Abstract
Sub-Saharan Africa (SSA), particularly Southern and East Africa, has the highest AIDS deaths and HIV-infected people in the world. Even though considerable effort has been made over the years to study HIV transmission risk behaviours of different population groups in SSA, there is little evidence of studies that have looked at pooled effects of associated HIV risk factors among men, particularly in Southern Africa. Thus, this study sought to fill this gap in knowledge by investigating the variations in HIV risk behaviours among men in the region. The study analysed cross-sectional data based on the most recent country Demographic and Health Survey (DHS) for six countries, namely Lesotho, Mozambique, Namibia, South Africa, Zambia and Zimbabwe. The study employed multivariate logistic regression models on a pooled dataset and individual country data to examine the relative risk of education and other factors on HIV risk behaviour indicators. It considered: (i) condom use during high risk-sex, (ii) multiple sexual partnerships, and (iii) HIV testing among men aged 15–59 years. Findings show that the proportion of men who engaged in HIV transmission risk behaviour was high in Southern Africa. Two-thirds of men reported non-use of a condom during last sex with most recent partners while 22% engaged in multiple sexual partnerships. The percentage of men who used condoms during sex with most recent partners ranged from 18% in Mozambique to 58% in Namibia. Age, residence, marital status and household wealth status were associated with HIV risk factors in the region. The study has established country variations in terms of how individual factors influence HIV transmission risk behaviour among men. Results show that the level of education was associated with increased use of condoms, only in Zambia and Mozambique. Delay in starting a sexual debut was associated with reduced odds of having multiple sexual partnerships in the region. Suggesting the need to strengthen comprehensive sexuality education among young men in school, to promote social behaviour change during adolescence age. The study presents important results to inform direct health policy, programme and government action to address HIV prevalence in the Southern region of Africa.
Subject terms: Social science, Sociology, Social policy
Background
As the world commemorates the Fortieth World AIDS Day, the Human Immunodeficiency Virus (HIV) statistics are still staggering around the globe. Since its discovery in the 1980s, HIV and acquired immune deficiency syndrome (AIDS) has globally claimed about 32 million people (UNAIDS, 2020a, 2013aa; WHO, 2015). The Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates show that about 37.9 million people were living with HIV in 2019, and of these, 28.5 million were in sub-Saharan Africa (UNAIDS, 2020a). UNAIDS estimates that over 70% of the global total of HIV-positive people live in sub-Saharan Africa (SAfAIDS, 2015; UNAIDS GH, 2019). HIV prevalence for males aged 15–59 years in Southern Africa is about 14% (UNAIDS, 2013ab). Even though the pandemic’s severe health effects have been managed by the advent of antiretroviral treatment (ART), many countries in Eastern and Southern Africa have not yet reached the HIV epidemic control level (UNAIDS, 2020b, 2013ab; WHO, 2015).
Epidemiological studies on HIV/AIDS published early in the late 1980s in sub-Saharan Africa reported formal education as a risk factor: educated sub-Saharan Africans had a higher chance of developing HIV/AIDS than their less educated counterparts (Baker et al., 2008; Leon et al., 2017). According to later demographic research, the effect of education on HIV transmission risk had reversed by the mid-1990s, later education functioned as a positive social factor helping men and women to have comprehensive knowledge of HIV prevention strategies (Baker et al., 2008; Gregson et al., 2007; Leon et al., 2017). Recent counter-evidence reveals a curvilinear pattern, with the link between educational attainment and HIV/AIDS infection risk, shifting from positive to negative as one progresses through the educational system (Gregson et al., 2001; Leon et al., 2017; Pettifor et al., 2008). However, this contracting finding in literature requires further repeated population level and longitudinal studies to validate the association between education level and other social independent correlates, conclusively.
Governments in the region, with support from the US President’s Emergency Plan for AIDS Relief (PEFFAR), United Nations Joint Programme on HIV/AIDS (UNAIDS), Global Fund and other international and local organisations have been implementing various HIV prevention programmes, targeted at ensuring that men have access to comprehensive information, encouraging abstinence from sex for the non-married, promote delay in initiation of sexual debut among school-going youths, encourage adults to stick to one equally faithful and uninfected sexual partner, and consistent use of condoms (SAfAIDS, 2015; Simona et al., 2022; UNAIDS, 2012, 2013ab; WHO, 2015) as well as the uptake of medical male circumcision (Garenne and Matthews, 2020; Phiri, 2011). Some studies have pointed out that promoting access to education is considered a priority intervention measure for reducing the risk of transmitting HIV among men. For instance, available research reveals a negative linear relationship between education attainment (years of education) and HIV infection rate (De Neve et al., 2015; Leon et al., 2017; Zuilkowski and Jukes, 2012). Despite Southern Africa having the highest HIV prevalence rates in the world, there is little research that has focused on understanding a comprehensive regional picture of the role of education and other social and behavioural factors in influencing HIV risk behaviour, especially in sexually active men. Considering the high prevalence of HIV rates among men in the Southern part of Africa, comparatively to other sub-regions in the continent and elsewhere (Awopegba et al., 2021; Johnson et al., 2017; Olakunde et al., 2020a), it is relevant that research aiming at investigating factors that predispose men to the risks of HIV infection should be of top priority in the region.
Although the prevalence of HIV in men appears to be falling over time globally, it remains unacceptably high in Southern and Eastern Africa (SAfAIDS, 2015; UNAIDS, 2020a; WHO, 2015). These levels of HIV prevalence in men, signify the fact that more effort in sexual and reproductive health programming at the health policy level needs to be done to stimulate a further reduction in HIV prevalence among men in Southern African (Baral and Phaswana-Mafuya, 2012; Hargreaves et al., 2008). Doing so requires an all-inclusive understanding of the determinants associated with HIV risk behaviour among men. Thus, the understanding of such factors affecting men is considerably lacking when we consider individual-level factors at the sub-regional level.
Therefore, this study was designed to fill the knowledge gap and come up with policy and programming suggestions aimed at further reducing HIV prevalence in men in Southern Africa. This study investigated factors associated with HIV transmission risk behaviour in men in the region. Furthermore, the study sought to establish if there were variations in HIV transmission risk behaviours across countries included in the analysis. Unlike similar studies conducted in SSA (Baker et al., 2008; Glynn et al., 2004; Leon et al., 2017; Lucas and Wilson, 2019), this study incorporates pooled comprehensive complex regional data to examine individual-level and contextual factors that influence HIV risk behaviours in men. The study achieved its objectives by making use of nationally representative cross-sectional surveys conducted in Southern African countries.
Methods and data
Data source
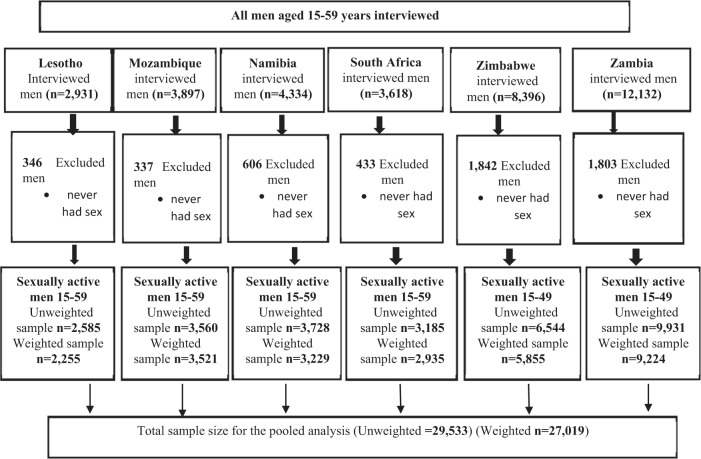
This study used data extracted from the recent Demographic and Health Survey (DHS) datasets from six countries in Southern Africa, namely Lesotho, Mozambique, Namibia, South Africa, Zambia, and Zimbabwe (Fig. 1). The DHS programme is conducted in many developing countries and draws national representative samples of households that are usually selected via a two-stage stratified cluster sampling technique (Croft et al., 2018). Women aged 15–49 and men aged 15–59 years are usually selected for interviews in all sampled households. To facilitate comparison of indicators across countries, interviews are usually conducted using three standard questionnaires, namely; household questionnaire, woman questionnaire and men questionnaire. Participants in the DHS survey were interviewed by field workers, who were well-versed in a wide range of topics, including sexuality, HIV and AIDS knowledge and awareness, fertility preferences and family planning, and other reproductive health topics. DHS data are typically weighted to account for the complexity of survey design and response bias, with the goal of ensuring that the sample represents the general population (DHS programme, 2021). The total number of men aged 15–59 years included in this study is 29,533 (weighted = 27,019). Figure 1 shows the sampling derivation steps.
Fig. 1.
Sample derivation diagram.
Sample description
Analysis samples for this study comprised males aged 15–59 years extracted from each country’s recent DHS. Data came from the men’s recode files (MR Datasets) for each country. The sample included all men who were sexually active at the time of the survey. This resulted in a pooled sample of 27,019 men included in the analysis. Weighted samples of sexually active men ranged from 2255 men in Lesotho to 9224 in Zambia (Fig. 1).
