Key Points
Question
Are children and adolescents, aged 4 to 14 years, able to adequately self-collect nasal swabs for SARS-CoV-2 testing after hearing and seeing brief and age-appropriate instructions?
Findings
In a cross-sectional study of 197 symptomatic children and adolescents, self-collected nasal swabs that were positive for SARS-CoV-2 agreed with results from health care worker–collected swabs in 97.8% of participants, while self-collected swabs that were negative agreed with health care worker–collected swabs in 98.1% of participants.
Meaning
SARS-CoV-2 detection in nasal swabs that were self-collected by school-aged children and adolescents, following simple instructions, demonstrated high agreement with results following collection by health care workers.
Abstract
Importance
Despite the expansion of SARS-CoV-2 testing, available tests have not received Emergency Use Authorization for performance with self-collected anterior nares (nasal) swabs from children younger than 14 years because the effect of pediatric self-swabbing on SARS-CoV-2 test sensitivity is unknown.
Objective
To characterize the ability of school-aged children to self-collect nasal swabs for SARS-CoV-2 testing compared with collection by health care workers.
Design, Setting, and Participants
Cross-sectional study of 197 symptomatic children and adolescents aged 4 to 14 years old. Individuals were recruited based on results of testing in the Children’s Healthcare of Atlanta system from July to August 2021.
Exposures
Children and adolescents were given instructional material consisting of a short instructional video and a handout with written and visual steps for self-swab collection. Participants first provided a self-collected nasal swab. Health care workers then collected a second specimen.
Main Outcomes and Measures
The primary outcome was SARS-CoV-2 detection and relative quantitation by cycle threshold (Ct) in self- vs health care worker–collected nasal swabs when tested with a real-time reverse transcriptase–polymerase chain reaction test with Emergency Use Authorization.
Results
Among the study participants, 108 of 194 (55.7%) were male and the median age was 9 years (IQR, 6-11). Of the 196 participants, 87 (44.4%) tested positive for SARS-CoV-2 and 105 (53.6%) tested negative by both self- and health care worker–collected swabs. Two children tested positive by self- or health care worker–collected swab alone; 1 child had an invalid health care worker swab. Compared with health care worker–collected swabs, self-collected swabs had 97.8% (95% CI, 94.7%-100.0%) and 98.1% (95% CI, 95.6%-100.0%) positive and negative percent agreement, respectively, and SARS-CoV-2 Ct values did not differ significantly between groups (mean [SD] Ct, self-swab: 26.7 [5.4] vs health care worker swab: 26.3 [6.0]; P = .65).
Conclusions and Relevance
After hearing and seeing simple instructional materials, children and adolescents aged 4 to 14 years self-collected nasal swabs that closely agreed on SARS-CoV-2 detection with swabs collected by health care workers.
This cross-sectional study examines the ability of school-aged children, aged 4 to 14 years, to self-collect nasal swabs for SARS-CoV-2 testing after hearing and seeing simple instructions compared with collection by health care workers.
Introduction
Testing has become widely available for children and adults who may have a SARS-CoV-2 infection. However, testing capacity remains insufficient for repeat testing of children and adolescents, particularly in group settings, such as camps and schools, that are less likely to have trained health care workers (HCWs) available for sample collection. Long-standing medical practice has involved sample collection by HCWs for respiratory virus testing.1,2 Yet little data exist to suggest that HCW collection is necessary, and it remains a barrier to expanded testing. Many SARS-CoV-2 tests have been authorized by the US Food and Drug Administration (FDA) under Emergency Use Authorizations (EUAs) for self-swabbing by adults and children aged 14 years and older and parental swab collection in children aged 2 years and older, with anterior nares (nasal) swabs being preferred because they are technically less complex.3,4 However, the age at which nasal self-swabbing would be successful and how it may affect SARS-CoV-2 test performance is unknown. As a result of this knowledge gap, no tests are authorized by the FDA under EUA for self-swabbing by children younger than 14 years.
The objective of the current study was to determine the agreement between self-collected nasal swabs, obtained after hearing and seeing simple instructions, and HCW-collected nasal swabs for SARS-CoV-2 testing of symptomatic children and adolescents.
