Abstract
Introduction:
The endocannabinoid (eCB) system plays a key role in maintaining homeostasis, including the regulation of metabolism and stress responses. Chronic stress may blunt eCB signaling, and disruptions in eCB signaling have been linked to stress-related psychiatric disorders and physical health conditions, including anxiety, depression, post-traumatic stress disorder (PTSD), diabetes, and obesity. Pharmacological and nonpharmacological behavioral interventions (e.g., exercise) that target the eCB system may be promising therapeutic approaches for the prevention and treatment of stress-related diseases. In this study, we perform a systematic review and the first meta-analysis to examine the impact of exercise on circulating eCB concentrations.
Materials and Methods:
We performed a review of the MEDLINE (PubMed) database for original articles examining the impact of exercise on eCBs in humans and animal models. A total of 262 articles were screened for initial inclusion.
Results:
Thirty-three articles (reporting on 57 samples) were included in the systematic review and 10 were included in the meta-analysis. The majority of samples that measured anandamide (AEA) showed a significant increase in AEA concentrations following acute exercise (74.4%), whereas effects on 2-arachidonoylglycerol (2-AG) were inconsistent. The meta-analysis, however, revealed a consistent increase in both AEA and 2-AG following acute exercise across modalities (e.g., running, cycling), species (e.g., humans, mice), and in those with and without pre-existing health conditions (e.g., PTSD, depression). There was substantial heterogeneity in the magnitude of the effect across studies, which may relate to exercise intensity, physical fitness, timing of measurement, and/or fasted state. Effects of chronic exercise were inconsistent.
Conclusions:
Potential interpretations and implications of exercise-induced mobilization of eCBs are discussed, including refilling of energy stores and mediating analgesic and mood elevating effects of exercise. We also offer recommendations for future work and discuss therapeutic implications for exercise in the prevention and treatment of stress-related psychopathology.
Keywords: anandamide, mental health, endocannabinoids, physical activity, running
Introduction
The endocannabinoid (eCB) system plays a key role in maintaining homeostasis throughout the brain and body, including regulating energy metabolism, cognition, sleep, inflammation, and stress responses.1–3 eCB receptors, including cannabinoid type-1 (CB1Rs) are located throughout the brain and play a neuromodulatory role in various neurotransmitter systems.4,5 Although they are widespread, CB1Rs are most densely located in limbic brain regions (e.g., including the prefrontal cortex, amygdala, and hippocampus)6 implicated in stress and emotion regulation, reward processes, fear learning, and extinction. Cannabinoid type-2 receptors (CB2Rs) are found on immune tissues and play an immunomodulatory role in cytokine release.7
The two most well-studied eCBs (i.e., endogenous ligands for cannabinoid receptors) are anandamide (AEA) and 2-arachidonoylglycerol (2-AG). These eCBs are synthesized “on demand” and released into the synapse from the post-synaptic neuron to inhibit pre-synaptic activity in a retrograde manner.8–10 Activity of AEA and 2-AG is primarily modulated by enzymes that synthesize (i.e., N-acylphosphatidylethanolamine phospholipase D [NAPE], diacylglycerol lipase [DAGL], respectively) or catabolize (i.e., fatty acid amide hydrolase [FAAH], monoacylglycerol lipase, respectively) eCBs.
In addition to regulating synaptic activity, eCBs can inhibit activity of the hypothalamo–pituitary–adrenocortical axis and the sympathetic nervous system.11,12 eCB concentrations are modulated in response to both acute and chronic stress exposure,13 and disruptions in eCB signaling are linked to several stress-related diseases, including anxiety, depression, post-traumatic stress disorder (PTSD), obesity, and diabetes.14–19
Given the critical role in regulating stress responses, the eCB system has emerged as a promising therapeutic target for the prevention and treatment of stress-related psychopathology.16,20 For example, pharmacologic interventions that enhance AEA concentrations through FAAH inhibition have been shown to attenuate autonomic stress reactivity and improve fear extinction memory recall in healthy adults.21 Similarly, administration of low doses of exogenous cannabinoids that act on CB1Rs, such as Δ9-tetrahydrocannabinol, reduces fear and anxiety-like behaviors in animal models,22 potentiates fear extinction memory recall in healthy adults,23 and reduces corticolimbic responses to threat in adults with PTSD.24 Several ongoing or recently completed clinical trials have examined drugs that target the eCB system for stress-related psychiatric disorders, including anxiety (NCT02432703), depression (NCT02498392), substance use disorders (SUDs; NCT03787628), and PTSD (NCT04080427).
Growing research suggests that nonpharmacological approaches—particularly exercise—can boost eCB signaling. Historically, the euphoric, analgesic, anxiolytic, and mood-elevating effects of exercise were thought to be mediated, at least in part, by the endogenous opioid system (i.e., exercise-induced increases in beta-endorphins).25,26 However, recent studies have challenged the role of endorphins in mediating the beneficial effects of exercise. For example, there is evidence that endorphins cannot cross the blood–brain barrier27 and blockage of endorphin signaling using an opioid receptor antagonist (e.g., naltrexone) does not block the post-exercise euphoric, analgesic, and anxiolytic effects.28,29
Rather, emerging data indicate that the eCB system plays a pivotal role in mediating some of the well-documented effects of exercise, including reductions in pain and anxiety, and improvements in cognitive functioning and mood.30,31 Indeed, studies suggest a role for eCBs in regulating hippocampal neurogenesis32,33; therefore, elevations in circulating eCBs may explain some of the reported beneficial effects of exercise on memory and cognitive functioning. There is also evidence that eCBs may contribute to the motivational or rewarding aspects of exercise through modulation of synaptic activity in reward-related brain regions.34,35 Furthermore, peripheral eCB levels are readily measured in lipid extracts of plasma or serum obtained from venous blood,36 making circulating eCBs an excellent potential biomarker for tracking the efficacy of exercise interventions.
While the number of studies examining the impact of exercise on eCB signaling has increased by 200% over the past 5 years (Supplementary Fig. S1), no meta-analyses to date have synthesized the current literature on the impact of exercise on eCB signaling. Furthermore, while previous reviews have been published on the eCB system in general,36 the role of eCBs in exercise focusing on regulation of skeletal muscle reponse to exercise,37 implications for the prevention and treatment of neurological disorders,37 and molecular and cellular pathways related to aerobic exercise,38 there have been no comprehensive reviews on the effects of exercise on eCB signaling and potential moderators of these effects.
To address this, we performed a systematic review and meta-analysis of all studies on acute (i.e., single bout) and chronic exercise measuring eCBs. Effects of acute and chronic exercise on eCB concentrations were examined separately, given evidence that they may have different effects.39 Finally, this is the first review to explore various factors that may influence eCB response to exercise, including acute versus chronic exercise, duration, intensity, modality, patient groups (i.e., with and without pre-existing health conditions), and species (i.e., humans, animal models).
Methodology
Search strategy
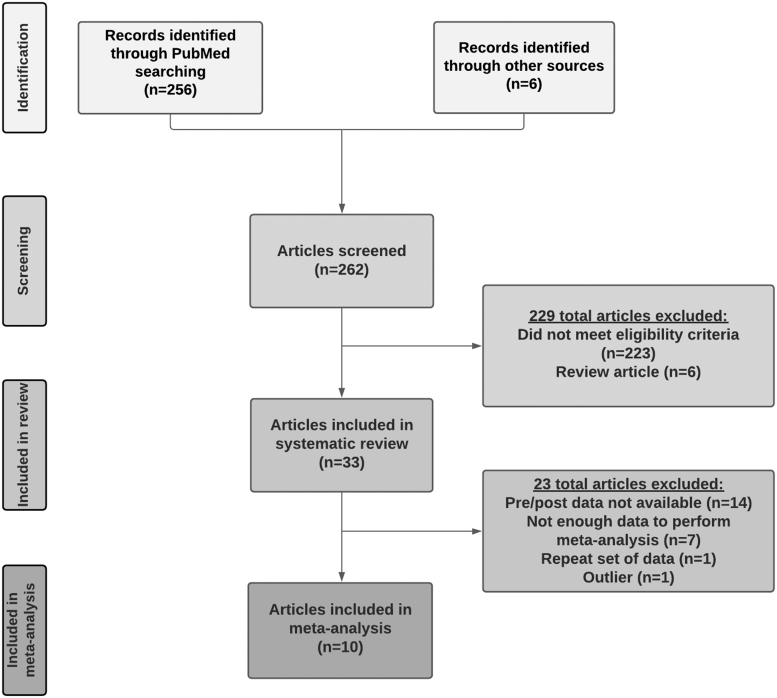
We searched the MEDLINE (PubMed) database for original articles published through December 31, 2020. Articles were included in the systematic review if they met the following inclusion criteria: (1) measured eCB concentrations in circulation or in tissue, (2) examined eCBs before and after exercise (i.e., within-subject design) or compared eCBs in an exercise versus a nonexercise control group (i.e., between-subject design), (3) examined effects of acute (i.e., single bout) or chronic exercise (e.g., 10-week running program). We included studies on aerobic (e.g., running, cycling, dancing) and resistance (e.g., isometric handgrip, resistance training, yoga) exercise. We included studies in humans, including those with and without pre-existing health conditions (e.g., depression, PTSD, SUDs, fibromyalgia), and animal models, of all ages. Although our search focused on AEA and 2-AG as the most well-characterized eCBs, we also examined eCB-like N-acylethanolamides (NAEs) and related compounds (e.g., oleoylethanolamide [OEA], palmitoylethanolamide [PEA], and 2-oleoylglycerol [2-OG]). See Supplementary Material for more information on search strategy and article screening, and Figure 1 for a flowchart of identified articles and exclusions.
FIG. 1.
Flowchart of literature search strategy, and identification of articles for the systematic review and meta-analysis.
A total of 33 articles were included in the systematic review. Of note, 12 articles included results of separate groups (PTSD vs. trauma-exposed controls, dogs vs. ferrets) and 8 included effects of separate exercise conditions (e.g., preferred vs. prescribed exercise),40 for a total of 57 samples/conditions. The earliest published study was in 2003.41 First, we summarize results from all articles on acute and chronic exercise. Next, we report results on subgroups, including (1) articles on healthy humans and (2) articles reporting on moderate-intensity aerobic exercise on humans.
Meta-analysis
The 33 articles included in the systematic review were subsequently examined for inclusion in the meta-analysis. Full data were available for 10 articles (reporting on 24 samples) that examined (1) eCB concentrations before and after acute exercise (within-subject design). There were not enough data (<5 articles) to perform meta-analyses on (2) within-subject effects of chronic exercise, (3) between-subject effects of chronic exercise, or (4) between-subject effects of acute exercise. Thus, we performed a meta-analysis on the articles that examine within-subject effects of acute exercise on eCBs. If an article reported effects in different samples separately (e.g., PTSD, healthy control), samples were considered as unique data points. Given the limited number of articles with data from multiple post-exercise time points (four articles), the meta-analysis focused on the first available measurement of eCBs following acute exercise.
