Abstract
Background:
Pharmacological management of chronic neuropathic pain (CNP) still represents a major clinical challenge. Collective harnessing of both the scientific evidence base and clinical experience (of clinicians and patients) can play a key role in informing treatment pathways and contribute to the debate on specific treatments (e.g., cannabinoids). A group of expert clinicians (pain specialists and psychiatrists), scientists, and patient representatives convened to assess the relative benefit–safety balance of 12 pharmacological treatments, including orally administered cannabinoids/cannabis-based medicinal products, for the treatment of CNP in adults.
Methods:
A decision conference provided the process of creating a multicriteria decision analysis (MCDA) model, in which the group collectively scored the drugs on 17 effect criteria relevant to benefits and safety and then weighted the criteria for their clinical relevance.
Findings:
Cannabis-based medicinal products consisting of tetrahydrocannabinol/cannabidiol (THC/CBD), in a 1:1 ratio, achieved the highest overall score, 79 (out of 100), followed by CBD dominant at 75, then THC dominant at 72. Duloxetine and the gabapentinoids scored in the 60s, amitriptyline, tramadol, and ibuprofen in the 50s, methadone and oxycodone in the 40s, and morphine and fentanyl in the 30s. Sensitivity analyses showed that even if the pain reduction and quality-of-life scores for THC/CBD and THC are halved, their benefit–safety balances remain better than those of the noncannabinoid drugs.
Interpretation:
The benefit–safety profiles for cannabinoids were higher than for other commonly used medications for CNP largely because they contribute more to quality of life and have a more favorable side effect profile. The results also reflect the shortcomings of alternative pharmacological treatments with respect to safety and mitigation of neuropathic pain symptoms. Further high-quality clinical trials and systematic comprehensive capture of clinical experience with cannabinoids is warranted. These results demonstrate once again the complexity and multimodal mechanisms underlying the clinical experience and impact of chronic pain.
Keywords: neuropathic pain, analgesics, cannabis-based medical products, CBMP, multicriteria decision analysis, MCDA
Introduction
Cannabis-based medical products (CBMPs) are now approved in >20 countries and so accessible to hundreds of millions of patients.1,2 But in some countries, notably the United Kingdom, there is very limited prescribing.1,2 Reasons for this are varied and complex, including considerable medicolegal and bureaucratic hurdles,2,3 but they also reflect a concern by the medical profession that the randomized control trial (RCT) evidence base for medical cannabis is limited and, for many of its possible indications, inconclusive. This attitude is opposite to that of many patient testimonies (both in the United Kingdom and in other countries) where medical cannabis is seen as an important addition to their treatment (UPA 2018). Similarly, clinicians with considerable practical experience with use of cannabis, including for pain management, also see this as an important and major addition to their armamentarium.4,5 The Centre for Medical Cannabis (CMC) estimates that in the United Kingdom more than one million patients are using cannabis,6 and almost all of them are obtaining it illegally, which presents them with significant legal and product quality risks.2 Moreover, these patients are less likely to involve their health care specialist in the process and, therefore, manage their own treatment unsupervised.
Surveys highlight that pain is one of the conditions patients most commonly treated with medical cannabis6,7 and a review by the National Academies of Sciences, Engineering, and Medicine (NASEM) in 2017 found the evidence base for chronic pain to be “substantial.”8 However, conclusions of recent meta-analyses and systematic reviews on the use of cannabinoids, cannabis and cannabis-based medicines to treat chronic neuropathic pain (CNP) have ranged from weakly positive to inconclusive or negative.9–15 These differing conclusions may be the result of including different trial designs, different standards to evaluate the quality of evidence, and different weighting of the outcomes of efficacy, tolerability, and safety.16,17 Thus, systematic reviews examining the same studies often arrived at different conclusions and recommendations.18 The scientific literature examining the efficacy of cannabinoids, cannabis, and cannabis-based medicines for CNP is, therefore, still developing. An expert Task Force of the European Pain Federation recently published a position article that concluded that the quantity and quality of evidence are such that cannabis-based medicines may be reasonably considered for CNP. For all other chronic pain conditions (cancer, non-neuropathic noncancer pain), the use of cannabis-based medicines should be regarded as an individual therapeutic trial.19 Recent RCTs examining the efficacy of cannabinoids, cannabis, and cannabis-based medicines for CNP are shown in Appendix Table A1. This study focuses exclusively on this area to narrow down the complexity of chronic pain.
Available data to date suggest that the use of cannabinoids for chronic pain is relatively safe, with little evidence for the increase of risk for experiencing serious adverse events, although nonserious adverse events may be common in the short-term period after use.20,21 Notably, despite the rapidly increasing multitude of patients, there has never been an overdose fatality directly attributed to cannabis use reported in medical literature. As many patients with chronic pain often suffer from multiple comorbidities and physical disability, and considering the numerous safety concerns of current pain pharmacotherapy,22–24 careful consideration of the safety profile is of crucial importance and improved harms assessment and reporting are needed in cannabinoid pain trials.25
This study was designed to explore the evidence base for the clinical utility of orally administered CBMPs (including cannabis extracts and cannabis-based medicines such as nabiximols [Sativex® is approved in 30 countries but not marketed in each] dronabinol, and nabilone) for management of CNP in adults by creating a multicriteria decision analysis (MCDA) model that compares these cannabinoid formulations with other drugs. The results of this modeling can provide prescribers and others with an updated viewpoint incorporating the current state of scientific knowledge as well as the cumulative clinical experience regarding the use of orally administered CBMPs for CNP in adults.
MCDA models about drugs often compare a single drug, or one drug at different doses, with a placebo as an approach for determining the extent to which benefits exceed risks.26,27 A recent trend is to compare one drug(s) to other drugs for the same medical condition.28,29 The rationale for this is that drugs for a given medical condition differ in their benefit–safety profiles, so making those profiles explicit and quantitative, and balancing them using a collective expert decision process coupled with sensitivity analyses, can reveal new insights and provide information for guiding prescribers, policy makers and patients to complement and augment that available from RCT data. The MCDA process is particularly pertinent to areas such as CBMPs for CNP, where, due to the psychoactivity of cannabis, it is challenging to achieve true blinding in RCTs, and where the real-world use of CBMPs has continued to advance rapidly and controversially, despite a limited and relatively low-quality RCT evidence base. The issue of blinding has been specifically addressed with nabiximols with positive assessment of its validity due to flavoring and low dosing avoiding overt psychoactive adverse events.30
Methods
Experts ranging from pain clinicians with and without prescribing expertise in CBMPs from the United Kingdom, Denmark, Israel, and Germany, psychiatrists, a neurologist, researchers with expertise in cannabinoid pharmacology, decision analysis, and patient representatives with personal experience of CBMPs (representing the United Patients Alliance) were selected and invited to take part in the MCDA modeling process. The meeting was facilitated by L.D.P. with support from D.J.N., but neither of them participated in the scoring. Participants did not benefit financially from participating in the MCDA.
A subgroup met on December 9, 2019 to begin the process of developing the MCDA model, which would enable participants in the subsequent (January 2020) decision conference to complete their work in 1 day. The subgroup suggested that the medical condition should be chronic pain and they developed a list of drugs, including CBMPs, used to treat chronic pain. They also identified favorable and unfavorable effects of all treatments and suggested definitions of all these effects. This preliminary overview of an MCDA model's structure was sent to all participants before the decision conference.
A decision conference is a facilitated workshop31 designed to resolve one or more issues of concern by building a quantitative model that incorporates the differing perspectives of the participants along with data and judgments about the relevance of the data to those issues of concern.32 At the decision conference, participants agreed that the potential for orally administered cannabis-based medicinal products to treat CNP lasting >3 months in adults, rather than the broader “chronic pain” definition, was a key issue largely because there is more published evidence for cannabis-based medicinal products alone or in comparison with other approved medicines for CNP than that exists for other pain syndromes.
The process of extending and developing the model at the decision conference followed the steps developed during the 2009–2011 Benefit–Risk Project sponsored by the European Medicines Agency (EMA)33 and by the 2009–2013 IMI-PROTECT administered by the EMA.34 The steps are fully described in Chapter 5 of Benefit–Risk Assessment in Pharmaceutical Research and Development,35 and follow the subheadings hereunder.