Study measures
Outcome variables
Three outcome variables of interest were used to measure HIV risk behaviour in men, these are condom use during sex with the most recent partner; multiple sexual partnerships and HIV testing in the last 12 months prior to the survey. Prior research has revealed that promoting condom use and understanding barriers to consistent condom use remain priorities, especially among sexually active men who have sex with multiple partners (Campbell et al., 2016). Further, fear of HIV testing has been identified to play an important role in HIV transmission in Africa (Asaolu et al., 2016a; Olakunde et al., 2020b). The DHS collected this information from all sexually active men aged 15–59 years who were interviewed in the survey. To facilitate analysis, the study classified the outcome variables as binary, with “0” showing non-occurrence of the outcome and “1” showing occurrence of the outcome of interest. We coded condom use as “0” representing non-use and “1” for using. The DHS collected a variable for multiple sexual partners as a numeric response. For the study analysis, we recoded this variable into binary to reflect “0” as having one sexual partner and “1” as having two or more sexual partners. HIV testing in the last 12 months was coded as “0” did not take an HIV test and “1” representing uptake on an HIV test.
Independent variables
Based on the review of existing literature on HIV and AIDS in SSA and elsewhere, the study identified correlates at individual and household levels that could be potentially associated with HIV transmission risk factors among men in the region. These variables were classified as socio-economic, demographic and contextual factors. The DHS reference materials and data collection forms were used to identify the independent variables of interest presented in this section. The following independent variables were included in the study analysis: age of a man categorised as (15–24, 25–34, 35–44, 45–54 and 55–59); current marital status (categorised as never married, currently married/living with a partner and formally married; place of residence (urban, rural); education level (no education, primary, secondary, tertiary); literacy (literate, illiterate); household wealth index, this variable is usually captured in the DHS using 5 response categories (poorest, poor, middle, rich, richest). For the analysis, we recoded this variable with the following categorisation (poor, middle, rich); employment status was categorised as (employed, unemployed); age at first sex was coded as (below 15 years, 15–24 years and 25+ years) and circumcision status (yes, no).
Statistical analysis
Data analysis was performed using Stata SE version 17.0 statistical software. All analyses applied “svy” command to account for complex survey design, which considered sample weight. Descriptive analyses were presented using percentages and counts. Cross tabulations with Chi-square tests were conducted on country-specific data to explore the bivariate association between explanatory factors and exposure variables and establish the significance of the relationships across countries. Multivariate binary logistic regression to examine the determinants of HIV risk behaviours among men in the region. The choice of this model was informed by the dichotomous distribution of the dependent variables. Further, a regression model was fitted with all independent correlates identified in the literature. Multivariate logistic regression models were performed to examine the influence of education on HIV risk factors within countries. Based on the regression model, the odds ratios (OR) were calculated, along with their respective 95% confidence intervals (95% CI).
Ethics
This study’s datasets were obtained from the DHS programme’s website (https://dhsprogram.com/), which is freely accessible to the public. Permission to use the dataset was obtained through the study’s registration with the DHS programme. The countries followed all ethical approvals before conducting their respective DHSs. The original DHS biomarker and survey protocols were approved by the respective countries’ Ethical Review Bodies and the Research Ethics Review Board of the Center for Disease Control and Prevention (CDC) Atlanta. There was no need for a separate ethical approval because the study used secondary datasets that did not contain any personally identifying information about the participants.
Results
Condom use during sex with most recent partner
Results show condom use during sex with the most recent partner varied across countries. The percentage ranged from 18.2% in Mozambique to 59.1% in Namibia. In almost all countries in Southern Africa, men with secondary education or higher were less likely to engage in HIV risk behaviour. However, it was not the case in Zimbabwe, where bivariate analysis reveal that education level was not associated with condom use during sex with the most recent partner. Age, residence, marital status and wealth status were statistically significant factors influencing condom use. Consistently, in all countries, older men were less likely to use a condom during sex with a recent partner. Men living in urban areas and those who were single had a higher probability of using a condom with a most recent partner. The study also observed that condom use was higher among men who belonged to rich households (Table 1). Our study further reveals that HIV prevalence rates for men aged 15–59 in Southern Africa range between 8.3% in Zambia to 19.6% in Lesotho. These rates are among the highest in sub-Saharan Africa. HIV prevalence rate is lower among men with secondary education or higher, except in Zambia, where the result is the opposite (Supplementary Table 1).
Table 1.
Percentage distribution of men who used condom with most recent partner by background characteristics, Southern Africa.
| DHS 2014 N = 2255 |
DHS 2011 N = 3521 |
DHS 2013 N = 3229 |
DHS 2016 N = 2935 |
DHS 2015 N = 5855 |
DHS 2018 N = 9224 |
|
|---|---|---|---|---|---|---|
| Background characteristics | Lesotho | Mozambique | Namibia | South Africa | Zimbabwe | Zambia |
| Age | *** | *** | *** | *** | *** | *** |
| 15–24 | 76.3 | 33.3 | 79.2 | 74.1 | 63.1 | 41.5 |
| 25–34 | 59.3 | 16.7 | 64.6 | 52.6 | 23.3 | 22.8 |
| 35–44 | 42.2 | 9.4 | 46.7 | 38.9 | 17.1 | 16.4 |
| 45–54 | 41.8 | 4.0 | 30.3 | 30.9 | 20.6 | 12.4 |
| 55–59 | 27.7 | 1.6 | 23.0 | 18.3 | – | 9.3 |
| Residence | *** | *** | ** | * | *** | *** |
| Urban | 66.8 | 34.9 | 61.4 | 48.7 | 34.1 | 28.8 |
| Rural | 53.8 | 8.5 | 55.7 | 53.9 | 27.3 | 20.7 |
| Marital status | *** | *** | *** | *** | *** | *** |
| Never married | 79.2 | 46.7 | 80.7 | 69.4 | 81.6 | 50.8 |
| Married | 40.9 | 8.3 | 30.5 | 25.2 | 11.9 | 12.6 |
| Formerly married | 56.8 | 22.2 | 61.4 | 59.0 | 70.8 | 42.1 |
| Age at first sex | *** | ** | ** | ns | *** | ** |
| Below 15 years | 66.7 | 26.8 | 67.6 | 55.4 | 40.3 | 27.1 |
| 15–24 years | 57.9 | 16.9 | 58.2 | 49.7 | 31.6 | 24.0 |
| 25+ | 41.7 | 16.4 | 52.4 | 45.4 | 14.1 | 17.2 |
| Education level | *** | *** | *** | *** | ns | *** |
| None | 35.6 | 2.0 | 49.4 | 31.9 | 15.3 | 16.6 |
| Primary | 52.3 | 11.1 | 51.3 | 37.2 | 28.7 | 16.8 |
| Secondary | 70.3 | 40.8 | 63.9 | 54.8 | 30.9 | 29.8 |
| Higher | 67.5 | 53.5 | 57.5 | 42.2 | 27.7 | 29.2 |
| Literacy | *** | *** | *** | ns | ns | *** |
| No | 36.9 | 5.1 | 42.9 | 42.9 | 32.0 | 17.2 |
| Yes | 62.8 | 24.5 | 60.9 | 50.7 | 29.8 | 25.6 |
| Wealth status | *** | *** | * | * | * | *** |
| Poor | 46.1 | 3.6 | 55.0 | 48.5 | 27.5 | 20.6 |
| Middle | 60.5 | 12.1 | 63.6 | 58.2 | 29.2 | 23.6 |
| Rich | 65.2 | 33.5 | 59.7 | 47.9 | 32.3 | 27.3 |
| Working status | ns | *** | *** | *** | *** | *** |
| No | 59.1 | 40.5 | 68.1 | 59.6 | 40.0 | 39.4 |
| Yes | 57.8 | 15.1 | 54.5 | 41.9 | 26.9 | 21.5 |
| Circumcision status | ns | * | ns | ns | *** | *** |
| No | 59.2 | 20.2 | 60.2 | 45.8 | 28.5 | 21.3 |
| Yes | 58.0 | 16.3 | 56.3 | 53.3 | 39.3 | 30.4 |
| Total | 58.3% | 18.2% | 59.1% | 50.2% | 29.9% | 24.0% |
***p < 0.0001; **p < 0.01; *p < 0.05; ns not significant.
Multiple sexual partnerships
The overall patterns in the prevalence of men who had multiple sexual partners were lowest in Namibia (13.7%) and highest in Lesotho (32.9%). The findings show that education was inversely related to having multiple sexual partnerships in men in Lesotho, Namibia, South Africa, and Zambia. An increase in the level of education was associated with a higher chance of having multiple sexual partners. Bivariate analysis shows that a man’s age and age at first sex were significantly associated with having multiple partnerships (p < 0.001) in all countries. Findings reveal that as age increases, the risk of engaging in multiple sexual partners reduces among men in the region. Men who began having sexual intercourse at an early age and those from rich households had a higher probability of engaging in multiple partnerships. Men who were living in rural areas from Namibia, South Africa, Zimbabwe and Zambia were engaging in multiple partnerships more than their counterparts in urban areas. In terms of marital status, the never-married men had higher proportions of engaging in multiple sexual relations.
