Abstract
Research over the past few decades has established a role for the endocannabinoid system in contributing to the neural and endocrine responses to stress exposure. The two endocannabinoid ligands, anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), both play roles in regulating the stress response and both exhibit dynamic changes in response to stress exposure. Most of this previous research, however, was conducted in male rodents. Given that, especially in rodents, the stress response is influenced by sex, an understanding of how these dynamic responses of endocannabinoids in response to stress is influenced by sex could provide insight into sex differences of the acute stress response. We exposed adult, Sprague Dawley rats to different commonly utilized acute stress modalities, specifically restraint, swim and foot shock stress. Thirty minutes following stress onset, we excised the amygdala, hippocampus and medial prefrontal cortex, corticolimbic brain regions involved in the stress response, to measure endocannabinoid levels. When AEA levels were altered in response to restraint and swim stress, they were reduced, whereas exposure to foot shock stress led to an increase in the amygdala. 2-AG levels, when they were altered by stress exposure were only increased, specifically in males in the amygdala following swim stress, and in the hippocampus and medial prefrontal cortex overall following foot shock stress. This increase in 2-AG levels following stress only in males was the only sex difference found in stress-induced changes in endocannabinoid levels. There were no consistent sex differences observed. Collectively, these data contribute to our further understanding of the interactions between stress and endocannabinoid function.
Keywords: Endocannabinoid, Stress, Stressor modality, Sex differences, Rats, Corticolimbic
Abbreviations: THC, delta-9-tetrahydrocannabinol; CB1, cannabinoid type 1 receptor; AEA, anandamide; 2-AG, 2-arachidonoyl glycerol; CRH, corticotropin releasing hormone; HPA, hypothalamic-pituitary-adrenal; SEM, standard error of the mean; FAAH, fatty acid amide hydrolase; CRH-R1, CRH receptor type 1
Highlights
-
•
Stressor modality influences acute stress-induced changes in endocannabinoid levels in corticolimbic brain regions.
-
•
There are minimal sex differences with regards to basal levels or stress-induced changes in endocannabinoid levels.
-
•
AEA decreased from acute restraint and swim stresses but increased from acute foot shock stress.
-
•
2-AG levels only showed increases when they were impacted by the different stress modalities.
1. Introduction
The endocannabinoid system was first identified as the molecular target through which delta-9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis, exerts its effects on the brain and body (Hillard, 2015). While the cannabinoid type 1 receptor (CB1) is established as the primary mediator of the effects of THC, this receptor is endogenously activated by a class of lipid signaling molecules known as endocannabinoids, with the arachidonate-derived anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) being the two primary endocannabinoid signaling molecules (Hillard, 2015). As reduction of stress and anxiety is often listed as a primary motivation for using cannabis (Halikas et al., 1971), it is not surprising that endocannabinoid signaling is well known to influence neural and endocrine responses to stress exposure (Morena et al., 2016).
Research over the past two decades has fleshed out the complex interactions that exist between stress and the endocannabinoid system (Morena et al., 2016). For example, the release of corticotropin releasing hormone (CRH) in response to stress exposure rapidly triggers hydrolysis and subsequent depletion of AEA signaling in brain regions such as the amygdala (Gray et al., 2015; Natividad et al., 2017). This loss of AEA signaling, in turn, results in an augmentation of excitatory neurotransmission within subregions of the amygdala (Natividad et al., 2017; Yasmin et al., 2020), which then contributes to the generation of a stress response and the transition into a behavioral state of anxiety (Bluett et al., 2014; Gray et al., 2015; Hill et al., 2009a). However, stress-induced release of glucocorticoid hormones mobilizes endocannabinoid signaling, primarily 2-AG, which then acts to reduce neural activity throughout stress-responsive neural circuits and contributes to the normative termination of the stress response (Di et al., 2003; Evanson et al., 2010; Hill et al., 2011; Ragozzino et al., 2020). These dynamic changes in endocannabinoid signaling in response to stress have been well characterized, and several translational studies have also demonstrated similar dynamic changes in endocannabinoid signaling in humans (Crombie et al., 2019; Hill et al., 2009b; Mayo et al., 2020a; Spohrs et al., 2022), and verified that the endocannabinoid system is also an important regulator of stress responses and affective states in humans (Gunduz-Cinar et al., 2013; Mayo et al., 2020a, 2020b).
To date, the overwhelming majority of research on endocannabinoids and stress has focused explicitly on male subjects. Particularly in rodents, stress reactivity is well documented to be influenced by the sex of the subject, predominately with respect to the activation of the hypothalamic-pituitary-adrenal (HPA) axis and the subsequent release of glucocorticoid hormones, such as corticosterone (Rincón-Cortés et al., 2019). As more research has begun to focus on sex differences, it has also become apparent that the endocannabinoid system is influenced by sex, both in terms of the impact that fluctuations in endocannabinoid signaling have on behavior and the dynamic changes that occur in endocannabinoid signaling in response to various challenges or external stimuli. For example, while elevating AEA signaling in males has typically been found to promote the extinction of fear, we recently reported that augmentation of AEA signaling appears to do the opposite and promote fear behaviors in female rodents (Morena et al., 2021). In a similar vein, chronic stress has been reliably found to downregulate CB1 receptors in males, however chronic stress in females was found to upregulate CB1 receptor expression (Reich et al., 2009). These sex differences are not surprising, however, given that cannabis itself can produce opposing or differential effects and there are sex differences in both the expression of CB1 receptors and endocannabinoid molecules in humans and rodents (Cooper and Craft, 2018).
As there are well established sex differences in stress reactivity and endocannabinoid function, we sought to examine if the effects of acute stress on dynamic changes in endocannabinoid function were influenced by sex. Moreover, given that the modality and nature of the stressor applied can influence many of the effects of stress, we performed a detailed analysis of changes in endocannabinoid content throughout stress-responsive corticolimbic structures following exposure to three different commonly used experimental models to induce stress: restraint, swim and foot shock. Our results indicate that both sex and stressor modality have a significant influence on dynamic changes in endocannabinoid function.
