Abstract
Hypothesis:
Administration of the phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC) will enhance brain repair and improve short-term spatial working memory in mice following controlled cortical impact (CCI) by upregulating granulocyte colony-stimulating factor (G-CSF) and other neurotrophic factors (brain-derived neurotrophic factor [BDNF], glial-derived neurotrophic factor [GDNF]) in hippocampus (HP), cerebral cortex, and striatum.
Materials and Methods:
C57BL/6J mice underwent CCI and were treated for 3 days with Δ9-THC 3 mg/kg intraperitoneally (i.p.). Short-term working memory was determined using the spontaneous alternations test during exploratory behavior in a Y-maze. Locomotor function was measured as latency to fall from a rotating drum (rotometry). These behaviors were recorded at baseline and 3, 7, and 14 days after CCI. Groups of mice were euthanized at 7 and 14 days. Extent of microgliosis, astrocytosis, and G-CSF, BDNF, and GDNF expression were measured at 7 and 14 days in cerebral cortex, striatum, and HP on the side of the trauma. Levels of the most abundant endocannabinoid (2-arachidonoyl-glycerol [2-AG]) was also measured at these times.
Results:
Δ9-THC-treated mice exhibited marked improvement in performance on the Y-maze indicating that treatment with the phytocannabinoid could reverse the deficit in working memory caused by the CCI. Δ9-THC-treated mice ran on the rotarod longer than vehicle-treated mice and recovered to normal rotarod performance levels at 2 weeks. Δ9-THC-treated mice, compared with vehicle-treated animals, exhibited significant upregulation of G-CSF as well as BDNF and GDNF in the cerebral cortex, striatum, and HP. Levels of 2-AG were also increased in the Δ9-THC-treated mice.
Conclusion:
Administration of the phytocannabinoid Δ9-THC promotes significant functional recovery from traumatic brain injury (TBI) in the realms of working memory and locomotor function. This beneficial effect is associated with upregulation of brain 2-AG, G-CSF, BDNF, and GDNF. The latter three neurotrophic factors have been previously shown to mediate brain self-repair following TBI and stroke.
Keywords: controlled cortical impact, traumatic brain injury, neurotrophic factors, BDNF, GDNF, G-CSF, endocannabinoid system, phytocannabinoids, working memory
Introduction
The endocannabinoid system (eCBS) plays a central role in modulating neuronal activity and maintaining homeostasis in health and disease.1,2 Importantly, the eCBS has been shown to mediate recovery from a spectrum of neurological injuries, including traumatic brain injury (TBI).3,4 Administration of phytocannabinoids (like Δ9-tetrahydrocannabinol [Δ9-THC]) or agents that increase levels of endogenous cannabinoid ligands, can improve dysfunction of the blood–brain barrier, reduce lesion volume, decrease neuronal death, and improve behavioral performance in rodent models of TBI.4–6 The endogenous cannabinoid system (eCS) comprises endogenous ligands N-arachidonoylethanolamine (AEA) and 2-arachidonoyl-glycerol (2-AG), cannabinoid receptors (CB1, CB2), as well as the proteins that transport, synthesize, and degrade these ligands.1,2 There are other potential receptors (RPV1 and GPR55) that mediate the eCB ligand effects.1 CB1 receptors are enriched in the nervous system but are also present in peripheral tissues. In neurons, CB1 receptors are primarily located on synaptic terminals, reflecting their major role in modulating synaptic transmission although they are also expressed at functionally important levels on neuronal somata and dendrites.2 CB2 receptors are primarily expressed in cells of immune origin, including microglia but may also be expressed in neurons especially in pathological states.2,7 Microglial CB2 receptor activation is considered to be primarily anti-inflammatory.2
Numerous studies on experimental models of brain toxicity, neuroinflammation, and trauma support the notion that the eCBs are part of the brain's compensatory or repair mechanisms.7 We have recently reported that delivery of mild-to-moderate controlled cortical impact (CCI) to mice resulted in downregulation of CB1-R expression and upregulation of CB2-R in cortex, striatum, and hippocampus.8 These changes in expression of CB receptors were similar to those reported in a weight drop mouse model of TBI.9 Moreover, treatment of these mice with granulocyte colony-stimulating factor (G-CSF), a hematopoietic cytokine with neurotrophic effects, reversed the changes in CB1 and CB2 receptor expression and enhanced recovery of locomotor function.8
Other researchers have reported that endogenous G-CSF levels can be significantly increased by administration of the phytocannabinoid Δ9-THC to normal mice.7 We have replicated this observation in mice that had sustained CCI.8 These mice exhibited upregulation of G-CSF expression in cerebral cortex, striatum, and hippocampus (HP) at 3, 7, and 14 days after the trauma. Administration of Δ9-THC for 3 days after CCI resulted in significant recovery of motor function and was associated with further upregulation of G-CSF expression in brain.8
Manipulation of the eCBS by using drugs that are able to increase levels of the principal cannabinoids AEA (anandamide) and 2-AG has been reported to enhance functional recovery from traumatic brain injury (TBI) in mice.10,11 AEA is mainly hydrolyzed by the fatty acid amide hydrolase (FAAH), whereas 2-AG is degraded primarily by monoacylglycerol lipase (MAGL) and to a lesser extent by alpha, beta-hydrolase domain 6 (ABHD6).12 Treatment of mice with an inhibitor of ABHD6 to increase levels of 2-AG, or with an inhibitor of FAAH to increase levels of AEA, has been reported to reverse motor functional deficits, but also to enhance recovery of hippocampal-dependent working memory.10,11
Earlier work in our laboratory has shown that G-CSF treatment restored hippocampal-dependent spatial learning using a modified Morris Water Maze after TBI, but we have not tested if Δ9-THC treatment would restore hippocampal-dependent learning deficits. For the present report, we opted to utilize the spontaneous alternation Y-maze as a measure of hippocampal-dependent working memory because the Morris Water Maze is better suited for studying spatial memory than it is for working memory. For example, the inhibitor of 2-AG hydrolysis (WWL70) was reported to have little to no effect on spatial learning and memory in the Morris Water Maze test, but improved TBI-induced deficits in working memory performance measured with the spontaneous alternation Y-maze test.11
The objectives for this study were: (1) to measure loss of hippocampal-dependent working memory following CCI to the right cerebral frontal cortex, (2) to test if a moderate dose of Δ9-THC administered for 3 days after CCI would reverse the working memory deficit, and (3) to measure changes in concentrations of 2-AG, levels of neurotrophic factors (BDNF, GDNF), and of the hematopoietic/neurotrophic cytokine G-CSF in HP (and other brain regions) 7 and 14 days after injury.
