Abstract
Introduction:
The hematopoietic cytokine granulocyte-colony stimulating factor (G-CSF) is well known to stimulate proliferation of blood stem/progenitor cells of the leukocyte lineage, but is also recognized as a neurotrophic factor involved in brain self-repair processes. G-CSF administration has been shown to promote recovery from experimental models of traumatic brain injury (TBI) and to modulate components of the endocannabinoid system (eCS). Conversely, Δ9-tetrahydrocannabinol (Δ9THC) treatment of normal mice has been shown to increase blood levels of G-CSF in the periphery.
Hypothesis:
Administration of the phytocannabinoid Δ9THC will enhance brain repair following controlled cortical impact (CCI) by upregulating G-CSF and other neurotrophic factors (brain-derived neurotrophic factor [BDNF] and glial-derived neurotrophic factor [GDNF]) in brain regions.
Materials and Methods:
C57BL/6J mice underwent CCI and were treated for 3 days with THC 3 mg/kg intraperitoneally. Motor function on a rotarod was recorded at baseline and 3, 7, and 14 days after CCI. Groups of mice were euthanized at 7 and 14 days. G-CSF, BDNF, and GDNF expression were measured at 7 and 14 days in cerebral cortex, striatum, and hippocampus on the side of the trauma.
Results:
Δ9THC-treated mice ran on the rotarod longer than vehicle-treated mice and recovered to normal rotarod performance levels at 2 weeks. These mice, compared to vehicle-treated animals, exhibited significant upregulation of G-CSF as well as BDNF and GDNF in cerebral cortex, striatum, and hippocampus.
Conclusion:
Administration of the phytocannabinoid Δ9THC promotes significant recovery from TBI and is associated with upregulation of brain G-CSF, BDNF, and GDNF, neurotrophic factors previously shown to mediate brain self-repair following TBI and stroke.
Keywords: controlled cortical impact, traumatic brain injury, neurotrophic factors, hematopoietic cytokines, endocannabinoid system, phytocannabinoids
Introduction
Traumatic brain injury (TBI) is a global public health problem and is recognized as the “signature injury” sustained by soldiers and civilians in foreign conflicts.1 In the United States, TBI is a major cause of death and disability.2 Those who survive a TBI can face effects that last a few days, or the rest of their lives. Effects of TBI can include impairments related to thinking or memory, movement, sensation (e.g., vision or hearing), or emotional functioning (e.g., personality changes and depression).
TBI triggers a complex pathophysiological process encompassing three overlapping phases: (1) primary injury to brain tissue and/or the cerebral vasculature; (2) the secondary injury, which includes physiological, neuroinflammatory, and biochemical processes triggered by the primary insult; and (3) regenerative responses, including enhanced proliferation of neural progenitor cells and endothelial cells.1 The secondary injury evolves over hours to days and may significantly contribute to chronic post-traumatic neurologic disability. Closely overlapping and following the secondary injury phase is the complex regenerative response that ultimately determines the extent of functional recovery from the injury.
Granulocyte-colony stimulating factor (G-CSF) has been identified as one of the many cytokines that modulate the secondary response to TBI.3,4 Evidence for the role of G-CSF in the brain's self-repair mechanism is growing. “Stab” lesions of the hippocampus made by insertion and removal of an electrode or needle triggered an acute local rise in G-CSF and other cytokines released at the sites of insertion in frontal cortex and hippocampus.5 Hippocampal neurogenesis was stimulated by the focal injury and was associated with upregulation of other neurotrophic factors as well as G-CSF.5 From these results and the reports of others on the effects of G-CSF in stroke,6,7 it was postulated that G-CSF plays an important role in the brain's repair response to injury.