2. Methods
2.1. Animals
Adult (∼post-natal 70 days old) male and female Sprague Dawley rats were utilized for this study. All rats were obtained from Charles River (Saint Constant, QC, Canada, RGD Cat# 734476, RRID:RGD_734476). Rats were acclimated for 1 week prior to experiments. Rats were pair housed; from each pair-housed duo, one rat was collected immediately for inclusion in the basal group while the other was used for the stress group. Rats were kept on a 12:12 light:dark cycle and had ad libitum access to food and water. All experiments were performed during the start of the light phase. All protocols were approved by the University of Calgary Animal Care Committee and Canadian Council for Animal Care. All experiments took place in the light portion of the light cycle.
2.2. Acute stress protocols
From each pair-housed duo of rats, one was placed in the stress apparatus and the other was immediately euthanized for use in the corresponding basal group.
2.2.1. Restraint stress
Rats were exposed to 30 min of acute restraint stress in a clear, Plexiglass restrainer. Immediately following restraint termination, blood and tissue samples were collected. Graphical representation of methodology is found in Fig. 1A (Created in Biorender).
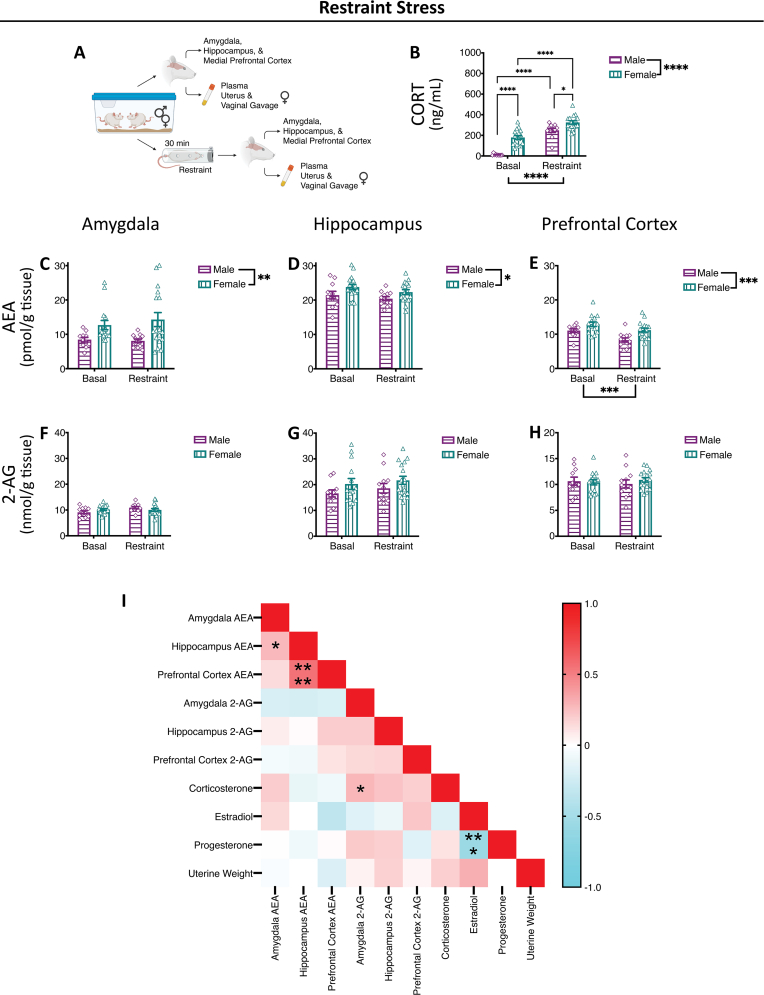
Fig. 1.
Restraint Stress-Induced Alterations in Endocannabinoid Levels.
(A) Representative methods schematic (created in Biorender). (B) Corticosterone (CORT) levels increased with restraint stress exposure and were greater in females compared to males both basally and in response to stress. Anandamide (AEA) levels were higher in females compared to males in the (C) amygdala, (D) hippocampus and (E) medial prefrontal cortex, but were only altered by stress in the (E) medial prefrontal cortex, where they were decreased. 2-arachidonoyl glycerol (2-AG) levels were not altered by sex or restraint stress exposure in the (F) amygdala, (G) hippocampus and (H) medial prefrontal cortex. (I) Correlation matrix. Estradiol, progesterone and uterine weights only compared in females. Significant bars inside the axes represent specific comparisons between groups, while those outside of the axes represent main effects (sex and stress). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Basal = left pair, Stress = right pair. In each pair, the left bars are the males (grape, horizontal lines, diamonds) and the right bars are the females (teal, vertical lines, triangle).
2.2.2. Swim stress
Rats were exposed to 15 min of acute swim stress in an opaque 5 gal container, containing 4 gal water, followed by 15 min in the home cage. Water temperature was 25 °C ± 1 °C. Graphical representation of methodology is found in Fig. 2A (Created in Biorender).
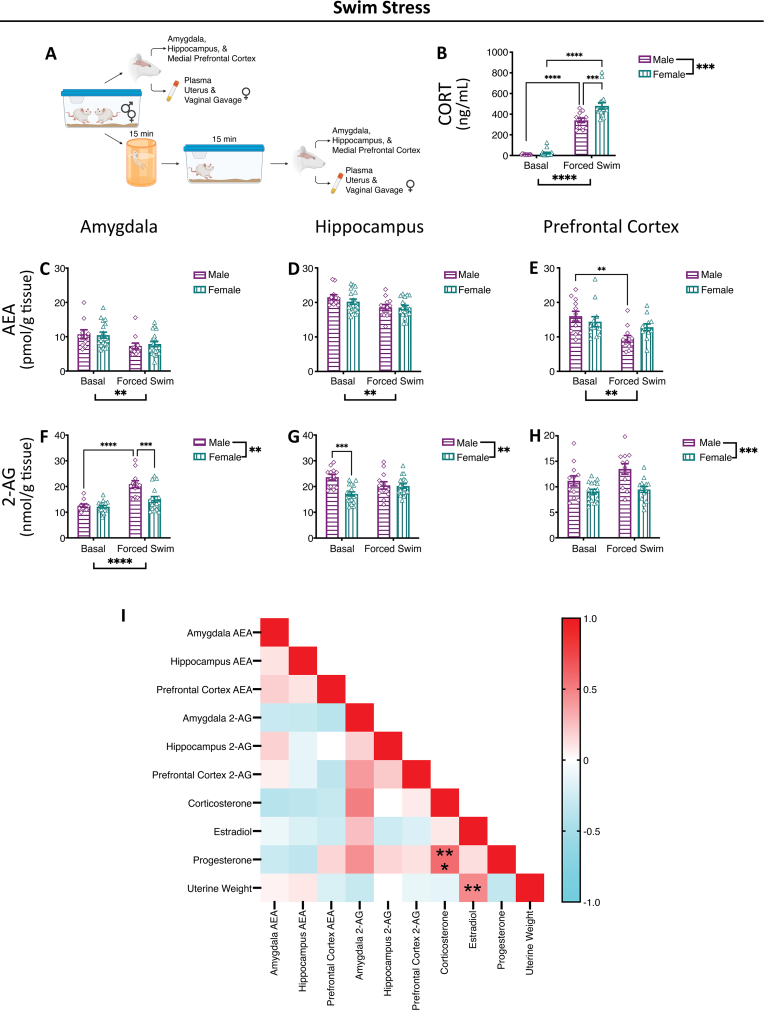
Fig. 2.