Effects and their definitions
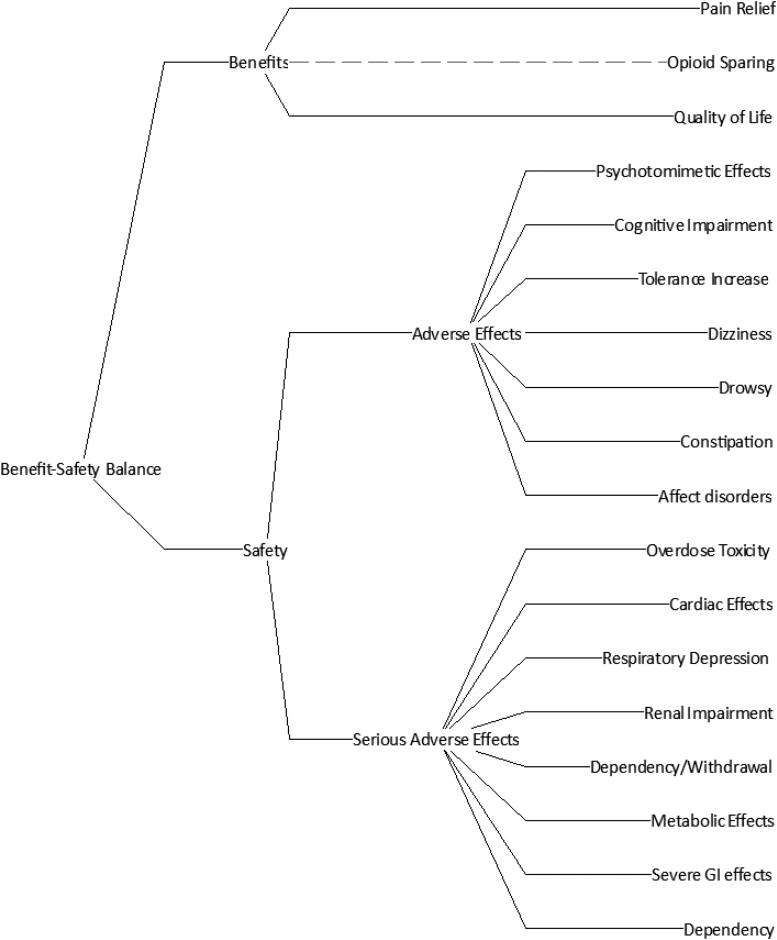
At the decision conference, participants reviewed the subgroup's benefits and safety effects of medical treatments for CNP. They agreed that pain relief and quality of life were the two benefits, and they added seven more safety effects. These are shown in the Effects Tree of Figure 1, which was created using Hiview3 software,36 with agreed definitions in Table 1.
FIG. 1.
The Effects Tree for assessing the relative benefit–safety of neuropathic pain pharmacotherapy.
Table 1.
Definitions of the Favorable and Unfavorable Effects
| Effect | Description | |
|---|---|---|
| Favorable effects | Pain relief | Proportion of patients reporting >30% reduction in neuropathic pain relief compared with baseline |
| Opioid sparing | Meaningful reduction in milligrams of 24-h morphine consumption | |
| Quality of life | Improvement in quality-of-life score | |
| Unfavorable effects Adverse events |
Psychotomimetic | Proportion of patients experiencing psychotomimetic effects |
| Cognitive impairment | Proportion of patients experiencing cognitive impairment | |
| Tolerance increase | Proportion of patients requiring more drug as tolerance increases | |
| Dizziness | Proportion of patients experiencing dizziness | |
| Drowsy | Proportion of patients experiencing drowsiness | |
| Constipation | Proportion of patients experiencing constipation | |
| Affect disorders | Proportion of patients experiencing affect disorders. Includes anxiety, depression, emotional blunting, decreased motivation, and disconnect | |
| Unfavorable effects Serious adverse events |
Overdose toxicity | The potential for toxic effects from accidental of deliberate overdosing |
| Cardiac effects | Proportion of patients experiencing cardiac effect | |
| Respiratory depression | Proportion of patients experiencing respiratory depression | |
| Renal impairment | Proportion of patients experiencing renal impairments | |
| Withdrawal | Proportion of patients experiencing drug withdrawal per se | |
| Metabolic effects | Proportion of patients experiencing metabolic effects. Includes hypoglycemic effects, diabetics, weight changes, libido, and osteoporosis. | |
| Gastrointestinal | Proportion of patients experiencing gastrointestinal effects. Includes bleeds and ulcers. | |
| Dependency | The likelihood of increasing the dosage |
Treatment options
The group considered 12 pharmacotherapies that are widely used in chronic pain syndromes. Three different types of CBMPs were distinguished: 1:1 ratio Δ9—tetrahydrocannabinol (THC): cannabidiol (CBD) products (e.g., Sativex or 1:1 THC:CBD extracts), CBD-dominant products (either purified CBD or a CBD-rich extract with very low or no THC) and THC-dominant products (either purified THC or a THC-rich extract with i.e., with very low or no CBD), administered orally or by sublingual routes. We chose to consider orally administered pharmacotherapies only, and not to include inhalation therapies (although the most commonly used) because of expert recommendations against smoking19 and health concerns relating to other methods of inhalation.
| 1 | THC/CBD 1:1 | 7 | Tramadol |
| 2 | CBD dominant | 8 | Ibuprofen |
| 3 | THC dominant | 9 | Methadone |
| 4 | Duloxetine | 10 | Oxycodone |
| 5 | Gabapentinoids | 11 | Morphine |
| 6 | Amitriptyline | 12 | Fentanyl |
Scoring the drugs on the criteria
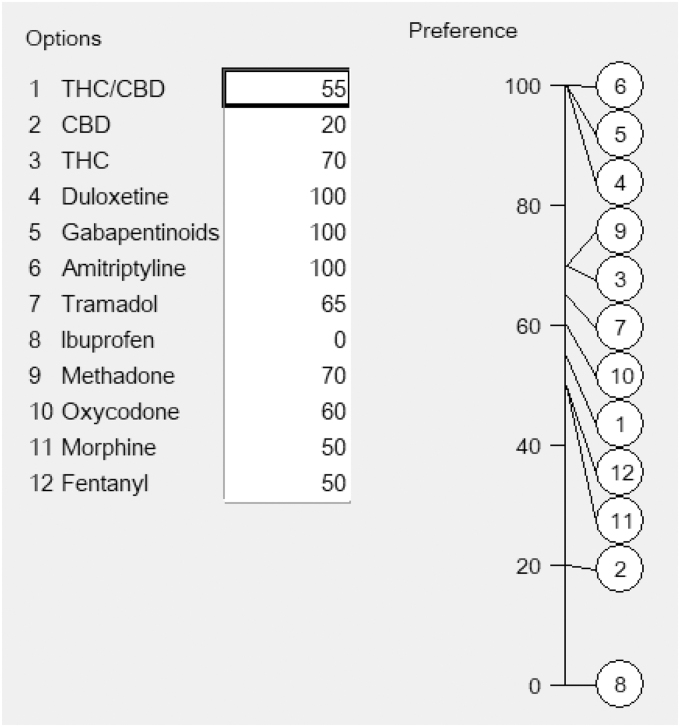
Participants evaluated the pharmacotherapies relative to each other on 0–100 scales, one scale for each effect criterion, similar to the one for pain relief shown in Figure 2. First, the group agreed which options were most preferred for their clinical value, and these were assigned an arbitrary score of 100. Second, they agreed the least preferred and assigned it a score of zero. Third, the group discussed, debated, and agreed scores between 0 and 100 for the remaining options. All numbers represent the judged strength of preference for the pharmacotherapies; higher numbers representing more effectiveness for the favorable effects, and better safety for the unfavorable effects.
FIG. 2.
Scores agreed by participants for pain relief.
The properties of these 0–100 scales are similar to those of a Celsius scale, whose 0 and 100 points are based on the freezing and boiling points of water at sea level. Zero does not represent no temperature and 100 is not a maximum temperature; the zero for ibuprofen simply means it has the least effect on pain control, whereas the three pharmacotherapies at 100 are tied for having the best effect.
In scoring the options, participants were given a few moments to think of an appropriate number, compared with the zero and 100, then to say what they thought, followed by group discussion. This “think, reveal, discuss” process was intended to prevent participants from anchoring on the first person to suggest a number in open discussion,37 an approach that minimizes bias in group assessments.
Consistency checks initiated by the facilitator were intended to ensure the internal consistency in the preference values assessed by the group. For example, the facilitator asked, “Morphine has been scored at 50, duloxetine at 100 and ibuprofen at 0 for pain relief. Is duloxetine really as much better than morphine as ibuprofen is worse?” This sort of question helps assessors to provide realistic numbers. It also avoids interpreting ratios of numbers. Duloxetine is not twice better than morphine for pain relief because ibuprofen can provide some pain relief to some patients, so its zero is merely defining a point on a relative scale, similar to zero degrees in Celsius temperature (Table 2).