This hypothesis was tested and confirmed in mice by administration of G-CSF for 3 days after animals sustained mild to moderate controlled cortical impact (CCI). G-CSF treatment significantly enhanced recovery of performance in the radial arm water maze (RAWM), a test of learning and memory.3,4 G-CSF treatment also modulated astrocytosis and microgliosis, increased expression of neurotrophic factors and anti-inflammatory cytokines, and generated new neurons in the hippocampus.4
Interestingly, endogenous G-CSF levels can be significantly increased by administration of the phytocannabinoid Δ9-tetrahydrocannabinol (Δ9THC) to normal mice.8 Δ9THC effects are mediated primarily by interaction with specific cannabinoid receptors (CB1 and CB2) of the endocannabinoid system (eCS).8,9 Given that Δ9THC treatment increases levels of G-CSF, we hypothesized that the beneficial effects of administration of G-CSF in promoting brain repair after injury may involve interaction with the eCS.
The eCS plays a central role in modulating neuronal activity and maintaining homeostasis.9,10 The eCS comprises endogenous ligands, cannabinoid receptors, and the proteins that transport, synthesize, and degrade these ligands.8,9 The two primary endogenous ligands are anandamide and 2-arachydonoylglycerol; CB1 and CB2 are the best-characterized cannabinoid receptors.10 CB1 receptors are particularly enriched in the nervous system, but are also present in peripheral tissues. In neurons, CB1 receptors are enriched on synaptic terminals, reflecting their major role in modulating synaptic transmission, although they are also expressed at functionally important levels on neuronal somata and dendrites.10 CB2 receptors are primarily expressed in cells of immune origin, including microglia, but may also be expressed in neurons, especially in pathological states.8,10 Microglial CB2 receptor activation is considered to be primarily anti-inflammatory.10
There is a large body of evidence showing that eCS is markedly increased in response to pathogenic events, including TBI.11 This fact, as well as numerous studies on experimental models of brain toxicity, neuroinflammation, and trauma, supports the notion that the eCS is part of the brain's compensatory or repair mechanisms.8
We have previously reported that mice that undergo mild to moderate CCI exhibit a downregulation of CB1-R expression and upregulation of CB2-R in cortex, striatum, and hippocampus.12 These changes in expression of CB receptors are similar to those reported in a weight-drop mouse model of TBI.13 Moreover, treatment of these mice with G-CSF reversed the changes in CB1 and CB2 receptor expression.12
The overall objective of these experiments was to test the hypothesis that enhanced brain repair promoted by a course of THC administration following TBI is mediated, in part, by upregulation of brain G-CSF and other neurotrophic factors involved in brain repair such as brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF). Given the importance of the eCS in mediating brain self-repair following TBI, experiments were conducted a) to test whether THC treatment alone could enhance recovery of locomotor function following CCI; b) to determine if G-CSF upregulation occurs beyond the level observed to be upregulated in untreated mice following CCI; and c) to determine if THC also upregulates neurotrophic factor expression (BDNF and GDNF).
Materials and Methods
Animals
This study was carried out in strict accordance with the National Institutes of Health guide for the care and use of laboratory animals. The protocol was approved by the institutional animal care and use committee of the University of South Florida. Adult C57BL/6J male, 3-month-old mice (25–30 g) were housed in standard laboratory cages and left undisturbed for 1 week after arrival at the animal facility. Animals had ad libitum access to water and laboratory chow and were maintained in a temperature- and humidity-controlled room on a 12-h light/12-h dark cycle with lights on at 7:00 AM. A total of nine groups of C57BL/6J mice were used in this study (n=6 per group, total of 54 mice). Seven groups underwent CCI to right frontal cortex; three CCI groups did not receive treatment and were euthanized 24, 48, and 72 h after CCI. Two of the CCI groups were treated with vehicle or Δ9THC 3 mg/kg×3-day treatment and euthanized 7 days after CCI. Two CCI groups were treated with vehicle or Δ9THC 3 mg/kg×3-day treatment and euthanized 14 days after CCI. Two groups served as controls (sham surgery) treated with vehicle and Δ9THC 3 mg/kg×3 day. Each brain was removed after perfusion with heparinized saline, and dissected into three regions (cerebral cortex, corpus striatum, and hippocampus) for analyses.