First, we extracted data from each of the 10 articles (24 samples) on eCB concentrations before (pre) and after (post) acute exercise. Extracted data included means, standard deviations (SDs), and sample size (N). Change scores were calculated as post-exercise mean minus pre-exercise mean; therefore positive scores represent increases while negative scores represent decreases in eCBs. SDs for the change scores were estimated from pre- and post-exercise SDs (SDpre and SDpost).42,43 See Supplementary Material for more information. Meta-analyses were conducted for AEA and 2-AG separately, using RevMan software v.5.4 (Cochrane Community, London, United Kingdom) and fixed effect models. Forest plots were generated for sample-specific effect sizes along with 95% confidence intervals (CIs) and pooled effects. Results were considered significant at p<0.05 and heterogeneity between studies was tested using a chi-square test for heterogeneity and the I2 statistic. Follow-up analyses were performed in the following subgroups: (1) articles on healthy humans (i.e., adults without a clinical diagnosis) and (2) articles reporting on moderate-intensity aerobic exercise on humans.
Results of Systematic Review
Overview of studies included in systematic review
Thirty-three articles reporting on 57 samples were included in the systematic review (n=1021 participants, see Table 1. Nine of the 33 articles included patients with pre-existing physical or mental health conditions (e.g., PTSD, fibromyalgia), 15 included strictly healthy volunteers (e.g., no current physical or mental health diagnosis), and 10 included animal models (Tables 1 and 2 and Supplementary Material).
Table 1.
Included samples on acute exercise (N=40 samples reported in N=22 articles)
| Type | First author (year) | Condition | Strain of animal | Sample size (n) | Age (mean±SD) | Gender (% female) | Race/ethnicity (%) | Type of exercise | Exercise modality | Exercise duration | Exercise intensity | eCB collection method | eCB collection timepoints | Significant reported change in AEA | Significant reported change in 2-AG | Significant reported change in other eCBs | Additional outcomesa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human studies | |||||||||||||||||
| Patients, including those with pre-existing conditions | |||||||||||||||||
| Crombie (2020)88 | Patients with PTSD | N/A | HC: n=12 TE: n=14 PTSD: n=14 |
HC: 21.4±3.9 TE: 23.0±5.4 PTSD: 27.6±6.6 |
HC: 100% F TE: 100% F PTSD: 100% F |
HC: 92% W, 8% B, 0% A, 0% M, 0% O TE: 86% W, 0% B, 7% A, 0% M, 7% O PSTD: 64% W, 21% B, 7% A, 0% M, 7% O |
Endurance | Treadmill running | 30 min of running on a treadmill at a moderate-intensity exercise with 5 min warm-up and cool-down | 70–75% AAMHR | Standard blood draw | Collected immediately before and after exercise | HC: AEA ↑ TE: AEA ↑ PTSD: AEA ↑ |
HC: 2-AG ↑ TE: 2-AG ↑ PTSD: 2-AG ↑ |
HC: OEA ↑ PEA ↑ 2-OG ↑ TE: OEA ↑ PEA ↑ 2-OG ↑ PTSD: OEA ↑ PEA ↑ 2-OG ↑ |
HC: Anxiety ↓ Fear ↓ Improvements in mood TE: Anxiety ↓ Fear ↓ Improvements in mood PTSD: Anxiety ↓ Fear ↓ Improvements in mood |
|
| Meyer (2019)58 | Patients with MDD | N/A | n=17 | 40.8±14.8 | 100% F | N/R | Endurance | Electronically braked cycle ergometer | 30 min of cycling, either (1) at a moderate intensity or (2) at a preferred intensity, each with 5 min warm-up and cool-down | Ratings of perceived exertion of “13” | Standard blood draw | Collected immediately before and as quickly as possible after the session ended, always within 10 min post-exercise | Prescribed: AEA ↑ Preferred: No AEA changes |
Prescribed: No 2-AG changes Preferred: No 2-AG changes |
Prescribed: OEA ↑ No PEA, 2-OG changes Preferred: No OEA, PEA, 2-OG changes |
Prescribed: Improvements in mood related to eCB changes Preferred: Improvements in mood not related to eCB changes |
|
| Crombie (2019)80 | Patients with PTSD | N/A | HC: n=10 PTSD: n=10 |
HC: 22.0±6.1 PTSD: 25.2±8.1 |
HC: 70% F PTSD: 80% F |
HC: 70% W, 0% B, 20% A, 10% M, 0% O PTSD: 90% W, 0% B, 10% A, 0% M, 0% O |
Endurance | Treadmill running or incline walking | 30 min of running or walking on a treadmill at a moderate-intensity exercise with 5 min warm-up and cool-down | 70–75% AAMHR | Standard blood draw | Collected immediately before and after exercise | HC: AEA ↑ PTSD: AEA ↑ |
HC: 2-AG ↑ PTSD: No 2-AG changes |
HC: OEA ↑ No PEA, 2-OG changes PTSD: OEA ↑ No PEA, 2-OG changes |
N/A | |
| Stensson (2019)49 | Patients with CNSP | N/A | HC: n=11 CNSP: n=21 |
HC: 37.7±15.9 CNSP: 50.8±12.9 |
HC: 55% F CNSP: 76% F |
N/R | Endurance | Arm cycling | A 30-min session of arm cycling | N/R | Standard blood draw | Collected immediately before and 60 min after exercise | HC: AEA ↓ CNSP: No AEA changes |
HC: No 2-AG changes CNSP: No 2-AG changes |
HC: No OEA, PEA, SEA changes CNSP: No OEA, PEA, SEA changes |
HC: Glutamate/AEA ratio ↑ CNSP: No glutamate/AEA ratio changes |
|
| Brellenthin (2019)44,b | Patients with Substance Abuse Disorder |
N/A | Treatment as usual with exercise: n=21 |
35.1±10.2 | 45% F | 64% W, 2% B, 0% A, 1% M, 1% O | Endurance | Treadmill incline walking | 30 min of moderate-intensity treadmill incline walking | 70–75% AAMHR | Standard blood draw | Collected within 5 min before and after exercise | AEA ↑ | No 2-AG changes | N/A | Acute improvements in mood and reductions in perceived stress and drug craving | |
| Crombie (2018)30 | Patients with PTSD | N/A | HC: n=12 PTSD: n=12 |
HC: 22.0±4.7 PTSD: 26.1±6.7 |
HC: 75% F PTSD: 75% F |
N/R | Endurance | Treadmill running or incline walking | 30 min of walking or running on a treadmill at a moderate intensity with 10 min warm-up | 70–75% AAMHR | Standard blood draw | Collected immediately before and after exercise | HC: AEA ↑ PTSD: AEA ↑ |
HC: 2-AG ↑ PTSD: 2-AG ↑ |
HC: OEA ↑ No PEA, 2-OG changes PTSD: OEA ↑ No PEA, 2-OG changes |
HC: Improvements in mood Pain ↓ PTSD: Improvements in mood Pain ↓ |
|
| Healthy volunteers | |||||||||||||||||
| Marin Bosch (2020)46 | Healthy volunteers | N/A | n=15 | 23.2±4.2 | 0% F | N/R | Endurance | Cycling | A 30-min session of cycling at a moderate intensity and 15 min of cycling at a high intensity the next day, each with a 3 min warm-up and cool-down |
Moderate intensity: 70% MHR High intensity: 80% MHR |
Standard blood draw | Collected immediately before and after exercise | Moderate intensity: AEA ↑ High intensity: AEA ↑ |
N/A | N/A | Hippocampus activation ↑ Motor sequence learning ↑ |
|
| Hughes (2020)89 | Healthy, recreationally active volunteers | N/A | n=12 | 29.0±6.0 | 17% F | N/R | Strength | Light load RE, BFR-RE, BFR-RE with high pressure, and heavy load RE | Light load RE, BFR-RE, BFR-RE with high pressure: 4 sets (30, 15, 15, and 15 repetitions, respectively) of unilateral leg press exercise with 30 sec interset rest periods. Heavy load RE: 4 sets of 10 repetitions of unilateral leg press exercise with 53 sec interset rest periods. |
Light load RE, BFR-RE, BFR-RE with high pressure: 30% one rep max Heavy load RE: 70% one rep max |
Standard blood draw | Collected immediately before, 5 min after, 10 min after, and 24 h after exercise | N/A | Light load RE, BFR-RE, BFR-RE with high pressure, and heavy load RE: No 2-AG changes (decrease for heavy load RE after 24 h) | N/A | Beta-endorphin concentration ↑ Pain ↓ |
|
| Stone (2018)90 | Healthy volunteers | N/A | n=9 | 61.0 | 100% F | N/R | Endurance | Cycling and dancing | One 30-min session of cycling and one 30 min session of dancing | N/R | Standard blood draw | Collected immediately before and after exercise | Cycling: No AEA changes Dancing: No AEA changes |
N/A | Cycling: OEA ↑, no PEA changes Dancing: no OEA changes, no PEA changes |
Cycling: ↓ hunger/appetite Dancing: Improved mood |
|
| Crombie (2018)91,c | Healthy volunteers (placebo condition) | N/A | n=58 | 21.0±3.0 | 50% F | 57% W, 17% B, 14% A, 12% M, 0% O | Strength | Isometric handgrip | Isometric handgrip exercise for 3 min | 25% MVC | Standard blood draw | Collected immediately before and within 5 min after exercise | AEA ↑ | 2-AG ↑ | OEA ↑ PEA ↑ 2-OG ↑ |
See Koltyn (2014)29 | |
| Brellenthin (2017)40 | Healthy volunteers | N/A | Low PA exercisers (preferred and prescribed): n=11 Moderate PA exercisers (preferred and prescribed): n=12 High PA exercisers (preferred and prescribed): n=13 |
Low PA exercisers (preferred and prescribed): 20.6±2.4 Moderate PA exercisers (preferred and prescribed): 19.8±1.1 High PA exercisers (preferred and prescribed): 22.6±5.5 |
Low PA exercisers (preferred and prescribed): 50% F Moderate PA exercisers (preferred and prescribed): 50% F High PA exercisers (preferred and prescribed): 50% F |