Materials and Methods
Animals
This study was carried out in strict accordance with the National Institutes of Health Guide for the care and use of laboratory animals. The protocol was approved by the Institutional Animal Care and Use Committee of the University of South Florida. Adult, 3-month-old, male C57BL/6J mice (25–30 g) were housed in standard laboratory cages and left undisturbed for 1 week after arrival at the animal facility. Animals had ad libitum access to water and laboratory chow and were maintained in a temperature- and humidity-controlled room on a 12-h light/12-h dark cycle with lights on at 7:00 AM. Groups of C57BL/6J mice (n=8 per group) sustained CCI to the right frontal cortex. One group served as controls (sham surgery, no drugs). The remaining six groups of mice were treated for 3 days after CCI with daily i.p. injections of Δ9-THC (3 mg/kg) or vehicle for 3 days. The dose of Δ9-THC (3 mg/kg) was chosen as the lowest dose capable of triggering significant upregulation of GCSF in normal C57BL mice.13 The first dose of Δ9-THC was administered 18 h after TBI, followed by dosing for two more days. A schedule of three daily doses of Δ9-THC after TBI was chosen to replicate the schedule followed when treating mice with G-CSF, a protocol that promoted recovery of motor and cognitive performance 7 and 14 days after TBI.14,15 An objective of the study was to determine the extent of behavioral recovery in a hippocampal-dependent memory task (Y-maze) and in motor function (rotometry) in the subacute period (3, 7, and 14 days after injury). No parameters of recovery were measured on day 1, although prior reports from our laboratory did note a rapid upregulation of hippocampal G-CSF expression at 6 and 12 h following an acute stab wound (brief insertion and withdrawal of needle to HP).16 Groups of mice treated with vehicle and THC were euthanized 3, 7, and 14 days after CCI. Each brain was removed after perfusion with heparinized saline and dissected into three regions (cerebral cortex, corpus striatum, and HP) for freezing for analyses of G-CSF, BDNF, GDNF, and 2-AG. Two brains from each group were dissected and processed for immunohistology to assess the extent of microgliosis (Iba1 immunostaining) and astrocytosis (GFAP immunostaining).
Y-maze data collection and analysis
The spontaneous alternation y-maze has been used to assess short-term spatial working memory. This tests spatial recognition memory at a rudimentary level using the natural exploratory behavior of rodents..17 Y-maze was purchased from Stoelting, catalog no. 60180), and the mouse version has dimensions 39.5×8.5×13 cm. The three arms of the maze are interconnected at an angle of 120°. Animals were habituated to the room for at least 30 min before testing, without the y-maze being visible. Animal behavior was tracked using a video camera recording, which facilitated scoring by two individuals blinded to experimental groups. The three main outputs from the spontaneous alternation y-maze analysis are the number of alternations and entries, as well as percent alternations. The number of alternations was calculated based on the sequence of arm entries. An alternation is defined as successive entries into three arms, on overlapping triplet sets. For example, entries into arms 1, 3, and 2 is considered an alternation. Whereas entries into arms 1, 2, and 1 would not be considered an alternation. Alternations have been used to measure short-term spatial memory in mice.18 An arm entry is completed when the hind paws of the mouse had been completely placed in the arm. The number of entries per arm is a measurement of activity and locomotion during the testing session and has been used to calculate the percent alternations. The percentage of alternation was calculated as the ratio of total number of alternations divided by the number of arms entered. The % alternations=total number of alternations/number of arms entered×100%.
Rotometry
The ability to run on a rotating drum (rotarod) was used as a measure of motor balance and coordination (Madel 47600 rotarod for mice; Ugo Basile, Gemonio, Italy). Data were generated by averaging the scores (total time spent on the rotating drum divided by three trials) for each animal during training and testing days. Each animal was placed in a neutral position on a cylinder, the rod was rotated, with the speed accelerated linearly from 4 to 40 rpm within 3 min, and the time spent on the rotarod was recorded automatically. For training, animals were given one trial before testing. For testing, animals were given three trials, and the average score on these three trials were used as the individual rotarod score. After baseline performance had been established, animals were randomly assigned to receive vehicle (n=8) or THC treatment (n=8) following CCI.
Surgery and CCI
Animals underwent an experimental TBI with a controlled cortical impactor (Pittsburgh Precision Instruments), as described previously.19 Animals initially received buprenorphine (0.05 mg/kg, s.c.) at the time of anesthesia induction (with 125 mg/kg ketamine, 12.5 mg/kg xylazine). After deep anesthesia had been achieved (verified by checking for pain reflexes), individual animals were fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). After exposing the skull, craniectomy (∼3 mm to accommodate the impactor tip) was performed over the right frontoparietal cortex (0.5 mm anteroposterior and 1.0 mm mediolateral to bregma). All mice received a “moderate” TBI. The pneumatically operated TBI device (with a convex tip diameter of 2 mm) impacts the brain at a velocity of 6.0 m/sec, reaching a depth of 1.0 mm below the dura mater layer and remains in the brain for 150 msec. The impactor rod was angled at 158° to the vertical to maintain a perpendicular position in reference to the tangential plane of the brain curvature at the impact surface. A linear variable displacement transducer (Macrosensors, Pennsauken, NJ) connected to the impactor measured velocity and duration to verify consistency. Bone wax was used to cover the region of craniectomy, and the skin incision sutured thereafter. A computer-operated thermal blanket pad and a rectal thermometer allowed maintenance of body temperature within normal limits. All animals were closely monitored until recovery from anesthesia and over the next 3 days.
