Abstract
After endocytosis, diverse cargos are sorted into endosomes and directed to various destinations, including extracellular macromolecules, membrane lipids, and membrane proteins. Some cargos are returned to the plasma membrane via endocytic recycling. In contrast, others are delivered to the Golgi apparatus through the retrograde pathway, while the rest are transported to late endosomes and eventually to lysosomes for degradation. Rab GTPases are major regulators that ensure cargos are delivered to their proper destinations. Rabs are localized to distinct endosomes and play predominant roles in membrane budding, vesicle formation and motility, vesicle tethering, and vesicle fusion by recruiting effectors. The cascades between Rabs via shared effectors or the recruitment of Rab activators provide an additional layer of spatiotemporal regulation of endocytic trafficking. Notably, several recent studies have indicated that disorders of Rab-mediated endocytic transports are closely associated with diseases such as immunodeficiency, cancer, and neurological disorders.
1. Introduction
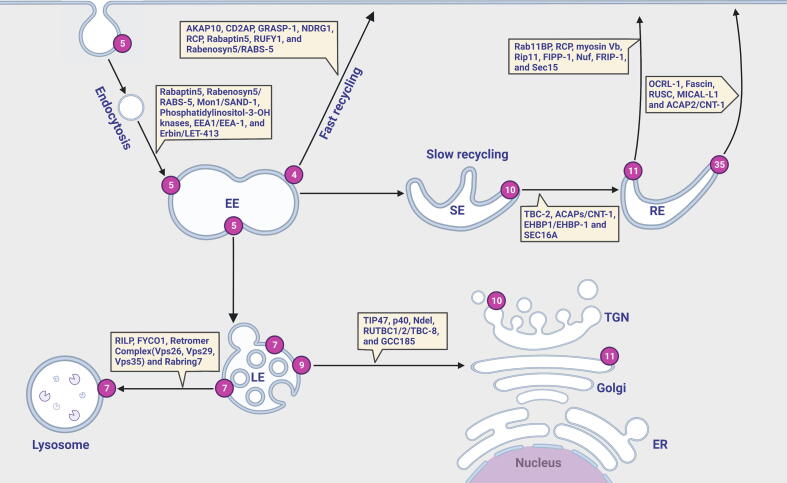
Cells can take in extracellular macromolecules, membrane phospholipids, and membrane proteins through endocytosis. As the primary method of intracellular transport, endocytic transport is involved in various processes, including nutrient uptake, cell polarity, cell migration, cell division, and synaptic transmission [43]. Intracellular transport involves endocytosis, sorting, recycling, and the degradation of macromolecules, phospholipids, and proteins; it is a highly complex regulatory network. Some membrane proteins and membrane phospholipids are transported to the lysosome for degradation. In contrast, the rest of the membrane proteins and membrane phospholipids are returned to the plasma membrane for reuse, allowing the plasma membrane to maintain structural and functional homeostasis [26]. The endocytic system consists of components such as Rab GTPases, cytoskeleton-based tracks for endocytic vesicle translocation, cytoskeleton-associated motor proteins, and vesicles that carry cargos. In eukaryotic cells, actin filaments and microtubules provide a pathway for endocytic transport. The myosin family drives vesicle movement along actin filaments, while the kinesin and dynein families cooperate to move vesicles along microtubules [75]. Rab GTPases are the most significant molecular switches in regulating endocytic transport. Notably, Rab GTPases are localized to diverse endosomal structures and regulate the functions of endosomes by recruiting various effectors (Fig. 1) [53]. A large body of evidence demonstrates that Rab proteins play pivotal roles in the formation, translocation, anchoring, and fusion of vesicles during endocytic transport [43].
Fig. 1.
Rab GTPases are molecular switches for endocytic trafficking. Rab4 medicates fast endocytic recycling directly from the early endosome to the plasma membrane. Rab5, which is localized to the early endosome, mediates endosomal fusion of clathrin-coated vesicles and the maturation of early endosomes. Rab11 and Rab35 regulate the slow endocytic recycling (that delivers the cargo back to the cell surface) through recycling endosomes. Rab7 modulates the transport from late endosomes to lysosomes. Rab9 functions in the pathway from late endosomes to the Golgi apparatus. Rab10 is localized to sorting endosomes and operates in the route from sorting endosomes to recycling endosomes. RE: early endosome; SE: sorting endosome; RE: recycling endosome; LE: late endosome; TGN: trans-Golgi network; ER: endoplasmic reticulum.
Rab GTPases are monomeric GTP-binding proteins of approximately 200 amino acids in all eukaryotic cells. The human genome encodes more than 60 Rabs [4], [66], while there are only 31 Rab-like genes in C. elegans [37]. Furthermore, Rab isoforms involved in various endocytic transport can be produced by selectively splicing Rab genes [38]. Upstream regulators control the activity of Rab proteins, which then direct downstream effector proteins to perform specific functions. Irregular Rab-mediated endocytic steps can cause aberrant distribution of functional proteins, leading to physiological malformation and diseases.
It is worth noting that newly synthesized Rabs are not modified by prenylation (farnesyl modification or geranylgeranyl modification). Once synthesized, the Rab is then transferred to farnesyl transferase or geranyl transferase, which will change the cysteines at the C-terminus of Rab proteins using farnesyl pyrophosphate or geranyl pyrophosphate, respectively [129]. The prenylated Rab is delivered to the target membrane, where the Rab is converted from the GDP-bound inactive form to the GTP-bound active form by the Rab guanylate exchange factor (GEF) and associates with the membranes of specific organelles. In contrast, those Rabs that GEF does not activate will reside in the cytoplasm by binding to the Rab GDP dissociation inhibitor (GDI) [98], [116]. The GTP-bound active form of Rab exerts its regulatory role in various ways by recruiting effectors. Multiple types of Rab effectors have been identified, including tether complexes [6], motor proteins and adapters [51], and proteins that mediate vesicle membrane fusion [92]. Of note, post-translational Rab modifications, such as phosphorylation, can regulate the interaction of Rabs with GDI, GEF, GTPase-activating proteins (GAPs), and effector proteins [96], [83], [49].
Rab4, Rab5, Rab7, Rab9, Rab10, Rab11, and Rab35 have been associated with endocytic trafficking. These Rabs are located in different endosomes or at different positions in the same endosome, where they regulate different steps of endocytic trafficking independently or cooperatively (Fig. 1) [93], [97]. For instance, two distinct Rab-mediated pathways can orchestrate the endocytic recycling of G protein-coupled receptors. Rab4 is mainly localized on early endosomes and regulates the recycling of receptors from early endosomes directly back to the plasma membrane (fast recycling pathway) [133]. Rab11 resides in the perinuclear recycling endosome, where it facilitates the delivery of receptors from the recycling endosome to the cell surface (slow recycling pathway) [130].
2. Rab4 is required for endosomal sorting and fast recycling
Rab4 is a well-known regulator of cargo sorting and fast recycling in the early endosome (Fig. 1) [132]. The binding of Rab4 to GTP or GDP and its affiliated functions are directly or indirectly regulated by other proteins or signaling molecules. Through a PI3K-independent route, the messenger molecule cAMP promotes Rab4 activation [114]. The phosphorylation of serine residue 213 of GDI can encourage the formation of the GDI-Rab4 complex and facilitate the functional cycle of Rab4 [77]. TBC1D16 was identified as a GAP of Rab4 that affects the localization of Rab4A in the endosomal membrane by enhancing the intrinsic GTP hydrolysis efficiency of Rab4A [42].
The Rab4 GTPase subfamily has three members: Rab4A, Rab4B, and Rab4C [38], whereas C. elegans has no ortholog or paralog. Rab4A and Rab4B mediate GLUT4 (Glucose Transporter 4) transport, thereby modulating glucose uptake [60]. According to researchers, Rab4B expression was significantly reduced in adipose tissue from obese diabetic patients and mice; Rab4B colocalizes with GLUT4 and affects insulin-stimulated glucose uptake in 3T3-L adipocytes. In contrast, Rab4A rarely overlaps with GLUT4, and downregulating its expression had little impact on basal or insulin-stimulated glucose uptake [60]. Therefore, Rab4A and Rab4B could have functional redundancy during recycling regulation, but they are likely to have different roles and mechanisms. The functional scenarios of Rab4C are still unknown and need further investigation.
