Abstract
The integrity of the skin is an important aspect of QOL. Whether caused by genetic deficiencies or environmental insults, disruption of the surface barrier allows irritants and allergens to penetrate the skin, which initiates inflammatory responses by immune cells that often lead to life-long allergies. In this study, eczema was induced on depilated mouse skin with topical lipopolysaccharide or a mixture of Staphylococcal enterotoxin B and an extract of house dust mites, which resulted in thickening of the epidermis, epidermal disruption, and abundant neutrophils in the dermis. Within 14 days of topical treatment with 1 μM svL4, a tetravalent peptide, neutrophils were absent, and the epidermis had returned to a normal morphology. The sequence of svL4 contains glutamine residues that serve as a cross-linking substrate for transglutaminase 2, which gains access to the skin surface where the epidermis becomes disrupted. In contrast, topical application of 1 μM svH1C, a peptide mimetic of sialic acid that lacks glutamine residues, or 1 μM dexamethasone was ineffective in restoring normal epidermal morphology. The data suggest that svL4 would be a powerful treatment for resolving severe eczema.
Abbreviations: Ca2+, calcium ion; Dex, dexamethasone; HDM, house dust mite; KC, keratinocyte; LPS, lipopolysaccharide; SEB, Staphylococcal enterotoxin B; TGM, transglutaminase
Graphical abstract
Introduction
Terminal differentiation of keratinocytes (KCs) in the epidermis produces a nearly impermeable barrier that separates the organism from its environment. Disruption of the barrier can lead to life-threatening water loss and vulnerability to serious allergen-induced inflammatory disease. The incidence of eczema (atopic dermatitis) in children is as high as 20% in some countries, peaks at ages 2‒3 years, and then declines to a few percent in adults (Nutten, 2015; Shaw et al., 2011). Genetic mutations (Bin et al., 2021; Ma et al., 2017) and environmental allergens (Sözener et al., 2020; Werfel et al., 2016) are risk factors for eczema. The prototypic genetic deficiency is loss-of-function mutations in the gene encoding the protein proFLG (Hoober and Eggink, 2022; Irvine et al., 2011; Smith et al., 2006). ProFLG is synthesized in the granular layer of the epidermis as a polyprotein that contains 10‒12 repeating domains in humans (Gan et al., 1990) but 20 repeats in the mouse (Rothnagel and Steinert, 1990) and rat (Haydock and Dale, 1990). The protein is proteolytically cleaved to monomers that facilitate the aggregation of keratin intermediate microfibrils and collapse of the corneocyte cytoplasm (Steinert et al., 1981), after which the protein is further degraded to amino acids that aid in maintaining moisture in the stratum corneum (Harding and Scott, 1983; Schwartz and Friedman, 2016; Thyssen et al., 2020). The absence of FLG leads to dry, flaky skin and a loosely packed, leaky stratum corneum (Irvine et al., 2011).
Kanemaru et al. (2019) discovered that the NC/Nga strain of mouse, which has a loss-of-function mutation in the Clec10a gene that encodes MGL1 (CD301a), a receptor expressed by macrophages in the dermis (Higashi et al., 2002), is particularly susceptible to dermatitis in response to allergens in house dust mites (HDMs). The major allergen in the HDM extract, Der f2 (Johannessen et al., 2005), induced the production of proinflammatory cytokines, which was mediated by toll-like receptor 4. This observation was followed by inducing inflammation with lipopolysaccharide (LPS), a well-known ligand for toll-like receptor 4, instead of the HDM extract. LPS, mediated by CD14, initiates a signaling pathway from toll-like receptor 4 that leads to the activation of NF-κB; release of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-8, and IL-12p40; and induction of the M1 macrophage phenotype (Ley, 2017; Liu et al., 2017; Orecchioni et al., 2019; Palma et al., 2018). A high-molecular-weight glycoprotein that is rich in terminal T (Galβ(1-3)-GalNAcα-OSer/Thr) and Tn (GalNAcα-OSer/Thr) antigens was purified from HDM and inhibited LPS-induced eczema in tape-stripped skin of wild-type but not of Clec10a‒/‒ mice. Terminal galactose is a ligand for MGL1, but whether the paralog MGL2 (CD301b), which is specific for N-acetylgalactosamine, plays a role in resistance to eczema remains unclear. MGL1 and MGL2 are markers for the M2a macrophage phenotype (Knudsen and Lee, 2016; Orecchioni et al., 2019; Westcott et al., 2009), which is essential for recovery from wounds (Greenlee-Wacker, 2016; Haas et al., 2021; Shook et al., 2016, 2018) and comprises nearly 60% of all cells in healthy murine dermis (Dupasquier et al., 2006, 2004). Humans express the C-type, N-acetylgalactosamine‒specific receptor CLEC10A, an ortholog of MGL2, but do not have an ortholog of MGL1.
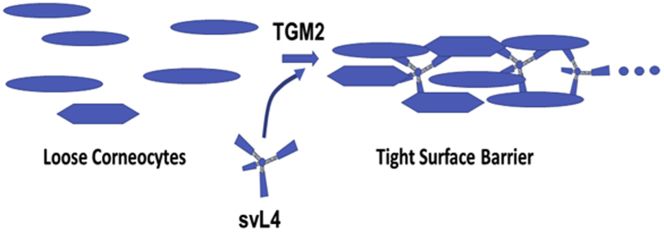
We initiated a study with a murine model of eczema to determine whether peptide mimetics of N-acetylgalactosamine, svL4 and sv6D (Eggink et al., 2018), have activity similar to that of the glycoprotein described by Kanemaru et al. (2019). However, we discovered that these peptides are also functional substrates of transglutaminase (TGM) 2, also called tissue TGM, and that structural disruption of the epidermis was repaired when these tetravalent peptides were applied topically as cross-linking substrates, regardless of possible interaction with MGL1 or MGL2. Peptides svC1 and sv6D, the N- and C-terminal halves of svL4, respectively, contain glutamine residues at the same position as svL4, but only sv6D, along with svL4, binds with high specificity and avidity to recombinant and native human CLEC10A (CD301) (Eggink et al., 2018; Hoober, 2020). Nevertheless, svC1 was also active in reducing the thickness of the epidermis. In contrast, peptide svH1C, a mimetic of sialic acid (Eggink et al., 2010, 2015), lacks glutamine and was not effective when applied topically. A low concentration of dexamethasone (Dex) was also not effective when applied alone, but treatment for only 5 days with a combination of the steroid and svL4 after induction of eczema with Staphylococcal enterotoxin B (SEB) and HDM extract significantly reduced the extent of disruptions and thickness of the epidermis. These data suggest that topical application of these peptides is an effective means to achieve the restoration of normal skin.
Results
Tetravalent peptides
The discovery and characterization of the 12-mer tetravalent peptide svL4 were described previously (Eggink et al., 2018). The arms of tetravalent sv6D contain the C-terminal half of the svL4 sequence (Table 1). Both peptides bind specifically to human CLEC10A (Hoober, 2020) and other N-acetylgalactosamine‒specific lectins (Eggink et al., 2018). svC1 contains the N-terminal half of svL4; although it does not bind to CLEC10A, it contains the same arrangement of glutamine residues one turn of an α-helix distant from each other as in svL4. svL4 and sv6D are basic peptides, with a positive charge of 12 at the acidic surface of normal skin (pH 5.0 ± 0.5) (Nguyen and Soulika, 2019; Xie et al., 2021). svH1C, a mimetic of sialic acid, binds to sialic acid‒specific plant lectins (Eggink et al., 2010); and recombinant human siglecs (Eggink et al., 2015).
Table 1.
Peptide Sequences
| Sequence | Code | MW |
|---|---|---|
| [(VQATQSNQHTPR-GGGS)2K2]K-NH2 | svL4 | 6,826 |
| [(NQHTPR-GGGS)2K2]K-NH2 | sv6D | 4,369 |
| [(VQATQS-GGGS)2K2]K-NH2 | svC1 | 3,893 |
| [(NPSHPLSG-GGGS)2K]2K-NH2 | svH1C | 4,592 |
Abbreviation: MW, molecular weight.
svL4 promoted the healing of epidermal disruptions induced by LPS
In our experiments, dorsal skin of mice was shaved, depilated with thioglycolate and sodium hydroxide (Nair), and washed with 70% ethanol. Treatment with 4% SDS as used by Kanemaru et al. (2019) caused extensive damage to the skin, which led us to treat the skin every third day with 1% SDS for 2 hours, which served as a penetration-enhancing treatment. The irritant LPS and peptides were applied daily to a 1-cm2 area of depilated skin (Figure 1a). At the end of the 14-day treatment period, the skin was excised and sectioned for immunohistochemical analysis to determine the extent of injury. Skin treated with LPS contained a thick epidermis, and most sections revealed one or more areas of extensive epidermal damage. Our designation of epidermal disruption included breaks in the integrity of the surface barrier; disarrangement of the epidermis with loss of the basement membrane; and occasionally, nearly complete loss of the epidermal epithelium. Because we considered a disruption of a thickened epidermis an extreme state of eczema and the repair of these areas an essential process in healing, we initially quantitated the effects of 1 μM solutions of the peptides when included in the topical application with LPS or administered by subcutaneous injection of 1 nmol/g. Shown in Figure 1b is the extent of surface disruption among the sections, which typically varied from a thickened epidermis with no disruption to skin with about one third of the surface areas containing extensive damage. This variability was also a function of the path taken by the section through the affected area. Topical treatment with svL4 restored the integrity of the skin surface, with no epidermal disruptions (Figure 1b); however, no recovery occurred when the peptide was injected subcutaneously. In contrast, svH1C was effective when injected subcutaneously but not when applied topically. Although lesions were absent after injections of svH1C, most areas of the skin contained a thickened epidermis and abundant neutrophils in the dermis (not shown).