Drugs
Δ9-tetrahydrocannabinol was dissolved in 100% ethanol to a concentration of 6 mg/mL. In a second tube, Kolliphor_ EL (synonym: Cremophor_ EL; Sigma-Aldrich, St. Louis, MO) was mixed with sterile 0.9% saline. The dissolved cannabinoid was then mixed with the Cremophor/NaCl solution to a final ratio of 1:1:18 (cannabinoid/ethanol:Cremophor:saline). The final concentration of each component in the injection solutions was 0.3 mg/mL cannabinoid, 5% ethanol, 5% Cremophor, and 0.81% NaCl. Mice were injected i.p. with 3 mg/kg of of Δ9-THC daily for 3 days. Controls received a vehicle consisting of 5% ethanol, 5% Cremophor, and 0.81% NaCl i.p. daily for 3 days after CCI. Δ9-THC was purchased from Sigma-Aldrich. This dose of Δ9-THC was chosen as the lowest dose capable of triggering significant upregulation of GCSF in normal C57BL mice.13
Mouse brain 2-AG, BDNF, GDNF, G-CSF enzyme-linked immunosorbent assay
Regions of brain were dissected as described above, and tissue was processed according to the protocol for the Enzyme-Linked Immunosorbent Assay (ELISA) Kit for mouse 2-AG (catalog no. CE0443Ge; Cloud-Clone Corp., Katy, TX). BDNF, GDNF, and G-CSF protein levels were measured by ELISA in brain tissue and G-CSF was measured in blood as well.
Statistics
Data are expressed as mean±standard error of mean (n=7–8 mice per data point). Prism 8 (GraphPad Software, Inc., San Diego, CA) was used to perform one-way or two-way analysis of variance, followed by correction for multiple comparisons. p-Values <0.05 were considered statistically significant.
Results
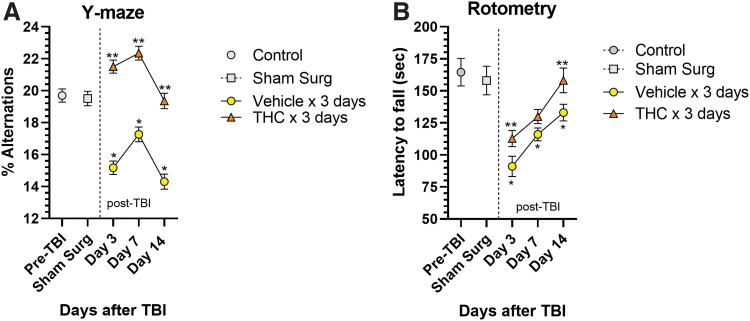
The effects of CCI on HP-dependent short-term spatial working memory was assessed using the spontaneous alternation Y-maze test.17 CCI significantly impaired working memory in vehicle-treated mice indicated by marked decreases in percent alternations in entering Y-maze arms during exploration at 3, 7, and 14 days (p<0.05) (Fig. 1A). Δ9-THC treatment for 3 days after CCI resulted in significantly improved working memory performance compared with vehicle-treated mice indicated by increased percent alternations at all three time points (p<0.05). Vehicle-treated mice never recovered to baseline and performed worse than pre-TBI baseline at 3, 7, and 14 days (p<0.05). Performance by the THC-treated group was significantly better than before surgery (or sham-surgery mice) on days 3 and 7, with best performance on day 7. By day 14, the THC group was still improved but had fallen to a normal pre-injury level of performance. The slight decline in performance from day 7 to 14 may be due to (1) elimination of the drug from brain and/or (2) a result of time-dependent intrinsic hippocampal repair/regenerative responses. Approximately 95% of the drug will have been eliminated from brain after 4–5 half-lives or about 10 h.20 We speculate that a sustained higher level of performance on day 14 might have been achieved if the Δ9-THC was given for 7–10 days. Curiously, the Y-maze performances plotted against time in both groups of mice appear to mirror each other in the decline of performance from day 7 to 14, although the vehicle-treated group performance always remained well below pre-TBI performance. Intrinsic brain repair and regenerative processes, especially the extent of hippocampal neurogenesis, is the primary determinant of performance over time in the Y-maze, a hippocampal-dependent spatial memory task.
FIG. 1.
Behavioral effects of CCI. (A) Y-maze was used to assess short-term hippocampal-dependent working memory. Following CCI, vehicle-treated mice (n=8) exhibited significant decreases in percent spontaneous alternations compared with pre-TBI and sham surgery controls at each time point (*p<0.05). Mice treated for 3 days with Δ9-THC exhibited significantly greater percent alternations than vehicle-treated mice (n=8) at all time points, indicating improvement in working memory (**p<0.05). Performance by the Δ9-THC group on days 3 and 7 was even better than in untreated controls and returned to pre-TBI level by day 14. Vehicle-treated mice performed worse than pre-TBI baseline at all 3 days. Two-way ANOVA showed treatment accounted for 37% of total variance (p<0.0001) and time accounted for 7% of total variance (p=0.07). Sidak's multiple comparisons test showed that the vehicle-treated mice performed significantly worse than pre-TBI performance at each time point (*p<0.05). The Δ9-THC treatment group performed significantly better than the vehicle treatment group at each time point (**p<0.05). (B) Rotometry: both the Δ9-THC-treated group (n=8 mice) and vehicle-treated group (n=8 mice) were trained to run on a rotometer before undergoing CCI. After CCI, treatment with Δ9-THC 3 mg/kg for 3 days resulted in a time-dependent recovery of motor function. Two-way ANOVA testing showed that both treatment (THC vs. vehicle) and time (days) after CCI contributed significantly to total variance (p<0.005). Sidak's correction for multiple comparisons revealed that THC significantly improved time on the rotometer compared with vehicle treatment on day 3 and 14 (**p<0.05). Vehicle-treated mice also gradually improved performance over time, but did not reach pre-TBI levels of performance. Sidak's correction for multiple comparisons showed performance in the vehicle-treated group remained significantly less compared with pre-TBI and sham surgery controls (*p<0.05). Δ9-THC, Δ9-tetrahydrocannabinol; ANOVA, analysis of variance; CCI, controlled cortical impact; TBI, traumatic brain injury.
