Abstract
Many patients return to cognitively demanding work after breast cancer treatments. This makes treatment-related cognitive decline an important research topic. Psychological resilience, cognitive reserve and better perceived general health may work as protective factors against cognitive decline. The aim of this study was to analyse whether these factors are associated with cognitive function among such women.
Data from 384 breast cancer survivors who underwent neuropsychological examination at follow-up 4–9 years after surgery were used. The neurocognitive domain variable Learning and Memory was computed from Wechsler Memory Scale-III subtests Learning and Delayed Recall. Another variable, Attention, Processing speed and Executive function, was computed from semantic and verbal fluency tests, Trail Making Test A and B, and Wechsler Adult Intelligence Test-IV subtest Coding. Psychological resilience was measured with Resilience Scale-14, and perceived general health with RAND-36 subitem General Health.
Results
showed that levels of cognitive performance and general health were statistically higher than population average. Resilience and general health in separate models were associated with Attention, Processing speed and Executive function (β = 0.14, p = 0.01; β = 0.13, p = 0.03, respectively). When added simultaneously in the same model, resilience was significant (β = 0.13, p = 0.04), but general health was not. These associations were nonsignificant after controlling for confounding factors. Learning and Memory was not associated with resilience or general health.
Future research should focus on longitudinal studies identifying patients at a high risk of developing cognitive decline after breast cancer treatments and on preventive and therapeutic approaches.
Keywords: Breast cancer, Cognitive reserve, General health, Neurocognitive performance, Psychological resilience
Highlights
-
•
401 women were neuropsychologically examined 4–9 years after breast cancer surgery.
-
•
Their cognitive performance was on average well preserved.
-
•
Psychological Resilience associated with better cognitive performance in one domain.
-
•
The association between General Health and cognition was less prominent.
-
•
High education level might protect against cognitive decline after breast cancer.
1. Introduction
Thanks to improved screening and treatment [1], mortality from breast cancer (BC) has decreased, and good quality of life after BC treatments has become an important goal [2]. Many patients return to cognitively demanding work, making treatment-related cognitive decline an important research topic. A recent review concluded that 24% of the patients have cognitive impairment immediately after chemotherapy and that it remains at a similar level at follow-up [3]. However, 25% of the patients already had cognitive impairment before chemotherapy [3], suggesting that other causes need to be considered as possible risk factors. Another meta-analysis showed that BC patients treated with chemotherapy demonstrated overall cognitive impairment (e.g. attention, executive function) when compared with healthy controls, but not when compared with BC survivors who had not received chemotherapy [4], suggesting that other factors associated with breast cancer and its treatment may have an effect.
Endocrine therapy may also be associated with impaired cognition, but its effects may emerge later [5] than after chemotherapy. Some patients improve over months or years after BC treatments but others experience long-term cognitive impairment, lasting up to 20 years after treatment [6]. It has been suggested that a subgroup of BC patients experience cognitive decline [7] and that those who develop long-term cognitive decline may have an underlying risk of, for example, dementia, which is exacerbated by chemotherapy or the stress and other psychological responses associated with BC [4,8]. For example, anxiety and depressive symptoms are more frequent among BC survivors [9], while fatigue [10], poor sleep [11], and persistent pain may also be associated with worse cognitive performance [12].
Psychological resilience is an individual's ability to maintain or recover relatively stable psychological and physical functioning during or after exposure to significant life adversities [13]. Among cancer patients, resilience is a dynamic process that promotes adaptation to cancer-related adversity [14]. Resilience is built from personal characteristics and protective factors, such as optimism, positive emotion, self-esteem, coping or social support [14]. Among BC patients, resilience is associated directly with psychological wellbeing [15,16] and physical symptom burden [17]. We have previously shown that resilience moderates the association between anxiety and pain experience in BC survivors [15]. Resilience might protect a person against cognitive decline by adding more psychological flexibility, activity, and adaptive coping skills throughout the lifespan. In this study, general health captures the patients' subjective perception of health. In a recent study on breast cancer patients, higher resilience associated with higher general health and had a greater effect through affective well-being than directly [18]. General health has been reported to be lower in women having BC than in the general population [19].
Cognitive reserve [20] refers to individual differences that can protect against or compensate for cognitive decline related to brain pathology or normal ageing. Education and cognitively challenging activities, such as hobbies, social activities, and information-seeking, can strengthen cognitive reserve through better compensatory mechanisms. There is some evidence that cognitive reserve can protect against cognitive impairment after BC treatments [[21], [22], [23]] and should therefore be controlled when studying cognitive performance. Lower cognitive reserve has been reported to be associated with depression and anxiety [24,25], which are more prevalent in BC patients than in the general population [26,27]. Objective cognitive performance in BC survivors has not been thoroughly studied with an extensive neuropsychological test battery with long follow up together with psychological protective factors.
The aim of the present study was to analyse how resilience and perceived general health associate with cognitive function. We hypothesised that higher resilience and better self-reported general health associate with better cognitive function among women treated for BC.
2. Methods
The study was approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District (reference number 149/13/03/00/14) and registered in ClinicalTrials.gov (NCT02487524). All participating patients gave written informed consent.
