Abstract
Global pollution is calling for advanced methods to remove contaminants from water and wastewater, such as TiO2-assisted photocatalysis. The environmental applications of titanium dioxide have started after the initial TiO2 application for water splitting by Fujishima and Honda in 1972. TiO2 is now used for self-cleaning surfaces, air and water purification systems, microbial inactivation and selective organic conversion. The synthesis of titanium dioxide nanomaterials with high photocatalytic activity is actually a major challenge. Here we review titanium dioxide photocatalysis with focus on mechanims, synthesis, and applications. Synthetic methods include sol-gel, sonochemical, microwave, oxidation, deposition, hydro/solvothermal, and biological techniques. Applications comprise the production of energy, petroleum recovery, and the removal of microplastics, pharmaceuticals, metals, dyes, pesticides, and of viruses such as the severe acute respiratory syndrome coronavirus 2.
Keywords: Titanium dioxide, Wastewater, Photocatalysis, Emerging pollutant, Photocatalyst
Introduction
The past several decades have seen an ever-increasing rate of scientific and engineering innovations which has also brought with it the undesirable effects of anthropogenic activities such as pollution, and the proliferation of antibiotic-resistant microbes (Ojemaye et al. 2020; Maganha de Almeida Kumlien et al. 2021) and their associated health hazards. While concerted efforts have been taken to decrease the detrimental effects of such pollutants on the health and wellbeing of the environment, much attention has been on pollution mitigation via adsorption or oxidative mineralization (Daghrir et al. 2013). To this end, nanoparticles have been successfully demonstrated to attenuate pollutants of varying characteristics such as trace, organic, inorganic and biological (Villaseñor and Ríos 2017). Numerous candidates are deployed in environmental endeavours including oxides of transition metals (zinc oxide, titanium oxide, iron oxide, aluminium oxide) (Udom et al. 2013; Fajardo et al. 2014; Haider et al. 2017; Hitam and Jalil 2020), sulphides (zinc sulphide, cadmium sulphide) (Lee and Wu 2017; Theerthagiri et al. 2017), nitrides (graphitic carbon nitrides) (Zhang et al. 2018; Zhu et al. 2020), carbon allotropes (Madima et al. 2020; Long et al. 2021), nanocomposites with noble metals (Liu et al. 2017; Prakash et al. 2018) and other transition metal compounds (López et al. 2021). However, the chief criterion for selecting the best nanoparticle for a certain application is based on the cost-effectiveness, chemical stability and recoverability. Consequently, nanosized titanium dioxide (TiO2) has become a chief candidate for pollution mitigation application as a photocatalyst, adsorbent or electrocatalyst.
Tremendous development in TiO2 nanoparticles synthesis techniques has enabled its application in an array of fields such as water purification (Jézéquel and Chu 2005)(Lee and Park 2013), carbon dioxide reduction (Ola and Maroto-Valer 2015), water splitting (Ismael 2020), air pollution mitigation (Toma et al. 2004) (Lyu et al. 2014), food (Boutillier et al. 2021) and microbial decontamination/surface disinfection (Laxma Reddy et al. 2017) with promising results. This has also partly been motivated by the eco-friendly, self-cleaning/sterilizing, stable and inert nature of titanium dioxide. In addition, bulk-scale titanium dioxide possesses little to no environmental application and is mostly employed as a dye/pigment (Weir et al. 2012).
A variety of TiO2 nanoparticles such as dendrites (Sun et al. 2011), anodic grids (de Freitas et al. 2011), nanodots (Zhang et al. 2017), powders (Yildiz et al. 2020), 2D nanosheets (Chen et al. 2020), hollow spheres (Su et al. 2020), nanoflowers (Harris et al. 2020), nanorods (Diao et al. 2021) and nanobelts have been synthesized through various techniques like sol–gel method, hydro (solvo)-thermal method and microwave methods. Considering the diversiform application areas of titanium dioxide nanoparticles in the environment, this work tries to provide a consolidated review of various synthesis techniques that can be applied for the production of nano-titanium dioxide and their application as photocatalysts for pollutants (heavy metals, dyes and microplastic) removal, electrocatalysts for energy production (water splitting and carbon dioxide reforming), antimicrobial agents, nanomedicine and oil recovery which will be discussed towards the latter sections of the work.
While many reviews currently exist on the various techniques for synthesis of TiO2 nanoparticles, some excellent reviews in this field include the works such as Daghrir et al. (2013), Wang et al. (2014), and Gopinath et al. (2020) that have covered numerous articles until recent past. However, with tremendous improvement in nanoparticle synthesis techniques, a latest and more comprehensive review on the synthesis and application of titanium dioxide is needed and this work has been framed towards addressing this endeavour.
Mechanism of titanium dioxide photocatalysis
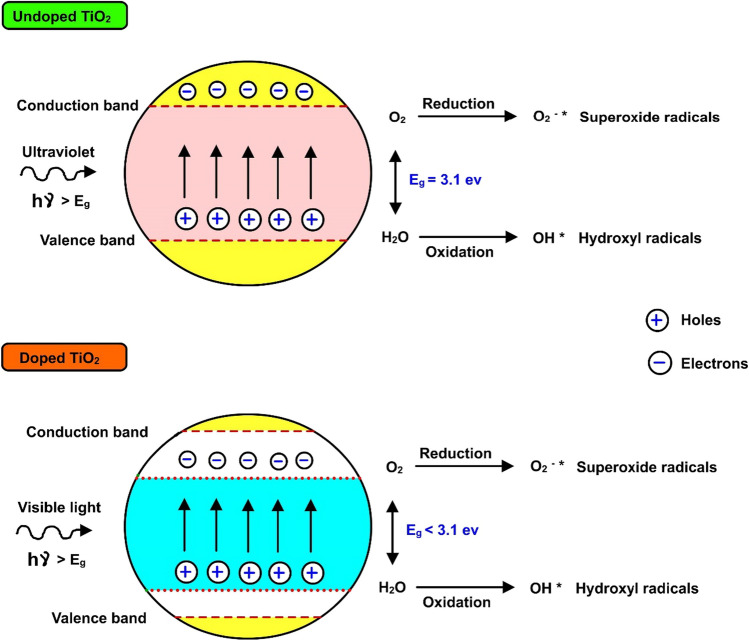
Being a semiconductor, titanium dioxide (TiO2) comprises a small energy difference between the conduction and valence bands. Electrons that are present in the valence band get excited to the conduction band when light (ultraviolet) falls on its surface. This phenomenon leads to the formation of both negative and positive charges on the semiconductor surface. Titanium dioxide has a band gap (3–3.2 eV) with a maximum absorption wavelength of 400 nm. The entire phenomenon of electron excitation is depicted in Fig. 1. Constant exposure to light results in the formation of high temperature on the titanium dioxide surface, and this, in turn, results in the degradation of pollutants in the water system. The degradation of pollutants with the help of dissolved oxygen present in the water is depicted in Eqs. (1–3). Hydroxyl radicals formed during these reactions also help in the degradation of pollutants via the photocatalytic degradation mechanism (Eq. 4) (Yao et al. 2017). Here they undergo protonation and end up in the formation of hydroperoxyl compounds, which help in scavenging undesired radicals and favoring slowness in electron and hole recombination.
| 1 |
| 2 |
| 3 |
| 4 |
The degradation of pollutants undergoes five major steps. In steps 1 and 2, pollutant molecules are transferred to the surface of the photocatalyst and followed by adsorption onto the active sites. In step 3, once the pollutants get attached to the active sites, the electrons get photoexcited and initiate the photocatalytic degradation process. In steps 4 and 5, once the pollutant is degraded, desorption occurs, and finally, the degraded molecules are released to the water surface (Wang et al. 2015; Gopinath et al. 2020). Additionally, the process of degradation leads to the possible formation of five different products. The process of photocatalytic degradation and end products depends upon the type of pollutant and photocatalyst choice. The possible products that can be resulted at the end of the photocatalytic process are dehalogenated compounds, oxides of alkali, isomerized and cyclized compounds, aromatic ring molecules and decarboxylated compounds (Prihod’ko and Soboleva 2013).
Fig. 1.
Comparison of energy band gap levels of undoped (pure) and doped titanium dioxide. Upon irradiation with light of suitable energy that corresponds to the bandgap, the electrons present in the valence band of titanium dioxide get excited and their concurrent transfer to the conduction band occurs. This phenomenon facilitates the generation of various reactive oxygen species leading to the degradation of pollutants present in the wastewater. H2O: water, O2: oxygen, Eg: energy gap, h : photon energy
: photon energy
Techniques for titanium dioxide synthesis
Works of literature have shown that the synthesis of titanium dioxide (TiO2) could be achieved through various methodologies including chemical, physical and green (Nabi et al. 2020a; Sharma et al. 2020; Wang et al. 2020b). Table 1 provides a detailed literature comparison on the synthesis routes of titanium dioxide-based catalysts for environmental applications. A variety of different techniques including vapour deposition (Singh et al. 2019a), electrodeposition (He et al. 2019), sol–gel method (Phattepur et al. 2019), hydrothermal method (Wang et al. 2020b), solvothermal method (Ramakrishnan et al. 2018), microwave method (Cabello et al. 2017) and sonochemical method (Moreira et al. 2020) has been extensively used in the past few years to produce nano- titanium dioxide. Though chemical synthesis of titanium dioxide nanoparticles has widely been used owing to the ease of synthesis and effective control of size and shape of nanoparticles, there still exist certain limitations including high cost, the requirement of extreme temperatures and pressures, and eco-toxicity. Consequently, these disadvantages limit titanium dioxide applications in different fields and their mass industrial production (Nadeem et al. 2018). Therefore, there has been a paradigm shift in recent times towards a more eco-friendlier way of titanium dioxide fabrication called the “green synthesis,” wherein reducing agents derived from biological extracts are used for synthesis (Edmundson et al. 2014; Subhapriya and Gomathipriya 2018). The upcoming subsection of the review paper covers a few of these most used techniques for titanium dioxide synthesis in detail.
Table 1.
Synthetic methods for titanium dioxide-based catalysts for environmental applications. TiO2: Titanium dioxide, Hg: mercury, CuO: copper oxide, NO: nitrous oxide, HCHO: formaldehyde, SO2: sulphur dioxide, BTEX: benzene, toluene, ethylbenzene, xylene
| Catalyst | Synthesis method | Intended application | Category of application | Initial concentration | Illumination condition | Optimal performance | References |
|---|---|---|---|---|---|---|---|
| TiO2 films | Sol–gel | Trichloroethylene degradation | Air pollution mitigation | 50 ppm | Ultraviolet lamp | Up to 90% conversion | Arconada et al. (2009) |
| TiO2-P25 | Commercial P25 | Nitrous oxide removal | Air pollution mitigation | 5 ppm | Ultraviolet lamp (25 W) | 70% conversion | Devahasdin et al. (2003) |
| TiO2-P25 | Commercial P25 | HCHO:SO2:BTEX | Air pollution mitigation | 1:1:1 ratio of gases at 1, 10 and 50 ppm | 6 W Ultraviolet lamp | 88% conversion | Ao et al. (2004) |
| TiO2-aluminium silicate | Sol–gel | SO2:NO:Hg0 | Air pollution mitigation | 400–1200 ppm SO2, 50–300 ppm NO, 50 µg/m3 Hg0 | 9 W Ultraviolet lamp | Up to 80% | Yuan et al. (2012) |
| TiO2nanofibre | Electrospinning | SO2 | Air pollution mitigation | 100–300 ppm in air | 300 W Xenon arc lamp | Up to 100% | Wang et al. (2017a) |
| TiO2 | Precursor hydrolysis | Arsenic removal | Water treatment–adsorption | Arsenic = 26.7 µm | – | 90% | Pena et al. (2005) |
| TiO2 | Precursor hydrolysis | Arsenic removal | Water treatment–adsorption | Arsenic = 0–80 ppm | – | 85% | Xu and Meng (2009) |
| TiO2 | Commercial P25 | Methylene blue removal | Water treatment–adsorption | Methylene blue = 56.5 ppm | – | 88.5% | Munjal et al. (2014) |
| TiO2/chitosan | Commercial TiO2 | Dye (thymol violet) removal and antimicrobial activity | Water treatment– adsorption | Thymol violet = 120 ppm | – | 88% | Kamal et al. (2016) |
| TiO2 nanotube | Anodization | Bacteria (E. coli) disinfection | Water treatment–microbial decontamination | 106 cpu/mL | Ultraviolet lamp | 95% disinfection | Ng et al. (2010) |
| TiO2 | Commercial P25 | Fungi (F. solani) disinfection | Water treatment–microbial decontamination | 103 cpu/mL | Sunlight | 99.99% removal | Fernández-Ibáñez et al. (2009) |
| TiO2 | Commercial P25 | Protozoa (C.parvum) disinfection | Water treatment–microbial decontamination | 15 × 106 oocytes | 1100 W Xenon arc lamp | 99.33% | Abeledo-Lameiro et al. (2016) |
| Bismuth/TiO2 | Sol–gel | Water splitting/methanol sacrificial agent | Sustainable energy | – | Ultraviolet lamp | 3.5 mL hydrogen production | Wu et al. (2009) |
| Platinum/Nitrogen/TiO2 | Hydrothermal | Water splitting/glycerol sacrificial agent | Sustainable energy | – | 250 W Ultraviolet lamp | 3200 µmol hydrogen production | Slamet et al. (2013) |
| Platinum/TiO2−xNx | Microemulsification | Water splitting/methanol sacrificial agent | Sustainable energy | – | 400 W halogen lamp | 130 µmol hydrogen production | Lin et al. (2009) |
| Nickel/TiO2 | Precipitation | Water splitting/methanol sacrificial agent | Sustainable energy | – | 3 W Ultraviolet light emitting diode | 3056 μmol h−1 g−1 | Yu et al. (2011) |
| Silver/TiO2 | Sol–gel synthesis | Carbon dioxide reduction/water sacrificial agent | Pollution mitigation/sustainable energy | – | Ultraviolet light |
Methane = 10.5 Methanol = 2 μmol/gcatalyst |
Krejčíková et al. (2012) |
| TiO2 | Wet impregnation | Carbon dioxide reduction/water sacrificial agent | Pollution mitigation/sustainable energy | – | 300 W Xenon arc lamp | Methane = 52 μmol/gcatalyst | Meng et al. (2014) |
| Palladium/TiO2 | Thermal hydrolysis | Carbon dioxide reduction/water sacrificial agent | Pollution mitigation/sustainable energy | – | 500 W Hg lamp |
Methane = 1.415 CO = 0.722 μmol/g.h |
Camarillo et al. (2017) |
| CuO/TiO2 | Sol–gel and wet impregnation | Carbon dioxide reduction/water sacrificial agent | Pollution mitigation/sustainable energy | – | 6 W Hg lamp | Methanol = 4120 μmol/g | Thamaraiselvi and Sivakumar (2017) |
Physical and chemical methods
Sol–gel technique
Sol–gel is a very versatile wet-chemical technique that has been extensively used in ceramic and material sciences engineering fields. The process proceeds through the conversion of a precursor mixture into an inorganic solid (usually inorganic metal salts or metal alkoxides) via polymerization reactions initiated by water (Nyamukamba et al. 2018). The hydrolysis step leads to the formation of a sol (aggregate of colloidal particles dispersed in a fluid) while the condensation reaction forms a gel. As mentioned earlier, the most common precursors for sol–gel are metal chlorides and metal alkoxides. The metal alkoxides are made up of an M–O–R linkage, where M stands for the metal, O stands for oxygen and R stands for an alkyl group. The incidence of a polarization reaction in the M–O bond makes the whole molecule susceptible to a nucleophilic substitution and in the presence of water molecules; the alkoxide group are substituted and replaced by hydroxyl ions from water (hydrolysis). Following this, the produced metal hydroxides inter-molecularly connect and generate a hydrated metal oxide network eventually forming small crystal nuclei (condensation) and this is how a typical sol–gel process occurs.