N/R | Endurance | Prescribed treadmill exercise and preferred intensity treadmill exercise. Order was randomized and counterbalanced across participants | Prescribed exercise: 45 min session of treadmill walking or running Preferred exercise: participants' choice of treadmill exercise intensity and duration |
Prescribed: 70–75% VO2max Low PA preferred: 74.7±7.8 Moderate PA preferred: 80.4±7.6 High PA preferred: 74.6±15.5 |
Standard blood draw | Collected immediately before and within 5 min after exercise | Low PA exercisers (preferred and prescribed): AEA ↑ Moderate PA exercisers (preferred and prescribed): AEA ↑ High PA exercisers (preferred and prescribed): AEA ↑ |
Low PA exercisers (preferred and prescribed): 2-AG ↑ Moderate PA exercisers (preferred and prescribed): 2-AG ↑ High PA exercisers (preferred and prescribed): 2-AG ↑ |
Low PA exercisers (preferred and prescribed): OEA ↑ PEA ↑ Moderate PA exercisers (preferred and prescribed): OEA ↑ PEA ↑ High PA exercisers (preferred and prescribed): OEA ↑ PEA ↑ |
Low PA exercisers (preferred and prescribed): Improvements in mood Moderate PA exercisers (preferred and prescribed): Improvements in mood High PA exercisers (preferred and prescribed): Improvements in mood |
|
| Cedernaes (2016)47 | Healthy volunteers | N/A | n=16 | 22.9±0.7 | 0% F | N/R | Endurance | Ergometer cycling | Ergometer cycling for 35 min | Watts equivalent to 75% of VO2 reserve | Standard blood draw | Collected immediately before, 15 min after, and 4 h after exercise | No AEA changes | 2-AG ↑ | OEA ↑ No PEA changes |
Sleep deprivation ↑2-AG levels but no modulation in exercise response | |
| Koltyn (2014)29 | Healthy volunteers (placebo condition only) | N/A | n=58 | 21±3.0 | 50% F | 57% W, 17% B, 14% A, 12% M, 0% O | Strength | Isometric handgrip | Isometric handgrip exercise for 3 min | 25% MVC | Standard blood draw | Collected immediately before and within 5 min after exercise | AEA ↑ | 2-AG ↑ | OEA ↑ PEA ↑ 2-OG ↑ DHEA ↑ |
PPT ↑ PPR ↓ |
|
| Raichlen (2013)31 | Healthy volunteers | N/A | n=10 | 31.9±12.1 | 40% F | N/R | Endurance | Treadmill running or walking | 30 min of treadmill walking or running at one of four intensities. Intensities were randomized across four separate laboratory visits. | Intensity 1: <50% AAMHR Intensity 2: 70% AAMHR Intensity 3: 80% AAMHR Intensity 4: 90% AAMHR |
Standard blood draw | Collected immediately before and after exercise | Intensity 1: No AEA changes Intensity 2: AEA ↑ Intensity 3: AEA ↑ Intensity 4: No AEA changes |
Intensity 1: No 2-AG changes Intensity 2: No 2-AG changes Intensity 3: No 2-AG changes Intensity 4: No 2-AG changes |
N/A | Improved positive affect | |
| Raichlen (2012)59 | Healthy volunteers | N/A | n=10 | N/R | N/R | N/R | Endurance | Treadmill running or walking | 30 min of treadmill walking on day 1 and 30 min of treadmill running on day 2 | Running: 72.5%±2.54% MHR (Froude of 0.71±0.03) Walking: 44.6%±1.25% MHR (Froude of 0.26±0.01) |
Standard blood draw | Collected immediately before and after exercise | Running: AEA ↑ Walking: No AEA changes |
Running: No 2-AG changes Walking: No 2-AG changes |
N/A | N/A | |
| Feuerecker (2012)48 | Healthy, trained hikers | N/A | n=12 | 27.6 | 0% F | N/R | Endurance | Hiking | Protocol A: Hiking at lower altitude for 4–4.5 h Protocol B: Hiking at higher altitude for 3.5–5.5 h |
Protocol A: Hiking at lower altitude of ∼1650 m Protocol B: Hiking at higher altitude of ∼3380 m (1780 m difference compared with lower altitude) |
Standard blood draw | Collected before, immediately after, 18 h after, and 24 h after exercise | Protocol A: AEA ↑ Protocol B: AEA ↑ |
Protocol A: No 2-AG changes Protocol B: No 2-AG changes |
N/A | Hypoxia (higher altitudes) ↑ eCB system activation | |
| Heyman (2012)56 | Healthy, trained cyclists | N/A | n=11 | 23.3±5.1 | 0% F | N/R | Endurance | Cycling | 60 min of pedaling on an ergometric bicycle, followed by predetermined amount of work equal to 30 min at higher intensity. The total exercise period was 90 min | 55% of maximal power output for 60 min, followed by 75% of maximal power output for 30 min | Standard blood draw | Collected at rest, after 60 min of exercise at 55% maximal power output, immediately after the 90 min time trial, and 15 min after exercise. | AEA ↑ after a 90-min exercise period | No 2-AG changes | N/A | BDNF levels ↑ significantly during exercise and ↓ during the 15 min of recovery BDNF and AEA concentrations correlated after exercise |
|
| Sparling (2003)41 | Healthy, trained volunteers | N/A | Running: n=8 Cycling: n=8 |
23.8±9.4 | Running: 0% F Cycling: 0% F |
N/R | Endurance | Running or cycling | 45 min of prescribed exercise | 70–80% MHR | Standard blood draw | Collected immediately before and after exercise | Running: AEA ↑ Cycling: AEA ↑ |
Running: No 2-AG changes Cycling: No 2-AG changes |
N/A | N/A | |
| Animal studies | |||||||||||||||||
| King-Himmelreich (2017)64 | Mice treated with CB1R antagonist and AMP-activated protein kinase | C57BL/6 mice | N/R | 6–8 weeks | N/R | N/R | Endurance | Treadmill running | 60 min of treadmill running | N/R | Standard blood draw | Collected immediately after, 30 min after, 2 h after, 4 h after, and 24 h after exercise | AEA ↑ | N/A | N/A | CB1 ↑, FAAH ↓ Found a significant ↓ in the nociceptive response after a single bout of 1 h treadmill running |
|
| Fuss (2015)92 | Mice | C57BL/6J mice | n=16 | 10 weeks | N/R | N/R | Endurance | Wheel running | 5 h of wheel running | N/R | Mice tissues (e.g., heart, lung, skeletal muscle, brain), cerebrospinal fluid, and plasma | Collected 5 h after exercise | AEA ↑ | 2-AG ↑ | OEA ↑ PEA ↑ AA ↑ |
Anxiety-like behavior ↓ Pain (thermal sensitivity) ↓ Absence of CB1R on GABAergic neurons and blockade of central and peripheral CBRs inhibits anxiolytic and analgesic effects, respectively. |
|
| Galdino (2014)93 | Rats injected with CB receptor inverse agonists, eCB metabolizing enzyme inhibitors and an AEA reuptake inhibitor | Male Wistar rats | N/R | N/R | 0% F | N/R | Strength | RE (i.e., electrical stimulation to the animal's tail causing them to flex their leg muscles) | 15 sets of 15 repetitions, with a 120 sec rest between each set. The total exercise period was 7.5 min | Load was 70% of one rep max | Cardiac puncture | Collected immediately after exercise | AEA ↑ | 2-AG ↑ | OEA ↑ PEA ↑ |
Injection of AM251 and AM630 prevents the antinociceptive effect induced by acute RE | |
| Galdino (2014)94 | Rats injected with CB receptor inverse agonists, eCB metabolizing enzyme inhibitors and an AEA reuptake inhibitor | Male Wistar rats | N/R | N/R | 0% F | N/R | Endurance | Treadmill running | Animals ran with a progressive speed at 20 m/min (average time of 49.06 min) and 0% inclination until fatigue | N/R | Cardiac puncture | Collected immediately after exercise | AEA ↑ | 2-AG ↑ | OEA ↑ PEA ↑ |
Injection of AM251 and AM630 prevents the antinociceptive effect induced by acute aerobic exercise | |
| Raichlen (2012)59,d | Dogs and ferrets | Mixed-breed dogs and ferrets | Dogs: n=8 Ferrets: n=8 |
N/R | N/R | N/R | Endurance | Treadmill running and walking | 30 min of walking on day 1 and 30 min of running on day 2 | Running (Dogs and Ferrets): Froude numbers of ∼0.70 Walking (Dogs and Ferrets): Froude numbers of ∼0.25 |
Standard blood draw | Collected immediately before and after exercise | Dogs: AEA ↑ Ferrets: No AEA changes |
Dogs: No 2-AG changes Ferrets: No 2-AG changes |
N/A | N/A | |
Reported sample size is only for the sample that performed the exercise condition.
Outcomes, including mood, anxiety, stress, pain, fear, hunger, cravings, etc. were all self-reported unless otherwise stated.
Also appears in chronic exercise section.
Same sample as in Koltyn (2014).29
Same article as human study by Raichlen (2012).31
2-AG, 2-arachidonoylglycerol; 2-OG, 2-oleoylglycerol; A, Asian; AA, arachidonic acid; AAMHR, age-adjusted maximum heart rate; AEA, anandamide; AMP, adenosine monophosphate; B, Black; BDNF, brain-derived neurotrophic factor; BFR-RE, blood flow restriction resistance exercise; CB1R, cannabinoid type 1 receptor; CNSP, chronic neck and shoulder pain; DHEA, docosahexaenoyl ethanolamide; eCB, endocannabinoid; F, female; FAAH, fatty acid amide hydrolase; HC, healthy control; M, mixed; MDD, major depressive disorder; MHR, maximum heart rate; MVC, maximum voluntary contraction; N/A, not applicable; N/R, not reported; O, other; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; PPR, pressure pain ratings; PPT, Pressure pain thresholds; PTSD, post-traumatic stress disorder; RE, resistance exercise; SD, standard deviation; TE, trauma-exposed individuals without PTSD; W, White.
Table 2.
