Abstract
Tubo-ovarian abscesses (TOAs) are a complicated form of pelvic inflammatory disease (PID). They are usually caused by Bacteroides species or Escherichia coli. A 35-year-old woman presented with TOA caused by an infection with the rare pathogen Edwardsiella tarda. Thus, in a suspected case of a TOA in a patient with PID, we recommend obtaining a culture to test for a wide variety of bacterial organisms. By identifying less common pathogenic causes of TOA earlier, more conservative treatments can be used to mitigate the negative consequences of TOA and the need for surgical intervention.
Keywords: Tubo-ovarian abscesses, Edwardsiella tarda, Pelvic inflammatory disease
Highlights
-
•
Edwardsiella tarda is an uncommon pathogen that typically presents as gastrointestinal infection.
-
•
It may cause tubo-ovarian abscess that can be treated with antibiotics with anaerobic coverage and surgical removal.
-
•
Edwardsiella tarda may cause tubo-ovarian abscess that histologically presents with xanthogranulomatous salpingitis.
1. Introduction
Edwarsiella tarda is a bacterium in the enterobacteriaceae family. E. tarda is a Gram-negative bacillus that is oxidase negative and catalase positive [1]. This pathogen is waterborne and rarely causes infections in humans [2]. It commonly infects water-living organisms, such as reptiles [2]. The rare infections in humans are mostly gastrointestinal, presenting with a fever, gastroenteritis, and diarrhea [1,2], and in tropical areas [1]. This distribution may be related to a greater consumption of infected raw fish in tropical areas. Thus, the entry of E. tarda into the human body is usually through oral digestion of infected seafood or aquatic animals [1]. More rare causes include trauma in which a wound was exposed or contaminated with a foreign body containing E. tarda [1]. Very few cases in the medical literature document E. tarda infections, and the entry of the bacterium is not understood well [1]. The present case concerns a 35-year-old woman who presented with a tubo-ovarian abscess (TOA) infected with E. tarda.
TOAs commonly present with unilateral abdominal pain, fever, and an adnexal mass on bimuanal examination, and are most often a late complication of pelvic inflammatory disease (PID), but they may also be caused by extension from an adjacent organ such as the appendix, or hematogenous spread. [3]. PID encompasses a wide array of disorders in the female genitalia, including endometritis, salpingitis and TOA [4]. Cases of PID caused by Chlamydia trachomatis or Neisseria gonorrhoeae do not commonly present as TOA [4]. The primary bacterial contributors to TOA secondary to PID are Escherichia coli, Bacteroides fragilis, other Bacteroides species, Peptostreptococcus, Peptococcus, and aerobic streptococci [5]. To our knowledge, a TOA caused by E. tarda has not been reported in the mainland United States but has been found in Hawaii according to a case study published in 1995 [6]. When TOA as a result of PID is suspected, it is recommended to use transvaginal ultrasound or magnetic resonance imaging to make a diagnosis [7]. Treatment for a TOA begins conservatively with antibiotic management [8]. However, if clinical improvement is not achieved with antibiotic therapy, then surgical intervention via laparoscopy or interventional radiology drainage may be required to resolve the abscess [8]. This case study is unique in that the cause of the TOA was infection with E. tarda; the route of entry remains unknown.
2. Case Presentation
The patient was a 35-year-old woman with a history of multiple episodes of PID managed in the outpatient setting, chlamydia, herpes simplex virus, abnormal pap smears and idiopathic pulmonary embolism (PE) (on apixaban), and a surgical history of a single cesarean section. She presented with 6 days of lower abdominal cramping, non-bloody diarrhea, nausea and emesis; all other reviews of systems were negative. The lower abdominal pain had started suddenly and gradually increased. The patient reported no aggravating or relieving factors, but commented on a recent change in diet, which did not include seafood, while visiting Arizona, where her initial symptoms began. After visiting two other emergency facilities, the patient was transferred and treated at a third emergency department. There, the patient's vital signs upon presentation were as follows: blood pressure 117/63 mmHg, heart rate 122 beats/min, temperature 38.1 °C, and oxygen saturation 96%. On the initial physical exam, the patient was not in acute distress and non-toxic appearing or diaphoretic. Physical exam demonstrated no abdominal distention nor guarding, but she had moderate tenderness to palpation in bilateral lateral abdomen. On bimanual exam, there was cervical motion tenderness, uterus was mobile and tender to palpation, and no masses were palpated, but the exam was limited due to patient discomfort. A bloody and purulent discharge was observed in the vaginal canal.