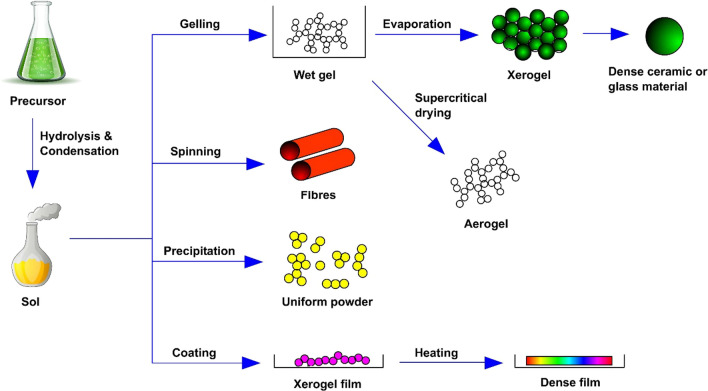
Among the various physical/chemical techniques, sol–gel is a very promising one since it allows the operation of the whole synthesis under low temperatures i.e. < 100 °C and provides a molecular level homogeneity in products (Malekshahi Byranvand et al. 2013). The other benefits of the sol–gel technique include easy monitoring and control of nanoparticle shape and size. Being a flexible technique, sol–gel process could be employed to produce a variety of products like spherical, fine and uniform-sized nanopowders. Works of literature show that sol–gel has been widely used for titanium dioxide synthesis from titanium (IV) alkoxides through acid catalysis. For example, in a study mesoporous titanium dioxide nanoparticles utilizing titanium (IV) isopropoxide using surfactant-mediated sol–gel were synthesized using the water technique (Nateq and Ceccato 2019). One of the outstanding features of the sol–gel technique is the possibility to shape the product into any desired form such as a film, a fibre or even a monodispersed powder. Mehrotra and Singh comprehensively studied and established the conditions and steps applied to achieve various morphological products from the process (Mehrotra and Singh 1997), as shown in Fig. 2.
Fig. 2.
Methodologies of the sol–gel process for the synthesis of titanium dioxide materials. Gelling, spinning, precipitation and coating are the most common techniques used in the sol–gel process. Dense ceramic material (xerogel) and aerogels are produced from the gelling process. Fibres, uniform powder and dense film (xerofilm) are produced from the other three methods
Sonochemical and microwave-assisted techniques
Ultrasound has been extensively used in the production of a wide range of nanosized materials in the past. The chemical effects arising from ultrasound do not result from direct interaction with the molecular species but instead from acoustic cavitation which involves the formation, growth and collapse of the bubbles within a liquid medium resulting in the creation of localized high pressures (~ 1000 atm) and high temperatures (~ 5000 K) (Chen 2009). The sonochemical technique has been employed to synthesize photoactive titanium dioxide nanoparticles through the hydrolysis of titanium tetraisopropoxide either in pure water or in a water/ethanol mixture using ultrasonic waves (Moreira et al. 2020).
Similar to sonochemical that employs ultrasound, the microwave-assisted technique uses microwaves (electromagnetic waves) with frequencies of 0.3–300 GHz and with wavelengths of 0.001–1 m to synthesize nano-titanium dioxide. It has been reported in a study that microwave heating involves two different mechanisms, namely ionic conduction and dipolar polarization (Zhu and Chen 2014). Any substance that contains a mobile electric charge, i.e. conducting ions or polar molecules, can be heated using microwaves. For polar molecules, the heating phenomenon occurs through the friction, rotation and collisions in between these molecules, which try to orient themselves to the rapidly alternating electric field, while for the conducting ions, the heat gets generated when these ions constantly move through the solution trying to orientate themselves with the electric field causing a local temperature rise as a result of friction and collision (Collins 2010). Microwaves have been applied to synthesize different titanium dioxide nanomaterials, especially in industrial processing due to their benefits of rapid heat transfer and selective heating (Nyamukamba et al. 2018). Cabello et al. (2017) synthesized nano-TiO2 particles with an average particle size of 73 nm and a pore diameter of 2.6 nm using microwaves under the conditions of 240 °C, 25 bar for 20 s indicating a low sintering time, thereby reducing the overall costs and energy requirements.
Oxidation techniques
The underlying principle behind these techniques is the oxidation of titanium metal to titanium dioxide either using oxidants or by anodization. Research has shown that crystalline titanium dioxide nanorods could be produced through the direct oxidation of titanium metal plates using hydrogen peroxide (Wu et al. 2005; Kumar and Pandey 2018). Archetypally, titanium dioxide nanorods could be obtained from a titanium plate when a pre-treated titanium plate is dissolved in the beaker containing 50 mL of 30 wt% hydrogen peroxide solution at 80 °C for 3 days. The formation of crystalline titanium dioxide initiates with the dissolution precipitation mechanism, followed by the addition of inorganic sodium salts (NaX; X = fluoride, sulphate, chloride ions) to control the crystalline phase of titanium dioxide nanorods. The addition of F− and SO4− forms of Na salt favours the formation of anatase while the addition of Cl− promotes the formation of rutile (Wu 2004). Apart from hydrogen peroxide pure oxygen, acetone and a mixture of argon and oxygen can alternatively be used as a source of oxygen for the oxidation of titanium (Chen and Mao 2007).
On the other hand, the anodization of titanium plate under voltages in the range of 5–20 V in a 0.5 wt% hydrogen fluoride solution leads to the production of titanium dioxide nanotubes, whose diameter could be controlled by varying the voltage applied (Chen 2009). In a recent study, Mohan et al. (2020) investigated the effects of anodization time and temperature on the formation of titanium dioxide nanotubes for biomedical purposes. The authors used titanium alloys in an electrolytic mixture of 50 mL of 1 M sulphuric acid and 50 mL of 0.08 M hydrogen fluoride at different temperatures ranging from 5 to 70 °C to prepare self-organized titanium dioxide nanotubes. They observed significant outcomes at 25 °C with titanium dioxide nanotube characteristics of 30 nm wall thickness, 250 nm length, 125 nm inner pore diameter and 35 nm inner tube space without any visible defects in their morphology unlike at other temperatures.
Deposition techniques
Deposition techniques are commonly utilized to form coatings, thereby altering the thermal, electrical, optical and mechanical properties of substrate materials. The characteristics of the product are fabricated by altering parameters like the geometry of the deposition chamber, composition, flow rate, temperature and pressure of deposition (Malekshahi Byranvand et al. 2013). Among the various deposition techniques, electrophoretic deposition and spray pyrolysis are the most favoured and employed techniques for nano-titanium dioxide synthesis (Irshad et al. 2021).
The electrophoretic deposition includes an electrolytic cell setup wherein charged particles from a suspension medium are deposited onto a substrate under the influence of the applied direct voltage (Cabanas-Polo and Boccaccini 2016) as shown in Fig. 3. When a direct voltage current is applied to the electrodes an electric field gets generated inside the cell, this field interacts with the surface charge of titanium dioxide, thereby creating a force that pulls these particles towards the electrode of opposite charge. Consequently, the accumulation of titanium dioxide onto the substrate (electrode) leads to the formation of a homogenous layer. The thickness of the layer could be adjusted by controlling the deposition parameters like deposition time, applied current/voltage, solvent type and suspension loading (Narayan and Raturi 2012). Researchers have employed various combinations of titanium dioxide thin film deposits for real-time applications like dye-sensitized solar cells (Xue et al. 2012), biomedical applications (Narkevica et al. 2017) and ceramic coatings (Ledwig et al. 2017). Nyongesa and Aduda (2017) employed electrophoretic deposition to deposit thin films of titanium dioxide onto glass substrates for application in water treatment. The authors concluded that ethanol was a better solvent to use for electrophoretic deposition compared to water, propanol and toluene, which was due to the high dielectric constant of ethanol (24.3). In general, water cannot be used as a suitable suspension medium due to the occurrence of water electrolysis, which leads to the accumulation of bubbles on electrode surfaces. The optimal parameters for the best adherence of titanium dioxide particles in the study were a pH of 3, an applied voltage of 20 V and solid loading of 4.0 wt% (Nyongesa and Aduda 2017). In a similar work performed by Nguu et al. (2018), the optimal time and voltage for achieving a uniform 5-μm titanium dioxide film were found to be 90 s and 35 V, respectively. The authors also discovered that a prolonged time > 90 s showed the agglomeration of the particles. On the contrary, Dhiflaoui et al. (2016) observed that titanium dioxide coatings on stainless steel substrates were the most homogenous at a voltage of 20 V and after a deposition time of 4 min.
Fig. 3.
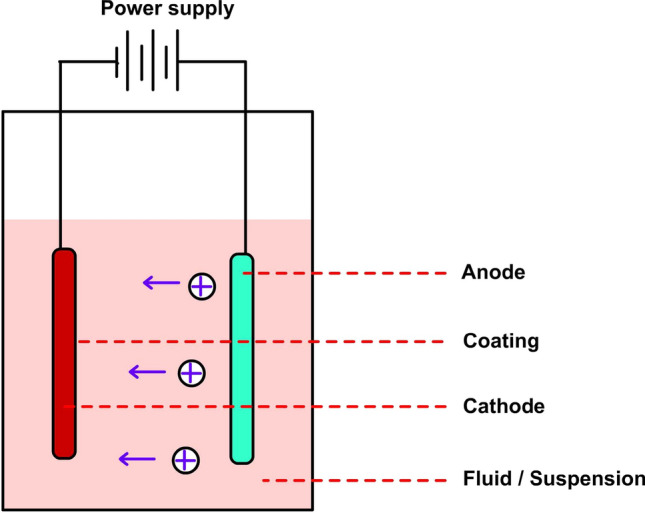
Cathodic electrophoretic deposition cell (Srikanth et al. 2017). The photocatalyst is suspended in the solvent phase with a steady direct voltage (25–400 V). Electrolysis produces ions, which migrate towards the oppositely charged electrode as a result of electrophoresis and gets deposited over the electrode support
While electrophoretic deposition solely allows the production of titanium dioxide films, the spray pyrolysis technique enables the fabrication of a variety of product forms including dense and fine dispersive powders (spray pyrolysis synthesis) and films (spray pyrolysis deposition). Schematic representations of different spray pyrolysis systems are presented in Fig. 4. The technique involves the spraying of a precursor’s solution across a direct flame source. This can be done in two ways: (i) by using supplemental burners mounted adjacent to the spray nozzle or (ii) by additional feeding of oxidants (i.e. air, oxygen) and the combustibles to the nozzle (Nyamukamba et al. 2018). The diameter of the spray droplets depends on the viscosity and surface tension of the precursor solution, the diameter of the nozzle tip, and the pressure difference before and after spraying (Kozhukharov and Tchaoushev 2013). The authors displayed the synthesis of ultrafine dispersive titanium dioxide powders through a swift rise in temperature inside the chamber, and during the process, the pre-formed solid particles were observed to undergo further splitting as a result of phase transitions and mechanical tensions (Kozhukharov and Tchaoushev 2011). The spray pyrolysis deposition has been reported to be performed both using a hot spray and a cold spray on a pre-heated substrate. For instance, Möllmann et al. (2019) prepared compact titanium dioxide layers using cold spray pyrolysis on a glass substrate preheated to 450 °C for the application in perovskite solar cells. The authors attempted to carry out the deposition process using substrates at low temperatures of 150 °C to reduce the overall cost of the process but observed the formation of a cracked titanium dioxide layer, which exhibited low light transmittance and reduced the device’s efficiency (Möllmann et al. 2019).
Fig. 4.
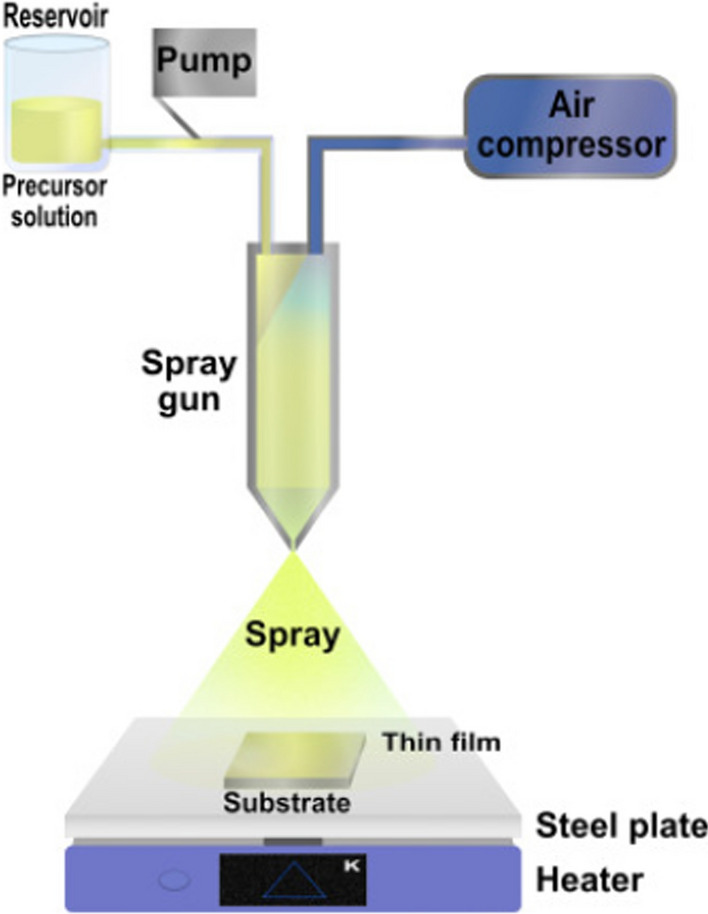
Spray pyrolysis. The spray gun provides the point for the substrate that was placed in the hot plate to get in contact with the precursor solution. Licensed from Elsevier books “Nanosensors for smart cities”, Han et al. (2020)
Hydro- and solvothermal techniques
Both these techniques hydrothermal and solvothermal are almost similar. In the hydrothermal technique, the substance is crystallized at high temperatures and vapour pressures using an aqueous suspension of the material (Reis et al. 2002). In general, this technique could be considered as crystal growth or crystal synthesis from materials that are commonly insoluble at customary pressure, i.e. < 1 atm, and temperature, i.e. 100 °C. This process is usually carried out in autoclaves under a controlled atmosphere, which in turn enables the utilization of temperature above the boiling point of water or an organic solution inside the chamber. Briefly, hydrothermal synthesis is described as a concoction response taking place in a dissolvable mixture at temperatures above the mixture’s breaking point and pressures over the bar. Since the essential properties of water including viscosity, heat capacity, thermal conductivity, dielectric constant and ionic product density are all temperature–pressure dependent, the hydrothermal technique exploits these properties, thereby tuning the synthesis factors and achieving specific solvent attributes. However, the technique has a few drawbacks including the high capital requirement for instrumentation, the inability to monitor crystal growth and the method which can only be performed under supercritical conditions of the solvent (Liu et al. 2014). Conversely, in solvothermal techniques, non-aqueous solvents with very high boiling points could be used and this consequently enables much better control over the properties of titanium dioxide particles during the synthesis. For instance, Kathirvel et al. (2020) produced titanium dioxide nanocrystals by treating titanium (IV) isopropoxide with various alcohol solvents (ethanol, propanol, isopropyl alcohol, butanol, tert-butyl alcohol and benzyl alcohol) at 150 °C for 8 h, while Li et al. (2020) used a one-step solvothermal technique to synthesize titanium dioxide from titanocene dichloride with uniform particle size without the use of any surfactants. The solvothermal technique allows better control over the size, shape, crystallinity and distribution of titanium dioxide nanoparticles compared to the hydrothermal, and these could be achieved through effective manipulation of reaction temperature, solvent and surfactant characteristics and reaction time (Stride and Tuong 2010). Additionally, the employment of organic solvents results in a product, which is free from extraneous anions because organic solvents tend to be devoid of ionic species and have relatively low permittivity (Nyamukamba et al. 2018).
