lawrie A.M., Lelliott A., Lwin M.N., Makinson R., Marchevsky N.G., Mujadidi Y., Munro A.P.S., Pacurar M., Plested E., Rand J., Rawlinson T., Rhead S., Robinson H., Ritchie A.J., Ross-Russell A.L., Saich S., Singh N., Smith C.C., Snape M.D., Song R., Tarrant R., Themistocleous Y., Thomas K.M., Villafana T.L., Warren S.C., Watson M.E.E., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Faust S.N., Pollard A.J., Oxford C.V.T.G. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.D.Y. Logunov, I.V. Dolzhikova, D.V. Shcheblyakov, A.I. Tukhvatulin, O.V. Zubkova, A.S. Dzharullaeva, A.V. Kovyrshina, N.L. Lubenets, D.M. Grousova, A.S. Erokhova, A.G. Botikov, F.M. Izhaeva, O. Popova, T.A. Ozharovskaya, I.B. Esmagambetov, I.A. Favorskaya, D.I. Zrelkin, D.V. Voronina, D.N. Shcherbinin, A.S. Semikhin, Y.V. Simakova, E.A. Tokarskaya, D.A. Egorova, M.M. Shmarov, N.A. Nikitenko, V.A. Gushchin, E.A. Smolyarchuk, S.K. Zyryanov, S.V. Borisevich, B.S. Naroditsky, A.L. Gintsburg, C.-V.V.T.G. Gam, Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia, Lancet (London, England), (2021). [DOI] [PMC free article] [PubMed]
- 196.J. Sadoff, M. Le Gars, G. Shukarev, D. Heerwegh, C. Truyers, A.M. de Groot, J. Stoop, S. Tete, W. Van Damme, I. Leroux-Roels, P.-J. Berghmans, M. Kimmel, P. Van Damme, J. de Hoon, W. Smith, K.E. Stephenson, S.C. De Rosa, K.W. Cohen, M.J. McElrath, E. Cormier, G. Scheper, D.H. Barouch, J. Hendriks, F. Struyf, M. Douoguih, J. Van Hoof, H. Schuitemaker, Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine, The New England journal of medicine, (2021). [DOI] [PMC free article] [PubMed]
- 197.Baden L.R., Stieh D., Sarnecki M., Walsh S.R., Tomaras G.D., Kublin J.G., McElrath M.J., Alter G., Ferrari G., Montefiori D., Mann P., Nijs S., Callewaert K., Goepfert P., Edupuganti S., Karita E., Langedijk J.P., Wegmann F., Corey L., Pau M.G., Barouch D.H., Schuitemaker H., Tomaka F., Traverse H. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet Hiv. 2020;7:E688–E698. doi: 10.1016/S2352-3018(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sadoff J., De Paepe E., DeVincenzo J., Gymnopoulou E., Menten J., Murray B., Bastian A.R., Vandebosch A., Haazen W., Noulin N., Comeaux C., Heijnen E., Eze K., Gilbert A., Lambkin-Williams R., Schuitemaker H., Callendret B. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tuthill M., Cappuccini F., Carter L., Pollock E., Poulton I., Verrill C., Evans T., Gillessen S., Attard G., Protheroe A., Hamdy F., Hill A.V.S., Redchenko I. Results from ADVANCE: A phase I/II open-label non-randomised safety and efficacy study of the viral vectored ChAdOx1-MVA 5T4 (VTP-800) vaccine in combination with PD-1 checkpoint blockade in metastatic prostate cancer. Ann. Oncol. 2020;31:S543. [Google Scholar]
- 200.Redman J., Gandhy S., Gatti-Mays M., Sater H.A., Tsai Y., Donahue R., Cordes L., Steinberg S., Marte J., McMahon S., Madan R., Karzai F., Bilusic M., Rabizadeh S., Lee J., Soon-Shiong P., Kim S., Marshall J., Weinberg B., Schlom J., Gulley J., Strauss J. A randomized phase II trial of mFOLFOX6-based standard of care alone or in combination with Ad-CEA vaccine plus avelumab in patients with previously untreated metastatic colorectal cancer. Ann. Oncol. 2020;31:S227. [Google Scholar]
- 201.Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 202.Sarivalasis A., Boudousquie C., Balint K., Stevenson B.J., Gannon P.O., Iancu E.M., Rossier L., Lluesma S.M., Mathevet P., Sempoux C., Coukos G., Dafni U., Harari A., BassaniSternberg M., Kandalaft L.E. A Phase I/II trial comparing autologous dendritic cell vaccine pulsed either with personalized peptides (PEP-DC) or with tumor lysate (OC-DC) in patients with advanced high-grade ovarian serous carcinoma. J. Translat. Med. 2019;17 doi: 10.1186/s12967-019-02133-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Avigan D.E., Shah N., Logan B., Zhu J.X., Bisharat L., Callander N.S., Chodon T., Dhakal B., Efebera Y.A., Geller N., Hematti P., Herman M., Lazarus H.M., McKenna D.H., Nelson C., Nooka A., O'Brien K., O'Donnell L.C., Rapoport A.P., Rosenblatt J., Soiffer R.J., Stroopinsky D., Torka P., Uhl L., Waller E.K., Wu J., Young J.W., Pasquini M.C., Chung D.J. Evaluation of tumor vaccine generation in a phase II multicenter trial of single autologous hematopoietic cell transplant (AutoHCT)followed by lenalidomide maintenance for multiple myeloma (MM) with or without vaccination with dendritic cell/ myeloma fusions (DC/MM fusion vaccine): blood and marrow transplant clinical trials network (BMT CTN) 1401. Biol. Blood Marrow Transplant. 2020;26:S62–S63. [Google Scholar]