Methods
This study was approved by the Emory University Institutional Review Board. Parents or guardians of all participants provided written informed consent. Children 6 years of age and older provided assent prior to participating.
A cohort of symptomatic SARS-CoV-2–positive and –negative children and adolescents was recruited based on a daily report generated of all individuals testing positive or negative by a standard of care nasopharyngeal swab within the Children’s Healthcare of Atlanta system in the previous 24 hours. Participants were enrolled either directly in the emergency department or urgent care, if they were still available, or parents were called and asked to return to a drive-through testing site set up at the Center for Advanced Pediatrics at Children’s Healthcare of Atlanta. Inclusion criteria were the following: entering kindergarten through eighth grade, with accepted ages from 0 to 17 years old, and willing to consent to the study. Individuals were excluded if self-swabbing was not feasible due to a medical condition, developmental delay precluded the child or adolescent from understanding the instructions in the opinion of the parent (or guardian), or the individual had a history of nosebleeds in the past 2 weeks.
On enrollment, parents completed a short questionnaire for demographic information, including selection of race and ethnicity, which was evaluated to place the participant population within the racial and ethnic makeup of the Atlanta area. Parents were asked to check 1 of the following options for race: Asian, Black/African American, White, other, or refuse to answer. Options for ethnicity included Hispanic, Non-Hispanic, and refuse to answer. Participants who identified with a race option were classified as such; those who did not identify with a race but did identify with Hispanic ethnicity were classified as Hispanic.
Once consented, a video of children teaching and demonstrating how to self-swab was shown on a tablet or smartphone. The video was presented along with a statement to watch the video “so you can see how easy it is to swab your own nose, and then we will ask you to do the same.” Participants were also provided with a laminated instructional handout with images on one side and images plus written instructions on the other (eAppendix in the Supplement). The handout was provided with a prompt, “Here are pictures and instructions if you need help.” The participant then performed the self-swab, followed by the HCW, at the site of enrollment. Swabbing was performed either in the examination room or in the back seat or passenger seat of the car at the drive-through site. If the participant had questions or appeared to need assistance, the HCW could encourage them to remember the video or remind them they could look at the handout. No physical assistance with the self-swab was provided by the HCW. Parents and guardians were present but not allowed to assist the child participant with swabbing.
Swab collections involved 4 rotations of a Nylon Flocked Swab (regular size with 30-mm breakpoint; Copan Diagnostics) in each naris. Then the swab was given to the HCW, who placed it in a sterile cryovial prefilled with 1 mL of saline. The same process was repeated by the HCW. The HCWs who conducted all swabbing for this study were highly skilled and experienced pediatric nurses (eTable 1 in the Supplement). Following collection, samples were immediately placed on ice and transported to the laboratory at the end of each day. Samples were stored at 4 °C for up to 72 hours prior to nucleic acid extraction and testing. If the expected duration of storage exceeded 72 hours, samples were stored at −80 °C.
SARS-CoV-2 Molecular Testing
All samples were extracted and tested with the Centers for Disease Control and Prevention’s (CDC) 2019 Novel Coronavirus Real-Time Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR) Diagnostic Panel (hereinafter referred to as the CDC EUA RT-PCR) according to the instructions for use.5 Briefly, 100 μL of sample was extracted on a Roche MagNA Pure 96 using the DNA and Viral NA Small Volume Kit. Nucleic acids were eluted in 100 μL and immediately tested for nucleocapsid 1 (N1), nucleocapsid 2 (N2), and ribonuclease P targets (the latter as a positive control) in separate 20-μL reactions of the TaqPath 1-Step RT-qPCR Master Mix (Thermo Fisher Scientific) on an ABI Fast DX Real-Time PCR system (Thermo Fisher Scientific). Results were interpreted according to the instructions for use,5 and cycle threshold (Ct) values were recorded and compared between self- and HCW-collected swabs. All samples with inconclusive results were retested by RT-PCR, and samples with invalid results were re-extracted and retested. A subset of samples with detectable SARS-CoV-2 RNA was also tested in a multiplex RT-PCR for specific spike mutations associated with variants of concern. This assay was performed as described.6,7
Usability Evaluation
A set of usability questions was developed to determine whether all tasks associated with self-swabbing were completed correctly, whether participants required any assistance, how participants felt about self-swabbing, and whether participants understood the instructions (eAppendix in the Supplement). A portion of the questions were HCW facing; that is, the HCW conducting the study observed participant actions and responded to questions accordingly. The remaining questions were participant facing. The usability questionnaire was completed immediately after sample collection by the child and HCW. The child received assistance with the questionnaire from the HCW, as needed. The vocabulary used in the questions was selected to be accessible to the age groups included in the study. Questions were designed to be nonleading and open-ended where possible, and used prompts such as “Can you tell me about…,” because these methods have been shown to elicit more complete and accurate information from children.8,9 Questions were designed to be answered with a yes/no selection, selection from a list of options, or, for participant-facing questions, 1 to 2 words summing up responses to open-ended questions.