Biological methods
Owing to the toxic nature of the chemical techniques towards the environment and the limitations encountered with the mass production, the search for a more sustainable and eco-friendly approach is greatly favoured. As an alternative, the green nanotechnology has gained a lot of attention lately for the production of titanium dioxide nanoparticles from naturally occurring biological resources (i.e. plants, fruit extracts, microorganisms and their waste materials) and for its cost-effect benefits (Nadeem et al. 2018; Singh et al. 2019b).
Plants contain nutrients like carbohydrates, proteins, alkaloids and nucleic acids, which can be used during particle synthesis for stabilization and reduction reactions. Moreover utilizing plant-based extracts as the key components for titanium dioxide production provides an additional safety compared to chemical/physical techniques (Irshad et al. 2021). Nabi et al. (2020a, b) prepared spherical-shaped anatase titanium dioxide nanoparticles through green synthesis using cinnamon powder. The overall method was reported to be feasible, simple and cost-effective. The product was found to have a band gap residing in the visible light zone along with the presence of oxygen vacancies and excitons necessary for its application in solar cells. In general, green synthesis commonly involves extracts obtained from leaves of plants as raw materials for nanoparticle synthesis since this section of plants is found to have high metabolites (Subhapriya and Gomathipriya 2018). For instance, Kumar used the leaf extracts of Syzygiumcumini and synthesized spherical aggregates of titanium dioxide using an eco-friendly, cheap and non-toxic technique (Kumar 2020). The so produced titanium dioxide was employed for treating lead-contaminated industrial waters and the authors reported a maximum removal of 82.5%.
Analogous to plants, microbial extracts from bacteria and fungi have also been utilized to biologically produce nano-titanium dioxide. These microbial extracts have gained considerable attention lately due to their low capital requirement, simple extraction leading to easier scale-up and larger surface of the synthesized product (Pantidos and Horsfall 2014). Peiris et al. (2018) identified the antimicrobial properties of baker’s yeast and used them to synthesize highly pure small-sized titanium dioxide particles (anatase) via a cost-efficient green technique. In a different study, Streptomyces species were used to synthesize less toxic and cheap but highly pure titanium dioxide nanoparticles (Ağçeli et al. 2020). The authors described the method to be simple and quick for achieving antibiofilm and antimicrobial properties-based products. Likewise, various shapes and sizes of titanium dioxide nanostructures have been reported to be synthesized from fungal extracts as they can produce a range of enzymes and other metabolites that can break down large salt molecules into elemental ions (Nadeem et al. 2018). For example, a study carried out by Rajakumar et al. (2012) established the fungus Aspergillus flavus as a novel species for biological titanium dioxide production. Aside from plants and microbes, various derivatives of biological origin like albumen, starch and cellulose have also shown some potential in producing titanium dioxide (Bao et al. 2012; Muniandy et al. 2017). However, nanoparticle titanium dioxide synthesis using these bio-derivatives has not been much explored like their counterparts. A comparison between the various techniques used for the synthesis of nano-TiO2 particles is summarized in Table 2 based on the technique’s major influencing factor, merits and demerits.
Table 2.
Physical, chemical and biological methods for the synthesis of titanium dioxide nanoparticles
| Method | Major factors | Advantage(s) | Disadvantage(s) | References | |
|---|---|---|---|---|---|
| Physical and Chemical method | Sol–gel | pH, time, temperature, agitation, nature of solvent and catalyst | Low operation temperature (< 100 °C), cheap and high yield | Slow process, difficult to synthesize monoliths, high precursors cost | Esposito (2019) |
| Sonochemical-assisted technique | Intensity of acoustic frequency, temperature, static pressure | Fast reaction rate, production of ultra-fine particles and no chemical addition | Low yield, high energy demand | Savun-Hekimouglu (2020) | |
| Microwave-assisted technique | Frequency and wavelength of microwave, temperature | Selective heating, short reaction time, easy handling and high yield | Expensive process, unfeasible to monitor reaction, difficult to scale up | Mikrovalov (2011) | |
| Oxidation techniques (anodization) | Electrolyte type and concentration, batch temperature, voltage, pH | Easy to scale up, time-efficient, facile, effective in synthesis of high quality 1D nanostructures | Limited mass production, employed mostly for nanotubes growth | Kaur et al. (2020) | |
| Electrophoretic deposition | Electrolyte type and nature, temperature, voltage | Simple, low cost equipment, high reproducibility, short product formation time | Requires high sintering temperatures, difficult to achieve fissure less coating, only films can be made | Chava et al. (2017) | |
| Spray Pyrolysis | Nature of precursor solution, pressure, properties of the instrument | Cost effective, do not require high quality reagents, morphology of product is easy to control | Hard to scale up, difficulties associated with determining growth temperature, oxidation of reagent when operated in air atmosphere | Gavrilović et al. (2018) | |
| Hydrothermal technique | Viscosity, heat capacity, thermal conductivity, dielectric constant, ionic product density | Easy handling, simple, production of high quality 1D nanostructures | Slow process, inability to monitor crystal growth, high equipment cost | Liu et al. (2014) | |
| Solvothermal technique | Viscosity, heat capacity, thermal conductivity, dielectric constant, ionic product density | Simple equipment, uniform production on larger area | Requirement of pure organic solvents, high pressure and temperatures are needed | Wang et al. (2017b) | |
| Biological method | Concentration, temperature, pH | Eco friendly, devoid of using any toxic chemicals, cheap and safe | Cell growth of organisms determine size of nano particles, low yield | Wu et al. (2019) | |
Applications of titanium dioxide
Removal of metals and dyes
In recent years, much attention has been paid by the research community to the simultaneous removal of dyes as well as heavy metals (Louangsouphom et al. 2019; de Lima et al. 2020; Izzudin et al. 2021). This methodology minimizes the processing time, operating cost as well as the use of chemicals in comparison with individual treatment methods. Titanium dioxide was found to be very effective in simultaneous removal of heavy metals and dyes. In some cases, the presence of one pollutant was found to enhance the removal of another pollutant. For instance, the presence of rhodamine B dye, during the transformation of aqueous metal chromium (IV) to chromium (III) using titanium dioxide nano-fibre membrane accelerated the removal rate (Zhang et al. 2021), and under optimized conditions, the removal efficiency of chromium (IV) was found to be 97.09%.
The efficacy of hetero-layered titanium dioxide incorporated on layered double hydroxide-molybdenum disulphide nanostructure was recently tested on the removal of organic cations and ionic dyes along with silver and lead ions and found that 97–99% dye removal was achieved (Panchal et al. 2021). Simultaneously, due to its excellent affinity and selectivity for heavy metal ions, it rapidly reduced the toxic lead from 10 mg/L to less than 0.8 µg/L and this substrate showed an enormous adsorption capacity of silver (421.88 mg/g). In recent years, different forms of nano-titanium dioxide were used which include one-dimensional nanowire, a nanobelt, two-dimensional nanotubes and nanosheets and three-dimensional nanoparticles as well as nanorods. The major advantages of one-dimensional nanobelt are lesser number of grain boundaries, fast charge transfer dynamics and high specific surface, which makes nanobelt more attractive (Pang et al. 2015).
The major disadvantages of one-dimensional nanobelt are (1) higher recombination rate of photoinduced electron–hole pairs due to the single-phase structure, and (2) pure titanium dioxide can only absorb ultraviolet irradiation because of its high band gap, which prevents this nanobelt from widespread applications (Tian et al. 2015). But these issues were resolved by researchers in recent years. For instance, less recombination of electron/hole pairs was observed during the use of novel hybrid bismuth subcarbonate (Bi2O2CO3) quantum dot/titanium dioxide nanobelt on the removal of rhodamine blue (Wang et al. 2021b). The removal efficiency of 95.43% under visible light, which is nine times larger than that of titanium dioxide nanobelts, was observed.
Literature related to the simultaneous removal of heavy metals and dyes is listed in Table 3. A mesoporous 2D-2D TiO2(B)-bismuth oxobromide heterojunction photocatalyst was synthesized and used for the degradation of mixed pollutants such as rhodamine blue, methyl orange, tetracycline hydrochloride and bisphenol A (Han et al. 2021). This substrate produced abundant superoxide radicals under visible light, which enabled the catalyst to accelerate the degradation process well. The removal of rhodamine blue, methyl orange tetracycline hydrochloride and bisphenol A using this synthesized substrate was found to be 4.7, 1.4, 23 and 16.4 times higher than that of using pure bismuth oxobromide, respectively. Recently, Karpuraranjith et al. (2022) synthesized a novel three-dimensional hybrid photocatalyst by embedding a porous molybdenum disulphide nano-box on graphitic carbon nitride nanosheets containing titanium dioxide nanoparticles. Around 97.5% photocatalytic removal of methylene blue dye was achieved using this hybrid photocatalyst, in the presence of visible light. Similarly, nanoflowers were widely employed in dye degradation and reported in recent studies (Huda et al. 2019; Shang et al. 2020; Wang et al. 2020a; Quyen et al. 2021) due to their high specific surface area, well-developed pore structure, enormous photocatalytic activity (Wu et al. 2014).
Table 3.
Applications of titanium dioxide based substrates on simultaneous removal of heavy metals and dyes. TiO2: Titanium dioxide, SiO2: silicon dioxide
| TiO2-based substrate | Pollutants considered | Removal efficiency | References |
|---|---|---|---|
| Polyvinyl alcohol and acrylamide incorporated on TiO2/SiO2 nanopowders | Basic blue 3 dye and copper (II) ions | Basic blue 3 dye = 93.5 and copper (II) = 95.2% after 7 h and 6 h, respectively | Elbarbary and Gad (2021) |
| Natural melamine/TiO2 hybrid | Methyl orange dye and chromium (IV) | Around 90% reduction on dye and 100% on chromium (IV), after 3 h of visible light irradiation | Xie et al. (2020) |
| TiO2/3-cyanopropyltriethoxysilane/metformin polyether sulfone nanocomposite membrane | Copper (II) ions and dye removal from liquorice extraction plant (LEP) wastewater | 90.1% removal of copper ions after 60 min and 88% chemical oxygen demand removal on LEP wastewater after 150 min | Barahimi et al. (2020) |
| Bismuth molybdenum oxide type-II loaded on TiO2 nanotubes | Methyl orange, rhodamine blue, methylene blue and chromium (VI) | Removal of nearly 100%, 75%, 100% and 100% for methyl orange, rhodamine blue, methylene blue and chromium (VI)ions, respectively, after 120 min of simulative sunlight irradiation | Liu et al. (2019) |
| TiO2/SiO2 doped with iron | Basic red 29, basic blue 41, basic yellow 51 and chromium (VI) | Reduction in total organic carbon = 74.39% and 78.04% under visible and solar light, respectively | Ghanbari et al. (2019) |
| Electro-spinning carbon nanofibers/TiO2 using polyacrylonitrile base | Methylene blue dye and different metal ions such as lead, copper and cadmium ions | A maximum rejection rate of 84%, 87%,73% and 66% for methylene blue, lead, copper and cadmium, respectively, after 1500 min | Kumar et al. (2018) |
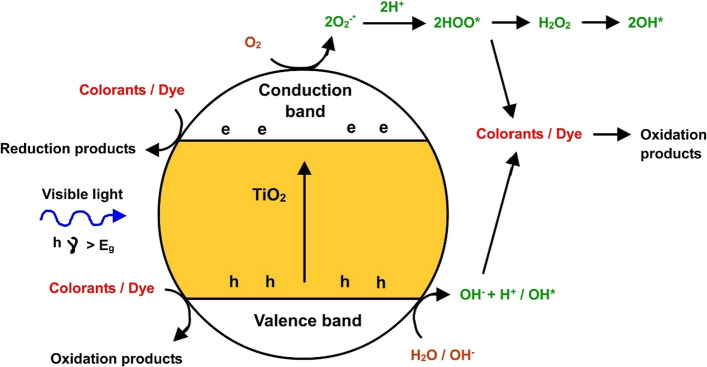
On the other hand, the photocatalytic efficacy of titanium dioxide was employed with a wide range of metal/metal oxides such as silver, cadmium, chromium, copper, manganese and nickel in the degradation of rhodamine blue (Le et al. 2021; Sengul and Asmatulu 2020). It was found that among these metal/metal oxides studied, silver- titanium dioxide produced maximum removal of 98.4% after 60 min, which follows second-order kinetics. All other metal/metal oxides coupled with titanium dioxide followed pseudo-first-order kinetics. Figure 5 provides the mechanistic pathway of dye degradation pathway with titanium dioxide as photocatalyst. A multi-metal oxide including catalyst (titanium dioxide–cadmium oxide-zinc oxide) loaded on silver nanoparticles in the presence of reduced graphene oxide was synthesized, and its photocatalytic efficacy on methylene blue degradation (Akyüz 2021) was explored and found 91% degradation within 15 min under ultraviolet irradiation, mainly attributed to decrease in the wide band gap of titanium dioxide due to the presence of multi-transition oxides. Similar strategy was used to degrade azo dye reactive violet 5 dye by titanium dioxide nanoparticles (Chung and Chen 2009).
Fig. 5.
Mechanism of dye degradation by titanium dioxide. Upon light induction the excitation of electrons occurs from valence band to conduction band. This triggers the formation of hydroxyls ions. Oxygen compounds also get converted into hydroxyl groups via hydrogen peroxide formation. This hydroxyl groups further help in formation of reduced and oxidized compounds upon dye degradation
Removal of microplastics
In our day-to-day life, plastics took an exceptional place due to their less weight, cheapness, versatility and high durability (Morin-Crini et al. 2022; Alqahtani and Zafar 2021). On the other hand, disposal of used plastics is a big challenge as only just 14% of used plastics are recycled while others enter into our environment in one way or another. More than 8 million metric tons of used plastics are entering into water bodies, every year (MacArthur 2017). Accumulation of these plastics in seas and oceans would surpass the amount of fish present in oceans in near future. These used plastics were decomposed due to physical degradation in the presence of sunlight, micro-organisms into micro-plastics (size less than 5 μm) and nano-plastics (size less than 1 μm) (Koelmans et al. 2015). Degradation of micro-plastics using conventional wastewater purification methodologies has difficulties in control and complete elimination (Moore 2008). TiO2 has been used widely in recent years to degrade these micro-plastics. The widely found micro-plastics such as polyethene and polystyrene can be completely degraded using Triton-based titanium dioxide nanoparticle films and ultraviolet light, through photocatalytic mineralization (Nabi et al. 2020b).
Wang et al. (2019) prepared a novel magnetic Au@Ni@TiO2 micromotor, by applying 10 nm thickness of nickel layer on titanium dioxide particles, covered with a 30-nm layer of gold, for the removal of microplastics present in water and hydrogen peroxide under ultraviolet light. This motor was found to have a velocity of 14.72 and 65.52 μm S−1 in water and 0.1% hydrogen peroxide solution, respectively. They showcased the efficacy of this micromotor on the degradation of microplastics from personal care products as well as river water and reported 67% of clearage efficiency in pure water. A faster elimination can be achieved by adding a low percentage of hydrogen peroxide to pure water.
The use of ultraviolet light, as well as hydrogen peroxide, makes this fuel-free micromotor less attractive. Some research works to overcome this issue were performed by researchers. The recent works dealing with titanium dioxide-based Janus micromotors are listed in Table 4. Recently, Janus cobalt oxide–titanium dioxide micro-swimmers driven by low intensity–ultraviolet free visible light present inside water were prepared and tested (Sridhar et al. 2020). These micro-swimmers propel by breaking down the water molecules into oxygen and oxide radicals, which enabled them to be applicable in photocatalytic reactions as well as drug delivery. The mean velocity of this micro-swimmer was found to be 11.5 μm S−1 at the intensity of 6 mW cm−2. It can also be accelerated under a magnetic field due to the presence of cobalt oxide.
Table 4.