Several Rab4 effectors have been identified, including AKAP10/D-AKAP2, CD2AP/CMS, GRASP-1, NDRG1, Rip11/RCP, Rabaptin5/RABEP1/Rbpt5, RUFY1/Rabip4s, and ZFYVE20/Rabenosyn5 (Table 1). Eggers et al. showed that the RGS domain of D-AKAP2 alters the morphology of the Rab11 compartment and affects the recycling of the transferrin receptor by interacting with Rab4 and Rab11 [31]. Along with Rab4, RUFY1/Rabip4s affect NIH 3T3 fibroblasts migration and adhesion by regulating integrin transport [135] and affect glucose uptake by regulating endocytosis and transport of GLUT4 in adipocytes [80]. Furthermore, Rab4 participates in a small GTPase cascade by promoting Arl1 recruitment to early endosomes, which supports the association of endosomal sorting sites with clathrin complexes and affects early endosomal sorting [27].
Table 1.
Rab proteins and their effectors in endocytic transport.
| Rab | Effector | Function | References |
|---|---|---|---|
| Rab4 | AKAP10/D-AKAP2 | Transferrin receptor recycling | [31] |
| CD2AP/CMS | Endosome morphology and lysosomal degradation | [25] | |
| GRASP-1 | Maturation of recycling endosome | [50] | |
| NDRG1 | Recycling of E-cadherin | [59] | |
| Rip11/RCP | Endosomal recycling | [71] | |
| Rabaptin5/RABEP1/Rbpt5 | Endosome maturation | [61] | |
| RUFY1/Rabip4s | Endocytic recycling | [135] | |
| ZFYVE20/Rabenosyn5 | Endocytosis | [126] | |
| Rab5 | Rabaptin5/RABEP1/Rbpt5 | Endosome maturation | [61] |
| ZFYVE20/Rabenosyn5 | PI3P level on early endosomes | [86] | |
| Mon1/SAND-1 | Early-to-late endosomes conversion | [63] | |
| EEA1/EEA-1 | Endosomal membrane fusion | [40] | |
| Erbin/LET-413 | RAB-10 activity | [74] | |
| Rab7 | RILP | Late endosome to lysosome trafficking | [11] |
| FYCO1 | Microtubule plus end-directed vesicle transport | [95] | |
| Retromer complex(Vps26, Vps29, and Vps35) | Late endosome to Golgi trafficking | [107] | |
| Rabring7 | EGF receptor degradation | [108] | |
| Rab9 | TIP47 | Receptor recruitment | [13] |
| p40 | Endosome-to-TGN transport | [28] | |
| Nde1 | Interaction between late endosomes and dynein | [154] | |
| RUTBC1/2/TBC-8 | N/A | [90], [91] | |
| GCC185 | Endosome-to-TGN transport | [102] | |
| Rab10 | TBC-2 | Endocytic recycling | [76] |
| ACAPs/CNT-1 | Endocytic recycling | [120] | |
| EHBP1/EHBP-1 | Endocytic recycling | [118], [136] | |
| SEC16A | Insulin-stimulated GLUT4 trafficking | [7] | |
| Rab11 | Rab11BP/Rabphilin-11 | Endocytic recycling | [78], [146] |
| Rip11/RCP | Endosomal recycling | [71] | |
| myosin Vb | Rab11-FIP2-dependent recycling | [46], [85] | |
| Rip11/pp75 | Apical recycling | [101] | |
| Rab11-FIP1/FIPP-1 | Rab11-dependent recycling | [46] | |
| Nuf/Rab11-FIP3/Arfophilin2 | Membrane traffic in cytokinesis | [12] | |
| Rab11-FIP4/RFIP-1 | Membrane traffic in cytokinesis | [33] | |
| Sec15 | Exocyst function in recycling | [151] | |
| Rab35 | OCRL/OCRL-1 | Membrane traffic in cytokinesis | [15] |
| Fascin | Actin Bundling during cell migration | [147] | |
| RUSC/NESCA | Rab35 activity | [35] | |
| MICAL-L1 | Membrane traffic during neurite outgrowth | [68] | |
| centaurin-β2/ACAP2/CNT-1 | Membrane traffic during neurite outgrowth | [67] |
Rab4 is influenced by multiple regulatory factors, regulates multiple effectors, and is involved in intracellular transport by affecting many intracellular biological functions. However, the molecular mechanisms of how Rab4 circulates in the endosome, how it precisely controls protein cargo-dependent transport pathways, how it cooperates with other GTPases to control vesicle aggregation, fusion, and targeted transport, how it controls metabolic and signaling pathways, and the temporal and spatial control of its function are all poorly understood. Current studies only partially explain these processes, and more research is needed to fully elucidate the mechanism of Rab4.
3. Rab5 directs biogenesis and functions of early endosomes
Rab5 plays a crucial role in endocytic transport, regulating vesicle transport from the plasma membrane to the endosome (Fig. 1). In particular, Rab5 promotes the fusion of nascent endocytic vesicles with early endosomes and fusion between early endosomes, so it is often used as a marker for early endosomes [141]. Studies in various systems have revealed multiple Rab5 GEF proteins, including RABX-5/Rabex-5 [109], [150], [149], [153], hRME-6/RME-6 [112], and Rin2/RIN-1 [29]. RABX-5/Rabex-5 is the first protein identified as a small GTPase GEF and is primarily localized at early endosomes [52]. During degradation transport from early to late endosomes, SAND-1 displaces RABX-5 from endosomal membranes and interacts with the core components of the HOPS (the homotypic fusion and vacuole protein sorting) complex to promote RAB-7/Rab7 activation and facilitate early-to-late endosome conversion [99]. RME-6, in concert with the adaptor protein APA-2, localizes to the clathrin-coated pits and vesicles, where it activates RAB-5 to promote the transit of cargos to early endosomes [112]. In C. elegans, the functions of RABX-5 and RME-6 were found to be partially redundant. Only simultaneous knockdown of RABX-5 and RME-6 led to almost complete dissociation of RAB-5 from early endosomes [112]. Studies on RIN-1 were primarily performed in the C. elegans nervous system. As an effector, RIN-1 also specifically binds to the active form of CED-10/Rac1 to regulate actin remodeling and directs the directional migration of neuronal cells and axon pathfinding [29]. Given that the overactivation of Rab proteins can adversely affect endocytic trafficking, it is crucial for TBC-2, a Rab5/RAB-5 GAP, to shut down RAB-5 activity in a timely manner [22]. Zhang et al. found that RAB-5 resides in discrete endosome subpopulations in the intestine of C. elegans, and under the oversight of CED-10, LET-502 synergizes with RABX-5 to revitalize RAB-5 on a subset of endosomes in the deep cytosol, ensuring the progress of basolateral recycling [150], [149], [153]. USP6NL/RN-tre is thought to be a GAP shared by Rab5 and Rab41, while TBC1D2/Armus/TBC-2 appears to function primarily as a GAP for Rab7 [22], [34].
Recent studies on endocytic trafficking indicated that Rab5/RAB-5 effectors range from Rabaptin5/RABEP1/Rbpt5, ZFYVE20/Rabenosyn5/RABS-5, Mon1/SAND-1, Erbin/LET-413, and EEA1 (Table 1). Among them, RABS-5(Rabenosyn5) and its binding protein VPS-45, as well as VPS-34, act during the endosome fusion process [41]. Vps34 is phosphatidylinositol 3-kinase (PI3K) that phosphorylates phosphatidylinositol to generate phosphatidylinositol-3-phosphate (PI3P) at early endosomes [84], [70]. In addition, Vps34 is known to form complexes with Beclin/BEC-1 and P150/VPS-15 to function during autophagy and other transport events [36]. Further work on C. elegans has revealed that RAB-5 recruits LET-413 to promote DENN-4 GEF activity toward RAB-10 on sorting endosomes [74]. As a Rab5 effector essential for endosome fusion, EEA1 is in a homodimeric configuration, forming a complex with Rab5 and specifically binding to PI3P [40]. As a common effector of Rab5 and Rab4, Rabaptin5/RABEP1/Rbpt5 interacts with the membrane remodeling protein EHD1 to regulate endosome morphology and contribute to endocytic transport [144], [61].