In addition to the impact of CCI on hippocampal-dependent working memory, locomotor function was significantly impaired as measured by the latency to fall from a rotating drum. Vehicle-treated mice exhibited shorter latency to fall, dropping from 164 sec pre-TBI to 91.1 sec on day 3 after CCI (Fig. 1B). These vehicle-treated mice exhibited a gradual recovery of locomotor performance but did not reach pre-TBI levels. In contrast, Δ9-THC treatment for 3 days after CCI enhanced recovery over time and restoration of performance reached pre-TBI baseline by day 14 (p<0.05).
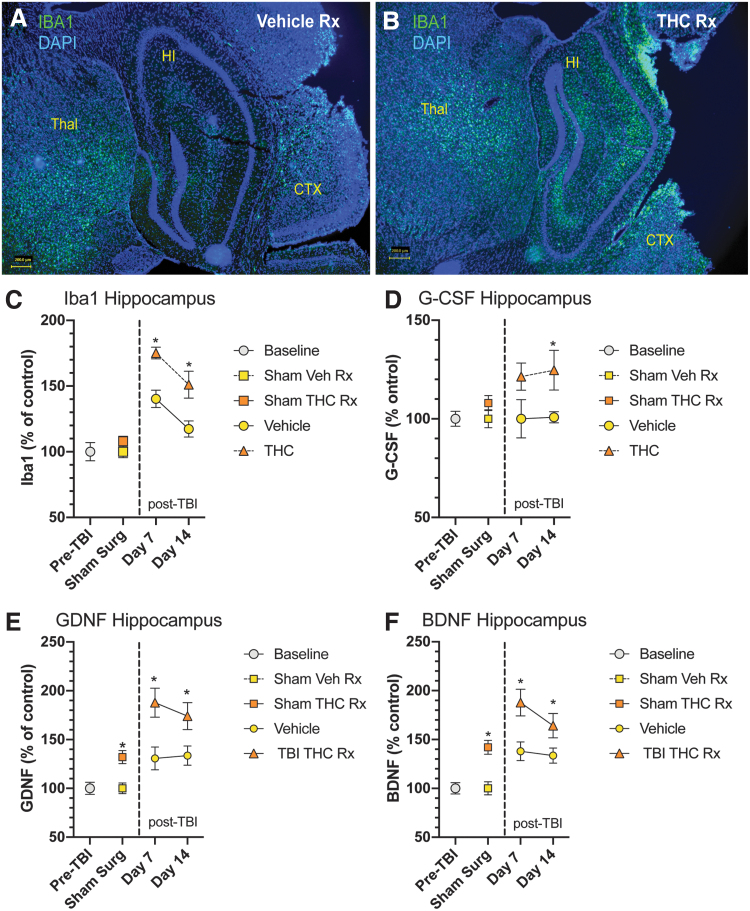
With the significant restoration of working memory on Y-maze testing, we examined changes in HP, a critical node in the neural network that mediates memory. The HP is viewed as an area supporting declarative long-term memory but it is also recruited during working memory,21 In an earlier report from our laboratory, we had found that CCI to the right frontal cortex resulted in increased microgliosis, astrocytosis, and upregulation of neurotrophic factor (BDNF and GDNF) expression in HP and other brain regions.14 In the present study, we have extended these observations by observing the effects of THC treatment on these parameters. Δ9-THC treatment for 3 days significantly increased microgliosis in HP (Fig. 2A, B) compared with vehicle-treated mice. To quantify the extent of microgliosis, Iba1 protein (marker of microglia) was measured using ELISA (Fig. 2C). Vehicle-treated mice exhibited increased hippocampal Iba1 levels at day 7 and 14. Δ9-THC-treated mice had significantly greater levels of Iba1 protein than vehicle-treated mice. Hippocampal GDNF and BDNF protein levels were significantly greater after Δ9-THC treatment compared with vehicle-treated mice on days 7 and 14 (Fig. 2E, F). G-GCSF protein was also elevated at days 7 and 14 compared with vehicle-treated mice (Fig. D).
FIG. 2.
Changes in HP after CCI. (A) Example of microglial activation in HP after CCI in vehicle-treated mouse (day 7 after CCI). Immunostaining for Iba1, a marker of microglia shows a mild degree of microgliosis in HP. (B) THC treatment for 3 days after CCI resulted in a significant increase in microgliosis in HP. (C) THC-treated mice had significantly increased Iba1 protein expression at both 7 and 14 days after CCI. Iba1 protein expression was also increased in the vehicle-treated mice compared with pre-TBI and sham surgery control groups. (D) Measurement of G-CSF protein in HP revealed a significant increase in G-CSF expression compared with vehicle-treated animals on day 14. (E, F) Both GDNF and BDNF protein expression were significantly increased in HP compared with vehicle-treated controls on days 7 and 14 after CCI. Two-way ANOVA showed that treatment, but not time accounted for most of the total variance (p<0.05). Sidak's multiple comparisons test showed THC treatment was significantly different than vehicle treatment at each time point (*p<0.05). BDNF, brain-derived neurotrophic factor; G-CSF, granulocyte colony-stimulating factor; GDNF, glial-derived neurotrophic factor; HP, hippocampus.
