SUMMARY
BACKGROUND:
Adolescents bear a large burden of TB but high-prevalence countries differ significantly in their approach to address the specific needs of adolescent patients. We explore the national approaches to TB care in adolescents and compare them to the recommendations of the WHO.
METHODS:
We conducted a scoping review to describe the country-level guidelines to TB care in adolescents in high-burden countries. These guidelines were obtained through open sources. Information on TB care in adolescents were extracted from guidelines and compared to WHO recommendations.
RESULTS:
We found a lack of consensus in defining adolescents and that many national guidelines do not address the special healthcare needs of adolescents nor align with the WHO recommendations. Recently updated country guidelines are more likely to recommend short-course regimens for TB preventive treatment and countries with a higher level of income were more likely to follow WHO guidance for microbiological confirmation of TB disease in adolescents.
CONCLUSION:
A clear understanding of the burden of TB in adolescents that is reflected in disaggregated data reported at the country level is imperative in order to address the specific challenges to care in this high-risk group
Keywords: tuberculosis, prevention, adolescents
TB programs have in the past defined childhood TB pragmatically as TB in people <15 years of age, which is inconsistent with the WHO’s proposed general definition of an adolescent: a person aged 10–19 years. However, within the WHO TB notification database, those aged 10–14 years are programmatically included as children within the 5–14 age band, while those aged 15–19 are included as adults within the 15–24 age band.1,2 Natural history studies describe a bimodal distribution of incident TB among pediatric populations after exposure, with risk of progression to TB disease following primary infection among young adolescents aged 11–15 years being 10–20%, which is as high as young children aged 1–2 years.3 However, by hemisecting the adolescent age band into children and adult categories in national reporting, the full understanding of the TB disease spectrum among adolescents may be diluted and potential interventions may be inadequately informed.
Recent estimates suggest that adolescents (age 10–19 years) may account for as much as 43% of cases in the 0–14 age group and 38% of cases in the 15–24 age group, depending on the country and WHO region.4 The WHO acknowledges the different health needs of adolescents who may experience TB disease similar to adults yet have unique non-adult challenges in engaging with the healthcare system. Such challenges may stem from adolescents’ natural focus on autonomy or prioritizing short-term gain over long-term health,5 in addition to variability in adherence and transition to adult care.6 These needs may be less likely to be addressed by national TB programs that have previously prioritized efforts among the <5-year old population.7
The United Nations and WHO have set a goal to end the TB pandemic by 2030 through Sustainable Development Goals 3 and WHO’s End TB strategy, both of which aim to achieve a reduction of 90% in TB deaths and 80% in TB incidence between 2015 and 2030, including all vulnerable populations.2 Adolescents may be a particularly vulnerable population, given the frequent lack of a defined source of medical care as they transition from pediatric to adult providers and higher rates of loss to follow-up that persist across income levels.8,9 Furthermore, unlike the younger children age group, which has experienced a significant reduction in TB incidence and mortality, many high-burden countries have not seen the same reduction in the older children and adolescent age groups.2,10,11 In this scoping review, we explore approaches towards TB care in adolescents in high TB burden countries, as outlined by their respective national TB guidelines.
METHODS
We followed the 5-stage-framework described by Arksey & O’Malley for scoping reviews.12 The 30 high TB burden countries were identified by the WHO in 2020; however, while the review was in process the WHO updated the high-burden list by adding Gabon, Mongolia and Uganda and moving Cambodia, the Russian Federation and Zimbabwe to the newly created “Global TB watchlist” due to a lower TB burden in the latter three countries.13 Thus, we elected to search the relevant TB guidelines for all 33 countries by identifying publicly available websites or the corresponding websites of the national TB control programs or ministries of health using the following terms: “National TB guidelines”, “Tuberculosis”, “Child TB guidelines”, “Guidelines” and “Tuberculosis control”. When a search using the English term did not return any fitting results, relevant translations were used. As needed, respective national TB control programs, the WHO national offices or embassies of high-burden countries in Washington, DC, USA, were contacted directly to obtain the country’s national guidelines.