Figure 1.
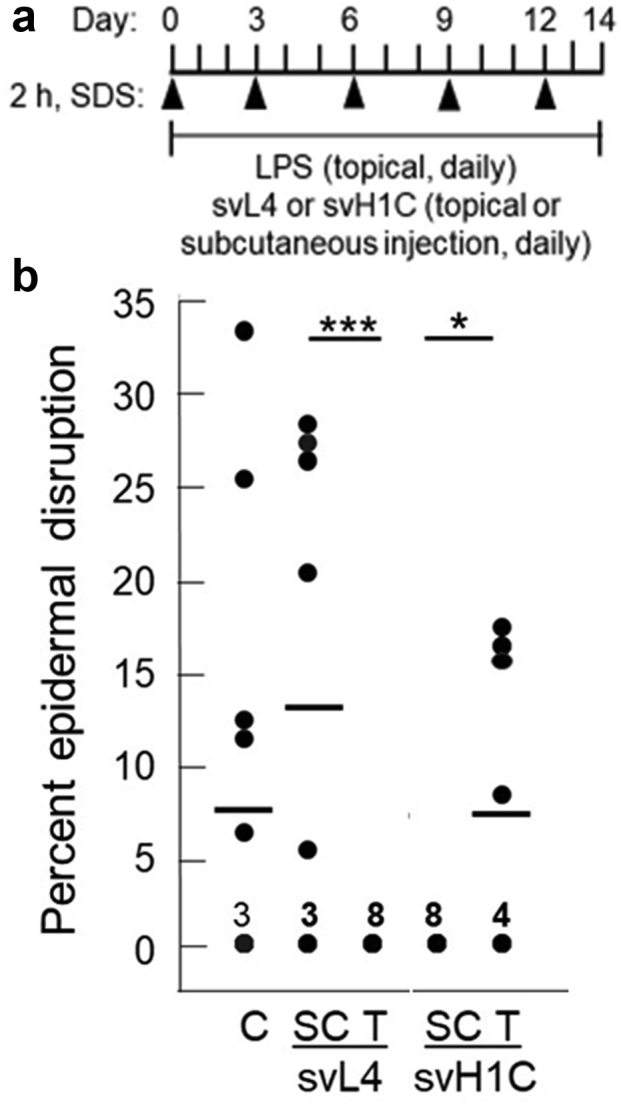
Extent of epidermal disruption after treatments with svL4 or svH1C. (a) Schematic of the experimental design after depilation of the skin. A 2-h treatment with 1% SDS was applied every 3 days. Eczema was induced by daily application of 200 μl of PBS containing 1 μg LPS per 1 cm2 area of the skin. svL4 or svH1C was added to the LPS solution to a final concentration of 1 μM for topical treatment (T). The peptides were injected subcutaneously at a dose of 1 nmol/g body weight (SC). (b) Two sections, one stained with H&E and the other with anti-Ly6G, were analyzed from each of the four mice in each group after 14 days of treatment (n = 8 for each group). The cumulative area of disruption of the epidermis is expressed as a percentage of the total length of 1-cm long sections. Dots indicate the values for each section, which ranged from zero to 33%. The number of sections that did not contain a disruption of the surface barrier and received a score of 0 is indicated for each group. Horizontal bars indicate mean values for each group. C depicts LPS-treated skin, which served as the control. Statistical data were analyzed, with all values per group, by one-way ANOVA test for pairs of treatment groups indicated by the bars at the top of the figure. ∗P = 0.022 and ∗∗∗P = 0.0056. C, control; h, hour; LPS, lipopolysaccharide; SC, subcutaneous; T, topical.
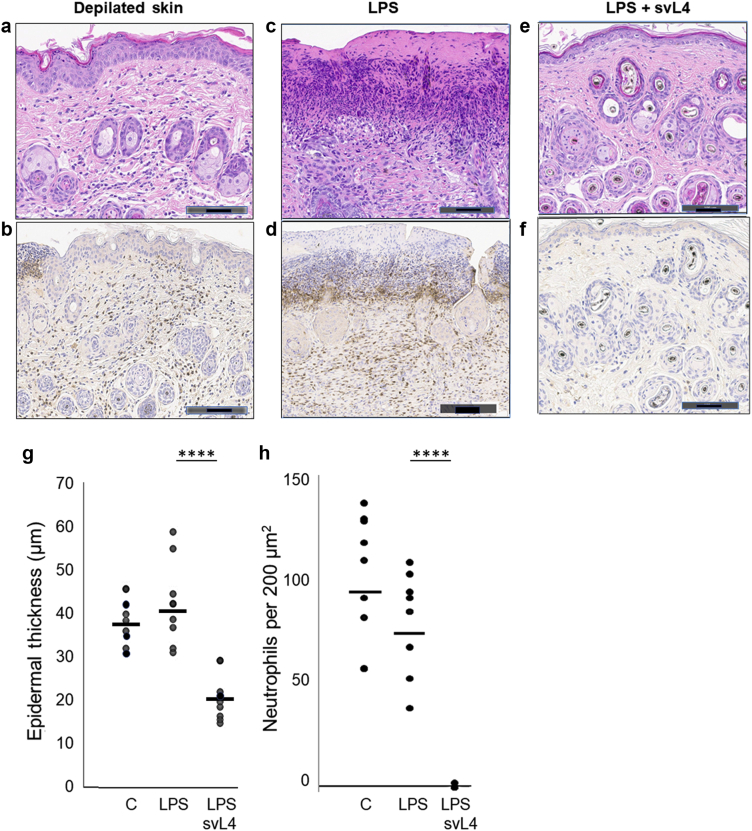
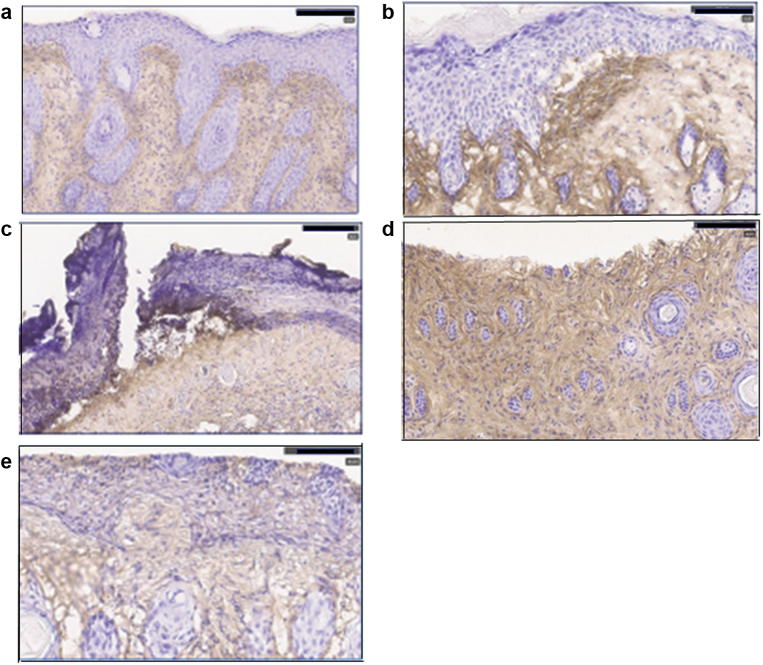
Immunohistochemical analyses of sections of treated skin showed that the process of depilation resulted in the thickening of the epidermis and frequent neutrophils in the dermis (Figure 2a and b). Daily treatment with LPS caused more extensive disruption of the epidermis and a high density of neutrophils in the dermis underlying these areas (Figure 2c and d). When 1 μM svL4 was included in the topical application with LPS, the epidermis at the end of the 14-day treatment was uniformly of normal thickness, and the dermis was nearly free of neutrophils (Figure 2e and f). Graphical representations of epidermal thickness and neutrophil frequency are presented (Figure 2g and h). Measurements of neutrophil frequency were made in areas of the dermis distant from those of the disruptions of the epidermis, which contained a very high abundance, as shown in Figure 2d. Although subcutaneous injections of svL4 did not reduce epidermal thickness, an increased density of dermal collagen was noted as thicker bundles with more intense eosin staining (not shown)
Figure 2.