3. Patients
This study is part of a larger project studying neuropathic pain following BC surgery (NeuroPain cohort). Participants in NeuroPain were recruited from a cohort of 1000 women treated for BC at Helsinki University Hospital 2006–2010 (BrePainGen cohort) [28]. For the original cohort, patients were eligible if younger than 75 years, with unilateral non-metastasised BC, treated with either breast-conserving surgery or mastectomy, and sentinel node biopsy and/or axillary clearance surgery. Oncological adjuvant treatments were given according to international and local guidelines [29]. These were radiotherapy, chemotherapy (docetaxel, 5-fluorouracil, epirubicin, and cyclophosphamide), trastuzumab, and endocrine therapy (anti-oestrogens, aromatase inhibitors, LHRH agonists or combinations), based on clinical indications.
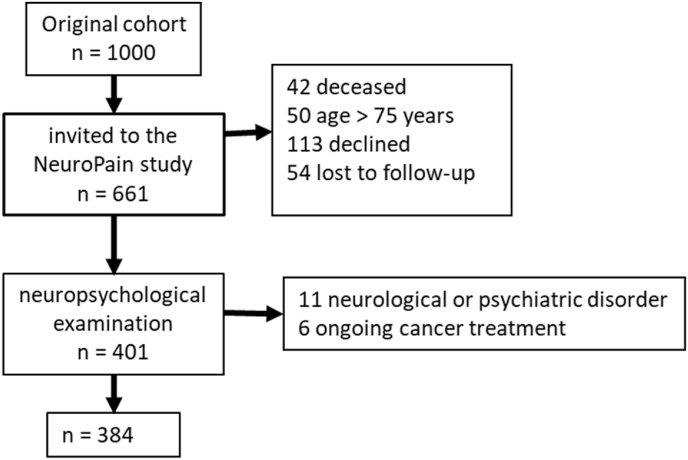
The patients in the NeuroPain study have previously been described in detail [30]: 401 patients underwent neuropsychological examination at the follow-up visit 4–9 (median 6) years after surgery; 17 were excluded from analyses due to certain neurological or psychiatric disorders or ongoing cancer treatments (Fig. 1).
Fig. 1.
Flow-chart of the paitents from the orginal cohort BrePainGen (consecutive cohort of women treated for breast cancer) to NeuroPain(study for pain following surgical nerve leasion) and the current cohort.
4. Tests and questionnaires
Wechsler Adult Intelligence Test-IV (WAIS-IV) subtest Coding [31], Wechsler Memory Scale-III (WMS-III) subtests Learning and Delayed Recall [32], semantic and phonemic verbal fluency tests [33,34], and Trail Making Test Parts A and B [35], were used to evaluate cognitive performance. Age is a well-known factor affecting neurocognitive performance and therefore age-corrected values of test scores are always used in clinical practice. Information about the average test performance in specific age groups in the Finnish population was used to convert each patient's raw test score into age-corrected normative values, describing how many standard deviations (SD) below or above the age group average a patient performed. These corrected values are also in the same scale (z-scores) with each other. Instead of multiple separate test scores, we decided to create two, more informative, neurocognitive domain variables: 1) Learning and Memory and 2) Attention, Processing speed and Executive function. Learning and Memory was computed as a mean z-score from WMS-III Learning and Delayed Recall. Attention, Processing speed and Executive function was computed as a mean z-score from semantic and verbal fluency, coding, Trail Making A and B. The scores from these domain variables tell how many SD above or below the person performs on average in tests measuring that cognitive domain in her age group.
Patients reported their worst pain intensity during the past week on a Numerical Rating Scale (NRS) from 0 to 10 (0 no pain, 10 worst possible pain). They also reported pain interference during the past week on an NRS (0–10: 0 not at all, 10 very much). Pain intensity NRS ≥4 is classified as clinically significant [36]. As pain interference is better associated with life-satisfaction and mood than with pain [37], we used pain interference as the covariate rather than pain intensity.
Insomnia symptoms were measured by Insomnia Severity Index (ISI), a 7-item questionnaire measuring the patient's perception of her sleep quality [38]. Patients answer on a Likert scale (0–4), the total varying from 0 to 28, a higher score indicating worse symptoms; the clinically significant level is ≥ 8 points (mild).
Depressive symptoms and anxiety were measured by Hospital Anxiety and Depression Scale (HADS), a 14-item questionnaire [39] answered on a 0–3 Likert scale. The total for the D and A subscales varies from 0 to 21, a higher score indicating more serious symptoms. The clinically significant level for both subscales is ≥ 8 points [39]. In this study, we calculated overall psychological distress by combining HADS-D and HADS-A as one variable.
We used the Modified Cognitive Reserve Scale (mCRS) [40] to measure cognitive reserve as lifelong attainment in leisure activities. This comprises 20 questions about studying and information-seeking, hobbies and social relationships. The patient indicates on a Likert scale (0–4) how often she participates in certain activities. The mCRS measures cognitive reserve in up to three age ranges: 18–35, 36–64, and 65 years and older (as appropriate to the participant's age). The mean of each item across the time points is then calculated, the total score varying from 0 to 80: a higher score indicates greater cognitive reserve. The mCRS was completed by 382 patients. Questionnaires with more than 20% of missing values were not analysed.