Table 2.
Input Preference Scores for Each of the Benefit and Safety Effects
| Weight | THC/CBD | CBD | THC | Duloxetine | Gabapentinoids | Amitriptyline | Tramadol | Ibuprofen | Methadone | Oxycodone | Morphine | Fentanyl | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain relief | 14.5 | 55 | 20 | 70 | 100 | 100 | 100 | 65 | 0 | 70 | 60 | 50 | 50 |
| Opioid sparing | 0 | 80 | 30 | 100 | 30 | 70 | 40 | 20 | 0 | 50 | 0 | 0 | 0 |
| Quality of life | 20.7 | 100 | 40 | 100 | 40 | 50 | 40 | 30 | 0 | 40 | 30 | 20 | 20 |
| Psychotomimetic | 5.1 | 50 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Cognitive impairment | 5.7 | 70 | 100 | 55 | 20 | 0 | 0 | 50 | 100 | 50 | 20 | 20 | 20 |
| Tolerance | 2.5 | 65 | 100 | 50 | 90 | 45 | 80 | 40 | 100 | 70 | 20 | 20 | 0 |
| Dizziness | 4.9 | 40 | 90 | 20 | 30 | 0 | 0 | 20 | 100 | 30 | 20 | 20 | 20 |
| Drowsy | 3.4 | 50 | 80 | 35 | 40 | 10 | 0 | 60 | 100 | 50 | 20 | 20 | 20 |
| Constipation | 3.4 | 100 | 100 | 100 | 90 | 90 | 75 | 40 | 100 | 10 | 10 | 0 | 20 |
| Affect disorders | 4.6 | 85 | 100 | 70 | 40 | 50 | 40 | 25 | 100 | 0 | 0 | 0 | 0 |
| Overdose toxicity | 8.4 | 100 | 100 | 90 | 60 | 70 | 20 | 40 | 60 | 10 | 20 | 20 | 0 |
| Cardiac effects | 5 | 70 | 100 | 40 | 40 | 90 | 0 | 70 | 80 | 40 | 80 | 80 | 80 |
| Respiratory depress | 6.3 | 100 | 100 | 100 | 90 | 60 | 80 | 60 | 100 | 30 | 20 | 20 | 0 |
| Renal impairment | 4.4 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 100 |
| Withdrawal | 1.5 | 50 | 100 | 20 | 10 | 0 | 30 | 40 | 80 | 0 | 10 | 10 | 0 |
| Metabolic effects | 2.5 | 75 | 90 | 50 | 75 | 10 | 50 | 75 | 100 | 0 | 0 | 0 | 0 |
| Severe GI effects | 4.4 | 100 | 100 | 100 | 100 | 100 | 100 | 90 | 0 | 100 | 100 | 100 | 100 |
| Dependency | 2.5 | 75 | 100 | 50 | 50 | 0 | 50 | 0 | 100 | 0 | 0 | 0 | 0 |
CBD, cannabidiol; GI, gastrointestinal; THC, tetrahydrocannabinol.
Weighting the effect criteria
The scoring process resulted in 17 0–100 scales, one for each effect criterion, but it is evident that not all scales represent the same ranges of added clinical value, so participants in the decision conference assigned weights to each. These are scale constants that equate the units of preference value across the scales. This weighting process is analogous to recognizing that both Fahrenheit and Celsius scales contain 0–100 portions, but the swing in temperature on the Celsius scale is a greater range of temperature: it takes 9 Fahrenheit degrees to equal 5 Celsius degrees, a ratio of 9:5.
In MCDA, the process is called “swing weighting” because it compares the swing from least to most preferred positions on one scale compared with another.38 It is not just the importance of the effect, rather it represents both the objective difference between least and most preferred positions on a scale, and how much the assessors care about that difference, which usually means judging the clinical relevance of the difference. The weighting process went through three stages: (1) relative weighting of the criteria for the benefit effects, (2) relative weighting comparing swings for the adverse safety effects, then for the serious adverse effects, and (3) relative weighting of the highest-weighted benefit effect against the highest-weighted safety effect.
The weighting process in MCDA preserves the ratios of all weights even though they are eventually normalized to ensure they sum to 1.00 (displayed in this report as 100) across all the criteria before multiplying by the scores to give the final weighted preference values. The group judged the clinical difference between the best and worst drug for quality of life to be the largest difference for this set of drugs, with pain relief the second largest best–worst difference.
Results
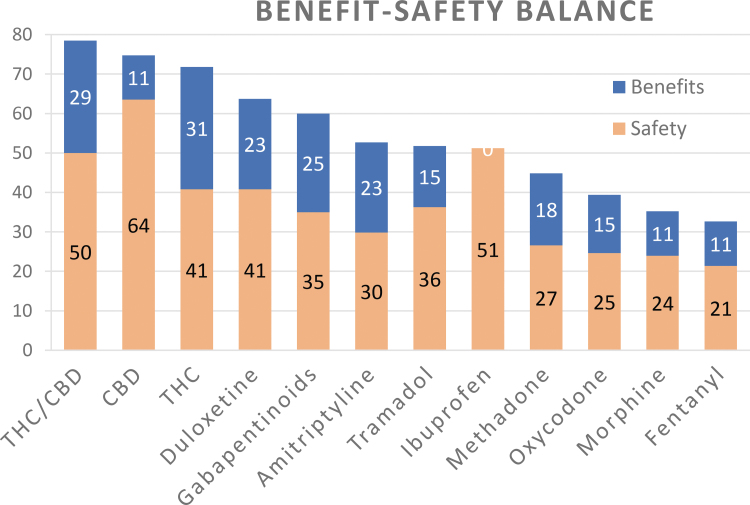
Multiplying preference values by the corresponding swing weights and summing those products for each drug gives the overall weighted preference values shown in Table 3. The figures in the white rows are now referred to as benefits and safety because they represent the weighted input evaluations separately for the two benefits and the 15 safety effects. The bottom total row shows the weighted average of the two weighted sums in each column, providing a single figure that represents each drug's benefit–risk balance. For example, the equation for THC/CBD is simply (81×0.352) + (78×0.648)=79. Figure 3 shows bar graphs of the separate weighted benefits and safety.
Table 3.
Weighted Benefits and Safety, and Their Weighted Totals
| Effect | Weight | THC/CBD | CBD | THC | Duloxetine | Gabapentinoids | Amitriptyline | Tramadol | Ibuprofen | Methadone | Oxycodone | Morphine | Fentanyl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benefits | 35.2 | 81 | 32 | 88 | 65 | 71 | 65 | 44 | 0 | 52 | 42 | 32 | 32 |
| Safety | 64.8 | 78 | 98 | 63 | 63 | 54 | 46 | 56 | 79 | 41 | 38 | 37 | 33 |
| Total | 100 | 79 | 75 | 72 | 64 | 60 | 53 | 52 | 51 | 45 | 40 | 36 | 33 |
FIG. 3.
The overall weighted preference values for the neuropathic pain pharmacotherapies. More blue means more benefit, more red indicates more safety.
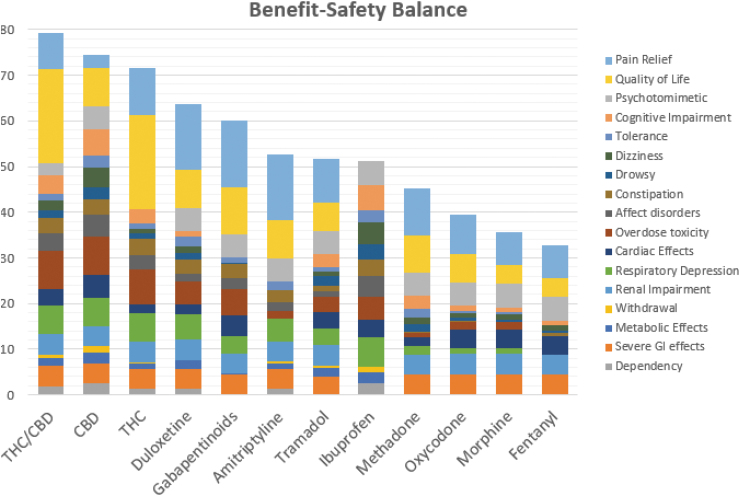
The breakdown of the weighted benefit and risks is shown in Figure 4, with a separate color associated with each of the 17 effect criteria. The figure is based on the weighted preference values shown in Table 4. This final table, called an Effects Table, provides the information for comparing the effects for any of the drugs as the weighting process has provided a common unit of preference value. Recall that only differences between the weighted preference values and/or their totals can be compared meaningfully, not their ratios.