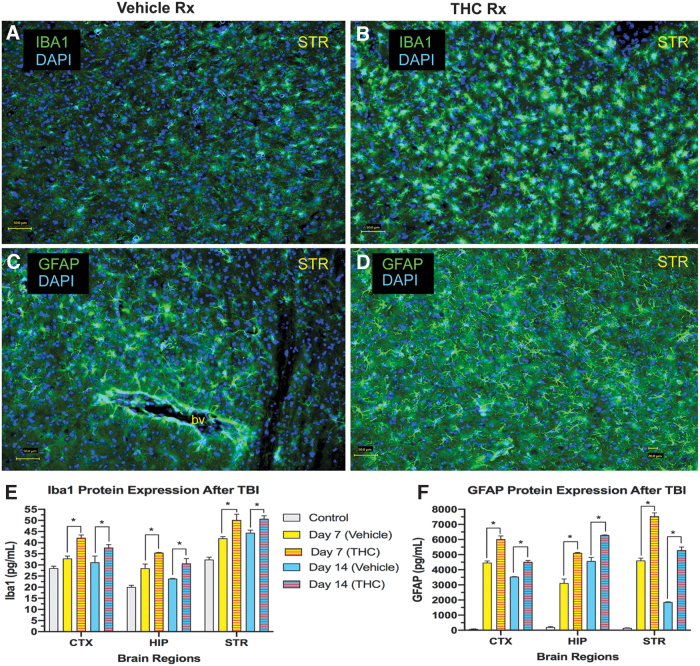
CCI also triggered microgliosis and astrocytosis in other brain regions on the side of the injury (Fig. 3). Immunolabeling of microglia with antibodies to Iba1 and astrocytes with antibodies to GFAP is illustrated in a striatal section in vehicle-treated and Δ9-THC-treated mice (Fig. 3A–D). Iba1 and GFAP protein, quantified with ELISA in 3 brain regions were significantly increased compared with pre-TBI control levels. Δ9-THC-treated mice exhibited greater levels of both Iba1 and GFAP compared with vehicle-treated mice in all 3 regions at 7 and 14 days after CCI (Fig. 3E, F).
FIG. 3.
Microgliosis and astrocytosis following CCI. (A) Example of striatal microgliosis in vehicle-treated mouse on day 7. (B) Microgliosis is increased in the THC-treated mouse. Scale bar in lower left=50 M. (C) Astrocytes in striatum in untreated control mouse. (D) Astrocytosis is increased in striatum in THC-treated mouse (day 7). Scale bar=50 M. (E) Summary of Iba1 protein expression in 3 brain regions at day 7 and 14 (CTX; HIP; STR; two-way ANOVA shows that treatment but not time contributed to most of the total variance in each brain region; p<0.05). (F) Summary of GFAP protein expression in 3 brain regions at day 7 and 14 (two-way ANOVA revealed that both treatment and time contributed significantly to total variance in each brain region; p<0.05). Sidak's correction for multiple comparisons revealed that THC treatment compared with vehicle treatment increased expression of both Iba1 and GFAP in all 3 brain regions on day 7 and 14 (*p<0.05). CTX, cortex; DAPI, nuclear stain; GFAP, astrocytic cell marker; HIP, hippocampus; Iba1, marker of microglial cells; STR, striatum.
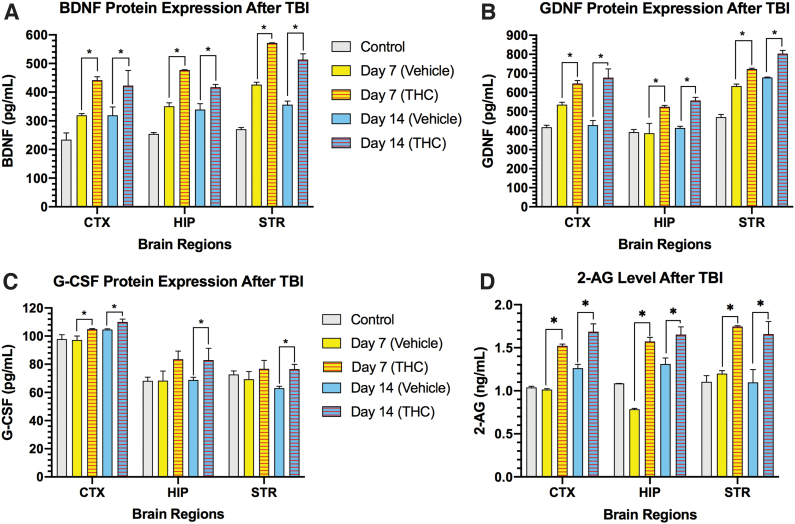
In addition to the activation of microglia and astrocytes, CCI markedly increased the neurotrophic factors BDNF and GDNF in cerebral cortex, striatum, and HP on days 7 and 14 (Fig. 4A, B). Treatment of mice with Δ9-THC for 3 days after CCI significantly increased levels of BDNF and GDNF compared with vehicle-treated mice in the 3 brain regions on days 7 and 14 (p<0.05). The pleiotropic neurotrophic factor G-CSF protein was not increased beyond pre-TBI levels in the 3 brain regions on days 7 and 14 (Fig. 4C). However, Δ9-THC-treated mice exhibited a significant increase in G-CSF expression in all 3 brain regions on day 14 (p<0.05). The major endogenous cannabinoid 2-AG was also found to be significantly upregulated by Δ9-THC treatment on days 7 and 14 in the 3 brain regions (p<0.05) (Fig. 4D).
FIG. 4.