Uptake of HIV testing
The overall pattern in the prevalence of men who had an HIV test in the last 12 months prior to the DHS was lowest in Mozambique (23%) and highest in Zambia (77.9%). Our findings show that education was positively related to the uptake of an HIV test among men in all the countries. Similarly, in almost all the countries, men who belonged to rich households had a higher proportion of HIV uptake. Living in urban areas was associated with a higher chance of taking an HIV test in all the countries (p < 0.000). Bivariate analysis shows that a man’s age and age at first sex were significantly associated with HIV uptake except for South Africa, where an HIV test uptake was not associated with age at first sex. Findings reveal that the uptake of HIV tests was higher among men who were circumcised in countries except for Mozambique. In terms of work status, men who were working had higher proportions of HIV uptake.
Determinants of HIV transmission risk behaviour among men
Multivariate binary logistic regressions were conducted on a pooled dataset to examine the influence of independent correlates of HIV transmission risk factors among sexually active men aged 15–59 years in Southern Africa. The study found that the age of a man, place of residence, household wealth status and employment status were significantly associated with all the three HIV risk factors examined in this study; that is condom use during sex with the most recent partner, multiple sexual partnerships and HIV testing.
Our study suggests that increasing age was associated with a higher HIV risk behaviour in men. The results show that men in older age groups had lower odds of using condoms during sex with a most recent partner (AOR = 0.63; 95% CI: (0.49–0.81); p < 0.001). Men living in rural areas (AOR = 0.72; 95% CI: (0.66–0.78); p < 0.001) and those who were married (AOR = 0.11; 95% CI: (0.10–0.12); p < 0.001) or formerly married were (AOR = 0.59; 95% CI: (0.50–0.69); p < 0.001), respectively, less likely to use a condom during sex with most recent partners than their counterparts in defined reference categories. Men with secondary or higher levels of education had higher odds of using a condom during a sexual encounter with the most recent partner, though the results were not significant. Similarly, men who belonged to middle or high wealth status families were more likely to use a condom during sex with most recent partners (AOR = 1.25; 95% CI: 1.13–1.39; p < 0.001) and (AOR = 1.30; 95% CI: 1.19–1.42; p < 0.001), respectively. Our study also found that men who were circumcised were more likely to use condoms during sexual intercourse with a recent partner (AOR = 1.17; 95% CI: 1.08–1.27; p < 0.001).
The study further reveals that older men had lower odds of engaging in multiple sexual partnerships in the region (p < 0.001) compared to young men. Men living in rural areas and those who were formerly married were more likely to have multiple sexual partnerships than their counterparts living in urban areas and the never married, respectively. The study further found that education level was not associated with engaging in multiple sexual partnerships. Study results also show that men who belonged to moderate, and those who belonged to rich households were more likely to have multiple sexual partnerships than those from poor households (AOR = 1.22; 95% CI: 1.10–1.35; p < 0.001) and (AOR = 1.36; 95% CI: 1.24–1.50; p < 0.001), respectively. Additionally, the study also found that men who were circumcised were more likely to have multiple sexual partnerships (AOR = 1.27; 95% CI: 1.17–1.37; p < 0.001).
Regarding HIV testing uptake, our study found that age, residence, marital status, education, household wealth status and employment status were associated with the uptake of HIV testing among sexually active men in the region. The odds of HIV testing were higher among men in the age groups 25–34 and 35–44 years (AOR = 2.04; 95% CI: 1.85–2.25; p < 0.001) and (AOR = 2.13; 95% CI: 1.89–2.39; p < 0.001), respectively. Men living in rural areas had lower odds (AOR = 0.83; 95% CI: 0.75–0.92) of taking an HIV test compared to their counterparts living in urban areas. Married men and those who were formerly married (AOR = 1.51; 95% CI: 1.38–1.66) and (AOR = 1.36; 95% CI: 1.16–1.59), respectively, were more likely to take an HIV test than those who never-married men. Men with secondary or higher levels of education were more likely to take an HIV test than men with no education. Similarly, men who belonged to rich households were more likely to take an HIV test (AOR = 1.21; 95% 1.10–1.34; p < 0.001) compared to those who belonged to a poor household. This study has established that being circumcised or being employed was not associated with the uptake of an HIV test among men (Tables 2–4).
Table 3.
Percentage distribution of men who tested for HIV and received results in the last 12 months by background characteristics, Southern Africa.
| DHS 2014 N = 2255 |
DHS 2011 N = 3521 |
DHS 2013 N = 3229 |
DHS 2016 N = 2935 |
DHS 2015 N = 5855 |
DHS 2018 N = 9224 |
|
|---|---|---|---|---|---|---|
| Background characteristics | Lesotho | Mozambique | Namibia | South Africa | Zimbabwe | Zambia |
| Age | *** | *** | *** | *** | *** | *** |
| 15–24 | 53.0 | 17.7 | 41.2 | 58 | 47.5 | 61.5 |
| 25–34 | 74.2 | 31.9 | 78.8 | 78.5 | 76.9 | 89.7 |
| 35–44 | 78.8 | 24.8 | 82.2 | 80.4 | 78.2 | 89.9 |
| 45–54 | 77.7 | 22.5 | 75.8 | 77.2 | 73.6 | 86.6 |
| 55–59 | 76.7 | 15.8 | 74.0 | 76.7 | – | 80.7 |
| Residence | *** | *** | *** | *** | *** | *** |
| Urban | 77.3 | 36.3 | 71.8 | 74.1 | 70.1 | 81.0 |
| Rural | 60.8 | 15.5 | 54.1 | 66.2 | 61.7 | 75.5 |
| Marital status | *** | *** | *** | *** | *** | *** |
| Never married | 55.6 | 18.1 | 71.8 | 64.1 | 47.1 | 62.1 |
| Married | 78.4 | 25 | 54.1 | 82.3 | 78.2 | 90.1 |
| Formerly married | 75.2 | 32.3 | 67.6 | 83.6 | 76.2 | 83.9 |
| Age at first sex | ** | ** | ** | ns | ** | *** |
| Below 15 years | 61.5 | 21.2 | 65.3 | 75.5 | 66 | 75.9 |
| 15–24 years | 70.9 | 25.0 | 71.8 | 76.5 | 73.6 | 85.1 |
| 25+ | 71.2 | 41.5 | 81.0 | 74.7 | 77.2 | 84.6 |
| Education level | *** | *** | *** | *** | *** | *** |
| None | 62.6 | 9.5 | 56.7 | 69.2 | 53.7 | 67.8 |
| Primary | 60.2 | 16.6 | 55.1 | 63.0 | 52.4 | 70.5 |
| Secondary | 71.0 | 41.1 | 65.4 | 71.5 | 65.8 | 82.1 |
| Higher | 85.0 | 77.6 | 85.7 | 84.2 | 84.7 | 92.8 |
| Literacy | *** | *** | *** | ** | *** | *** |
| No | 55.1 | 11.8 | 49.5 | 61.2 | 46.2 | 68.2 |
| Yes | 68.7 | 28.5 | 65.7 | 72.4 | 65.9 | 80.1 |
| Wealth status | *** | *** | *** | * | ns | ns |
| Poor | 59.5 | 8.2 | 54.6 | 68.9 | 64.0 | 76.9 |
| Middle | 60.2 | 14.0 | 62.1 | 71.6 | 64.6 | 76.7 |
| Rich | 73.9 | 39.8 | 72.1 | 74.8 | 65.3 | 79.3 |
| Working status | ** | * | *** | *** | *** | *** |
| No | 62.4 | 19.5 | 47.3 | 65.2 | 53.6 | 61.8 |
| Yes | 69.2 | 24.0 | 76.6 | 79.0 | 70.5 | 83.2 |
| Circumcision status | *** | ns | *** | *** | *** | *** |
| No | 54.6 | 24.9 | 60.7 | 65.8 | 61.5 | 76.3 |
| Yes | 70.9 | 21.5 | 73.7 | 76.4 | 83.7 | 81.7 |
| Total | 66.4% | 23.% | 64.0% | 71.6% | 64.7% | 77.9% |
***p < 0.0001; **p < 0.01; *p < 0.05; ns not significant.
Table 2.