Changes in the morphology of skin during treatment with svL4. Images were obtained at the end of a 14-day treatment. Depilation resulted in (a) a thick epidermis and (b) frequent Ly6G+ neutrophils in the dermis, which were identified by their brown stain. (c) A 2-hour treatment with 1% SDS every 3 days and daily application of LPS resulted in a thick epidermis, areas where the definition of the epidermis and the basement membrane were lost and d) an abundance of neutrophils underlying these disruptions. Inclusion of 1 μM svL4 in the topical LPS-containing dressing resulted in (e) normal morphology with thin epidermis and (f) a dermis that was essentially free of neutrophils. Bars = 100 μm. Graphical representations of the images show (g) epidermal thickness under the stratum corneum measured at 10 sites along 1-cm sections of the skin stained with H/E or anti-Ly6G. Two sections from each of the four mice per group were analyzed (n = 8). Dots represent the mean of measurements for each section. (h) Neutrophils were counted within two defined areas of the dermis on each section stained with anti-Ly6G. The neutrophil abundance for each area is indicated by a dot. The horizontal bars indicate the mean for each group. C depicts the depilated skin treated with PBS. Statistical data were calculated for pairs of treatment groups indicated by the bars at the top of the figure. ∗∗∗∗P = 0.000048 for g and 0.00001 for h. LPS, lipopolysaccharide.
svL4 reduced disrupted areas after induction with HDM and SEB
To determine whether svL4 is effective against irritants other than LPS, for example, serve as a general treatment, eczema was also induced with a combination of HDM extract and SEB, which are commonly encountered allergens (Kawakami et al., 2007). The design of the experiment is shown in Figure 3a. The allergens were applied to depilated skin for 4 days, whereafter the skin was treated with 2 μM svL4 for another 5 days alone or in combination with 1 μM Dex. With this experiment, we sought to determine the extent of repair of the epidermis during this short period of treatment and also whether svL4 actually reduced the severity of eczema when applied to inflamed skin or simply inhibited the action of irritants and allergens. As shown in Figure 3b, the application of svL4 dramatically reduced epidermal disruptions after the allergens had been removed. Topically applied Dex resulted in no reduction in epidermal disruption compared with that of untreated skin. However, disruptions were detected in only a few sections from animals treated with Dex plus svL4. The efficacy of treatment with svL4 alone was similar to that of the combination of svL4 and Dex, although several sections had small disruptions. The number of sections without a disruption in a group suggests a progression of healing from the Dex-treated group to the groups treated with the combination or svL4 alone (Figure 3).
Figure 3.
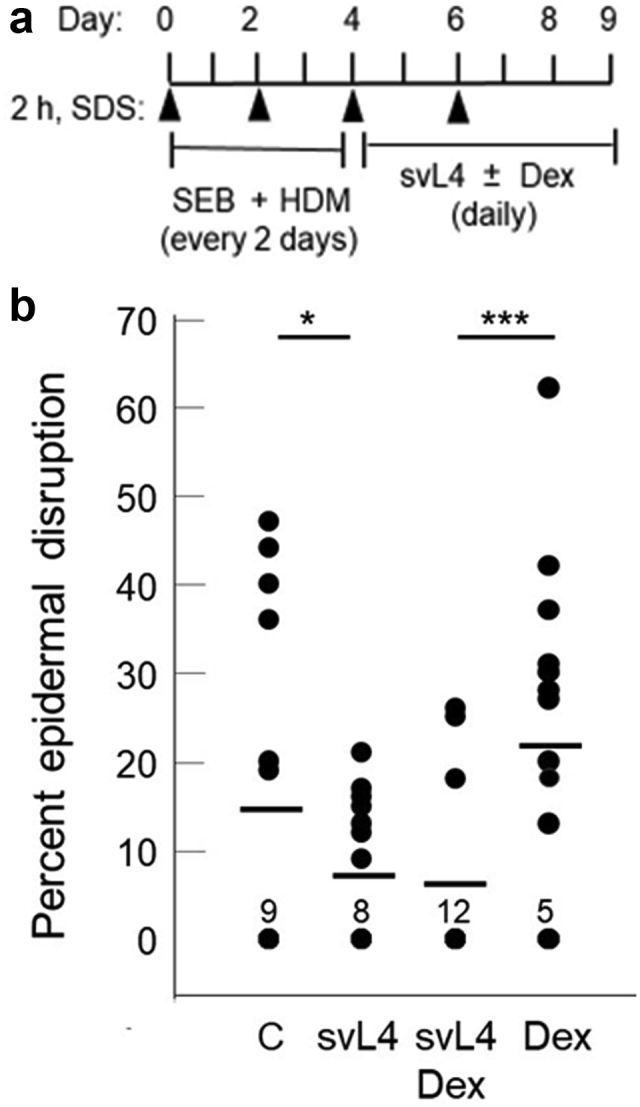
The extent of epidermal disruptions after topical treatment with SEB and HDM. (a) Schematic of the experimental design after depilation of the skin. A 2-h treatment with 1% SDS was applied every other day. SEB (100 μg) and HDM extract (10 μg) were applied in 200 μl of PBS to a 1-cm2 area of skin on days 0 and 2. The allergens were removed after 4 days of induction of eczema, and then svL4 (2 μM) was applied topically alone in 200 μl of PBS or in combination with 1 μM Dex for another 5 days to the affected areas. The topical dressings were replaced every day. (b) The extent of disrupted areas was determined on three 1-cm long sections from each of the five mice in each group at the end of the 9-day treatment (n = 15). Dots indicate the values for sections with disruptions of the epidermis, which are expressed as a percentage of the length of the section. The number of sections that did not contain a disruption of the surface barrier and received a score of 0 is indicated for each group. C depicts the allergen-induced control group treated with PBS. Horizontal bars indicate the mean for each group. Statistical data were calculated for the paired groups indicated at the top of b. ∗P = 0.052 and ∗∗∗P = 0.0041. Dex, dexamethasone; h, hour; HDM, house dust mite; SEB, Staphylococcal enterotoxin B.
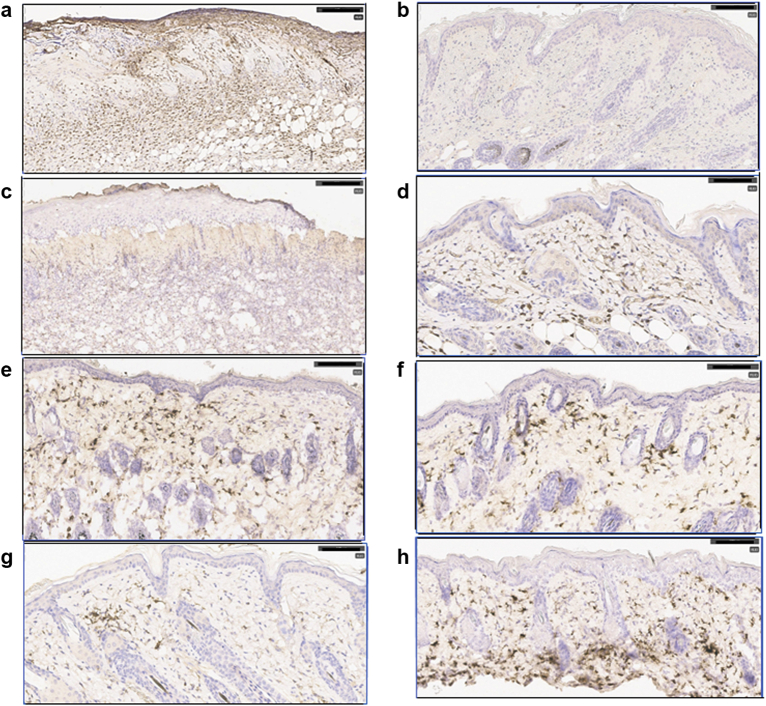
Similar to the results shown in Figure 2, skin from animals treated with HDM and SEB contained a thickened epidermis and extensive loss of structural integrity. The basement membrane between the epidermis and dermis was often obliterated, and dense populations of neutrophils were evident within damaged areas (Figure 4a). Neutrophils were essentially absent in the dermis underlying a thin epidermis after treatment with svL4 (Figure 4b). Because CD301b+ macrophages are essential for healing, sections of skin were stained with antibodies against murine MGL1 (CD301a) and MGL2 (CD301b), C-type lectin receptors expressed by M2a macrophages, and dendritic cells (Higashi et al., 2002; Krzyszczyk et al., 2018; Palma et al., 2018; Zhang et al., 2011). CD301a+ and CD301b+ cells were absent in the dermis underlying areas where the integrity of the epidermis was lost (Figure 4c) but were abundant in the dermis of svL4-treated skin (Figure 4d‒f). Whereas the CD301b+ cells were more frequent near the epidermis after treatment with svL4, these cells were more abundant in the lower dermis in the skin treated with Dex (Figure 4h). The reciprocal relationship between neutrophils and CD301b+ macrophages was a definitive marker of healing. The dermis contained a paucity of CD11c+ dendritic cells (Figure 4g), which also express CD301b.
Figure 4.