Patients’ levels of resilience were measured with the Resilience Scale (short version) (RS-14) [41]. Patients addressed 14 items on a 1–7 Likert scale, the total score ranging from 14 to 98 points (higher scores representing greater resilience).
Level of education was recorded as “low” (primary school), “medium” (secondary or vocational school), or “high” (university of applied sciences or higher).
We used the “General Health” sub-item from RAND-36 (a health-related quality of life measurement questionnaire) to evaluate patients’ subjectively perceived general health [42]. Adjusted for age and gender, the general health varies from 0 to 100, higher numbers indicating better perceived general health.
5. Statistical analyses
One sample t-tests were used to test if cognitive domains differed from the expectation (z-score 0). Independent sample t-tests were used to compare cognitive performances of groups of patients as continuous variables. Pearson's Chi-Squared test was used when comparing groups in categorical variables. Associations between continuous variables were estimated with Pearson's correlations.
Patients were allocated into groups according to the clinical significance of their reported insomnia (ISI ≥8 points), depressive symptoms (HADS-D ≥ 8 points), anxiety symptoms (HADS-A ≥ 8 points) or pain (NRS ≥4). We then selected patients with clinically significant symptoms in all four areas mentioned (burden). Independent sample t-tests were used to compare patients with clinically significant symptoms to those without.
Linear regression was used to test the effect of resilience and general health on cognitive performance measured as two cognitive domains (Model 1): a) Learning and Memory and b) Attention, Processing speed and Executive function. In the second step (Model 2), models were controlled by factors known to affect cognitive performance: cognitive reserve, pain interference, insomnia symptoms, depressive symptoms, and anxiety were added to the models. Variance Inflator Factor and tolerance and the variance proportions from collinearity diagnostics were checked for possible multicollinearity of the independent variables. Regression residual scatter plots were checked to make sure that the model fitted the data. The statistically significance level used for all analyses was 0.05. Statistical analyses were performed using SPSS 25.0 version for Windows (SPSS INC, Chicago, IL).
6. Results
6.1. Descriptive statistics
Descriptive statistics for the whole sample are presented in Table 1 and the associations between the variables in Table 2.
Table 1.
Descriptive statistics of the study cohort (N = 384).
| Mean | SD | n | |
| Age | 61.75 | 8.05 | 384 |
| BMI | 25.76 | 3.98 | 384 |
| Psychological distress | 7.45 | 5.87 | 382 |
| Cognitive Reserve | 36.28 | 7.78 | 360 |
| Resilience | 78.45 | 12.72 | 382 |
| General Health | 73.92 | 19.74 | 328 |
| Time since chemotherapy |
72.56 |
14.36 |
252 |
| Median |
IQR |
n |
|
| Pain Intensity | 3 | 1–5 | 384 |
| Pain Interference | 1 | 0–4 | 384 |
| Insomnia symptoms |
6 |
3–11 |
379 |
| n |
% |
||
| Pain sites | |||
| 0 | 66 | 17 | |
| 1-2 | 178 | 46 | |
| 3 or more | 140 | 36 | |
| Education | |||
| Low | 53 | 14 | |
| Medium | 87 | 23 | |
| High | 244 | 64 | |
| Adjuvant treatments | |||
| Chemotherapy | 253 | 66 | |
| Radiotherapy | 260 | 68 | |
| Endocrine therapy | 285 | 75 | |
Age in years; BMI = Body Mass Index; Psychological distress (HADS, [0–42]); Cognitive Reserve (CRS, [0–80]); Resilience (RS-14, [14–98]); General Health (General Health subdimension in SF-36, [0–100]); Time since chemotherapy (months); Insomnia Symptoms (ISI, [0–28]); Pain Intensity NRS (0–10); Pain interference NRS (0–10); Interquartile range (IQR).
Table 2.
Correlation table of the study variables N = 384.
| Attention, Processing speed and Executive function | Learning and Memory | Resilience | General Health | Cognitive Reserve | Psychological distress | Insomnia Symptoms | Pain Interference | |
|---|---|---|---|---|---|---|---|---|
| Attention, Processing speed and Executive function | 1 | .37** | .16* | .13* | 0.11 | −.13* | −0.08 | 0.03 |
| Learning and Memory | 1 | 0.03 | 0.08 | 0.03 | −0.01 | −0.03 | 0.09 | |
| Resilience | 1 | .41** | .28** | −.67** | −.30** | −0.07 | ||
| General Health | 1 | 0.11 | −.59** | −.41** | −.38** | |||
| Cognitive Reserve | 1 | −0.11 | −0.02 | 0.05 | ||||
| Psychological distress | 1 | .46** | .28** | |||||
| Insomnia Symptoms | 1 | .33** | ||||||
| Pain Interference | 1 |
Resilience (RS-14); General Health (General Health subdimension of SF-36); Cognitive Reserve (CRS); Psychological distress (HADS); Insomnia Symptoms (ISI); Pain Interference NRS (0–10): mild = 1–3; moderate to severe = 4–10. * correlation significant at the 0.05 level (2-tailed); **correlation significant at the 0.001 level (2-tailed).