Relevant guidelines were reviewed specifically looking for information within three main categories: 1) the definitions of “child” and “adolescent”, 2) the approach to TB disease evaluation and management, including the topics of emphasis on bacteriologic confirmation, role of tuberculin skin test (TST) or interferon-gamma release assay (IGRA) in diagnosis, role of chest radiograph (CXR) in diagnosis, dosing of anti-TB medications for active TB disease for children/adolescents and HIV screening for people diagnosed with TB, and 3) the approach to TB prevention, including the topics of contact tracing, recognition of the ability for adolescents to transmit TB, preventive treatment and regimens used for prevention (including those for contacts of people with drug-resistant TB), adherence among adolescents, and recommendations on bacilli Calmette-Guérin (BCG) vaccination. These data were tabulated and summarized to generate the results and recommendations of this review. At least two authors reviewed each document and areas of disagreement were discussed until consensus was reached. Data were compared to the relevant guidelines from the WHO.7,14 Where separate guidelines for TB management in adults or school children were available, these documents were reviewed to obtain additional information when the children-specific guidelines were not sufficient to answer the review questions.
Information on BCG status was obtained from the BCG World Atlas when the national guidelines made no specific mention of the vaccine.15 Information on the country’s income level was obtained from the Stop TB Partnership.16 Trends among topics were compared based on the country’s income level or the year of guideline publication.
In this review, the following definitions were used unless otherwise explicitly defined in the text: adolescents were defined as those aged 10–19 years; if guidelines mentioned “older children” we considered this to include adolescents. The approach to TB disease confirmation refers to TB disease diagnostic approach in adolescents. TB preventive treatment (TPT) is meant to address TB prevention in contacts after exposure to active pulmonary TB disease, and a country is considered to endorse latent TB infection (LTBI) treatment rather than TPT only if a test (either TST or IGRA) was required prior to therapy regardless of exposure history. Percentages were rounded to the nearest whole number, and thus may not add up to 100 percent.
Web-based and app-based translations were used when guidelines were in a language other than English, with consultation with native language speakers. MS Excel 2019 (Microsoft, Redmond, WA, USA) and Google Sheets (Google, Mountain View, CA, USA) were used to tabulate data.
This work was deemed exempt from institutional review board review, as it did not involve human subjects.
RESULTS
Guidelines were available for 28 of the total 33 countries on the high TB burden list and the global TB watchlist. Guidelines for Angola, Liberia, Sierra Leone, Gabon, and Republic of the Congo were not available. The Supplementary Table shows a list of the countries with available guidelines, the country’s level of income and the reference to the guideline. Bangladesh, China, India, Nigeria, and South Africa had more than one document, which were used to extract information. Most countries (89%) had updated their guidelines after the WHO’s 2014 publication of the childhood TB guidelines (second edition), except for three countries: Cambodia (2008), Mozambique (2013), and South Africa (2013).