Statistical Analysis
Target enrollment was 10 COVID-19–positive and 10 COVID-19–negative children and adolescents at each year of age from approximately 5 to 14 years (representing grades kindergarten to eighth grade). This sampling plan was designed after discussions with the FDA to provide adequate representation while maintaining feasibility. For analysis of test outcomes, concordant pairs were defined as specimen pairs that generated the same qualitative result with the CDC EUA RT-PCR. Usability data were evaluated by age and when binned as 8 years or younger and older than 8 years, based on initial review of the results. Descriptive statistics for the study were reported as medians and IQRs for continuous variables and counts with percentages for categorical variables. Shapiro-Wilk tests were used to check normality of continuous data. Two-group comparisons were conducted using 2-sided t tests for normally distributed continuous data. Two-sided Wilcoxon rank-sum tests were used for nonnormal continuous data. Categorical data were compared using 2-sided χ2 tests or Fisher exact tests for expected cell counts less than 5. Statistical significance was assessed at the .05 level (2-sided). If data were missing for a variable, that participant was excluded from the group comparison test of that variable. Complete outcome data were available for each participant. As such, no measures had to be taken to address missingness. All statistical analyses were conducted using SAS version 9.4 (SAS Institute).
Results
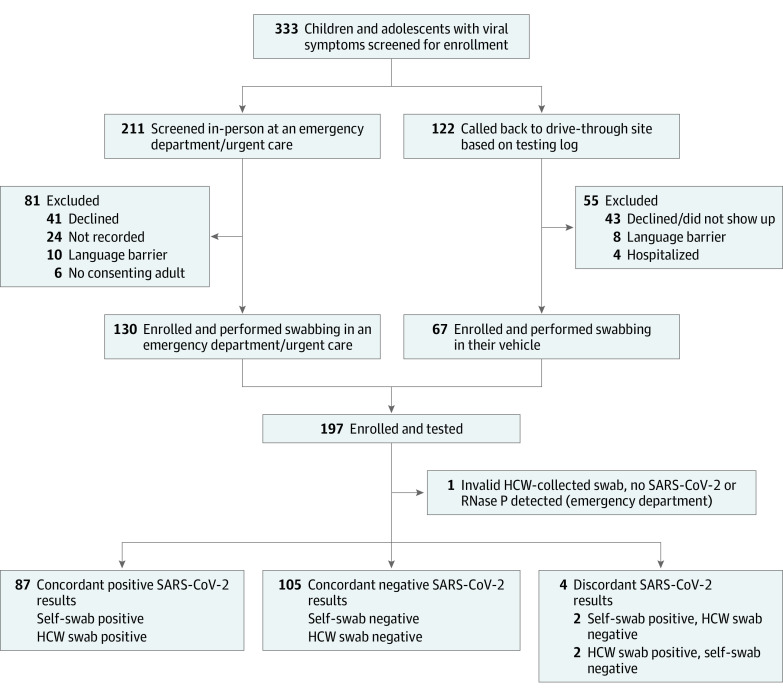
From July to August 2021, 197 symptomatic children and adolescents, aged 4 to 14 years, were enrolled. A total of 130 of 197 participants (66.0%) were enrolled in the emergency department or urgent care out of 211 individuals approached. The remaining 67 participants (34%) were enrolled by callback (Figure 1, Table 1).
Figure 1. Study Flowchart of Children and Adolescents Enrolled in a Study of Self-swabbing for SARS-CoV-2 Testing.