Patients included in this study (n = 384) were compared with patients from the original cohort who had declined participation (n = 119). There was no difference in descriptive statistics for the main variables, but patients who declined had a lower proportion with a high educational level (35% vs 60%, p < 0.05).
Patient-reported general health was higher than for Finnish women on average (73.9 [19.7] vs 65.4 [19.4], p ≤ 0.001), even though the scores were controlled for age.
The average z-score in Learning and Memory, 0.14 (0.83), exceeded that of the female population average (t = 3.33, p < 0.001, n = 383). For Attention, Processing speed and Executive function, the average z-score was 0.82 (0.72), also higher than the population average (t = 22.16, p = 0.001, n = 383).
We defined cognitive decline as performance 1.5 SD or more below the population average. The performance of 16 patients (4.2%) suggested cognitive decline in the Learning and Memory domain; for Attention, Processing speed and Executive function, all were in the normal range.
Patients who had received chemotherapy (n = 253) did not differ from those who had not (n = 128) for Learning and Memory (0.14 (0.84) vs 0.14 (0.80), t = −0.03, p = 0.97) and Attention, Processing speed and Executive function (0.80 (0.73) vs (0.84 (0.72), t = −0.46, p = 0.65). Time since chemotherapy (n = 252) was not associated with Learning and Memory (F = 0.33, p = 0.57) or Attention, Processing speed and Executive function (F = 0.03, p = 0.86). Patients who had received endocrine therapy (n = 285) did not differ from those who had not (n = 96) for Learning and Memory (0.16 (0.83)) vs 0.08 (0.84), t = −0.83, p = 0.41) and Attention, Processing speed and Executive function (0.85 (0.71) vs. 0.71 (0.76), t = - 1.69, p = 0.09).
6.2. Resilience, general health and cognitive performance
In the linear regression models (Table 3), the dependent variables Learning and Memory and Attention, Processing speed and Executive function were predicted by the independent variables resilience and general health (Model 1). The models were then controlled for cognitive reserve, psychological distress, insomnia symptoms, and pain interference (Model 2). Resilience was associated with Attention, Processing speed and Executive function, but this did not remain significant after controlling for confounding factors (Table 3a). Similarly, general health was associated with Attention, Processing speed and Executive function, but this did not remain significant after controlling for confounding factors (Table 3b). When resilience and general health were added to the same model (Table 3c), resilience was a significant predictor, but general health was not. Again, the association did not remain significant when controlled for confounding factors.
Table 3.
Linear regression models: (a) resilience and cognitive performance, n = 355. (b) General Health and cognitive performance, n = 308. (c) resilience, General Health, and cognitive performance, n = 308.
| Learning and Memory | Attention, Processing speed and Executive function | ||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | ||||||
| β | p | Β | p | β | P | β | p | ||
| a | |||||||||
| Resilience | 0.03 | 0.57 | 0.01 | 0.91 | 0.14 | 0.01 | 0.06 | 0.40 | |
| Cognitive reserve | 0.06 | 0.33 | 0.09 | 0.11 | |||||
| Psychological distress | 0.02 | 0.76 | −0.06 | 0.42 | |||||
| Insomnia Symptoms | −0.09 | 0.15 | −0.07 | 0.29 | |||||
| Pain Interference: mild | 0.01 | 0.83 | 0.08 | 0.20 | |||||
| Pain Interference: moderate to severe | 0.09 | 0.18 | 0.10 | 0.13 | |||||
| Model | 0.57 | 0.55 | 0.01 | 0.04 | |||||
| Adj R2 |
0.00 |
0.00 |
0.02 |
0.02 |
|||||
| b | |||||||||
| General Health | 0.08 | 0.15 | 0.14 | 0.06 | 0.13 | 0.03 | 0.10 | 0.19 | |
| Cognitive reserve | 0.01 | 0.88 | 0.09 | 0.13 | |||||
| Psychological distress | 0.07 | 0.35 | −0.07 | 0.34 | |||||
| Insomnia Symptoms | −0.05 | 0.43 | −0.05 | 0.49 | |||||
| Pain Interference; mild | 0.02 | 0.80 | 0.06 | 0.39 | |||||
| Pain Interference: moderate to severe | 0.13 | 0.08 | 0.13 | 0.08 | |||||
| Model | 0.15 | 0.31 | 0.03 | 0.06 | |||||
| Adj R2 | 0.00 | 0.00 | 0.01 | 0.02 | |||||
| c | |||||||||
|
Resilience |
−0.01 |
0.94 |
0.02 |
0.76 |
0.13 |
0.04 |
0.09 |
0.24 |
|
| General Health | 0.08 | 0.18 | 0.14 | 0.07 | 0.07 | 0.23 | 0.09 | 0.21 | |
| Cognitive reserve | 0.00 | 0.96 | 0.07 | 0.27 | |||||
| Psychological distress | 0.09 | 0.35 | −0.01 | 0.91 | |||||
| Insomnia Symptoms | −0.05 | 0.43 | −0.05 | 0.50 | |||||
| Pain Interference: mild | 0.02 | 0.81 | 0.06 | 0.43 | |||||
| Pain Interference: moderate to severe | 0.13 | 0.09 | 0.12 | 0.11 | |||||
| Model | 0.35 | 0.41 | 0.01 | 0.07 | |||||
| Adj R2 | 0.00 | 0.00 | 0.02 | 0.02 | |||||
Resilience (RS-14); Cognitive Reserve (CRS); Psychological distress (HADS); Insomnia Symptoms (ISI); Pain Interference NRS (0–10): mild 1–3; moderate to severe 4–10; General Health (General Health subdimension in SF-36); Standardized Beta Coefficient (β) Adjusted R-squared (Adj R2).