Induction of eczema with SEB and HDM. Shown in the Figure are sections of skin treated for 5 days with svL4 or svL4 plus dexamethasone after a 4-day induction of eczema with SEB and HDM. Sections from all mice in the experiment shown in Figure 3 were viewed to select representative images for this figure. (a) Section stained with anti-Ly6G from a mouse treated with PBS after induction of eczema revealed extensive epidermal damage and a dense population of neutrophils. (b) svL4-treated skin had a restored epidermis and a dermis essentially free of neutrophils. (c) Section from the same mouse as in a stained with ER-MP23 (anti-CD301a/b), which revealed the absence of macrophages in areas that lacked a definition of the epidermal/dermal boundary. (d) Section from the same mouse as in b treated with svL4, which had a thin epidermis and an abundance of ER-MP23-stained macrophages. (e, f) svL4-treated skin stained with anti-CD301b. (g) Section of skin from a mouse treated with dexamethasone and stained with anti-CD11c, which contained a cluster of dendritic cells. (h) Section from a mouse treated with dexamethasone and stained with anti-CD301b. Positive staining is indicated by the brown color. The images are representative of mice in each group (n = 5). Bar = 100 μm. HDM, house dust mite; SEB, Staphylococcal enterotoxin B.
svL4 is a substrate for TGM2
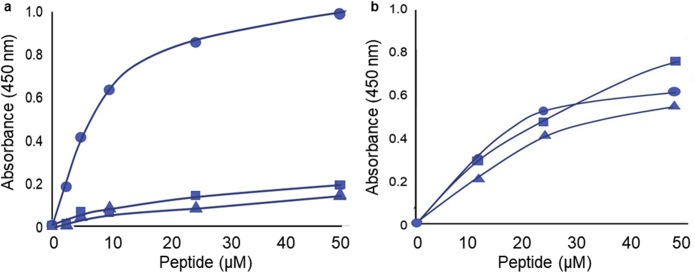
We then considered the possible mechanisms by which svL4 could provide a therapeutic action. An important aspect was the efficacy when applied topically but not when given subcutaneously. A particularly intriguing feature of the peptide is the arrangement of glutamine residues. We therefore asked whether the peptide is a substrate for TGM2. As shown in Figure 5a, TGM2 actively catalyzed a reaction in which biotinylated svL4 was linked to polylysine. A Km of approximately 7 μM was calculated, with svL4-biotin as a substrate, which compares favorably with the Km of 1 μM for a standard peptide, biotinyl-TVQQEL (Trigwell et al., 2004). We found that biotinylated sv6D and svC1 were poor substrates when assayed immediately after dissolving commercial lyophilized TGM2 in water containing 1 mM EDTA, pH 7.5, and 10 mM dithiothreitol (Figure 5a), but surprisingly, when the solution of TGM2 was stored over several days at 5 ºC in this medium, the enzyme lost over half of the activity with svL4 but gained the ability to use sv6D and, to a lesser extent, svC1 as substrates (Figure 5b). This observation suggested that the enzyme unfolds to a less specific conformation over time. The concentration dependence also suggests higher Km values for svC1 and sv6D with the aged enzyme than for svL4.
Figure 5.
Assay of svL4, svC1, and sv6D as substrates for TGM2. The cross-linking reaction was performed in microtiter wells with bound polylysine as acceptor. The results shown with biotinylated svL4 (circles), svC1 (triangles), and sv6D (squares) are representative of the results of the three assays for each panel. (a) The assays were performed with enzyme solutions freshly prepared from a lyophilized sample from the supplier. (b) The assays were performed after storage of the enzyme solution for 6 days at 5 ºC. TGM2, transglutaminase 2.
The tissue distribution of TGM2 was determined by immunohistochemical analysis of frozen sections after induction of eczema for 4 days with a combination of HDM extract and SEB and 5 days of treatment with svL4 (the experiment shown in Figure 3). In depilated control samples, TGM2 was detected under the epidermal/dermal boundary and around hair follicles (Figure 6a). After induction of eczema, intense staining with anti-TGM2 was often restricted to the dermis in the skin with a thickened epidermis (Figure 6b). However, where the epidermis was severely damaged, the layer of tissue containing TGM2 was exposed on the surface (Figure 6c). TGM2 was also detected among KCs in areas in which an early (Figure 6d) and later (Figure 6e) phase of re-epithelialization appeared to have occurred. The diffused appearance of the antibody stain suggests the presence of an extracellular enzyme.
Figure 6.
Immunohistochemical localization of TGM2. Eczema was induced on depilated mouse skin with SEB/HDM for 4 days as described in the legend of Figure 3. The induction period was followed by treatment with (a) PBS or (b‒e) 2 μM svL4 in PBS for another 5 days. Accumulation of TGM2 is indicated by the brown stain on frozen sections that were incubated with anti-TGM2. Bar = 180 μm for a‒c and 100 μm for in d and e. HDM, house dust mite; SEB, Staphylococcal enterotoxin B; TGM2, transglutaminase 2.
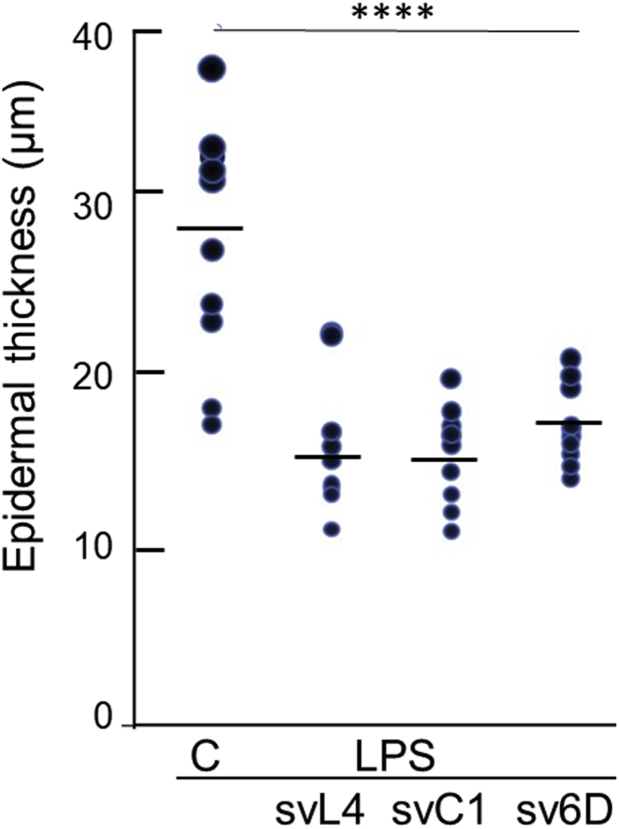
We then asked whether svC1 and sv6D have activity similar to that of svL4 when applied topically to the skin. In this experiment, the skin was treated topically with 2 μM peptides along with LPS for 14 days. Sections were then stained with anti-Ly6G, and epidermal thickness was measured. As shown in Figure 7, the three peptides were essentially equally effective in reducing the epidermis to the approximate average normal thickness for murine viable epidermis, excluding the stratum corneum, of 17.5 μm (Wei et al., 2017). The mean values of the thicknesses of peptide-treated mice were 16.1 μm for svL4, 15.5 μm for svC1, and 17.1 μm for sv6D. The dermis of peptide-treated skin also lacked neutrophils (not shown).
Figure 7.
Reduction in epidermal thickness by topical treatment with peptides. The experimental design was similar to the schematic shown in Figure 1. Peptides (2 μM) were added to LPS treatment for 14 days. Measurements of thickness under the stratum corneum were made at 20 sites along 1-cm long sections stained with anti-Ly6G. Two sections were analyzed from each of the five mice in each group (n = 10). Dots represent the mean values for each section. C depicts the depilated LPS-treated control. Statistical data were calculated for peptide-treated epidermis compared with the LPS control. ∗∗∗∗P = 0.00018 for svL4, 0.000056 for svC1, and 0.0002 for sv6D. LPS, lipopolysaccharide.
Discussion
A role for TGM2
Yokouchi et al. (2015) described a vicious cycle in which impairment of the surface barrier allows the penetration of allergens, which causes chronic inflammation, disturbed KC differentiation, and altered tight junction formation with further disruption of the barrier. Elias (2018) and Lee (2020) also argued that the inflammatory immune responses in eczema are secondary to the disruption of the surface barrier of the skin. Elias (1983) introduced the concept of the stratum corneum as a wall composed of corneocyte bricks sealed together with a lipid mortar composed of a mixture of ceramide, cholesterol, and fatty acids, which was replicated as a topical barrier-repair therapy for eczema (Elias, 2022; Yang et al., 1995). However, integral to barrier function is the strength of the interaction between the bricks. In addition to allowing penetration of allergens, loss of the barrier function can lead to dehydration and death shortly after birth (Kasparek et al., 2017; Leyvraz et al., 2005). Restoration of the surface barrier allows the epidermal calcium ion (Ca2+) gradient to form, which is critical for KC differentiation and cornification (Lee, 2020; Lee and Lee, 2018; Teshima et al., 2020).
TGM activities are essential for terminal differentiation of the epidermis and formation of the cornified layer (Lorand and Graham, 2003). Three isozymes of the Ca2+-dependent TGM family—TGM1, TGM3, and TGM5—are expressed in the epidermis and function within terminally differentiating KCs (Eckert et al., 2005). Although confined to the cellular interior, these TGMs could possibly be released from KCs as a result of trauma (Gross et al., 2003). However, unlike other members of the family, TGM2 is ubiquitously expressed and is secreted into the extracellular space (Chou et al., 2011; Griffin et al., 2002; Johnson et al., 1998; Zemskov et al., 2011). The activity of TGM2 is dependent on Ca2+, with an activation constant, KA, of 6.9 mM (Folk et al., 1967). A concentration this high is achieved at the peak of the Ca2+ gradient in the granular layer of the epidermis (Elias et al., 2002; Lee and Lee, 2018; Mauro et al., 1998), but the gradient is dissipated by disruption of the barrier. However, sufficient Ca2+ is apparently retained for some level of activity of TGMs.