Laboratory tests were remarkable for leukocytosis (WBCs 10.46 103/uL), anemia (Hgb 10.8 g/dL), mild transaminitis (AST 41 U/L) and hyperbilirubinemia (1.7 mg/dL). The patient's wet mount was positive for yeast infection, and she was treated with fluconazole. CT conducted at one of the other hospitals prior to transfer and admission at the third facility showed complex, serpiginous, fluid-filled structures on both sides of the pelvis, intimately associated with the uterus, without discernable ovaries bilaterally. The patient was admitted and started on intravenous cefoxitin, doxycycline and metronidazole. Image-guided right TOA drainage was conducted, when 5 cc of fluid was removed and a culture was acquired. Gram stain was positive for a few Gram-negative rods and many white blood cells (greater than 25 per 100×). The culture showed heavy growth of E. tarda at 48 h. The patient had had two positive blood cultures for Gram-negative rods from the previous facility, but the species was not reported. Repeat blood cultures drawn three days after the initial positive blood culture were negative.
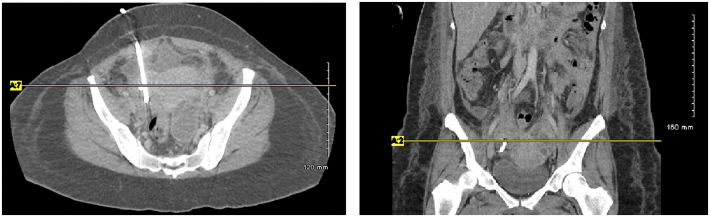
The patient improved with drainage and antibiotics and was discharged home on intravenous ertapenem daily via a PICC line for 4 weeks. Four weeks after discharge, repeat CT revealed new ovarian cysts in the right adnexa 4.6 × 3.2 × 3.6 cm and new cystic masses developing in the left adnexa (Fig. 1). Due to the patient's persistent TOAs refractory to both medical management and drainage, the patient was counseled on definitive management via surgical resection. She expressed frustration with continued pelvic pain and fear of recurrent hospitalization due to sepsis, therefore desired a total abdominal hysterectomy, bilateral salpingo-oophorectomy, rather than maintaining fertility with continued conservative management. After completion of the four weeks of ertapenem, she was switched to oral cefdinir until her scheduled operation. She was seen for pre-operative risk stratification by her hematologist and a CT pulmonary angiogram revealed a new non-occlusive pulmonary embolism in the lingula, and an inferior vena cava filter was placed two days prior to scheduled hysterectomy.
Fig. 1.
Complex loculated left adnexal cystic structure with peripheral enhancement measuring approximately 7.4 × 5.6 × 5.7 cm. Right pigtail drainage catheter seen within the region of the right adnexa without surrounding fluid.
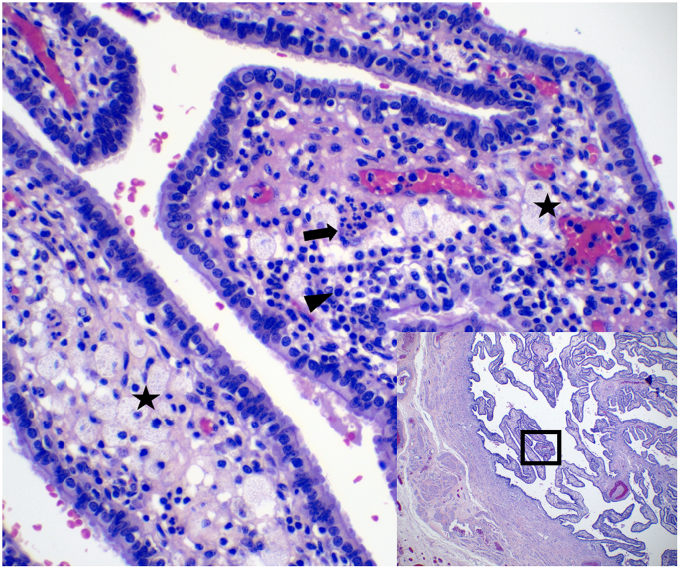
She underwent a total abdominal hysterectomy, bilateral salpingo-oophorectomy (BSO), when bilateral tubo-ovarian abscesses were noted densely adherent to bowel and bilateral ureters. Oral cefdinir was continued for 2 weeks post-operatively. Gross pathology and histology showed secretory endometrium, leiomyomata, serosal endometriosis, mild chronic cervicitis and erosion, multiple possible corpora lutea with areas of hemorrhage, and bilateral fallopian tubes with xanthogranulomatous salpingitis (XGS) (Fig. 2). Xanthogranuloma is a histological finding that most commonly occurs in the kidneys and is characterized as an abnormal accumulation of macrophages [9,10]. There have been very few reports of XGS in the literature, and it is considered a rare sequalae of chronic PID [9,10].
Fig. 2.
Xanthogranulomatous salpingitis. There are lipid-containing macrophages (stars) and mixed inflammatory infiltrate including plasma cells (arrowhead) and neutrophils (arrow).
Following BSO, the patient was place on cefdinir oral 300 mg daily for two weeks. For hormonal replacement therapy, the patient started estradiol (0.05 mg/24 h via patch placed twice a week).
