Abstract
Rationale & Objective
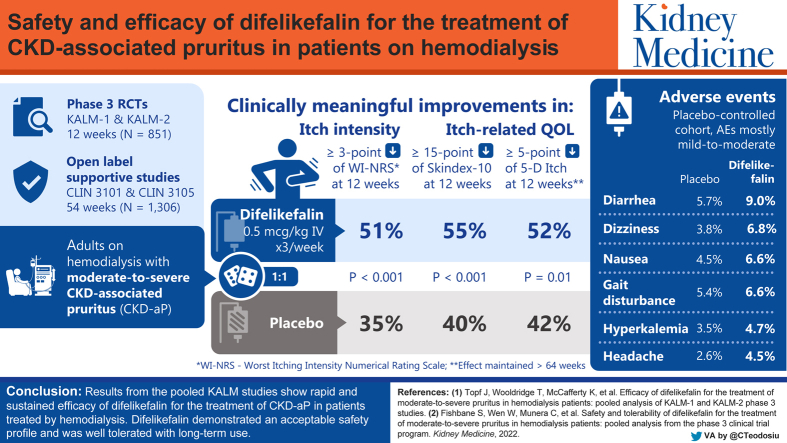
We report a pooled safety analysis of intravenous difelikefalin in participants with moderate to severe chronic kidney disease–associated pruritus (CKD-aP) treated by hemodialysis in 4 phase 3 clinical studies.
Study Design
KALM-1 and KALM-2 were randomized, double-blind, placebo-controlled, pivotal phase 3 studies; CLIN3101 (52 weeks) and CLIN3105 (12 weeks) were open-label studies.
Setting & Participants
Adults with moderate to severe CKD-aP treated by hemodialysis in North America, Europe, and the Asia-Pacific region.
Intervention
At least 1 intravenous placebo or difelikefalin dose of 0.5 mcg/kg for up to 64 weeks.
Outcomes
Safety.
Results
Safety analyses were conducted with 848 participants in the placebo-controlled cohort (424 participants each in the difelikefalin and placebo groups) and in 1,306 participants in the all-difelikefalin-exposure cohort. In the placebo-controlled cohort, the most commonly reported treatment-emergent adverse events (TEAEs), occurring in ≥2% of participants receiving difelikefalin and with a ≥1% higher incidence than placebo, were diarrhea (9.0% and 5.7%, respectively); dizziness (6.8% and 3.8%, respectively); nausea (6.6% and 4.5%, respectively); gait disturbances, including falls (6.6% and 5.4%, respectively), hyperkalemia (4.7% and 3.5%, respectively); headache (4.5% and 2.6%, respectively); somnolence (4.2% and 2.4%, respectively); and mental status changes (3.3% and 1.4%, respectively). These were mostly mild or moderate, with few leading to discontinuation. Incidence rates of TEAEs, serious TEAEs, and discontinuations because of TEAEs did not increase with long-term exposure. Three participants (0.7%) in the difelikefalin group and 5 participants (1.2%) in the placebo group died during the study.
Limitations
Pooled data from studies with different designs.
Conclusions
Intravenous difelikefalin demonstrated an acceptable safety profile, was generally well tolerated with long-term use, and may address the unmet treatment need for patients with CKD-aP treated by hemodialysis.
Funding
Cara Therapeutics, Inc.
Trial Registration
KALM-1 is registered as NCT03422653, KALM-2 as NCT03636269, CLIN3101 as NCT03281538, and CLIN3105 as NCT03998163.
Index Words: Chronic kidney disease, difelikefalin, κ-opioid receptor, pruritus, safety
Visual Abstract
Plain-Language Summary.
Patients with chronic kidney disease treated by hemodialysis (HD) often experience pruritus (intense itching) that negatively impacts their quality of life. Difelikefalin is an intravenous drug that reduces pruritus in patients who are treated by HD. Here we report pooled data from 4 phase 3 clinical trials to examine difelikefalin safety and tolerability. The most common adverse events that occurred in participants receiving difelikefalin included diarrhea, dizziness, nausea, gait disturbances (including falls), hyperkalemia, headache, somnolence, and changes in mental status. Most adverse events were mild to moderate in severity. Difelikefalin was well tolerated, with an acceptable safety profile for up to 64 weeks, and may address an unmet need for patients with chronic kidney disease–associated pruritus treated by HD.