Included studies on chronic exercise (N=18 samples reported in N=12 articles)
| Type | First author (year) | Condition | Strain of animal | Sample size (n) | Age (mean±SD) | Gender (% female) | Race/ethnicity (%) | Type of exercise | Exercise modality | Exercise duration | Exercise intensity | eCB collection method | eCB collection timepoints | Significant reported change in AEA | Significant reported change in 2-AG | Significant reported change in other eCBs | Additional outcomesa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human studies | |||||||||||||||||
| Patients, including those with pre-existing conditions | |||||||||||||||||
| Stensson (2020)45 | Patients with FM | N/A | HC: n=33 FM: n=37 |
HC: 50.3±12.8 FM: 50.1±10.5 |
HC: 100% F FM: 100% F |
N/R | Strength | RE | 60-min sessions of RE, 2 times/week for 15 weeks | Weeks 1–2: 40% of one rep max, 15–20 reps, 1–2 sets Weeks 3–5: 60% one rep max, 10–12 reps, 1–2 sets Weeks 6–15: 80% one rep max, 5–8 reps, 1–2 sets |
Standard blood draw | Collected before (within 1–7 days) the start of the 15-week exercise interventions and after the intervention (within 1–7 days) | HC: No AEA changes FM: AEA ↑ |
HC: No 2-AG changes FM: No 2-AG changes |
HC: No OEA, PEA changes SEA ↑ FM: No OEA, PEA changes SEA ↓ |
HC: No pain, depression scorings, or muscle strength changes FM: Pain ↓ Depression scorings ↓ Muscle strength ↑ |
|
| Belitardo de Oliveira (2019)95 | Patients with Episodic Migraines | N/A | HC: n=12 Migraines: n=13 |
HC: 34.7±12.0 Migraines: 37.4±13.8 |
HC: 83% F Migraine: 85% F |
HC: 92% W, 0% B, 8% A, 0% M, 0% O Migraines: 92% W, 0% B, 8% A, 0% M, 0% O |
Endurance | Treadmill jogging or walking | 40-min sessions of walking or jogging on a treadmill, 3 times/week for 12 weeks | Heart-rate equivalent to each individual's ventilatory threshold—additional information N/R | Standard blood draw | Collected before exercise and between 2 and 5 days after the last exercise session or 48 h after the last exercise session within the late follicular phase of the menstrual cycle | HC: AEA ↓ Migraines: AEA ↓ |
N/A | N/A | HC: No migraine attacks or mood changes Migraines: Migraine attacks ↓ Improvements in mood |
|
| Brellenthin (2019)44 | Patients with Substance Abuse Disorder | N/A | Treatment as usual with exercise: n=21 | 35.1±10.2 | 45% F | 64% W, 2% B, 0% A, 1% M, 1% O | Endurance | Treadmill incline walking | 30 min of moderate-intensity treadmill incline walking, 3 times/week for 6 weeks | 70–75% AAMHR | Standard blood draw | N/A | N/R | N/R | N/A | Acute improvements in mood and reductions in perceived stress and drug craving | |
| Fernández-Aranda (2014)96 | Patients who are obese | N/A | HC: n=16 Obese: n=30 Morbid obese: n=43 |
HC: 27.6±7.9 Obese: 44.9±12.9 Morbid obese: 43.5±10.2 |
HC: 100% F Obese: 100% F Morbid obese: 100% F |
N/R | Endurance | PA | PA was monitored using accelerometry for 6 days | Time accumulated in MVPA during waking hours | Standard blood draw | Collected between 8 and 9 am after at least 12 h of fasting | AEA ↑ (all groups combinedb) | No 2-AG changes | OEA ↑ (all groups combinedb) | High PA associated with low harm avoidance and high novelty seeking (temperament trait) | |
| Healthy volunteers | |||||||||||||||||
| Koay (2021)97 | Healthy, newly enlisted soldiers | N/A | n=52 | 22.0±4.0 | 0% F | N/R | Endurance and strength | Combined strength and endurance program and occupational specific activities such as marching. | 80-day physical training program with about 1.3 h of PA each day | 68% of the PA in the program was of moderate intensity (4.3–6 METs), while 32% was high intensity (METS >6) | Standard blood draw | Collected after an 80-day program | N/A | 2-AG ↓ | N/A | Indole-3-propionate ↑ Fatty acids and ketone body substrates ↓ Increases in dimethylguanidino valeric acid associated with increases in several cardiovascular risk factors Shifts in metabolism across many different substrates |
|
| Sadhasivam (2020)98 | Healthy volunteers | N/A | n=148 | ≥18.0 | Blood draws only: N/R Full sample: 49% F |
16% W, 2% B, 74% A, 2% M, 6% O | Balance | Yoga (BSP) | 4-day, 3-night yoga retreat | N/R | Standard blood draw | Collected before (up to 2 days before program began) and after exercise (within 2 days after program ended) | AEA ↑ | 2-AG ↑ | DEA ↑ 1-AG ↑ OEA ↑ |
BDNF levels ↑ Depression ↓ Anxiety ↓ Happiness ↑ |
|
| Belitardo de Oliveira (2019)99 | Healthy volunteers | N/A | n=17 | 39.0±13.4 | 60% F | 41% W, 1% B, 1% A, 0% M, 35% O | Endurance | Treadmill running or walking | 40 min of treadmill running or walking, 3 times/week for 12 weeks | Heart rate equivalent to each individual's ventilatory threshold—additional information N/R) | Standard blood draw | Collected before program and within 2–5 days after the last exercise session in program | AEA ↓ | N/A | N/A | Reduction in AEA associated with weight loss Improvements in mood |
|
| Animal studies | |||||||||||||||||
| Dos Santos (2019)100 | Mice that received carrageenan injection | C57BL/6 female mice | Exercise: n=6 Cg+ exercise: n=6 |
8–10 weeks | Exercise: 100% F Cg+ exercise: 100% F |
N/R | Endurance | Swimming | 5 days/week of swimming for 3 weeks: Week 1: 15 min Week 2: 30 min Week 3: 45 min |
N/R | Mice spinal cord tissue | Collected immediately after exercise program | Exercise: No AEA changes Cg+ exercise: AEA ↑ |
N/A | N/A | Spinal CB2R participate in exercise-induced antinociception in mice | |
| Thompson (2017)101 | Mice from HR lines | Trained mice from HR lines | HC: n=50 HR: n=50 |
N/R | HC: 50% F HR: 50% F |
N/R | Endurance | Wheel running | Wheel running access for exercise group for 6 days | N/R | Cardiac puncture | Collected after exercise program | HC: AEA ↑ HR: AEA ↓ |
HC: 2-AG ↓ HR: 2-AG ↓ |
N/A | HC: Female mice had ↓2-AG levels and ↑ AEA levels compared with male mice HR: Female mice had ↓2-AG levels and ↑ AEA levels compared with male mice |
|
| Biedermann (2016)39 | Mice | C57BL/6 mice | Runners: n=10 Restricted runners: n=10 |
10 weeks | Runners: 0% F Restricted runners: 0% F |
N/R | Endurance | Wheel running | Free access wheel running (all day) or restricted access wheel running (6 h/day) for 9 weeks | N/R | Cardiac puncture | Collected after exercise program | Runners: AEA ↓ Restricted runners: AEA ↓ |
Runners: No 2-AG changes Restricted runners: No 2-AG changes |
Runners: No OEA, PEA, 1-AG, and AA changes Restricted Runners: No OEA, PEA, 1-AG, and AA changes |
Runners: Hippocampal gray matter volume ↑ Immature hippocampal neurons ↑ Restricted runners: Immature hippocampal neurons ↑ |
|
| Gamelin (2016)102 | Rats | Male Winstar rats | HC: n=7 HFD: n=7 |
15 weeks | HC: 0% F HFD: 0% F |
N/R | Endurance | Treadmill running | Treadmill running 1 h/day, 5 days/week for 12 weeks | 70–80% of the maximal aerobic velocity | Cardiac puncture | Collected after exercise program | HC: No AEA changes HFD: No AEA changes |
HC: No 2-AG changes HFD: No 2-AG changes |
HC: No OEA and PEA changes HFD: No OEA and PEA changes |
HC: No CB1R, CB2R, or FAAH changes HFD: No CB1R, CB2R, or FAAH changes |
|
| Hill (2010)32 | Rats (treated with CB1R antagonist in experiment 2) | Sprague/Dawley rats | n=35 | 12 weeks | 0% F | N/R | Endurance | Wheel running | Access to wheel for 8 days | N/R | Brain tissue in rats | Collected after exercise program | AEA ↑ | No 2-AG changes | N/A | Voluntary exercise ↑ proliferation of progenitor cells in the dentate gyrus of the hippocampus Effect of proliferation was prevented following AM251 treatment |
|
Outcomes, including mood, anxiety, stress, pain, fear, hunger, cravings, etc., were all self-reported unless otherwise stated.
Did not report separate results for groups, treated as one sample.
1-AG, 1-arachidonoylglycerol; BSP, Bhava Spandana Program; CBR2, cannabinoid type 2 receptor; DEA, docosatetraenylethanolamide; FM, fibromyalgia; HFD, high-fat diet; HR, high runner; MVPA, moderate-vigorous physical activity; OEA, oleoylethanolamide; PA, physical activity; SEA, stearoylethanolamide.
The majority of the 33 articles (n=22 articles, 66.7%) focused on the effects of acute exercise on eCB concentrations (Table 1), and 12 articles examined chronic exercise (Table 2). One article examined both acute and chronic exercise.44 The most common modalities were walking and running, followed by cycling (Supplementary Fig. S2 and Tables 1 and 2). For human studies of acute exercise, the most frequently tested exercise duration (excluding warm-up and cool-down) was 30 min (55.6%).
Of the 33 articles, 93.9% reported on AEA concentrations, 81.8% reported on 2-AG, 51.5% on OEA, 51.5% on PEA, 18.2% on 2-OG, and few (<7%) examined other related molecules (Tables 1 and 2). The majority of articles (n=25) measured eCBs in circulation (plasma and/or serum) using a blood draw with standard venipuncture procedures (75.8%). For articles on acute exercise, the majority (n=21, 63.6%) measured eCBs immediately after or within 5 min following acute exercise. However, eCBs were also measured 15 min–24 h later. For articles on chronic exercise, the time that eCBs were measured varied from within 5 min44 to within 1–7 days post-exercise.45 All studies included adults (i.e., ages 18+) or mature/adult animals. Around 33.3% of studies reported male-only participants and subjects, 39.4% reported both, and 15.2% reported female only (12.1% did not report). For further description of included studies, see the Supplementary Material.
Systematic review results for AEA concentrations
Acute exercise
Twenty-one of the 22 of the articles (39 samples) that examined the effects of acute exercise measured AEA concentrations. Twenty-nine of the 39 (74.4%) samples demonstrated a significant increase in AEA, 1 (2.6%) showed a significant decrease, and 9 (23.1%) revealed no change in AEA.
Acute exercise: healthy individuals only
Sixteen of the 22 articles on acute exercise examined solely healthy humans or included a healthy control group and 15 of these (25 samples) measured AEA concentrations. Eighteen samples (72%) reported an increase in AEA compared with baseline levels, six samples (24.0%) reported no AEA changes, and one sample (4.0%) reported a decrease in AEA concentrations.
Acute exercise: moderate-intensity aerobic exercise: humans only
Twelve of the 22 acute exercise articles (18 samples) measuring AEA concentrations included a moderate-intensity aerobic acute exercise condition (i.e., running or bicycling between 70% and 80% maximum heart rate [MHR]). Seventeen samples (94.4%) reported an increase in AEA, one reported no AEA changes (5.6%), and none reported a decrease in AEA.