Rotometry
The ability to run on a rotating drum (rotarod) was used as a measure of motor balance and coordination (Madel 47600 rotarod for mice; Ugo Basile, Gemonio, Italy). Data were generated by averaging the scores (total time spent on the rotating drum divided by three trials) for each animal during training and testing days. Each animal was placed in a neutral position on a cylinder, the rod was rotated, with the speed accelerated linearly from 4 to 40 rpm within 3 min, and the time spent on the rotarod was recorded automatically. For training, animals were given one trial before testing. For testing, animals were given three trials, and the average score on these three trials was used as the individual rotarod score. After baseline performance had been established, animals were randomly assigned to receive vehicle (n=6) or THC treatment (n=6) following CCI.
Surgery and CCI
Animals underwent an experimental TBI with a controlled cortical impactor (Pittsburgh Precision Instruments), as described previously.14 Animals initially received buprenorphine (0.05 mg/kg, s.c.) at the time of anesthesia induction (with 125 mg/kg ketamine and 12.5 mg/kg xylazine). After deep anesthesia had been achieved (verified by checking for pain reflexes), individual animals were fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). After exposing the skull, craniectomy (∼3 mm to accommodate the impactor tip) was performed over the right frontoparietal cortex (0.5 mm anteroposterior and 1.0 mm mediolateral to bregma). All mice received a “moderate” TBI. The pneumatically operated TBI device (with a convex tip diameter of 2 mm) impacts the brain at a velocity of 6.0 m/sec, reaching a depth of 1.0 mm below the dura mater layer, and remains in the brain for 150 msec. The impactor rod was angled at 158° to the vertical to maintain a perpendicular position in reference to the tangential plane of the brain curvature at the impact surface. A linear variable displacement transducer (Macrosensors, Pennsauken, NJ) connected to the impactor measured velocity and duration to verify consistency. Bone wax was used to cover the craniectomized region, and the skin incision sutured thereafter. A computer-operated thermal blanket pad and a rectal thermometer allowed maintenance of body temperature within normal limits. All animals were closely monitored until recovery from anesthesia and over the next 3 days.
Drugs
Δ9THC a dose of 3 mg/kg dissolved in 100% ethanol to a concentration of 6 mg/mL. In a second tube, Kolliphor_ EL (synonym: Cremophor_EL; Sigma-Aldrich, St. Louis, MO) was mixed with sterile 0.9% saline. The dissolved cannabinoid was then mixed with the Cremophor/NaCl solution to a final ratio of 1:1:18 (cannabinoid/ethanol:Cremophor: saline). The final concentration of each component in the injection solutions was 0.3 mg/mL cannabinoid, 5% ethanol, 5% Cremophor, and 0.81% NaCl. Mice were injected intraperitoneally (i.p.) with 3 mg/kg of the indicated drug daily for 3 days. Controls received a vehicle consisting of 5% ethanol, 5% Cremophor, and 0.81% NaCl i.p. daily for 3 days after CCI. Δ9THC was purchased from Sigma-Aldrich. This dose of Δ9THC was chosen as the lowest dose capable of triggering significant upregulation of GCSF in normal C57BL mice.15
Quantitative real-time polymerase chain reaction of G-CSF receptors
The mouse brain tissue samples were homogenized, and total RNA was purified using the RNeasy Mini Kit (Qiagen, Germantown, MD). One microgram of total RNA was used to synthesize the complementary cDNA with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. The real-time polymerase chain reaction (PCR) reaction was performed using 2 μL of cDNA with 1 μL of 20×Taqman Mouse G-CSF primer/probe set (Assay ID no. Mm00438334_m1) (Applied Biosystems, Foster City, CA), 10 μL of TaqMan® Fast Advanced Master Mix (Applied Biosystems), and 7 μL of nuclease-free water for a total volume of 20 μL. As an internal control, a second real-time PCR reaction was performed using 2 μL of cDNA with 1 μL 20×Taqman Mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primer/probe set (Assay ID no. Mm99999915_g1) (Applied Biosystems), 10 μL of 2×TaqMan Fast Advanced Master Mix, and 7 μL of nuclease-free water for a total volume of 20 μL. Thermocycling conditions were as follows: 50°C, 2 min; 95°C, 10 min; and 40 cycles of 95°C for 15 sec and 60°C for 1 min using Applied BioSystems ViiA 7 System. Mouse G-CSF mRNA relative quantification (RQ) to GAPDH was calculated from the quantitative PCR cycle threshold (Ct) data by formula, 2−ddCt−1 (or RQ-1), where ddCt=(Ctgcsf−Ctgapdh)treatment−(Ctgcsf−Ctgapdh)control, Each sample was run in duplicate.