Percentage distribution of men with multiple sexual partnerships by background characteristics, Southern Africa.
| DHS 2014 N = 2255 |
DHS 2011 N = 3521 |
DHS 2013 N = 3229 |
DHS 2016 N = 2935 |
DHS 2015 N = 5855 |
DHS 2018 N = 9224 |
|
|---|---|---|---|---|---|---|
| Background characteristics | Lesotho | Mozambique | Namibia | South Africa | Zimbabwe | Zambia |
| Age | *** | *** | *** | *** | *** | *** |
| 15–24 | 37.2 | 33.2 | 16.9 | 32.4 | 25.0 | 21.2 |
| 25–34 | 41.1 | 36 | 16.2 | 21.4 | 21.7 | 22.6 |
| 35–44 | 26.5 | 31.5 | 9.8 | 12.1 | 17.6 | 18 |
| 45–54 | 18.6 | 23.2 | 8.3 | 6.6 | 16.5 | 16.8 |
| 55–59 | 11.3 | 20.6 | 6.7 | 5.3 | – | 12.9 |
| Residence | * | ns | * | ** | ** | *** |
| Urban | 38.6 | 33.7 | 12.3 | 17.4 | 22.6 | 16.3 |
| Rural | 29.9 | 30.5 | 15.7 | 23.5 | 19.2 | 22.3 |
| Marital status | ** | * | *** | *** | *** | ns |
| Never married | 37.3 | 35.3 | 18.8 | 26.5 | 26.9 | 21.6 |
| Married | 29.5 | 30.2 | 7.1 | 9.7 | 17.9 | 19.2 |
| Formerly married | 31.5 | 35.2 | 10.9 | 22 | 30.7 | 19.5 |
| Age at first sex | *** | *** | ** | *** | *** | *** |
| Below 15 years | 45.9 | 45.7 | 20.3 | 28.9 | 32.2 | 29.2 |
| 15–24 years | 32.3 | 29.8 | 12.8 | 18 | 21.8 | 19.1 |
| 25+ | 12.6 | 10.2 | 9.7 | 5.7 | 7.1 | 6.7 |
| Education level | ** | ns | * | *** | ns | * |
| None | 22.1 | 34.8 | 8.6 | 9.1 | 13.6 | 16.6 |
| Primary | 30.0 | 28.0 | 12.4 | 11.6 | 19.5 | 19.9 |
| Secondary | 37.7 | 38.1 | 14.1 | 20.7 | 21.3 | 20.9 |
| Higher | 41.9 | 32.4 | 18.1 | 21.0 | 18.6 | 16.2 |
| Literacy | *** | ns | * | ** | ns | ns |
| No | 23.7 | 30.4 | 9.6 | 14.4 | 17.4 | 18.5 |
| Yes | 34.9 | 32.3 | 14.1 | 19.5 | 20.7 | 20.2 |
| Wealth status | * | ** | ns | ** | ns | ** |
| Poor | 29.2 | 27.2 | 14.2 | 17.0 | 18.9 | 17.3 |
| Middle | 29.3 | 32.4 | 12.9 | 26.0 | 20.0 | 21.5 |
| Rich | 36.9 | 35.3 | 13.7 | 18.0 | 22.1 | 21.5 |
| Working status | ** | ** | ns | * | ns | ns |
| No | 28.8 | 24.2 | 14.7 | 21.5 | 19.7 | 21.5 |
| Yes | 35.2 | 32.8 | 13.2 | 17.1 | 20.7 | 19.6 |
| Circumcision status | ns | ns | ns | ** | ns | ns |
| No | 28.4 | 30.4 | 14.2 | 15.0 | 20.4 | 19.7 |
| Yes | 34.4 | 32.9 | 12.4 | 22.1 | 20.9 | 20.4 |
| Total | 32.9% | 31.7% | 13.7% | 19.2% | 20.5% | 19.9% |
***p < 0.0001; **p < 0.01; *p < 0.05; ns not significant.
Table 4.
Results of pooled multivariate analyses examining the effect of individual-level factors on HIV risk behaviours among men in Southern Africa.
| Condom use with most recent partner | Multiple partnerships | HIV testing in last 12 months | ||||
|---|---|---|---|---|---|---|
| Background characteristics | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI |
| Age | ||||||
| 15–24 | 1 | 1 | 1 | |||
| 25–34 | 1.14* | (1.02–1.28) | 1.05 | (0.94–1.18) | 2.04*** | (1.85–2.25) |
| 35–44 | 1.07 | (0.94–1.21) | 0.75*** | (0.66–0.85) | 2.13*** | (1.89–2.39) |
| 45–54 | 0.92 | (0.80–1.07) | 0.63*** | (0.54–0.73) | 1.80*** | (1.58–2.04) |
| 55–59 | 0.63*** | (0.49–0.81) | 0.45*** | (0.35–0.59) | 1.51*** | (1.25–1.83) |
| Residence | ||||||
| Urban | 1 | 1 | 1 | |||
| Rural | 0.72*** | (0.66–0.78) | 1.24*** | (1.13–1.36) | 0.83*** | (0.75–0.92) |
| Marital status | ||||||
| Never married | 1 | 1 | 1 | |||
| Married | 0.11*** | (0.10–0.12) | 0.89* | (0.80–1.00) | 1.51*** | (1.38–1.66) |
| Formerly married | 0.59*** | (0.50–0.69) | 1.25* | (1.05–1.49) | 1.36*** | (1.16–1.59) |
| Age at first sex | ||||||
| Below 15 years | 1 | 1 | 1 | |||
| 15–24 years | 1.04 | (0.93–1.16) | 0.60*** | (0.55–0.66) | 1.04 | (0.94–1.15) |
| 25+ | 1.04 | (0.85–1.26) | 0.22*** | (0.17–0.28) | 1.02 | (0.84–1.22) |
| Education level | ||||||
| None | 1 | 1 | 1 | |||
| Primary | 0.81* | (0.67–0.97) | 0.89 | (0.74–1.08) | 1.44*** | (1.24–1.66) |
| Secondary | 1.11 | (0.91–1.36) | 0.87 | (0.71–1.05) | 3.10*** | (2.64–3.64) |
| Higher | 1.04 | (0.82–1.32) | 0.83 | (0.66–1.05) | 5.19*** | (4.18–6.45) |
| Literacy | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 1.69*** | (1.47–1.94) | 1.09 | (0.96–1.23) | 1.35*** | (1.22–1.49) |
| Wealth status | ||||||
| Poor | 1 | 1 | 1 | |||
| Middle | 1.25*** | (1.13–1.39) | 1.22*** | (1.10–1.35) | 1.03 | (0.93–1.14) |
| Rich | 1.30*** | (1.19–1.42) | 1.36*** | (1.24–1.50) | 1.21*** | (1.10–1.34) |
| Working status | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 0.73*** | (0.66–0.81) | 1.22*** | (1.12–1.34) | 1.00 | (0.92–1.09) |
| Circumcision status | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 1.17*** | (1.08–1.27) | 1.27*** | (1.17–1.37) | 1.03 | (0.95–1.11) |
***p < 0.0001; **p < 0.01; *p < 0.05.
Factors associated with HIV risk behaviour among men in the individual countries
Tables 5 and 6 show country-level multivariate analysis of several independent correlates that are associated with condom use and HIV testing. The findings in Table 5 show that there are country-level variations in the way independent correlates influence condom use during sex with a most recent sexual partner among men in Southern African countries. In the full regression model, education was significantly associated with condom use among men only in Mozambique and Zimbabwe. An increase in the level of education was associated with higher odds of using condoms among men in the two countries. Findings show that an increase in the age of men was associated with lower odds of using condoms in Lesotho, Mozambique, Namibia and South Africa. The results were the opposite in Zimbabwe, where older men were more likely to use a condom during sexual debut with most recent partners compared to young men. In Zambia, age was not significantly associated with condom use. The odds of condom use were lower among men living in rural areas in all the countries except for South Africa. Compared to the poor, men in Lesotho from a middle wealth quantile (AOR = 1.47, 95% CI = 1.07–2.00) and those from both moderate (AOR = 2.79, 95% CI = 1.68–4.68) and rich households (AOR = 4.90, 95% CI = 2.93–8.20) in Mozambique were more likely to use condoms during sexual intercourse with most recent partners. Our study also found that the level of education was not significantly associated with having multiple sexual partnerships in all the countries. Age at first sex was found to be inversely associated with having multiple partnerships; men who initiate sexual debut late were less likely to have multiple sexual partnerships (Supplementary file 2: Table 2).
Table 5.