Acute exercise: effects of intensity
Seven of the 22 articles (16 samples) on acute exercise examined the impact of various exercise intensities on AEA concentrations. All seven studies (100%) indicated that moderate-intensity exercise was associated with greater increases in AEA as compared with less-intense exercise (i.e., <50% MHR). Of note, Marin Bosch et al.46 found that exercise performed at 80% MHR produces greater increases in AEA concentrations as compared with exercise performed at 70% MHR. However, one study by Raichlen et al.31 also examined vigorous-intensity exercise (i.e., >90% age adjusted MHR). In that study, vigorous-intensity exercise elicited lower AEA as compared with moderate exercise intensities (70–80% MHR). Two studies compared preferred and prescribed moderate exercise conditions (see Supplementary Material for discussion).
Chronic exercise
Eleven of 12 articles (17 samples) examined the effects of chronic exercise on AEA concentrations. However, one44 did not report AEA before and following the intervention (i.e., pre and post-exercise eCB concentrations averaged across acute exercise sessions throughout [baseline and week 6] the intervention were reported). Therefore, only 10 articles (16 samples) were considered in this section. In particular, we report on AEA concentrations before and after chronic exercise. Six samples (37.5%) revealed a significant increase in AEA, six (37.5%) demonstrated a significant decrease, and four (25%) showed no change in AEA.
Chronic exercise: healthy individuals only
Five of the 12 articles on chronic exercise solely examined healthy humans or included healthy control groups, and four of these (4 samples) measured AEA concentrations. One sample reported an increase in AEA, two reported a decrease in AEA, and one reported no changes in AEA following the exercise program.
Chronic exercise: moderate-intensity aerobic exercise
There were not enough data to evaluate.
Systematic review results for 2-AG
Acute exercise
Nineteen articles (35 samples) reported 2-AG concentrations following acute exercise. Twenty samples (57.1%) showed no 2-AG changes, 15 samples (42.9%) exhibited a significant increase in 2-AG following acute exercise, and no studies showed a decrease.
Acute exercise: healthy individuals only
Sixteen of the 22 articles on acute exercise solely examined healthy humans or included a healthy control group and 14 of these (22 samples) measured 2-AG conentrations. Thirteen samples (59.1%) reported no 2-AG changes compared with baseline levels, nine samples (40.9%) reported an increase in 2-AG, and no samples reported decreases in 2-AG concentrations.
Acute exercise: moderate-intensity aerobic exercise: humans only
Eleven of the 22 acute exercise articles (17 samples) used a moderate-intensity aerobic exercise (e.g., running or bicycling between 70% and 80% MHR) and measured 2-AG concentrations. Nine samples reported no 2-AG changes (59.1%), eight samples reported an increase in 2-AG (40.9%), and no samples reported a decrease in 2-AG.
Acute exercise: effects of intensity
Seven of the 22 articles (14 samples) examined the impact of exercise intensity on 2-AG concentrations. None of the 14 samples demonstrated significant effects of exercise intensity on 2-AG concentrations.
Chronic exercise
Eight articles (12 samples) reported 2-AG concentrations following chronic exercise. One sample (8.3%) demonstrated a significant increase in 2-AG following chronic exercise, three (25.0%) showed a significant decrease, and eight (66.7%) showed no changes.
Chronic exercise: healthy individuals only
Four of the 12 articles on chronic exercise solely examined healthy humans or included a healthy control group and 3 of these (3 samples) measured 2-AG concentrations. One sample reported an increase in 2-AG, one reported a decrease in 2-AG, and one reported no 2-AG changes following an exericse program.
Chronic exercise: moderate-intensity aerobic exercise
There were not enough data to evaluate.
Systematic review for eCB-like NAEs and related compounds
Seventeen articles measured OEA and PEA (13 articles acute exercise, 4 chronic exercise). Other eCB-like compounds (e.g., 2-OG, SEA, 1-AG) were measured infrequently. Results for these compounds were inconsistent and showed no clear trend for increases, decreases, or no changes in concentrations. However, one study reported elevations in OEA that lasted 4 h after an acute (30 min) cycling session.47 See Supplementary Material and Table 1 for full summary.
Results of Meta-Analysis: Within-Subject Effects of Acute Exercise
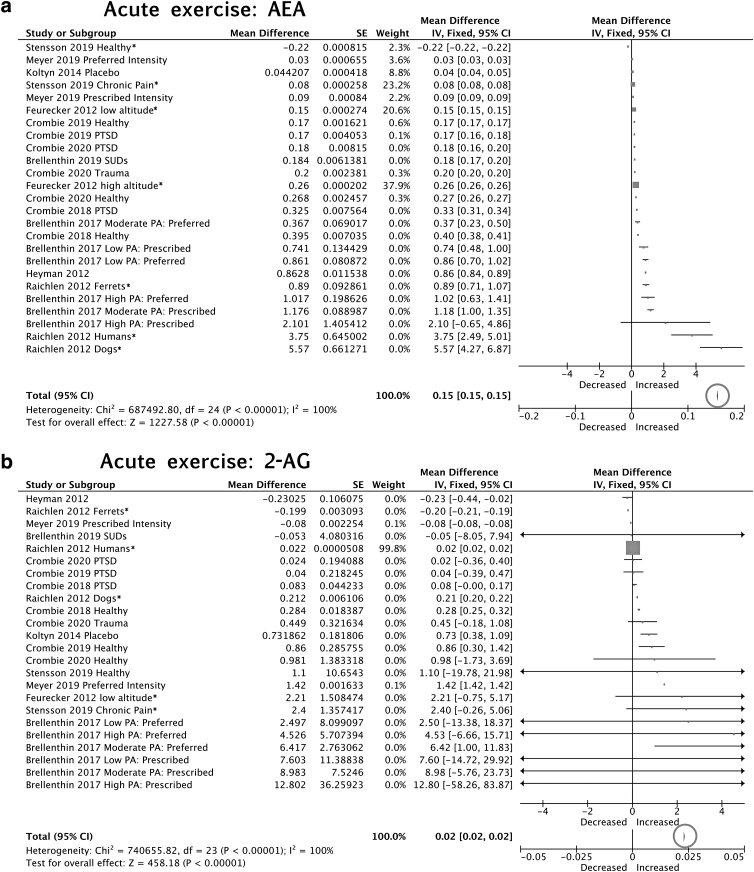
Acute exercise AEA
Eleven articles (24 samples) were included in the meta-analysis (n=335 participants). The meta-analysis revealed a significant increase (mean difference=0.15 a.u., 95% CIs=0.15 to 0.15) in AEA concentrations following acute exercise (z=1227.58, p<0.00001; Fig. 2a). Using the studies that reported AEA concentrations in units of pmol/mL (Fig. 2a), this mean difference was 0.03 pmol/mL (95% CIs=0.02 to 0.03). However, there was also substantial heterogeneity in the effect (I2=100%), suggesting that the magnitude of the increase varied across articles. Inspection of the effect sizes (Fig. 2a) suggests that moderate-intensity exercise is associated with greater increases in AEA as compared with low-intensity exercise, as revealed by the systematic review.48 Consistent with observations from the systematic review, in general, prescribed moderate-intensity exercise was associated with greater increases in AEA as compared with preferred (Fig. 2a). In addition to comparing preferred versus prescribed, Brellenthin et al.40 examined the impact of regular physical activity on exercise-related changes in eCBs across three groups: low (≤60 min MVPA per week), moderate (150–299 min MVPA per week), and high (≥300 MVPA per week) physical activity groups. Although the authors reported no significant main effects or interactions with group40 inspection of effect sizes (Fig. 2a) for moderate prescribed sessions suggest that the high moderate vigorous physical activity (MVPA) group demonstrated the greatest exercise-related increases in AEA, followed by the moderate, then low MVPA group. Importantly, participants in that study were asked to abstain from exercise within 24 h of testing to minimize potential carryover effects.40 Therefore, although acute exercise increased AEA among inactive to highly active individuals, prior exercise history may further augment eCB system activation in response to exercise. Post-exercise collection time may also contribute to the observed heterogeneity. While most studies measured post-exercise eCB concentrations immediately after or within 15 min following exercise, Stensson and Grimby-Ekman49 collected the post-exercise blood sample 60 min after finishing the exercise and demonstrated smaller effect sizes (Fig. 2a). We observed no other patterns related to modality, collection method, subject group, or other factors.
FIG. 2.
Forest plots for meta-analyses on effects of acute exercise on (a) AEA and (b) 2-AG concentrations (pmol/mL). Note: some articles reported effects in different samples separately (e.g., PTSD and healthy control groups); therefore, these articles are entered more than once in the meta-analysis and treated as separate subgroups. The total effects (bottom) are reported on a different scale than the study effects. Individual studies show mean difference scores and the pooled results show weighted mean differences and 95% confidence intervals. The size of the block indicates the weight assigned to that study in the meta-analysis and the horizontal line depicts the confidence interval. Thus, larger block sizes indicate studies with larger weight (typically with narrower confidence intervals), which contribute more to the calculation of the summary result. The summary result is presented as a diamond at the bottom. The total around that diamond is circled to draw attention to the total effect. 2-AG, 2-arachidonoylglycerol; AEA, anandamide; PTSD, post-traumatic stress disorder.
Acute exercise: healthy humans only
We repeated the meta-analysis using 15 samples that included healthy humans and found a significant increase (mean difference=0.18 a.u., 95% CIs=0.18 to 0.18) in AEA concentrations following acute exercise (z=1245.84, p<0.00001). Using the studies that reported AEA concentrations in units of pmol/mL, this mean difference was 0.2 pmol/mL (95% CIs=0.2 to 0.2). Heterogeneity remained high (I2=100%).
Acute exercise: moderate-intensity aerobic exercise: humans only
We repeated the meta-analysis using 15 samples that reported effects of a moderate-intensity aerobic exercise condition. There was a significant increase (mean difference=0.14 a.u., 95% CIs=0.13 to 0.14) in AEA concentrations following acute moderate exercise (z=205.11, p<0.00001). Using the studies that reported AEA concentrations in units of pmol/mL, this mean difference was 0.14 pmol/mL (95% CIs=0.14 to 0.14). Heterogeneity remained high (I2=100%).