In addition to regulating the fusion of early endosomes, Rab5 also plays a vital role in upstream events of the endocytosis process. Clathrin-dependent endocytosis (CDE) starts with clathrin and AP2 complex coated vesicles (CCVs), which are formed by the invagination of the plasma membrane. After uncoating clathrin, these vesicles fuse with similar vesicles or early endosomes. The AP2 complex is a heterotetramer (subunits α/β2/µ2/σ2) and acts as a significant adaptor in CDE. μ2-adaptin specifically recognizes the intracellular region of cargo proteins and can be phosphorylated by μ2 kinase bound to α-adaptin [64], [54]. After being phosphorylated, μ2-adaptin can strongly bind to phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) and encourage AP2 assembly [106]. It has been reported that dominant-negative Rab5(S34N) overexpression or the small siRNA-mediated knockdown of hRME-6 enhances AP2 levels while reducing the efficiency of CCV uncoating [117]. However, the loss of another Rab5 GEF, Rabex-5, does not lead to this phenotype. Further mechanistic dissection demonstrated that hRME-6 could competitively bind to the site on μ2 kinase AAK1 that interacts with α-adaptin. This interaction dissociates AAK1 from α-adaptin, thereby reducing the phosphorylation level of μ2-adaptin and promoting the uncoating of the AP2 complex [117]. It should be noted that overexpression of Rab5(S34N) also increases the level of PI(4,5)P2 in CCVs and thus impairs AP2 uncoating [111], [20]. Together, Rab5 and its GEF protein hRME-6 regulate AP2 uncoating by modulating μ2-adaptin dephosphorylation and PI(4,5)P2 levels, suggesting that Rab5 plays a role in regulating AP2 complex dissociation from CCVs.
By recruiting its effectors, Rab5 influences the internalization and intracellular translocation of several signal transduction receptors, including receptor tyrosine kinases (RTKs), G protein-coupled receptors (GPCRs), and antigen recognition receptors. Gene transcription could be impacted by the aberrant function or expression of Rab5, which could then affect cell shape, proliferation, differentiation, and apoptosis—any of which could result in illness [58]. Continued research on Rab5 has improved our understanding of the endocytic system, particularly the dynamic sorting regulatory mechanism of sorting endosomes. The endosome is not a static compartment used to transport cargo; rather, it is a sophisticated and finely regulated dynamic network system. However, there are still many unanswered concerns regarding Rab5′s regulatory processes, including which endosomal sorting tasks rely on it, how to coordinate these functions, and how it contributes to diseases. By addressing these fundamental issues, we can gain a better understanding of the regulatory system that controls vesicular transport, shed light on how Rab5 functions during both development and pathological condition, and create new opportunities for therapeutic interventions in related diseases.
4. Rab7 and Rab9 are associated with the regulation of late endosome function
The mutation or dysfunction of Rab7 is associated with various diseases, such as neurological disorders, cancer, and lipid metabolic disorders [147]. Rab7 is stably present on late endosomes (Fig. 1), and the SAND-1/CCZ-1 complex functions as a GEF for Rab7/RAB-7 [100], [87], [89]. It has been demonstrated that Rab7 is a critical regulator of endosomal degradation, which mediates the maturation of early endosomes to late endosomes along with its effector HOPS complex [3]. In addition, Rab7 is involved in the biogenesis of lysosomes, phagosomes, autophagosomes, and other lysosome-associated organelles (LRO) [8], [55]. By interacting with the Rab-interacting lysosomal protein (RILP), Rab7 also affects the morphology and spatial distribution of lysosomes by regulating the cytoskeleton [137].
In addition to Rab7, late endosomes often carry another Rab member, Rab9 (Fig. 1). However, Rab7 and Rab9 are recruited to the endosomal membrane via different mechanisms and are present in different subdomains of the same endosome [125]. Mammalian Rab9 has two isoforms, Rab9A and Rab9B, which share 87 % homology, and their GTP-bound forms display nearly identical structures [154]. There is no homolog of Rab9 in C. elegans. Notably, Rab9 is also known to play a role in lysosomal enzymes sorting to late endosomes, as well as lysosomes and LRO biogenesis, even though it is not directly involved in endosome maturation [103], [65]. TIP47, GCC185, p40, RUTBC1/2, and NdeI are Rab9 effectors (Table 1). Notably, Rab9A increases the affinity of TIP47 for CI-MPRs by promoting the recruitment of TIP47 to endosomes containing mannose-6-phosphate receptors (CI-MPRs) [13]. Likewise, GCC185 and Rab9A are required to transport CI-MPRs from late endosomes to the trans-Golgi network [102]. Recently, Nde1 was revealed to mediate the interaction of Rab9A with Lis1, dynein, and dynactin for the retrograde trafficking of late endosomes to TGN [154]. Rab9 is also involved in the Golgi targeting of glycosphingolipids internalized via caveolae [23].
In addition, Rab9-mediated fusion of the isolation membrane with TGN and late endosome vesicles is required for autophagosome formation in unconventional macroautophagy [88]. Likewise, Rab7 has been implicated in promoting the maturation of autophagosomes [140]. However, it is unclear how Rab7 and Rab9 are differentiated and recovered, as well as which protein complexes are involved in Rab9-mediated cargo protein transport. Rab7 plays an integral role in neurons [56], and neuronal homeostasis can be disrupted when Rab7 activity or expression is altered, leading to the development of multiple neurological disorders, such as AD, Parkinson's disease, Huntington's disease, and Lewy body dementia [140]. However, the precise function of Rab7 in the pathophysiology of various illnesses and the processes by which it contributes to the emergence of disease are still poorly understood.
5. Rab10 is a molecular switch for polarized sorting and recycling transport
Rab10 is mainly located on sorting endosomes and regulates polarized transport in epithelial cells (Fig. 1) [2], [115]. Interestingly, Rab10 was also found in perinuclear Golgi/TGN in nonpolar fibroblastic baby hamster kidney (BHK) and Chinese hamster ovary (CHO) cells [19]. Rab10 mediates transport from basolateral sorting endosomes to common endosomes in polarized MDCK cells [2]. The expression of activated Rab10(Q68L) was shown to prevent translocation of the vesicular stomatitis virus G glycoprotein (VSV-G) to the basolateral membrane in subsequent research. Instead, VSV-G was missorted to the apical membrane in MDCK cells [115]. Similarly, Rab10 and its GAP protein, AS160, regulate the trafficking of GLUT4 to the plasma membrane in response to insulin in 3T3-L1 adipocytes [110]. By regulating lipid synthesis, Rab10 also contributes to the development of membrane tubules and the maintenance of ER (endoplasmic reticulum) dynamics [17].
In a recent study in C. elegans, it was discovered that LET-413/Erbin, a RAB-5 effector, can promote RAB-10 activation by releasing the autoinhibitory configuration of RAB-10 GEF DENN-4 during endocytic recycling [74]. Numerous effectors, including CNT-1/ACAPs/Arf-GAP and EHBP-1, were discovered by studying Rab10/RAB-10 [118], [120]. In C. elegans, RAB-10 recruits CNT-1 to basolateral recycling endosomes, where CNT-1 negatively regulates ARF-6/Arf6 activity and reduces the abundance of the ARF-6 effector PI5K on the endosomal membrane, lowering the PI(4,5)P2 levels in recycling endosomes [120], [119], [18]. In recycling endosomes, RAB-10 also promotes membrane budding by regulating F-actin bundling via its effector EHBP-1 [118], [136]. A subsequent study further demonstrated that EHBP-1 facilitates recycling endosomal tubule fission by recruiting SID-3 and DYN-1/dynamin sequentially [39]. With the assistance of RAB-10 and AMPH-1/Amphiphyin, TBC-2/RAB-5-GAP, an effector of CED-10 [127], can also be recruited to endosomes for recycling regulation [76]. Additionally, recent research has revealed that the interaction between myosin Vb and Rab10 can control the vesicular transport from the trans-Golgi [45].
Numerous cancers, including hepatocellular carcinoma, glioma, cervical cancer, osteosarcoma, etc., exhibit high expression of Rab10 [57], [138], [47], [150], [149], [153], which affects the prognosis of tumors and controls the apoptosis, proliferation, migration, invasion, and autophagy processes of tumor cells [155]. In addition, the aberrant phosphorylation of Rab10 may contribute to the altered vesicular transport in Alzheimer's disease. It is unclear how the activity of Rab10 is turned off in time or what controls the aberrant phosphorylation of Rab10. The mechanism of Rab10 in both physiological states and pathological conditions, as well as its potential clinical applications, requires further study.
6. Rab11 is a key regulator of the slow recycling route
As a major regulator of the dynamics of endocytic transport, functional irregularities in Rab11 can cause tumor progression and invasion [32]. The human Rab11 gene family has three isoforms: Rab11A, Rab11B, and Rab25/Rab11C. RAB-11.1 and RAB-11.2 are present in C. elegans. Rab11A is present in most human tissues, while Rab11B and Rab25 are exclusively enriched in specific organs [139]. All three isoforms of Rab11 are subject to post-translational modifications, including isoprenylation, phosphorylation, and ubiquitination. The isoprenylation of the Rab11 C-terminus assists in targeting Rab11 to the endosomal membrane, while phosphorylation and ubiquitination regulate the activity and degradation of Rab11, respectively [139].