Editorial, XXX
Introduction
Patients with dialysis-dependent chronic kidney disease (CKD) treated by hemodialysis (HD) often experience pruritus, which can negatively impact their quality of life and their physical and mental health, causing sleep disturbances, chronic fatigue, and an increased incidence of depression.1, 2, 3, 4, 5 These physical and mental health changes can lead to a higher likelihood of dizziness or faintness as the severity of pruritus increases.6 In the international Dialysis Outcomes and Practice Patterns Study, patients treated by HD who reported being extremely bothered by pruritus also had higher rates of all-cause mortality (hazard ratio, 1.24; 95% confidence interval, 1.08-1.41), infection-related mortality (hazard ratio, 1.44; 95% confidence interval, 1.05-1.96), and cardiovascular-related mortality (hazard ratio, 1.29; 95% confidence interval, 1.06-1.57) compared with patients treated by HD who were not bothered by pruritus.6,7
Current management of CKD-associated pruritus (CKD-aP) includes dialysis optimization, the use of emollients, and off-label therapies, including topical corticosteroids, antihistamines, and gabapentin or pregabalin.8,9 Although evidence is lacking for antihistamines, several small, randomized trials of varying quality support the use of gabapentinoids as a useful treatment for pruritus; however, there are limitations with respect to tolerability that must be considered in this population.9 Of note, gabapentinoids are not approved for the treatment of pruritus anywhere in the world. Nalfurafine, a centrally acting κ-opioid receptor agonist with partial μ-agonist activity,10 has been approved in Japan and South Korea for the treatment of pruritus in patients treated by HD. Until the approval of difelikefalin by the US Food and Drug Administration in 2021,11 and by the European Medicines Agency in 2022,12 there were no approved medications for CKD-aP in the United States or Europe.13, 14, 15
Difelikefalin, indicated in the United States and Europe for the treatment of moderate to severe CKD-aP in adults treated by HD,11,12 is a novel, selective κ-opioid receptor agonist that works by activating κ-opioid receptors on peripheral sensory neurons and immune cells.16, 17, 18 In the pivotal, phase 3 KALM-1 and KALM-2 studies, difelikefalin was shown to significantly reduce itch intensity in HD participants with moderate to severe pruritus.17,19 Here, we report the pooled safety data from the placebo-controlled periods in KALM-1 and KALM-2, as well as data from up to 64 weeks of exposure to difelikefalin from the placebo-controlled and open-label extension periods of these 2 pivotal studies and from 2 additional open-label, phase 3 supportive studies. A pooled analysis of difelikefalin efficacy is reported in a companion article.20
Methods
Study Design and Participants
This analysis reports data from the following 4 phase 3 clinical studies of intravenous (IV) difelikefalin in adults with moderate to severe pruritus associated with CKD treated by HD: KALM-119 (NCT03422653), KALM-217 (NCT03636269), CLIN3101 (NCT03281538), and CLIN3105 (NCT03998163).
KALM-1 and KALM-2 were similarly designed, randomized, double-blind, placebo-controlled, pivotal, phase 3 studies that evaluated the efficacy and safety of difelikefalin.17,19 KALM-1 was conducted in the United States and KALM-2 was conducted in North America (United States and Canada), Europe (Czech Republic, Germany, United Kingdom, Hungary, and Poland), and the Asia-Pacific region (Australia, New Zealand, South Korea, and Taiwan). Eligible participants for these studies were randomized (1:1) to receive IV difelikefalin at 0.5 mcg/kg or placebo 3 times per week at the end of each dialysis session for 12 weeks, and participants could continue into an open-label extension period of up to 52 weeks, during which all participants received IV difelikefalin at 0.5 mcg/kg 3 times per week.
CLIN3101 was a long-term (up to 52 weeks), open-label, supportive study in the United States that evaluated the safety of IV difelikefalin. CLIN3105 was an open-label, supportive study (up to 12 weeks) that evaluated the safety and effectiveness of IV difelikefalin at 0.5 mcg/kg in adults and was conducted in the United States, Czech Republic, Hungary, and Poland. Participants had a safety follow-up visit 7-10 days after the end of treatment or early termination.