Swim Stress-Induced Alterations in Endocannabinoid Levels.
(A) Representative methods schematic (created in Biorender). (B) Corticosterone (CORT) levels increased with swim stress exposure and were greater in females compared to males in response to stress. Anandamide (AEA) levels were reduced in the (C) amygdala, (D) hippocampus and (E) medial prefrontal cortex following swim stress and this was primarily driven by males in the (E) medial prefrontal cortex. 2-arachidonoyl glycerol (2-AG) levels were lower in females compared to males in the (F) amygdala, (G) hippocampus and (H) medial prefrontal cortex. 2-AG levels were increased with swim stress in the (F) amygdala and this was primarily driven by males, with no stress-induced changes in the (G) hippocampus or (H) medial prefrontal cortex. (I) Correlation matrix. Estradiol, progesterone and uterine weights only compared in females. Significant bars inside the axes represent specific comparisons between groups, while those outside of the axes represent main effects (sex and stress). **p < 0.01, ***p < 0.001, ****p < 0.0001. Basal = left pair, Stress = right pair. In each pair, the left bars are the males (grape, horizontal lines, diamonds) and the right bars are the females (teal, vertical lines, triangle).
2.2.3. Foot shock stress
Foot shock stress consisted of ten foot-shocks (0.65 mA, 2 s) delivered in 15 min using MED Associates fear-conditioning chambers (St. Albans, VT, USA). Rats were returned to their home cage for 15 min after stress termination, before collection of blood and brain samples. Graphical representation of methodology is found in Fig. 3A (Created in Biorender).
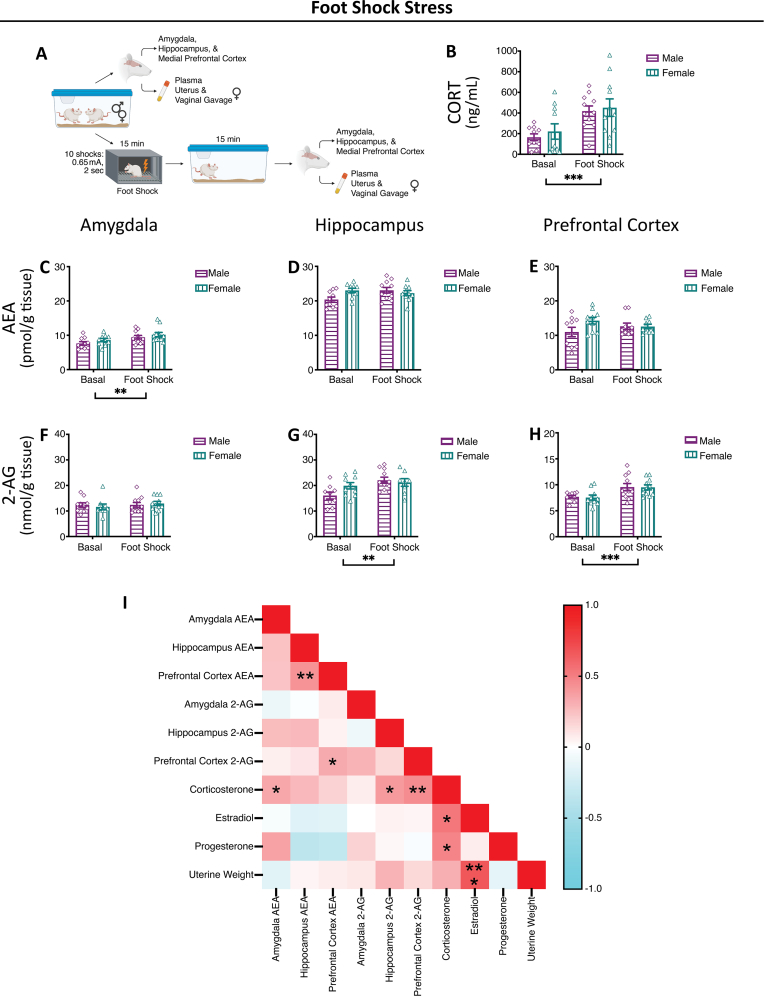
Fig. 3.
Foot Shock Stress-Induced Alterations in Endocannabinoid Levels.
(A) Representative methods schematic (created in Biorender). (B) Corticosterone (CORT) levels increased with foot shock stress exposure. Anandamide (AEA) levels were increased in the (C) amygdala following foot shock stress with no effect of sex. Sex or stress exposure did not alter AEA levels in the (D) hippocampus or (E) medial prefrontal cortex. 2-arachidonoyl glycerol (2-AG) levels were increased in the (G) hippocampus and (H) medial prefrontal cortex with exposure to foot shock stress with no effect of sex. Sex or stress did not alter 2-AG levels in the (F) amygdala. (I) Correlation matrix. Estradiol, progesterone and uterine weights only compared in females. Significant bars inside the axes represent specific comparisons between groups, while those outside of the axes represent main effects (sex and stress). *p < 0.05, **p < 0.01, ***p < 0.001. Basal = left pair, Stress = right pair. In each pair, the left bars are the males (grape, horizontal lines, diamonds) and the right bars are the females (teal, vertical lines, triangle).