FIG. 4.
Contributions to the totals by each of the 17 effects. The top blue (pain relief) and yellow (quality of life) sections of each bar show the magnitude of benefits; the rest show safety.
Table 4.
Effects Table of Weighted Preference Values
| Effects | Weight | THC/CBD | CBD | THC | Duloxetine | Gabapentinoids | Amitriptyline | Tramadol | Ibuprofen | Methadone | Oxycodone | Morphine | Fentanyl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benefits | |||||||||||||
| Pain relief | 14.5 | 8.0 | 2.9 | 10.2 | 14.5 | 14.5 | 14.5 | 9.4 | 0.0 | 10.2 | 8.7 | 7.3 | 7.3 |
| Quality of life | 20.7 | 20.7 | 8.3 | 20.7 | 8.3 | 10.4 | 8.3 | 6.2 | 0.0 | 8.3 | 6.2 | 4.1 | 4.1 |
| Safety—AEs | |||||||||||||
| Psychotomimetic | 5.1 | 2.6 | 5.1 | 0 | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 |
| Cognitive Impairment | 5.7 | 4.0 | 5.7 | 3.1 | 1.1 | 0 | 0 | 2.9 | 5.7 | 2.9 | 1.1 | 1.1 | 1.1 |
| Tolerance | 2.5 | 1.6 | 2.5 | 1.3 | 2.3 | 1.1 | 2.0 | 1.0 | 2.5 | 1.8 | 0.5 | 0.5 | 0 |
| Dizziness | 4.9 | 2.0 | 4.4 | 1.0 | 1.5 | 0 | 0 | 1.0 | 4.9 | 1.5 | 1.0 | 1.0 | 1.0 |
| Drowsy | 3.4 | 1.7 | 2.7 | 1.2 | 1.4 | 0.3 | 0 | 2.0 | 3.4 | 1.7 | 0.7 | 0.7 | 0.7 |
| Constipation | 3.4 | 3.4 | 3.4 | 3.4 | 3.1 | 3.1 | 2.6 | 1.4 | 3.4 | 0.3 | 0.3 | 0 | 0.7 |
| Affect disorders | 4.6 | 3.9 | 4.6 | 3.2 | 1.8 | 2.3 | 1.8 | 1.2 | 4.6 | 0 | 0 | 0 | 0 |
| Safety—SAEs | |||||||||||||
| Overdose toxicity | 8.4 | 8.4 | 8.4 | 7.6 | 5.0 | 5.9 | 1.7 | 3.4 | 5.0 | 0.8 | 1.7 | 1.7 | 0 |
| Cardiac effects | 5.0 | 3.5 | 5.0 | 2.0 | 2.0 | 4.5 | 0 | 3.5 | 4.0 | 2.0 | 4.0 | 4.0 | 4.0 |
| Respiratory depress | 6.3 | 6.3 | 6.3 | 6.3 | 5.7 | 3.8 | 5.0 | 3.8 | 6.3 | 1.9 | 1.3 | 1.3 | 0 |
| Renal impairment | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 0 | 4.4 | 4.4 | 4.4 | 4.4 |
| Withdrawal | 1.5 | 0.8 | 1.5 | 0.3 | 0.2 | 0 | 0.5 | 0.6 | 1.2 | 0 | 0.2 | 0.2 | 0 |
| Metabolic effects | 2.5 | 1.9 | 2.3 | 1.3 | 1.9 | 0.3 | 1.3 | 1.9 | 2.5 | 0 | 0 | 0 | 0 |
| Severe GI effects | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.4 | 4.0 | 0 | 4.4 | 4.4 | 4.4 | 4.4 |
| Dependency | 2.5 | 1.9 | 2.5 | 1.3 | 1.3 | 0 | 1.3 | 0 | 2.5 | 0 | 0 | 0 | 0 |
Boldface values identify best benefit and worst safety.
Trade-offs between benefits and safety
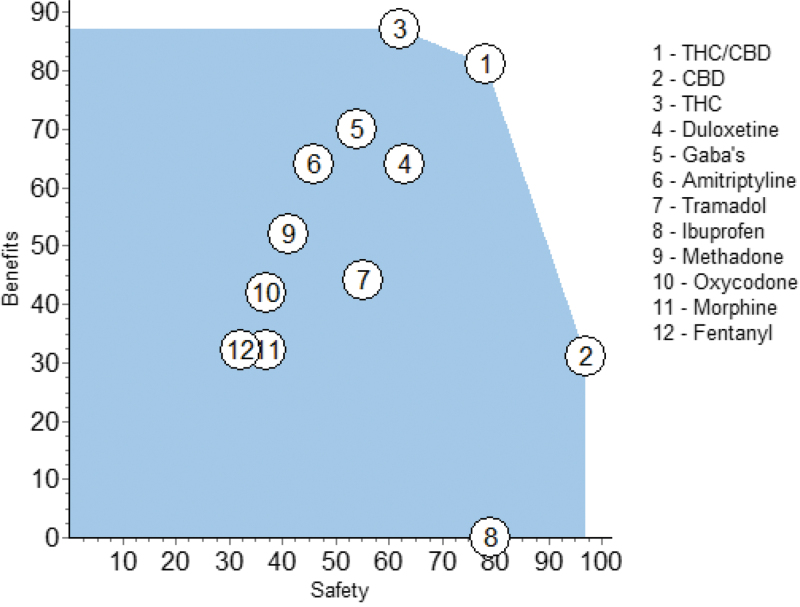
At this point it would be useful to see the separate weighted preference values for the benefits and for the safety effects without regard for the trade-off weight between those two nodes. This is shown in Figure 5. The circles are located at the values of benefits and safety from the corresponding rows of Table 3.
FIG. 5.
Benefit preference values versus the safety preference values shown in Table 3.
The ideal position on this graph would be the upper right, a beneficial safe pharmacotherapy, scoring 100 and 100, but there is no drug that is best in overall benefits and safety. THC/CBD is the best compromise between the higher benefits but less safety of THC, on the one hand, or the safer but lesser benefits of CBD, on the other hand.
Those three drugs define what is known as the “efficient frontier;” there is no better drug outside the blue shading. However, close examination reveals a unique feature: THC/CBD and THC are better in benefits and safety than all the noncannabinoid pharmacotherapies. The only exception is ibuprofen, whose safety score is 79 compared with 78 for THC/CBD. For all the other noncannabinoids, at least one of the three CBMPs is better. If a regression line were fit through the noncannabinoids, excepting ibuprofen, it would be tilted from lower left to upper right, which shows a trend for delivering more benefit at greater safety, an interesting feature.
Sensitivity analyses
Some participants were surprised to see the CBMPs dominating the other drugs and requested that sensitivity analyses be performed to address any potential bias (or perceived bias) in the group's scores and weights. These sensitivity analyses involved making changes to the inputs, both during and after the decision conference, to demonstrate the extent to which results would change with different scores or weights.
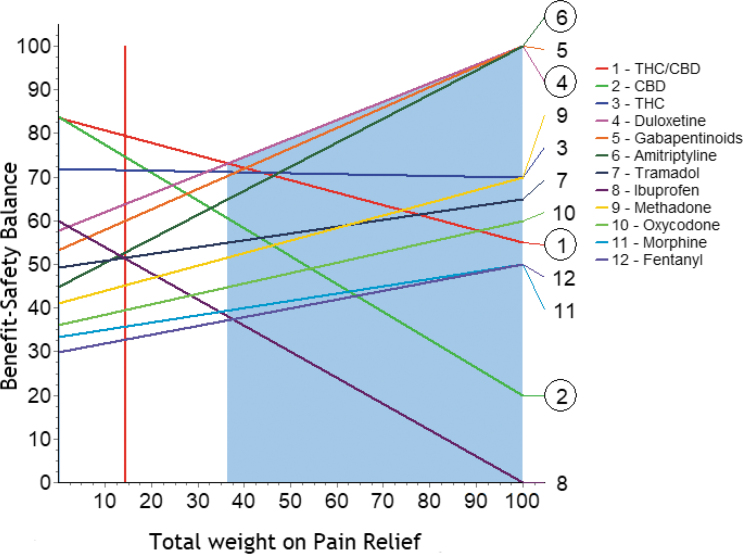
Changes in the weights were explored for all the effect criteria and the nodes in the Effect Tree. Nearly all showed that the dominance of the CBMPs remained, although not necessarily as strongly, over plausible ranges for weights. The only effect that breaks the dominance is shown in Figure 6.