The 4 studies enrolled adults with end-stage kidney disease who were treated by HD 3 times per week for at least 3 months and had moderate to severe CKD-aP at baseline. Eligible participants were aged 18 years or above in KALM-1 and CLIN3101 and 18-85 years in KALM-2 and CLIN3105. Participants eligible for CLIN3101 either enrolled in the study de novo or had previously participated in the phase 2 CLIN2005 (NCT02229929) or CLIN2101 (NCT02858726) studies.21 The dispositions of study participants for CLIN3101, CLIN3105, KALM-1 (double-blind and open-label), and KALM-2 (double-blind and open-label) are shown in Figs S1-S6.
Treatment with stable doses of antihistamines, glucocorticoids, opioids, pregabalin, and gabapentin for pruritus was permitted during the double-blind period in KALM-1 and KALM-2 and during the CLIN3105 study if established before screening; the use of new antipruritic medications after screening was not allowed. There were no antipruritic medication restrictions for CLIN3101 or during the open-label extension periods of KALM-1 and KALM-2.
All 4 phase 3 studies were conducted in accordance with ethical principles founded in the Declaration of Helsinki, International Council for Harmonization principles of Good Clinical Practice, and applicable regulations of the countries in which the studies were conducted. Institutional review boards or independent ethics committees reviewed and approved the protocols before the studies commenced (approval numbers were as follows: CLIN3101, Schulman IRB 201703410; CLIN3105, WCG IRB 20190622; KALM-1, Schulman IRB 201709004; KALM-2, WCB IRB 20181326). Participants provided written informed consent before participating in the studies.
Assessments and Analyses
Normally distributed baseline characteristics were summarized using the mean ± standard deviation, and skewed baseline characteristics were summarized in terms of percentiles. Safety was evaluated based on adverse events (AEs) and safety assessments (ie, physical examinations, vital signs, clinical laboratory tests, and electrocardiograms).
The placebo-controlled cohort included participants from the 12-week, pivotal studies (KALM-1 and KALM-2) who received IV difelikefalin at 0.5 mcg/kg or placebo 3 times per week.
The all-difelikefalin-exposure cohort included all participants who received 1 or more doses of IV difelikefalin at 0.5 mcg/kg for up to 64 weeks from the placebo-controlled periods of the pivotal studies (if randomized to difelikefalin) and from the open-label extension periods (up to 52 weeks) of these studies, as well as participants from the 2 additional open-label, phase 3 supportive studies (CLIN3101, for up to 52 weeks; and CLIN3105, for up to 12 weeks). Incidence rates were calculated as 1,000 times the number of events, divided by the total person-years (PYs). PYs were calculated as the sum of the individual participant risks for all participants in the cohort, times the number of days from their first dose to the last day of the period when an event would be deemed to be treatment emergent. Safety analyses were summarized descriptively.
Results
Participants
Safety analyses were conducted with 848 participants in the placebo-controlled cohort (424 participants each in the difelikefalin and placebo groups) and 1,306 participants in the all-difelikefalin-exposure cohort (Table 1). The most common reasons for treatment discontinuation in the placebo-controlled cohort were AEs (difelikefalin group, 6.4%; placebo group, 3.8%), followed by withdrawal by participant (difelikefalin group, 3.1%; placebo group, 1.7%). In the all-difelikefalin-exposure cohort, the most common reasons for treatment discontinuation were AEs (9.3%), other reasons (6.8%), and withdrawal by participant (5.1%).
Table 1.