Patients with clinically significant depressive symptoms, insomnia symptoms, or pain, were compared with those without: cognitive function did not differ (Table 4). Finally, groups where all these vulnerability factors were present at clinically significant levels were compared with patients not belonging to any of these groups: cognitive performance did not differ between these groups.
Table 4.
Cognitive performance in subgroups of clinically significant depressive symptoms (a), anxiety (b), insomnia symptoms (c), pain (d), and all these factors present simultaneously (e).
| clinically significant depressive symptoms | depressive symptoms below clinical significance | |||||||
| Mean | SD | n | Mean | SD | n | t | p | |
| a. Depressive symptoms (HADS-D ≥ 8) | ||||||||
| Learning and Memory | 0.05 | 1.01 | 34 | 0.15 | 0.81 | 384 | 0.56 | 0.58 |
| Attention, Processing speed and Executive function |
0.72 |
0.90 |
34 |
0.83 |
0.71 |
384 |
0.70 |
0.49 |
| clinically significant anxiety | anxiety below clinical significance | |||||||
| Mean |
SD |
n |
Mean |
SD |
n |
t |
p |
|
| b. Anxiety (HADS-A ≥ 8) | ||||||||
| Learning and Memory | 0.14 | 0.98 | 59 | 0.15 | 0.80 | 323 | 0.01 | 0.99 |
| Attention, Processing speed and Executive function |
0.76 |
0.88 |
59 |
0.83 |
0.69 |
323 |
0.60 |
0.55 |
| clinically significant insomnia | insomnia below clinical significance | |||||||
| Mean |
SD |
n |
Mean |
SD |
n |
t |
p |
|
| c. Insomnia symptoms (ISI ≥ 8) | ||||||||
| Learning and Memory | 0.14 | 0.84 | 160 | 0.14 | 0.82 | 219 | −0.03 | 0.98 |
| Attention, Processing speed and Executive function |
0.78 |
0.69 |
160 |
0.85 |
0.74 |
219 |
0.89 |
0.37 |
| clinically significant pain | pain below clinical significance | |||||||
| Mean |
SD |
n |
Mean |
SD |
n |
t |
p |
|
| d. Pain (NRS ≥ 4) | ||||||||
| Learning and Memory | 0.17 | 0.77 | 151 | 0.12 | 0.87 | 233 | −0.49 | 0.63 |
| Attention, Processing speed and Executive function |
0.83 |
0.74 |
151 |
0.81 |
0.72 |
233 |
−0.19 |
0.85 |
| burden | no burden | |||||||
| Mean |
SD |
n |
Mean |
SD |
n |
t |
p |
|
| e. Clinically significant symptoms of depression, anxiety, insomnia and pain | ||||||||
| Learning and Memory | 0.09 | 0.84 | 13 | 0.88 | 0.74 | 144 | −0.24 | 0.81 |
| Attention, Processing speed and Executive function | 1.01 | 0.86 | 13 | 0.15 | 0.83 | 144 | 0.60 | 0.55 |
Clinically significant depressive symptoms: HADS-D ≥ 8; clinically significant anxiety symptoms: HADS-A ≥ 8; clinically significant insomnia: ISI ≥8; clinically significant pain NRS (0–10) ≥ 4; burden: clinically significant depressive symptoms, anxiety, insomnia symptoms and pain.
7. Discussion
We found that cognitive performance 4–9 years after BC surgery was on average within normal range according to epidemiological studies on population performance. Higher resilience and perceived general health were associated with better performance on the cognitive domain Attention, Processing speed and Executive function. Even in the presence of clinically significant vulnerability factors (pain, insomnia, depressive or anxiety symptoms), the women in the present study were able to perform well in cognitive testing. BC treatments were not associated with cognitive performance in this cohort.
The overall level of cognitive performance in this study was good and the incidence of patients with cognitive decline was small. These are more positive results than has previously been reported. Follow-up times have usually been much shorter than in our study. In a recent review, 21% of the patients treated for BC showed cognitive decline up to 1 year after treatments [3]. Typically, follow-up times have been around 1 year, although neurocognitive impairments have been reported even up to 10 [43] or 17 years [44] after treatments. In our study, we found an association of Attention, Processing speed and Executive function with resilience and general health, which might be more sensitive to very subtle changes than is memory processing.
We studied objective neurocognitive performance in BC patients in general and also the role of chemotherapy. Previous studies on BC and cognitive impairment are heterogeneous in whether perceived or objective cognitive performance, the role of BC in general or the effect of chemotherapy, has been assessed. The correlation between perceived and objective cognitive performances is rather weak and it has been suggested that they measure different phenomena [45]. Subjective cognitive complaints are reported by BC survivors [46] more frequently than the objectively observed prevalence of neurocognitive decline [47].