2.3. Endocannabinoid ligand level quantification
Corticolimbic brain regions (amygdala, hippocampus and medial prefrontal cortex) were excised as previously described (Hill et al., 2010a), immediately snap frozen and stored at −80 °C. Specifically, excised brain regions were defined as: amygdala (central, basolateral, medial and cortical nuclei); hippocampus (both dorsal and ventral, including all sub-regions); and, medial prefrontal cortex and anterior cingulate cortex (dorsal to the anterior olfactory nucleus and medial to the corpus collosum and claustrum) (Hill et al., 2010a). Samples were homogenized for analysis of the bulk tissue level of AEA and 2-AG using liquid chromatography/tandem mass spectrometry as previously described (Morena et al., 2019; Qi et al., 2015; Vecchiarelli et al., 2021). Samples were homogenized in 2 mL of acetonitrile with 5 pmol d8-AEA (Cayman Chemical Company, Ann Arbor, Michigan, USA, #390050) and 5 nmol d8-2-AG (Cayman Chemical Company, #362160) in a borosilicate glass tube with a glass rod. Samples were sonicated, incubated overnight at −20 °C, centrifuged at 1500×g, supernatants containing lipids were isolated, evaporated with nitrogen gas, washed with acetonitrile and evaporated with nitrogen gas again. Final reconstitution for liquid chromatography/tandem mass spectrometry was in 200 μL of acetonitrile before storage at −80 °C.
2.4. Plasma corticosterone level quantification
Plasma corticosterone levels were determined using an ELISA as previously described, according to the manufacturer's protocol (Cayman Chemical Company, Ann Arbor, Michigan, USA, #500655, RRID:AB_2868564—for restraint and swim stress cohorts (Vecchiarelli et al., 2015); Arbor Assays, Ann Arbor, Michigan, USA, C#K014–H, RRID:AB_2877626—for the foot shock stress cohort (DeVuono et al., 2020)).
2.5. Estrus cycle determination
The phase of the estrus cycle that females were in was determined by using a composite of plasma estradiol, plasma progesterone, uterine weight and vaginal cytology all taken immediately post-mortem concomitantly with brain tissue collection. Plasma estradiol (Alpco Diagnostics, Salem, New Hampshire, USA, #55-ESTRT-E01) and progesterone (Alpco Diagnostics, #55-PROMS-E01) levels were ascertained using ELISAs per manufacturer's protocols. For analysis, rats that were in the proestrus phase were compared to rats in remaining phases. However, there were no overall significant effects of estrus cycle phase on endocannabinoid levels, so the female data were collapsed for analysis.
2.6. Statistical analysis
Statistical analysis was performed using Prism v9 (GraphPad, San Diego, California, USA). For all experiments, statistical analysis was performed using a two-way ANOVA, analyzing for an interaction between stress and sex, as well as, main effects for stress and sex, followed by post-hoc comparisons with Bonferroni corrections between relevant groups. For all non-correlative data, F-values and p-values are reported in Supplemental Table 1; all data are represented as mean±standard error of the mean (SEM). Correlations were assessed between all parameters within each stressor using Pearson r; r-, F- and p-values are reported in Supplemental Table 1.
3. Results
3.1. Restraint stress
Plasma corticosterone levels in the restraint cohort were increased with exposure to restraint stress (Fig. 1B); furthermore, plasma corticosterone levels were higher in females than males, both basally and in response to restraint stress exposure (Fig. 1B). In the restraint cohort, females had higher basal levels of AEA than males in the amygdala, hippocampus and medial prefrontal cortex (Fig. 1C–E). Overall, in the medial prefrontal cortex, there was a reduction in AEA levels with acute restraint stress (Fig. 1E), but there was no main effect of acute restraint stress on AEA levels in the amygdala or hippocampus (Fig. 1C and D). With restraint, there was no an effect of sex, acute restraint stress or the interaction of the two, on 2-AG levels in the amygdala, hippocampus or medial prefrontal cortex (Fig. 1F–H).
3.2. Swim stress
Plasma corticosterone levels in the swim stress cohort were increased with exposure to swim stress (Fig. 2B); furthermore, plasma corticosterone levels were higher in females than males in response to swim stress exposure (Fig. 2B). With exposure to swim, there was an overall reduction in AEA levels across the amygdala, hippocampus and medial prefrontal cortex (Fig. 2C–E); this was perhaps driven by males in the medial prefrontal cortex, as in this region males had significantly lower levels of AEA with swim stress (Fig. 2E). Amygdala 2-AG levels were increased with stress exposure in males, but not females (Fig. 2F). There was also an overall effect of sex on 2-AG levels in this cohort, with males across groups having greater 2-AG levels than females in the amygdala, hippocampus and medial prefrontal cortex (Fig. 2F–H); in the hippocampus, this may have been driven by significant sex differences specifically in the basal group, with the basal male group having higher levels than basal female group (Fig. 2G).
3.3. Foot shock stress
Plasma corticosterone levels in the foot shock stress cohort were increased with exposure to stress (Fig. 3B); there were no observed sex differences in plasma corticosterone levels, basally or as a response to stress exposure. Following acute foot shock in the amygdala, there was an increase in AEA levels (Fig. 3C), and no changes in the hippocampus or medial prefrontal cortex (Fig. 3D and E). With foot shock stress, there was an increase in 2-AG levels in the hippocampus and medial prefrontal cortex (Fig. 3G and H), but there were no changes in 2-AG levels in the amygdala (Fig. 3F).
3.4. Correlations
While there were no significant effects of estrous cycle on endocannabinoid ligand levels, basally or following stress exposure, it was possible that individual aspects of our composition score, i.e. estradiol, progesterone, uterine weight, had effects on endocannabinoids. We correlated, across all groups, between endocannabinoid levels, corticosterone, estradiol, progesterone and uterine weights, in the restraint stress cohort (Fig. 1I), swim stress cohort (Fig. 2I) and foot shock stress (Fig. 3I). There were no significant correlations between female endocannabinoid ligand levels and estradiol, progesterone or uterine weight (Supplemental Table 1). When looking at any overall patterns, there were generally negative correlations between plasma estradiol and progesterone and AEA levels; progesterone was also generally negatively correlated with 2-AG levels. While each cohort had some significant correlations, there were no significant correlations across all three stressors.