Regional brain changes in expression of neurotrophic factors BDNF, GDNF, G-CSF, and the endocannabinoid 2-AG. CCI triggered increases in BDNF protein (A) and GDNF protein expression (B) compared with normal control mice in the 3 brain regions on days 7 and 14. Treatment with THC further increased expression of BDNF and GDNF compared with vehicle-treated mice in all 3 brain regions on days 7 and 14. (C) G-CSF protein was increased in all 3 brain regions in THC-treated mice compared with vehicle-treated mice on day 14. The increase in G-CSF expression was observed on day 7 only in the cortex. (D) Levels of 2-AG were significantly increased in all 3 brain regions in the THC-treated mice compared with vehicle treatment. For each brain region in the four panels, two-way ANOVA revealed that both treatment and time each contributed significantly to total variance in the specific neurotrophic factor and cannabinoid 2-AG; p<0.05. Sidak's correction for multiple comparisons revealed that THC treatment compared with vehicle treatment increased expression of BDNF, GDNF, and 2-AG in all 3 brain regions on day 7 and 14 (*p<0.05). In the case of G-CSF, the increase did not reach statistical significance on day 7 in HP and striatum. 2-AG, 2-arachidonoyl-glycerol.
Discussion
Administration of the phytocannabinoid Δ9-THC following CCI promoted recovery of cognitive and locomotor function and significantly increased expression of neurotrophic factors, BDNF, GDNF, and G-CSF in HP, cerebral cortex, and striatum. The repair response to injury occurred on a substrate of significant microgliosis (indicated by Iba1 expression) and astrocytosis (indicated by GFAP expression) in the 3 brain tissues studied. Δ9-THC treatment further amplified microgliosis, which is the common denominator and pathophysiological hallmark of the inflammatory response to various brain insults, including trauma. Acutely injured neurons release a spectrum of factors, including glutamate that activate an array of receptors expressed on microglia.22 In turn, activated microglia modulate the repair response by releasing a profile of inflammatory and anti-inflammatory cytokines and growth factors, including G-CSF, BDNF, and GDNF.14,15 Another research team has previously reported that Δ9-THC treatment of TBI in a mouse model results in further upregulation of G-CSF expression by massive expansion of a population of CD11b+Gr-1+ myeloid-derived suppressor cells (MDSC) in the peritoneal cavity and spleen.13 Induction of MDSC by Δ9-THC was associated with a long-lasting increase in G-CSF which, as previously described, mediates brain repair.14,15 The beneficial effects of Δ9-THC appear to be mediated in part by G-CSF, which is a powerful immunomodulator that increases expression of anti-inflammatory cytokines, while also dampening expression of proinflammatory cytokines.23 Taken all together, Δ9-THC does not simply serve as an “anti-inflammatory” agent, but balances the profile of inflammatory and anti-inflammatory cytokines to promote repair and recovery of function.
These findings replicate recently published observations from our laboratory demonstrating the association between recovery of locomotor function in a mouse model of TBI and the upregulation of expression of neurotrophic factors.8 The present report on the beneficial effect of Δ9-THC treatment extends to reversal of the deficit in short-term spatial working memory caused by the trauma. Δ9-THC treatment for 3 days after CCI resulted in significantly improved working memory performance compared with vehicle-treated mice indicated by increased percent alternations in the Y-maze test at all three time points. Vehicle-treated mice never recovered to baseline and performed worse than pre-TBI baseline at 3, 7, and 14 days (p<0.05).
The spontaneous alternation Y-maze test has been used to assess short-term spatial working memory using the natural exploratory behavior of rodents..17 Similar to our findings reported in this study, treatment of mice after TBI with an agent that increased levels of the endogenous cannabinoids AEA (anandamide) and 2-AG resulted in reversal of short-term memory deficits.10 These authors assessed the mice with the same Y-maze test protocol we employed to measure short-term spatial working memory.
Past research in both humans and animals have revealed that two brain areas, the HP and medial prefrontal cortex (mPFC), are essential for the encoding and retrieval of episodic memories (see review in Jin and Maren24). Bidirectional interactions between the HP and mPFC are also involved in working memory in animals and humans. Working memory has been characterized as a short-term repository for task-relevant information that is critical for the successful completion of complex tasks.25 For example, in a spatial working memory task, animals must hold in memory the location of food rewards to navigate to those locations after a delay. Disconnection of the HPC and mPFC with asymmetrical lesions has been shown to disrupt spatial working memory.26,27
In the present report, CCI to the right frontal cortex resulted in significant deficits in short-term spatial working memory that was improved by administration of the phytocannabinoid Δ9-THC for 3 days. When compared with vehicle-treated animals, Δ9-THC-treated mice exhibited significant reversal of the deficit in spatial working memory. Improvement in working memory was associated with upregulation of BDNF, GDNF, and G-CSF in cerebral cortex, striatum, and HP. In addition, levels of the most abundant endocannabinoid ligand, 2-AG, were increased in the Δ9-THC-treated mice compared with controls.
Each of the upregulated neurotrophic factors and the endocannabinoid 2-AG have been shown by us and others to enhance brain repair after a variety of insults ranging from stroke, neurotoxicity, and trauma. BDNF acts on certain neurons, especially glutamatergic neurons of the central nervous system to support survival of existing neurons, and promoting growth and differentiation of new neurons and synapses.28–30 High levels of BDNF mRNA are found in pyramidal and granule cells of the HP and specific regions of the cerebral cortex. In many neural circuits, synaptically released BDNF is essential for structural and functional long-term potentiation, two prototypical cellular models of learning and memory formation.31 Hence it is possible that the enhanced recovery of hippocampal-dependent short-term working memory promoted by Δ9-THC was facilitated by amplification of BDNF expression in hippocampal–frontal cortical neural networks.