Notably, three isoforms of Rab11 perform distinct cellular functions. Rab11A regulates the endocytic transport from or through the recycling endosome to the plasma membrane [130], [81]. Additionally, Rab11A mediates lipid transport from sorting endosomes to the recycling endosome [10]. Rab11A is also observed in TGN, where it regulates the secretory transport to the apical and basolateral membranes [32]. Rab11B is highly enriched in sorting and recycling endosomes in the brain. However, the loss of Rab11B failed to cause an accumulation of transferrin receptors [128]. Interestingly, Rab11A and Rab11B could play opposite roles in the same cellular context, with Rab11B controlling the recycling of PAR-1 from endosomes to the plasma membrane and Rab11A promoting PAR-1 degradation by delivering this signaling receptor to the autophagic pathway [44]. In addition, Rab11B mediates secretory transport in polarized epithelial cells and neurons [62], [122]. Rab25 interacts directly with the α5β1 integrin located at the pseudopod of migrating cells and confines it to the pseudopod site, thus promoting the invasive migration of tumor cells [14]. In addition, Rab25 and CLIC3 (chloride channel protein 3) synergistically regulated integrins from the late endosome to the dorsal surface of migrating cells, thus promoting tumor cell invasion [30].
Rab11BP/Rabphilin11, the first discovered Rab11A effector (Table 1), has been associated with transferrin recycling [78], [146]. Myosin Vb, the Rab11A-interacting effector, was screened from a rabbit cDNA library [46]. Consistently, Myosin Vb is involved in recycling transport by mediating the movement of vesicles along actin filaments [85]. Moreover, six Rab11 family interacting proteins (Rab11-FIPs) have been identified. Class I FIPs consist of Rip11 (also known as pp75 or FIP5), RCP (Rab11 coupling protein), and FIP2. Rip11 is recruited by endosomal Rab11 and cooperates with RCP to regulate transport from recycling endosomes to the apical membrane [101], [72]. Class II members (FIPs, FIP3/Arfophilin/Eferin, and FIP4) are involved in regulating transport from recycling endosomes to the cleavage furrow, facilitating cytoplasmic division [33]. FIP1, which colocalizes with Rab11 and likely assists Rab11 in specific recycling transport, is currently the only class III member [46].
Rab11 is a known overexpressed protein that has a role in the proliferation and invasion of malignant tumors, such as rectal, gastric, esophageal, skin, and breast cancers [5], [143], [152], [94]. However, the mechanism governing the involvement of Rab11 in tumor cell proliferation and apoptosis inhibition is not well defined.
7. Rab35 is a regulator of cargo recycling and actin remodeling
It has been demonstrated that Rab35 regulates the endocytic recycling of various protein cargos and actin dynamics [24], [79]. Unlike Rab11, Rab35 regulates a slow recycling route that coordinates cell adhesion and migration (Fig. 1) [1], making Rab35 a new area of interest for endosomal research.
Rab35 is localized to the clathrin-coated pits/vesicles and endosomal membranes, regulating the endocytic recycling of cargo proteins [69]. Rab35 has also been involved in recycling synaptic vesicles [131]. Moreover, Rab35 promotes actin reorganization during filopodia formation and protrusive membrane extension in cultured cells [148], [79]. A study using PC12 cells revealed a role for Rab35 in the maintenance of PI(4,5)P2 level [67]. The recruitment of PI(4,5)P2-binding proteins involved in membrane bending and fission is specifically regulated by the Rab35-to-Arf6 cascade, which also controls endosomal PI(4,5)P2 levels. Arf6(GTP) can negatively regulate Rab35 activity in human cells by interacting with the Rab35 GAP EPI64B [21]. Indeed, a similar Rab-to-Arf cascade has been discovered in the regulation of recycling transport in C. elegans [120], [119]. These findings suggested that crosstalk between different types of small GTPases is a conserved mechanism that could be significant for endocytic transport. In C. elegans, RAB-35 is required for clathrin-based receptor-mediated endocytosis of yolk proteins [113].
The importance of fully comprehending the molecular pathways regulated by Rab35 is highlighted by the fact that Rab35 or its regulators are associated with diseases such cancer [16]. Through a cooperative relationship between membrane trafficking mediated by Rab family proteins and activation of oncogenic signals, Rab35 controls the development of tumors [145], [134]. While Rab35 is implicated in the regulation of a number of cancers, its exact function is not yet clear and requires further research.
8. Two-way cascades between Rab proteins
The identity of distinct membrane compartments is defined by specific Rab proteins. To ensure effective membrane trafficking, it is important to convert the identity of the endosomal compartment [99]. Of note, Rab cascades are involved in converting endosomal compartment identities [99], [53]. GEFs of downstream Rabs are recruited by upstream Rabs. In turn, upstream Rab GAPs are then recruited by downstream Rabs after their activation, which stops the activity of the upstream Rab. Therefore, the two-way Rab cascades (Rab-GEF forward cascade and Rab-GAP reverse cascade) can change the molecular composition of the membrane and, thus, the properties of endosomal compartments. For instance, the HOPS complex, an effector of Rab5, is recruited to early endosomes by binding Rab5. Then, Rab7 is activated by the HOPS complex via its VPS39/GEF subunit [142], [9]. Furthermore, active RAB-7/Rab7 has been shown to recruit the effector TBC-2/RAB-5-GAP to inactivate upstream RAB-5/Rab5 [22].
In C. elegans, similar cascade-mediated regulation has been found to sustain endocytic recycling. TBC-2 is capable of binding to the active RAB-10(GTP) but not the inactive RAB-10(GDP), and the interface mediating TBC-2 binding to RAB-10 is not the GAP domain of TBC-2 [76]. RAB-10, a key regulator of early-to-recycling endosomal transport, shuts down upstream RAB-5 activity by recruiting TBC-2, forming a GAP reverse cascade [76]. Recently, in C. elegans intestinal cells, LET-413/Erbin was found to act as an effector of RAB-5/Rab5, synergizing with DENN-4/RAB-10-GEF to activate downstream RAB-10 [74]. This study provides a forward GEF cascade mechanism that further clarifies the regulatory process of recycling transport.
9. Post-translational modifications affect Rabs efficacy
Although the function of Rabs is typically coordinated by regulators such as GAPs and GEFs, recent studies have revealed that some Rabs are phosphorylated by Rab kinases in the switch II region, inhibiting binding to Rab interactors [124]. Furthermore, the conformational change of the switch II region depends on nucleotide binding, so the phosphorylation described above can significantly affect the structure and function of the switch region. Indeed, new evidence is emerging that phosphorylation regulates Rabs function, some of which are implicated in diseases such as Parkinson’s disease and cancer [156], [105].
Given the importance of Rabs post-translational modifications, biochemical assays such as mass spectrometry and proximity-dependent biotinylation allow us to learn more about the functional mechanisms of Rabs by finding new types of post-translational modifications. In addition, cryo-electron microscopy analysis of non-crystalline protein structure makes it possible to study high molecular weight Rab-effector complexes. Future studies are required to elucidate the details of post-translational modifications in regulating Rabs function, thereby helping to understand the role of Rab kinases in the interaction between disease-related signaling and endocytic trafficking.
10. Conclusions
Rabs act as regulatory switches for endocytic transport, directly or indirectly affecting endosomal functions by recruiting various effector proteins (Fig. 1; Table 1). For example, the well-studied Rab5 recruits the PI3K to synthesize PI3P and then recruits PI3P-binding proteins to form the functional endosome domain (membrane property regulation) [121]. Rab5 also recruits Rabenosyn-5 and EEA1 to facilitate endosomal fusion (membrane fusion regulation) and activates downstream Rab7 to promote endosome maturation (Rab cascade) [123], [86], [104]. Moreover, Rab5 is essential for clathrin-mediated TfR endocytosis and clathrin-coated vesicle formation (membrane budding regulation) [82]. Subsequently, endosomal Rab5 recruits the phosphatases OCRL and Inpp5b to reduce PI(4,5)P2 levels and encourages membrane tubule fission (membrane fission regulation) [111]. Rab5 also indirectly affects the function of microtubule motor kinesin by regulating PI3P levels (vesicle motility regulation) [48]. Rab5 recruits the effector Rabaptin-5, which cooperates with Rabex-5 to promote its own activity (Rab activity regulation) [73]. Compared with Rab5, studies on other Rabs have primarily focused on elaborating a specific working mode, and many functional nodes are missing in the regulatory networks. Subsequent research should use multicellular in vivo models to identify new Rab-binding proteins and associated regulatory elements, promoting a comprehensive and systematic understanding of endocytic transport's operational process and regulation.