4. Discussion
This study builds on previous work investigating the impact of acute stress on endocannabinoid dynamics in the brain by comparing a host of stressors of differing modalities, as well as exploring sex differences in the responses by utilizing both male and female subjects. Generally consistent with previous reports, we found stressor- and sex-specific changes in endocannabinoid levels across corticolimbic brain regions. Surprisingly, there was no consistent effect of all three stressors employed on any endocannabinoid change in any brain region, suggesting that these changes produced by stress are heavily influenced by the nature of the stressor being employed. More so, we did not detect any reliable sex difference, either in basal or stress-induced changes in endocannabinoid contents in any brain region examined.
Surprisingly, exposure to restraint stress had very little impact on endocannabinoid levels, with a stress-induced reduction in AEA content in the medial prefrontal cortex in both sexes (although largely carried by males) being the only significant outcome. While stress-induced reductions in amygdalar AEA levels are also typically reported after acute restraint, this effect in rats is typically quite subtle (∼10–15% reduction in rats) (Hill et al., 2009a) and is typically found to be more robust in mice (Bedse et al., 2017; Mayo et al., 2020a; Patel et al., 2005), suggesting that there may be species differences in terms of the magnitude of this response. More so, previous work has indicated that some of the restraint-induced changes in endocannabinoid levels, particularly with respect to 2-AG levels, do not become apparent until later time points following stress cessation (Hill et al., 2011); however, analysis of the temporal kinetics of stressors was beyond the scope of this study and we focused on the time point associated with peak neuroendocrine responses to stress. We focused on 30 min post-stress onset for all of our analysis, as this is when corticosterone levels generally peak in the brain. Given that the other stress groups were given a period of 15 min following stress cessation in their home cage, this could have contributed to differences seen across cohorts. Additionally, longer exposure to other stress protocols could have introduced confounding variables to the interpretation, potentially. Repeated exposure to restraint in both rats and mice has found to be more reliable in producing bidirectional changes in endocannabinoid levels (reduced AEA and elevated 2-AG) (Dubreucq et al., 2012; Hill et al., 2008, 2010b, 2013; Jennings et al., 2016; Patel et al., 2005; Rademacher et al., 2008), particularly in the amygdala, further supporting that there may be a temporal or stress volume component to these changes, which will require further exploration.
Swim stress produced the most consistent and robust effects on endocannabinoid levels, which aligns well with previous work (Jennings et al., 2016; Mayo et al., 2020a; McLaughlin et al., 2012). Specifically, swim stress caused widespread reductions in amygdalar, hippocampal and prefrontal AEA levels as well as elevations in amygdalar 2-AG levels in males. This bidirectional change in endocannabinoid molecules following stress is consistent with many previous reports (Morena et al., 2016). Although the swim stress was 15 min, versus the restraint being 30 min, the time point examined post-stress onset was the same. Swim stress duration was chosen to exclude confounds due to physical exhaustion and to ensure a stress response (Abel, 1993; Commons et al., 2017; Cordova et al., 1990). Given that the corticosterone response to swim stress was considerably more robust than restraint stress, this also suggests the possibility of a stress intensity threshold to produce detectable changes in endocannabinoid levels, such that the level of stress produced by restraint in this study was not sufficient to result in changes in amygdalar endocannabinoid levels, but for swim stress it was. Given that we and others have previously identified CRH signaling as the primary mechanism by which stress compromises AEA signaling, through an increase in the activity of AEA's primary metabolic enzyme, fatty acid amide hydrolase (FAAH) (Gray et al., 2015; Natividad et al., 2017), perhaps the degree to which a stressor can influence AEA signaling is related to the ability of the stressor to mount a robust elevation in CRH signaling. The current data support previous work indicating that stress-induced corticosterone release may serve as somewhat of a proxy of central CRH release given that swim stress evoked larger corticosterone responses than restraint, and also produced more robust changes in corticolimbic endocannabinoid content.
In our results, males exhibited a greater reduction of AEA following swim stress exposure compared to females (Fig. 2E), despite females mounting a greater corticosterone response following swim stress. There are differences in CRH levels and CRH receptor type 1 (CRH-R1) expression and binding between males and females in regions we examined (Bangasser, 2013; Bangasser and Wiersielis, 2018), including in the amygdala (Lim et al., 2005; Weathington and Cooke, 2012) and prefrontal cortex (Daiwile et al., 2021; Weathington et al., 2014). There is also a difference in CRH-R1 G-protein signaling between males and females, with CRH-R1 in females being preferentially coupled to Gs proteins (Bangasser et al., 2010; Bangasser and Wiersielis, 2018). Given that the intracellular mechanisms linking CRH-R1 and FAAH have yet to be elucidated, this could indicate that specific intracellular cascades activated by CRH-R1, which trigger FAAH activity, may differ in males and females or stress-induced changes in AEA levels occur at a different time course in males than females.
Foot shock has previously been reported to be the one stressor that results in an increase in AEA signaling (Hohmann et al., 2005; Morena et al., 2014), as opposed to a decrease, and consistent with those reports we similarly found that repeated shock exposure increased AEA levels within the amygdala. In line with previous reports, and consistent with other stressors(Morena et al., 2016), foot shock was also found to increase 2-AG levels in the hippocampus and prefrontal cortex. Previous work using a shock paradigm has indicated that this mobilization of endocannabinoid signaling could be involved in the generation of stress-induced analgesia (Hohmann et al., 2005), which suggests that the inclusion of a noxious component to the stressor itself could alter endocannabinoid dynamics, as the presence of a painful stimuli could recruit endocannabinoid signaling as an endogenous analgesic mechanism. Future work is required to dissociate the impacts of pain and stress on endocannabinoid signaling and determine the underlying mechanisms that drive these responses.