Baseline Demographics and Baseline Characteristics of Participants in the Placebo-Controlled and All-Difelikefalin-Exposure Cohorts
| Characteristic | Placebo-Controlled Cohort n = 848 |
All-Difelikefalin-Exposure Cohort n = 1,306 | |
|---|---|---|---|
| Placebo n = 424 | Difelikefalin n = 424 | ||
| Age, mean ± SD, y | 58.4 ± 13.5 | 59.0 ± 12.3 | 58.3 ± 12.8 |
| Age ≥65 y, n (%) | 135 (31.8) | 143 (33.7) | 425 (32.5) |
| Male, n (%) | 257 (60.6) | 247 (58.3) | 767 (58.7) |
| Race, n (%) | |||
| White | 262 (61.8) | 253 (59.7) | 692 (53.0) |
| Black or African American | 113 (26.7) | 135 (31.8) | 494 (37.8) |
| Asian | 27 (6.4) | 18 (4.2) | 57 (4.4) |
| Other | 20 (4.7) | 17 (4.0) | 58 (4.4) |
| Unknown or not reported | 2 (0.5) | 1 (0.2) | 5 (0.4) |
| Dry body weight, mean ± SD, kg | 82.2 ± 20.3 | 83.5 ± 20.0 | 84.4 ± 21.5 |
| Time since diagnosis of ESKD, median (IQR), y | 4.1 (5.3) | 3.8 (4.8) | 4.3 (5.4) |
| Duration of pruritus, median (IQR), y | 2.5 (3.2) | 2.1 (3.2) | 2.6 (3.5) |
| Years on chronic HD, median (IQR), y | 3.9 (5.0) | 3.5 (4.8) | 4.0 (5.2) |
| Prior anti-itch medications, n (%) | 190 (44.8) | 176 (41.5) | 517 (39.6) |
| Most commonly used anti-itch medications (≥2%) | |||
| Diphenhydramine | 100 (23.6) | 104 (24.5) | 321 (24.6) |
| Hydroxyzine | 52 (12.3) | 42 (9.9) | 131 (10.0) |
| Hydrocortisone | 14 (3.3) | 11 (2.6) | 30 (2.3) |
| Cetirizine | 10 (2.4) | 7 (1.7) | 20 (1.5) |
| Clemastine | 10 (2.4) | 7 (1.7) | 18 (1.4) |
Abbreviations: ESKD, end-stage kidney disease; HD, hemodialysis; IQR, interquartile range; SD, standard deviation.
Baseline characteristics in the placebo-controlled cohort and the all-difelikefalin-exposure cohort were comparable (Table 1). Mean ages of participants were 58.7 years and 58.3 years in the placebo-controlled and all-difelikefalin-exposure cohorts, respectively; the proportion of adults aged 65 years or above was 33% across cohorts. Most participants were men and White, and approximately one-third of participants were Black or African American. The mean HD duration was 4.6 years in both cohorts, and the mean durations of pruritus were 3.2 years and 3.6 years in the placebo-controlled and the all-difelikefalin-exposure cohorts, respectively. The most common comorbid conditions based on medical history were hypertension, anemia of CKD, and diabetes in the placebo-controlled cohort and hypertension, hyperphosphatemia, secondary hyperparathyroidism, and diabetes in the all-difelikefalin-exposure cohort. Approximately 40% of participants in both cohorts reported the use of an antipruritic medication at baseline. The most commonly used anti-itch medications were diphenhydramine, hydroxyzine, hydrocortisone, cetirizine, and clemastine. Gabapentinoids were used for itch by <2% of participants.
Safety Analyses
Exposure
In the placebo-controlled cohort, most participants in the difelikefalin (79.7%) and placebo (85.1%) groups had a treatment duration of ≥3 months. Among the 1,306 participants in the all-difelikefalin-exposure cohort, the median duration of continuous difelikefalin exposure was 6.9 months; 711 participants had exposure of ≥6 months and 400 participants had ≥12 months of cumulative difelikefalin exposure. Total exposure in the all-difelikefalin-exposure cohort was 811.3 PY.
Overview of AEs
In the placebo-controlled cohort, 71.2% of participants in the difelikefalin group and 65.3% of participants in the placebo group reported ≥1 treatment-emergent AE (TEAE; Table 2). The most commonly reported TEAEs (≥2%) occurring in participants in the difelikefalin group and with a ≥1% higher incidence than placebo were diarrhea; dizziness; nausea; gait disturbances, including falls; hyperkalemia; headache; somnolence; and mental status changes (Table 2). In the majority of participants, these events were mild or moderate in severity (≥65% for any of the events), with few leading to study-drug discontinuation (≤4 participants in either treatment group). Dizziness occurred within the first 3 weeks of treatment and was generally transient, with a median duration of 1 day. Somnolence also occurred within the first 3 weeks of treatment and tended to subside with continued dosing. The incidence of somnolence was higher in difelikefalin participants aged 65 years or above (7.0%) than in difelikefalin participants younger than 65 (2.8%) and was comparable in both age groups in the placebo group (≥65 years, 3.0%; <65 years, 2.1%). Participants receiving difelikefalin who used anti-itch medications had a higher incidence of somnolence (6.3%; predominately sedating antihistamines) compared with those who did not use anti-itch medications (3.0%); in the placebo group, the incidence of somnolence was lower in participants who used anti-itch medications (1.2%) versus participants who did not use anti-itch medications (3.1%). Rates of the commonly reported TEAEs did not increase during long-term treatment with difelikefalin (Table 2).