Data were not systematically recorded for all potential callback participants who were not enrolled, including those with incorrect contact information, no-answers, and immediate refusals without listening to the complete telephone script. HCW indicates health care worker; RNase, ribonuclease.
Table 1. Characteristics of 196 Symptomatic Children and Adolescents With Interpretable SARS-CoV-2–Positive vs SARS-CoV-2–Negative Samples.
| Category | SARS-CoV-2, No./total (%) | |
|---|---|---|
| Yes (n = 91) | No (n = 105) | |
| Age, median (IQR), y | 9 (7-11) | 8 (6-11) |
| Age group, y | ||
| 4-5 | 9 (9.9) | 17 (16.2) |
| 6 | 9 (9.9) | 15 (14.3) |
| 7 | 10 (11.0) | 14 (13.3) |
| 8 | 9 (9.9) | 10 (9.5) |
| 9 | 13 (14.3) | 8 (7.6) |
| 10 | 10 (11.0) | 6 (5.7) |
| 11 | 9 (9.9) | 14 (13.3) |
| 12 | 9 (9.9) | 9 (8.6) |
| 13-14 | 13 (14.3) | 12 (11.4) |
| Sex | ||
| Female | 43/89 (48.3) | 43 (41.0) |
| Male | 46/89 (51.7) | 62 (59.1) |
| Race and ethnicitya | ||
| Black/African American | 54/87 (62.1) | 66/100 (66.0) |
| Hispanic | 4/87 (4.6) | 5/100 (5.0) |
| White | 26/87 (29.9) | 27/100 (27.0) |
| Other | 3/87 (3.5) | 2/100 (2.0) |
| Days after symptom onset, median (IQR) | 3 (1-4) | 2 (1-3) |
| Required assistance with swabb | 11/88 (12.5) | 16/101 (15.8) |
| Self-swab collection correctly completedc | 62/88 (70.5) | 79/100 (79.0) |
| Enrollment locationd | ||
| Emergency department/urgent care | 43 (36.3) | 86 (81.9) |
| Drive-through site | 48 (52.7) | 19 (18.1) |
Participants were asked to check an option for race (Asian, Black/African American, White, other, or refuse to answer) and ethnicity (Hispanic, non-Hispanic, and refuse to answer). Participants who identified with a race option were classified as such; those who did not identify with a race but did identify with Hispanic ethnicity were classified as Hispanic. Participants who refused to answer for both the race and ethnicity questions were considered missing.
Observing health care worker was asked “Did the participant require assistance?”
Observing health care worker was asked “Was the swab collection completed correctly?”
See eTable 1 in the Supplement for characteristics of health care workers involved in the study.
A single HCW-collected swab was invalid (no detectable SARS-CoV-2 or ribonuclease P RNA as a positive control), and this participant was removed from further analysis (self-swab, negative). Of 196 children and adolescents, 87 (44.4%) tested positive and 105 (53.6%) tested negative for SARS-CoV-2 by both self- and HCW-collected swabs (positive and negative concordant samples, respectively). Two participants each tested positive by self- or HCW-collected swab, but negative by the alternate swab. Positive and negative percent agreements were 97.8% (95% CI, 94.7%-100.0%) and 98.1% (95% CI, 95.6%-100.0%), respectively (κ, 0.96 [95% CI, 0.92 to 0.99]; Table 2). Children and adolescents with concordant positive SARS-CoV-2 RT-PCR results presented 1 day later than those who tested negative (median days after symptom onset, 3 [IQR, 1-4] vs 2 [IQR, 1-3], respectively; P = .002). These 2 groups were otherwise similar (Table 1). N2 Ct values obtained with the CDC EUA RT-PCR did not differ between self- and HCW-collected swabs among the 87 participants with SARS-CoV-2 detected in both samples (mean [SD] Ct: self-swab, 26.7 [5.4] vs HCW swab, 26.3 [6.0]; P = .65; Figure 2A).