Substrate specificity of TGM
As KCs terminally differentiate into corneocytes, an early step in the formation of the cornified envelope is an attachment of involucrin to the inner surface of the cell membrane in a reaction catalyzed by the essential enzyme, TGM1 (Candi et al., 2005; Eckert et al., 2005; Kalinin et al., 2001; Lee, 2020). Highlighting the specificity of TGM1, involucrin contains 39 repeats of a 10 amino acid sequence, with each containing three glutamine residues (Eckert and Green, 1986), but only one glutamine residue within this 585-amino acid protein—in the sequence ELPEQQVGQP (reactive Q is in bold)—is reactive in vitro unless the protein is proteolytically degraded (Simon and Green, 1988). The activity of TGM1 is required for the formation of cross-links between structural proteins and loricrin during the formation of the intracellular cornified envelope (Candi et al., 2005; Eckert et al., 2005; Ishitsuka and Roop, 2020; Kalinin et al., 2001; Lee, 2020).
Monovalent peptides were identified as specific substrates for several TGM isozymes (Fukui et al., 2013; Sugimura et al., 2008; Tanabe et al., 2019). TGM2 is also remarkably specific for the glutamine residues that provide the substrate for the first step in the cross-linking reaction. The sequences glutamine-X (any amino acid)-proline (QXP), QQ, and QXXQ are preferred targets for TGM2, whereas QP and QXXP are poor substrates (Fleckenstein et al., 2002; Vader et al., 2002). The sequence in sv6D (QXXP, see Table 1) is unfavorable, whereas svC1 contains a favorable sequence (QXXQ), but both were poor substrates in vitro with freshly prepared enzyme solutions. However, both contain an active Q in position 2 of the peptide (Hitomi et al., 2009; Pastor et al., 1999). The short arms of sv6D and svC1 may be hindered from entering the active site by the remainder of the tetravalent peptides. Alternatively, the low activity of sv6D and svC1 with freshly prepared enzyme may result from Km values for these peptides much higher than that of 7 μM for svL4 when assayed under the same conditions. For example, a frequently used substrate to study TGM2, carbobenzoxy-L-glytaminylglycine, has a Km of 22 mM (Folk and Cole, 1965). The kinetics of the reaction suggest that glutamines that appear unreactive may simply react more slowly (Christensen et al., 2014).
The acceptor amine group for the second step in the cross-linking reaction is less restrictive, although N-terminal amino groups of peptides are not active acceptors. Primary alkyl amines such as putrescine, spermidine, or the side chain of lysine are active second substrates (Schrode and Folk, 1978). The reaction catalyzed by TGM2, shown in Figure 5, demonstrated that the enzyme can use glutamine from the arms of svL4, svC1, and sv6D to link with the ε-amine groups of lysine residues to form ε(γ-glutamyl)lysine isopeptide bonds. This reaction with the peptides would form cross-links without the restrictive specificity for endogenous glutamine.
Possible functions of other TGM
Expression of TGM2 is induced in inflamed and wounded tissues (Haroon et al., 1999; Griffin et al., 2002), by Dex (Johnson et al., 1998), and in KCs under inflammatory conditions (Bowness and Tarr, 1997; Eckert et al., 2005) and is reported to facilitate wound healing (Eckert et al., 2005; Griffin et al., 2002; Gross et al., 2003; Haroon et al., 1999; Stephens et al., 2004; Verderio et al., 2004). Knockout of TGM2 normally has no significant physiological consequence, which suggests that other members of the TGM family compensate for the absence of TGM2 (De Laurenzi and Melino, 2001; Nanda et al., 2001; Odii and Coussons, 2014), although closure of wounds was significantly impaired in Tgm2-knockout mice (Iismaa et al., 2009; Mearns et al., 2002; Sarang et al., 2009). Furthermore, processes required for wound healing and extracellular matrix remodeling are dramatically reduced when fibroblasts and macrophages are rendered TGM2 deficient by transfection with antisense RNA (Stephens et al., 2004; Telei and Griffin, 2006). TGM2 normally occurs in an inactive, closed conformation but is rapidly converted to the open, active form by injury (Griffin et al., 2002; Pinkas et al., 2007).
Although we have emphasized a possible role for TGM2 in recovery from eczema, TGM3 may also be important. TGM2 and TGM3 are expressed in all layers of the epidermis in humans (Chermnykh et al., 2020; Su et al., 2017), but TGM2 is expressed predominantly in the dermis of mice (Figure 6). As with TGM2, knockout mutation of Tgm3 causes no significant physiological effect in mice, but in contrast to Tgm2, this mutation has no obvious effect on wound healing (John et al., 2012). TGM3 is expressed at high levels in KCs in inflamed skin. Liedén et al. (2012) and Su et al. (2020) showed a dramatic increase in the expression of TGM in the epidermis of patients with atopic dermatitis. TGM3 has also been detected extracellularly and is present in the cornified layer (John et al., 2012). The lack of apparent involvement of TGM3 in the formation of the epidermal barrier may result from a paucity of suitable glutamine-containing substrates.
The absence of TGM1 in mice leads to death within a few hours after birth (Candi et al., 2005; Matsuki et al., 1998) and causes congenital ichthyosis, a severe skin disease, in humans. Remarkably, therapy by topical replacement of TGM1 corrected skin architecture and dramatically reduced transepidermal water loss (Aufenvenne et al., 2013).
A possible target for cross-linking
A possible target to which TGMs may form cross-links with svL4 is lysine residues in corneodesmosin, a major component along with desmoglein 1 and desmocollin 1 of corneodesmosomes (Caubet et al., 2004; Igawa et al., 2013), which occur at the tips of villus-like structures on the surface of corneocytes (Riethmüller et al., 2015). Corneodesmosin is synthesized in the lower granular layer of the epidermis and is secreted in vesicles from KCs as a glycoprotein (Guerrin et al., 1998; Simon et al., 1997). Loops in the N-terminal domain are exposed on the cell surface and are binding sites for colonizing Staphylococcus aureus in atopic dermatitis lesions (Towell et al., 2021). Several lysine residues lie within the sequence near these loops and conceivably would provide the amino groups for cross-linking. Whereas corneodesmosomes occur at the periphery of flattened wild-type corneocytes, they cover the entire surface of loosely packed homozygous FLG-deficient (FLG‒/‒) mutant cells. Moreover, these structures are approximately five-fold more abundant on FLG‒/‒ cells than on wild-type cells (Riethmüller et al., 2015). The villus projections extend up to several hundreds of nanometers into the extracellular space between cells and conveivably would be readily available to engage in cross-linking.
To our knowledge, this is a previously unreported use of tetravalent peptides as a treatment for eczema. The efficacy of topical application of svL4 (Figure 1) supports a role for the physical presence of the peptide on the surface of the skin. The most likely role of the peptide is to provide a glutamine-containing cross-linking substrate for TGMs. Further analysis of the details of the mechanism of the svL4/TGM2 effect in the reconstruction of the surface barrier, and questions that this study has raised regarding the involvement of the immune system require additional research.
In summary, our data suggest that the ability of svL4 to serve as a treatment for eczema is provided by the tetravalent properties of the peptide, which allow cross-linking of multiple proteins and cells catalyzed by extracellular TGMs. TGM2 was exposed on the skin surface in areas where the epidermis was disrupted or disarranged and was expressed in epidermal tissue during re-epithelialization. Without exogenous glutamine residues provided by the peptides, repair of the epidermis did not occur within the 2-week period of treatment. Foremost among the observations during treatment with svL4 was the repair of areas of structural disruption of the epidermis, reduction of the thickness of the epidermis, and a loss of neutrophils in the dermis. Efficacy of topical svL4 supports a role for the peptide on the skin surface. Thus, we propose that the primary role of the peptides in resolving eczema is related to their ability to serve as a substrate for TGMs to achieve cross-linking of structural proteins or cells to reconstruct a functional stratum corneum. Toxicity and antigenicity have not been detected in our studies with the peptides in animals (Eggink et al., 2018).
Materials and Methods
Peptides
Synthesis and purification of endotoxin-negative svL4, svC1, and sv6D (Eggink et al., 2018) and svH1C (Eggink et al., 2015) were described previously.
Animals
The animal studies were conducted at Biomodels LLC (Waltham, MA), an Association for Assessment and Accreditation of Laboratory Animal Care‒accredited facility in Watertown, MA. Approval for this work was obtained from Biomodels LLC Institutional Animal Care and Use Committee (protocol number 17-0613-4). Female C57BL/6J mice aged 10 weeks were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed at Biomodels LLC.
Induction of disease
LPS
On day minus-1, all mice were anesthetized with inhaled isoflurane, and the hair was removed from the dorsal skin using electric clippers followed by depilation with thioglycolate and sodium hydroxide (Nair). The skin was then washed with 70% ethanol followed by PBS to ensure no depilation reagent remained. On day 0, mice were again anesthetized with inhaled isoflurane, and a 1-cm2 square was drawn on the center of the back skin to direct the placement of gauze. A 1% SDS solution was applied to a 1-cm2 sterile piece of gauze secured to the hair-free back skin with a bio-occlusive dressing (Tegaderm). Two hours after SDS treatment, mice were again anesthetized with inhaled isoflurane, and the Tegaderm and gauze were removed. The skin was blotted dry with sterile gauze. For treatment groups, 0.1 ml of a 10 μg/ml solution of LPS (E. coli 0111:B4, catalog number L2630, MilliporeSigma, Burlington, MA) and 0.1 ml of PBS or 2 μM peptide in PBS were applied to a 1-cm2 sterile piece of gauze secured to the hair-free back skin with a bio-occlusive dressing (Tegaderm). Every 3 days, the SDS application was repeated 2 hours before topical gauze application of the treatment solution, which was replaced every 24 hours with fresh LPS and peptide. On day 14, all mice were killed by carbon dioxide inhalation, and skin was collected for histological analysis.