Table 2.
Overall Safety Summary and Frequent TEAEs for the Placebo-Controlled Cohort and the All-Difelikefalin-Exposure Cohort
| Placebo-Controlled Cohort |
All-Difelikefalin-Exposure Cohort |
|||||
|---|---|---|---|---|---|---|
| Placebo n = 424 101.1 PY |
Difelikefalin n = 424 98.0 PY |
Difelikefalin n = 1,306 811.3 PY |
||||
| n (%) | IR/1,000 PY | n (%) | IR/1,000 PY | n (%) | IR/1,000 PY | |
| Overview of TEAEs | ||||||
| ≥1 TEAE | 277 (65.3) | 9,597.8 | 302 (71.2) | 10,862.9 | 1,022 (78.3) | 8,115.5 |
| ≥1 Nonfatal, serious TEAE | 96 (22.6) | 1,860.2 | 107 (25.2) | 2,040.0 | 542 (41.5) | 1,824.3 |
| AEs leading to death | 5 (1.2) | 49.5 | 3 (0.7) | 30.6 | 56 (4.3) | 69.0 |
| TEAEs leading to discontinuation | 17 (4.0) | 395.8 | 29 (6.8) | 428.4 | 121 (9.3) | 196.0 |
| Commonly reported TEAEsa | ||||||
| Diarrhea | 24 (5.7) | 267.2 | 38 (9.0) | 469.2 | 158 (12.1) | 266.2 |
| Dizziness | 16 (3.8) | 188.0 | 29 (6.8) | 316.2 | 103 (7.9) | 151.6 |
| Nausea | 19 (4.5) | 207.8 | 28 (6.6) | 326.4 | 147 (11.3) | 225.6 |
| Gait disturbancesb | 23 (5.4) | 237.5 | 28 (6.6) | 336.6 | 152 (11.6) | 267.5 |
| Hyperkalemia | 15 (3.5) | 158.3 | 20 (4.7) | 234.6 | 108 (8.3) | 157.8 |
| Headache | 11 (2.6) | 118.7 | 19 (4.5) | 214.2 | 78 (6.0) | 106.0 |
| Somnolence | 10 (2.4) | 98.9 | 18 (4.2) | 204.0 | 29 (2.2) | 39.4 |
| Mental status changec | 6 (1.4) | 59.4 | 14 (3.3) | 142.8 | 58 (4.4) | 80.1 |
Note: IR is calculated as 1,000 times the number of events divided by the total patient-years of exposure.
Abbreviations: AE, adverse event; IR, incidence rate; PY, person-year; TEAE, treatment-emergent adverse event.
Preferred terms of TEAEs reported in ≥2% of difelikefalin participants with an incidence ≥1 percentage point higher than in placebo participants.
Gait disturbances include preferred terms of falls and gait disturbances.
Mental status change includes preferred terms of confusional state and mental status change.
Four participants in the difelikefalin group and 3 participants in the placebo group experienced TEAEs of falls that occurred concurrent with TEAEs of dizziness, somnolence, or gait disturbances. The time to first onset of these falls ranged from 10-82 days and times were similar between the difelikefalin and placebo participants. The incidence rate of falls in participants in the difelikefalin group in the placebo-controlled cohort was 265.2/1,000 PY and did not increase during long-term treatment with difelikefalin (255.1/1,000 PY).