Interestingly, there was no consistent effect of sex on either basal or stress-induced changes in endocannabinoid signaling across these studies. Previous work has suggested that endocannabinoid levels, particularly that of AEA, may be sensitive to reproductive hormones such as estrogen (Hill et al., 2007; Maccarrone et al., 2002; Maia et al., 2017; Tabatadze et al., 2015). In the cohort of rats used for the restraint study, we found that females had globally higher AEA then males, regardless of stress condition; however, this was not seen in either of the other two cohorts. Additionally, in the cohort of rats used for the swim stress study, females exhibited globally lower 2-AG levels than males, which again was not observed in the other cohorts. We did not perform serial lavages to establish cycle stage in the days preceding the stress exposure (due to the potential confounds this introduces to the study given that the lavage itself is mildly stressful, and is only performed in females and not males, and thus represents a confounding variable that could independently influence female stress responses). We used a combination of estrogen/progesterone levels at time of tissue collection, uterine weight and a single post-mortem lavage to identify estrus stage and we did not see any association with differences in estrus stage (proestrus versus other stages) or estradiol levels that could explain the differences in endocannabinoid levels seen in one cohort but not the other. We also saw no significant correlations between estradiol or progesterone levels or uterine weights and endocannabinoid levels. We did see differences in basal corticosterone across the cohorts, however, which could be reflective of either life stress history (in breeding facility or during shipping) or current differences in ambient stress in the housing facility in which they reside, suggesting that perhaps additional variables beyond our control could be interacting with sex to produce spurious differences in endocannabinoid levels that are being attributed explicitly to sex. Regardless of the reason as to why we saw inconsistent sex effects across the cohorts, our data generally do not suggest that there are robust sex differences across modalities in stress-induced endocannabinoid dynamics within corticolimbic brain structures.
Collectively, these data add to the growing body of literature regarding the interactions between stress and endocannabinoid function. Our data generally support previous findings suggesting that stressors act to dampen AEA and augment 2-AG signaling, but suggest that the impacts of these effects may be modified by stressor modality, with more mixed or physical modalities producing more robust and consistent changes in endocannabinoid levels. More so, the inclusion of a noxious component to the stressor could influence the directionality of endocannabinoid changes due to its co-involvement in central pain networks. Surprisingly, biological sex of the subject seemed to have minimal or inconsistent impact on basal or stress-induced endocannabinoid changes. Several previous studies have investigated sex differences in the context of the impacts of pharmacological modulation of endocannabinoid signaling on the neuroendocrine response to stress (Atkinson et al., 2010; Roberts et al., 2014), and similar to what we saw here the overall conclusion from those studies is minimal or inconsistent. Interestingly, previous work has found that there are, however, sex differences in the impact of endocannabinoid signaling on stress-related affective behaviors such as fear and anxiety (Albrechet-Souza et al., 2021; Bowers and Ressler, 2016; Morena et al., 2021; Simone et al., 2018). Given that pharmacological tools which target endocannabinoid signaling are well into clinical development for the treatment of stress-related and affective disorders (Mayo et al., 2020b; Paulus et al., 2021; Schmidt et al., 2021), understanding the parameters by which stress regulates endocannabinoid signaling is important to establish. Future work will need to focus on the influence of sex on endocannabinoid dynamics to chronic or repeated stress exposure, as this may be particularly relevant for disease states, and endocannabinoid signaling has already been identified to play an important role in stress adaptation and plasticity of the HPA axis.
Funding
This work was supported by a foundation grant from the Canadian Institutes of Health Research (CIHR) FDN333950 to MNH; HAV received stipend funding from CIHR (Vanier CGS), the University of Calgary (UofC; Killam Pre-doctoral Laureate), Alberta Innovates-Health Solutions (AIHS) and Branch Out Neurological Foundation (BONF); MM received fellowship support from AIHS and CIHR; ASN received stipend funding from CIHR (CGS-M), and the UofC (Mathison Centre Graduate Recruitment Scholarship in Mental Health and Cumming School of Medicine Graduate Scholarship); RJA received stipend funding from the UofC (Mathison Centre Graduate Recruitment Scholarship in Mental Health); JMG received stipend funding from CIHR (CGS-D) and fellowship support from AIHS; MNH was the recipient of a Tier II Canada Research Chair in the Neurobiology of Stress.
Funding agencies had no influence on the design, execution or publishing of this work.
CRediT authorship contribution statement
Haley A. Vecchiarelli: Conceptualization, Formal analysis, Investigation, Writing – original draft, Visualization. Maria Morena: Conceptualization, Formal analysis, Investigation, Writing – original draft. Tiffany T.Y. Lee: Conceptualization, Investigation, Writing – review & editing. Andrei S. Nastase: Investigation, Writing – review & editing. Robert J. Aukema: Investigation, Writing – review & editing. Kira D. Leitl: Investigation, Writing – review & editing. J. Megan Gray: Investigation, Writing – review & editing. Gavin N. Petrie: Investigation, Writing – review & editing. Kristin J. Tellez-Monnery: Investigation, Writing – review & editing. Matthew N. Hill: Conceptualization, Methodology, Investigation, Resources, Writing – original draft, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare no conflict of interest. Our funding acknowledgement statement can be found above, and funding agencies had no influence on the design, execution or publishing of this work.
Acknowledgements
This manuscript is dedicated to the memory of Dr. Bruce McEwen.
We acknowledge that this work was conducted on the traditional territories of the people of the Treaty 7 region in Southern Alberta, which includes the Blackfoot Confederacy (comprising the Siksika, Piikani, and Kainai First Nations), as well as the Tsuut'ina First Nation, and the Stoney Nakoda (including the Chiniki, Bearspaw, and Wesley First Nations). The City of Calgary is also home to Métis Nation of Alberta, Region III.