Results of multivariate analyses examining the effect of individual-level factors on HIV risk behaviour among men by country in Southern Africa countries.
| Condom use with most recent partner | ||||||
|---|---|---|---|---|---|---|
| Lesotho | Mozambique | Namibia | South Africa | Zimbabwe | Zambia | |
| Background characteristics | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) |
| Age | ||||||
| 15–24 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25–34 | 0.94 (0.66–1.34) | 1.07 (0.79–1.45) | 0.88 (0.67–1.16) | 0.88 (0.67–1.16) | 1.06 (0.79–1.42) | 1.12 (0.89–1.41) |
| 35–44 | 0.63* (0.66–1.34) | 0.79 (0.52–1.18) | 0.65* (0.47–0.89) | 0.65* (0.47–0.89) | 1.67** (1.25–2.24) | 1.13 (0.88–1.44) |
| 45–54 | 0.70 (0.66–1.34) | 0.28*** (0.14–0.53) | 0.42*** (0.28–0.62) | 0.42*** (0.28–0.63) | 2.20*** (1.51–3.20) | 0.86 (0.66–1.13) |
| 55–59 | 0.37** (0.66–1.34) | 0.16*** (0.06–0.41) | 0.38** (0.21–0.68) | 0.24*** (0.14–0.43) | – | 0.69 (0.43–0.10) |
| Residence | ||||||
| Urban | 1 | 1 | 1 | 1 | 1 | 1 |
| Rural | 0.69* (0.50–0.94) | 0.53** (0.36–0.76) | 0.69** (0.55–0.87) | 0.97 (0.77–1.23) | 0.75** (0.62–0.90) | 0.84* (0.73–0.97) |
| Marital status | ||||||
| Never married | 1 | 1 | 1 | 1 | 1 | 1 |
| Married | 0.22*** (0.16–0.31) | 0.21*** (0.15–0.29) | 0.14*** (0.10–0.18) | 0.23*** (0.17–0.30) | 0.02*** (0.02–0.03) | 0.15*** (0.12–0.18) |
| Formerly married | 0.48** (0.30–0.79) | 0.59** (0.37–0.93) | 0.53* (0.33–0.85) | 1.00 (0.62–1.60) | 0.43*** (0.30–0.61) | 0.75 (0.55–1.02) |
| Age at first sex | ||||||
| Below 15 years | 1 | 1 | 1 | 1 | 1 | 1 |
| 15–24 years | 1.15 (0.83–1.58) | 0.75 (0.55–1.02) | 0.96 (0.71–1.31) | 1.08 (0.78–1.49) | 1.14 (0.80–1.62) | 1.05 (0.87–1.25) |
| 25+ years | 0.48 (0.63–1.85) | 1.88 (0.39–0.10) | 1.93* (1.08–3.44) | 1.94* (1.03–3.65) | 0.77 (0.49–1.23) | 0.94* (0.67–1.31) |
| Education level | ||||||
| No education | 1 | 1 | 1 | 1 | 1 | 1 |
| Primary | 0.74 (0.45–1.23) | 2.94* (1.28–6.75) | 0.71 (0.46–1.08) | 0.77 (0.42–1.43) | 2.58* (1.08–6.16) | 0.79 (0.55–1.12) |
| Secondary | 1.01 (0.59–1.74) | 5.62*** (2.28–3.89) | 0.83 (0.53–1.30) | 1.05 (0.56–2.00) | 2.41* (1.01–5.75) | 1.23 (0.84–1.81) |
| Tertiary | 0.95 (0.47–1.89) | 10.23*** (3.89–16.89) | 0.79 (0.45–1.37) | 0.74 (0.37–1.50) | 2.27 (0.93–5.54) | 1.31 (0.84–2.05) |
| Literacy | ||||||
| Illiterate | 1 | 1 | 1 | 1 | 1 | 1 |
| Literacy | 2.10** (1.07–3.27) | 1.34 (0.85–2.10) | 1.97** (1.27–3.06) | 0.95 (0.62–1.45) | 1.29 (0.86–1.94) | 1.21** (0.97–1.49) |
| Wealth status | ||||||
| Poor | 1 | 1 | 1 | 1 | 1 | 1 |
| Moderate | 1.47* (1.07–2.00) | 2.79*** (1.68–4.63) | 1.20 (0.90–1.60) | 1.42 (1.03–1.98) | 0.86 (0.68–1.09) | 1.06 (0.90–1.25) |
| Rich | 1.29 (0.94–1.77) | 4.90*** (2.93–8.20) | 0.99 (0.74–1.33) | 1.25 (0.96–1.63) | 0.93 (0.74–1.15) | 1.13 (0.97–1.31) |
| Working status | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.07 (0.83–1.39) | 0.75 (0.53–1.04) | 0.84 (0.67–1.06) | 0.89 (0.69–1.15) | 0.89 (0.72–1.09) | 0.92 (0.74–1.14) |
| Circumcision status | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.09 (0.83–1.42) | 0.74* (0.58–0.94) | 1.06 (0.84–1.34) | 1.17 (0.92–1.50) | 0.92 (0.69–1.22) | 1.10 (0.95–1.27) |
***p < 0.0001; **p < 0.01; *p < 0.05.
Table 6.
Results of multivariate analyses examining the effect of individual-level factors on HIV risk behaviour among men by country in Southern Africa countries.
| HIV testing in the last 12 months prior to survey | ||||||
|---|---|---|---|---|---|---|
| Lesotho | Mozambique | Namibia | South Africa | Zimbabwe | Zambia | |
| Background Characteristics | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) |
| Age | ||||||
| 15–24 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25–34 | 2.15 (1.60–2.90)*** | 1.67 (1.22–2.30)** | 3.11 (2.35–4.12)*** | 1.56 (1.15–2.11)* | 1.41 (1.14–1.74)** | 1.83 (1.50–2.23)*** |
| 35–44 | 2.96 (1.89–4.62)*** | 1.23 (0.87–1.74) | 4.04 (3.02–5.40)*** | 1.61 (1.13–2.31)* | 1.18 (0.92–1.52) | 1.57 (1.23–2.02)*** |
| 45–54 | 3.02 (1.87–4.89)*** | 1.01 (0.70–1.45) | 2.68 (1.85–3.88)*** | 1.29 (0.84–1.99) | 0.90 (0.68–1.20) | 1.23 (0.95–1.59) |
| 55–59 | 3.34 (1.83–6.11)*** | 0.77 (0.45–1.32)*** | 2.72 (1.59–4.68)*** | 1.25 (0.74–2.11) | _ | 0.74 (0.50–1.08) |
| Residence | ||||||
| Urban | 1 | 1 | 1 | 1 | 1 | 1 |
| Rural | 0.56 (0.42–0.77)*** | 0.97 (0.72–1.30) | 0.70 (0.56–0.88) | 0.85 (0.67–1.09) | 1.00 (0.86–1.18) | 0.81 (0.65–1.01) |
| Marital status | ||||||
| Never married | 1 | 1 | 1 | 1 | 1 | 1 |
| Married | 1.80 (1.33–2.44)*** | 2.22 (1.61–3.07)*** | 1.23 (10.97–1.56) | 1.73 (1.29–2.31)*** | 2.45 (2.02–2.97)*** | 3.70 (2.94–4.66)*** |
| Formerly married | 1.37 (0.84–2.24) | 2.66 (1.67–4.00)*** | 0.62 (0.38–0.99)* | 1.99 (1.13–3.51)* | 2.24 (1.62–3.10)*** | 1.97 (1.39–2.80)*** |
| Age at first sex | ||||||
| Below 15 years | 1 | 1 | 1 | 1 | 1 | 1 |
| 15–24 years | 0.93 (0.68–1.27) | 1.14 (0.87–1.49) | 1.07 (0.80–1.42) | 0.92 (0.68–1.24) | 1.19 (0.91–1.56) | 1.18 (0.98–1.42) |
| 25+ years | 0.72 (0.42–1.25) | 2.59 (0.98–6.81) | 1.32 (0.76–2.29) | 0.86 (0.42–1.74) | 1.09 (0.77–1.53) | 0.70 (0.48–1.02) |
| Education level | ||||||
| No education | 1 | 1 | 1 | 1 | 1 | 1 |
| Primary | 0.93 (0.60–1.43) | 1.59 (1.04–2.42)* | 1.10 (0.78–1.55) | 0.77 (0.46–1.31) | 0.78 (0.42–1.46) | 1.09 (0.79–1.50) |
| Secondary | 1.64 (0.99–2.71) | 4.41 (2.68–7.26)*** | 1.65 (1.14–2.40)* | 1.09 (0.60–1.98) | 1.31 (0.70–2.46) | 1.93 (1.37–2.72)*** |
| Tertiary | 1.99 (1.01–3.92)* | 15.52 (7.61–31.67)*** | 2.35 (1.30–4.25)* | 1.42 (0.69–2.91) | 2.82 (1.45–5.50)** | 2.48 (1.48–4.15)** |
| Literacy | ||||||
| Illiterate | 1 | 1 | 1 | 1 | 1 | 1 |
| Literacy | 1.99 (1.42–2.79)*** | 1.99 (1.42–2.79)*** | 1.79 (1.32–2.43)*** | 1.51 (0.99–2.30) | 1.30 (0.99–1.72) | 1.43 (1.22–1.68)*** |
| Wealth status | ||||||
| Poor | 1 | 1 | 1 | 1 | 1 | 1 |
| Moderate | 0.86 (0.65–1.15) | 1.73 (1.19–2.53)* | 0.98 (0.78–1.23) | 1.18 (0.85–1.66) | 1.01 (0.83–1.22) | 1.03 (0.85–1.24) |
| Rich | 1.15 (0.85–1.54) | 5.13 (3.64–7.25)*** | 1.25 (0.94–1.67) | 1.43 (1.09–1.88)* | 1.10 (0.93–1.29) | 1.33 (1.11–1.58)** |
| Working status | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 0.85 (0.67–1.08) | 0.98 (0.71–1.36) | 1.61 (1.30–2.00)*** | 1.02 (0.80–1.29) | 1.03 (0.88–1.22) | 1.11 (0.96–1.30) |
| Circumcision status | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 2.05 (1.61–2.61)*** | 0.54 (0.42–0.70)*** | 1.48 (1.18–1.84)** | 1.60 (1.28–2.00)*** | 3.12 (2.25–4.34)*** | 1.40 (1.20–1.63)*** |
***p < 0.0001; **p < 0.01; *p < 0.05
Findings in Table 6 show that uptake of HIV testing among sexually-active men varies across countries in Southern Africa. Multivariate regression analysis shows that education was significantly associated with HIV testing among men in Lesotho, Mozambique, Namibia, Zimbabwe and Zambia. An increase in the level of education was associated with higher odds of HIV testing uptake among men in these countries. Results further show that in Lesotho and Namibia, older men were more likely to take an HIV test compared to young men aged 15–24. In Mozambique and Zambia, men aged 55–59 were less likely to take an HIV test compared to men aged 15–24. The odds of HIV testing uptake were lower among men living in rural areas only in Lesotho. Compared to men from poor households, those from both moderate (AOR = 1.73, 95% CI = 1.19–2.53) and rich households (AOR = 5.13, 95% CI = 3.64–7.25) in Mozambique, were more likely to take an HIV test. Furthermore, men from rich households (AOR = 1.43, 95% CI = 1.09–1.88) in South Africa, were more likely to take an HIV test. The study found that age at first sex was not associated with undertaking an HIV test among sexually active men in all the countries. Additionally, in all the countries except for Mozambique, circumcised men were more likely to undertake an HIV test.