Definition of adolescents and children
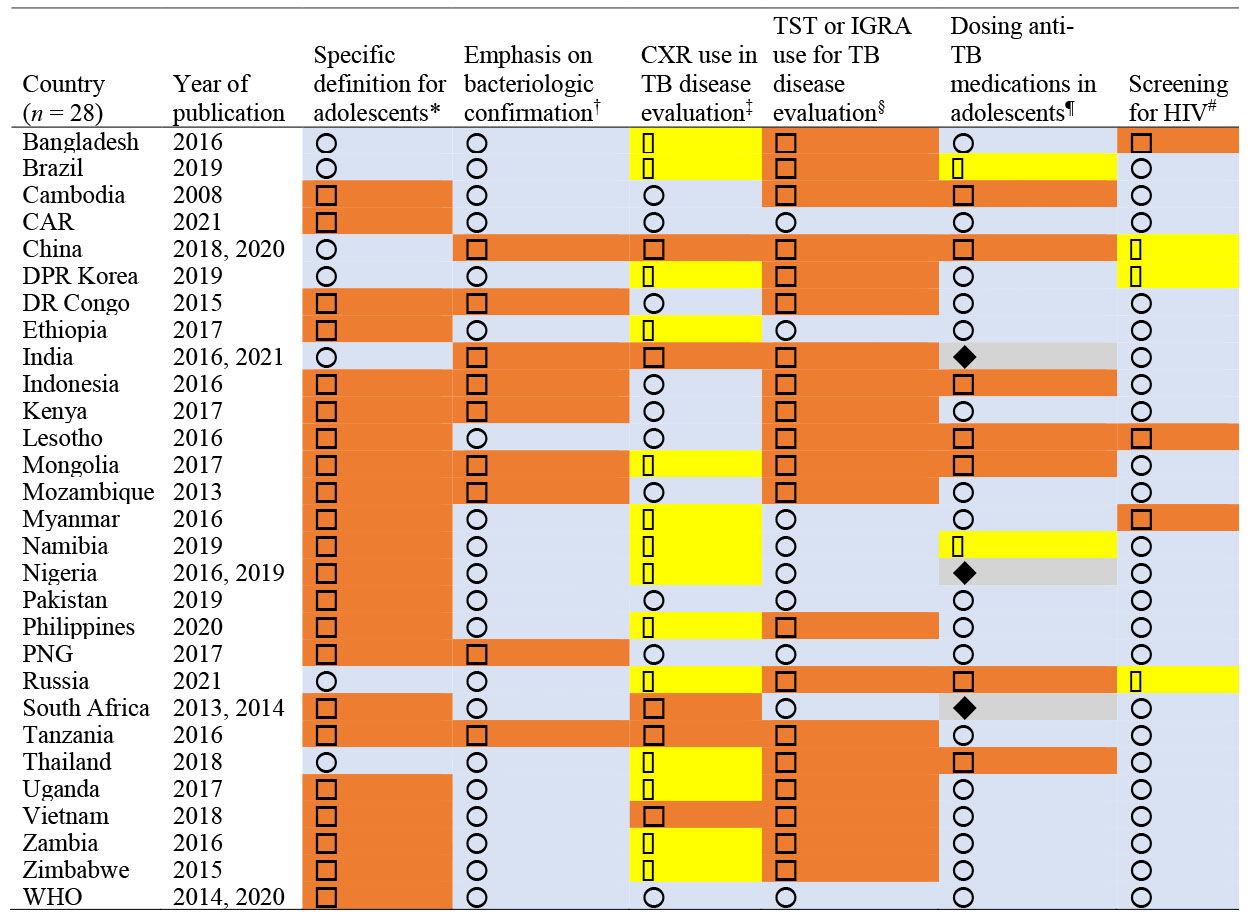
Table 1 gives the approaches to defining adolescents. Twenty-one countries (75%) did not explicitly define adolescents and only provided a definition for children as those aged 0–14 years, thus conforming to WHO TB reporting systems. The remaining seven countries (25%) had different definitions. Bangladesh defined adolescents as those aged 10–19 years, but approached individuals ≥8 years as adults when it came to TB disease evaluation. Brazil and North Korea defined children as those aged 0–9 years and adolescents as those ≥10 years. The Russian Federation defined children as those aged 0–17 years, but provided separate statistics for those aged 15–17 years, and Thailand defined children as age 0–18 years. China’s TB guidelines for schools covers ages 3–24 years, and India groups ages 5–19 years together for nutritional assessment for TB.
Table 1.
Individual approaches to defining adolescents and TB disease evaluation and management
|
 = country had a definition of adolescent separate from that of children;
= country had a definition of adolescent separate from that of children;  = country defined children 0–14 without separation from adolescents.
= country defined children 0–14 without separation from adolescents.
 = required/highly recommended bacteriologic confirmation for TB disease diagnosis;
= required/highly recommended bacteriologic confirmation for TB disease diagnosis;  = allowed clinical diagnosis without emphasis on bacteriologic confirmation.
= allowed clinical diagnosis without emphasis on bacteriologic confirmation.
 = CXR regarded as an important supplemental test;
= CXR regarded as an important supplemental test;  = CXR recommended as an initial test;
= CXR recommended as an initial test;  = CXR recommended if bacteriologic confirmation unavailable.
= CXR recommended if bacteriologic confirmation unavailable.
 = TST (or IGRA) is not recommended as part of TB disease evaluation;
= TST (or IGRA) is not recommended as part of TB disease evaluation;  = TST (or IGRA) is recommended as part of TB disease evaluation.
= TST (or IGRA) is recommended as part of TB disease evaluation.
 = transition to adult dosing if weight >25 kg;
= transition to adult dosing if weight >25 kg;  = only maximum doses are mentioned;
= only maximum doses are mentioned;  = transition to adult dosing based on age cut-off;
= transition to adult dosing based on age cut-off;  = transition to adult dosing based on a combination of age and/or weight cut-off.
= transition to adult dosing based on a combination of age and/or weight cut-off.
 = HIV screening is recommended for all TB patients;
= HIV screening is recommended for all TB patients;  = HIV screening is recommended for TB patients with risk factors for HIV or in high prevalence areas;
= HIV screening is recommended for TB patients with risk factors for HIV or in high prevalence areas;  = no specific mention.
= no specific mention.