FIG. 6.
Sensitivity analysis for pain relief.
The vertical red line is located at the current weight for pain relief, 14.5 (from Fig. 3). All the other lines represent their total score as the weight on pain relief is changed. The intersection of the vertical red line with each of the other lines occurs at their total values shown in Table 3. As the weight is increased, THC/CBD declines in preference, with duloxetine emerging as better when the weight exceeds 35, followed closely by the gabapentinoids and amitriptyline, then opioids.
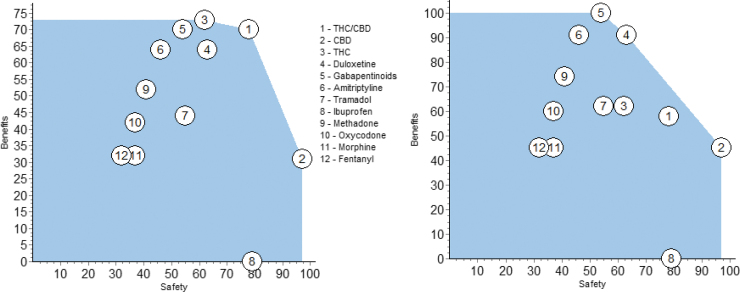
After the decision conference, one participant suggested we had scored THC/CBD and THC too high on pain relief and quality of life and suggested those two scores be decreased by half. The result is shown in the benefit versus safety plot of Figure 7.
FIG. 7.
The results of sensitivity analyses on the input preference scores for THC/CBD and THC. Halving the Pain Control scores gives the left plot. An additional halving of the quality-of-life scores is shown in the right plot. CBD, cannabidiol; THC, tetrahydrocannabinol.
Halving just the pain control scores shows that THC/CBD and THC still dominate the pharmacotherapies, but less so than shown in Figure 5. In addition, if the quality-of-life scores for the two cannabinoids are halved, the right plot shows that duloxetine and the gabapentinoids move up to the efficient frontier, with amitriptyline slightly less good.
Differences
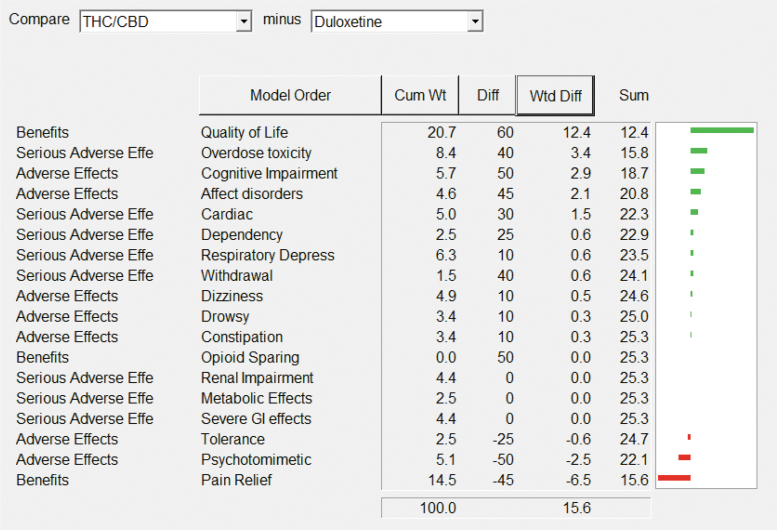
The aforementioned plots made no assumption about the trade-off between benefits and safety. Taking account of that trade-off makes it possible to compare the performance between pharmacotherapies because the scores are based on a common unit of preference value. The aforementioned analyses show that decisions about the drug will be different depending on whether a patient or clinician is more concerned about pain relief or about quality of life, as seen most clearly in Figure 8.
FIG. 8.
THC/CBD is better for quality of life, but duloxetine is better for pain relief. This pattern is similar for gabapentinoids and amitriptyline as comparators with THC/CBD.
The weighted difference (Wtd Diff) column shows the result of multiplying the cumulative weight (Cum Wt) for each effect by the difference in preference values (Diff) between the two drugs shown in the top white fields. The effects have been put in order of the Wtd Diffs of the pair of pharmacotherapies, with the Wtd Diff sum equal to the difference in their overall preference values (negative signs favor the right-listed pharmacotherapies over the left one). The green horizontal bar graphs show the relative advantages of THC:CBD, and the red bars are the advantages of duloxetine. The sums of those positive and negative weighted scores equals the 15-point difference in their total scores shown in Table 3.
Discussion
This is the first time that CBMPs have been subjected to an MCDA and compared with each other and against other neuropathic pain medications, based on benefit–safety balances. In this study, we compared three cannabinoid options and nine noncannabinoid medications for their beneficial effects on pain relief and quality of life, against seven adverse effects, and eight serious adverse effects. The results showed the best benefit–safety balance, 79, for THC/CBD (1:1), and least good balance for fentanyl, 33. The 46-point difference in performance is substantial, with about two-thirds of the difference contributed by better quality of life and lack of overdose toxicity and respiratory depression.
The dominance analysis showed that two CBMPs options, THC/CBD and THC, are more beneficial and safer than any of the alternatives. However, that generalization is only valid at the level of the combined benefits and combined safety effects. The Effects Table at Figure 4 gives the weighted preference values for all 204 combinations of the 12 pharmacotherapies and 17 effects. The table shows duloxetine, gabapentinoids, and amitriptyline as best for pain relief, with THC/CBD and THC as best for quality of life. The Effects Table also highlights the worst side effect for each drug. Only THC/CBD, CBD, and duloxetine avoid worst scores for side effects, whereas THC has only one worst score, for psychotomimetic effects.
Sensitivity analyses—conducted jointly during the MCDA process—helped to resolve uncertainty about the data, such as it is, and disagreements about weights. Fortunately, MCDA models are fairly robust to imprecision in the inputs,39 which was borne out for this MCDA on CBMPs The benefit versus safety graphs showed that THC/CBD remained the most preferred pharmacotherapy over wide ranges of weights on the safety node and the individual effects. Since this emerged from a multitude of questions and comparisons, rather than straightforward simple scoring, the final dominance at the benefit–safety level of the cannabinoids over all the other medicines was initially surprising to the group. However, this became reasonable and accepted by the group in light of the recognition that CBMPs scores were driven by their beneficial effects on quality of life, combined with their superior side effects profile. The 15-point superiority of THC/CBD over duloxetine, for example, was due to better quality of life, less overdose toxicity, cognitive impairment, and less disturbance on affect, which provided a net benefit for the CBMPs over duloxetine's better pain relief and psychotomimetic effects. This profile was similar to the difference between THC/CBD and gabapentinoids and amitriptyline.
Many pain patients seek medical attention precisely because their pain interferes with some or all aspects of their quality of life.40 Simple pain scores, although providing important information, do not capture the patient's total pain experience, which includes the effect on quality of life.41 In our study, the improvement in quality of life was considered a major therapeutic target by the two patient representatives a view supported by the expert clinicians within the group and by the research of Almeida et al.42
This complex multimodal analgesia exerted by THC is compatible with the ubiquitous distribution of CB1 receptors in the CNS and the fact that cannabinoids act as complex neuromodulators. It has also been shown that the analgesic effects of THC may be more attributable to its effects on higher cognitive emotional brain mechanisms than its effects on somatosensory processing (antinociception).43 Clinically, this has been scarcely explored. In one of the two medical cannabis studies including quality of life, Toth et al.44 highlight that flexible-dose nabilone (1–4 mg/day) was effective in relieving neuropathic pain symptoms, improving disturbed sleep, and overall quality of life. In another small study of 23 participants, Ware et al.45 found no statistically significant effects on quality of life of smoking cannabis with THC potencies of 0%, 2.5%, and 6%. However, at 9.4% sleep improved. The absence of quality research on quality-of-life studies is disappointing and highlights the need to collect better evidence.
Recognizing the relative paucity of high-quality published data on the three cannabinoid pharmacotherapies, the group conducted a sensitivity analysis whereby scores on pain relief for THC/CBD and THC were halved; but these two cannabinoids still dominated the alternatives. In contrast, when the input preference scores on quality of life were also halved, then duloxetine, the gabapentinoids, and amitriptyline clustered together near or on the efficient curve, leaving the CBMPs well inside.