CRediT authorship contribution statement
Jing Zhang: Conceptualization, Validation, Writing – original draft, Writing – review & editing. Zongyan Jiang: Visualization. Anbing Shi: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key R&D Program of China (2021YFA1300302), the National Natural Science Foundation of China (32130027), the National Science Fund for Distinguished Young Scholars (31825017), and the Major Research Plan of the Natural Science Foundation of China (91954001) to A. Shi.
Reference
- 1.Allaire P.D., Seyed Sadr M., Chaineau M., Seyed Sadr E., Konefal S., Fotouhi M., et al. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci. 2013;126(Pt 3):722–731. doi: 10.1242/jcs.112375. [DOI] [PubMed] [Google Scholar]
- 2.Babbey C.M., Ahktar N., Wang E., Chen C.-C.-H., Grant B.D., Dunn K.W. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17(7):3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balderhaar H.J., Ungermann C. CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion. J Cell Sci. 2013;126(Pt 6):1307–1316. doi: 10.1242/jcs.107805. [DOI] [PubMed] [Google Scholar]
- 4.Bock J.B., Matern H.T., Peden A.A., Scheller R.H. A genomic perspective on membrane compartment organization. Nature. 2001;409(6822):839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 5.Boulay P.L., Mitchell L., Turpin J., Huot-Marchand J., Lavoie C., Sanguin-Gendreau V., et al. Rab11-FIP1C Is a Critical Negative Regulator in ErbB2-Mediated Mammary Tumor Progression. Cancer Res. 2016;76(9):2662–2674. doi: 10.1158/0008-5472.CAN-15-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bröcker C., Engelbrecht-Vandré S., Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol. 2010;20(21):R943–R952. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Bruno J., Brumfield A., Chaudhary N., Iaea D., McGraw T.E. SEC16A is a RAB10 effector required for insulin-stimulated GLUT4 trafficking in adipocytes. J Cell Biol. 2016;214(1):61–76. doi: 10.1083/jcb.201509052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucci C., Thomsen P., Nicoziani P., McCarthy J., van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11(2):467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera M., Ostrowicz C.W., Mari M., LaGrassa T.J., Reggiori F., Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20(7):1937–1948. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campa C.C., Margaria J.P., Derle A., Del Giudice M., De Santis M.C., Gozzelino L., et al. Rab11 activity and PtdIns(3)P turnover removes recycling cargo from endosomes. Nat Chem Biol. 2018;14(8):801–810. doi: 10.1038/s41589-018-0086-4. [DOI] [PubMed] [Google Scholar]
- 11.Cantalupo G., Alifano P., Roberti V., Bruni C.B., Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. Embo j. 2001;20(4):683–693. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J., Albertson R., Riggs B., Field C.M., Sullivan W. Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J Cell Biol. 2008;182(2):301–313. doi: 10.1083/jcb.200712036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll K.S., Hanna J., Simon I., Krise J., Barbero P., Pfeffer S.R. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 2001;292(5520):1373–1376. doi: 10.1126/science.1056791. [DOI] [PubMed] [Google Scholar]
- 14.Caswell P.T., Spence H.J., Parsons M., White D.P., Clark K., Cheng K.W., et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13(4):496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Cauvin C., Rosendale M., Gupta-Rossi N., Rocancourt M., Larraufie P., Salomon R., et al. Rab35 GTPase Triggers Switch-like Recruitment of the Lowe Syndrome Lipid Phosphatase OCRL on Newborn Endosomes. Curr Biol. 2016;26(1):120–128. doi: 10.1016/j.cub.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Chaineau M., Ioannou M.S., McPherson P.S. Rab35: GEFs, GAPs and effectors. Traffic. 2013;14(11):1109–1117. doi: 10.1111/tra.12096. [DOI] [PubMed] [Google Scholar]
- 17.Chang J., Blackstone C. Rab10 joins the ER social network. Nat Cell Biol. 2013;15(2):135–136. doi: 10.1038/ncb2682. [DOI] [PubMed] [Google Scholar]
- 18.Chen D., Yang C., Liu S., Hang W., Wang X., Chen J., et al. SAC-1 ensures epithelial endocytic recycling by restricting ARF-6 activity. J Cell Biol. 2018;217(6):2121–2139. doi: 10.1083/jcb.201711065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.-T., Holcomb C., Moore H. Expression and localization of two low molecular weight GTP-binding proteins, Rab8 and Rab10, by epitope tag. Proc Natl Acad Sci. 1993;90(14):6508–6512. doi: 10.1073/pnas.90.14.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Schmid S.L. Evolving models for assembling and shaping clathrin-coated pits. J Cell Biol. 2020;219(9) doi: 10.1083/jcb.202005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesneau L., Dambournet D., Machicoane M., Kouranti I., Fukuda M., Goud B., et al. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr Biol. 2012;22(2):147–153. doi: 10.1016/j.cub.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 22.Chotard L., Mishra A.K., Sylvain M.A., Tuck S., Lambright D.G., Rocheleau C.E. TBC-2 regulates RAB-5/RAB-7-mediated endosomal trafficking in Caenorhabditis elegans. Mol Biol Cell. 2010;21(13):2285–2296. doi: 10.1091/mbc.E09-11-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhury A., Dominguez M., Puri V., Sharma D.K., Narita K., Wheatley C.L., et al. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J Clin Invest. 2002;109(12):1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua C.E., Lim Y.S., Tang B.L. Rab35–a vesicular traffic-regulating small GTPase with actin modulating roles. FEBS Lett. 2010;584(1):1–6. doi: 10.1016/j.febslet.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 25.Cormont M., Metón I., Mari M., Monzo P., Keslair F., Gaskin C., et al. CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic. 2003;4(2):97–112. doi: 10.1034/j.1600-0854.2003.40205.x. [DOI] [PubMed] [Google Scholar]
- 26.Cullen P.J., Steinberg F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol. 2018;19(11):679–696. doi: 10.1038/s41580-018-0053-7. [DOI] [PubMed] [Google Scholar]
- 27.D'Souza R.S., Semus R., Billings E.A., Meyer C.B., Conger K., Casanova J.E. Rab4 orchestrates a small GTPase cascade for recruitment of adaptor proteins to early endosomes. Curr Biol. 2014;24(11):1187–1198. doi: 10.1016/j.cub.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Díaz E., Schimmöller F., Pfeffer S.R. A novel Rab9 effector required for endosome-to-TGN transport. J Cell Biol. 1997;138(2):283–290. doi: 10.1083/jcb.138.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi M., Minematsu H., Kubota Y., Nishiwaki K., Miyamoto M. The novel Rac effector RIN-1 regulates neuronal cell migration and axon pathfinding in C. elegans. Development. 2013;140(16):3435–3444. doi: 10.1242/dev.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dozynkiewicz M.A., Jamieson N.B., Macpherson I., Grindlay J., van den Berghe P.V., von Thun A., et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22(1):131–145. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggers C.T., Schafer J.C., Goldenring J.R., Taylor S.S. D-AKAP2 interacts with Rab4 and Rab11 through its RGS domains and regulates transferrin receptor recycling. J Biol Chem. 2009;284(47):32869–32880. doi: 10.1074/jbc.M109.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferro E., Bosia C., Campa C.C. RAB11-Mediated Trafficking and Human Cancers: An Updated Review. Biology (Basel) 2021;10(1) doi: 10.3390/biology10010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fielding A.B., Schonteich E., Matheson J., Wilson G., Yu X., Hickson G.R., et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. Embo J. 2005;24(19):3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda M. TBC proteins: GAPs for mammalian small GTPase Rab? Biosci Rep. 2011;31(3):159–168. doi: 10.1042/BSR20100112. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda M., Kobayashi H., Ishibashi K., Ohbayashi N. Genome-wide investigation of the Rab binding activity of RUN domains: development of a novel tool that specifically traps GTP-Rab35. Cell Struct Funct. 2011;36(2):155–170. doi: 10.1247/csf.11001. [DOI] [PubMed] [Google Scholar]