Titanium dioxide-based Janus micromotors propelled by different propellants for the removal of microplastics
| Substrate | Conditions | Results | Reference |
|---|---|---|---|
| Titanium dioxide/water-soluble conjugated polyelectrolyte/glucose oxidase | Visible light; hydrogen peroxide produced through the decomposition of glucose using glucose oxidase | Maximum velocity of 7.49 μm S−1 observed under visible light | Noh et al. (2021) |
| Hedgehog-shaped titanium dioxide/functional multiwall carbon nanotubes | Ultraviolet light illumination | Velocity of 8.9 μm S−1 is achieved under 160 mW cm−2 ultraviolet light illumination | Jiang et al. (2021) |
| Titanium dioxide/gold nanowire-based motor | Ultraviolet irradiation | A velocity of 5.6 ± 1.5 μm S−1 is achieved in deionized water while it was 2.41 ± 0.53 µm S−1 and 2.27 ± 0.35 µm S−1, respectively at pH = 4 and 10 | Chen et al. (2021a) |
| Titanium dioxide/manganese dioxide | Hydrogen peroxide decomposed to oxygen bubbles, which propels the micromotor acts as fuel | Maximum speed of 48.1 μm S−1 at [hydrogen peroxide] = 30% with maximum instantaneous velocity of 135 μm S−1 | Ge et al. (2019) |
Electrocatalysis for energy
Currently, heteroatom doping technology has emerged as an intriguing technique to enhance electrocatalytic efficiency (Ochedi et al. 2021; Kumar et al. 2021). An inexpensive and novel catalyst without using noble metals, namely carbon, nitrogen–zeolitic imidazolate framework/TiFe (that is carbon and nitrogen derived from zeolitic imidazolate framework-8 embedded in titanium dioxide/ferric oxide) was found to exhibit superior electrocatalytic performance towards water splitting, oxygen evolution reactions and hydrogen evolution reactions (Vattikuti et al. 2021). This synthesized catalyst has more excellent catalytic power than that of zeolitic imidazolate framework/TiFe and TiFe nanostructures, towards oxygen evolution reactions and hydrogen evolution reactions. The overpotential was found to be 290 mV and 291 mV for oxygen evolution reactions and hydrogen evolution reactions, respectively, to deliver the benchmark current density of 10 mA cm−2 under alkaline conditions. This was attributed to the addition of highly active carbon and nitrogen from zeolitic imidazolate framework with titanium dioxide/ferric oxide, which enhanced the rate of water splitting.
Similarly, numerous researchers made innovations in making novel catalysts to enhance the electrocatalytic processes and some of these novel catalysts recently reported are listed in Table 5. Another novel catalyst using reduced titanium dioxide-supported ruthenium nanocatalyst was developed with more oxygen vacancies for hydrogen evolution (Chen et al. 2021b). This nanocatalyst required very less overpotential (15 mV) to deliver the current density of 10 mA cm−2 under alkaline conditions. This superior performance was attributed to synergistic water splitting and weakening of OH· adsorption.
Table 5.
Titanium dioxide-based substrates for electrocatalysis for energy. Nb-MoS2: niobium-molybdenum disulfide, TiO2: titanium dioxide, Ti3C2Tx: titanium carbide, Co3O4: cobalt oxide, Ag3PO4: Tri-silver phosphate, Bi2WO6: bismuth tungstate
| Catalyst | Overpotential and other remarks | References |
|---|---|---|
| Cobalt, Nb-MoS2 nanosheets shelled in micro-TiO2 hollow spheres |
• Hydrogen evolution reaction = 58.8 mV and oxygen evolution reaction = 260.0 mV to deliver current density of 10 mA cm−2 • Catalyst needed an operating voltage of 1.57 V to achieve this current density |
Nguyen et al. (2021) |
| Ag3PO4-Bi2WO6-TiO2 |
• Oxygen evolution reaction = 360 mV, this nanohybrid was stable and exhibited a remarkable efficiency of 99.8% for the oxygen evolution reaction • Highly efficient in comparison with individual components from where it was derived |
Mandari et al. (2021) |
| TiO2/Co3O4 composite |
• Oxygen evolution reaction = 270 mV (at 10 mA cm−2), with a Tafel slope of 60 mV dec−1 and found that overpotential decreases with an increase in titania content in the composite • This composite is highly stable for 45 h |
Aftab et al. (2021) |
| Ti3C2Tx (MXene) decorated by phosphorus-doped TiO2 (P–TiO2@Ti3C2) |
• Less overpotential of 97 mV (at 10 mA cm−2) and a low Tafel slope of 48.4 mV dec−1, which performed more efficient than that in darkness • The catalyst was found to be more stable for more than 50 h under light irradiation and alkaline conditions. Outperformed than Mxene and corresponding derived materials |
Deng et al. (2021) |
| Palladium@TiO2–(hollow) core–shell |
• This novel catalyst (with a trace of Pd—0.05 wt%) required the lowest overpotential of 0.43 V at 10 mA cm−2 with a Tafel slope of 63 mV dec−1 • Outperformed that of the commercial TiO2 (0.92 V, 636 mV dec−1) |
Shu et al. (2021) |
Antimicrobial activity
In recent years, the spreading of infectious diseases such as Ebola, and severe acute respiratory syndrome coronavirus 2 (SARS), is very rapid (COVID-19 in particular) and the health department across the world is working hard to contain these diseases. So, it is highly needed to disinfect the high touch surfaces, such as sinks, faucets, handrails, doorknobs and in public places, as these are acknowledged as a pool of agents causing a wide range of infections (Huslage et al. 2010). Numerous antibacterial/antiviral chemicals were used to sanitize these high touch surfaces, which needs more manpower and chemical use. Thus, the use of self-disinfecting materials on these surfaces is one of the best options to prevent the spreading of COVID-19 and other infectious diseases (Mathew et al. 2020). Many studies made in this decade elucidated the antibacterial efficacy of photocatalysts (Mahmood et al. 2012; Lin et al. 2013; McEvoy and Zhang 2014). These photocatalyst-based substrates gained more attention from the research community due to the direct utilization of sunlight or in some cases, ultraviolet irradiation. Among the photocatalysts employed, TiO2 is the most extensively used photocatalyst for antimicrobial applications (Foster et al. 2011; Yousef et al. 2015; Mathew et al. 2018).
Very recently, the effective use of titanium dioxide on disinfecting the SARS-CoV-2 virion (severe acute respiratory syndrome coronavirus 2) present in the air, as well as the liquid, was tested (Matsuura et al. 2021). The study elucidated the efficacy of titanium dioxide-mediated photocatalytic reaction on this virion that about 99% reduction in infectivity in aerosol after 20 min of treatment while it took 120 min for achieving this level in liquids. This disinfection happened due to the damage caused to the viral proteins and the genome through the mechanistic effects of titanium dioxide. Similarly, the research community performed numerous innovative works since 2020 to contain the covid-19 pandemic across the world and some of these works using titanium dioxide-based substrates are listed in Table 6.
Table 6.
Methodologies to remove SAR-CoV-2 using titanium dioxide-based substrates. TiO2: titanium dioxide, Al2O3: Aluminium oxide IC50: inhibitory concentration, CC50: cytotoxic concentration, SARS: severe acute respiratory syndrome, CoV: coronavirus
| Target organism | Materials employed | Methodology | Results | References |
|---|---|---|---|---|
| SARS-CoV-2 spike protein | TiO2 and Al2O3 | Adsorption | Adsorption on TiO2 proceeds about one order of magnitude faster than that of Al2O3 | Xin et al. (2021) |
|
SARS-CoV-2: (1) Spike pseudo-typed virions (2) fully infectious virus |
TiO2 and TiO2-silver coated on wall tiles | Free radical attack on viruses | Spike viral load was decreased by four orders of magnitude after 1 h, while no active virus was found after 5 h, no significant difference was observed between TiO2 and TiO2-silver | Micochova et al. (2021) |
| SARS-CoV-2 pseudo-virus | TiO2 supported silver—single atom nano enzyme (silver-TiO2 SAN) | Adsorption and then reactive oxygen species attack | Silver-TiO2 SAN produced maximum adsorption (99.65%) than nano-TiO2, silver | Wang et al. (2021a) |
| A broad range of pathogens including SAR-CoV-2, Hepatitis C | Nanosized TiO2 | Hydroxyl attack on viral Ribo nucleic acid genome | At low irradiation, TiO2 inhibited SAR-CoV-2, Hepatitis C and other pathogens | Tong et al. (2021) |
| SAR-CoV-2 cells | TiO2 nanoparticles and nanotubes | Reactive oxygen species and titanium radicals damaged proteins, Deoxy Ribonucleic acids and lipid | TiO2-nanoparticles and nanotubes have potent antiviral activity at very low concentration (IC50 = 568.6 ng/mL), with a weak cytotoxic effect on the cellular host (CC50 = 399.1 ng/mL), where CC50and IC50 denotes cytotoxic half concentration and half maximal inhibitory concentration, respectively | Hamza et al. (2021) |
A significant enhancement in the antibacterial property of titanium dioxide was observed with the addition of chalcogens such as sulphur, selenium and tellurium (Mathew et al. 2020). Among these three chalcogens, tellurium-doped titanium dioxide stood first on antimicrobial behaviour against E. coli as complete disinfection was achieved within 70 min of light irradiation while the other two chalcogens when doped with titanium dioxide produced little less activity (took 90 min for complete disinfection).
Similarly, cerium-doped titanium dioxide nanoparticles deposited on reduced graphene oxide were found to enhance the microbial activity as well as photocatalytic behaviour under visible light (Behera et al. 2021). This is attributed to the fact that the presence of cerium increases the absorption of titanium from ultraviolet to visible light. This novel substrate containing cerium was found to be more effective, under visible light, in disinfecting human pathogenic bacteria as well as degradation of two pesticides, namely quinalphos and imidacloprid, in comparison with the conventional titanium dioxide catalyst.
The use of zinc oxide, titanium dioxide and silicon dioxide-based nanomaterials on creating protective coatings against biodeterioration was getting momentum in recent years. In another study, titanium dioxide induces membrane rupture (Ranjan and Ramalingam 2016). Therefore, there is a need to compare the efficacy of these substates on antimicrobial activity. In a recent study, these nanomaterials were tested and compared against eight different types of micro-organisms that are commonly found on building material surfaces such as Bacillus subtilis, Aspergillus niger, Aspergillus terreus, Aureobasidium pullulans, Cladosporium cladosporioides, Penicillium ochrochloron, Trichoderma viride, Paecilomyces variotii (Dyshlyuk et al. 2020). Among these three nanomaterials studied, zinc oxide with particle sizes 2–7 mm was found to be most efficient against these micro-organisms.
Nanomedicine
Nanoparticle-based drugs have been introduced in recent years to increase the efficiency of treatment strategies in oncology in treating cancer cells (Umapathi et al. 2021; Patel et al. 2021; Sharma et al. 2017). In a recent study, the autophagic potential of titanium dioxide nanoparticles was explored in increasing the chemotherapeutic effect of 5-fluorouracil in human AGS gastric cells (human gastric adenocarcinoma cell line) (Azimee et al. 2020). The production of reactive oxygen species by titanium dioxide impaired the lysosomal function, which leads to a block in autophagy flux in AGS cells. A microlevel addition (1 μg/ml) of these titanium dioxide nanoparticles enhanced the cytotoxic as well as apoptotic effects of 5-fluorouracil.
A case study was performed by a team of researchers on the treatment of diabetic foot ulcers using catalytic nanomedicine containing copper/titanium dioxide–silicon dioxide as it was estimated that around 20% of diabetic patients will develop foot ulcers over their lifetime (López-Goerne et al. 2019). These works observed a significant improvement healing process on the first application itself, and the infection was limited. Further, the regeneration of tissue was enhanced due to its use.
In dentistry, the research community is in search of an efficient method to reduce bacterial adhesion on archwires and around the orthodontic appliance to restrict enamel decalcification as well as periodontal diseases. In this area, one study was performed to clinically test the efficacy of titanium dioxide nanostructure coating on stainless steel orthodontic wires towards the growth of most found bacteria in dentistry, namely Streptococcus mutans (Mollabashi et al. 2020). It was found that decreased adhesion of S. mutans was found on tested orthodontic wires which is attributed to the fact that, the damage in the cell wall of this micro-organism due to titanium dioxide leads to changes in osmotic pressure; subsequently, the cell organelles were destroyed. However, further studies are needed in this area to investigate the mechanical stability of titanium dioxide-coated stainless steel orthodontic wires under mechanical loading during the dental treatment.
Removal of pharmaceutical and personal care products
Pharmaceuticals and personal care products (PPCPs) are substances that are man-made for human and/or animal healthcare and medical purposes (Jiang et al. 2013; Omar et al. 2016; Yang et al. 2017). These substances can be classified into a different wide range of products such as antibiotics, antifungal, antiemetics, antineoplastics, vaccinations, contrast agents, sedatives, anticonvulsants, nonsteroidal anti-inflammatory drugs, hormones, lipid regulators, painkillers, b-blockers, preservatives, disinfectants, insect repellents, fungicides, soaps and detergents, fragrances, sunscreen ultraviolet filters (Yang et al. 2017). More than 3000 pharmaceuticals and personal care products have been produced as of now (Šauer et al. 2019; Chen et al. 2016) and the constant development of new chemical compounds to contain different newly arising diseases such as COVID-19, Ebola, severe acute respiratory syndrome, led to an increasing abundance and variety of pharmaceuticals and personal care products in the environment. Several methodologies were implemented to reduce the load caused by pharmaceuticals and personal care products, and some of the titanium dioxide-based methodologies proposed in recent studies are listed in Table 7 (Saravanan et al. 2020; Ajiboye et al. 2021; He et al. 2021).
Table 7.
Advanced titanium dioxide-based substrates for the removal of pharmaceutical and personal care products. PPCPs: pharmaceutical and personal care products, C3N4: carbon nitride, TiO2: titanium dioxide, Fe3O4: iron oxide, SiO2: silicon dioxide, BiOCl: bismuth oxychloride
| TiO2 based substrate | Target Pollutant | Results | References |
|---|---|---|---|
| 2,5-Bis (tributylstannyl) thiophene-perylene diimide-T@TiO2 (Bis-PDI-T@TiO2) composite | Carbamazepine (CBZ) (a typical PPCP) | Complete degradation of CBZ (dosage: 5 ppm) achieved after 30 min under visible light, in presence of persulfate | Yang et al. (2021) |
| Copper oxide/TiO2 nanoparticle coated ceramic ultrafiltration membrane | Phthalates and parabens from synthetic systems (10–1000 ppb concentration) | More than 99% removal achieved | Bhattacharya et al. (2021) |
| Ultra-thin, defect rich copper-doped TiO2nanosheets with rich oxygen vacancies | Tetracycline and acetaminophen | Nearly 100% removal was achieved with both the pollutants using photocatalyst containing 4% copper, after 100 min of visible light irradiation | Qu et al. (2021) |
| BiOCl; TiO2 | Atenolol and ibuprofen | BiOCl degraded ibuprofen 15 times faster than TiO2 while TiO2 degraded atenolol 2.2 times faster than BiOCl, under ultraviolet irradiation (254 nm) | Speller (2021) |
| Ternary film of Fe2O3–TiO2 Polyvinyl pyrrolidine coated on a glass tube | Triclosan | 83.27% of degradation efficiency was observed at optimum conditions under solar irradiation | Pragada and Thalla (2021) |
| Terephthalic acid-functionalized g–C3N4/TiO2/Fe3O4@SiO2 heterojunction nano-photocatalyst | Ibuprofen, benzophenone-3, carbamazepine in Real sewage effluent | Ibuprofen after 120 min = 97%, benzophenone – 3 after 150 min = 94%, carbamazepine after 240 min = 94%; under visible light irradiation | Kumar et al. (2020) |
Oil recovery
Many oilfields are employing alkali/surfactant/polymer to enhance the oil recovery across the world, which produces much better results than water flooding. Production of highly stable emulsion (which contains residual oil in the water phase) leads to a decrease in the efficacy of the recovery process. The presence of titanium dioxide was found to enhance the oil recovery due to many reasons. (1) Titanium dioxide is intrinsically hydrophilic (Choi et al. 2017; Kameya and Yabe 2019), the attraction of water molecules increased the water layer and thereby the residual emulsion decreased; (2) improved coalescence between adjacent oil droplets; and (3) improved behaviour of oil/water interfacial film (Kang et al. 2012). Khan et al. (2021) observed 75% clarity in the separated aqueous phase in the presence of titanium dioxide while it was 45% without titanium dioxide, which leads to a 19% overall increase in oil/water separation due to the presence of titanium dioxide.