HDM and SEB
A similar experiment was conducted with 0.1 ml of 1 mg/ml SEB (100 μg, catalog number BT202, Toxin Technology, Sarasota, FL) and 0.1 ml of 0.1 mg/ml HDM extract (10 μg, catalog number B81, Greer Laboratories, Lenoir, NC), in which eczema was induced by a 2-hour treatment with 0.2 ml of 1% SDS followed by replacement of the allergen mixture in 0.2 ml for 2 days. The SDS/allergen treatment was then repeated for a second 2-day period. The skin was then washed with PBS. The 2-hour SDS treatment was continued every other day, with 2 μM svL4 in PBS alone or mixed with 1 μM Dex sodium phosphate (catalog number 2480645, Henry Schein, Melville, NY) in 0.2 ml of PBS applied daily for another 5 days.
Histological analyses
At the end of the treatment period, mice were killed by carbon dioxide inhalation, and a 1-cm2 portion of the skin was excised. One half was fixed in formalin for histopathological analysis, whereas the other half was flash frozen. Histological analysis by H&E staining, measurement of epidermal thickness, and immunostaining were performed by Inotiv Boulder (Boulder, CO) with the Leica Bond RX/RXm platform using standard chromogenic methods or by Dallas Tissue Research (Farmers Branch, TX). Neutrophils were stained on fixed sections with monoclonal anti-Ly6G, whereas frozen samples were embedded in optimal cutting temperature compound, and sections were stained with monoclonal anti-CD301b or anti-TGM2 (See Table 2 for the antibodies used in this study). Image capture and analysis were performed with Aperio’s ImageScope program or with EasyScan Pro 6 digital slide scanner.
Table 2.
Antibodies used for Immunohistochemistry
| Ab Target | Cell Type | Clone | Catalog Number | Vendor |
|---|---|---|---|---|
| Ly6G | Neutrophil | 1A8 | 551459 | BD Biosciences |
| CD301a | Macrophage | ER-MP231 | NB100-64874 | Novus Biologicals |
| CD301b | Macrophage | 11A10-B7 | 50-3011-82 | Thermo Fisher Scientific |
| CD11c | Dendritic cell | D1V9Y | 97585S | Cell Signaling Technology |
| TGM2 | D11A6 | 3557S | Cell Signaling Technology |
Abbreviation: Ab, antibody.
ER-MP23 reacts with a common epitope in CD301a and CD301b (Denda-Nagai et al., 2010).
Analysis of tissue sections
The cumulative length of epidermal disruptions on 1-cm long sections of fixed tissues was measured at a magnification of ×20 and related to the total length of the section. Epidermal thickness of each sample was measured at 10 or 20 sites along 1-cm long, H&E- or anti-Ly6G‒stained sections without histological artifacts, perpendicular to the long axis of the sections, encompassing the basal layer through all cell layers, excluding the stratum corneum, and averaged to obtain the mean thickness for each section. The number of neutrophils in square areas of the dermis, 200 μm on a side, was counted with a magnification of ×320 on images of sections stained with anti-Ly6G.
TGM assay
The assay was performed in polylysine-coated microtiter wells. The reaction mixture contained, in 50 μl, 100 mM Tris hydrogen chloride, pH 7.5, 10 mM calcium chloride, 5 mM dithiothreitol, 1 mM EDTA, 150 mM sodium chloride, and 0.05% Tween-20. Lyophilized TGM2 from guinea pig liver (1.5 units/mg, catalog number T5398, Sigma-Aldrich, St. Loius, MO) was dissolved in 1 mM EDTA, pH 7.5, containing 10 mM dithiothreitol. Freshly prepared TGM2 was assayed with 10 ng protein/well, whereas 40 ng protein/well was used with enzyme stored at 5 ⁰C for 6 days. After a 2-minute activation period, biotinylated peptides were added to provide a series of concentrations from 0.5 to 50 μM. After 30 minutes of incubation, biotin bound in the wells was detected with streptavidin conjugated with horseradish peroxidase and 3,3’,5,5’-tetramethylbenzidine. The reaction was stopped with 1 N sulfuric acid and read immediately at 450 nm.
Statistical analyses
Quantitation of disrupted areas, epidermal thickness, and neutrophil abundance was analyzed by the one-way ANOVA test. Significance was set at P ≤ 0.05 for all tests.
Data availability statement
No large datasets were generated or analyzed during this study. Minimal datasets necessary to interpret and/or replicate data in this paper are available on request to the corresponding author.
Conflict of Interest
LLE and JKH declare that they are inventors of the technology contained in this report. Intellectual property has been assigned to Susavion Biosciences, in which the authors hold shares.
ORCIDs
Laura L. Eggink: http://orcid.org/0000-0002-9928-1644
J. Kenneth Hoober: http://orcid.org/0000-0003-0250-9970
Acknowledgments
This work was supported by Susavion Biosciences. The research received no external funding.
Author Contributions
Conceptualization: LLE, JKH; Data Curation: JKH; Formal Analysis: JKH; Investigation: LLE, JKH; Project Administration: JKH; Writing – Original Draft Preparation: JKH; Writing – Review and Editing: LLE, JKH
Disclaimer
The sponsors had no role in the design of the study; collection, analysis, or interpretation of data; the writing of this article; or the decision to submit it for publication.
accepted manuscript published online XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2022;X:100142
References
- Aufenvenne K., Larcher F., Hausser I., Duarte B., Oji V., Nikolenko H., et al. Topical enzyme-replacement therapy restores transglutaminase 1 activity and corrects architecture of transglutaminase-1-deficient skin grafts. Am J Hum Genet. 2013;93:620–630. doi: 10.1016/j.ajhg.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin L., Malley C., Taylor P., Preethi Boorgula M.P., Chavan S., Daya M., et al. Whole genome sequencing identifies novel genetic mutations in patients with eczema herpeticum. Allergy. 2021;76:2510–2523. doi: 10.1111/all.14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowness J.M., Tarr A.H. Increase in transglutaminase and its extracellular products in response to an inflammatory stimulus by lipopolysaccharide. Mol Cell Biochem. 1997;169:157–163. doi: 10.1023/a:1006846400478. [DOI] [PubMed] [Google Scholar]
- Candi E., Schmidt R., Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Caubet C., Jonca N., Brattsand M., Guerrin M., Bernard D., Schmidt R., et al. Degradation of corneodesmosome proteins by two serine proteases of the Kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- Celebi Sözener Z.C., Cevhertas L., Nadeau K., Akdis M., Akdis C.A. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145:1517–1528. doi: 10.1016/j.jaci.2020.04.024. [DOI] [PubMed] [Google Scholar]
- Chermnykh E.S., Alpeeva E.V., Vorotelyak E.A. Transglutaminase 3: the involvement in epithelial differentiation and cancer. Cells. 2020;9:1996. doi: 10.3390/cells9091996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.Y., Streets A.J., Watson P.F., Huang L., Verderio E.A.M., Johnson T.S. A crucial sequence for transglutaminase type 2 extracellular trafficking in renal tubular epithelial cells lies in its N-terminal β-sandwich domain. J Biol Chem. 2011;286:27825–27835. doi: 10.1074/jbc.M111.226340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B., Zachariae E.D., Scavenius C., Thybo M., Callesen M.M., Kløverpris S., et al. Identification of transglutaminase reactive residues in human osteopontin and their role in polymerization. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laurenzi V., Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001;21:148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda-Nagai K., Aida S., Saba K., Suzuki K., Moriyama S., Oo-puthinan S., et al. Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem. 2010;285:19193–19204. doi: 10.1074/jbc.M110.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupasquier M., Stoitzner P., van Oudenaren A., Romani N., Leenen P.J.M. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol. 2004;123:876–879. doi: 10.1111/j.0022-202X.2004.23427.x. [DOI] [PubMed] [Google Scholar]
- Dupasquier M., Stoitzner P., Wan H., Cerqueira D., van Oudenaren A., Voerman J.S.A., et al. The dermal microenvironment induces the expression of the alternative activation marker CD301/mMGL in mononuclear phagocytes, independent of IL-4/IL-13 signaling. J Leukoc Biol. 2006;80:838–849. doi: 10.1189/jlb.1005564. [DOI] [PubMed] [Google Scholar]
- Eckert R.L., Green H. Structure and evolution of the human involucrin gene. Cell. 1986;46:583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- Eckert R.L., Sturniolo M.T., Broome A.M., Ruse M., Rorke E.A. Transglutaminase function in epidermis. J Invest Dermatol. 2005;124:481–492. doi: 10.1111/j.0022-202X.2005.23627.x. [DOI] [PubMed] [Google Scholar]
- Eggink L.L., Roby K.F., Cote R., Kenneth Hoober J.K. An innovative immunotherapeutic strategy for ovarian cancer: CLEC10A and glycomimetic peptides. J Immunother Cancer. 2018;6:28. doi: 10.1186/s40425-018-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggink L.L., Salas M., Hanson C.V., Hoober J.K. Peptide sugar mimetics prevent HIV type 1 replication in peripheral blood mononuclear cells in the presence of HIV-positive antiserum. AIDS Res Hum Retroviruses. 2010;26:149–160. doi: 10.1089/aid.2009.0155. [DOI] [PubMed] [Google Scholar]