Mouse brain BDNF, GDNF, and G-CSF enzyme-linked immunosorbent assay
Regions of brain were dissected as described above, the mouse brain tissue samples were homogenized in T-PER Tissue Protein Reagent (Thermo-Fisher, PI-#78510; Waltham, MA) with Protease and Phosphatase inhibitor cocktail (PI-78443), and each brain sample's protein concentration was measured by a BCA kit (Fisher Scientific no. 23225; Pittsburgh, PA). Levels of Mouse BDNF and GDNF were measured using a mouse BDNF and GDNF enzyme-linked immunosorbent assay (ELISA) kit from Boster Biological Technology (Fremont, CA. Cat no. EK0309 and no. EK0935) following kit instructions. Results of brain BDNF and GDNF levels in brain tissue using these kits have been previously reported.4,16 The mouse G-CSF ELISA kit was from Abcam (Cat no. Ab 197743; Cambridge, MA) and followed the kit instructions. Results using this kit for brain tissue G-CSF have been previously reported.5 Each sample was run in duplicate. The ELISA 96-well plate was read at a plate reader (BioTek Synergy) at optical density absorbance at 450 nm. The cytokine concentration was expressed at unit pg/mL with sample total protein concentration at 1 mg/mL.
Statistics
Data are expressed as mean±standard error of mean (n=6 mice per treatment in the rotarod data and n=6 per treatment group, with n=3 or 4 for each time point in measures of GCSF, BDNF, and GDNF expression). Prism 7 (GraphPad Software, Inc., San Diego, CA) was used to perform 1-way or 2-way analysis of variance (ANOVA) followed by correction for multiple comparisons. p-Values <0.05 were considered statistically significant.
Results
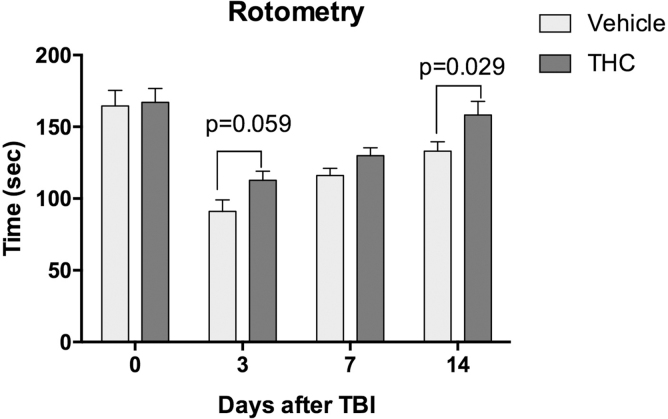
Animals that sustained CCI to the right frontal cortex exhibited a significant decline in performance on the rotarod. Latency to fall decreased to 55% of baseline 3 days after the injury and gradually improved without treatment to 67% of baseline after 2 weeks (Fig. 1). Mice treated with Δ9THC showed a significantly greater improvement over time compared to vehicle-treated mice, reaching 95% of baseline performance at 14 days (p<0.05).
FIG. 1.
Effects of THC treatment for 3 days following CCI. Both the THC-treated group (n=6 mice) and vehicle-treated group (n=6 mice) were trained to run on a rotometer before undergoing CCI. After CCI, treatment with THC 3 mg/kg for 3 days resulted in a time-dependent recovery of motor function. Two-way ANOVA testing showed that both treatment (THC vs. vehicle) and time (days) after CCI contributed significantly to total variance (p<0.005). Sidak's correction for multiple comparisons revealed that THC significantly improved time on the rotometer compared to vehicle treatment on day 14 (p=0.029). ANOVA, analysis of variance; CCI, controlled cortical impact; THC, tetrahydrocannabinol.