GDNF is found in both the peripheral and central nervous system (CNS) and is secreted by astrocytes, oligodendrocytes, Schwann cells, and motor neurons.32 GDNF produced by activated microglia/macrophages can promote repair of CNS injuries. After striatal mechanical injury and spinal cord injury, activated microglia and macrophages express GDNF, thereby inducing axonal sprouting and locomotor improvements.33
G-CSF has been identified as one of the many cytokines that modulate the secondary response to traumatic brain injury (TBI).14,15 Evidence for the role of G-CSF in the brain's self-repair mechanism is growing. “Stab” lesions of the HP made by insertion and removal of an electrode or needle triggered an acute local rise in G-CSF and other cytokines released at the sites of insertion in frontal cortex and hippocampus.16 Hippocampal neurogenesis was stimulated by the focal injury and was associated with upregulation of other neurotrophic factors as well as G-CSF.34 From these results and the reports of others on the beneficial effects of G-CSF in stroke and in neurodegenerative diseases, it is clear that G-CSF plays an important role in the brain's repair response to injury.35–40
After injury to the mouse brain, the eCS is involved in mediating brain repair.4 We recently reported that treatment of mice with agents that inhibit FAAH, or treatment with direct CB1 and CB2 receptor agonists for 3 days after CCI significantly increased levels of the major eCS ligand, 2-AG, in cortex and striatum compared with levels in vehicle-treated mice.8 The present report extends this observation to include treatment with the phytocannabinoid Δ9-THC, which was found to increase levels of 2-AG significantly in all 3 brain regions on day 7 and 14 after CCI. Others have reported conflicting results regarding elevation of AEA and 2-AG. After closed head injury (CHI) in mice, the level of endogenous 2-AG was significantly elevated in ipsilateral brain from 1 to 24 h with elevations as high as 10-fold.5 Administration of synthetic 2-AG to mice after CHI resulted in the reduction of brain edema, better clinical recovery, reduced infarct volume, and reduced hippocampal cell death compared with controls.6 However, concussive head trauma in rats resulted in modest increases of AEA (anandamide) levels in ipsilateral cortex, but no change in 2-AG levels.41 These results were similar to those of another researcher who reported a 1.5-fold increase of anandamide levels at 3 days post-TBI in ipsilateral mouse brain, and with no change in 2-AG levels.10 More research will be required to determine whether species differences, the model used to elicit neurotrauma, and/or other procedural considerations contribute to the differential elevation of these eCBs.42 In any case, increased brain levels of 2-AG, produced by inhibiting its breakdown, are reported to mediate recovery of working memory and fine motor function in a mouse model of TBI.11
The present report that exogenous administration of Δ9-THC significantly increased expression of 2-AG was unexpected in the context of classical drug receptor pharmacology. Typically, chronic activation of a neurotransmitter receptor with an exogenous agonist results in downregulation of the endogenous ligand to maintain synaptic homeostasis. However, there are reports consistent with our finding. Administration of a synthetic CB receptor agonist JWH-018 resulted in increased level of the endocannabinoids, AEA, and 2- (2-AG).7 The increase of endocannabinoid levels in response to JWH-018 could be inhibited by coadministration of AM251, a CB1 receptor antagonist. Further analysis revealed that this was the result of suppression of two hydrolases involved in endocannabinoid degradation (FAAH and MAGL).7 There is also evidence, although in an ex vivo placental model that Δ9-THC treatment inhibits FAAH and increases the biosynthetic enzyme for AEA resulting in increased levels of AEA and 2-AG.43
Conclusions
Treatment of mice with Δ9-THC (3 mg/kg i.p. for 3 days) following CCI resulted in time-dependent recovery of short-term spatial working memory in the spontaneous alternations Y-maze test and motor performance on the rotarod. Δ9-THC -treated animals exhibited significant increases in expression of the neurotrophic factors BDNF, GDNF, and G-CSF in cerebral cortex, striatum, and HP at 7 and 14 days after CCI. Levels of the endocannabinoid ligand 2-AG was also increased in all 3 brain regions. Recovery of both short-term working memory and locomotor function by Δ9-THC is associated with upregulation of the neurotrophic factors, BDNF, GDNF, G-CSF, and with increased levels of 2-AG. Further research is required to elucidate the role of the eCS in mediating the recovery from injury.
Abbreviations Used
- Δ9-THC
Δ9-tetrahydrocannabinol
- 2-AG
2-arachidonoyl-glycerol
- ABHD6
alpha, beta-hydrolase domain 6
- AEA
N-arachidonoylethanolamine
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- CB
cannabinoid
- CCI
controlled cortical impact
- CHI
closed head injury
- CNS
central nervous system
- eCBS
endocannabinoid system
- eCS
endogenous cannabinoid system
- ELISA
enzyme-linked immunosorbent assay
- FAAH
fatty acid amide hydrolase
- G-CSF
granulocyte colony-stimulating factor
- GDNF
glial-derived neurotrophic factor
- HP
hippocampus
- MAGL
monoacylglycerol lipase
- MDSC
myeloid-derived suppressor cells
- mPFC
medial prefrontal cortex
- TBI
traumatic brain injury
Authors' Contributions
S.S., X.K., and B.W. performed the experiments and collected the data. S.S. and J.S.-R. designed the experiments, analyzed the data, and wrote the article.
Disclaimer
The opinions presented in this study do not represent the U.S. government.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was funded by the Veteran Affairs Merit Review grant BX004037-01A1 to Shijie Song, MD.
Cite this article as: Song S, Kong X, Wang B, Sanchez-Ramos J (2022) Administration of Δ9-tetrahydrocannabinol following controlled cortical impact restores hippocampal-dependent working memory and locomotor function, Cannabis and Cannabinoid Research 7:4, 424–435, DOI: 10.1089/can.2021.0053.