Few studies have assessed cognitive reserve in BC patients, with the evidence so far suggesting a role of CR as a compensatory mechanism against cognitive impairment after BC treatments [[21], [22], [23]]. Lower CR has been suggested as a risk factor for future Alzheimer's disease among chemotherapy-treated BC patients with a particular profile of brain structure [22].
In our study, we did not find cognitive reserve to have a protective effect on cognitive performance. This could be explained by the fact that in the highly educated and cognitively well-performing patients in our cohort, a “ceiling effect” of cognitive reserve and cognitive performance had been reached. The scores on the CRS in this study were lower than in a previous study in neurologically healthy Finnish-speaking participants (38% women). However, the difference is clinically very minor. The CRS is a new measuring tool, not previously used among BC-treated women, especially in countries with high education levels such as Finland.
The level of resilience in this study cohort was slightly lower than in previous studies among BC-treated women [48,49]. In researching resilience in BC patients, the focus has been on a strong association with anxiety, depression, health-related quality of life, coping styles, and perceived social support [16]. Earlier clinical stages of BC and its treatment type (chemotherapy, conservative surgery) is associated with higher resilience while number of courses of adjuvant therapy, symptom burden or severity, and greater physical symptom distress are associated with lower resilience [16]. These results may be understood in the multifactorial context of resilience. More robust trait-like resilience may affect health behavior, with highly resilient people seeking medical care more rapidly when symptoms first emerge. On the other hand, more reactive state-like resilience may decrease as a reaction to worsening clinical progression. Research into associations between time since diagnosis or treatment and level of resilience have had mixed and inconsistent results [16] and no such association was found in our study.
In general, resilience has been shown to be positively associated with perceived general health in BC patients [19], in line with our results. In a recent study of BC-treated women [19], patient-reported general health was slightly lower than in our study, but the associations between perceived general health and resilience were very similar.
7.1. Clinical implications
According to our results, women who survive BC and its treatments mostly manage cognitively well after treatments, though it is possible that there is a fragile subgroup of women who are more affected. In clinical practice, if cognitive impairment is suspected, referral to a neuropsychologist for further evaluation is recommended. It would be important to identify patients with low resilience who are therefore at risk of deteriorating general health when cancer is diagnosed and who therefore need more intense psychological support in addition to standard BC treatment.
In a recent review, most of the resilience-enhancing intervention programmes for BC patients were found to be effective in improving participants’ quality of life [16]. These included adaptive coping strategies, social support, education, and stress management skills [16]. Resilience-enhancing interventions could also be beneficial for cognitive performance as the current study shows this to be partly associated with resilience.
7.2. Strengths and limitations
A strength of this study is the use of a thorough neuropsychological test battery, giving more robust information about the patient's cognitive functioning than a single test. Neuropsychological tests are more sensitive to subtle changes in cognitive functioning than shorter cognitive screening tools (e.g. Mini-Mental State Examination) [50]. Our study assessed a large and unselected cohort of BC-treated women, with a longer follow-up time than in similar studies. It is possible that cognitive problems after cancer treatments are short term and ameliorate with time, as seen with the longer follow-up.
A limitation is the cross-sectional study design. A longitudinal design would be preferable in future BC studies since cognitive decline has also been reported prior to treatments, possibly cancer-related or caused by other mechanisms [3], and this design would provide more detailed information about such cognitive changes [7]. The current study represents women with a high level of education which can also be considered as a limitation even though our cohort represents the general population of Finnish women being treated for breast cancer.
8. Conclusion
This study has shown that cognitive function is on average at a good level some years after BC treatments. In addition, we have shown that higher psychological resilience and perceived general health are associated with better cognitive performance. This is important information both for patients and for clinicians involved in the treatment of BC. Future research should focus on longitudinal studies of patients at a higher risk of cognitive decline after BC treatments. Further, more sensitive neurocognitive tests need to be developed to capture more subtle changes in cognitive performance.
Research funding
This study was supported by a grant from the European Union Seventh Framework Program (FP7) under grant agreement no. 602891.
Ethical approval
The study was approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District (reference number 149/13/03/00/14).
Declaration of competing interest
The authors have declared that no competing interests exist.