- Eggink L.L., Spyroulias G.A., Jones N.G., Hanson C.V., Hoober J.K. A peptide mimetic of 5-acetylneuraminic acid-galactose binds with high avidity to siglecs and NKG2D. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P.M. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80:44s–49s. [PubMed] [Google Scholar]
- Elias P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp Dermatol. 2018;27:847–851. doi: 10.1111/exd.13693. [DOI] [PubMed] [Google Scholar]
- Elias P.M. Optimizing emollient therapy for skin barrier repair in atopic dermatitis. Ann Allergy Asthma Immunol. 2022;128:505–511. doi: 10.1016/j.anai.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P.M., Ahn S.K., Brown B.E., Crumrine D., Feingold K.R. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002;119:1269–1274. doi: 10.1046/j.1523-1747.2002.19622.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Molberg Ø., Qiao S.W., Schmid D.G., von der Mülbe F., Elgstøen K., et al. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J Biol Chem. 2002;277:34109–34116. doi: 10.1074/jbc.M204521200. [DOI] [PubMed] [Google Scholar]
- Folk J.E., Cole P.W. Structural requirements of specific substrates for guinea pig liver transglutaminase. J Biol Chem. 1965;240:2951–2960. [PubMed] [Google Scholar]
- Folk J.E., Mullooly J.P., Cole P.W. Mechanism of action of guinea pig liver transglutaminase. II. The role of metal in enzyme activation. J Biol Chem. 1967;242:1838–1844. [PubMed] [Google Scholar]
- Fukui M., Kuramoto K., Yamasaki R., Shimizu Y., Itoh M., Kawamoto T., et al. Identification of a highly reactive substrate peptide for transglutaminase 6 and its use in detecting transglutaminase activity in the skin epidermis. FEBS Journal. 2013;280:1420–1429. doi: 10.1111/febs.12133. [DOI] [PubMed] [Google Scholar]
- Gan S.Q., McBride O.W., Idler W.W., Markova N., Steinert P.M. Organization, structure, and polymorphisms of the human profilaggrin gene [published correction appears in Biochemistry 1991;30:5814] Biochemistry. 1990;29:9432–9440. doi: 10.1021/bi00492a018. [DOI] [PubMed] [Google Scholar]
- Greenlee-Wacker M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273:357–370. doi: 10.1111/imr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M., Casadio R., Bergamini C.M. Transglutaminases: nature’s biological glues. Biochem J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S.R., Balklava Z., Griffin M. Importance of tissue transglutaminase in repair of extracellular matrices and cell death of dermal fibroblasts after exposure to a solarium ultraviolet A source. J Invest Dermatol. 2003;121:412–423. doi: 10.1046/j.1523-1747.2003.12353.x. [DOI] [PubMed] [Google Scholar]
- Guerrin M., Simon M., Montézin M., Haftek M., Vincent C., Serre G. Expression cloning of human corneodesmosin proves its identity with the product of the S gene and allows improved characterization of its processing during keratinocyte differentiation. J Biol Chem. 1998;273:22640–22647. doi: 10.1074/jbc.273.35.22640. [DOI] [PubMed] [Google Scholar]
- Haas M.R., Nguyen D.V., Shook B.A. Recovery of altered diabetic myofibroblast heterogeneity and gene expression are associated with CD301b+ macrophages. Biomedicines. 2021;9:1752. doi: 10.3390/biomedicines9121752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.R., Scott I.R. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983;170:651–673. doi: 10.1016/s0022-2836(83)80126-0. [DOI] [PubMed] [Google Scholar]
- Haroon Z.A., Hettasch J.M., Lai T.S., Dewhirst M.W., Greenberg C.S. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J. 1999;13:1787–1795. doi: 10.1096/fasebj.13.13.1787. [DOI] [PubMed] [Google Scholar]
- Haydock P.V., Dale B.A. Filaggrin, an intermediate filament-associated protein: structural and functional implications from the sequence of a cDNA from rat. DNA Cell Biol. 1990;9:251–261. doi: 10.1089/dna.1990.9.251. [DOI] [PubMed] [Google Scholar]
- Higashi N., Fujioka K., Denda-Nagai K., Hashimoto S., Nagai S., Sato T., et al. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem. 2002;277:20686–20693. doi: 10.1074/jbc.M202104200. [DOI] [PubMed] [Google Scholar]
- Hitomi K., Kitamura M., Sugimura Y. Preferred substrate sequences for transglutaminase 2: screening using a phage-displayed peptide library. Amino Acids. 2009;36:619–624. doi: 10.1007/s00726-008-0126-6. [DOI] [PubMed] [Google Scholar]
- Hoober J.K. ASGR1 and its enigmatic relative, CLEC10A. Int J Mol Sci. 2020;21:4818. doi: 10.3390/ijms21144818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J.K., Eggink L.L. The discovery and function of filaggrin. Int J Mol Sci. 2022;23:1455. doi: 10.3390/ijms23031455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa S., Kishibe M., Honma M., Murakami M., Mizuno Y., Suga Y., et al. Abberrant distribution patterns of corneodesmosomal components of tape-stripped corneocytes in atopic dermatitis and related skin conditions (ichthyosis vulagris, Netherton syndrome and peeling skin syndrome type B) J Dermatol Sci. 2013;72:54–60. doi: 10.1016/j.jdermsci.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Iismaa S.E., Mearns B.M., Lorand L., Graham R.M. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- Irvine A.D., McLean W.H.I., Leung D.Y.M. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Ishitsuka Y., Roop D.R. Loricrin: past, present, and future. Int J Mol Sci. 2020;21:2271. doi: 10.3390/ijms21072271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen B.R., Skov L.K., Kastrup J.S., Kristensen O., Bolwig C., Larsen J.N., et al. Structure of the house dust mite allergen Der f 2: implications for function and molecular basis of IgE cross-reactivity. FEBS Lett. 2005;579:1208–1212. doi: 10.1016/j.febslet.2004.11.115. [DOI] [PubMed] [Google Scholar]
- John S., Thiebach L., Frie C., Mokkapati S., Bechtel M., Nischt R., et al. Epidermal transglutaminase (TGase 3) is required for proper hair development, but not the formation of the epidermal barrier. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.S., Scholfield C.I., Parry J., Griffin M. Induction of tissue transglutaminase by dexamethasone: its correlation to receptor number and transglutaminase-mediated cell death in a series of malignant hamster fibrosarcomas. Biochem J. 1998;331:105–112. doi: 10.1042/bj3310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin A., Marekov L.N., Steinert P.M. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001;114:3069–3070. doi: 10.1242/jcs.114.17.3069. [DOI] [PubMed] [Google Scholar]
- Kanemaru K., Noguchi E., Tahara-Hanaoka S., Mizuno S., Tateno H., Denda-Nagai K., et al. Clec10a regulates mite-induced dermatitis [published correction appears in Sci Immunol 2020;5:eabg0688] Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aax6908. eaax6908. [DOI] [PubMed] [Google Scholar]
- Kasparek P., Ileninova Z., Zbodakova O., Kanchev I., Benada O., Chalupsky K., et al. KLK5 and KLK7 ablation fully rescues lethality of netherton syndrome-like phenotype. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Yumoto K., Kawakami T. An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of Tec family kinases. Allergol Int. 2007;56:403–409. doi: 10.2332/allergolint.O-07-486. [DOI] [PubMed] [Google Scholar]
- Knudsen N.H., Lee C.H. Identity crisis: CD301b(+) mononuclear phagocytes blur the M1-M2 macrophage line. Immunity. 2016;45:461–463. doi: 10.1016/j.immuni.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.Y. Molecular mechanism of epidermal barrier dysfunction as primary abnormalities. Int J Mol Sci. 2020;21:1194. doi: 10.3390/ijms21041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Lee S.H. Skin barrier and calcium. Ann Dermatol. 2018;30:265–275. doi: 10.5021/ad.2018.30.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. M1 means kill; M2 means heal. J Immunol. 2017;199:2191–2193. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- Leyvraz C., Charles R.P., Rubera I., Guitard M., Rotman S., Breiden B., et al. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–496. doi: 10.1083/jcb.200501038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedén A., Winge M.C.G., Sääf A., Kockum I., Ekelund E., Rodriguez E., et al. Genetic variation in the epidermal transglutaminase genes is not associated with atopic dermatitis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Graham R.M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Ma C.A., Stinson J.R., Zhang Y., Abbott J.K., Weinreich M.A., Hauk P.J., et al. Germline hypomorphic CARD11 mutations in severe atopic disease. Nat Genet. 2017;49:1192–1201. doi: 10.1038/ng.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki M., Yamashita F., Ishida-Yamamoto A., Yamada K., Kinoshita C., Fushiki S., et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase) Proc Natl Acad Sci USA. 1998;95:1044–1049. doi: 10.1073/pnas.95.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro T., Bench G., Sidderas-Haddad E., Feingold K., Elias P., Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXe. J Invest Dermatol. 1998;111:1198–1201. doi: 10.1046/j.1523-1747.1998.00421.x. [DOI] [PubMed] [Google Scholar]
- Mearns B., Nanda N., Michalicek J., Iismaa S., Graham R. Impaired wound healing and altered fibroblast cytoskeletal dynamics in Gh knockout mice. Minerva Biotecnol. 2002;14:218. [Google Scholar]