The Effects Table provides the collective views of the experts, clinicians, and patients who participated in the decision conference, so could be of use to a prescriber wishing to make a better-informed decision for an individual patient. As a general example, if a patient and their clinician are more concerned about quality of life than pain relief, then Table 4 shows that THC/CBD or THC could be the best pharmacotherapy. For patients who are more concerned about pain relief, then duloxetine might be the best choice, as it has a better safety profile than the gabapentinoids and amitriptyline. The Effects Table makes clear that there is a trade-off to be considered between pain relief and quality of life measures, and that side effect profiles need to be considered. The Effects Table makes all this explicit in a form made possible by MCDA modeling. The data supplied regarding subscores may, therefore, further serve to consider specific desired or undesired effects in patients with specific symptom constellations and/or medical comorbidities.
A main limitation of this study is in the shortage of published data for some of the pharmacotherapies, particularly for the CBMPs (and CBD alone in particular), requiring heavy reliance on participants' judgments about scores. That paucity of data was not only anticipated but was actually the rationale for holding this MCDA meeting and benefitting from the real-world views of an expert panel. Accordingly, participants were carefully chosen for their medical and scientific expertise about neuropathic pain and its treatment, clinical experience in treating patients, knowledge about cannabinoids and CBMPs, and incorporating representatives of patients with CNP who have direct experience themselves with the pharmacotherapies reviewed and were also acquainted with the experiences of other patients using them.
At the end of the decision conference, participants were asked for their views of the MCDA/decision conferencing approach. Most considered the results were a very good way of reaching a joint result about an issue for which there is little data. Several participants thought the group might be biased in favor of the CBMPs. Some participants also thought the group might be biased against opioids although they are widely used (although not usually guideline recommended) in treatment of CNP. It was felt that this may have been particularly influenced by the patient representatives' opinions expressing negative views. Bias is indeed a concern in any study relying on responses from panel members. Fortunately, minimizing or eliminating bias is built into the decision conferencing process by careful selection of participants with diverse views on the key issues and the application by the facilitator of bias-minimizing techniques in eliciting participants' judgments, as had been explained elsewhere.46,47 We would welcome studies aiming to replicate this research with different groups of experts in different countries. Finally, although we fully acknowledge that this MCDA approach is not a substitute for high-quality clinical trials, it may serve to responsibly share valuable clinical experience, and provide much-needed guidance to prescribers and patients while awaiting publications of better evidence in the field.
Conclusions
What is known about this topic
The value of CBMPs for pain management remains controversial. Some patients and clinicians prefer it to other medications but in the United Kingdom most doctors are reluctant to prescribe because high-quality evidence-based recommendations are lacking, and clinical experience, education, and support are limited. In the absence of persuasive evidence, and in the face of growing clinical use, it is important to obtain the best specialist estimates of the value of the available cannabis-based medicinal products. This is ideally performed by applying MCDA using evidence from RCTs and judgments about the clinical relevance of the evidence to determine the benefit–safety balance of drugs.48
What this study has contributed
Our study, the first of its kind, revealed clinically relevant differences in efficacy and adverse effects ratings for a range of different pharmacotherapies used for neuropathic pain. Overall CBMPs of THC/CBD, in a 1:1 ratio, achieved the highest overall score, followed by CBD dominant at 75, then THC dominant at 72. When only benefit measures are considered and not safety measures, the antidepressants (duloxetine and amitriptyline) and the gabapentinoids were rated best for pain control, whereas the THC-containing products scored best for improving quality of life. When safety benefits alone were considered CBD/ibuprofen and THC/CBD came out best.
The current disparity between prescribers' and patients' beliefs in CBMPs medical cannabis may reflect different weightings being given to the relief of pain compared with quality of life. The impact of CBMPs on improving patients' quality of life is increasingly recognized.49,50 As it emerges that cannabinoids are relatively safe options, certainly compared with other substances used for CNP management, they should be included in the armamentarium of clinical specialists, in line with the recent position article from the European Pain Federation Task Force on the topic.19 The decision regarding specific patients should take into account existing literature and professional guidelines and, in the face of partial evidence, collective clinical experience. This option should be patient tailored, that is, explored in detail by prescribers and patients together when pharmacological treatment of neuropathic pain is being considered.
Acknowledgments
We thank all delegates for their participation in our study. B.B. is a specialist in anesthesia and pain management, and member of NICE guidance on CBMP (2019). M.P.B. is a consultant with prescribing experience of medical cannabis. H.V.C. is a professor of psychopharmacology. A.F. is a consultant in anesthesia and pain medicine. D.P.F. is a professor of pharmacology and therapeutics and research scientist with expertise in cannabinoids, the endocannabinoid system, and pain neurobiology. T.H. is a pain specialist and medical cannabis prescriber at Clinic Horsted. J.M. is a physician with clinical experience in prescribing medicinal cannabis at Clinic Horsted. L.D.P. is a decision analyst. D.J.N. is a psychiatrist and neuropsychopharmacologist, as well as a medical cannabis researcher with previous prescribing experience of medical cannabis. C.S. is a psychiatrist and was Drug Science's Project Twenty21 clinical director at the time of writing this article. S.E.O'S. is a professor of pharmacology and works as an independent consultant on cannabinoid pharmacology and cannabis-based medicines. A.K.S. is a psychologist and medical cannabis researcher. Prof. Willem Scholten is a consultant on medicines and controlled substances. H.S. is a specialist in internal medicine and pain management and a neuroscientist at the Sagol Brain Institute in Tel Aviv with extensive clinical and research experience in cannabis and cannabis-based medicinal products. T.W. is a psychiatrist with substance misuse specialism. G.Z. is a medical cannabis expert and teacher at the University of Padova. Our two patient representatives from the United Patient Alliance had experience in treating their chronic neuropathic pain with all pain medications included in our study. David Badcock is CEO of Drug Science. James Bunn is communications manager of Drug Science.
Abbreviations Used
- AEs
adverse events
- CBMPs
cannabis-based medical products
- CI
confidence interval
- CMC
Centre for Medical Cannabis
- CNP
chronic neuropathic pain
- DDS
descriptor differential scale
- DPN
diabetic peripheral neuropathy
- EMA
European Medicines Agency
- GI
gastrointestinal
- IQR
interquartile range
- MCDA
multicriteria decision analysis
- NASEM
National Academies of Sciences, Engineering and Medicine
- NNT
number needed to treat
- NRS
numerical rating scale
- PNP
peripheral neuropathic pain
- RCT
randomized control trial
- SAEs
serious adverse events
- SGIC
Subject Global Impression of Change
- THC/CBD
tetrahydrocannabinol/cannabidiol
- VAS
visual analog scale
Appendix Table A1. Randomized Control Trials on the Use of Medical Cannabis for Chronic Neuropathic Pain
| Title | Year | Authors | CBMP | Condition | Results |
|---|---|---|---|---|---|
| A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms | 2003 | Wade DT, Robson P, House H, Makela P, Aram J. | Whole-plant extracts of THC, CBD, 1:1 CBD:THC, or matched placebo | Mixed: multiple sclerosis (18), spinal cord injury (4), brachial plexus damage (1), and limb amputation due to neurofibromatosis (1) | Pain relief associated with both THC and CBD was significantly superior to placebo. |
| Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial | 2004 | Berman JS, Symonds C, Birch R. | Sativex (THC:CBD) or THC | Neuropathic pain from brachial plexus avulsion | The pain rating index and VAS were significantly improved by THC (p=0.04) but not Sativex. The pain review score, sleep quality and sleep disturbance scores were significantly improved by both THC and Sativex. |
| Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1' studies | 2004 | Nottcut W, Price M, Miller R, Newport S, Phillips C, Simmons S, Sansom C. | THC, CBD, 1:1 CBD:THC | CNP | Extracts which contained THC proved most effective in symptom control. |
| Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis | 2005 | Rog D, Nurmikko T, Friede T, Young C. | Whole plant THC:CBD delivered through oromucosal spray, each spray delivered 2.7 mg of THC and 2.5 of CBD. | Central pain in multiple sclerosis | CBMP was superior to placebo in reducing the mean intensity of pain (CBMP mean change −2.7, 95% CI: −3.4 to −2.0, placebo −1.4 95% CI: −2.0 to −0.8, comparison between groups, p=0.005) and sleep disturbance (CBMP mean change −2.5, 95% CI: −3.4 to −1.7, placebo −0.8, 95% CI: −1.5 to −0.1, comparison between groups, p=0.003). |
| Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial | 2007 | Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. | Sativex (THC:CBD) | Unilateral PNP and allodynia | The mean reduction in pain intensity scores (primary outcome measure) was greater in patients receiving Sativex than placebo (mean adjusted scores −1.48 points vs. −0.52 points) on a 0–10 Numerical Rating Scale (p=0.004; 95% CI: −1.59 to −0.32). Improvements in Neuropathic Pain Scale composite score (p=0.007), sleep NRS (p=0.001), dynamic allodynia (p=0.042), punctate allodynia (p=0.021), Pain Disability Index (p=0.003) and Patient's Global Impression of Change (p<0.001) were similarly greater on Sativex versus placebo. |
| Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial | 2007 | Abrams D, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. | Smoked cannabis | Painful HIV-associated sensory neuropathy | Smoked cannabis reduced daily pain by 34% (median reduction; IQR=−71 to −16) versus 17% (IQR=−29 to 8) with placebo (p=0.03). Greater than 30% reduction in pain was reported by 52% in the cannabis group and by 24% in the placebo group (p=0.04). The first cannabis cigarette reduced chronic pain by a median of 72% versus 15% with placebo (p<0.001). Cannabis reduced experimentally induced hyperalgesia to both brush and von Frey hair stimuli (p≤0.05) but appeared to have little effect on the painfulness of noxious heat stimulation. |
| A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain | 2008 | Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. | Cannabis cigarettes | Neuropathic pain | No effect on evoked pain was seen. |
| Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial | 2009 | Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley | Cannabis cigarettes | HIV-associated distal sensory predominant polyneuropathy | Among the completers, pain relief was greater with cannabis than placebo (median difference in DDS pain intensity change, 3.3 points, effect size=0.60; p=0.016). The proportions of subjects achieving at least 30% pain relief with cannabis versus placebo were 0.46 (95% CI: 0.28 to 0.65) and 0.18 (0.03 to 0.32). |
| Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor | 2010 | Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. | Sativex (THC:CBD) | Painful diabetic neuropathy | There was significant improvement in pain scores in both groups, but mean change between groups was not significant. Depression was a major confounder and may have important implications for future trials on painful DPN. |
| Smoked cannabis for chronic neuropathic pain: a randomized controlled trial | 2010 | Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, Collet JP. | Smoked cannabis | CNP: (post-traumatic or postsurgical) | The average daily pain intensity, measured on the 11-point numeric rating scale, was lower on the prespecified primary contrast of 9.4% versus 0% THC (5.4 vs. 6.1, respectively; difference=0.7, 95% CI: 0.02 to 1.4). Preparations with intermediate potency yielded intermediate but nonsignificant degrees of relief. Participants receiving 9.4% THC reported improved ability to fall asleep (easier, p=0.001; faster, p<0.001; more drowsy, p=0.003) and improved quality of sleep (less wakefulness, p=0.01) relative to 0% THC. |
| An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain | 2012 | Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, Garven A, Bestard J, Korngut L. | Nabilone (THC) | Diabetic PNP | For nabilone run-in-phase responders, there was an improvement in the change in mean end-point neuropathic pain versus placebo (mean treatment reduction of 1.27; 95% CI: 2.29 to 0.25, p=0.02), with an average nabilone dose at end-point of 2.9±1.1 mg/day, and improvements from baseline for the anxiety subscale of the Hospital Anxiety and Depression Scale, the Medical Outcomes Study sleep scale problems index, and the European Quality of Life-5-Domains index score (each p<0.05). Nabilone run-in-phase responders reported greater global end-point improvement with nabilone than with placebo (100% vs. 31%; p<0.05). |
| Low-dose vaporized cannabis significantly improves neuropathic pain | 2013 | Wilsey B, Marcotte TD, Deutsch R, Gouaux B, Sakai S, Donaghe H. | Vaporized cannabis | Experiencing neuropathic pain despite traditional treatment | A treatment effect was noted with cumulative dosing, with the magnitude of differences between the doses changing over time (treatment by time interaction: p=0.0133). Significant separation occurred at 120 min (p=0.0002). The NNT to achieve 30% pain reduction was 3.2 for placebo versus low dose, 2.9 for placebo versus medium dose, and 25 for medium versus low dose. |
| A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment | 2014 | Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, Ehler E | Sativex (THC:CBD) | PNP associated with mechanical allodynia | At the 30% responder level, there were statistically significant treatment differences in favor of THC/CBD spray in the full analysis (intention-to-treat) data set (p=0.034; 95% CI: 1.05 to 3.70). There was also a reduction in mean PNP 0–10 NRS scores in both treatment groups that was numerically higher in the THC/CBD spray group, but which failed to reach statistical significance. Secondary measures of sleep quality 0–10 NRS score (p=0.0072) and SGIC (p=0.023) also demonstrated statistically significant treatment differences in favor of THC/CBD spray treatment. |
| The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: a phase 1a study | 2014 | Eisenberg E, Ogintz M, Almog S. | Cannabis flower | Sufferers of neuropathic pain of any type | A significant 45% reduction in pain intensity was noted 20 min postinhalation (p=0.001), turning back to baseline within 90 min. |
| A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain | 2015 | Hoggart B, Ratcliffe S, Ehler E, Simpson KH, Hovorka J, Lejčko J, Taylor L | Sativex (THC:CBD) | PNP associated with diabetes or allodynia | Decrease in pain score over time. At least half of patients had a 30% improvement in pain at all time points. Sustained improvements from baseline were also observed in NPS and sleep quality scores. Also no evidence of a tolerance developing toward THC/CBD spray. |
| An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease | 2016 | Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. | Vaporized cannabis | Individuals with injury or disease of the spinal cord | Using an 11-point numerical pain intensity rating scale as the primary outcome, a mixed effects linear regression model showed a significant analgesic response for vaporized cannabis. When subjective and psychoactive side effects (e.g., good drug effect and feeling high) were added as covariates to the model, the reduction in pain intensity remained significant above and beyond any effect of these measures (all p<0.0004). |
CBMP, cannabis-based medical product; CBD, cannabidiol; CI, confidence interval; CNP, chronic neuropathic pain; DDS, descriptor differential scale; DPN, diabetic peripheral neuropathy; IQR, interquartile range; NNT, number needed to treat; NRS, numerical rating scale; PNP, peripheral neuropathic pain; SGIC, Subject Global Impression of Change; THC, tetrahydrocannabinol; VAS, visual analog scale.
Author Disclosure Statement
M.P.B. is director of Maple Tree Consultants. D.P.F reports an Industry-Academia research grant from Alkermes, Inc., and Science Foundation Ireland outside of the submitted study. He also reports research grants in the area of cannabinoids or the endocannabinoid system from Shionogi Ltd. (Shionogi Science Programme), from B. Braun Ltd. jointly with Science Foundation Ireland, and from the Irish Research Council, CNPq Brazil, and EU INTERREG Programmes. T.H. is director of Clinic Horsted. J.M. is scientific advisor to Thedrug.store, a retailer of CBD products and natural supplements, and she also runs an online blog writing about cannabinoid science and general health and well-being. The blog does not generate an income and does not run advertisements. S.E.O'S. is scientific advisor for Artelo Biosciences, Dragonfly Biosciences, FSDPharma, Therapix Biosciences, and MJResults, and Science Lead of The Centre for Medicinal Cannabis. H.S. is on the scientific advisory board of Cellen Inc. D.J.N. is chair of the charity Drug Science. A.K.S. is head of research of the charity Drug Science. Drug Science receives an unrestricted educational grant from a consortium of medical cannabis companies. C.S. was clinical director of Drug Science's Project Twenty21. G.Z. is chief scientific officer and scientific board member at Cannaray Ltd., advisory board member and teacher at Masterclass Medicinal Cannabis, and scientific board member at Portugal Medical Cannabis (PTMC). All other authors declare no competing interests.
Funding Information
No funding was received for the writing of this article.
Cite this article as: Nutt DJ, Phillips LD, Barnes MP, Brander B, Curran HV, Fayaz A, Finn DP, Horsted T, Moltke J, Sakal C, Sharon H, O'Sullivan SE, Williams T, Zorn G, Schlag AK (2022) A multicriteria decision analysis comparing pharmacotherapy for chronic neuropathic pain, including cannabinoids and cannabis-based medical products, Cannabis and Cannabinoid Research 7:4, 482–500, DOI: 10.1089/can.2020.0129.