Table 2. Comparison of Qualitative SARS-CoV-2 Detection by RT-PCR From Self- and Health Care Worker–Collected Nasal Swabs in Symptomatic Children and Adolescentsa.
| Health care worker–collected swab | |||
|---|---|---|---|
| Positive | Negative | Total | |
| Self-collected swab | |||
| Positive | 87 | 2 | 89 |
| Negative | 2 | 105 | 107 |
| Total | 89 | 107 | 196 |
Abbreviation: RT-PCR, real-time reverse transcriptase–polymerase chain reaction.
Results by age group are shown in the eFigure in the Supplement.
Figure 2. Comparison of Nucleocapsid 2 (N2) Cycle Threshold (Ct) Values From Self- and Health Care Worker (HCW)–Collected Swabs.
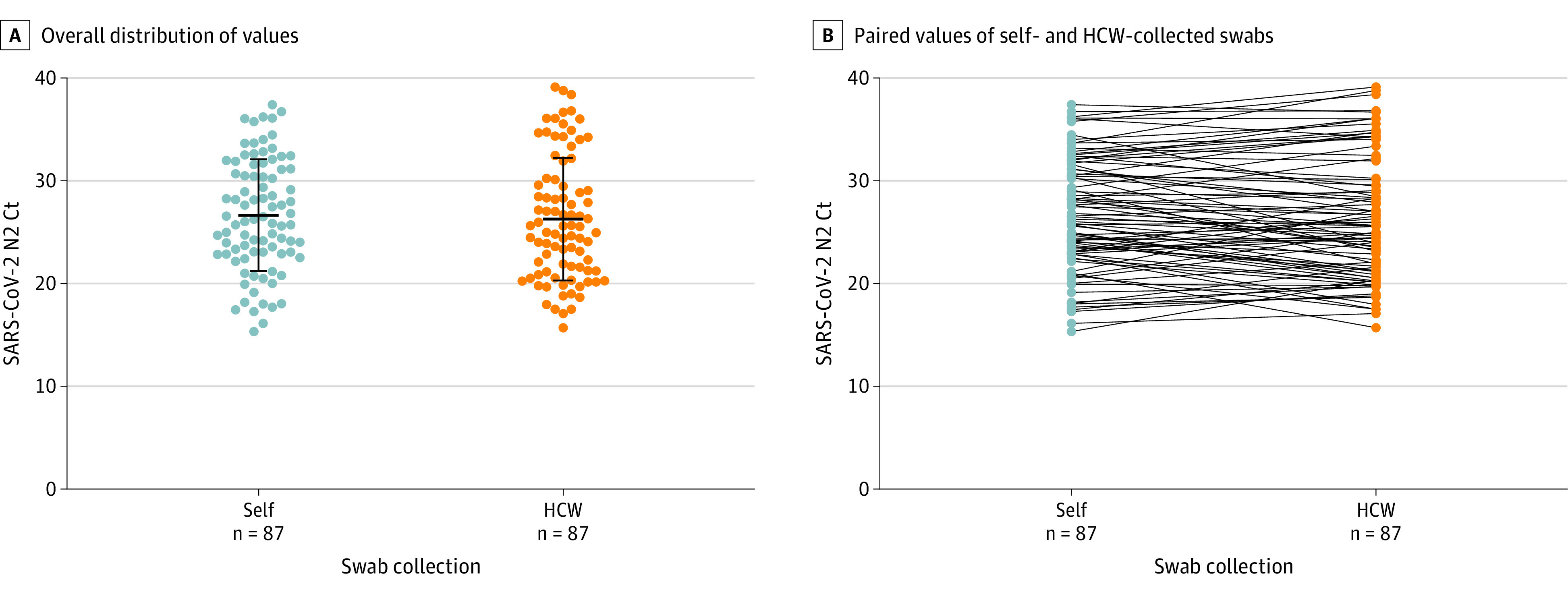
A, Overall distribution of SARS-CoV-2 N2 target Ct values (bars display means and SDs). Ct values compared by 2-sided t test yielding a P value of .65. B, Paired SARS-CoV-2 N2 Ct values for self- and HCW-collected swabs. All displayed results were obtained with the Centers for Disease Control and Prevention’s Emergency Use Authorization real-time reverse transcriptase–polymerase chain reaction (RT-PCR) test and interpreted according to the instructions for use. Ct values are defined as the RT-PCR cycle at which an amplification curve crosses the defined signal threshold. This defines a positive result, with lower Ct values indicating higher concentrations of viral RNA in the sample.