Acknowledges
Proofreading Les Hearn.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. S0140-6736(17)32154-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodai B.I., Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19(2):48–79. doi: 10.7812/TPP/14-241. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dijkshoorn A.B.C., van Stralen H.E., Sloots M., Schagen S.B., Visser-Meily J.M.A., Schepers V.P.M. Prevalence of cognitive impairment and change in patients with breast cancer: a systematic review of longitudinal studies. Psycho Oncol. 2021 doi: 10.1002/pon.5623. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein L.J., McCreath G.A., Komeylian Z., Rich J.B. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis. Neurosci Biobehav Rev. 2017;83:417–428. doi: 10.1016/j.neubiorev.2017.10.028. S0149-7634(17)30135-5 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Wagner L.I., Gray R.J., Sparano J.A., et al. Patient-reported cognitive impairment among women with early breast cancer randomly assigned to endocrine therapy alone versus chemoendocrine therapy: results from TAILORx. J Clin Oncol. 2020;38(17):1875–1886. doi: 10.1200/JCO.19.01866. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koppelmans V., Breteler M.M., Boogerd W., Seynaeve C., Gundy C., Schagen S.B. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30(10):1080–1086. doi: 10.1200/JCO.2011.37.0189. [doi] [DOI] [PubMed] [Google Scholar]
- 7.Vardy J., Wefel J.S., Ahles T., Tannock I.F., Schagen S.B. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19(4):623–629. doi: 10.1093/annonc/mdm500. S0923-7534(19)41436-1 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Ahles T.A., Root J.C., Ryan E.L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martino G., Catalano A., Agostino R.M., et al. Quality of life and psychological functioning in postmenopausal women undergoing aromatase inhibitor treatment for early breast cancer. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu Y.H., Chen V.C., Hsieh C.C., et al. Subjective and objective cognitive functioning among patients with breast cancer: effects of chemotherapy and mood symptoms. Breast Cancer. 2021;28(1):236–245. doi: 10.1007/s12282-020-01168-y. [doi] [DOI] [PubMed] [Google Scholar]
- 11.Xu S., Thompson W., Ancoli-Israel S., Liu L., Palmer B., Natarajan L. Cognition, quality-of-life, and symptom clusters in breast cancer: using Bayesian networks to elucidate complex relationships. Psycho Oncol. 2018;27(3):802–809. doi: 10.1002/pon.4571. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Dyk K., Bower J.E., Crespi C.M., Petersen L., Ganz P.A. Cognitive function following breast cancer treatment and associations with concurrent symptoms. NPJ Breast Cancer. 2018;4 doi: 10.1038/s41523-018-0076-4. 25-2018-0076-4. eCollection 2018. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonanno G.A., Westphal M., Mancini A.D. Resilience to loss and potential trauma. Annu Rev Clin Psychol. 2011;7:511–535. doi: 10.1146/annurev-clinpsy-032210-104526. [doi] [DOI] [PubMed] [Google Scholar]
- 14.Eicher M., Matzka M., Dubey C., White K. Resilience in adult cancer care: an integrative literature review. Oncol Nurs Forum. 2015;42(1):E3–E16. doi: 10.1188/15.ONF.E3-E16. [doi] [DOI] [PubMed] [Google Scholar]
- 15.Liesto S., Sipilä R., Aho T., Harno H., Hietanen M., Kalso E. Psychological resilience associates with pain experience in women treated for breast cancer. Scand J Pain. 2020;20(3):545–553. doi: 10.1515/sjpain-2019-0137. [doi] [DOI] [PubMed] [Google Scholar]
- 16.Aizpurua-Perez I., Perez-Tejada J. Resilience in women with breast cancer: a systematic review. Eur J Oncol Nurs. 2020;49 doi: 10.1016/j.ejon.2020.101854. S1462-3889(20)30134-4 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Fradelos E.C., Papathanasiou I.V., Veneti A., et al. Psychological distress and resilience in women diagnosed with breast cancer in Greece. Asian Pac J Cancer Prev. 2017;18(9):2545–2550. doi: 10.22034/APJCP.2017.18.9.2545. APJCP-18-2545 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerezo M.V., Álvarez-Olmo A., Rueda P. General health and resilience of breast cancer patients: the Mediator role of affective well-being. Int J Environ Res Public Health. 2022 Apr 28;19(9):5398. doi: 10.3390/ijerph19095398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Å Mohlin, Bendahl P.O., Hegardt C., Richter C., Hallberg I.R., Rydén L. Psychological resilience and health-related quality of life in 418 Swedish women with primary breast cancer: results from a prospective longitudinal study. Cancers. 2021;13(9):2233. doi: 10.3390/cancers13092233. 10.3390/cancers13092233 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 21.Perrier J., Viard A., Levy C., et al. Longitudinal investigation of cognitive deficits in breast cancer patients and their gray matter correlates: impact of education level. Brain Imaging Behav. 2020;14(1):226–241. doi: 10.1007/s11682-018-9991-0. [doi] [DOI] [PubMed] [Google Scholar]
- 22.Kesler S.R., Rao V., Ray W.J., Rao A., Alzheimer's Disease Neuroimaging Initiative Probability of Alzheimer's disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimers Dement (Amst) 2017;9:67–75. doi: 10.1016/j.dadm.2017.10.002. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahles T.A., Saykin A.J., McDonald B.C., et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gómez-Beldarrain M., Anton-Ladislao A., Aguirre-Larracoechea U., Oroz I., García-Moncó J.C. Low cognitive reserve is associated with chronic migraine with medication overuse and poor quality of life. Cephalalgia. 2015;35(8):683–691. doi: 10.1177/0333102414553822. [doi] [DOI] [PubMed] [Google Scholar]