A newly developed titanium dioxide/silicon dioxide/poly(acrylamide) nanocomposites using pomegranate seeds has shown better results in the enhanced oil recovery process in carbonate reservoirs (Ali et al. 2021). These nanocomposites (1500 ppm) when mixed with smart nanofluid (5000 ppm of calcium sulphate and calcium chloride ions) demonstrated the highest performance in oil recovery from 36% to 46.53% original oil in place due to a reduction in interfacial tension and wettability alteration.
In addition to enhanced oil recovery, research broadened the application of titanium dioxide in different areas such as (1) it can also be used as an excellent insulator which leads to better energy saving in the oil recovery process; (2) it can be used in the purification of recovered oily organic substances from waste liquids or accidental oil discharges in marine or continental situations.
The titanium dioxide–silicon dioxide composite was found to have excellent infrared radiation shield capability, and thus the composite can be used as a thermal insulator for high-temperature steam pipes used in oil recovery. This glass fibre/titanium dioxide–silicon dioxide composite significantly reduces the thermal conductivity by 13.1% and 23.9% at 300 °C and 400 °C, respectively (Liu et al. 2021).
Recently, super-hydrophobic F-TiO2@polypropylene membranes were prepared from polypropylene, trifluorofluorooctyl methacrylate and sol–gel-derived titanium dioxide with vinyltriethoxysilane by Zhu et al. (2021) to recover oily organic components from waste liquids. This membrane was found to enhance the water contact angle up to 157°, and thus, the water removing efficiency was found to be more than 99.7%.
Degradation of pesticides
The residues of pesticides even at minute levels can cause severe harmful effects on human and animal health (Sakkas et al. 2005; Bamba et al. 2008; Colombo et al. 2012; Kaur and Goyal 2019), and the condition still worsens with bioaccumulation of these pesticides in long run (Garcia et al. 2006; Bamba et al. 2008). Many studies (Kaur and Goyal 2019) were performed in recent years to degrade this pesticide, and some recent work is listed in Table 8.
Table 8.
Degradation of pesticides using titanium dioxide-based substrates. Fe3O4: ferric oxide, TiO2: titanium dioxide, LaFeO3: lanthanum iron oxide
| Pollutant | Substrate | Results | References |
|---|---|---|---|
| Atrazine and dimethoate residues in three samples as deionized water, wastewater and agricultural wastewater containing these residues | TiO2 in the presence of ultraviolet irradiation of (1) 254 nm and (2) 306 nm | Complete degradation is achieved after 12 h under 306 nm ultraviolet irradiation; dimethoate degrades more easily than atrazine | EL-Saeid et al. (2021) |
| Monocrotophos (dimethyl (E)-1-methyl-2-(methyl carbamoyl)vinyl phosphate) | MIL-88(IRON) anchored TiO2-chitosan(2 dimensional) hybrid nanocomposite | 98.79% degradation observed at optimized conditions (within 30 min) under visible light irradiation | Vigneshwaran et al. (2021) |
| Atenolol removal from domestic wastewater effluent | Iron doped with TiO2 nanoparticles, synthesized using Acacia Catechu pods | A maximum of 85% of atenolol was degraded under visible light for 105 min at pH = 9 | Bhuvaneswari et al. (2021) |
| Herbicide 2,4-dichlorophenoxyacetic acid (2,4D), and the insecticide imidacloprid (1-(6-chloro-3-pyridinylmethyl)-Nnitro-2-imidazolidinimine) (IM) | Molecularly imprinted (MI) TiO2 powder with pesticides (TiO2 MI/2,4-D; TiO2 MI/IM) | At 368 nm ultraviolet irradiation, TiO2 MI/2,4-D produced 6 times faster degradation of 2,4-D in comparison with bare TiO2, while TiO2 IM/IM produced 2 times faster degradation of IM in comparison with bare TiO2 | Fiorenza et al. (2020) |
| Fungicide myclobutanil (C15H17ClN4) | LaFeO3@TiO2 heterojunction photocatalysts |
• Complete removal achieved after 180 min under solar light • 85% removal was observed after 240 min using each pure substrate, viz. LaFeO3 and TiO2 |
Garcia-Muñoz et al. (2020) |
| Zoxamide [3, 5-dichloro-N-(3-chloro, 1-ethyl, 1-methyl, 2-oxopropyl)-4 methyl benzamide] | Titanium dioxide nanoparticles (TiO2nanoparticles) Synthesized using aqueous leaf extract of Trema Orientalis |
• The lowest concentration (105 mg/L) of zoxamide exhibits the highest degradation rate (0.32048 h−1) • Degradation follows pseudo-first-order kinetics under ultraviolet irradiation |
Purkait et al. (2020) |
| Atrazine degradation | Magnetic Fe3O4-TiO2/graphene oxides nanocomposite |
• This nano-enzyme has a dual role, as highly selective in detecting and degrading atrazine • Within 40 min, 100% degradation was achieved in acidic conditions (pH = 3) under sunlight |
Boruah and Das (2020) |
Conclusion
Photocatalysis with titanium dioxide (TiO2) is highly recommended for effective wastewater treatment as this technique removes pollutants to a greater extent than conventional physical techniques such as adsorption and coagulation. Titanium dioxide nano-catalysts are commonly synthesized by physiochemical, e.g. solgel technique, sonochemical and microwave-assisted technique, oxidation technique, deposition technique, hydro/solvothermal techniques, and biological methods. Physical characteristics of titanium dioxide nanoparticles are strongly dependent on the synthesis technique adopted.
Acknowledgements
Authors wish to thank Sathyabama Institute of Science and Technology for the support.
Abbreviations
- BTEX
Benzene toluene ethylbenzene and xylene
- SARS
Severe acute respiratory syndrome
- PPCP
Pharmaceutical and personal care products
Declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abeledo-Lameiro MJ, Ares-Mazás E, Gómez-Couso H. Evaluation of solar photocatalysis using TiO2 slurry in the inactivation of Cryptosporidium parvum oocysts in water. J Photochem Photobiol B Biol. 2016;163:92–99. doi: 10.1016/j.jphotobiol.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Aftab U, Tahira A, Gradone A, et al. Two step synthesis of TiO2–Co3O4 composite for efficient oxygen evolution reaction. Int J Hydrogen Energy. 2021;46:9110–9122. doi: 10.1016/j.ijhydene.2020.12.204. [DOI] [Google Scholar]
- Ağçeli G, Hammachi H, Kodal S, et al. A novel approach to synthesize TiO2 nanoparticles: Biosynthesis by using Streptomyces sp. HC1. J Inorg Organomet Polym Mater. 2020;30:3221–3229. doi: 10.1007/S10904-020-01486-W. [DOI] [Google Scholar]
- Ajiboye TO, Oyewo OA, Onwudiwe DC (2021) Photocatalytic removal of parabens and halogenated products in wastewater: a review. Environ Chem Lett 1–31
- Akyüz D. rGO-TiO2-CdO-ZnO-Ag photocatalyst for enhancing photocatalytic degradation of methylene blue. Opt Mater (amst) 2021;116:111090. doi: 10.1016/j.optmat.2021.111090. [DOI] [Google Scholar]
- Ali JA, Kolo K, Manshad AK, Stephen KD. Emerging applications of TiO2/SiO2/poly (acrylamide) nanocomposites within the engineered water EOR in carbonate reservoirs. J Mol Liq. 2021;322:114943. doi: 10.1016/j.molliq.2020.114943. [DOI] [Google Scholar]
- Alqahtani FK, Zafar I. Plastic-based sustainable synthetic aggregate in Green Lightweight concrete—a review. Constr Build Mater. 2021;292:123321. doi: 10.1016/J.Conbuildmat.2021.123321. [DOI] [Google Scholar]
- Ao CH, Lee SC, Yu JZ, Xu JH. Photodegradation of formaldehyde by photocatalyst TiO2: effects on the presences of NO, SO2 and VOCs. Appl Catal B Environ. 2004;54:41–50. doi: 10.1016/J.apcatB.2004.06.004. [DOI] [Google Scholar]
- Arconada N, Durán A, Suárez S, et al. Synthesis and photocatalytic properties of dense and porous TiO2-anatase thin films prepared by sol–gel. Appl Catal B Environ. 2009;86:1–7. doi: 10.1016/J.apcatB.2008.07.021. [DOI] [Google Scholar]
- Azimee S, Rahmati M, Fahimi H, Moosavi MA. TiO2 nanoparticles enhance the chemotherapeutic effects of 5-fluorouracil in human AGS gastric cancer cells via autophagy blockade. Life Sci. 2020;248:117466. doi: 10.1016/j.lfs.2020.117466. [DOI] [PubMed] [Google Scholar]
- Bamba D, Atheba P, Robert D, et al. Photocatalytic degradation of the diuron pesticide. Environ Chem Lett. 2008;6:163–167. doi: 10.1007/S10311-007-0118-X/TABLES/2. [DOI] [Google Scholar]
- Bao S, Lei C, Xu M, et al. Environment-friendly biomimetic synthesis of TiO2 nanomaterials for photocatalytic application. Nanotechnology. 2012 doi: 10.1088/0957-4484/23/20/205601. [DOI] [PubMed] [Google Scholar]
- Barahimi V, Taheri RA, Mazaheri A, Moghimi H. Fabrication of a novel antifouling TiO2/CPTES/metformin-PES nanocomposite membrane for removal of various organic pollutants and heavy metal ions from wastewater. Chem Pap. 2020;74:3545–3556. doi: 10.1007/s11696-020-01178-2. [DOI] [Google Scholar]
- Behera L, Barik B, Mohapatra S. Improved photodegradation and antimicrobial activity of hydrothermally synthesized 0.2 Ce-TiO2/RGO under visible light. Colloids Surfaces A Physicochem Eng Asp. 2021;620:1265. doi: 10.1016/j.colsurfa.2021.126553. [DOI] [Google Scholar]
- Bhattacharya P, Mukherjee D, Deb N, et al. Indigenously developed CuO/TiO2 coated ceramic ultrafiltration membrane for removal of emerging contaminants like phthalates and parabens: toxicity evaluation in PA-1 cell line. Mater Chem Phys. 2021;258:123920. doi: 10.1016/j.matchemphys.2020.123920. [DOI] [Google Scholar]
- Bhuvaneswari R, Jeyanthi J, Kumar M. Visible light assisted degradation of atenolol by Fe-TiO2: synthesis, characterization, optimization and mechanism. Optik (stuttg) 2021;239:166658. doi: 10.1016/j.ijleo.2021.166658. [DOI] [Google Scholar]
- Boruah PK, Das MR. Dual responsive magnetic Fe3O4-TiO2/graphene nanocomposite as an artificial nanozyme for the colorimetric detection and photodegradation of pesticide in an aqueous medium. J Hazard Mater. 2020;385:121516. doi: 10.1016/j.jhazmat.2019.121516. [DOI] [PubMed] [Google Scholar]
- Boutillier S, Fourmentin S, Laperche B (2021) History of titanium dioxide regulation as a food additive: a review. Environ Chem Lett 1–17
- Cabanas-Polo S, Boccaccini AR. Electrophoretic deposition of nanoscale TiO2: technology and applications. J Eur Ceram Soc. 2016;2:265–283. doi: 10.1016/J.Jeurceramsoc.2015.05.030. [DOI] [Google Scholar]
- Cabello G, Davoglio RA, Pereira EC. Microwave-assisted synthesis of anatase-TiO2 nanoparticles with catalytic activity in oxygen reduction. J Electroanal Chem. 2017;794:36–42. doi: 10.1016/J.Jelechem.2017.04.004. [DOI] [Google Scholar]
- Camarillo R, Tostón S, Martínez F, et al. Enhancing the photocatalytic reduction of CO2 through engineering of catalysts with high pressure technology: Pd/TiO2 photocatalysts. J Supercrit Fluids. 2017;123:18–27. doi: 10.1016/J.Supflu.2016.12.010. [DOI] [Google Scholar]
- Chava RK, Lee W-M, Oh S-Y, et al. Improvement in light harvesting and device performance of dye sensitized solar cells using electrophoretic deposited hollow TiO2 NPs scattering layer. Sol Energy Mater Sol Cells. 2017;161:255–262. doi: 10.1016/j.solmat.2016.11.037. [DOI] [Google Scholar]
- Chen X. Titanium dioxide nanomaterials and their energy applications. Chin J Catal. 2009;30:839–851. doi: 10.1016/S1872-2067(08)60126-6. [DOI] [Google Scholar]
- Chen X, Mao SS. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev. 2007;107:2891–2959. doi: 10.1021/CR0500535. [DOI] [PubMed] [Google Scholar]
- Chen Y, Vymazal J, Březinová T, et al. Occurrence, removal and environmental risk assessment of pharmaceuticals and personal care products in rural wastewater treatment wetlands. Sci Total Environ. 2016;566:1660–1669. doi: 10.1016/j.scitotenv.2016.06.069. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang M, Han J, Guo R. TiO2 nanosheet/NiO nanorod hierarchical nanostructures: p–n heterojunctions towards efficient photocatalysis. J Colloid Interface Sci. 2020;562:313–321. doi: 10.1016/J.JCIS.2019.12.031. [DOI] [PubMed] [Google Scholar]
- Chen B, Liu L, Liu K, et al. Photoelectrochemical TiO2-Au-nanowire-based motor for precise modulation of single-neuron activities. Adv Funct Mater. 2021;31:2008667. doi: 10.1002/ADFM.202008667. [DOI] [Google Scholar]
- Chen LN, Wang SH, Zhang PY, et al. Ru nanoparticles supported on partially reduced TiO2 as highly efficient catalyst for hydrogen evolution. Nano Energy. 2021;88:106211. doi: 10.1016/J.NANOEN.2021.106211. [DOI] [Google Scholar]
- Choi SK, Son HA, Kim HT, Kim JW. Nanofluid enhanced oil recovery using hydrophobically associative zwitterionic polymer-coated silica nanoparticles. Energy Fuels. 2017;31:7777–7782. doi: 10.1021/acs.energyfuels.7b00455. [DOI] [Google Scholar]
- Chung Y-C, Chen C-Y. Degradation of azo dye reactive violet 5 by TiO2 photocatalysis. Environ Chem Lett. 2009;7:347–352. doi: 10.1007/s10311-008-0178-6. [DOI] [Google Scholar]
- Collins M. Future trends in microwave synthesis. Future Med Chem. 2010;2:151–155. doi: 10.4155/FMC.09.133. [DOI] [PubMed] [Google Scholar]
- Colombo A, Cappelletti G, Ardizzone S, et al. Bisphenol A endocrine disruptor complete degradation using TiO2 photocatalysis with ozone. Environ Chem Lett. 2012;10:55–60. doi: 10.1007/S10311-011-0328-0. [DOI] [Google Scholar]
- Daghrir R, Drogui P, Robert D. Modified TiO2 for environmental photocatalytic applications: a review. Ind Eng Chem Res. 2013;52:3581–3599. doi: 10.1021/IE303468T. [DOI] [Google Scholar]
- de Freitas AM, Sirtori C, Peralta-Zamora P. Photoelectrocatalytic degradation of camphor on TiO2/RuO2 electrodes. Environ Chem Lett. 2011;9:97–102. doi: 10.1007/S10311-009-0252-8. [DOI] [Google Scholar]