However, while the mean values were similar, there were important differences in N2 Ct values for a substantial proportion of the participants, in divergent directions. For 28 of 87 participants (32.2%), N2 Ct values differed by more than 3.3 cycles between self- and HCW-collected swabs, consistent with a 1.0 log10 difference in viral RNA concentration. Of those 87 participants, 12 (13.8%) had lower Ct values (greater RNA) in self-collected swabs and 16 (18.4%) had lower Ct values in HCW-collected swabs (Figure 2B). Children with lower Ct values with the self-collected swab presented earlier in the course of illness than those with lower Ct values in the HCW-collected swab (median days after symptom onset, 1 [IQR, 1-3] vs 4 [IQR, 1-4], respectively; P = .04; eTable 2 in the Supplement). Four participants (aged 4, 10, 12, and 14 years) had discordant qualitative SARS-CoV-2 results: 2 self-swab positive only (aged 12 and 14 years) and 2 HCW swab positive only (aged 4 and 10 years). The 4 participants with discordant samples had N2 Ct values greater than 30.0 from the positive sample. Twenty-one samples were tested for spike receptor-binding domain mutations; of these, 18 (85.7%) had interpretable results and evidence of infection with the Delta variant (K417, L452R, T478K).
Based on the usability questionnaires, 38 of 196 participants (19.4%) were rated by the HCW as having difficulty completing swab collection and 27 of 196 (13.8%) as requiring assistance; 47 of 196 (24.0%) were rated as having not completed collection correctly (eTables 3 and 4 in the Supplement), with younger children rated lower (ie, 12/24 children aged 4-5 years had “significant difficulties” completing the swab collection and 13/24 did not complete the swab collection correctly). Children 8 years and younger were more likely than those older than 8 years to be scored as requiring assistance (19/90 [21.1%] vs 8/99 [8.1%]; P = .01), and they were less likely to be rated as having completed sample collection correctly (60/90 [66.7%] vs 81/98 [82.7%]; P = .01; eTable 4 in the Supplement). Despite these findings, the proportion of concordant samples was similar for children 8 years or younger and older than 8 years (92/93 [98.9%] vs 100/103 [97.1%], respectively; eFigure in the Supplement).
Discussion
When provided with video and printed instructions, symptomatic children and adolescents 4 to 14 years old were able to self-collect nasal samples that closely agreed on SARS-CoV-2 detection with swabs collected by HCWs. Children younger than 8 years had more difficulty with sample collection, but this did not affect the concordance with HCW-collected samples. For the very youngest children, who had difficulty with swab collection and for whom swab collection was not completed correctly, this approach should be used with caution.
One prior study investigated the feasibility of nasal swab self-collection by school-aged children based on the rate of observed deviations from a standard collection protocol, but results of molecular testing were not reported.10 In contrast, the studies presented here characterized the ability of school-aged children and adolescents to self-collect nasal samples based on the results of SARS-CoV-2 molecular testing and evaluated the usability of self-collection from both the child and HCW perspectives. There was no significant bias or improvement in performance based on collected demographic variables. These findings support that supervised pediatric self-collection can be used in existing SARS-CoV-2 testing strategies. Additionally, the results support the potential for nontraditional testing schemas for children, and future studies should investigate unsupervised self-collection and sample drop off at schools, prior to events, and testing at home.
This study was planned and initiated during a nadir in COVID-19 cases in Georgia. Low rates of infection during the initial phase of the study highlight the need to identify surrogate measures of specimen concordance for evaluating the performance of specimen collection and testing protocols for seasonal infectious diseases, such as SARS-CoV-2 and other respiratory viruses.
Limitations
This study had a number of limitations. First, data comparing SARS-CoV-2 detection in self- vs HCW-collected samples were limited to symptomatic participants. Second, there was not sufficient statistical power to detect small differences in SARS-CoV-2 detection by year of age. Third, this study was performed during the Delta variant wave. Fourth, participation in the study was voluntary, which may create selection bias for children less likely to object to self-swabbing. Fifth, there was individual variability in correlation for the Ct data such that only 68% had similar N2 Ct values.