3. Discussion and Conclusion
To date, no cases of TOA caused by E. tarda have been identified in the mainland United States. E. tarda is a waterborne infection and may be a greater threat to populations living near contaminated water supplies or populations eating a diet more likely to contain raw or infected seafood. TOAs are classically diagnosed by transvaginal ultrasound and managed by an IV antibiotic regimen with resolution achieved in 93.4% of cases [8]. The patient received a classic antibiotic regimen of cefoxitin, doxycycline and metronidazole, as well as drain placement, and did not have resolution of the TOA. Her history of multiple genitourinary infections may have contributed to the refractory TOA. For the recurring problem of TOA, the patient desired a total abdominal hysterectomy, bilateral salpingo-oophorectomy to eliminate this medical issue and cease worrying about recurring infections. Medical history provided no information as to how E. tarda had entered a sterile site. To her knowledge, the patient had had no open trauma or contact with a foreign body. Furthermore, presentation of the TOA with E. tarda suggests the bacterium was not acquired via the oral route. It remains unclear if the source of TOA was hematogenous or PID, as she was septic at presentation to the first hospital and there was no evident source of exposure such as raw seafood or swimming. The previous reported case of TOA with E. tarda was resolved with drainage and antibiotics; therefore, surgical biopsy and histological evaluation was not required. The presence of XGS suggests that the current case was likely complicated by a longstanding history of pelvic infections. In the few case reports of XGS, it was associated with malignancy, long-standing endometriosis, or chronic PID. It is thought that the week's delay in the present case from initial symptoms to receiving antibiotics at the third emergency department may have contributed to the poor response to conservative management. Furthermore, this case demonstrates the importance of drainage and culture of TOAs which are not responding to empiric therapy to improve patient comfort and to treat non-resolving TOAs.
Acknowledgments
Contributors
Kaitlyn Cox wrote all aspects of the paper.
Marci Crowley helped write all aspects of the paper.
Kimberly Fryer aided in the revision and editing of all aspects of the paper, and was also involved in direct patient care.
All authors approved the final submitted manuscript
Funding
No funding from an external source supported the publication of this case report.
Patient consent
Obtained.
Provenance and peer review
This article was not commissioned and was peer reviewed.
Acknowledgments
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this case report.
Contributor Information
Kaitlyn Cox, Email: coxk4@usf.edu.
Marci Crowley, Email: marcicrowley@usf.edu.
Kimberly Fryer, Email: kfryer@usf.edu.
References
- 1.Janda J.M., Abbott S.L. Infections associated with the genus Edwardsiella: the role of Edwardsiella tarda in human disease. Clin. Infect. Dis. 1993;17(4):742–748. doi: 10.1093/clinids/17.4.742. [DOI] [PubMed] [Google Scholar]
- 2.Hirai Y., et al. Edwardsiella tarda bacteremia. A rare but fatal water- and foodborne infection: review of the literature and clinical cases from a single Centre. Can. J. Infect. Dis. Med. Microbiol. 2015;26(6):313–318. doi: 10.1155/2015/702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curry A., Williams T., Penny M.L. Pelvic inflammatory disease: diagnosis, management, and prevention. Am. Fam. Physician. 2019;100(6):357–364. [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention Pelvic inflammatory disease (PID) - STI treatment guidelines. 2021 November 9. https://www.cdc.gov/std/treatment-guidelines/pid.htm 2021 February 9, 2022; Available from:
- 5.Mohseni M., Simon L.V., Sheele J.M. Epidemiologic and clinical characteristics of tubo-ovarian abscess, hydrosalpinx, pyosalpinx, and oophoritis in emergency department patients. Cureus. 2020;12(11) doi: 10.7759/cureus.11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pien F.D., Jackson M.T. Tuboovarian abscess caused by Edwardsiella tarda. Am. J. Obstet. Gynecol. 1995;173(3 Pt 1):964–965. doi: 10.1016/0002-9378(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 7.Workowski K.A., et al. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 8.Gjelland K., Ekerhovd E., Granberg S. Transvaginal ultrasound-guided aspiration for treatment of tubo-ovarian abscess: a study of 302 cases. Am. J. Obstet. Gynecol. 2005;193(4):1323–1330. doi: 10.1016/j.ajog.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Idrees M., Zakashansky K., Kalir T. Xanthogranulomatous salpingitis associated with fallopian tube mucosal endometriosis: a clue to the pathogenesis. Ann. Diagn. Pathol. 2007;11(2):117–121. doi: 10.1016/j.anndiagpath.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Chiesa-Vottero A. Xanthogranulomatous Salpingitis. Int. J. Gynecol. Pathol. 2020;39(5):468–472. doi: 10.1097/PGP.0000000000000625. [DOI] [PubMed] [Google Scholar]