Discussion
This study sought to analyse the influence of education and other individual correlates on HIV transmission risk behaviour in men, and examine differentials of the risk factors across countries in Southern Africa. The study utilised the most recent cross-sectional data collected in the respective countries. The study focused on the influence of individual-level factors on HIV risk behaviour among men in the Southern African region, and also at the country level. Our review of the literature reveals that there is little or no known evidence of a comprehensive study of this nature that has been conducted to inform HIV risk behaviour among sexually active men in the region. Thus, analysis done using pooled data bolsters the importance of the findings to inform regional HIV and AIDS public health policy and programmes to further reduce the regional HIV prevalence.
Even though the use of condoms is one of the most effective ways to reduce the sexual transmission of HIV, the rate at which condoms are used in countries in the region is still unacceptably low. The study findings show that overall, condom use during sex with most recent partners is not influenced by the level of education among men. Prior studies have identified multiple sexual relations as a major hindrance to reducing HIV transmission in SSA (Deeks et al., 2016; Mutinta, 2014; Nalukwago et al., 2018; Onoya et al., 2015). Peer influence, parental and societal indifference to polygamy and male dominance in sexual relation decisions as major factors influencing men’s decision to engage in multiple sexual relations. Our study found that education had no influence on men’s decision to engage in multiple sexual partnerships. However, education level showed a positive association with the uptake of HIV testing among men. The study also shows that there are major disparities in the prevalence of HIV testing among men in different educational sub-categories across countries. Furthermore, we found that married men and those who were formerly married were more likely to use a condom and take an HIV test than never-married men. This could imply that this positive sexual behaviour being adopted by married men is meant to protect their spouses within the marriage institution. The findings of this study have implications for both shaping public health policy and re-designing sexual reproductive health programmes in the region, considering the COVID-19 pandemic that may pose significant challenges in accessing sexual reproductive health services, especially among marginalised communities.
Recent studies on education and HIV/AIDS in SSA have shown sound evidence of the association between education and reduced risk of HIV transmission, suggesting the need for countries to invest in the education sector in order to maximise social behaviour change in men (De Neve et al., 2015; Leon et al., 2017; Pettifor et al., 2008). However, it is important to note that because of the socio-cultural heterogeneity of sub-Saharan African countries, the influence of education on HIV risk behaviour among men may not be similar in all the countries. Our study findings further reveal that, at the regional level, an increasing level of education was not associated with a higher probability of using condoms during a sexual encounter with most recent partners among men. This means that in the pooled analysis, the educational level of a man did not play a significant role in determining a positive decision to avoid HIV transmission risk. Individual country-level analysis shows that an increasing level of education was associated with increased odds of using condoms among men in Mozambique and Zimbabwe. This, therefore, shows a positive association between education and condom use in these two countries, implying that promoting education should be a priority in influencing safer sex behaviour among men in Mozambique and Zimbabwe.
Other studies have also reported that men who are well educated are better able to seek more accurate information with which to assess their risks and develop preventative measures (Exavery et al., 2012; Jung et al., 2013; Leon et al., 2017; Manlove et al., 2008; Zhao et al., 2012). This, therefore, suggests that men with low levels of education may be at high risk of contracting the HIV virus. Related studies confirm this finding in other SSA countries. A study conducted in 2017 in four countries (Ghana, Kenya, Tanzania and Cameroon) found that people with no or primary education had a greater risk of acquiring HIV than those with secondary education or higher (Leon et al., 2017). Theoretically, multiple sexual partnerships can play a negative role in the spread of HIV in most societies through multiple sexual networks (Lurie and Rosenthal, 2010). Though no conclusive evidence is available on its relationship with HIV prevalence, multiple sexual partnerships might increase the risk of acquiring HIV if coupled with inconsistent condom use or non-safe sex practices (Maher et al., 2011; SAfAIDS, 2015). Some recent studies have reported multiple sexual partnerships and inconsistent condom use as among the key drivers of HIV transmission among men in sub-Saharan Africa (Manjengwa et al., 2019). However, this study found that educational level was not associated with men’s decision to engage in multiple sexual partnerships in the region. There were no differences in proportions of men engaging in multiple sexual relations by education level. The decision to engage in multiple sexual relations appears to be a personal intrinsic choice to satisfy one’s sexual desire rather than a decision informed by education. This finding has implications for the success of behaviour change campaigns, which aim at encouraging men to stick to one faithful partner. A significant component of social behaviour change campaigns in SSA has a target to reduce multiple partnerships in order to reduce exposure to HIV infection (Khidir et al., 2020; SAfAIDS, 2015).
HIV testing is regarded as one important step in the prevention of HIV transmission, especially among men with more than a sexual partner (Awopegba et al., 2021; Olakunde et al., 2020a). It is expected that men who know their HIV status should make an informed decision about prevention measures; that is correct and consistent use of condoms to avoid spreading the HIV virus or preventing infection of their partners, if HIV positive. Studies conducted in South Africa, Nigeria and Ghana on HIV testing coverage found that men with a higher level of education were more likely to test for HIV (Awopegba et al., 2021; Nyarko and Sparks, 2020; Olakunde et al., 2020a). These studies are consistent with the findings of our study. Other studies have shown that educated persons are highly likely to be knowledgeable about disease preventive measures and be able to make informed decisions to present themselves early for HIV treatment (Hachfeld et al., 2019; Mulemena et al., 2020). Non-acceptance of HIV testing among men with lower levels of education has an implication for the early initiation of HIV-positive clients on antiretroviral treatment (ART). Community and school-based HIV prevention campaigns championing HIV self-testing will be key to reaching universal HIV testing coverage.
Community and school-based interventions supporting self-HIV testing campaigns have been documented to be among the successful approaches to enhancing acceptance and uptake of HIV testing in Tanzania, Uganda, Malawi and Kenya (Chamie et al., 2014; Harper et al., 2018; Kumwenda et al., 2019; Kurth et al., 2015; Mangenah et al., 2019; Njau et al., 2021). Njau et al. (2021) in Tanzania found that HIV self-testing initiatives were workable to implement in settings where the majority of community members had positive attitudes and supportive perceived norms towards HIV prevention. In South Africa, Mokgatle (2017) proposed extending school-based self-HIV testing interventions to communities because of its effectiveness in promoting acceptance of HIV testing. In Kenya, Harper et al. (2018) reported that school-based HIV testing campaigns had the potential to become institutionalised in school settings in order to maintain their long-term sustainability (Harper et al., 2018; Mokgatle and Madiba, 2017; Njau et al., 2021).
The reviewed literature and analysis of this study show that education plays a part in HIV transmission among men in some countries in Southern Africa. However, the influence of education on various HIV risk factors is not uniform. Despite similarities in socio-economic and cultural contexts among countries in the region, analyses in this study present heterogeneity in the influence of education on different HIV risk factors. Other studies on SSA have found that other factors (i.e. age, residence, household wealth status, religious affiliation and employment status) were equally important factors influencing HIV transmission in SSA (Asaolu et al., 2016b; Dake et al., 2012; Devine-Wright et al., 2015; Nyarko and Sparks, 2020; Oster, 2012; Voisin et al., 2012). Detailed decomposition analysis of factors’ contribution to the reduction in HIV prevalence at the country level would be useful to inform adequate conclusions on key factors influencing HIV risk behaviour and transmissions in Southern African countries.