Conclusions
After hearing and seeing simple instructional materials, children and adolescents aged 4 to 14 years self-collected nasal swabs that closely agreed on SARS-CoV-2 detection with swabs collected by HCWs.
eAppendix. Self-swab Evaluation Questionnaire
eFigure. Comparison of Qualitative SARS-CoV-2 Detection by rRT-PCR From Self- and HCW- collected Nasal Swabs by Participant Age for Participants ≤8 or >8 Years Old and Each Age Group
eTable 1. Characteristics of HCWs Involved in Participant Swabbing and Completion of the Useability Questionnaire
eTable 2. Analysis of the Direction and Degree of SARS-CoV-2 N2 Ct Differences in the CDC EUA rRT-PCR Between Self- and HCW-Collected Swabs
eTable 3. Complete Usability Questionnaire Results From Symptomatic Children and Adolescents, Stratified by Age
eTable 4. Complete Usability Questionnaire Results Binned By Age
References
- 1.Altamirano J, Govindarajan P, Blomkalns AL, et al. Assessment of sensitivity and specificity of patient-collected lower nasal specimens for severe acute respiratory syndrome coronavirus 2 testing. JAMA Netw Open. 2020;3(6):e2012005. doi: 10.1001/jamanetworkopen.2020.12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan SY, Tey HL, Lim ETH, et al. The accuracy of healthcare worker versus self collected (2-in-1) oropharyngeal and bilateral mid-turbinate (OPMT) swabs and saliva samples for SARS-CoV-2. PLoS One. 2020;15(12):e0244417. doi: 10.1371/journal.pone.0244417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson KE, Barker AP, Hillyard DR, et al. Self-collected anterior nasal and saliva specimens versus health care worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2. J Clin Microbiol. 2020;58(11):e01824-e20. doi: 10.1128/JCM.01824-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RA, Herigon JC, Benedetti A, Pollock NR, Denkinger CM. Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: a systematic review and meta-analysis. J Clin Microbiol. 2021;59(5):e02881-e20. doi: 10.1128/JCM.02881-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Real-time RT-PCR panel for detection 2019-novel coronavirus, instructions for use. Accessed September 1, 2021. https://stacks.cdc.gov/view/cdc/84526
- 6.Babiker A, Immergluck K, Stampfer SD, et al. Single-amplicon multiplex real-time reverse transcription-PCR with tiled probes to detect SARS-CoV-2 spike mutations associated with variants of concern. J Clin Microbiol. 2021;59(12):e0144621. doi: 10.1128/JCM.01446-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez M, Nguyen P-V, Su M, et al. SARS-CoV-2 variants in Paraguay: detection and surveillance with an economical and scalable molecular protocol. Viruses. 2022;14(5):873. doi: 10.3390/v14050873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waterman AH, Blades M, Spencer C. Interviewing children and adults: the effect of question format on the tendency to speculate. Appl Cogn Psychol. 2001;15(5):521-531. doi: 10.1002/acp.741 [DOI] [Google Scholar]
- 9.Cameron H. Asking the tough questions: a guide to ethical practices in interviewing young children. Early Child Dev Care. 2005;175(5):597-610. doi: 10.1080/03004430500131387 [DOI] [Google Scholar]
- 10.Altamirano J, Lopez M, Robinson IG, et al. Feasibility of specimen self-collection in young children undergoing SARS-CoV-2 surveillance for in-person learning. JAMA Netw Open. 2022;5(2):e2148988. doi: 10.1001/jamanetworkopen.2021.48988 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Self-swab Evaluation Questionnaire
eFigure. Comparison of Qualitative SARS-CoV-2 Detection by rRT-PCR From Self- and HCW- collected Nasal Swabs by Participant Age for Participants ≤8 or >8 Years Old and Each Age Group
eTable 1. Characteristics of HCWs Involved in Participant Swabbing and Completion of the Useability Questionnaire
eTable 2. Analysis of the Direction and Degree of SARS-CoV-2 N2 Ct Differences in the CDC EUA rRT-PCR Between Self- and HCW-Collected Swabs
eTable 3. Complete Usability Questionnaire Results From Symptomatic Children and Adolescents, Stratified by Age
eTable 4. Complete Usability Questionnaire Results Binned By Age