References
- 1. Nutt D. Why medical cannabis is still out of patients' reach—an essay by David Nutt. BMJ. 2019;365:l1903. [DOI] [PubMed] [Google Scholar]
- 2. Schlag A, Baldwin D, Barnes M, et al. Medical cannabis in the UK: from principle to practice. J Psychopharmacol. 2020;34:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlag A. An evaluation of regulatory regimes of medical cannabis: what lessons can be learned for the UK? Med Cannabis Cannabinoids. 2020;3:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharon H, Goldway N, Goor-Aryeh I, et al. Personal experience and attitudes of pain medicine specialists in Israel regarding the medical use of cannabis for chronic pain. J Pain Res. 2018;11:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mirelman D, Waissengrin B, Goldway N, et al. Use of medical cannabis: perceptions of Israeli oncologists. Lancet Oncol. 2019;20:475–477. [DOI] [PubMed] [Google Scholar]
- 6. Couch D. Left behind: the scale of illegal cannabis use for medicinal intent in the UK2020. https://www.thecmcuk.org/left-behind-the-scale-of-illegal-cannabis-use-for-medicinal-intent-in-the-uk (last accessed March 10, 2020).
- 7. United Patients' Alliance (UPA). UPA Patients' Survey 2018. https://www.upalliance.org/patient-survey-2018 (last accessed April 18, 2020).
- 8. National Academies of Sciences Engineering and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press: Washington, DC, 2017. [PubMed] [Google Scholar]
- 9. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29:7–14. [DOI] [PubMed] [Google Scholar]
- 10. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petzke F, Enax-Krumova EK, Hauser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmertz. 2016;30:62–88. [DOI] [PubMed] [Google Scholar]
- 12. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473. [DOI] [PubMed] [Google Scholar]
- 13. Stockings E, Campbell G, Hall W, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159:1932–1954. [DOI] [PubMed] [Google Scholar]
- 14. Fisher E, Moore RA, Fogarty AE, et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain. 2020. In Press. [DOI] [PubMed] [Google Scholar]
- 15. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, et al. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27:672–680. [DOI] [PubMed] [Google Scholar]
- 16. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:CD012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore RA, Fisher E, Finn DP, et al. Cannabinoids, cannabis, and cannabis-based medicines for pain management: an overview of systematic reviews. Pain. 2020. [Epub ahead of print]; DOI: 10.1097/j.pain.0000000000001941. [DOI] [PubMed] [Google Scholar]
- 18. Sharon H, Brill S. Cannabis-based medicines for chronic pain management: current and future prospects. Curr Opin Anaesthesiol. 2019;32:623–628. [DOI] [PubMed] [Google Scholar]
- 19. Häuser W, Finn DP, Kalso E, et al. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain. 2018;22:1547–1564. [DOI] [PubMed] [Google Scholar]
- 20. Johal H, Devji T, Chang Y, et al. Cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohiuddin M, Blyth FM, Degenhardt L, et al. General risks of harm with cannabinoids, cannabis, and cannabis-based medicine possibly relevant to patients receiving these for pain management: an overview of systematic reviews. Pain. 2020. [Epub ahead of print]; DOI: 10.1097/j.pain.0000000000002000. [DOI] [PubMed] [Google Scholar]
- 22. Fine P, Cheatle MD. Common adverse effects and complications of long-term opioid therapy. Pain Med. 2015;16 Suppl 1:S1–S2. [DOI] [PubMed] [Google Scholar]
- 23. Onakpoya IJ, Thomas ET, Lee JJ, et al. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohiuddin MM, Mizubuti GB, Haroutounian S, et al. Adherence to Consolidated Standards of Reporting Trials (CONSORT) Guidelines for reporting safety outcomes in trials of medical cannabis and cannabis-based medicines for chronic noncancer pain: a systematic review. Clin J Pain. 2020;36:302–319. [DOI] [PubMed] [Google Scholar]
- 26. Leong J, Salek S, Walker S. Benefit-risk assessment of medicines: the development and application of a universal framework for decision-making and effective communication. Wiley-Blackwell: Chichester, 2012. [Google Scholar]
- 27. Phillips LD, Fasolo B, Zafiropoulos N, et al. Is quantitative benefit-risk modelling of drugs desirable or possible? Drug Discov Today Technol. 2011;8:e3–e10. [DOI] [PubMed] [Google Scholar]
- 28. Moore R, Crossley A, Ng B, et al. Use of multicriteria decision analysis (MCDA) for assessing the benefit and risk of over-the-counter analgesics. J Pharm Pharmacol. 2017;69:1364–1373. [DOI] [PubMed] [Google Scholar]
- 29. Vermersch P, Martinelli V, Pfleger C, et al. Benefit-risk assessment of cladribine using multi-criteria decision analysis (MCDA) for patients with relapsing-remitting multiple sclerosis. Clin Ther. 2019;41:249–260. [DOI] [PubMed] [Google Scholar]
- 30. Wright S, Duncombe P, Altman DG. Assessment of blinding to treatment allocation in studies of a cannabis-based medicine (Sativex®) in people with multiple sclerosis: a new approach. Trials. 2012;13:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franco LA, Montibeller G. Facilitated modelling in operational research. Eur J Oper Res. 2010;205:489–500. [Google Scholar]
- 32. Phillips LD. Decision conferencing. In: Edwards W, Miles RF, von Winterfeldt D, eds. Advances in decision analysis: from foundations to applications. Cambridge University Press: Cambridge, 2007. [Google Scholar]
- 33. European Medicines Agency. Benefit-risk methodology project; Work package 4 report: benefit-risk tools and processes. European Medicines Agency: London, 2012. [Google Scholar]
- 34. Hughes D, Waddingham E, Mt-Isa S, et al. Recommendations for benefit-risk assessment methodologies and visual representations. Pharmacoepidemiol Drug Saf. 2016;25:251–262. [DOI] [PubMed] [Google Scholar]
- 35. Phillips LD. Benefit-risk modeling of medicinal products: methods and applications. In: Sashegyi A, Felli J, Noel R, eds. Benefit-risk assessment in pharmaceutical research and development. CRC Press: Boca Raton, FL, 2014: pp. 59–96. [Google Scholar]
- 36. LSE/Catalyze. 2011. Hiview3 www.catalyzeconsulting.com. (last accessed October 1, 2019).
- 37. Montibeller G, von Winterfeldt D. Cognitive and motivational biases in decision and risk analysis. Risk Anal. 2015;25:1230–1251. [DOI] [PubMed] [Google Scholar]
- 38. Tervonen T, Gelhorn H, Bhashyam SS, et al. MCDA swing weighting and discrete choice experiments for elicitation of patient benefit-risk preferences: a critical assessment. Pharmacoepidemiol Drug Saf. 2017;26:1483–1491. [DOI] [PubMed] [Google Scholar]
- 39. von Winterfeldt D, Edwards W. Decision analysis and behavioral research. Cambridge University Press: Cambridge, 1986. [Google Scholar]
- 40. Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- 41. Rowbotham DJ. Neuropathic pain and quality of life. Eur J Pain. 2002;6 Suppl B:19–24. [DOI] [PubMed] [Google Scholar]
- 42. Almeida FC, Castilho A, Cesarino CB, et al. Correlation between neuropathic pain and quality of life. Br J Pain. 2018;1:349–353. [Google Scholar]
- 43. Weizman L, Dayan L, Brill S, et al. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology. 2018;91:e1285–e1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toth C, Mawani S, Brady S, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. 2012;153:2073–2082. [DOI] [PubMed] [Google Scholar]
- 45. Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182:E694–E701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nutt DJ, Phillips LD, Balfour D, et al. E-cigarettes are less harmful than smoking. Lancet. 2016;387:1160–1161. [DOI] [PubMed] [Google Scholar]
- 47. Phillips LD. Best practice for MCDA in healthcare. In: Marsh K, Goetghebeur M, Thokala P, Baltussen R, eds. Multi-criteria decision analysis to support healthcare decisions. Springer International Publishing AG, New York, 2017: pp. 311–329. [Google Scholar]
- 48. Thokala P, Devlin N, Marsh K, et al. Multiple criteria decision analysis for health care decision making—an introduction: report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19:1–13. [DOI] [PubMed] [Google Scholar]
- 49. Lavie-Ajayi M, Shvartzman P. Restored self: a phenomenological study of pain relief by cannabis. Pain Med. 2018;20:2086–2093. [DOI] [PubMed] [Google Scholar]
- 50. Russo EB. Cannabis and pain. Pain Med. 2019;20:2083–2085. [DOI] [PubMed] [Google Scholar]