- de Lima BRM, do Nascimento NMP, Zamian JR, et al. Higher dye degradation using a visible-light photocatalyst made of mesoporous graphitic carbon nitride prepared with the Tween-40 surfactant. Environ Chem Lett. 2020;18:1413–1422. doi: 10.1007/S10311-020-01008-7. [DOI] [Google Scholar]
- Deng L, Chang B, Shi D, et al. MXene decorated by phosphorus-doped TiO2 for photo-enhanced electrocatalytic hydrogen evolution reaction. Renew Energy. 2021;170:858–865. doi: 10.1016/j.renene.2021.02.040. [DOI] [Google Scholar]
- Devahasdin S, Fan C, Li K, Chen DH. TiO2 photocatalytic oxidation of nitric oxide: transient behavior and reaction kinetics. J Photochem Photobiol A Chem. 2003;156:161–170. doi: 10.1016/S1010-6030(03)00005-4. [DOI] [Google Scholar]
- Dhiflaoui H, Khlifi K, Cheikh larbi A. Effect of deposition parameters on electrophoretically deposited TiO2. Res Rev J Mater Sci. 2016;4:7–15. doi: 10.4172/2321-6212.1000148. [DOI] [Google Scholar]
- Diao W, Xu J, Rao X, Zhang Y. Facile synthesis of fluorine doped rutile TiO2 nanorod arrays for photocatalytic removal of formaldehyde. Catal Lett. 2021;2021(1):1–11. doi: 10.1007/S10562-021-03700-X. [DOI] [Google Scholar]
- Dyshlyuk L, Babich O, Ivanova S, et al. Antimicrobial potential of ZnO, TiO2 and SiO2 nanoparticles in protecting building materials from biodegradation. Int Biodeterior Biodegrad. 2020;146:104821. doi: 10.1016/j.ibiod.2019.104821. [DOI] [Google Scholar]
- Edmundson M, Capeness M, Horsfall L. Exploring the potential of metallic nanoparticles within synthetic biology. N Biotechnol. 2014;31:572–578. doi: 10.1016/J.NBT.2014.03.004. [DOI] [PubMed] [Google Scholar]
- EL-Saeid MH, Alotaibi M, Alshabanat M, et al. Impact of photolysis and TiO2 on pesticides degradation in wastewater. Water. 2021;13:655. doi: 10.3390/w13050655. [DOI] [Google Scholar]
- Elbarbary AM, Gad YH (2021) Radiation synthesis and characterization of poly (vinyl alcohol)/acrylamide/TiO2/SiO2 nanocomposite for removal of metal ion and dye from wastewater. J Inorg Organomet Polym Mater 1–23
- Esposito S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials (basel) 2019;12:668. doi: 10.3390/ma12040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo C, Saccà ML, Costa G, et al. Impact of Ag and Al2O3 nanoparticles on soil organisms: in vitro and soil experiments. Sci Total Environ. 2014;473–474:254–261. doi: 10.1016/J.SCITOTENV.2013.12.043. [DOI] [PubMed] [Google Scholar]
- Fernández-Ibáñez P, Sichel C, Polo-López MI, et al. Photocatalytic disinfection of natural well water contaminated by Fusarium solani using TiO2 slurry in solar CPC photo-reactors. Catal Today. 2009;144:62–68. doi: 10.1016/J.CATTOD.2009.01.039. [DOI] [Google Scholar]
- Fiorenza R, Di Mauro A, Cantarella M, et al. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. Chem Eng J. 2020;379:122309. doi: 10.1016/j.cej.2019.122309. [DOI] [Google Scholar]
- Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Muñoz P, Fresno F, Ivanez J, et al. Activity enhancement pathways in LaFeO3@ TiO2 heterojunction photocatalysts for visible and solar light driven degradation of myclobutanil pesticide in water. J Hazard Mater. 2020;400:123099. doi: 10.1016/j.jhazmat.2020.123099. [DOI] [PubMed] [Google Scholar]
- Garcia A, Amat AM, Arques A, et al. Detoxification of aqueous solutions of the pesticide “Sevnol” by solar photocatalysis. Environ Chem Lett. 2006;3:169–172. doi: 10.1007/s10311-005-0026-x. [DOI] [Google Scholar]
- Gavrilović T V, Jovanović DJ, Dramićanin MD (2018) Synthesis of multifunctional inorganic materials: from micrometer to nanometer dimensions. In: Nanomaterials for green energy. Elsevier, pp 55–81
- Ge Y, Wang T, Zheng M, et al. Controlled one-sided growth of Janus TiO2/MnO2 nanomotors. Nanotechnology. 2019;30:315702. doi: 10.1088/1361-6528/ab19c7. [DOI] [PubMed] [Google Scholar]
- Ghanbari S, Givianrad MH, Aberoomand Azar P. Simultaneous reduction of Cr (VI) and degradation of azo dyes by F-Fe-codoped TiO2/SiO2 photocatalysts under visible and solar irradiation. Can J Chem. 2019;97:659–671. doi: 10.1139/cjc-2018-0529. [DOI] [Google Scholar]
- Gopinath KP, Madhav NV, Krishnan A, et al. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: a review. J Environ Manag. 2020;270:110906. doi: 10.1016/j.jenvman.2020.110906. [DOI] [PubMed] [Google Scholar]
- Haider AJ, Al-Anbari RH, Kadhim GR, Salame CT. Exploring potential environmental applications of TiO2 nanoparticles. Energy Procedia. 2017;119:332–345. doi: 10.1016/J.EGYPRO.2017.07.117. [DOI] [Google Scholar]
- Hamza RZ, Gobouri AA, Al-Yasi HM, et al. A new sterilization strategy using TiO2 nanotubes for production of free radicals that eliminate viruses and application of a treatment strategy to combat infections caused by emerging SARS-CoV-2 during the COVID-19 pandemic. Coatings. 2021;11:680. doi: 10.3390/coatings11060680. [DOI] [Google Scholar]
- Han B, Tomer V, Nguyen TA, et al. Nanosensors for smart cities. Elsevier; 2020. [Google Scholar]
- Han L, Li B, Wen H, et al. Photocatalytic degradation of mixed pollutants in aqueous wastewater using mesoporous 2D/2D TiO2(B)-BiOBr heterojunction. J Mater Sci Technol. 2021;70:176–184. doi: 10.1016/J.JMST.2020.08.036. [DOI] [Google Scholar]
- Harris J, Silk R, Smith M, et al. Hierarchical TiO2 nanoflower photocatalysts with remarkable activity for aqueous methylene blue photo-oxidation. ACS Omega. 2020;5:18919–18934. doi: 10.1021/ACSOMEGA.0C02142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Kai T, Ding P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: a review. Environ Chem Lett. 2021;19(6):4563–4601. doi: 10.1007/S10311-021-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Cao D, Wang Y, et al. Preparation of Co–P–TiO2 nanocomposite coatings via a pulsed electrodeposition process. Surf Eng. 2019;36:975–981. doi: 10.1080/02670844.2019.1693172. [DOI] [Google Scholar]
- Hitam CNC, Jalil AA. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J Environ Manag. 2020;258:110050. doi: 10.1016/J.JENVMAN.2019.110050. [DOI] [PubMed] [Google Scholar]
- Huda A, Suman PH, Torquato LDM, et al. Visible light-driven photoelectrocatalytic degradation of acid yellow 17 using Sn3O4 flower-like thin films supported on Ti substrate (Sn3O4/TiO2/Ti) J Photochem Photobiol A Chem. 2019;376:196–205. doi: 10.1016/j.jphotochem.2019.01.039. [DOI] [Google Scholar]
- Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect Control Hosp Epidemiol. 2010;31:850–853. doi: 10.1086/655016. [DOI] [PubMed] [Google Scholar]
- Irshad MA, Nawaz R, ur Rehman MZ, et al. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: a review. Ecotoxicol Environ Saf. 2021;212:111978. doi: 10.1016/J.ECOENV.2021.111978. [DOI] [PubMed] [Google Scholar]
- Ismael M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol Energy. 2020;211:522–546. doi: 10.1016/J.SOLENER.2020.09.073. [DOI] [Google Scholar]
- Izzudin NM, Jalil AA, Aziz FFA et al (2021) Simultaneous remediation of hexavalent chromium and organic pollutants in wastewater using period 4 transition metal oxide-based photocatalysts: a review. Environ Chem Lett 1–29
- Jézéquel H, Chu KH. Enhanced adsorption of arsenate on titanium dioxide using Ca and Mg ions. Environ Chem Lett. 2005;3:132–135. doi: 10.1007/s10311-005-0018-x. [DOI] [Google Scholar]
- Jiang H, He X, Ma Y, et al. Isotropic hedgehog shape TiO2 functional multiwall carbon nanotube micromotors with phototactic motility in fuel free environments. ACS Appl Mater Interfaces. 2021;13:5406–5417. doi: 10.1021/acsami.0c19606. [DOI] [PubMed] [Google Scholar]
- Jiang JQ, Zhou Z, Sharma VK. Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in waste water—a review from global views. Microchem J. 2013;110:292–300. doi: 10.1016/J.MICROC.2013.04.014. [DOI] [Google Scholar]
- Kamal T, Anwar Y, Khan SB, et al. Dye adsorption and bactericidal properties of TiO2/chitosan coating layer. Carbohydr Polym. 2016;148:153–160. doi: 10.1016/J.CARBPOL.2016.04.042. [DOI] [PubMed] [Google Scholar]
- Kameya Y, Yabe H. Optical and superhydrophilic characteristics of TiO2 coating with subwavelength surface structure consisting of spherical nanoparticle aggregates. Coatings. 2019;9:547. doi: 10.3390/coatings9090547. [DOI] [Google Scholar]
- Kang W, Guo L, Fan H, et al. Flocculation, coalescence and migration of dispersed phase droplets and oil–water separation in heavy oil emulsion. J Pet Sci Eng. 2012;81:177–181. doi: 10.1016/j.petrol.2011.12.011. [DOI] [Google Scholar]
- Karpuraranjith M, Chen Y, Rajaboopathi S, et al. Three-dimensional porous MoS2 nanobox embedded g-C3N4@ TiO2 architecture for highly efficient photocatalytic degradation of organic pollutant. J Colloid Interface Sci. 2022;605:613–623. doi: 10.1016/j.jcis.2021.07.133. [DOI] [PubMed] [Google Scholar]
- Kathirvel S, Pedaballi S, Su C, et al. Morphological control of TiO2 nanocrystals by solvothermal synthesis for dye-sensitized solar cell applications. Appl Surf Sci. 2020;519:146082. doi: 10.1016/J.APSUSC.2020.146082. [DOI] [Google Scholar]
- Kaur R, Goyal D. Toxicity and degradation of the insecticide monocrotophos. Environ Chem Lett. 2019;17(3):1299–1324. doi: 10.1007/S10311-019-00884-Y. [DOI] [Google Scholar]
- Kaur N, Singh M, Moumen A, et al. 1D Titanium dioxide: achievements in chemical sensing. Materials (basel) 2020;13:2974. doi: 10.3390/ma13132974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MKA, Khan JA, Ullah H, et al. De-emulsification and gravity separation of micro-emulsion produced with enhanced oil recovery chemicals flooding. Energies. 2021;14:2249. doi: 10.3390/en14082249. [DOI] [Google Scholar]
- Koelmans AA, Besseling E, Shim WJ (2015) Nanoplastics in the aquatic environment. Critical review. Mar Anthropog Litter 325–340
- Kozhukharov S, Tchaoushev S. Perspectives for development and industrial application of spray pyrolysis method. Annu Proceeds“angel Kanchev” Univ Ruse. 2011;50:46–50. [Google Scholar]
- Kozhukharov S, Tchaoushev S. Spray pyrolysis equipment for various applications. Tchaoushev J Chem Technol Metall. 2013;48:111–118. [Google Scholar]
- Krejčíková S, Matějová L, Kočí K, et al. Preparation and characterization of Ag-doped crystalline titania for photocatalysis applications. Appl Catal B Environ. 2012;111–112:119–125. doi: 10.1016/J.APCATB.2011.09.024. [DOI] [Google Scholar]
- Kumar P. The influence of Azadirachta indica, Melaleuca alternifolia, and Cocos nucifera on Candida albicans strain in tissue conditioner at varying time intervals. J Indian Prosthodont Soc. 2020;20:171. doi: 10.4103/JIPS.JIPS_366_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Pandey G. Different methods used for the synthesis of TiO2 based nanomaterials: a review. Am J Nano Res Appl. 2018;6:1. doi: 10.11648/J.NANO.20180601.11. [DOI] [Google Scholar]
- Kumar PS, Venkatesh K, Gui EL, et al. Electrospun carbon nanofibers/TiO2-PAN hybrid membranes for effective removal of metal ions and cationic dye. Environ Nanotechnol Monit Manag. 2018;10:366–376. [Google Scholar]
- Kumar A, Khan M, He J, Lo IMC. Visible light driven magnetically recyclable terephthalic acid functionalized g- C3N4/TiO2 heterojunction nanophotocatalyst for enhanced degradation of PPCPs. Appl Catal B Environ. 2020;270:118898. doi: 10.1016/j.apcatb.2020.118898. [DOI] [Google Scholar]
- Kumar A, Shandilya P, Vo D-VN, et al. Metallic and bimetallic phosphides-based nanomaterials for photocatalytic hydrogen production and water detoxification: a review. Environ Chem Lett. 2021;2021(1):1–36. doi: 10.1007/S10311-021-01331-7. [DOI] [Google Scholar]
- Laxma Reddy PV, Kavitha B, Kumar Reddy PA, Kim KH. TiO2-based photocatalytic disinfection of microbes in aqueous media: a review. Environ Res. 2017;154:296–303. doi: 10.1016/J.ENVRES.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Le AT, Tan ZH, Sivakumar R, Pung SY. Predicting the photocatalytic performance of metal/metal oxide coupled TiO2 particles using response surface methodology (RSM) Mater Chem Phys. 2021;269:124739. doi: 10.1016/J.MATCHEMPHYS.2021.124739. [DOI] [Google Scholar]
- Ledwig P, Kot M, Moskalewicz T, Dubiel B. Electrophoretic deposition of nc-TiO2/chitosan composite coatings on X2CrNiMo17-12-2 stainless steel. Arch Metall Mater. 2017;62:405–410. doi: 10.1515/AMM-2017-0063. [DOI] [Google Scholar]
- Lee SY, Park SJ. TiO2 photocatalyst for water treatment applications. J Ind Eng Chem. 2013;19:1761–1769. doi: 10.1016/J.JIEC.2013.07.012. [DOI] [Google Scholar]
- Lee GJ, Wu JJ. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—a review. Powder Technol. 2017;318:8–22. doi: 10.1016/J.POWTEC.2017.05.022. [DOI] [Google Scholar]
- Li G, Song J, Meng C, et al. Single source, surfactant-free, and one-step solvothermal route synthesized TiO2 microspheres for highly efficient mesoscopic perovskite solar cells. Sol RRL. 2020 doi: 10.1002/SOLR.202000519. [DOI] [Google Scholar]
- Lin W-C, Yang W-D, Huang I-L, et al. Hydrogen production from methanol/water photocatalytic decomposition using Pt/TiO2−xNx catalyst. Energy Fuels. 2009;23:2192–2196. doi: 10.1021/EF801091P. [DOI] [Google Scholar]
- Lin ZH, Roy P, Shih ZY, et al. Synthesis of anatase Se/Te-TiO2 nanorods with dominant 100 facets: photocatalytic and antibacterial activity induced by visible light. ChemPlusChem. 2013;78:302–309. doi: 10.1002/CPLU.201200281. [DOI] [Google Scholar]
- Liu N, Chen X, Zhang J, Schwank JW. A review on TiO2-based nanotubes synthesized via hydrothermal method: formation mechanism, structure modification, and photocatalytic applications. Catal Today. 2014;225:34–51. doi: 10.1016/J.CATTOD.2013.10.090. [DOI] [Google Scholar]