Conclusion
The study showed that education matters in explaining the uptake of HIV testing among sexually active men in Southern African countries. Country-level variations exist in terms of how education influences HIV transmission risk behaviour, such as condom use and HIV testing, among men. Age of men, place of residence, marital status, wealth status and circumcision status were found to be significantly associated with HIV risk behaviour in men. Therefore, investing in education could be one of the potential long-term cost-effective interventions to reduce HIV infections among men in some countries in the region, as it has shown potential to improve HIV testing uptake in men. The study has also established that HIV prevention campaigns targeting men at the country level are yielding different results in the region. Therefore, there is the need to re-evaluate country-level HIV prevention programmes across the region to identify best practices. There is also a need for countries in the sub-region to consider strengthening community social behaviour change communication (SBCC) programmes, aimed at discouraging cultural practices that predispose men to engage in multiple sexual partnerships. Furthermore, empowering young men with comprehensive sexual education through the integration of sexuality education into primary and secondary school curricula could yield long-term benefits in reducing HIV risk behaviour among men. There is a need for qualitative research to explore socio-cultural factors that could explain why educated men engage in risky behaviour, such as multiple sexual partnerships.
Study limitations
There are several limitations to the DHS studies. First, because the data is cross-sectional, therefore, the researchers could not do causality analyses, which limits the ability to understand the complexities of men’s experiences regarding the risk of HIV transmission behaviours through their life cycle. As a result, the findings highlight the need for additional research, particularly qualitative and longitudinal research, to further the understanding of the complex interplay between the various individual and community factors that shape men’s HIV risk behaviours. However, the male datasets in the DHS programme are comprehensive enough to understand men’s sexual behaviour in detail. Data for other countries in the region was not available on the DHS programme website.
Supplementary information
Acknowledgements
We appreciate the support from the DHS programme for authorising DHS datasets for six countries in the Southern African region. No funding was received.
Author contributions
MP developed the concept for this study, performed data analysis and wrote data interpretation text for the analysis. ML prepared the methods section for the manuscript. VK prepared the background section. CC and MP wrote the “Discussion” and “Conclusion” sections. ML performed an overall review and editing of the manuscript for intellectual content. All authors have read and approved the final version of this manuscript.
Data availability
Data used in this study are publicly available upon request from the DHS programme website: https://dhsprogram.com/.
Competing interests
The authors declare no competing interests.
Ethical approval
The study utilised secondary data extracted from recent country DHS datasets for six countries in Southern Africa. The DHS programme allowed for permission to use survey datasets. The datasets have no personal identities for research participants. All DHSs study protocols were approved by the respective country ethical review boards and the Centers for Disease Control and Prevention (CDC) Atlanta.
Informed consent
The DHS data collection procedures required informed consent from participants aged 18 and older. The survey protocol also required authorisation from parents/guardians for all participants aged 15–17 years before seeking accents from teenagers before an interview was conducted.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1057/s41599-022-01312-3.
References
- Asaolu IO, Gunn JK, Center KE, Koss MP, Iwelunmor JI, Ehiri JE. Predictors of HIV testing among youth in sub-Saharan Africa: a cross-sectional study. PLoS ONE. 2016;11(10):e0164052. doi: 10.1371/journal.pone.0164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaolu IO, Gunn JK, Center KE, Koss MP, Iwelunmor JI, Ehiri JE. Predictors of HIV testing among youth in sub-Saharan Africa: a cross-sectional study. PLoS ONE. 2016;11(10):e0164052. doi: 10.1371/journal.pone.0164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awopegba OE, Ologunowa TO, Ajayi AI. HIV testing and self‐testing coverage among men and women in South Africa: an exploration of related factors. Trop Med Int Health. 2021;26(2):214–227. doi: 10.1111/tmi.13514. [DOI] [PubMed] [Google Scholar]
- Baker DP, Collins JM, Leon J. Risk factor or social vaccine? The historical progression of the role of education in HIV and AIDS infection in Sub-Saharan Africa. PROSPECTS. 2008;38(4):467–486. doi: 10.1007/s11125-009-9097-y. [DOI] [Google Scholar]
- Baral S, Phaswana-Mafuya N. Rewriting the narrative of the epidemiology of HIV in sub-Saharan Africa. SAHARA-J: J Soc Asp HIV/AIDS. 2012;9(3):127–130. doi: 10.1080/17290376.2012.743787. [DOI] [PubMed] [Google Scholar]
- Campbell ANC, Brooks AJ, Pavlicova M, Hu M-C, Hatch-Maillette MA, Calsyn DA, Tross S. Barriers to condom use: results for men and women enrolled in HIV risk reduction trials in outpatient drug treatment. J HIV/AIDS Soc Serv. 2016;15(2):130–146. doi: 10.1080/15381501.2016.1166090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, Balzer LB, Petersen ML, Thirumurthy H, Charlebois ED, Kamya MR, Havlir DV. Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PLoS ONE. 2014;9(1):e84317. doi: 10.1371/journal.pone.0084317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft TN, Aileen MJM, Courtney KA. Guide to DHS statistics. Rockville, MD: ICF; 2018. [Google Scholar]
- Dake JA, Price JH, Maziarz L, Ward B. Prevalence and correlates of sexting behavior in adolescents. Am J Sex Educ. 2012;7(1):1–15. doi: 10.1080/15546128.2012.650959. [DOI] [Google Scholar]
- De Neve J-W, Fink G, Subramanian SV, Moyo S, Bor J. Length of secondary schooling and risk of HIV infection in Botswana: evidence from a natural experiment. Lancet Global Health. 2015;3(8):e470–e477. doi: 10.1016/S2214-109X(15)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo Y-R, Kuritzkes D, Margolis D, Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F, Alter G, Auerbach J, Autran B, Zack J. International AIDS Society global scientific strategy: towards an HIV cure. Nat Med. 2016;22(8):839–850. doi: 10.1038/nm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine-Wright H, Abraham C, Onya H, Ramatsea S, Themane M, Aarø LE. Correlates of condom use and condom-use motivation among young South Africans. J Appl Soc Psychol. 2015;45(12):674–683. doi: 10.1111/jasp.12328. [DOI] [Google Scholar]
- DHS program (2021) The DHS program [Online]. www.dhsprogram.com
- Exavery A, Kanté AM, Jackson E, Noronha J, Sikustahili G, Tani K, Mushi HP, Baynes C, Ramsey K, Hingora A, Phillips JF. Role of condom negotiation on condom use among women of reproductive age in three districts in Tanzania. BMC Public Health. 2012;12(1):1097. doi: 10.1186/1471-2458-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garenne M, Matthews A. Voluntary medical male circumcision and HIV in Zambia: expectations and observations. J Biosoc Sci. 2020;52(4):560–572. doi: 10.1017/S0021932019000634. [DOI] [PubMed] [Google Scholar]
- Glynn JR, Carael M, Buve A, Anagonou S, Zekeng L, Kahindo M, Musonda R, for the Study Group on Heterogeneity of HIV Epidemics in African Cities* Does increased general schooling protect against HIV infection? A study in four African cities. Trop Med Int Health. 2004;9(1):4–14. doi: 10.1046/j.1365-3156.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- Gregson S, Nyamukapa C, Lopman B, Mushati P, Garnett GP, Chandiwana SK, Anderson RM. Critique of early models of the demographic impact of HIV/AIDS in sub-Saharan Africa based on contemporary empirical data from Zimbabwe. Proc Natl Acad Sci USA. 2007;104(37):14586–14591. doi: 10.1073/pnas.0611540104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson S, Waddell H, Chandiwana S. School education and HIV control in sub-Saharan Africa: from discord to harmony. J Int Dev. 2001;13(4):467–485. doi: 10.1002/jid.798. [DOI] [Google Scholar]
- Hachfeld A, Darling K, Calmy A, Ledergerber B, Weber R, Battegay M, Wissel K, Di Benedetto C, Fux C, Tarr P, Kouyos R, Ruggia L, Furrer H, Wandeler G, the Swiss HIV Cohort Study, Aubert V, Battegay M, Bernasconi E, Böni J, … Yerly S (2019) Why do sub‐Saharan Africans present late for HIV care in Switzerland? HIV Med hiv.12727. 10.1111/hiv.12727 [DOI] [PubMed]
- Hargreaves JR, Morison LA, Kim JC, Bonell CP, Porter JDH, Watts C, Busza J, Phetla G, Pronyk PM. The association between school attendance, HIV infection and sexual behaviour among young people in rural South Africa. J Epidemiol Community Health. 2008;62(2):113–119. doi: 10.1136/jech.2006.053827. [DOI] [PubMed] [Google Scholar]