- 36.Funderburk S.F., Wang Q.J., Yue Z. The Beclin 1–VPS34 complex – at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20(6):355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallegos M.E., Balakrishnan S., Chandramouli P., Arora S., Azameera A., Babushekar A., et al. The C. elegans rab family: identification, classification and toolkit construction. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galvez T., Gilleron J., Zerial M., O'Sullivan G.A. SnapShot: Mammalian Rab proteins in endocytic trafficking. Cell. 2012;151(1):234–234.e232. doi: 10.1016/j.cell.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Gao J., Zhao L., Luo Q., Liu S., Lin Z., Wang P., et al. An EHBP-1-SID-3-DYN-1 axis promotes membranous tubule fission during endocytic recycling. PLoS Genet. 2020;16(5) doi: 10.1371/journal.pgen.1008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaullier J.M., Simonsen A., D'Arrigo A., Bremnes B., Stenmark H., Aasland R. FYVE fingers bind PtdIns(3)P. Nature. 1998;394(6692):432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 41.Gengyo-Ando K., Kuroyanagi H., Kobayashi T., Murate M., Fujimoto K., Okabe S., et al. The SM protein VPS-45 is required for RAB-5-dependent endocytic transport in Caenorhabditis elegans. EMBO Rep. 2007;8(2):152–157. doi: 10.1038/sj.embor.7400882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goueli B.S., Powell M.B., Finger E.C., Pfeffer S.R. TBC1D16 is a Rab4A GTPase activating protein that regulates receptor recycling and EGF receptor signaling. Proc Natl Acad Sci U S A. 2012;109(39):15787–15792. doi: 10.1073/pnas.1204540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant B.D., Donaldson J.G. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grimsey N.J., Coronel L.J., Cordova I.C., Trejo J. Recycling and Endosomal Sorting of Protease-activated Receptor-1 Is Distinctly Regulated by Rab11A and Rab11B Proteins. J Biol Chem. 2016;291(5):2223–2236. doi: 10.1074/jbc.M115.702993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta K., Mukherjee S., Sen S., Sonawane M. Coordinated activities of Myosin Vb isoforms and mTOR signaling regulate epithelial cell morphology during development. Development. 2022;149(6) doi: 10.1242/dev.199363. [DOI] [PubMed] [Google Scholar]
- 46.Hales C.M., Griner R., Hobdy-Henderson K.C., Dorn M.C., Hardy D., Kumar R., et al. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276(42):39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 47.Han H., Shao Q., Liu X. LINC00441 promotes cervical cancer progression by modulating miR-450b-5p/RAB10 axis. Cancer Cell Int. 2020;20:368. doi: 10.1186/s12935-020-01400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoepfner S., Severin F., Cabezas A., Habermann B., Runge A., Gillooly D., et al. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121(3):437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Homma Y., Hiragi S., Fukuda M. Rab family of small GTPases: an updated view on their regulation and functions. Febs J. 2021;288(1):36–55. doi: 10.1111/febs.15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoogenraad C.C., Popa I., Futai K., Martinez-Sanchez E., Wulf P.S., van Vlijmen T., et al. Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol. 2010;8(1) doi: 10.1371/journal.pbio.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horgan C.P., McCaffrey M.W. Rab GTPases and microtubule motors. Biochem Soc Trans. 2011;39(5):1202–1206. doi: 10.1042/BST0391202. [DOI] [PubMed] [Google Scholar]
- 52.Horiuchi H., Lippé R., McBride H.M., Rubino M., Woodman P., Stenmark H., et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90(6):1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 53.Hutagalung A.H., Novick P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91(1):119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson A.P., Flett A., Smythe C., Hufton L., Wettey F.R., Smythe E. Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase. J Cell Biol. 2003;163(2):231–236. doi: 10.1083/jcb.200304079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jäger S., Bucci C., Tanida I., Ueno T., Kominami E., Saftig P., et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117(Pt 20):4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 56.Jain N., Ganesh S. Emerging nexus between RAB GTPases, autophagy and neurodegeneration. Autophagy. 2016;12(5):900–904. doi: 10.1080/15548627.2016.1147673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang W., Liu J., Xu T., Yu X. MiR-329 suppresses osteosarcoma development by downregulating Rab10. FEBS Lett. 2016;590(17):2973–2981. doi: 10.1002/1873-3468.12337. [DOI] [PubMed] [Google Scholar]
- 58.Jin N., Bi A., Lan X., Xu J., Wang X., Liu Y., et al. Identification of metabolic vulnerabilities of receptor tyrosine kinases-driven cancer. Nat Commun. 2019;10(1):2701. doi: 10.1038/s41467-019-10427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kachhap S.K., Faith D., Qian D.Z., Shabbeer S., Galloway N.L., Pili R., et al. The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin. PLoS ONE. 2007;2(9) doi: 10.1371/journal.pone.0000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaddai V., Gonzalez T., Keslair F., Grémeaux T., Bonnafous S., Gugenheim J., et al. Rab4b is a small GTPase involved in the control of the glucose transporter GLUT4 localization in adipocyte. PLoS ONE. 2009;4(4) doi: 10.1371/journal.pone.0005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kälin S., Hirschmann D.T., Buser D.P., Spiess M. Rabaptin5 is recruited to endosomes by Rab4 and Rabex5 to regulate endosome maturation. J Cell Sci. 2015;128(22):4126–4137. doi: 10.1242/jcs.174664. [DOI] [PubMed] [Google Scholar]
- 62.Khvotchev M.V., Ren M., Takamori S., Jahn R., Südhof T.C. Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis. J Neurosci. 2003;23(33):10531–10539. doi: 10.1523/JNEUROSCI.23-33-10531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinchen J.M., Ravichandran K.S. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464(7289):778–782. doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 65.Kloer D.P., Rojas R., Ivan V., Moriyama K., van Vlijmen T., Murthy N., et al. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;285(10):7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klöpper T.H., Kienle N., Fasshauer D., Munro S. Untangling the evolution of Rab G proteins: implications of a comprehensive genomic analysis. BMC Biol. 2012;10:71. doi: 10.1186/1741-7007-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi H., Fukuda M. Rab35 regulates Arf6 activity through centaurin-β2 (ACAP2) during neurite outgrowth. J Cell Sci. 2012;125(Pt 9):2235–2243. doi: 10.1242/jcs.098657. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi H., Fukuda M. Rab35 establishes the EHD1-association site by coordinating two distinct effectors during neurite outgrowth. J Cell Sci. 2013;126(Pt 11):2424–2435. doi: 10.1242/jcs.117846. [DOI] [PubMed] [Google Scholar]
- 69.Kouranti I., Sachse M., Arouche N., Goud B., Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16(17):1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Law F., Seo J.H., Wang Z., DeLeon J.L., Bolis Y., Brown A., et al. The VPS34 PI3K negatively regulates RAB-5 during endosome maturation. J Cell Sci. 2017;130(12):2007–2017. doi: 10.1242/jcs.194746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindsay A.J., Hendrick A.G., Cantalupo G., Senic-Matuglia F., Goud B., Bucci C., et al. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem. 2002;277(14):12190–12199. doi: 10.1074/jbc.M108665200. [DOI] [PubMed] [Google Scholar]
- 72.Lindsay A.J., McCaffrey M.W. Characterisation of the Rab binding properties of Rab coupling protein (RCP) by site-directed mutagenesis. FEBS Lett. 2004;571(1–3):86–92. doi: 10.1016/j.febslet.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 73.Lippe R., Miaczynska M., Rybin V., Runge A., Zerial M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell. 2001;12(7):2219–2228. doi: 10.1091/mbc.12.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu H., Wang S., Hang W., Gao J., Zhang W., Cheng Z., et al. LET-413/Erbin acts as a RAB-5 effector to promote RAB-10 activation during endocytic recycling. J Cell Biol. 2018;217(1):299–314. doi: 10.1083/jcb.201705136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J.J. Regulation of dynein-dynactin-driven vesicular transport. Traffic. 2017;18(6):336–347. doi: 10.1111/tra.12475. [DOI] [PubMed] [Google Scholar]
- 76.Liu O., Grant B.D. Basolateral endocytic recycling requires RAB-10 and AMPH-1 mediated recruitment of RAB-5 GAP TBC-2 to endosomes. PLoS Genet. 2015;11(9) doi: 10.1371/journal.pgen.1005514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu W., Yuen E.Y., Yan Z. The stress hormone corticosterone increases synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors via serum- and glucocorticoid-inducible kinase (SGK) regulation of the GDI-Rab4 complex. J Biol Chem. 2010;285(9):6101–6108. doi: 10.1074/jbc.M109.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mammoto A., Ohtsuka T., Hotta I., Sasaki T., Takai Y. Rab11BP/Rabphilin-11, a downstream target of rab11 small G protein implicated in vesicle recycling. J Biol Chem. 1999;274(36):25517–25524. doi: 10.1074/jbc.274.36.25517. [DOI] [PubMed] [Google Scholar]