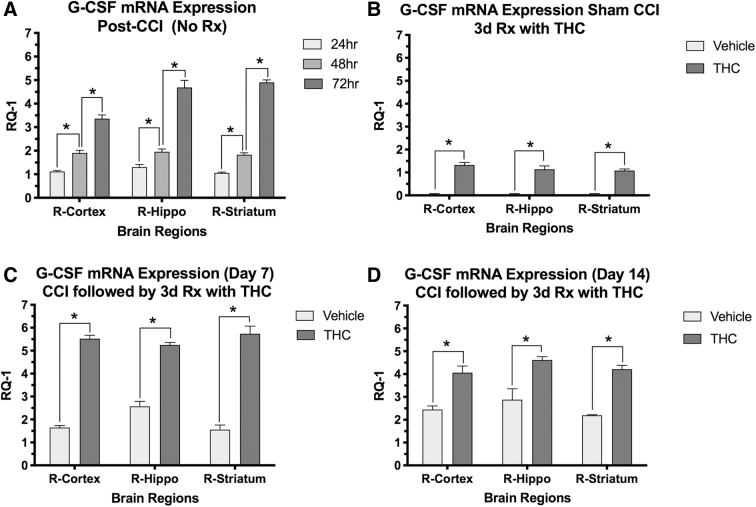
Following CCI, there was a time-dependent upregulation of G-CSF mRNA expression in three brain regions in vehicle-treated mice (Fig. 2A). Δ9THC treatment in sham surgery controls also increased G-CSF expression in all three brain regions (Fig. 2B), similar to that produced by CCI alone at 24 h (Fig. 2A). Compared to vehicle-treated mice, animals treated with Δ9THC significantly upregulated G-CSF mRNA expression at 7 and 14 days in cortex, striatum, and hippocampus (Fig. 2A, B). G-CSF protein levels in cortex and striatum were also significantly increased in the sham control group, and 7 and 14 days after CCI in mice treated with Δ9THC (Fig. 3A–C).
FIG. 2.
Effects of THC treatment on G-CSF mRNA expression in brain regions on the side of injury (right hemisphere; n=4 mice per group). X-axis=brain regions, Y-axis is change in mRNA expression (RQ-1). (A) G-CSF expression increased in the three brain regions measured in a time-dependent manner after CCI in untreated mice. Two-way ANOVA revealed that time contributed more than brain region to total variance; Sidaks correction for multiple comparison showed significant differences between times after CCI in each region (*<0.001); (B) THC treatment for 3 days in animals that had sham surgery (no CCI) also resulted in increased mRNA expression of G-CSF in the three brain regions measured on day 3. Two-way ANOVA revealed that time, but not brain region contributed significantly to total variance. G-CSF mRNA in each brain region was significantly increased by THC compared to vehicle treatment (*p<0.001); (C, D) G-CSF mRNA was also significantly increased by THC treatment compared to controls in the three brain regions on days 7 and 14 after CCI. Two-way ANOVA revealed that treatment contributed more than brain region to total variance. Sidak's correction for multiple comparisons showed that all three brain regions had significantly increased G-CSF mRNA upregulation on days 7 and 14 (*p<0.05). G-CSF, granulocyte-colony stimulating factor; RQ-1, relative quantification-1.
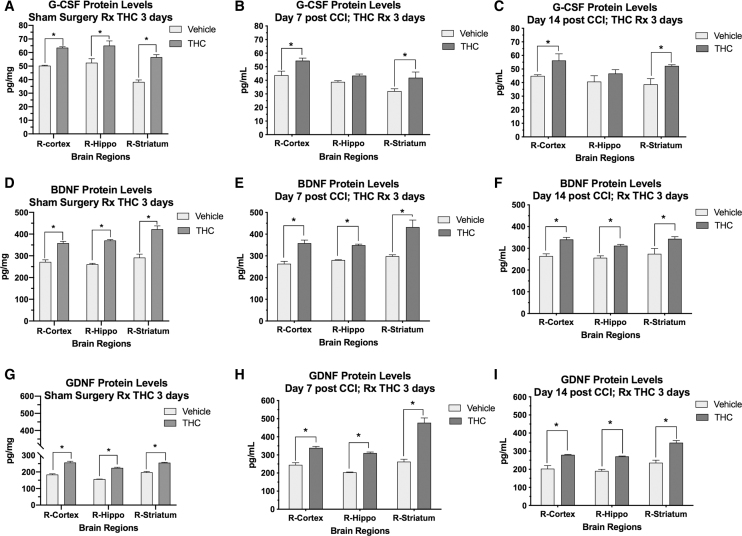
FIG. 3.