- Liu H, Feng J, Jie W. A review of noble metal (Pd, Ag, Pt, Au)–zinc oxide nanocomposites: synthesis, structures and applications. J Mater Sci Mater Electron. 2017;28(22):16585–16597. doi: 10.1007/S10854-017-7612-0. [DOI] [Google Scholar]
- Liu Z, Song Y, Wang Q, et al. Solvothermal fabrication and construction of highly photoelectrocatalytic TiO2 NTs/Bi2MoO6 heterojunction based on titanium mesh. J Colloid Interface Sci. 2019;556:92–101. doi: 10.1016/j.jcis.2019.08.038. [DOI] [PubMed] [Google Scholar]
- Liu S, Wu X, Li Y, et al. Hydrophobic in-situ SiO2-TiO2 composite aerogel for heavy oil thermal recovery: Synthesis and high temperature performance. Appl Therm Eng. 2021;190:116745. doi: 10.1016/j.applthermaleng.2021.116745. [DOI] [Google Scholar]
- Long C, Jiang Z, Shangguan J, et al. Applications of carbon dots in environmental pollution control: a review. Chem Eng J. 2021;406:126848. doi: 10.1016/J.CEJ.2020.126848. [DOI] [Google Scholar]
- López-Goerne T, Ramírez-Olivares P, Pérez-Dávalos LA, et al. Catalytic nanomedicine. Cu/TiO2 & SiO2 nanoparticles as treatment of diabetic foot ulcer: a case report. Curr Nanomed. 2019;10:290–295. doi: 10.2174/2468187309666190906121924. [DOI] [Google Scholar]
- López YC, Viltres H, Gupta NK, et al. Transition metal-based metal–organic frameworks for environmental applications: a review. Environ Chem Lett. 2021;19(2):1295–1334. doi: 10.1007/S10311-020-01119-1. [DOI] [Google Scholar]
- Louangsouphom B, Wang X, Song J, Wang X. Low-temperature preparation of a N-TiO2/macroporous resin photocatalyst to degrade organic pollutants. Environ Chem Lett. 2019;17:1061–1066. doi: 10.1007/S10311-018-00827-Z/FIGURES/3. [DOI] [Google Scholar]
- Lyu J, Zhu L, Burda C. Considerations to improve adsorption and photocatalysis of low concentration air pollutants on TiO2. Catal Today. 2014;225:24–33. doi: 10.1016/J.CATTOD.2013.10.089. [DOI] [Google Scholar]
- MacArthur E. Beyond plastic waste. Science. 2017;358:843. doi: 10.1126/science.aao6749. [DOI] [PubMed] [Google Scholar]
- Madima N, Mishra SB, Inamuddin I, Mishra AK. Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater. A review. Environ Chem Lett. 2020;18:1169–1191. doi: 10.1007/s10311-020-01001-0. [DOI] [Google Scholar]
- Maganha de Almeida Kumlien AC, Borrego CM, Balcázar JL (2021) Antimicrobial resistance and bacteriophages: an overlooked intersection in water disinfection. Trends Microbiol 29:517–527.10.1016/J.TIM.2020.12.011 [DOI] [PubMed]
- Mahmood MA, Baruah S, Anal AK, Dutta J. Heterogeneous photocatalysis for removal of microbes from water. Environ Chem Lett. 2012;10:145–151. doi: 10.1007/s10311-011-0347-x. [DOI] [Google Scholar]
- Malekshahi Byranvand M, Nemati Kharat A, Fatholahi L, Malekshahi Beiranvand Z. A review on synthesis of nano-TiO2 via different methods. J Nanostructures. 2013;3:1–9. doi: 10.7508/JNS.2013.01.001. [DOI] [Google Scholar]
- Mandari KK, Son N, Kang M. Ag3PO4-Bi2WO6-TiO2 as a high performance electrocatalyst for oxygen evolution reaction. Appl Surf Sci. 2021;566:150681. doi: 10.1016/j.apsusc.2021.150681. [DOI] [Google Scholar]
- Mathew S, Ganguly P, Rhatigan S, et al. Cu-doped TiO2: visible light assisted photocatalytic antimicrobial activity. Appl Sci. 2018;8:2067. doi: 10.3390/app8112067. [DOI] [Google Scholar]
- Mathew S, Ganguly P, Kumaravel V, et al. Effect of chalcogens (S, Se, and Te) on the anatase phase stability and photocatalytic antimicrobial activity of TiO2. Mater Today Proc. 2020;33:2458–2464. doi: 10.1016/j.matpr.2020.01.336. [DOI] [Google Scholar]
- Matsuura R, Lo C-W, Wada S, et al. SARS-CoV-2 disinfection of air and surface contamination by TiO2 photocatalyst mediated damage to viral morphology, RNA, and protein. Viruses. 2021;13:942. doi: 10.3390/v13050942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy JG, Zhang Z. Antimicrobial and photocatalytic disinfection mechanisms in silver-modified photocatalysts under dark and light conditions. J Photochem Photobiol C Photochem Rev. 2014;19:62–75. doi: 10.1016/j.jphotochemrev.2014.01.001. [DOI] [Google Scholar]
- Mehrotra RC, Singh A. Recent trends in metal alkoxide chemistry. Prog Inorg Chem. 1997;46:239–454. doi: 10.1002/9780470166475.CH4. [DOI] [Google Scholar]
- Meng X, Ouyang S, Kako T, et al. Photocatalytic CO2 conversion over alkali modified TiO2 without loading noble metal cocatalyst. Chem Commun. 2014;50:11517–11519. doi: 10.1039/C4CC04848B. [DOI] [PubMed] [Google Scholar]
- Micochova P, Chadha A, Hesseloj T, et al. Rapid inactivation of SARS-CoV-2 by titanium dioxide surface coating. Wellcome Open Res. 2021;6:56. doi: 10.12688/wellcomeopenres.16577.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikrovalov V. Microwave-assisted non-aqueous synthesis of ZnO nanoparticles. Mater Tehnol. 2011;45:173–177. [Google Scholar]
- Mohan L, Dennis C, Padmapriya N, et al. Effect of electrolyte temperature and anodization time on formation of TiO2 nanotubes for biomedical applications. Mater Today Commun. 2020;23:101103. doi: 10.1016/J.MTCOMM.2020.101103. [DOI] [Google Scholar]
- Mollabashi V, Farmany A, Alikhani MY, et al. Effects of TiO2-coated stainless steel orthodontic wires on streptococcus mutans bacteria: a clinical study. Int J Nanomed. 2020;15:8759. doi: 10.2147/IJN.S258440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllmann A, Gedamu D, Vivo P, et al. Highly compact TiO2 films by spray pyrolysis and application in perovskite solar cells. Adv Eng Mater. 2019;21:1801196. doi: 10.1002/ADEM.201801196. [DOI] [Google Scholar]
- Moore CJ. Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res. 2008;108:131–139. doi: 10.1016/j.envres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Moreira AJ, Campos LO, Maldi CP, et al. Photocatalytic degradation of Prozac mediated by TiO2 nanoparticles obtained via three synthesis methods: sonochemical, microwave hydrothermal, and polymeric precursor. Environ Sci Pollut Res. 2020;27(21):27032–27047. doi: 10.1007/S11356-020-08798-X. [DOI] [PubMed] [Google Scholar]
- Morin-Crini N, Lichtfouse E, Fourmentin M et al (2022) Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ Chem Lett 1–43
- Muniandy SS, Kaus NHM, Jiang Z-T, et al. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017;7:48083–48094. doi: 10.1039/C7RA08187A. [DOI] [Google Scholar]
- Munjal G, Dwivedi G, ANB (2014) Adsorption studies of methylene blue on TiO2 nanoparticles: experimental and mathematical modeling. 10.7763/IPCBEE
- Nabi G, Raza W, Tahir MB. Green synthesis of TiO2 nanoparticle using cinnamon powder extract and the study of optical properties. J Inorg Organomet Polym Mater. 2020;30:1425–1429. doi: 10.1007/S10904-019-01248-3. [DOI] [Google Scholar]
- Nabi I, Li K, Cheng H, et al. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. Iscience. 2020;23:101326. doi: 10.1016/j.isci.2020.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem M, Tungmunnithum D, Hano C, et al. The current trends in the green synthesis of titanium oxide nanoparticles and their applications. Green Chem Lett Rev. 2018;11:492–502. doi: 10.1080/17518253.2018.1538430. [DOI] [Google Scholar]
- Narayan MR, Raturi A. Deposition and characterisation of titanium dioxide films formed by electrophoretic deposition. Int J Mater Eng Innov. 2012;3:17–31. doi: 10.1504/IJMATEI.2012.044447. [DOI] [Google Scholar]
- Narkevica I, Stradina L, Stipniece L, et al. Electrophoretic deposition of nanocrystalline TiO2 particles on porous TiO2-x ceramic scaffolds for biomedical applications. J Eur Ceram Soc. 2017;37:3185–3193. doi: 10.1016/J.JEURCERAMSOC.2017.03.053. [DOI] [Google Scholar]
- Nateq MH, Ceccato R. Sol-Gel synthesis of TiO2 nanocrystalline particles with enhanced surface area through the reverse micelle approach. Adv Mater Sci Eng. 2019 doi: 10.1155/2019/1567824. [DOI] [Google Scholar]
- Ng J, Zhang X, Zhang T, et al. Construction of self-organized free-standing TiO2 nanotube arrays for effective disinfection of drinking water. J Chem Technol Biotechnol. 2010;85:1061–1066. doi: 10.1002/JCTB.2395. [DOI] [Google Scholar]
- Nguu J, Nyongesa F, Musembi R, Aduda B. Electrophoretic deposition and characterization of TiO2/Nb2O5 composite thin films for dye sensitized solar cells. J Mater Phys Chem. 2018;6:1–8. [Google Scholar]
- Nguyen DC, Doan TLL, Prabhakaran S, et al. Hierarchical Co and Nb dual-doped MoS2 nanosheets shelled micro-TiO2 hollow spheres as effective multifunctional electrocatalysts for HER, OER, and ORR. Nano Energy. 2021;82:105750. doi: 10.1016/j.nanoen.2021.105750. [DOI] [Google Scholar]
- Noh W, Jo S, Kim J, Lee TS (2021) Visible light driven asymmetric TiO2 based photocatalytic micromotor hybridized with a conjugated polyelectrolyte and glucose oxidase. Langmuir [DOI] [PubMed]
- Nyamukamba P, Okoh O, Mungondori H, et al. Synthetic methods for titanium dioxide nanoparticles: a review. Titan Dioxide Mater Sustain Environ. 2018 doi: 10.5772/INTECHOPEN.75425. [DOI] [Google Scholar]
- Nyongesa F, Aduda B. Electrophoretic deposition of titanium dioxide thin films for photocatalytic water purification systems. Adv Mater. 2017;6:31. doi: 10.11648/J.AM.20170604.11. [DOI] [Google Scholar]
- Ochedi FO, Liu D, Yu J, et al. Photocatalytic, electrocatalytic and photoelectrocatalytic conversion of carbon dioxide: a review. Environ Chem Lett. 2021;19:941–967. doi: 10.1007/s10311-020-01131-5. [DOI] [Google Scholar]
- Ojemaye MO, Adefisoye MA, Okoh AI. Nanotechnology as a viable alternative for the removal of antimicrobial resistance determinants from discharged municipal effluents and associated watersheds: a review. J Environ Manag. 2020;275:111234. doi: 10.1016/J.JENVMAN.2020.111234. [DOI] [PubMed] [Google Scholar]
- Ola O, Maroto-Valer MM. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J Photochem Photobiol C Photochem Rev. 2015;24:16–42. doi: 10.1016/J.JPHOTOCHEMREV.2015.06.001. [DOI] [Google Scholar]
- Omar TFT, Ahmad A, Aris AZ, Yusoff FM. Endocrine disrupting compounds (EDCs) in environmental matrices: Review of analytical strategies for pharmaceuticals, estrogenic hormones, and alkylphenol compounds. TrAC Trends Anal Chem. 2016;85:241–259. doi: 10.1016/j.trac.2016.08.004. [DOI] [Google Scholar]
- Panchal D, Sharma A, Mondal P, et al. Heterolayered TiO2@layered double hydroxide-MoS2 nanostructure for simultaneous adsorption-photocatalysis of co-existing water contaminants. Appl Surf Sci. 2021;553:149577. doi: 10.1016/J.APSUSC.2021.149577. [DOI] [Google Scholar]
- Pang LX, Wang XY, De TX. Enhanced photocatalytic performance of porous TiO2 nanobelts with phase junctions. Solid State Sci. 2015;39:29–33. doi: 10.1016/J.SOLIDSTATESCIENCES.2014.11.004. [DOI] [Google Scholar]
- Pantidos N, Horsfall L. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J Nanomed Nanotechnol. 2014 doi: 10.4172/2157-7439.1000233. [DOI] [Google Scholar]
- Patel G, Patra C, Srinivas SP, et al. Methods to evaluate the toxicity of engineered nanomaterials for biomedical applications: a review. Environ Chem Lett. 2021;19:4253–4274. doi: 10.1007/s10311-021-01280-1. [DOI] [Google Scholar]
- Peiris M, Gunasekara T, Jayaweera P, Fernando S. TiO2 nanoparticles from baker’s yeast: a potent antimicrobial. J Microbiol Biotechnol. 2018;28:1664–1670. doi: 10.4014/JMB.1807.07005. [DOI] [PubMed] [Google Scholar]
- Pena ME, Korfiatis GP, Patel M, et al. Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res. 2005;39:2327–2337. doi: 10.1016/J.WATRES.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Phattepur H, Siddaiah GB, Ganganagappa N. Synthesis and characterisation of mesoporous TiO2 nanoparticles by novel surfactant assisted sol-gel method for the degradation of organic compounds. Period Polytech Chem Eng. 2019;63:85–95. doi: 10.3311/PPCH.11789. [DOI] [Google Scholar]
- Pragada SC, Thalla AK. Polymer-based immobilized Fe2O3–TiO2/PVP catalyst preparation method and the degradation of triclosan in treated greywater effluent by solar photocatalysis. J Environ Manag. 2021;296:113305. doi: 10.1016/j.jenvman.2021.113305. [DOI] [PubMed] [Google Scholar]
- Prakash J, Sun S, Swart HC, Gupta RK. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications. Appl Mater Today. 2018;11:82–135. doi: 10.1016/J.APMT.2018.02.002. [DOI] [Google Scholar]
- Prihod’ko R V, Soboleva NM (2013) Photocatalysis: oxidative processes in water treatment. J Chem 2013
- Purkait P, Bhattacharyya A, Roy S, et al. Green synthesis of TiO2 nanoparticle: its characterization and potential application in Zoxamide photodegradation. J Water Environ Nanotechnol. 2020;5:191–203. [Google Scholar]
- Qu X, Lin J, Chaudhary JP, et al. Defect enrich ultrathin TiO2 nanosheets for rapid adsorption and visible light mediated PPCPs degradation. Chemosphere. 2021;268:128782. doi: 10.1016/j.chemosphere.2020.128782. [DOI] [PubMed] [Google Scholar]
- Quyen VT, Ha LTT, Thanh DM, et al. Advanced synthesis of MXene-derived nanoflower-shaped TiO2@Ti3C2 heterojunction to enhance photocatalytic degradation of Rhodamine B. Environ Technol Innov. 2021;21:101286. doi: 10.1016/J.ETI.2020.101286. [DOI] [Google Scholar]
- Rajakumar G, Rahuman AA, Priyamvada B, et al. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater Lett. 2012;68:115–117. doi: 10.1016/J.MATLET.2011.10.038. [DOI] [Google Scholar]
- Ramakrishnan VM, Natarajan M, Santhanam A, et al. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater Res Bull. 2018;97:351–360. doi: 10.1016/J.MATERRESBULL.2017.09.017. [DOI] [Google Scholar]
- Ranjan S, Ramalingam C. Titanium dioxide nanoparticles induce bacterial membrane rupture by reactive oxygen species generation. Environ Chem Lett. 2016;14:487–494. doi: 10.1007/s10311-016-0586-y. [DOI] [Google Scholar]