- Harper GW, Muthigani A, Neubauer LC, Simiyu D, Murphy AG, Ruto J, Suleta K, Muthiani P (2018) The development and evaluation of a national school-based HIV prevention intervention for primary school children in Kenya. J HIV AIDS 4(1) 10.16966/2380-5536.150 [DOI] [PMC free article] [PubMed]
- Johnson LF, Dorrington RE, Moolla H. HIV epidemic drivers in South Africa: a model-based evaluation of factors accounting for inter-provincial differences in HIV prevalence and incidence trends. South Afr J HIV Med. 2017;18(1):9. doi: 10.4102/sajhivmed.v18i1.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Arya M, Viswanath K. Effect of media use on HIV/AIDS-related knowledge and condom use in sub-Saharan Africa: a cross-sectional study. PLoS ONE. 2013;8(7):e68359. doi: 10.1371/journal.pone.0068359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khidir H, Mosery N, Greener R, Milford C, Bennett K, Kaida A, Psaros C, Safren SA, Bangsberg DR, Smit JA, Matthews LT. Sexual relationship power and periconception HIV-Risk behavior among HIV-infected men in serodifferent relationships. AIDS Behav. 2020;24(3):881–890. doi: 10.1007/s10461-019-02536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumwenda MK, Johnson CC, Choko AT, Lora W, Sibande W, Sakala D, Indravudh P, Chilongosi R, Baggaley RC, Nyirenda R, Taegtmeyer M, Hatzold K, Desmond N, Corbett EL. Exploring social harms during distribution of HIV self-testing kits using mixed-methods approaches in Malawi. J Int AIDS Soc. 2019;22(S1):e25251. doi: 10.1002/jia2.25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth AE, Lally MA, Choko AT, Inwani IW, Fortenberry JD. HIV testing and linkage to services for youth. J Int AIDS Soc. 2015;18:19433. doi: 10.7448/IAS.18.2.19433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Baker DP, Salinas D, Henck A. Is education a risk factor or social vaccine against HIV/AIDS in Sub-Saharan Africa? The effect of schooling across public health periods. J Popul Res. 2017;34(4):347–372. doi: 10.1007/s12546-017-9192-5. [DOI] [Google Scholar]
- Lucas AM, Wilson NL. Schooling, wealth, risky sexual behaviour, and HIV/AIDS in sub-Saharan Africa. J Dev Stud. 2019;55(10):2177–2192. doi: 10.1080/00220388.2018.1493195. [DOI] [Google Scholar]
- Lurie MN, Rosenthal S (2010) Concurrent partnerships as a driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited. AIDS Behav 14(1) 10.1007/s10461-009-9583-5 [DOI] [PMC free article] [PubMed]
- Maher D, Waswa L, Karabarinde A, Baisley K. Concurrent sexual partnerships and associated factors: a cross-sectional population-based survey in a rural community in Africa with a generalised HIV epidemic. BMC Public Health. 2011;11(1):651. doi: 10.1186/1471-2458-11-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangenah C, Mwenge L, Sande L, Ahmed N, d’Elbée M, Chiwawa P, Chigwenah T, Kanema S, Mutseta MN, Nalubamba M, Chilongosi R, Indravudh P, Sibanda EL, Neuman M, Ncube G, Ong JJ, Mugurungi O, Hatzold K, Johnson CC, Terris-Prestholt F. Economic cost analysis of door-to-door community-based distribution of HIV self-test kits in Malawi, Zambia and Zimbabwe. J Int AIDS Soc. 2019;22(S1):e25255. doi: 10.1002/jia2.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjengwa PG, Mangold K, Musekiwa A, Kuonza LR (2019) Cognitive and behavioural determinants of multiple sexual partnerships and condom use in South Africa: results of a national survey. South Afr J HIV Med 20(1). 10.4102/sajhivmed.v20i1.868 [DOI] [PMC free article] [PubMed]
- Manlove J, Ikramullah E, Terry-Humen E. Condom use and consistency among male adolescents in the United States. J Adolesc Health. 2008;43(4):325–333. doi: 10.1016/j.jadohealth.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Mokgatle MM, Madiba S. High acceptability of HIV self-testing among technical vocational education and training college students in Gauteng and North West Province: what are the implications for the scale up in South Africa? PLoS ONE. 2017;12(1):e0169765. doi: 10.1371/journal.pone.0169765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulemena D, Phiri M, Mutombo N (2020) Fertility intentions among HIV-positive and HIV-negative mothers in Zambia: analysis of 2013–14 and 2018 DHS data [Preprint]. (in press) 10.21203/rs.3.rs-58160/v1
- Mutinta G. Multiple sexual partnerships and their underlying risk influences at the University of KwaZulu-Natal. J Hum Ecol. 2014;46(2):147–155. doi: 10.1080/09709274.2014.11906715. [DOI] [Google Scholar]
- Nalukwago J, Alaii J, Borne BVD, Bukuluki PM, Crutzen R (2018) Application of core processes for understanding multiple concurrent sexual partnerships among adolescents in Uganda. Front Public Health 6 10.3389/fpubh.2018.00371 [DOI] [PMC free article] [PubMed]
- Njau B, Mhando G, Jeremiah D, Mushi D. Correlates of sexual risky behaviours, HIV testing, and HIV testing intention among sexually active youths in Northern Tanzania. EA Health Res J. 2021;5(2):151–158. doi: 10.24248/eahrj.v5i2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyarko SH, Sparks C. Levels and determinants of HIV testing uptake among Ghanaian men. Afr J AIDS Res. 2020;19(1):40–47. doi: 10.2989/16085906.2019.1679851. [DOI] [PubMed] [Google Scholar]
- Olakunde BO, Adeyinka DA, Olawepo JO, Pharr JR. HIV testing among men in Nigeria: a comparative analysis between young people and adults. AIDS Care. 2020;32(2):155–162. doi: 10.1080/09540121.2019.1622642. [DOI] [PubMed] [Google Scholar]
- Olakunde BO, Adeyinka DA, Olawepo JO, Pharr JR. HIV testing among men in Nigeria: a comparative analysis between young people and adults. AIDS Care. 2020;32(2):155–162. doi: 10.1080/09540121.2019.1622642. [DOI] [PubMed] [Google Scholar]
- Onoya D, Zuma K, Zungu N, Shisana O, Mehlomakhulu V. Determinants of multiple sexual partnerships in South Africa. J Public Health. 2015;37(1):97–106. doi: 10.1093/pubmed/fdu010. [DOI] [PubMed] [Google Scholar]
- Oster E. HIV and sexual behavior change: Why not Africa? J Health Econ. 2012;31(1):35–49. doi: 10.1016/j.jhealeco.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Pettifor AE, Levandowski BA, MacPhail C, Padian NS, Cohen MS, Rees HV. Keep them in school: the importance of education as a protective factor against HIV infection among young South African women. Int J Epidemiol. 2008;37(6):1266–1273. doi: 10.1093/ije/dyn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiri M (2011) Awareness, knowledge, attitudes and up-take of male circumcision as an HIV prevention strategy among youth in Zambia: a case study of Lusaka District. University of Zambia, p. 74.
- SAfAIDS (2015) Multiple and concurrent partnerships: driving Southern Africa’s HIV epidemic [Advocacy report]. SAfAIDS, p. 16 www.safaids.net
- Simona S, Lumamba C, Moyo F, Ng’andu E, Phiri M (2022) The influence of contextual factors on maternal healthcare utilization in sub-Saharan Africa: a scoping review of multilevel models [Preprint]. Sexual and reproductive Health. 10.1101/2022.03.15.22272437
- UNAIDS (2012) How women living with HIV will help the world end AIDS. UNAIDS https://www.unaids.org
- UNAIDS (2020a) 2020 Global AIDS update—seizing the moment—tackling entrenched inequalities to end epidemics. UNAIDS https://www.unaids.org/en/resources/documents/2020/global-aids-report
- UNAIDS (2020b) UNAIDS report on the global AIDS epidemic shows that 2020 targets will not be met because of deeply unequal success; COVID-19 risks blowing HIV progress way off course. UNAIDS https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2020/july/20200706_global-aids-report
- UNAIDS (2013aa) Report on HIV epidemic in Eastern and Southern Africa [Epidemic update]. UNAIDS https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa.
- UNAIDS (2013ab) Report on HIV epidemic in Eastern and Southern Africa [Epidemic update]. UNAIDS https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa.
- UNAIDS GH (2019) AIDS statistics—2019 fact sheet [fact sheet]. UNAIDS.
- Voisin DR, Hong JS, King K. Ecological factors associated with sexual risk behaviors among detained adolescents: a systematic review. Children Youth Services Rev. 2012;34(10):1983–1991. doi: 10.1016/j.childyouth.2012.07.003. [DOI] [Google Scholar]
- WHO (2015) World health statistics. Health statistics. WHO
- Zhao J, Song F, Ren S, Wang Y, Wang L, Liu W, Wan Y, Xu H, Zhou T, Hu T, Bazzano L, Sun Y. Predictors of condom use behaviors based on the health belief model (HBM) among female sex workers: a cross-sectional study in Hubei Province, China. PLoS ONE. 2012;7(11):e49542. doi: 10.1371/journal.pone.0049542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuilkowski SS, Jukes MCH. The impact of education on sexual behavior in sub-Saharan Africa: a review of the evidence. AIDS Care. 2012;24(5):562–576. doi: 10.1080/09540121.2011.630351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are publicly available upon request from the DHS programme website: https://dhsprogram.com/.