Effects of THC treatment on G-CSF, BDNF, and GDNF protein concentrations in brain regions following CCI and sham surgery. X-axis=brain regions, and Y-axis=tissue concentration of the protein in pg/mg (A–C). G-CSF protein levels were significantly increased by 3 days of THC treatment in the sham surgery control group (A). Following CCI, G-CSF levels were significantly increased on day 7 and 14 in cortex and striatum, but not hippocampus (B, C). (D–F) BDNF levels were increased in both the sham control group (D) and following CCI on days 7 and 14 (E, F). (G–I) THC treatment resulted in increased GDNF levels in both the sham control group (G) and on day 7 and 14 after CCI in animals treated with THC compared to vehicle (H, I). Data analysis: 2-way ANOVA was performed for data set on each graph revealing that both time and brain region contributed significantly to total variance. Sidak's correction for multiple comparisons was performed to detect significant differences indicated by *p<0.05). BDNF, brain-derived neurotrophic factor; GDNF, glial-derived neurotrophic factor.
To determine if the improved performance on rotarod was associated with increased expression of the neurotrophic factors BDNF and GDNF, protein levels were measured by ELISA in three regions of brain at 7 and 14 days (Fig. 3). Animals treated with Δ9THC exhibited a significant increase in both BDNF and GDNF protein expression in cortex, striatum, and hippocampus at 7 and 14 days after CCI (p<0.05). In the sham control group, THC treatment for 3 days also increased protein levels of BDNF and GDNF (Fig. 3D, G).
Discussion
Administration of Δ9THC at a moderate dose for 3 days after CCI resulted in enhanced recovery of motor performance evidenced by longer latencies to fall from a rotating drum (rotarod). Similar improvements in motor function were observed when G-CSF was administered after CCI.3 In this report, we found that Δ9THC administration triggered a significant upregulation of brain G-CSF mRNA and protein expression that was temporally associated with improved recovery of motor function. In addition to upregulation of G-CSF expression, two neurotrophic factors (BDNF and GDNF) were significantly increased. BDNF and GDNF have both been implicated in enhancing survival of injured neurons and promoting hippocampal neurogenesis.17–19
The recognition that some hematopoietic growth factors like G-CSF act directly on neural tissue has stimulated research on the therapeutic applications of these agents for neurodegenerative diseases, stroke, and brain trauma.4,20–26 Systemic G-CSF administration to rats that had sustained TBI resulted in significantly improved recovery of motor function compared to the control group.27 In a separate study in mice, G-CSF treatment following CCI resulted in significantly better performance on the rotarod at 1 week, and in the RAWM after 1 and 2 weeks.4
We have recently reported that administration of G-CSF for 3 days following CCI mitigated or reversed the trauma-induced CB1-R downregulation and CB2-R upregulation in the three brain regions.12 That study suggested enhanced recovery was not completely dependent on cannabinoid receptor interactions, but was a result of direct activation of the brain's G-CSF receptors.12
Given that G-CSF treatment also upregulated expression of BDNF and GDNF, it is likely these neurotrophic factors play an important role in brain repair. BDNF has been reported to be involved in neuroprotection and neuronal repair and recovery after TBI.28 Recently, the prognostic value of increased expression of BDNF protein levels in serum has received attention in some brain disorders, including TBI and recovery from stroke17,29 Acute serum concentrations of BDNF predicted severity and outcome of a TBI, and patients with the lowest BDNF concentrations had the highest odds of incomplete recovery.17 Unfortunately, exogenous administration of BDNF is limited by a lack of blood–brain barrier permeability, poor half-life, and rapid degradation.30
A recent report has shown that activation of CB1 and CB2 receptors by administration of the phytocannabinoid Δ9THC into normal (noninjured) mice greatly stimulated generation of myeloid-derived suppressor cells (MDSC).15 These MDSCs comprise a heterogenous population of immature macrophages, granulocytes, dendritic cells, and other myeloid cells, which exert immunosuppressive actions. Moreover, induction of MDSC by THC administration was associated with an increase in serum G-CSF levels.15 Interestingly, G-CSF itself will also enhance proliferation of peripheral monocytes, promote recruitment of bone marrow-derived cells to the site of injury, and act directly in brain as a neurotrophic factor to enhance recovery from the injury.3,4
From a clinical perspective, there is a need to study the neuroprotective effects of exogenously administered cannabinoids. Interestingly, users of cannabis admitted to hospital following TBI exhibited less severe injury and better recovery than nonusers of cannabis.31 Unfortunately, there are no high quality controlled clinical trials on the use of cannabinoids to promote recovery from TBI or to minimize damage when the cannabinoid is used prophylactically. Additional pre-clinical research on mechanisms of recovery triggered by the interaction of G-CSF and other neurotrophic factors with the eCS will facilitate translation to clinical trials in patients with TBI.