Serious TEAEs
In the placebo-controlled cohort, the incidences of nonfatal, serious TEAEs were similar between the difelikefalin and placebo groups, at 25.2% and 22.6%, respectively. The most commonly reported serious TEAEs in both groups were chest pain (difelikefalin group, 1.9%; placebo group, 0.9%), hyperkalemia (difelikefalin group, 1.9%; placebo group, 1.9%), pneumonia (difelikefalin group, 1.4%; placebo group, 1.7%), and sepsis (difelikefalin group, 1.2%; placebo group, 1.7%).
In the all-difelikefalin-exposure cohort, 41.5% of participants experienced at least 1 nonfatal, serious TEAE, with the incidence rate of 1,824.3/1,000 PY showing no increase compared with the placebo-controlled cohort (2,040/1,000 PY for difelikefalin; Table 2).
In the placebo-controlled cohort, there were 3 deaths (0.7%) in the difelikefalin group and 5 deaths (1.2%) in the placebo group. Cardiac failure, cardiac arrest, and staphylococcal sepsis were reported as the causes of death for participants on difelikefalin, and septic shock (2 participants), sepsis, cardiac arrest, and an unknown cause were reported as the causes of death for participants on placebo. All events were considered by the investigator not to be related to the study drug.
A total of 56 deaths occurred among the 1,306 participants in the all-difelikefalin-exposure cohort, with an incidence rate of 69.0/1,000 PY (Table 2). All TEAEs leading to death were considered by the investigator to be not related to the study drug. The most common system organ class of TEAEs leading to death was cardiac disorders (2.0%; 32.0/1,000 PY). The most commonly reported TEAEs leading to death included cardiac arrest (0.8%; 13.6/1,000 PY), death (0.5%; 7.4/1,000 PY), congestive cardiac failure (0.3%; 4.9/1,000 PY), myocardial infarction (0.3%; 4.9/1,000 PY), and sepsis (0.3%; 4.9/1,000 PY).
TEAEs Leading to Discontinuation
In the placebo-controlled cohort, 6.8% of participants in the difelikefalin group and 4.0% in the placebo group reported at least 1 TEAE leading to study-drug discontinuation, with dizziness being the most common reason for participants discontinuing difelikefalin (difelikefalin group, 0.9%; placebo group, 0.2%). Events of dizziness leading to discontinuation (0.5%) did not increase with long-term difelikefalin use.
Clinical laboratory results, vital signs, and electrocardiogram parameters were similar between the difelikefalin and placebo groups and showed no trends for change over time.
Discussion
Despite the impacts on patient health and quality of life, until recently, no approved treatments were available for patients with CKD-aP in the United States. In August 2021, IV difelikefalin was approved by the US Food and Drug Administration for the treatment of moderate to severe pruritus in adults with CKD treated by HD.11 To our knowledge this is the first report of a pooled analysis of the long-term safety of difelikefalin in HD patients with moderate to severe pruritus. The analysis of pooled safety data from the IV difelikefalin phase 3 clinical trial program allowed for a comprehensive characterization of difelikefalin safety for up to 64 weeks in patients with CKD-aP treated by HD. In the KALM studies, common TEAEs with difelikefalin included diarrhea; dizziness; nausea; gait disturbances, including falls; hyperkalemia; headache; somnolence; and mental status changes. Most TEAEs were mild or moderate in severity and did not increase in incidence over time; few participants discontinued study-drug use due to AEs.
Dizziness and somnolence, which are among the most common TEAEs with difelikefalin, occurred within the first 3 weeks of treatment. Dizziness was generally transient, and somnolence tended to subside with continued dosing. The incidences of somnolence were higher in difelikefalin participants aged 65 years or above and in participants on difelikefalin who used sedating antihistamines. Although falls were a commonly reported TEAE, these events were serious in <1% of participants receiving difelikefalin.
Nonfatal, serious TEAEs were balanced between the difelikefalin and placebo groups. The incidence of these events is reflective of this susceptible population of patients, who present with many clinically significant, coexisting conditions.22 In addition, the natures of the events are generally similar to common reasons for hospitalization among patients treated by HD.23
CKD treated by dialysis is associated with increased all-cause mortality relative to the general population; the death rate in patients treated by HD is 165 events per 1,000 PY.23,24 The most common comorbid condition in patients treated by HD is cardiovascular disease, which occurs in up to 76.5% of patients, with the most prevalent cardiovascular conditions being heart failure, coronary artery disease, and peripheral arterial disease.23 In addition, patients treated by HD who report being moderately to extremely bothered by pruritus have a higher prevalence of heart failure, as well as increased rates of cardiovascular-related mortality, compared with patients treated by HD who do not have severe pruritus.6,25 In the current study, most deaths that occurred (3 [0.7%] in the difelikefalin group and 5 [1.2%] in the placebo group) were caused by cardiac- or sepsis-related events, and the incidence rate of death observed in the all-difelikefalin-exposure cohort was 69.0/1,000 PY.