We acknowledge the work of the University of Calgary Health Sciences Animal Research Centre for care of the rats, particularly Krista Jensen and Brittany Munro.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100470.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abel E.L. Physiological correlates of the forced swim test in rats. Physiol. Behav. 1993;54:309–317. doi: 10.1016/0031-9384(93)90116-w. [DOI] [PubMed] [Google Scholar]
- Albrechet-Souza L., Nastase A.S., Hill M.N., Gilpin N.W. Amygdalar endocannabinoids are affected by predator odor stress in a sex-specific manner and modulate acoustic startle reactivity in female rats. Neurobiol. Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson H.C., Leggett J.D., Wood S.A., Castrique E.S., Kershaw Y.M., Lightman S.L. Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology. 2010;151:3720–3727. doi: 10.1210/en.2010-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A. Sex differences in stress-related receptors: ″micro″ differences with ″macro″ implications for mood and anxiety disorders. Biol. Sex Differ. 2013;4:2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.a.S., Bethea T.T., Parastatidis I., Ischiropoulos H., Van Bockstaele E.J., Valentino R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15(877):896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Wiersielis K.R. Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones (Basel) 2018;17:5–13. doi: 10.1007/s42000-018-0002-z. [DOI] [PubMed] [Google Scholar]
- Bedse G., Hartley N.D., Neale E., Gaulden A.D., Patrick T.A., Kingsley P.J., Uddin M.J., Plath N., Marnett L.J., Patel S. Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol. Psychiatr. 2017;82:488–499. doi: 10.1016/j.biopsych.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett R.J., Gamble-George J.C., Hermanson D.J., Hartley N.D., Marnett L.J., Patel S. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl. Psychiatry. 2014;4:e408. doi: 10.1038/tp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers M.E., Ressler K.J. Sex-dependence of anxiety-like behavior in cannabinoid receptor 1 (Cnr1) knockout mice. Behav. Brain Res. 2016;300:65–69. doi: 10.1016/j.bbr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons K.G., Cholanians A.B., Babb J.A., Ehlinger D.G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper Z.D., Craft R.M. Sex-Dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology. 2018;43:34–51. doi: 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A., Gimenez M., Escanero J.F. Effect of swimming to exhaustion, at low temperatures, on serum Zn, Cu, Mg and Ca in rats. Physiol. Behav. 1990;48:595–598. doi: 10.1016/0031-9384(90)90197-c. [DOI] [PubMed] [Google Scholar]
- Crombie K.M., Leitzelar B.N., Brellenthin A.G., Hillard C.J., Koltyn K.F. Loss of exercise- and stress-induced increases in circulating 2-arachidonoylglycerol concentrations in adults with chronic PTSD. Biol. Psychol. 2019;145:1–7. doi: 10.1016/j.biopsycho.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Daiwile A.P., Jayanthi S., Cadet J.L. Sex- and brain region-specific changes in gene expression in male and female rats as consequences of methamphetamine self-administration and abstinence. Neuroscience. 2021;452:265–279. doi: 10.1016/j.neuroscience.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVuono M.V., Hrelja K.M., Petrie G.N., Limebeer C.L., Rock E.M., Hill M.N., Parker L.A. Nausea-induced conditioned gaping reactions in rats produced by high-dose synthetic cannabinoid, JWH-018. Cannabis Cannabinoid Res. 2020;5:298–304. doi: 10.1089/can.2019.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S., Malcher-Lopes R., Halmos K.C., Tasker J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S., Matias I., Cardinal P., Häring M., Lutz B., Marsicano G., Chaouloff F. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37:1885–1900. doi: 10.1038/npp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson N.K., Tasker J.G., Hill M.N., Hillard C.J., Herman J.P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.M., Vecchiarelli H.A., Morena M., Lee T.T.Y., Hermanson D.J., Kim A.B., McLaughlin R.J., Hassan K.I., Kühne C., Wotjak C.T., Deussing J.M., Patel S., Hill M.N. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 2015;35:3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., MacPherson K.P., Cinar R., Gamble-George J., Sugden K., Williams B., Godlewski G., Ramikie T.S., Gorka A.X., Alapafuja S.O., Nikas S.P., Makriyannis A., Poulton R., Patel S., Hariri A.R., Caspi A., Moffitt T.E., Kunos G., Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatr. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halikas J.A., Goodwin D.W., Guze S.B. Marihuana effects. A survey of regular users. JAMA. 1971;217:692–694. doi: 10.1001/jama.217.5.692. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Carrier E.J., McLaughlin R.J., Morrish A.C., Meier S.E., Hillard C.J., Gorzalka B.B. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J. Neurochem. 2008;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Karacabeyli E.S., Gorzalka B.B. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Karatsoreos I.N., Hillard C.J., McEwen B.S. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010;35:1333–1338. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Kumar S.A., Filipski S.B., Iverson M., Stuhr K.L., Keith J.M., Cravatt B.F., Hillard C.J., Chattarji S., McEwen B.S. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatr. 2013;18:1125–1135. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Bingham B., Shrestha L., Lee T.T.Y., Gray J.M., Hillard C.J., Gorzalka B.B., Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Morrish A.C., Viau V., Floresco S.B., Hillard C.J., Gorzalka B.B. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Pan B., Fitzgerald M.L., Roberts C.J., Lee T.T.-Y., Karatsoreos I.N., Mackie K., Viau V., Pickel V.M., McEwen B.S., Liu Q., Gorzalka B.B., Hillard C.J. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Miller G.E., Carrier E.J., Gorzalka B.B., Hillard C.J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J. The endocannabinoid signaling system in the CNS: a primer. Int. Rev. Neurobiol. 2015;125:1–47. doi: 10.1016/bs.irn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A.G., Suplita R.L., Bolton N.M., Neely M.H., Fegley D., Mangieri R., Krey J.F., Walker J.M., Holmes P.V., Crystal J.D., Duranti A., Tontini A., Mor M., Tarzia G., Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Jennings E.M., Okine B.N., Olango W.M., Roche M., Finn D.P. Repeated forced swim stress differentially affects formalin-evoked nociceptive behaviour and the endocannabinoid system in stress normo-responsive and stress hyper-responsive rat strains. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;64:181–189. doi: 10.1016/j.pnpbp.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Lim M.M., Nair H.P., Young L.J. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J. Comp. Neurol. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M., Bari M., Battista N., Finazzi-Agrò A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- Maia J., Almada M., Silva A., Correia-da-Silva G., Teixeira N., Sá S.I., Fonseca B.M. The endocannabinoid system expression in the female reproductive tract is modulated by estrogen. J. Steroid Biochem. Mol. Biol. 2017;174:40–47. doi: 10.1016/j.jsbmb.2017.07.023. [DOI] [PubMed] [Google Scholar]