- 79.Marat A.L., Ioannou M.S., McPherson P.S. Connecdenn 3/DENND1C binds actin linking Rab35 activation to the actin cytoskeleton. Mol Biol Cell. 2012;23(1):163–175. doi: 10.1091/mbc.E11-05-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mari M., Monzo P., Kaddai V., Keslair F., Gonzalez T., Le Marchand-Brustel Y., et al. The Rab4 effector Rabip4 plays a role in the endocytotic trafficking of Glut 4 in 3T3-L1 adipocytes. J Cell Sci. 2006;119(Pt 7):1297–1306. doi: 10.1242/jcs.02850. [DOI] [PubMed] [Google Scholar]
- 81.Maxfield F.R., McGraw T.E. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5(2):121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 82.McLauchlan H., Newell J., Morrice N., Osborne A., West M., Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8(1):34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 83.Müller M.P., Goody R.S. Molecular control of Rab activity by GEFs, GAPs and GDI. Small GTPases. 2018;9(1–2):5–21. doi: 10.1080/21541248.2016.1276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murray J.T., Panaretou C., Stenmark H., Miaczynska M., Backer J.M. Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic. 2002;3(6):416–427. doi: 10.1034/j.1600-0854.2002.30605.x. [DOI] [PubMed] [Google Scholar]
- 85.Nedvetsky P.I., Stefan E., Frische S., Santamaria K., Wiesner B., Valenti G., et al. A Role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic. 2007;8(2):110–123. doi: 10.1111/j.1600-0854.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 86.Nielsen E., Christoforidis S., Uttenweiler-Joseph S., Miaczynska M., Dewitte F., Wilm M., et al. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 2000;151(3):601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nieto C., Almendinger J., Gysi S., Gómez-Orte E., Kaech A., Hengartner M.O., et al. ccz-1 mediates the digestion of apoptotic corpses in C. elegans. J Cell Sci. 2010;123(Pt 12):2001–2007. doi: 10.1242/jcs.062331. [DOI] [PubMed] [Google Scholar]
- 88.Nishida Y., Arakawa S., Fujitani K., Yamaguchi H., Mizuta T., Kanaseki T., et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461(7264):654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 89.Nordmann M., Cabrera M., Perz A., Brocker C., Ostrowicz C., Engelbrecht-Vandre S., et al. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20(18):1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Nottingham R.M., Ganley I.G., Barr F.A., Lambright D.G., Pfeffer S.R. RUTBC1 protein, a Rab9A effector that activates GTP hydrolysis by Rab32 and Rab33B proteins. J Biol Chem. 2011;286(38):33213–33222. doi: 10.1074/jbc.M111.261115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nottingham R.M., Pusapati G.V., Ganley I.G., Barr F.A., Lambright D.G., Pfeffer S.R. RUTBC2 protein, a Rab9A effector and GTPase-activating protein for Rab36. J Biol Chem. 2012;287(27):22740–22748. doi: 10.1074/jbc.M112.362558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novick P., Medkova M., Dong G., Hutagalung A., Reinisch K., Grosshans B. Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans. 2006;34(Pt 5):683–686. doi: 10.1042/BST0340683. [DOI] [PubMed] [Google Scholar]
- 93.Numrich J., Ungermann C. Endocytic Rabs in membrane trafficking and signaling. Biol Chem. 2014;395(3):327–333. doi: 10.1515/hsz-2013-0258. [DOI] [PubMed] [Google Scholar]
- 94.Osaki F., Matsui T., Hiragi S., Homma Y., Fukuda M. RBD11, a bioengineered Rab11-binding module for visualizing and analyzing endogenous Rab11. J Cell Sci. 2021;134(7) doi: 10.1242/jcs.257311. [DOI] [PubMed] [Google Scholar]
- 95.Pankiv S., Alemu E.A., Brech A., Bruun J.A., Lamark T., Overvatn A., et al. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188(2):253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfeffer S.R. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25(4):414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pfeffer S.R. Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol Biol Cell. 2017;28(6):712–715. doi: 10.1091/mbc.E16-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pfeffer S.R., Dirac-Svejstrup A.B., Soldati T. Rab GDP dissociation inhibitor: putting rab GTPases in the right place. J Biol Chem. 1995;270(29):17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- 99.Poteryaev D., Datta S., Ackema K., Zerial M., Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141(3):497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 100.Poteryaev D., Fares H., Bowerman B., Spang A. Caenorhabditis elegans SAND-1 is essential for RAB-7 function in endosomal traffic. Embo J. 2007;26(2):301–312. doi: 10.1038/sj.emboj.7601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prekeris R., Klumperman J., Scheller R.H. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6(6):1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 102.Reddy J.V., Burguete A.S., Sridevi K., Ganley I.G., Nottingham R.M., Pfeffer S.R. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell. 2006;17(10):4353–4363. doi: 10.1091/mbc.E06-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riederer M.A., Soldati T., Shapiro A.D., Lin J., Pfeffer S.R. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol. 1994;125(3):573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rink J., Ghigo E., Kalaidzidis Y., Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 105.Ritter J.L., Zhu Z., Thai T.C., Mahadevan N.R., Mertins P., Knelson E.H., et al. Phosphorylation of RAB7 by TBK1/IKKε Regulates Innate Immune Signaling in Triple-Negative Breast Cancer. Cancer Res. 2020;80(1):44–56. doi: 10.1158/0008-5472.CAN-19-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rohde G., Wenzel D., Haucke V. A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J Cell Biol. 2002;158(2):209–214. doi: 10.1083/jcb.200203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rojas R., van Vlijmen T., Mardones G.A., Prabhu Y., Rojas A.L., Mohammed S., et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183(3):513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakane A., Hatakeyama S., Sasaki T. Involvement of Rabring7 in EGF receptor degradation as an E3 ligase. Biochem Biophys Res Commun. 2007;357(4):1058–1064. doi: 10.1016/j.bbrc.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 109.Sann S.B., Crane M.M., Lu H., Jin Y. Rabx-5 regulates RAB-5 early endosomal compartments and synaptic vesicles in C. elegans. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0037930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sano H., Eguez L., Teruel M.N., Fukuda M., Chuang T.D., Chavez J.A., et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5(4):293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 111.Sarantis H., Balkin D.M., De Camilli P., Isberg R.R., Brumell J.H., Grinstein S. Yersinia entry into host cells requires Rab5-dependent dephosphorylation of PI(4,5)P₂ and membrane scission. Cell Host Microbe. 2012;11(2):117–128. doi: 10.1016/j.chom.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sato M., Sato K., Fonarev P., Huang C.-J., Liou W., Grant B.D. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nat Cell Biol. 2005;7(6):559. doi: 10.1038/ncb1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sato M., Sato K., Liou W., Pant S., Harada A., Grant B.D. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. Embo J. 2008;27(8):1183–1196. doi: 10.1038/emboj.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schonhoff C.M., Thankey K., Webster C.R., Wakabayashi Y., Wolkoff A.W., Anwer M.S. Rab4 facilitates cyclic adenosine monophosphate-stimulated bile acid uptake and Na+-taurocholate cotransporting polypeptide translocation. Hepatology. 2008;48(5):1665–1670. doi: 10.1002/hep.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schuck S., Gerl M.J., Ang A., Manninen A., Keller P., Mellman I., et al. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8(1):47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 116.Seabra M.C., Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol. 2004;16(4):451–457. doi: 10.1016/j.ceb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 117.Semerdjieva S., Shortt B., Maxwell E., Singh S., Fonarev P., Hansen J., et al. Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J Cell Biol. 2008;183(3):499–511. doi: 10.1083/jcb.200806016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi A., Chen C.-C.-H., Banerjee R., Glodowski D., Audhya A., Rongo C., et al. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol Biol Cell. 2010;21(16):2930–2943. doi: 10.1091/mbc.E10-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi A., Grant B.D. Interactions between Rab and Arf GTPases regulate endosomal phosphatidylinositol-4,5-bisphosphate during endocytic recycling. Small GTPases. 2013;4(2):106–109. doi: 10.4161/sgtp.23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi A., Liu O., Koenig S., Banerjee R., Chen C.