Although this pooled analysis presents important information on the safety profile of IV difelikefalin, there are some limitations to consider. Data have been pooled from 4 different phase 3 studies, of which 2 are open-label studies. The absence of a placebo control group makes the interpretation of open-label safety data more difficult.
In conclusion, IV difelikefalin was well tolerated, with an acceptable safety profile for up to 64 weeks in participants with CKD-aP treated by HD. No unexpected safety signals emerged during long-term difelikefalin treatment. The natures and rates of the reported safety events are consistent with published morbidity and mortality data for patients treated by HD. Together with the clinically meaningful improvements in pruritus intensity observed with IV difelikefalin in the phase 3, pivotal KALM studies, the current safety analyses demonstrate that IV difelikefalin is suitable for long-term use (up to 64 weeks) and has the potential to address an unmet need for patients with moderate to severe pruritus treated by HD.
Article Information
Authors’ Full Names and Academic Degrees
Steven Fishbane, MD, Warren Wen, PhD, Catherine Munera, PhD, Rong Lin, MD, Sukirti Bagal, MD, Kieran McCafferty, MD, Frédérique Menzaghi, PhD, and Joana Goncalves, MD.
Authors’ Contributions
Study design: WW, FM; data analysis: CM, RL, JG; data interpretation: SF, WW, CM, RL, FM, SB, KM, JG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The research was sponsored by Cara Therapeutics, Inc. Cara Therapeutics, Inc, employees contributed to the study design, data analysis and interpretation, drafting of the report, and approval to submit the report for publication.
Financial Disclosure
Dr Fishbane has received grants and is an investigator for Cara Therapeutics, Inc. Drs Wen, Munera, Bagal, Menzaghi, and Goncalves are employed at Cara Therapeutics, Inc. Dr Lin is a consultant for Cara Therapeutics, Inc. Dr McCafferty holds grants from AstraZeneca; and has received speaker honoraria and travel sponsorship and is an advisory board member for AstraZeneca, Napp, Pharmacosmos, and Vifor Fresenius.
Acknowledgments
The authors thank the study investigators and patients who participated in these studies. The authors gratefully acknowledge Hilary Wall, MS, Jiaan Illidge, MS, Xinyuan Duan, PhD, and Harper Yu, MS, for the statistical analysis. The authors also gratefully acknowledge Callie Grimes, PhD, and Illyce Nuñez, PhD (Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ), for medical writing and editorial support, which was funded by Cara Therapeutics, Inc, under the direction of the authors.
Data Sharing
Please contact Cara Therapeutics, Inc for data inquiries.
Peer Review
Received January 5, 2022. Evaluated by 3 external peer reviewers, with direct editorial input by an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form June 12, 2022. The involvement of an Acting Editor-in-Chief was to comply with Kidney Medicine’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
Figure S1: Participant flowchart for CLIN3101.
Figure S2: Participant flowchart for CLIN3105.
Figure S3: Participant flowchart for the KALM-1 double-blind phase.
Figure S4: Participant flowchart for the KALM-1 open-label phase.
Figure S5: Participant flowchart for the KALM-2 double-blind phase.
Figure S6: Participant flowchart for the KALM-2 open-label phase.
Supplementary Material
Figures S1-S6.