Conclusions
Treatment of mice with Δ9THC (3 mg/kg i.p. for 3 days) following CCI resulted in complete recovery of motor performance on the rotarod by 2 weeks.
Δ9THC-treated animals exhibited a significant increase in expression of G-CSF mRNA in cerebral cortex, striatum, and hippocampus at 7 and 14 days after CCI.
Increased expression of the neurotrophic factors BDNF and GDNF was also significantly upregulated in the same brain regions at 7 and 14 days.
Recovery of motor function promoted by Δ9THC appears to be mediated, in part, by upregulation of G-CSF expression and the neurotrophic factors BDNF and GDNF.
Acknowledgment
Supported by VA Merit Review Grant to S. Song.
Abbreviations Used
- 2-AG
arachidonoylglycerol
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- CB
cannabinoid
- CCI
controlled cortical impact
- eCS
endocannabinoid system
- G-CSF
granulocyte-colony stimulating factor
- GDNF
glial-derived neurotrophic factor
- i.p.
intraperitoneally
- MDSC
myeloid-derived suppressor cell
- NIH
National Institutes of Health
- RAWM
radial arm water maze
- RQ-1
relative quantification-1
- TBI
traumatic brain injury
- Δ9THC
Δ9-tetrahydrocannabinol
Authors' Contributions
S.S., X.K., and B.W. performed the experiments and collected the data. S.S. and J.S.-R. designed the experiment, analyzed the data, and wrote the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Veteran Affairs Merit Review Grant BX004037-01A1.
Cite this article as: Song S, Kong X, Wang B, Sanchez-Ramos J (2022) Recovery from traumatic brain injury following treatment with Δ9-tetrahydrocannabinol is associated with increased expression of granulocyte-colony stimulating factor and other neurotrophic factors, Cannabis and Cannabinoid Research 7:4, 415–423, DOI: 10.1089/can.2020.0119.