-C.-H., Eimer S., et al. RAB-10-GTPase–mediated regulation of endosomal phosphatidylinositol-4, 5-bisphosphate. Proc Natl Acad Sci. 2012;109(35):E2306–E2315. doi: 10.1073/pnas.1205278109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shin H.W., Hayashi M., Christoforidis S., Lacas-Gervais S., Hoepfner S., Wenk M.R., et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol. 2005;170(4):607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Silvis M.R., Bertrand C.A., Ameen N., Golin-Bisello F., Butterworth M.B., Frizzell R.A., et al. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell. 2009;20(8):2337–2350. doi: 10.1091/mbc.E08-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simonsen A., Lippe R., Christoforidis S., Gaullier J.M., Brech A., Callaghan J., et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394(6692):494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 124.Sobu Y., Wawro P.S., Dhekne H.S., Yeshaw W.M., Pfeffer S.R. Pathogenic LRRK2 regulates ciliation probability upstream of tau tubulin kinase 2 via Rab10 and RILPL1 proteins. Proc Natl Acad Sci U S A. 2021;118(10) doi: 10.1073/pnas.2005894118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soldati T., Rancaño C., Geissler H., Pfeffer S.R. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J Biol Chem. 1995;270(43):25541–25548. doi: 10.1074/jbc.270.43.25541. [DOI] [PubMed] [Google Scholar]
- 126.Stockler S., Corvera S., Lambright D., Fogarty K., Nosova E., Leonard D., et al. Single point mutation in Rabenosyn-5 in a female with intractable seizures and evidence of defective endocytotic trafficking. Orphanet J Rare Dis. 2014;9:141. doi: 10.1186/s13023-014-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun L., Liu O., Desai J., Karbassi F., Sylvain M.-A., Shi A., et al. CED-10/Rac1 regulates endocytic recycling through the RAB-5 GAP TBC-2. PLoS Genet. 2012;8(7) doi: 10.1371/journal.pgen.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takahashi S., Kubo K., Waguri S., Yabashi A., Shin H.-W., Katoh Y., et al. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J Cell Sci. 2012;125(17):4049–4057. doi: 10.1242/jcs.102913. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka D., Kameyama K., Okamoto H., Doi M. Caenorhabditis elegans Rab escort protein (REP-1) differently regulates each Rab protein function and localization in a tissue-dependent manner. Genes Cells. 2008;13(11):1141-1157. doi: 10.1111/j.1365-2443.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 130.Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135(4):913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uytterhoeven V., Kuenen S., Kasprowicz J., Miskiewicz K., Verstreken P. Loss of Skywalker Reveals Synaptic Endosomes as Sorting Stations for Synaptic Vesicle Proteins. Cell. 2011;145(1):117–132. doi: 10.1016/j.cell.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 132.van der Sluijs P., Hull M., Webster P., Male P., Goud B., Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70(5):729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- 133.Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 1991;88(14):6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Villagomez F.R., Medina-Contreras O., Cerna-Cortes J.F., Patino-Lopez G. The role of the oncogenic Rab35 in cancer invasion, metastasis, and immune evasion, especially in leukemia. Small GTPases. 2020;11(5):334–345. doi: 10.1080/21541248.2018.1463895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vukmirica J., Monzo P., Le Marchand-Brustel Y., Cormont M. The Rab4A Effector Protein Rabip4 Is Involved in Migration of NIH 3T3 Fibroblasts*. J Biol Chem. 2006;281(47):36360–36368. doi: 10.1074/jbc.M602920200. [DOI] [PubMed] [Google Scholar]
- 136.Wang P., Liu H., Wang Y., Liu O., Zhang J., Gleason A., et al. RAB-10 Promotes EHBP-1 Bridging of Filamentous Actin and Tubular Recycling Endosomes. PLoS Genet. 2016;12(6) doi: 10.1371/journal.pgen.1006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang T., Wong K.K., Hong W. A unique region of RILP distinguishes it from its related proteins in its regulation of lysosomal morphology and interaction with Rab7 and Rab34. Mol Biol Cell. 2004;15(2):815–826. doi: 10.1091/mbc.E03-06-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang W., Jia W.D., Hu B., Pan Y.Y. RAB10 overexpression promotes tumor growth and indicates poor prognosis of hepatocellular carcinoma. Oncotarget. 2017;8(16):26434–26447. doi: 10.18632/oncotarget.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Welz T., Wellbourne-Wood J., Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 2014;24(7):407–415. doi: 10.1016/j.tcb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 140.Wen H., Zhan L., Chen S., Long L., Xu E. Rab7 may be a novel therapeutic target for neurologic diseases as a key regulator in autophagy. J Neurosci Res. 2017;95(10):1993–2004. doi: 10.1002/jnr.24034. [DOI] [PubMed] [Google Scholar]
- 141.Woodman P.G. Biogenesis of the sorting endosome: the role of Rab5. Traffic. 2000;1(9):695–701. doi: 10.1034/j.1600-0854.2000.010902.x. [DOI] [PubMed] [Google Scholar]
- 142.Wurmser A.E., Sato T.K., Emr S.D. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151(3):551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu C.L., Wang J.Z., Xia X.P., Pan C.W., Shao X.X., Xia S.L., et al. Rab11-FIP2 promotes colorectal cancer migration and invasion by regulating PI3K/AKT/MMP7 signaling pathway. Biochem Biophys Res Commun. 2016;470(2):397–404. doi: 10.1016/j.bbrc.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 144.Yamamoto H., Koga H., Katoh Y., Takahashi S., Nakayama K., Shin H.W. Functional cross-talk between Rab14 and Rab4 through a dual effector, RUFY1/Rabip4. Mol Biol Cell. 2010;21(15):2746–2755. doi: 10.1091/mbc.E10-01-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ye B., Duan B., Deng W., Wang Y., Chen Y., Cui J., et al. EGF Stimulates Rab35 Activation and Gastric Cancer Cell Migration by Regulating DENND1A-Grb2 Complex Formation. Front Pharmacol. 2018;9:1343. doi: 10.3389/fphar.2018.01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng J., Ren M., Gravotta D., De Lemos-Chiarandini C., Lui M., Erdjument-Bromage H., et al. Identification of a putative effector protein for rab11 that participates in transferrin recycling. Proc Natl Acad Sci U S A. 1999;96(6):2840–2845. doi: 10.1073/pnas.96.6.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang J., Fonovic M., Suyama K., Bogyo M., Scott M.P. Rab35 controls actin bundling by recruiting fascin as an effector protein. Science. 2009;325(5945):1250–1254. doi: 10.1126/science.1174921. [DOI] [PubMed] [Google Scholar]
- 148.Zhang M., Chen L., Wang S., Wang T. Rab7: roles in membrane trafficking and disease. Biosci Rep. 2009;29(3):193–209. doi: 10.1042/BSR20090032. [DOI] [PubMed] [Google Scholar]
- 149.Zhang W., Wang S., Yang C., Hu C., Chen D., Luo Q., et al. LET-502/ROCK Regulates Endocytic Recycling by Promoting Activation of RAB-5 in a Distinct Subpopulation of Sorting Endosomes. Cell Reports. 2020;32(12) doi: 10.1016/j.celrep.2020.108173. [DOI] [PubMed] [Google Scholar]
- 150.Zhang W., Wang S., Yang C., Hu C., Chen D., Luo Q., et al. LET-502/ROCK Regulates Endocytic Recycling by Promoting Activation of RAB-5 in a Distinct Subpopulation of Sorting Endosomes. Cell Rep. 2020;32(12) doi: 10.1016/j.celrep.2020.108173. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X.-M., Ellis S., Sriratana A., Mitchell C.A., Rowe T. Sec15 Is an Effector for the Rab11 GTPase in Mammalian Cells*. J Biol Chem. 2004;279(41):43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 152.Zhang X., Peng Y., Huang Y., Deng S., Feng X., Hou G., et al. Inhibition of the miR-192/215-Rab11-FIP2 axis suppresses human gastric cancer progression. Cell Death Dis. 2018;9(7):778. doi: 10.1038/s41419-018-0785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X., Wang S., Lin G., Wang D. Down-regulation of circ-PTN suppresses cell proliferation, invasion and glycolysis in glioma by regulating miR-432-5p/RAB10 axis. Neurosci Lett. 2020;735 doi: 10.1016/j.neulet.2020.135153. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Y., Chen Z., Wang F., Sun H., Zhu X., Ding J., et al. Nde1 is a Rab9 effector for loading late endosomes to cytoplasmic dynein motor complex. Structure. 2022;30(3):386–395.e385. doi: 10.1016/j.str.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y.J., Pan Q., Yu Y., Zhong X.P. microRNA-519d Induces Autophagy and Apoptosis of Human Hepatocellular Carcinoma Cells Through Activation of the AMPK Signaling Pathway via Rab10. Cancer Manag Res. 2020;12:2589–2602. doi: 10.2147/CMAR.S207548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]