- 25.Evans I.E.M., Llewellyn D.J., Matthews F.E., Woods R.T., Brayne C., Clare L. Social isolation, cognitive reserve, and cognition in older people with depression and anxiety. Aging Ment Health. 2019;23(12):1691–1700. doi: 10.1080/13607863.2018.1506742. [doi] [DOI] [PubMed] [Google Scholar]
- 26.Hashemi S., Rafiemanesh H., Aghamohammadi T., et al. Prevalence of anxiety among breast cancer patients: a systematic review and meta-analysis. Breast Cancer. 2020;27(1261):166–178. doi: 10.1007/s12282-019-01031-9. [DOI] [PubMed] [Google Scholar]
- 27.Pilevarzadeh M., Amirshahi M., Afsargharehbagh R., Rafiemanesh H., Hashemi S., Balouchi A. Global prevalence of depression among breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(2605):519–533. doi: 10.1007/s10549-019-05271-3. [DOI] [PubMed] [Google Scholar]
- 28.Meretoja T. Pain at 12 months after surgery for breast cancer. JAMA. 2014;311(1):90–92. doi: 10.1001/jama.2013.278795. [DOI] [PubMed] [Google Scholar]
- 29.Goldhirsch A., Wood W.C., Gelber R.D., et al. Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18(7):1133–1144. doi: 10.1093/annonc/mdm271. S0923-7534(19)41220-9 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Mustonen L., Aho T., Harno H., Sipila R., Meretoja T., Kalso E. What makes surgical nerve injury painful? A 4-year to 9-year follow-up of patients with intercostobrachial nerve resection in women treated for breast cancer. Pain. 2018 doi: 10.1097/j.pain.0000000000001398. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wechsler D. Psykologien kustannus Oy; Helsinki: 2012. WAIS-IV Käsikirja (The WAIS-IV. A Finnish manual) [Google Scholar]
- 32.Wechsler D. Psykologien Kustannus Oy; Helsinki: 2012. WMS-III Käsikirja (The WMS-III. A Finnish manual) [Google Scholar]
- 33.Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. fifth ed. Oxford University Press.; 2012. Neuropsychological assessment. [Google Scholar]
- 34.Kivisaari S., Kuha A., Poutiainen E. Suomen neuropsykologinen yhdistys ry; 2009. Sanasujuvuustehtävien suomalainen viitearvoaineisto. [verkkojulkaisu] [Google Scholar]
- 35.Poutiainen E., Kalska H., Laasonen M., et al., editors. Trail—making—testi. Käsikirja (The trail-making test. A Finnish manual) Psykologien Kustannus Oy; Helsinki: 2010. [Google Scholar]
- 36.Gerbershagen H.J., Rothaug J., Kalkman C.J., Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth. 2011;107(4):619–626. doi: 10.1093/bja/aer195. [doi] [DOI] [PubMed] [Google Scholar]
- 37.Sipilä R., Kalso E., Lötsch J. Machine-learned identification of psychological subgroups with relation to pain interference in patients after breast cancer treatments. Breast. 2020;50:71–80. doi: 10.1016/j.breast.2020.01.042. S0960-9776(20)30057-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morin C.M., Belleville G., Belanger L., Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. doi: S0022399901002963 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Relander K., Mäki K., Soinne L., García-García J., Hietanen M. Active lifestyle as a reflection of cognitive reserve: the modified cognitive reserve scale. Nordic Psychol. 2021;73(3):242–252. doi: 10.1080/19012276.2021.1902846. [DOI] [Google Scholar]
- 41.Wagnild G.M., Young H.M. Development and psychometric evaluation of the resilience scale. J Nurs Meas. 1993;1(2):165–178. [PubMed] [Google Scholar]
- 42.Hays R.D., Sherbourne C.D., Mazel R.M. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 43.de Ruiter M.B., Reneman L., Boogerd W., et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32(8):1206–1219. doi: 10.1002/hbm.21102. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada T.H., Denburg N.L., Beglinger L.J., Schultz S.K. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22(1):48–54. doi: 10.1176/appi.neuropsych.22.1.48. 22/1/48 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morse R., Rodgers J., Verrill M., Kendell K. Neuropsychological functioning following systemic treatment in women treated for breast cancer: a review. Eur J Cancer. 2003;39(16):2288–2297. doi: 10.1016/s0959-8049(03)00600-2. doi: S0959804903006002 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Janelsins M.C., Heckler C.E., Peppone L.J., et al. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol. 2018;36(32) doi: 10.1200/JCO.2018.78.6624. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchinson A.D., Hosking J.R., Kichenadasse G., Mattiske J.K., Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Bazzi A.R., Clark M.A., Winter M.R., Ozonoff A., Boehmer U. Resilience among breast cancer survivors of different sexual orientations. LGBT Health. 2018;5(5):295–302. doi: 10.1089/lgbt.2018.0019. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamen C., Jabson J.M., Mustian K.M., Boehmer U. Minority stress, psychosocial resources, and psychological distress among sexual minority breast cancer survivors. Health Psychol. 2017;36(6):529–537. doi: 10.1037/hea0000465. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung Y.T., Tan E.H., Chan A. An evaluation on the neuropsychological tests used in the assessment of postchemotherapy cognitive changes in breast cancer survivors. Support Care Cancer. 2012;20(7):1361–1375. doi: 10.1007/s00520-012-1445-4. [doi] [DOI] [PubMed] [Google Scholar]