Acute exercise 2-AG
Twenty-four samples were included in the 2-AG meta-analysis (n=335). Similar to results for AEA, the meta-analysis showed a significant increase in 2-AG (mean difference=0.02 a.u., 95% CI=0.02 to 0.02) following acute exercise (z=458.18, p<0.00001; Fig. 2b). Using the studies that reported 2-AG concentrations in units of pmol/mL (Fig. 2b), this mean difference was 0.9 pmol/mL (95% CIs=0.9 to 0.9). There was also substantial heterogeneity across samples (I2=100%). Brellenthin et al.40 examined both preferred and moderate prescribed exercise conditions and reported larger effect sizes for prescribed relative to preferred conditions (Fig. 2b). However, the authors reported no group-by-time interactions in 2-AG concentrations.40 Breakdown of studies by modality, duration, sample, and timing of sample collection did not reveal any clear patterns in terms of effects on 2-AG (Fig. 2b).
Acute exercise: healthy humans only
We repeated the meta-analysis using 15 samples that included healthy humans and found a significant increase (mean difference=0.02 a.u., 95% CIs=0.02 to 0.02) in 2-AG concentrations following acute exercise (z=459.95, p<0.00001). Using the studies that reported 2-AG concentrations in units of pmol/mL, this mean difference was 0.02 pmol/mL (95% CIs=0.02 to 0.02). Heterogeneity remained high (I2=100%).
Acute exercise: moderate-intensity aerobic exercise: humans only
We repeated the meta-analysis using 15 samples that reported effects of a moderate-intensity aerobic exercise condition. There was a significant increase (mean difference=0.02 a.u., 95% CIs=0.02 to 0.02) in 2-AG concentrations following acute moderate exercise (z=205.18, p<0.00001). Using the studies that reported 2-AG concentrations in units of pmol/mL, this mean difference was −0.07 pmol/mL (95% CIs=−0.08 to −0.07). Heterogeneity remained high (I2=97%).
ECB-like NAEs and related compounds
Eight articles (19 samples) reported PEA concentrations, 7 articles (13 samples) reported OEA, and 6 articles (10 samples) reported 2-OG. Acute exercise was associated with a significant increase in both PEA (z=681.21, p<0.00001) and OEA concentrations (z=75.79, p<0.00001); but these results showed significant heterogeneity (I2=100%). In contrast, acute exercise was associated with a significant decrease in 2-OG concentrations (z=26.78, p<0.00001) with lower heterogeneity (I2=28%). See Supplementary Figures S1–S3 for full results and forest plots.
Discussion
Our systematic review revealed a consistent increase in AEA concentrations following acute exercise, which was confirmed by our meta-analysis. This increase in AEA was observed across modalities (e.g., running, cycling, resistance exercise) as well as, across pre-clinical and clinical studies, including patients with and without pre-existing health conditions (e.g., depression, PTSD). However, there was substantial variation in magnitude of the increase across samples, which may be related to exercise intensity, timing of sample collection, or other factors. These possibilities are discussed below, and we provide some recommendations for future studies.
With regard to 2-AG, effects of acute exercise were less consistent. In the systematic review, half of the samples demonstrated a significant increase in 2-AG concentrations following acute exercise, while half reported no significant changes. However, the meta-analysis supported a small but significant increase in 2-AG concentrations following acute exercise, with substantial heterogeneity across samples. Both the increase in AEA and 2-AG remained significant when considering the subgroup of healthy adults and moderate-intensity aerobic exercise subgroup, only. Overall, the effects of chronic exercise on circulating eCB concentrations were inconsistent, which may be due to the smaller number of studies examining chronic exercise and substantial heterogeneity across studies.
In this study, we provide the first meta-analytic evidence to support the notion that acute exercise mobilizes eCBs.36 Post-exercise eCB mobilization in the periphery is thought in part to be related to the need to replenish energy stores,36 given that CB1Rs are widely distributed in adipose tissues, liver, and skeletal muscle. Centrally, eCB mobilization may contribute to the wide range of cognitive, emotional, and physiological processes associated with exercise, through modulation of the HPA axis, neural progenitor cell proliferation, and glutamate and GABA signaling (for a comprehensive review, see Forteza et al.38). Indeed, studies included in this review frequently report that elevations in eCBs are associated with, or accompanied by, mood elevations, reductions in stress, anxiety, and pain, and enhanced cognitive functioning (Tables 1 and 2).
Given evidence that skeletal muscle expresses enzymes that synthesize eCBs,50,51 the observed increase in circulating eCBs following acute exercise may be produced by skeletal muscle. Our meta-analysis revealed that acute exercise increases AEA, PEA, and OEA concentrations (but not 2-OG, which showed a significant decrease). This is consistent with prior studies showing that PEA and OEA are cosynthesized with AEA (NAPE), but not with 2-OG.52,53 Although PEA and OEA do not bind to CB1Rs, they may be involved in other well-documented effects of exercise, such as appetite suppression, reduced inflammation, and reduced nociception.54,55 In addition, exercise has been shown to increase circulating glucocorticoids, which may increase AEA concentrations in the brain.32 Circulating concentrations of other biomarkers (e.g., brain-derived neurotrophic factor [BDNF]) have also been found to be elevated following acute exercise,56 which may contribute (independently or in conjunction with eCBs) to neuroplastic and antidepressant effects of exercise.
Although the overall effect size was much smaller than for AEA, our meta-analysis revealed an increase in 2-AG concentrations following acute exercise (Fig. 3). The small effect size may explain some of the inconsistencies in reported results in the literature, particularly for studies with smaller sample sizes. 2-AG biosynthesis occurs separately from AEA (e.g., DAGL) and plays a role in reducing HPA axis activation in response to stressors.57 A few studies reported that elevations in 2-AG (but not AEA) were associated with reductions in tension, mood disturbance, and negative affect following acute exercise (Tables 1 and 2). Therefore, elevations in 2-AG may have additional or complementary benefits from AEA.
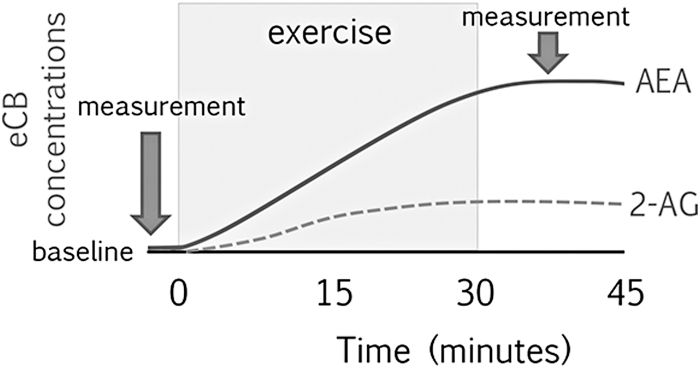
FIG. 3.
Proposed increase in AEA and 2-AG concentrations following moderate acute exercise.
Variation in exercise intensity may explain some of the observed heterogeneity in AEA (but not 2-AG). Indeed, several studies report that moderate-intensity exercise is associated with greater increases in AEA as compared with other intensities (Fig. 3).31,40,46,48,56,58,59 There is also initial evidence for an inverted U-shaped effect of exercise intensity on AEA concentrations, such that moderate intensity is associated with the greatest increases; however, this notion is based on results of one study that examined high-intensity exercise (i.e., ∼90% age-adjusted MHR).31 However, the idea that the optimal eCB response may be at moderate-intensity exercise fits with prior data on other outcomes. Indeed, prior studies that compare effects of exercise intensity commonly report greater improvements in mood and cognitive performance, reductions in stress, and enhanced neurobiological reward response following moderate-intensity compared with low- or high-intensity exercise.60–62 This also fits with the dual-mode model, proposed by Ekkekakis et al., which suggests that moderate-intensity exercise seems to be optimal for improvements in affect.63
There is also some evidence that AEA concentrations continue to increase to 15 mins post-exercise.31 One study in mice suggest that AEA concentrations remain elevated (with respect to a no-exercise control group) for up to 2 h.64 However, the duration, shape, and recovery of eCB concentrations following exercise remain open questions (Fig. 3). In addition, we did not find consistent effects of chronic exercise on eCB concentrations. Thus, the potential for chronic exercise to modulate basal eCB concentrations or eCB responses to acute stress—including exercise stress—is unclear.
In addition to exercise intensity, several other factors may contribute to the observed variation in eCB mobilization following acute exercise. We found initial evidence that the timing of the post-exercise eCB measurement is important, with at least one study measuring eCBs after a 60-min rest period demonstrating smaller effect sizes than studies that measured eCBs immediately after exercise. Exercise history may also modify eCB responses, with initial evidence that more physically active individuals may show a more robust AEA response to exercise as compared with those with low physical activity. eCB tone or response to stress may also be affected by fasted state, last meal timing and content, recent exercise, circadian timing of exercise, BMI, sex, gender, pre-existing health conditions, genetic variance in the eCB system (e.g., FAAH, CNR1), sleep restriction, psychotropic medications, oral contraceptives, menstrual stage, recent cannabis or alcohol use, injury or inflammation, or racial/ethnic background.30,65–73
Future studies should characterize eCB system functioning at baseline and responses to exercise in various patient or risk groups (e.g., trauma-exposed, first-degree relative with depression), which may inform the pathophysiology of these disorders or inform exercise interventions. Future studies should also standardize the duration, timing of measurements and exercise, time-of-day, fed state (e.g., fasted or standard breakfast), and ask participants to refrain from exercising or using substances (e.g., cannabis, alcohol) before the session. Furthermore, future studies are needed to identify the most effective modalties and durations of exercise for enahcing eCB concentrations, as most of the modalties examined in this review were primarily aerobic in nature. We also found no studies that examine the impact of exercise on children, adolescents, or juvenile animals. Thus, the observed effects of acute exercise on eCBs in adults may not generalize to younger populations.
The involvement of stress in the neurobiology of anxiety, depression, PTSD, and SUDs is well described,74–76 and emerging data implicate eCB system hypoactivity in the pathophysiology and/or maintenance of these disorders.16,77,78 Indeed, pre-clinical studies suggest that acute, repeated psychological stress exposure is associated with increased 2-AG and reduced AEA concentrations in the brain. Clinical studies have reported increases in circulating concentrations of 2-AG in response to acute social stress.65,79,80 However, lower basal 2-AG concentrations have been reported in individuals with PTSD as compared with trauma-exposed healthy controls, and lower basal AEA and 2-AG concentrations in unmedicated depressed women as compared with controls.79,81,82 Given the critical role of eCB signaling in regulating anxiety, stress, and fear-related behavior, the eCB system has garnered attention as a potential therapeutic target for the prevention and treatment of stress-related psychopathology.