References
- 1. Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Surveillance report of traumatic brain injury related emergency department visits, hospitalization, and deaths. U.S. Department of Health and Human Services: Atlanta, GA, 2019. [Google Scholar]
- 3. Song S, Kong X, Acosta S, et al. . Granulocyte colony-stimulating factor promotes behavioral recovery in a mouse model of traumatic brain injury. J Neurosci Res. 2016;94:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song S, Kong X, Acosta S, et al. . Granulocyte-colony stimulating factor promotes brain repair following traumatic brain injury by recruitment of microglia and increasing neurotrophic factor expression. Restor Neurol Neurosci. 2016;34:415–431. [DOI] [PubMed] [Google Scholar]
- 5. Song S, Song S, Cao C, et al. . Hippocampal neurogenesis and the brain repair response to brief stereotaxic insertion of a microneedle. Stem Cells Int. 2013;2013:205878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Six I, Gasan G, Mura E, et al. . Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003;458:327–328. [DOI] [PubMed] [Google Scholar]
- 7. Schabitz WR, Kollmar R, Schwaninger M, et al. . Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. [DOI] [PubMed] [Google Scholar]
- 8. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9–29. [DOI] [PubMed] [Google Scholar]
- 9. Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu H-C, Mackie K. Review of the endocannabinoid system. Biol Psychiatry CNNI. 2020; DOI: 10.1016/j.bpsc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shohami E, Cohen-Yeshurun A, Magid L, et al. . Endocannabinoids and traumatic brain injury. Br J Pharmacol. 2011;163:1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song S, Kong X, Borlongan C, et al. . Granulocyte-colony stimulating factor enhances brain repair following traumatic brain injury without requiring activation of cannabinoid receptors. Cannabis Cannabinoid Res. 2020; DOI: 10.1089/can.2019.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, et al. . Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One. 2015;10:e0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu S, Kaneko Y, Bae E, et al. . Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–163. [DOI] [PubMed] [Google Scholar]
- 15. Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40:3358–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weng L, Guo X, Li Y, et al. . Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur J Pharmacol. 2016;774:50–54. [DOI] [PubMed] [Google Scholar]
- 17. Failla MD, Conley YP, Wagner AK. Brain-derived neurotrophic factor (BDNF) in traumatic brain injury-related mortality: interrelationships between genetics and acute systemic and central nervous system BDNF profiles. Neurorehabil Neural Repair. 2016;30:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cacialli P, Palladino A, Lucini C. Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish. Neural Regen Res. 2018;13:941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonafina A, Trinchero MF, Ríos AS, et al. . GDNF and GFRalpha1 are required for proper integration of adult-born hippocampal neurons. Cell Rep. 2019;29:4308..e4–4319.e4. [DOI] [PubMed] [Google Scholar]
- 20. Sanchez-Ramos J, Song S, Cao C, et al. . The potential of hematopoietic growth factors for treatment of Alzheimer's disease: a mini-review. BMC Neurosci. 2008;9(Suppl. 2):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitzer C, Krüger C, Plaas C, et al. . Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 2008;131(Pt 12):3335–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchez-Ramos J, Cimino C, Avila R, et al. . Pilot study of granulocyte-colony stimulating factor for treatment of Alzheimer's disease. J Alzheimers Dis. 2012;31:843–855. [DOI] [PubMed] [Google Scholar]
- 23. Sanchez-Ramos J, Song S, Sava V, et al. . Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice. Neuroscience. 2009;163:55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchez-Ramos J, Sava V, Rowe A, et al. . The hematopoietic growth factor G-CSF enhances recovery in the MPTP mouse model of PD. Neurology. 2010;74:A82. [Google Scholar]
- 25. Schabitz WR, Schneider A. New targets for established proteins: exploring G-CSF for the treatment of stroke. Trends Pharmacol Sci. 2007;28:157–161. [DOI] [PubMed] [Google Scholar]
- 26. Rowe A, Eberhard J, Sanchez-Ramos J. Hematopoietic growth factors: novel therapeutic strategy for Alzheimer's disease. Drugs Future. 2010;34:1–13. [Google Scholar]
- 27. Yang DY, Chen YJ, Wang MF, et al. . Granulocyte colony-stimulating factor enhances cellular proliferation and motor function recovery on rats subjected to traumatic brain injury. Neurol Res. 2010;32:1041–1049. [DOI] [PubMed] [Google Scholar]
- 28. Rostami E, Krueger F, Plantman S, et al. . Alteration in BDNF and its receptors, full-length and truncated TrkB and p75(NTR) following penetrating traumatic brain injury. Brain Res. 2014;1542:195–205. [DOI] [PubMed] [Google Scholar]
- 29. Stanne TM, David Åberg N, Nilsson S, et al. . Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke. 2016;47:1943–1945. [DOI] [PubMed] [Google Scholar]
- 30. Houlton J, Abumaria N, Hinkley SFR, et al. . Therapeutic potential of neurotrophins for repair after brain injury: a helping hand from biomaterials. Front Neurosci. 2019;13:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen BM, Kim D, Bricker S, et al. . Effect of marijuana use on outcomes in traumatic brain injury. Am Surg. 2014;80:979–983. [PubMed] [Google Scholar]