- Reis KP, Ramanan A, Whittingham S. Hydrothermal synthesis of sodium tungstates. Chem Mater. 2002;2:219–221. doi: 10.1021/CM00009A003. [DOI] [Google Scholar]
- Sakkas VA, Dimou A, Pitarakis K, et al. TiO2 photocatalyzed degradation of diazinon in an aqueous medium. Environ Chem Lett. 2005;3:57–61. doi: 10.1007/S10311-004-0091-6/TABLES/2. [DOI] [Google Scholar]
- Saravanan A, Kumar PS, Vo DVN, et al. Photocatalysis for removal of environmental pollutants and fuel production: a review. Environ Chem Lett. 2020;19(1):441–463. doi: 10.1007/S10311-020-01077-8. [DOI] [Google Scholar]
- Šauer P, Stará A, Golovko O et al (2019) Determining the potential of progestins to induce progestagenic activities in the aquatic environment. Environ Pollut Progestins Occur Horm Act Eff Fish
- Savun-Hekimouglu B (2020) A review on sonochemistry and its environmental applications. In: Acoustics, pp 766–775
- Sengul AB, Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett. 2020;18:1659–1683. doi: 10.1007/s10311-020-01033-6. [DOI] [Google Scholar]
- Shang Q, Gao S, Dai G, et al. Structure and photocatalytic activity of Ti3+ self-doped TiO2 flower shaped nanospheres. Surfaces Interfaces. 2020;18:100426. doi: 10.1016/j.surfin.2019.100426. [DOI] [Google Scholar]
- Sharma G, Thakur B, Naushad M, et al. (2017) Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ Chem Lett. 2017;161(16):113–146. doi: 10.1007/S10311-017-0671-X. [DOI] [Google Scholar]
- Sharma R, Sarkar A, Jha R, et al. Sol-gel–mediated synthesis of TiO2 nanocrystals: Structural, optical, and electrochemical properties. Int J Appl Ceram Technol. 2020;17:1400–1409. doi: 10.1111/IJAC.13439. [DOI] [Google Scholar]
- Shu C, Du H, Pu W, et al. Trace amounts of palladium-doped hollow TiO2 nanosphere as highly efficient electrocatalyst for hydrogen evolution reaction. Int J Hydrogen Energy. 2021;46:1923–1933. doi: 10.1016/j.ijhydene.2020.10.034. [DOI] [Google Scholar]
- Singh AK, Chaudhary V, Singh AK, Sinha SRP. Effect of TiO2 nanoparticles on electrical properties of chemical vapor deposition grown single layer graphene. Synth Met. 2019 doi: 10.1016/J.SYNTHMET.2019.116155. [DOI] [Google Scholar]
- Singh J, Kumar S, Alok A, et al. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J Clean Prod. 2019;214:1061–1070. doi: 10.1016/J.JCLEPRO.2019.01.018. [DOI] [Google Scholar]
- Slamet, Tristantini D, Valentina, Ibadurrohman M (2013) Photocatalytic hydrogen production from glycerol–water mixture over Pt-N-TiO2 nanotube photocatalyst. Int J Energy Res 37:1372–1381.10.1002/ER.2939
- Speller KA (2021) The photocatalytic degradation of ibuprofen and atenolol using bismuth oxychloride and titanium dioxide
- Sridhar V, Park B-W, Guo S, et al. Multiwavelength steerable visible light driven magnetic CoO-TiO2 microswimmers. ACS Appl Mater Interfaces. 2020;12:24149–24155. doi: 10.1021/acsami.0c06100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth B, Goutham R, Badri Narayan R, et al. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J Environ Manag. 2017;200:60–78. doi: 10.1016/J.JENVMAN.2017.05.063. [DOI] [PubMed] [Google Scholar]
- Stride JA, Tuong NT. Controlled synthesis of titanium dioxide nanostructures. Solid State Phenom. 2010;162:261–294. doi: 10.4028/WWW.SCIENTIFIC.NET/SSP.162.261. [DOI] [Google Scholar]
- Su J, Yu S, Xu M, et al. Enhanced visible light photocatalytic performances of few-layer MoS2@TiO2 hollow spheres heterostructures. Mater Res Bull. 2020;130:110936. doi: 10.1016/J.MATERRESBULL.2020.110936. [DOI] [Google Scholar]
- Subhapriya S, Gomathipriya P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb Pathog. 2018;116:215–220. doi: 10.1016/J.MICPATH.2018.01.027. [DOI] [PubMed] [Google Scholar]
- Sun Z, Kim JH, Zhao Y, et al. Rational design of 3D dendritic TiO2 nanostructures with favorable architectures. J Am Chem Soc. 2011;133:19314–19317. doi: 10.1021/JA208468D. [DOI] [PubMed] [Google Scholar]
- Thamaraiselvi K, Sivakumar T. Photocatalytic reduction of carbon dioxide by using bare and copper oxide impregnated nano titania catalysts. J Nanosci Nanotechnol. 2017;17:313–322. doi: 10.1166/JNN.2017.12421. [DOI] [PubMed] [Google Scholar]
- Theerthagiri J, Senthil RA, Senthilkumar B, et al. Recent advances in MoS2 nanostructured materials for energy and environmental applications—a review. J Solid State Chem. 2017;252:43–71. doi: 10.1016/J.JSSC.2017.04.041. [DOI] [Google Scholar]
- Tian J, Leng Y, Cui H, Liu H. Hydrogenated TiO2 nanobelts as highly efficient photocatalytic organic dye degradation and hydrogen evolution photocatalyst. J Hazard Mater. 2015;299:165–173. doi: 10.1016/j.jhazmat.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Toma FL, Bertrand G, Klein D, Coddet C. Photocatalytic removal of nitrogen oxides via titanium dioxide. Environ Chem Lett. 2004;2:117–121. doi: 10.1007/s10311-004-0087-2. [DOI] [Google Scholar]
- Tong Y, Shi G, Hu G, et al. Photo-catalyzed TiO2 inactivates pathogenic viruses by attacking viral genome. Chem Eng J. 2021;414:128788. doi: 10.1016/j.cej.2021.128788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udom I, Ram MK, Stefanakos EK, et al. One dimensional-ZnO nanostructures: synthesis, properties and environmental applications. Mater Sci Semicond Process. 2013;16:2070–2083. doi: 10.1016/J.MSSP.2013.06.017. [DOI] [Google Scholar]
- Umapathi A, Kumawat M, Daima HK (2021) Engineered nanomaterials for biomedical applications and their toxicity: a review. Environ Chem Lett 1–24
- Vattikuti SVP, Devarayapalli KC, Reddy Nallabala NK, et al. Onion ring like carbon and nitrogen from ZIF-8 on TiO2/Fe2O3 nanostructure for overall electrochemical water splitting. J Phys Chem Lett. 2021;12:5909–5918. doi: 10.1021/acs.jpclett.1c01497. [DOI] [PubMed] [Google Scholar]
- Vigneshwaran S, Sirajudheen P, Karthikeyan P, et al. Immobilization of MIL-88(Fe) anchored TiO2-chitosan(2D/2D) hybrid nanocomposite for the degradation of organophosphate pesticide: characterization, mechanism and degradation intermediates. J Hazard Mater. 2021;406:124728. doi: 10.1016/J.JHAZMAT.2020.124728. [DOI] [PubMed] [Google Scholar]
- Villaseñor MJ, Ríos Á. Nanomaterials for water cleaning and desalination, energy production, disinfection, agriculture and green chemistry. Environ Chem Lett. 2017;16(1):11–34. doi: 10.1007/S10311-017-0656-9. [DOI] [Google Scholar]
- Wang Y, He Y, Lai Q, Fan M. Review of the progress in preparing nano TiO2: an important environmental engineering material. J Environ Sci. 2014;26:2139–2177. doi: 10.1016/J.JES.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Wang D, Li Y, Puma GL, et al. Mechanism and experimental study on the photocatalytic performance of Ag/AgCl chiral TiO2 nanofibers photocatalyst: the impact of wastewater components. J Hazard Mater. 2015;285:277–284. doi: 10.1016/j.jhazmat.2014.10.060. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao Y, Zhang J. Photochemical removal of SO2 over TiO2-Based nanofibers by a dry photocatalytic oxidation process. Energy Fuels. 2017;31:9905–9914. doi: 10.1021/ACS.ENERGYFUELS.7B01514. [DOI] [Google Scholar]
- Wang X, Ahmad M, Sun H. Three-dimensional ZnO hierarchical nanostructures: Solution phase synthesis and applications. Materials (basel) 2017;10:1304. doi: 10.3390/ma10111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kaeppler A, Fischer D, Simmchen J. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Appl Mater Interfaces. 2019;11:32937–32944. doi: 10.1021/acsami.9b06128. [DOI] [PubMed] [Google Scholar]
- Wang J, Li Y, Luo YN, et al. New mixed-crystal nanostructure of K3TiOF5/TiO2 with dominant facets for efficient removal of anionic dyes. Ceram Int. 2020;46:25598–25602. doi: 10.1016/J.CERAMINT.2020.07.032. [DOI] [Google Scholar]
- Wang Z, Ali Haidry A, Xie L, et al. Acetone sensing applications of Ag modified TiO2 porous nanoparticles synthesized via facile hydrothermal method. Appl Surf Sci. 2020;533:147383. doi: 10.1016/J.APSUSC.2020.147383. [DOI] [Google Scholar]
- Wang D, Zhang B, Ding H, et al. TiO2 supported single Ag atoms nanozyme for elimination of SARS-CoV2. Nano Today. 2021;40:101243. doi: 10.1016/J.NANTOD.2021.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li J, Wang B, et al. Bi2O2CO3/TiO2 hybrid with 0D/1D nanostructure: design, synthesis and photocatalytic performance. New J Chem. 2021;45:6247–6253. doi: 10.1039/D1NJ00281C. [DOI] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, et al. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242–2250. doi: 10.1021/ES204168D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JM. Low-temperature preparation of titania nanorods through direct oxidation of titanium with hydrogen peroxide. J Cryst Growth. 2004;269:347–355. doi: 10.1016/J.JCRYSGRO.2004.05.023. [DOI] [Google Scholar]
- Wu J-M, Zhang T-W, Zeng Y-W, et al. Large-scale preparation of ordered titania nanorods with enhanced photocatalytic activity. Langmuir. 2005;21:6995–7002. doi: 10.1021/LA0500272. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lu G, Li S. The doping effect of Bi on TiO2 for photocatalytic hydrogen generation and photodecolorization of Rhodamine B. J Phys Chem C. 2009;113:9950–9955. doi: 10.1021/JP9009433. [DOI] [Google Scholar]
- Wu Z, Wu Q, Du L, et al. Progress in the synthesis and applications of hierarchical flower-like TiO2 nanostructures. Particuology. 2014 doi: 10.1016/j.partic.2013.04.003. [DOI] [Google Scholar]
- Wu F, Zhou Z, Hicks AL. Life cycle impact of titanium dioxide nanoparticle synthesis through physical, chemical, and biological routes. Environ Sci Technol. 2019;53:4078–4087. doi: 10.1021/acs.est.8b06800. [DOI] [PubMed] [Google Scholar]
- Xie W, Pakdel E, Liang Y, et al. Natural melanin/TiO2 hybrids for simultaneous removal of dyes and heavy metal ions under visible light. J Photochem Photobiol A Chem. 2020;389:112292. doi: 10.1016/j.jphotochem.2019.112292. [DOI] [Google Scholar]
- Xin Y, Grundmeier G, Keller A. Adsorption of SARS-CoV-2 spike protein S1 at oxide surfaces studied by high-speed atomic force microscopy. Adv Nanobiomed Res. 2021;1:2000024. doi: 10.1002/anbr.202000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Meng X. Size effects of nanocrystalline TiO2 on As(V) and As(III) adsorption and As(III) photooxidation. J Hazard Mater. 2009;168:747–752. doi: 10.1016/J.JHAZMAT.2009.02.084. [DOI] [PubMed] [Google Scholar]
- Xue Z, Zhang W, Yin X, et al. Enhanced conversion efficiency of flexible dye-sensitized solar cells by optimization of the nanoparticle size with an electrophoretic deposition technique. RSC Adv. 2012;2:7074–7080. doi: 10.1039/C2RA20542D. [DOI] [Google Scholar]
- Yang Y, Ok YS, Kim K-H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ. 2017;596:303–320. doi: 10.1016/j.scitotenv.2017.04.102. [DOI] [PubMed] [Google Scholar]
- Yang L, Hao X, Yu D, et al. High visible-light catalytic activity of Bis-PDI-T@ TiO2 for activating persulfate toward efficient degradation of carbamazepine. Sep Purif Technol. 2021;263:118384. doi: 10.1016/j.seppur.2021.118384. [DOI] [Google Scholar]
- Yao J, Chen H, Jiang F, et al. Titanium dioxide and cadmium sulfide co-sensitized graphitic carbon nitride nanosheets composite photocatalysts with superior performance in phenol degradation under visible-light irradiation. J Colloid Interface Sci. 2017;490:154–162. doi: 10.1016/j.jcis.2016.11.051. [DOI] [PubMed] [Google Scholar]
- Yildiz T, Yatmaz HC, Öztürk K. Anatase TiO2 powder immobilized on reticulated Al2O3 ceramics as a photocatalyst for degradation of RO16 azo dye. Ceram Int. 2020;46:8651–8657. doi: 10.1016/J.CERAMINT.2019.12.098. [DOI] [Google Scholar]
- Yousef A, El-Halwany MM, Barakat NAM, et al. CuO-doped TiO2 nanofibers as potential photocatalyst and antimicrobial agent. J Ind Eng Chem. 2015;26:251–258. doi: 10.1016/j.jiec.2014.11.036. [DOI] [Google Scholar]
- Yu J, Hai Y, Cheng B. Enhanced photocatalytic H2-production activity of TiO2 by Ni(OH)2 cluster modification. J Phys Chem C. 2011;115:4953–4958. doi: 10.1021/JP111562D. [DOI] [Google Scholar]
- Yuan Y, Zhang J, Li H, et al. Simultaneous removal of SO2, NO and mercury using TiO2-aluminum silicate fiber by photocatalysis. Chem Eng J. 2012;192:21–28. doi: 10.1016/J.CEJ.2012.03.043. [DOI] [Google Scholar]
- Zhang Y, Ma H, Yi M, et al. Magnetron-sputtering fabrication of noble metal nanodots coated TiO2 nanoparticles with enhanced photocatalytic performance. Mater Des. 2017;125:94–99. doi: 10.1016/J.MATDES.2017.03.084. [DOI] [Google Scholar]
- Zhang Y, Wang L, Zhang N, Zhou Z. Adsorptive environmental applications of MXene nanomaterials: a review. RSC Adv. 2018;8:19895–19905. doi: 10.1039/C8RA03077D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yan M, Sun G, Liu K. Simultaneous removal of Cu (II), Cd (II), Cr (VI), and rhodamine B in wastewater using TiO2 nanofibers membrane loaded on porous fly ash ceramic support. Sep Purif Technol. 2021;272:118888. doi: 10.1016/j.seppur.2021.118888. [DOI] [Google Scholar]
- Zhu Y, Chen F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem Rev. 2014;114:6462–6555. doi: 10.1021/CR400366S. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Feng Y, Chen S, et al. Carbon nitride nanotube-based materials for energy and environmental applications: a review of recent progresses. J Mater Chem A. 2020;8:25626–25648. doi: 10.1039/D0TA08892G. [DOI] [Google Scholar]
- Zhu Y, Lu Y, Yu H, et al. Super-hydrophobic F-TiO2@ PP membranes with nano-scale “coral”-like synapses for waste oil recovery. Sep Purif Technol. 2021;267:118579. doi: 10.1016/j.seppur.2021.118579. [DOI] [Google Scholar]