- Mayo L.M., Asratian A., Lindé J., Holm L., Nätt D., Augier G., Stensson N., Vecchiarelli H.A., Balsevich G., Aukema R.J., Ghafouri B., Spagnolo P.A., Lee F.S., Hill M.N., Heilig M. Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice. Mol. Psychiatr. 2020;25:993–1005. doi: 10.1038/s41380-018-0215-1. [DOI] [PubMed] [Google Scholar]
- Mayo L.M., Asratian A., Lindé J., Morena M., Haataja R., Hammar V., Augier G., Hill M.N., Heilig M. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol. Psychiatr. 2020;87:538–547. doi: 10.1016/j.biopsych.2019.07.034. [DOI] [PubMed] [Google Scholar]
- McLaughlin R.J., Hill M.N., Bambico F.R., Stuhr K.L., Gobbi G., Hillard C.J., Gorzalka B.B. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur. Neuropsychopharmacol. 2012;22:664–671. doi: 10.1016/j.euroneuro.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Aukema R.J., Leitl K.D., Rashid A.J., Vecchiarelli H.A., Josselyn S.A., Hill M.N. Upregulation of anandamide hydrolysis in the basolateral complex of amygdala reduces fear memory expression and indices of stress and anxiety. J. Neurosci. 2019;39:1275–1292. doi: 10.1523/JNEUROSCI.2251-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Nastase A.S., Santori A., Cravatt B.F., Shansky R.M., Hill M.N. Sex-dependent effects of endocannabinoid modulation of conditioned fear extinction in rats. Br. J. Pharmacol. 2021;178:983–996. doi: 10.1111/bph.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Patel S., Bains J.S., Hill M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Roozendaal B., Trezza V., Ratano P., Peloso A., Hauer D., Atsak P., Trabace L., Cuomo V., McGaugh J.L., Schelling G., Campolongo P. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc. Natl. Acad. Sci. U. S. A. 2014;111:18333–18338. doi: 10.1073/pnas.1420285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad L.A., Buczynski M.W., Herman M.A., Kirson D., Oleata C.S., Irimia C., Polis I., Ciccocioppo R., Roberto M., Parsons L.H. Constitutive increases in amygdalar corticotropin-releasing factor and fatty acid amide hydrolase drive an anxious phenotype. Biol. Psychiatr. 2017;82:500–510. doi: 10.1016/j.biopsych.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Roelke C.T., Rademacher D.J., Hillard C.J. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur. J. Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B., Simmons A.N., Risbrough V.B., Halter R., Chaplan S.R. The effects of FAAH inhibition on the neural basis of anxiety-related processing in healthy male subjects: a randomized clinical trial. Neuropsychopharmacology. 2021;46:1011–1019. doi: 10.1038/s41386-020-00936-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M., Morena M., Vecchiarelli H.A., Hill M.N., Schriemer D.C. A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun. Mass Spectrom.: RCM (Rapid Commun. Mass Spectrom.) 2015;29 doi: 10.1002/rcm.7277. [DOI] [PubMed] [Google Scholar]
- Rademacher D.J., Meier S.E., Shi L., Ho W.-S.V., Jarrahian A., Hillard C.J. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Ragozzino F.J., Arnold R.A., Kowalski C.W., Savenkova M.I., Karatsoreos I.N., Peters J.H. Corticosterone inhibits vagal afferent glutamate release in the nucleus of the solitary tract via retrograde endocannabinoid signaling. Am. J. Physiol. Cell Physiol. 2020;319:C1097. doi: 10.1152/ajpcell.00190.2020. –C1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich C.G., Taylor M.E., McCarthy M.M. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M., Herman J.P., Lupien S., Maguire J., Shansky R.M. Stress: influence of sex, reproductive status and gender. Neurobiol. Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C.J., Stuhr K.L., Hutz M.J., Raff H., Hillard C.J. Endocannabinoid signaling in hypothalamic-pituitary-adrenocortical axis recovery following stress: effects of indirect agonists and comparison of male and female mice. Pharmacol. Biochem. Behav. 2014;117:17–24. doi: 10.1016/j.pbb.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.E., Liebowitz M.R., Stein M.B., Grunfeld J., Van Hove I., Simmons W.K., Van Der Ark P., Palmer J.A., Saad Z.S., Pemberton D.J., Van Nueten L., Drevets W.C. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: a double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology. 2021;46:1004–1010. doi: 10.1038/s41386-020-00888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone J.J., Baumbach J.L., McCormick C.M. Sex-specific effects of CB1 receptor antagonism and stress in adolescence on anxiety, corticosterone concentrations, and contextual fear in adulthood in rats. Int. J. Dev. Neurosci. 2018;69:119–131. doi: 10.1016/j.ijdevneu.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Spohrs J., Prost M., Ulrich M., Plener P.L., Bindila L., Abler B. Endocannabinoid system reactivity during stress processing in healthy humans. Biol. Psychol. 2022;169 doi: 10.1016/j.biopsycho.2022.108281. [DOI] [PubMed] [Google Scholar]
- Tabatadze N., Huang G., May R.M., Jain A., Woolley C.S. Sex differences in molecular signaling at inhibitory synapses in the Hippocampus. J. Neurosci. 2015;35:11252–11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli H.A., Gandhi C.P., Gray J.M., Morena M., Hassan K.I., Hill M.N. 2015. Divergent Responses of Inflammatory Mediators within the Amygdala and Medial Prefrontal Cortex to Acute Psychological Stress. Brain, behavior, and immunity. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli H.A., Morena M., Keenan C.M., Chiang V., Tan K., Qiao M., Leitl K., Santori A., Pittman Q.J., Sharkey K.A., Hill M.N. Comorbid anxiety-like behavior in a rat model of colitis is mediated by an upregulation of corticolimbic fatty acid amide hydrolase. Neuropsychopharmacology. 2021;46:992–1003. doi: 10.1038/s41386-020-00939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington J.M., Cooke B.M. Corticotropin-releasing factor receptor binding in the amygdala changes across puberty in a sex-specific manner. Endocrinology. 2012;153:5701–5705. doi: 10.1210/en.2012-1815. [DOI] [PubMed] [Google Scholar]
- Weathington J.M., Hamki A., Cooke B.M. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J. Comp. Neurol. 2014;522:1284–1298. doi: 10.1002/cne.23475. [DOI] [PubMed] [Google Scholar]
- Yasmin F., Colangeli R., Morena M., Filipski S., van der Stelt M., Pittman Q.J., Hillard C.J., Teskey G.C., McEwen B.S., Hill M.N., Chattarji S. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc. Natl. Acad. Sci. U. S. A. 2020;117:650–655. doi: 10.1073/pnas.1910322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.