- 204.Chiappori A.A., Williams C.C., Gray J.E., Tanvetyanon T., Haura E.B., Creelan B., Thapa R., Chen D.T., Simon G.R., Bepler G., Gabrilovich D.I., Antonia S.J. Randomized-controlled phase II trial of salvage chemotherapy after immunization with a TP53-transfected dendritic cell-based vaccine (Ad.p53-DC) in patients with recurrent small cell lung cancer. Can. Immunol. Immunoth. 2019;68:517–527. doi: 10.1007/s00262-018-2287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Karaulov A.V., Bykov A.S., Volkova N.V. Rewiew of grippol family vaccine studies and modern adjuvant development. Epidemiologiya i vaktsinoprofilaktika. 2019;18:101–124. [Google Scholar]
- 206.van den Berg A.I.S., Yun C.-O., Schiffelers R.M., Hennink W.E. Polymeric delivery systems for nucleic acid therapeutics: approaching the clinic. J. Controlled Release: Off. J. Controlled Release Soc. 2021;331:121–141. doi: 10.1016/j.jconrel.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 207.Aldosari B.N., Alfagih I.M., Almurshedi A.S. Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou J.R., Kroll A.V., Holay M., Fang R.H., Zhang L.F. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2020;32 doi: 10.1002/adma.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shemesh C.S., Hsu J.C., Hosseini I., Shen B.-Q., Rotte A., Twomey P., Girish S., Wu B. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol. Therap.: J. Am. Soc. Gene Therapy. 2021;29:555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Esterhazy D., Canesso M.C.C., Mesin L., Muller P.A., de Castro T.B.R., Lockhart A., ElJalby M., Faria A.M.C., Mucida D. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature. 2019;569:126-+. doi: 10.1038/s41586-019-1125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eisenbarth S.C. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Soni R., Heindl S.E., Wiltshire D.A., Vahora I.S., Khan S. Antigenic variability a potential factor in assessing relationship between guillain barre syndrome and influenza vaccine - up to date literature review. Cureus. 2020;12 doi: 10.7759/cureus.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Barbuto J.A., Ensina L.F., Neves A.R., Bergami-Santos P., Leite K.R., Marques R., Costa F., Martins S.C., Camara-Lopes L.H., Buzaid A.C. Dendritic cell-tumor cell hybrid vaccination for metastatic cancer. Can. Immunol. Immunother. 2004;53:1111–1118. doi: 10.1007/s00262-004-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li S.P., Jiang Q., Liu S.L., Zhang Y.L., Tian Y.H., Song C., Wang J., Zou Y.G., Anderson G.J., Han J.Y., Chang Y., Liu Y., Zhang C., Chen L., Zhou G.B., Nie G.J., Yan H., Ding B.Q., Zhao Y.L. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018;36:258-+. doi: 10.1038/nbt.4071. [DOI] [PubMed] [Google Scholar]
- 216.Gutierrez-Zevallos J.D., Espiritu-Martinez L.B. COVID-19: vaccination in a developing country. J. Public Health (Oxf) 2021;43:e362–e363. doi: 10.1093/pubmed/fdab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Weissburg R.P., Berman P.W., Cleland J.L., Eastman D., Farina F., Frie S., Lim A., Mordenti J., Nguyen T.T., Peterson M.R. Characterization of the MN gp120 HIV-1 vaccine: antigen binding to alum. Pharm. Res. 1995;12:1439–1446. doi: 10.1023/a:1016266916893. [DOI] [PubMed] [Google Scholar]
- 218.Shi Y., HogenEsch H., Hem S.L. Change in the degree of adsorption of proteins by aluminum-containing adjuvants following exposure to interstitial fluid: freshly prepared and aged model vaccines. Vaccine. 2001;20:80–85. doi: 10.1016/s0264-410x(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 219.Liu Z.H., Huang L.H., Xue W. pH-responsive vaccine delivery systems for improving cellular immunity. Prog. Nat. Sci.-Mater. 2020;30:609–617. [Google Scholar]
- 220.Whitworth H.S., Gallagher K.E., Howard N., Mounier-Jack S., Mbwanji G., Kreimer A.R., Basu P., Kelly H., Drolet M., Brisson M., Watson-Jones D. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. 2020;38:1302–1314. doi: 10.1016/j.vaccine.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 221.N. Marasini, K. Ghaffar, M. Skwarczynski, I. Toth, Liposomes as a vaccine delivery system, Micro and Nanotechnology in vaccine Development, Elsevier2017, pp. 221-239.
- 222.Xu A., Freywald A., Xiang J. Novel T-cell-based vaccines via arming polyclonal CD4+ T cells with antigen-specific exosomes. Future Medicine. 2016 doi: 10.2217/imt-2016-0094. [DOI] [PubMed] [Google Scholar]
- 223.Wang R., Xu A., Zhang X., Wu J., Freywald A., Xu J., Xiang J. Novel exosome-targeted T-cell-based vaccine counteracts T-cell anergy and converts CTL exhaustion in chronic infection via CD40L signaling through the mTORC1 pathway. Cell. Mol. Immunol. 2017;14:529–545. doi: 10.1038/cmi.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]