References
- 1.Satti M.Z., Arshad D., Javed H., et al. Uremic pruritus: prevalence and impact on quality of life and depressive symptoms in hemodialysis patients. Cureus. 2019;11(7):e5178. doi: 10.7759/cureus.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehman I.U., Lai P.S.M., Lim S.K., Lee L.H., Khan T.M. Sleep disturbance among Malaysian patients with end-stage renal disease with pruritus. BMC Nephrol. 2019;20(1):102. doi: 10.1186/s12882-019-1294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirazian S., Aina O., Park Y., et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11–26. doi: 10.2147/IJNRD.S108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel T.S., Freedman B.I., Yosipovitch G. An update on pruritus associated with CKD. Am J Kidney Dis. 2007;50(1):11–20. doi: 10.1053/j.ajkd.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Verduzco H.A., Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep. 2020;5(9):1387–1402. doi: 10.1016/j.ekir.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sukul N., Karaboyas A., Csomor P.A., et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 2021;3(1):42–53.e1. doi: 10.1016/j.xkme.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisoni R.L., Wikström B., Elder S.J., et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21(12):3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 8.Eusebio-Alpapara K.M.V., Castillo R.L., Dofitas B.L. Gabapentin for uremic pruritus: a systematic review of randomized controlled trials. Int J Dermatol. 2020;59(4):412–422. doi: 10.1111/ijd.14708. [DOI] [PubMed] [Google Scholar]
- 9.Hercz D., Jiang S.H., Webster A.C. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst Rev. 2020;12(12):CD011393. doi: 10.1002/14651858.CD011393.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan N.Q., Lotts T., Antal A., Bernhard J.D., Ständer S. Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review. Acta Derm Venereol. 2012;92(5):555–560. doi: 10.2340/00015555-1353. [DOI] [PubMed] [Google Scholar]
- 11.Korsuva (Difelikefalin) [Package Insert]. Cara Therapeutics, Inc.; 2021. Accessed April 14, 2022. https://www.korsuva.com/pi
- 12.Kapruvia Summary of Product Characteristics 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/kapruvia
- 13.Świerczyńska K., Białynicki-Birula R., Szepietowski J.C. Chronic intractable pruritus in chronic kidney disease patients: prevalence, impact, and management challenges –a narrative review. Ther Clin Risk Manag. 2021;17:1267–1282. doi: 10.2147/TCRM.S310550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayner H.C., Larkina M., Wang M., et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000–2007. doi: 10.2215/CJN.03280317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozono H., Yoshitani H., Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch® capsules 2.5 μg) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis. 2018;11:9–24. doi: 10.2147/IJNRD.S145720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer R.H., Lewis M.E., Stauffer J.W., Mathur V.S., Menzaghi F. Antipruritic effect of the long-acting peripheral kappa opioid receptor agonist CR845: a novel approach for the treatment of uremic pruritus in hemodialysis patients [abstract] J Am Soc Nephrol. 2016;27:338A. [Google Scholar]
- 17.Wooldridge T.D., Mccafferty K., Schoemig M., et al. Efficacy and safety of difelikefalin for moderate-to-severe CKD–associated pruritus: a global phase 3 study in hemodialysis patients (KALM-2) [abstract FR-OR24] J Am Soc Nephrol. 2020;31(suppl):22–23. [Google Scholar]
- 18.Albert-Vartanian A., Boyd M.R., Hall A.L., et al. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther. 2016;41(4):371–382. doi: 10.1111/jcpt.12404. [DOI] [PubMed] [Google Scholar]
- 19.Fishbane S., Jamal A., Munera C., Wen W., Menzaghi F. KALM-1 Trial Investigators. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222–232. doi: 10.1056/NEJMoa1912770. [DOI] [PubMed] [Google Scholar]
- 20.Topf J., Wooldridge T., McCafferty K. Efficacy of difelikefalin for the treatment of moderate-to-severe pruritus in hemodialysis patients: pooled analysis of KALM-1 and KALM-2 phase 3 studies. Kidney Med. 2022 doi: 10.1016/j.xkme.2022.100512. ●●●. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishbane S., Mathur V., Germain M.J., et al. Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep. 2020;5(5):600–610. doi: 10.1016/j.ekir.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combs S.A., Teixeira J.P., Germain M.J. Pruritus in kidney disease. Semin Nephrol. 2015;35(4):383–391. doi: 10.1016/j.semnephrol.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States.https://adr.usrds.org/2020/ [Google Scholar]
- 24.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukul N., Speyer E., Tu C., et al. Pruritus and patient reported outcomes in non-dialysis CKD. Clin J Am Soc Nephrol. 2019;14(5):673–681. doi: 10.2215/CJN.09600818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S6.