Exercise may be a potent nonpharmacological intervention for a variety of stress-related disorders that could benefit from elevations in eCBs and associated effects on mood, stress, anxiety, pain, cognitive functioning, inflammation, sleep, and appetite. Exercise may also be beneficial for conditions associated with eCB system hyperactivity, for example, diabetes, obesity, and inflammation. Given that the eCB system responds to stimuli (i.e., stressors), and due to the fact that exercise is a form of physical stress, administering an acute bout of exercise may be useful for probing eCB system stress reactivity in those at risk for psychopathology.30 Furthermore, eCB signaling is considered to be critical for fear extinction learning and recall, and prior studies indicate that individuals with stress-related disorders (e.g., PTSD) have impaired fear extinction learning and/or recall.83 Therefore, the ability of exercise to augment eCB signaling may have significant benefits for the management of stress-related disorders, for example, as an adjunct to extinction-based exposure therapy.84–87
Limitations should be mentioned. First, there were not enough data to perform meta-analysis on the effects of chronic exercise on eCB concentrations, or to examine studies on acute exercise separately by species, modality, duration, measurement timing, sex, or other factors. We did, however, examine the potential impact of various factors on results of the systematic review and meta-analysis, to identify potential patterns for future study. Furthermore, we focused on the impact of exercise on eCB concentrations; future studies should also examine the role of endorphins, BDNF, or other signaling systems in mediating or moderating acute exercise effects either in isolation from or in conjuction with the eCB system.
Conclusions
In this study, we provide evidence from a systematic review and meta-analysis that acute exercise activates the eCB system, that is, increases circulating concentrations of eCBs. Given that disruptions in eCB signaling are linked to several stress-related diseases (e.g., anxiety, depression, PTSD, obesity, and diabetes) exercise is a promising nonpharmacological approach for the prevention and treatment of stress-related psychopathology through its potential impacts on eCB signaling. However, the wide heterogeneity observed across studies to-date suggests that there is a critical need for standardized, well-powered studies to examine (1) the usefulness of exercise as a probe for eCB system functioning in disease or at-risk states, (2) the shape of the eCB response and recovery following acute exercise, (3) factors that modify eCB response to exercise, (4) ability for chronic exercise to modulate basal eCB concentrations and eCB response to stress, and (5) exercise as a preventive or therapeutic intervention.
Supplementary Material
Abbreviations Used
- 1-AG
1-arachidonoylglycerol
- 2-AG
2-arachidonoylglycerol
- 2-OG
2-oleoylglycerol
- A
Asian
- AA
arachidonic acid
- AAMHR
age-adjusted maximum heart rate
- AEA
anandamide
- AMP
adenosine monophosphate
- B
Black
- BDNF
brain-derived neurotrophic factor
- BFR-RE
blood flow restriction resistance exercise
- BSP
Bhava Spandana Program
- CB1R
cannabinoid type 2 receptor
- CB2R
cannabinoid type 2 receptor
- CIs
confidence intervals
- CNSP
chronic neck and shoulder pain
- DAGL
diacylglycerol lipase
- DEA
docosatetraenylethanolamide
- DHEA
docosahexaenoyl ethanolamide
- eCB
endocannabinoid
- F
female
- FAAH
fatty acid amide hydrolase
- FM
fibromyalgia
- HC
healthy control
- HFD
high-fat diet
- HR
high runner
- M
mixed
- MDD
major depressive disorder
- MHR
maximum heart rate
- MVC
maximum voluntary contraction
- MVPA
moderate-vigorous physical activity
- N/A
not applicable
- N/R
not reported
- NAE
N-acylethanolamide
- NAPE
N-acylphosphatidylethanolamine phospholipase D
- O
other
- OEA
oleoylethanolamide
- PA
physical activity
- PEA
palmitoylethanolamide
- PPR
pressure pain ratings
- PPT
pressure pain thresholds
- PTSD
post-traumatic stress disorder
- RE
resistance exercise
- SD
standard deviation
- SEA
stearoylethanolamide
- SUDs
substance use disorders
- TE
trauma-exposed individuals without PTSD
- W
White
Authors' Contributions
S.D., B.B., and H.M. performed the systematic review. C.C. and H.M. performed the analyses. S.D., B.B., C.C., K.C., C.R., M.H., and H.M. contributed to writing and reviewing. All authors approved the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Ms. Desai received an Undergraduate Research Creative Project (UROP) award from Wayne State University. Dr. Marusak is supported by the National Institute of Mental Health award K01MH119241 and Children's Foundation award R1-2021-31. Funding sources had no role in this review.
Supplementary Material
Cite this article as: Desai S, Borg B, Cuttler C, Crombie KM, Rabinak CA, Hill MN, Marusak HA (2022) A systematic review and meta-analysis on the effects of exercise on the endocannabinoid system, Cannabis and Cannabinoid Research 7:4, 388–408, DOI: 10.1089/can.2021.0113.
References
- 1. Hillard CJ. Stress regulates endocannabinoid-CB1 receptor signaling. Semin Immunol. 2014;26:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kruk-Slomka M, Dzik A, Budzynska B, et al. . Endocannabinoid system: the direct and indirect involvement in the memory and learning processes—a short review. Mol Neurobiol. 2017;54:8332–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kesner AJ, Lovinger DM. Cannabinoids, endocannabinoids and sleep. Front Mol Neurosci. 2020;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castillo PE, Younts TJ, Chávez AE, et al. . Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burns HD, Van Laere K, Sanabria-Bohórquez S, et al. . [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A. 2007;104:9800–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 2009;11:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci. 2016;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herkenham M, Lynn AB, Johnson MR, et al. . Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marsicano G, Kuner R. Anatomical distribution of receptors, ligands and enzymes in the brain and in the spinal cord: circuitries and neurochemistry. In: Köfalvi A (ed.) Cannabinoids and the Brain. Boston, MA: Springer, 2008, pp. 161–201. [Google Scholar]
- 11. Dean C. Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am J Physiol Regul Integr Comp Physiol. 2011;300:R771–R779. [DOI] [PubMed] [Google Scholar]
- 12. Cota D. The role of the endocannabinoid system in the regulation of hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol. 2008;20(Suppl 1):35–38. [DOI] [PubMed] [Google Scholar]
- 13. deRoon-Cassini TA, Stollenwerk TM, Beatka M, et al. . Meet your stress management professionals: the endocannabinoids. Trends Mol Med. 2020;26:953–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engeli S. Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol. 2008;20(Suppl 1):110–115. [DOI] [PubMed] [Google Scholar]
- 15. Pagotto U, Vicennati V, Pasquali R. The endocannabinoid system and the treatment of obesity. Ann Med. 2005;37:270–275. [DOI] [PubMed] [Google Scholar]
- 16. Hill MN, Campolongo P, Yehuda R, et al. . Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43:80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charytoniuk T, Zywno H, Konstantynowicz-Nowicka K, et al. . Can physical activity support the endocannabinoid system in the preventive and therapeutic approach to neurological disorders? Int J Mol Sci. 2020;21:4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. [DOI] [PubMed] [Google Scholar]
- 19. Cheung KAK, Peiris H, Wallace G, et al. . The interplay between the endocannabinoid system, epilepsy and cannabinoids. Int J Mol Sci. 2019;20:6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mayo LM, Rabinak CA, Hill MN, et al. Targeting the endocannabinoid system in the treatment of PTSD: a promising case of preclinical-clinical translation? Biol Psychiatry. 2021. [Epub ahead of print]; DOI: 10.1016/j.biopsych.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mayo LM, Asratian A, Lindé J, et al. . Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol Psychiatry. 2020;87:538–547. [DOI] [PubMed] [Google Scholar]
- 22. Patel S, Roelke CT, Rademacher DJ, et al. . Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. [DOI] [PubMed] [Google Scholar]
- 23. Rabinak CA, Angstadt M, Sripada CS, et al. . Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rabinak CA, Blanchette A, Zabik NL, et al. . Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: a preliminary study. Psychopharmacology (Berl). 2020;237:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan WP. Affective beneficence of vigorous physical activity. Med Sci Sports Exerc. 1985;17:94–100. [PubMed] [Google Scholar]
- 26. Dishman RK, O'Connor PJ. Lessons in exercise neurobiology: the case of endorphins. Ment Health Phys Act. 2009;2:4–9. [Google Scholar]
- 27. Pardridge WM, Triguero D, Buciak JL. Beta-endorphin chimeric peptides: transport through the blood-brain barrier in vivo and cleavage of disulfide linkage by brain. Endocrinology. 1990;126:977–984. [DOI] [PubMed] [Google Scholar]
- 28. Siebers M, Biedermann SV, Bindila L, et al. . Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173. [DOI] [PubMed] [Google Scholar]
- 29. Koltyn KF, Brellenthin AG, Cook DB, et al. . Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15:1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crombie KM, Brellenthin AG, Hillard CJ, et al. . Psychobiological responses to aerobic exercise in individuals with posttraumatic stress disorder. J Trauma Stress. 2018;31:134–145. [DOI] [PubMed] [Google Scholar]
- 31. Raichlen DA, Foster AD, Seillier A, et al. . Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2013;113:869–875. [DOI] [PubMed] [Google Scholar]
- 32. Hill MN, Titterness AK, Morrish AC, et al. . Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolf SA, Bick-Sander A, Fabel K, et al. . Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun Signal. 2010;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dubreucq S, Koehl M, Abrous DN, et al. . CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224:106–113. [DOI] [PubMed] [Google Scholar]
- 35. Dubreucq S, Durand A, Matias I, et al. . Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry. 2013;73:895–903. [DOI] [PubMed] [Google Scholar]
- 36. Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schönke M, Martinez-Tellez B, Rensen PC. Role of the endocannabinoid system in the regulation of the skeletal muscle response to exercise. Curr Opin Pharmacol. 2020;52:52–60. [DOI] [PubMed] [Google Scholar]
- 38. Forteza F, Giorgini G, Raymond F. Neurobiological processes induced by aerobic exercise through the endocannabinoidome. Cells. 2021;10;938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biedermann SV, Auer MK, Bindila L, et al. . Restricted vs. unrestricted wheel running in mice: effects on brain, behavior and endocannabinoids. Horm Behav. 2016;86:45–54. [DOI] [PubMed] [Google Scholar]
- 40. Brellenthin AG, Crombie KM, Hillard CJ, et al. . Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med Sci Sports Exerc. 2017;49:1688–1696. [DOI] [PubMed] [Google Scholar]
- 41. Sparling PB, Giuffrida A, Piomelli D, et al. . Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. [DOI] [PubMed] [Google Scholar]