References
- 1. Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu HC, Mackie K. Review of the endocannabinoid system. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shohami E, Cohen-Yeshurun A, Magid L, et al. . Endocannabinoids and traumatic brain injury. Br J Pharmacol. 2011;163:1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schurman LD, Lichtman AH. Endocannabinoids: a promising impact for traumatic brain injury. Front Pharmacol. 2017;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panikashvili D, Simeonidou C, Ben-Shabat S, et al. . An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. [DOI] [PubMed] [Google Scholar]
- 6. Panikashvili D, Shein NA, Mechoulam R, et al. . The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264. [DOI] [PubMed] [Google Scholar]
- 7. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9–29. [DOI] [PubMed] [Google Scholar]
- 8. Song S, Kong X, Borlongan C, et al. . Granulocyte colony-stimulating factor enhances brain repair following traumatic brain injury without requiring activation of cannabinoid receptors. Cannabis Cannabinoid Res. 2021;6:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, et al. . Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One. 2015;10:e0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tchantchou F, Tucker LB, Fu AH, et al. . The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology. 2014;85:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tchantchou F, Zhang Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J Neurotrauma. 2013;30:565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savinainen JR, Saario SM, Laitinen JT. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf). 2012;204:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40:3358–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song S, Kong X, Acosta S, et al. . Granulocyte colony-stimulating factor promotes behavioral recovery in a mouse model of traumatic brain injury. J Neurosci Res. 2016;94:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song S, Kong X, Acosta S, et al. . Granulocyte-colony stimulating factor promotes brain repair following traumatic brain injury by recruitment of microglia and increasing neurotrophic factor expression. Restor Neurol Neurosci. 2016;34:415–431. [DOI] [PubMed] [Google Scholar]
- 16. Song S, Song S, Cao C, et al. . Hippocampal neurogenesis and the brain repair response to brief stereotaxic insertion of a microneedle. Stem Cells Int. 2013;2013:205878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prieur EAK, Jadavji NM. Assessing spatial working memory using the spontaneous alternation Y-maze test in aged male mice. Bio-Protocol. 2019;9:e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology (Berl). 1988;94:491–495. [DOI] [PubMed] [Google Scholar]
- 19. Yu S, Kaneko Y, Bae E, et al. . Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–163. [DOI] [PubMed] [Google Scholar]
- 20. Torrens A, Vozella V, Huff H, et al. . Comparative pharmacokinetics of delta(9)-tetrahydrocannabinol in adolescent and adult male mice. J Pharmacol Exp Ther. 2020;374:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leszczynski M. How does hippocampus contribute to working memory processing? Front Hum Neurosci. 2011;5:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaminska B, Mota M, Pizzi M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim Biophys Acta. 2016;1862:339–351. [DOI] [PubMed] [Google Scholar]
- 23. Hartung T. Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol. 1998;5:221–225. [DOI] [PubMed] [Google Scholar]
- 24. Jin J, Maren S. Prefrontal-hippocampal interactions in memory and emotion. Front Syst Neurosci. 2015;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baddeley A. Working memory and language: an overview. J Commun Disord. 2003;36:189–208. [DOI] [PubMed] [Google Scholar]
- 26. Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res. 2011;225:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Acheson A, Conover JC, Fandl JP, et al. . A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. [DOI] [PubMed] [Google Scholar]
- 29. Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. [DOI] [PubMed] [Google Scholar]
- 30. Yamada A, Irie K, Deguchi-Tawarada M, et al. . Nectin-dependent localization of synaptic scaffolding molecule (S-SCAM) at the puncta adherentia junctions formed between the mossy fibre terminals and the dendrites of pyramidal cells in the CA3 area of the mouse hippocampus. Genes Cells. 2003;8:985–994. [DOI] [PubMed] [Google Scholar]
- 31. Sasi M, Vignoli B, Canossa M, et al. . Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017;469:593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cintron-Colon AF, Almeida-Alves G, Boynton AM, et al. . GDNF synthesis, signaling, and retrograde transport in motor neurons. Cell Tissue Res. 2020;382:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duarte Azevedo M, Sander S, Tenenbaum L. GDNF, a neuron-derived factor upregulated in glial cells during disease. J Clin Med. 2020;9:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song S, Kong X, Sava V, et al. . Transient micro-needle insertion into hippocampus triggers neurogenesis and decreases amyloid burden in a mouse model of Alzheimer's disease. Cell Transplant. 2016;25:1853–1861. [DOI] [PubMed] [Google Scholar]
- 35. Schneider A, Krüger C, Steigleder T, et al. . The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–1757. [DOI] [PubMed] [Google Scholar]
- 37. Schabitz WR, Schneider A. New targets for established proteins: exploring G-CSF for the treatment of stroke. Trends Pharmacol Sci. 2007;28:157–161. [DOI] [PubMed] [Google Scholar]
- 38. Schabitz WR, Krüger C, Pitzer C, et al. . A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J Cereb Blood Flow Metab. 2008;28:29–43. [DOI] [PubMed] [Google Scholar]
- 39. Song S, Sava V, Rowe A, et al. . Granulocyte-colony stimulating factor (G-CSF) enhances recovery in mouse model of Parkinson's disease. Neurosci Lett. 2011;487:153–157. [DOI] [PubMed] [Google Scholar]
- 40. Sanchez-Ramos J, Song S, Sava V, et al. . Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice. Neuroscience. 2009;163:55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hansen HS, Moesgaard B, Petersen G, et al. . Putative neuroprotective actions of N-acyl-ethanolamines. Pharmacol Ther. 2002;95:119–126. [DOI] [PubMed] [Google Scholar]
- 42. Gilgun-Sherki Y, Melamed E, Mechoulam R, et al. . The CB1 cannabinoid receptor agonist, HU-210, reduces levodopa-induced rotations in 6-hydroxydopamine-lesioned rats. Pharmacol Toxicol. 2003;93:66–70. [DOI] [PubMed] [Google Scholar]
- 43. Maia J, Midão L, Cunha SC, et al. . Effects of cannabis tetrahydrocannabinol on endocannabinoid homeostasis in human placenta. Arch Toxicol. 2019;93:649–658. [DOI] [PubMed] [Google Scholar]