- Nanda N., Iismaa S.E., Owens W.A., Husain A., Mackay F., Graham R.M. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276:20673–20678. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- Nguyen A.V., Soulika A.M. The dynamics of the skin’s immune system. Int J Mol Sci. 2019;20:1811. doi: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- Odii B.O., Coussons P. Biological functionalities of transglutaminase 2 and the possibility of its compensation by other members of the transglutaminase family. ScientificWorldJournal. 2014;2014:714561. doi: 10.1155/2014/714561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orecchioni M., Ghosheh Y., Pramod A.B., Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M29LPS‒) vs. alternatively activated macrophages [published correction appears in Front Immunol 2020;11:234. Front Immunol. 2019;10:1084. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma A., Jarrah A.S., Tieri P., Cesareni G., Castiglione F. Gene regulatory network modeling of macrophage differentiation corroborates the continuum hypothesis of polarization states. Front Physiol. 2018;9:1659. doi: 10.3389/fphys.2018.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor M.T., Diez A., Pérez-Payá E., Abad C. Addressing substrate glutamine requirements for tissue transglutaminase using substance P analogues. FEBS Lett. 1999;451:231–234. doi: 10.1016/s0014-5793(99)00572-4. [DOI] [PubMed] [Google Scholar]
- Pinkas D.M., Strop P., Brunger A.T., Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmüller C., McAleer M.A., Koppes S.A., Abdayem R., Franz J., Haftek M., et al. Filaggrin breakdown products determine corneocyte conformation in patients with atopic dermatitis. J Allergy Clin Immunol. 2015;136:1573–1580.e2. doi: 10.1016/j.jaci.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothnagel J.A., Steinert P.M. The structure of the gene for mouse filaggrin and a comparison of the repeating units. J Biol Chem. 1990;265:1862–1865. [PubMed] [Google Scholar]
- Sarang Z., Tóth B., Balajthy Z., Köröskényi K., Garabuczi E., Fésüs L., et al. Some lessons from the tissue transglutaminase knockout mouse. Amino Acids. 2009;36:625–631. doi: 10.1007/s00726-008-0130-x. [DOI] [PubMed] [Google Scholar]
- Schrode J., Folk J.E. Transglutaminase-catalyzed cross-linking through diamines and polyamines. J Biol Chem. 1978;253:4837–4840. [PubMed] [Google Scholar]
- Schwartz J., Friedman A.J. Exogenous factors in skin barrier repair. J Drugs Dermatol. 2016;15:1289–1294. [PubMed] [Google Scholar]
- Shaw T.E., Currie G.P., Koudelka C.W., Simpson E.L. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook B., Xiao E., Kumamoto Y., Iwasaki A., Horsley V. CD301b+ macrophages are essential for effective skin wound healing. J Invest Dermatol. 2016;136:1885–1891. doi: 10.1016/j.jid.2016.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook B.A., Wasko R.R., Rivera-Gonzalez G.C., Salazar-Gatzimas E., López-Giráldez F., Dash B.C., et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science. 2018;362:909. doi: 10.1126/science.aar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Green H. The glutamine residues reactive in transglutaminase-catalyzed cross-linking of involucrin. J Biol Chem. 1988;263:18093–18098. [PubMed] [Google Scholar]
- Simon M., Montézin M., Guerrin M., Durieux J.J., Serre G. Characterization and purification of human corneodesmosin, an epidermal basic glycoprotein associated with corneocyte-specific modified desmosomes. J Biol Chem. 1997;272:31770–31776. doi: 10.1074/jbc.272.50.31770. [DOI] [PubMed] [Google Scholar]
- Smith F.J.D., Irvine A.D., Terron-Kwiatkowski A., Sandilands A., Campbell L.E., Zhao Y., et al. Los-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- Steinert P.M., Cantieri J.S., Teller D.C., Lonsdale-Eccles J.D., Dale B.A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci USA. 1981;78:4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P., Grenard P., Aeschlimann P., Langley M., Blain E., Errington R., et al. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci. 2004;117:3389–3403. doi: 10.1242/jcs.01188. [DOI] [PubMed] [Google Scholar]
- Su C.C., Su T.R., Lai J.C., Tsay G.J., Lin H.K. Elevated transglutaminase-2 expression in the epidermis of psoriatic skin and its role in the skin lesion development. J Dermatol. 2017;44:699–702. doi: 10.1111/1346-8138.13742. [DOI] [PubMed] [Google Scholar]
- Su H., Luo Y., Sun J., Liu X., Ling S., Xu B., et al. Transglutaminase 3 promotes skin inflammation in atopic dermatitis by activating monocyte-derived dendritic cells via DC-SIGN. J Invest Dermatol. 2020;140:370–379.e8. doi: 10.1016/j.jid.2019.07.703. [DOI] [PubMed] [Google Scholar]
- Sugimura Y., Hosono M., Kitamura M., Tsuda T., Yamanishi K., Maki M., et al. Identification of preferred substrate sequences for transglutaminase 1—development of a novel peptide that can efficiently detect cross-linking enzyme activity in the skin. FEBS Journal. 2008;275:5667–5677. doi: 10.1111/j.1742-4658.2008.06692.x. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Yamane M., Kato M., Teshima H., Kuribayashi M., Tatsukawa H., et al. Studies on differentiation-dependent expression and activity of distinct transglutaminases by specific substrate peptides using three-dimensional reconstituted epidermis. FEBS Journal. 2019;286:2536–2548. doi: 10.1111/febs.14832. [DOI] [PubMed] [Google Scholar]
- Telei D., Griffin M. Tissue transglutaminase (TG2) – a wound response enzyme. Front Biosci. 2006;11:867–882. doi: 10.2741/1843. [DOI] [PubMed] [Google Scholar]
- Teshima H., Kato M., Tatsukawa H., Hitomi K. Analysis of the expression of transglutaminases in the reconstructed human epidermis using a three-dimensional cell culture. Anal Biochem. 2020;603 doi: 10.1016/j.ab.2020.113606. [DOI] [PubMed] [Google Scholar]
- Thyssen J.P., Jakasa I., Riethmüller C., Schön M.P., Braun A., Haftek M., et al. Filaggrin expression and processing deficiencies impair corneocyte surface texture and stiffness in mice. J Invest Dermatol. 2020;140:615–623.e5. doi: 10.1016/j.jid.2019.07.716. [DOI] [PubMed] [Google Scholar]
- Towell A.M., Feuillie C., Vitry P., Da Costa T.M., Mathelié-Guinlet M., Kezic S., et al. Staphylococcus aureus binds to the N-terminal region of corneodesmosin to adhere to the stratum corneum in atopic dermatitis. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2014444118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigwell S.M., Lynch P.T., Griffin M., Hargreaves A.J., Bonner P.L.R. An improved colorimetric assay for the measurement of transglutaminase (type II)-(γ-glutamyl) lysine cross-linking activity. Anal Biochem. 2004;330:164–166. doi: 10.1016/j.ab.2004.03.068. [DOI] [PubMed] [Google Scholar]
- Vader L.W., de Ru A., van der Wal Y., Kooy Y.M.C., Benckhuijsen W., Mearin M.L., et al. Specificity of tissue transglutaminase explains cereal Toxicity in celiac disease. J Exp Med. 2002;195:643–649. doi: 10.1084/jem.20012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio E.A.M., Johnson T., Griffin M. Tissue transglutaminase in normal and abnormal wound healing: review article. Amino Acids. 2004;26:387–404. doi: 10.1007/s00726-004-0094-4. [DOI] [PubMed] [Google Scholar]
- Wei J.C.J., Edwards G.A., Martin D.J., Huang H., Crichton M.L., Kendall M.A.F. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for Micro-Medical device translation: from mice, rats, rabbits, pigs to humans. Sci Rep. 2017;7:15885. doi: 10.1038/s41598-017-15830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werfel T., Allam J.P., Biedermann T., Eyerich K., Gilles S., Guttman-Yassky E., et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–349. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Westcott D.J., DelProposto J.B., Geletka L.M., Wang T., Singer K., Saltiel A.R., et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., McKenzie C.I., Qu X., Mu Y., Wang Q., Bing N., et al. pH and proton sensor GPR65 determine susceptibility to atopic dermatitis. J Immunol. 2021;207:101–109. doi: 10.4049/jimmunol.2001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Mao-Qiang M., Taljebini M., Elias P.M., Feingold K.R. Topical stratum corneum lipids accelerate barrier repair after tape stripping, solvent treatment and some but not all types of detergent treatment. Brit J Dermatol. 1995;133:679–685. doi: 10.1111/j.1365-2133.1995.tb02738.x. [DOI] [PubMed] [Google Scholar]
- Yokouchi M., Kubo A., Kawasaki H., Yoshida K., Ishii K., Furuse M., et al. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J Dermatol Sci. 2015;77:28–36. doi: 10.1016/j.jdermsci.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Zemskov E.A., Mikhailenko I., Hsia R.C., Zaritskaya L., Belkin A.M. Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xu W., Xiong S. Macrophage differentiation and polarization via phosphatidylinositol 3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P component. J Immunol. 2011;187:1764–1777. doi: 10.4049/jimmunol.1002315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No large datasets were generated or analyzed during this study. Minimal datasets necessary to interpret and/or replicate data in this paper are available on request to the corresponding author.