- 42. Forbes SC, Candow DG, Krentz JR, et al. . Changes in fat mass following creatine supplementation and resistance training in adults ≥50 years of age: a meta-analysis. J Funct Morphol Kinesiol. 2019;4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cumpston M, Li T, Page MJ, et al. . Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brellenthin AG, Crombie KM, Hillard CJ, et al. . Psychological and endocannabinoid responses to aerobic exercise in substance use disorder patients. Subst Abus. 2019;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stensson N, Gerdle B, Ernberg M, et al. . Increased anandamide and decreased pain and depression after exercise in fibromyalgia. Med Sci Sport Exerc. 2020;52:1617–1628. [DOI] [PubMed] [Google Scholar]
- 46. Marin Bosch B, Bringard A, Logrieco MG, et al. . Effect of acute physical exercise on motor sequence memory. Sci Rep. 2020;10:15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cedernaes J, Fanelli F, Fazzini A, et al. . Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology. 2016;74:258–268. [DOI] [PubMed] [Google Scholar]
- 48. Feuerecker M, Hauer D, Toth R, et al. . Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur J Appl Physiol. 2012;112:2777–2781. [DOI] [PubMed] [Google Scholar]
- 49. Stensson N, Grimby-Ekman A. Altered relationship between anandamide and glutamate in circulation after 30 min of arm cycling: a comparison of chronic pain subject with healthy controls. Mol Pain. 2019;15:1744806919898360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hutchins-Wiese HL, Li Y, Hannon K. Hind limb suspension and long-chain omega-3 PUFA increase mRNA endocannabinoid system levels in skeletal muscle. J Nutr Biochem. 2012;23:986–993. [DOI] [PubMed] [Google Scholar]
- 51. Crespillo A, Suárez J, Bermúdez-Silva FJ, et al. . Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade. Biochem J. 2011;433:175–185. [DOI] [PubMed] [Google Scholar]
- 52. Sagar DR, Gaw AG, Okine BN, et al. . Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol Pain. 2009;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ho W-S V, Barrett DA, Randall MD. “Entourage” effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mattace Raso G, Russo R, Calignano A, et al. . Palmitoylethanolamide in CNS health and disease. Pharmacol Res. 2014;86:32–41. [DOI] [PubMed] [Google Scholar]
- 55. Hazell TJ, Islam H, Townsend LK, et al. . Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: potential mechanisms. Appetite. 2016;98:80–88. [DOI] [PubMed] [Google Scholar]
- 56. Heyman E, Gamelin FX, Goekint M, et al. . Intense exercise increases circulating endocannabinoid and BDNF levels in humans—possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. [DOI] [PubMed] [Google Scholar]
- 57. Hillard CJ, Beatka M, Sarvaideo J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal axis. Compr Physiol. 2017;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meyer JD, Crombie KM, Cook DB, et al. . Serum endocannabinoid and mood changes after exercise in major depressive disorder. Med Sci Sport Exerc. 2019;51:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Raichlen DA, Foster AD, Gerdeman GL, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner's high.” J Exp Biol. 2012;215(Pt 8):1331–1336. [DOI] [PubMed] [Google Scholar]
- 60. Ensari I, Sandroff BM, Motl RW. Intensity of treadmill walking exercise on acute mood symptoms in persons with multiple sclerosis. Anxiety Stress Coping. 2017;30:15–25. [DOI] [PubMed] [Google Scholar]
- 61. Paolucci EM, Loukov D, Bowdish DME, et al. . Exercise reduces depression and inflammation but intensity matters. Biol Psychol. 2018;133:79–84. [DOI] [PubMed] [Google Scholar]
- 62. Nakagawa T, Koan I, Chen C, et al. . Regular moderate- to vigorous-intensity physical activity rather than walking is associated with enhanced cognitive functions and mental health in young adults. Int J Environ Res Public Health. 2020;17:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ekkekakis P, Hall EE, Petruzzello SJ. Variation and homogeneity in affective responses to physical activity of varying intensities: an alternative perspective on dose-response based on evolutionary considerations. J Sports Sci. 2005;23:477–500. [DOI] [PubMed] [Google Scholar]
- 64. King-Himmelreich TS, Möser CV, Wolters MC, et al. . AMPK contributes to aerobic exercise-induced antinociception downstream of endocannabinoids. Neuropharmacology. 2017;124:134–142. [DOI] [PubMed] [Google Scholar]
- 65. Dlugos A, Childs E, Stuhr KL, et al. . Acute stress increases circulating anandamide and other n-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hassanzadeh P, Rahimpour S. The cannabinergic system is implicated in the upregulation of central NGF protein by psychotropic drugs. Psychopharmacology (Berl). 2011;215:129–141. [DOI] [PubMed] [Google Scholar]
- 67. Hanlon EC. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology. 2020;111:104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hanlon EC, Tasali E, Leproult R, et al. . Circadian rhythm of circulating levels of the endocannabinoid 2 arachidonoylglycerol. J Clin Endocrinol Metab. 2015;100:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dincheva I, Drysdale AT, Hartley CA, et al. . FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Côté M, Matias I, Lemieux I, et al. . Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes. 2007;31:692–699. [DOI] [PubMed] [Google Scholar]
- 71. Monteleone P, Piscitelli F, Scognamiglio P, et al. . Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97:E917–E924. [DOI] [PubMed] [Google Scholar]
- 72. El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril. 2010;93:1989–1996. [DOI] [PubMed] [Google Scholar]
- 73. McPartland JM, Guy GW, Di Marzo V. Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One. 2014;9:e89566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ferrari F, Villa RF. The neurobiology of depression: an integrated overview from biological theories to clinical evidence. Mol Neurobiol. 2017;54:4847–4865. [DOI] [PubMed] [Google Scholar]
- 75. Maeng LY, Milad MR. Post-traumatic stress disorder: the relationship between the fear response and chronic stress. Chronic Stress (Thousand Oaks). 2017;1:2470547017713297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Patriquin MA, Mathew SJ. The neurobiological mechanisms of generalized anxiety disorder and chronic stress. Chronic Stress (Thousand Oaks). 2017;1:2470547017703993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Silveira KM, Wegener G, Joca SRL. Targeting 2-arachidonoylglycerol signalling in the neurobiology and treatment of depression. Basic Clin Pharmacol Toxicol. 2021;129:3–14. [DOI] [PubMed] [Google Scholar]
- 78. Black N, Stockings E, Campbell G, et al. . Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hill MN, Miller GE, Carrier EJ, et al. . Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Crombie KM, Leitzelar BN, Brellenthin AG, et al. . Loss of exercise- and stress-induced increases in circulating 2-arachidonoylglycerol concentrations in adults with chronic PTSD. Biol Psychol. 2019;145:1–7. [DOI] [PubMed] [Google Scholar]
- 81. Schaefer C, Enning F, Mueller JK, et al. . Fatty acid ethanolamide levels are altered in borderline personality and complex posttraumatic stress disorders. Eur Arch Psychiatry Clin Neurosci. 2014;264:459–463. [DOI] [PubMed] [Google Scholar]
- 82. Hill MN, Bierer LM, Makotkine I, et al. . Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the world trade center attacks. Psychoneuroendocrinology. 2013;38:2952–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology 2015;41:58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Keyan D, Bryant RA. The capacity for acute exercise to modulate emotional memories: a review of findings and mechanisms. Neurosci Biobehav Rev. 2019;107:438–449. [DOI] [PubMed] [Google Scholar]
- 85. Tanner MK, Hake HS, Bouchet CA, et al. . Running from fear: exercise modulation of fear extinction. Neurobiol Learn Mem. 2018;151:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Crombie KM, Sartin-Tarm A, Sellnow K, et al. . Aerobic exercise and consolidation of fear extinction learning among women with posttraumatic stress disorder. Behav Res Ther. 2021;142:103867. [DOI] [PubMed] [Google Scholar]
- 87. Crombie KM, Sartin-Tarm A, Sellnow K, et al. . Exercise-induced increases in Anandamide and BDNF during extinction consolidation contribute to reduced threat following reinstatement: preliminary evidence from a randomized controlled trial. Psychoneuroendocrinology. 2021;132:105355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Crombie KM, Cisler JM, Hillard CJ, et al. . Aerobic exercise reduces anxiety and fear ratings to threat and increases circulating endocannabinoids in women with and without PTSD. Ment Health Phys Act. 2021;20:100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hughes L, Patterson SD. The effect of blood flow restriction exercise on exercise-induced hypoalgesia and endogenous opioid and endocannabinoid mechanisms of pain modulation. J Appl Physiol. 2020;128:914–924. [DOI] [PubMed] [Google Scholar]
- 90. Stone NL, Millar SA, Herrod PJJ, et al. . An analysis of endocannabinoid concentrations and mood following singing and exercise in healthy volunteers. Front Behav Neurosci. 2018;12:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Crombie KM, Brellenthin AG, Hillard CJ, et al. . Endocannabinoid and opioid system interactions in exercise-induced hypoalgesia. Pain Med. 2018;19:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fuss J, Steinle J, Bindila L, et al. . A runner's high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A. 2015;112:13105–13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Galdino G, Romero T, Silva JF, et al. . Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth Analg. 2014;119:702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Galdino G, Romero TR, Silva JF, et al. . The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology 2014;77:313–324. [DOI] [PubMed] [Google Scholar]
- 95. Oliveira AB, Ribeiro RT, Mello MT, et al. . Anandamide is related to clinical and cardiorespiratory benefits of aerobic exercise training in migraine patients: a randomized controlled clinical trial. Cannabis Cannabinoid Res. 2019;4:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fernández-Aranda F, Sauchelli S, Pastor A, et al. . Moderate-vigorous physical activity across body mass index in females: moderating effect of endocannabinoids and temperament. PLoS One. 2014;9:e104534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Koay YC, Stanton K, Kienzle V, et al. . Effect of chronic exercise in healthy young male adults: a metabolomic analysis. Cardiovasc Res. 2021;117:613–622. [DOI] [PubMed] [Google Scholar]
- 98. Sadhasivam S, Alankar S, Maturi R, et al. . Inner engineering practices and advanced 4-day isha yoga retreat are associated with cannabimimetic effects with increased endocannabinoids and short-term and sustained improvement in mental health: a prospective observational study of meditators. Evid Based Complement Altern Med. 2020;2020:8438272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Belitardo de Oliveira A, de Mello MT, Tufik S, et al. . Weight loss and improved mood after aerobic exercise training are linked to lower plasma anandamide in healthy people. Physiol Behav. 2019;201:191–197. [DOI] [PubMed] [Google Scholar]
- 100. Dos Santos RS, Sorgi CA, Peti APF, et al. . Involvement of spinal cannabinoid CB(2) receptors in exercise-induced antinociception. Neuroscience. 2019;418:177–188. [DOI] [PubMed] [Google Scholar]
- 101. Thompson Z, Argueta D, Garland Jr. T, et al. Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol Behav. 2017;170:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gamelin FX, Aucouturier J, Iannotti FA, et al. . Exercise training and high-fat diet elicit endocannabinoid system modifications in the rat hypothalamus and hippocampus. J Physiol Biochem. 2016;73:335–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.