CXR = chest X-ray; TST = tuberculin skin test; IGRA = interferon-gamma release assay; CAR = Central African Republic; DPR = Democratic People’s Republic; DR = Democratic Republic; PNG = Papua New Guinea.
Approach to TB disease evaluation and management
Table 1 also shows the national approaches to TB disease diagnosis and treatment. With regard to emphasis on bacteriologic confirmation in older children and adolescents, 19 countries (68%) followed the WHO recommendation to require or highly recommend bacteriologic confirmation for TB disease, while nine countries (32%) allowed clinical diagnosis without strong emphasis on attempting bacteriologic confirmation. Figure 1 shows that bacteriologic confirmation was more likely to be recommended in countries with higher levels of income; it was recommended by 83% of upper middle-income countries compared to 67% of lower middle-income countries and 57% of low-income countries. Additional diagnostic work-up was included in the guidelines, with all countries recognizing CXR as an important tool in TB disease evaluation. Five countries (China, India, Tanzania, South Africa, and Vietnam, 18%) recommended CXR as part of the initial approach to presumptive TB cases. Nine other countries (32%) recognized the importance of CXR as a supplemental test and the remaining 14 countries (50%) recommended CXR when TB was strongly suspected and bacteriologic testing was either negative or not available.
Figure 1.
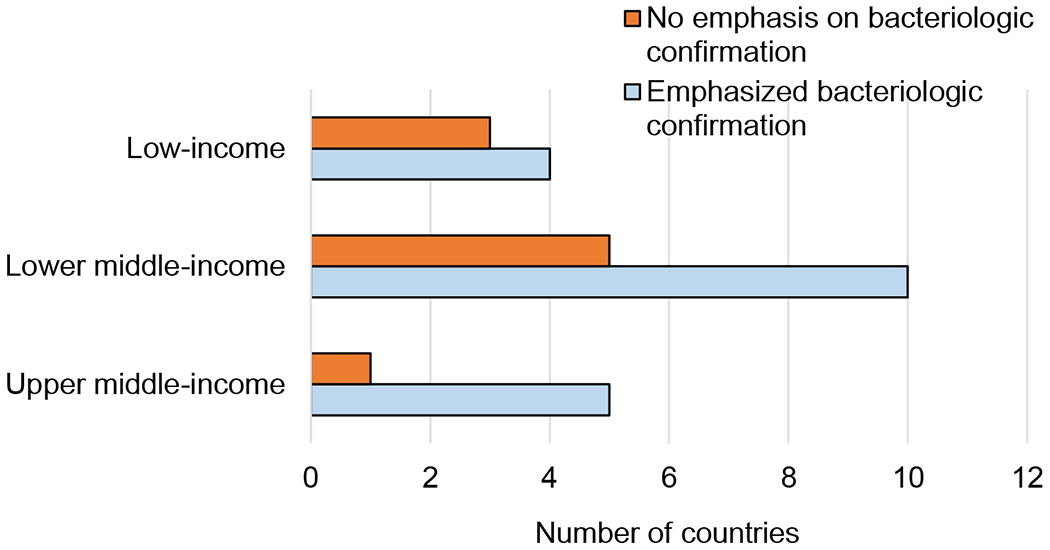
Diagnostic approach per income level: break down of countries with high TB burden based on whether their TB guidelines emphasized the importance of bacteriologic confirmation for TB disease evaluation in older children and adolescents.
Either TST or IGRA were included in the diagnostic algorithm in 20 countries (71%), while eight countries (29%) conformed to WHO guidance, which cautions against using these tests as part of active TB disease evaluation. Table 2 also shows the heterogeneity in approaches to dosing anti-TB medications for active TB disease in adolescents, with various criteria being used, including age, weight, or both. Sixteen countries (57%) followed WHO guidelines with using adult dosing for weight over 25 kg, seven countries (25%) mentioned a maximum dose only, two countries (7%) used age-based cut-off, and three (11%) countries used a combination of weight and/or age cut-offs. Most guidelines recommended HIV screening for all people diagnosed with TB (22 countries, 79%). Three additional guidelines (Bangladesh, Lesotho and Myanmar, 11%) only recommended HIV screening when risk factors for HIV were present or in areas with high HIV prevalence and guidelines of the remaining three countries (China, Democratic People’s Republic [DPR] of Korea and Russian Federation, 11%) did not specifically mention HIV screening for people diagnosed with TB.
Table 2.
Individual approaches to including adolescents in TB preventive services
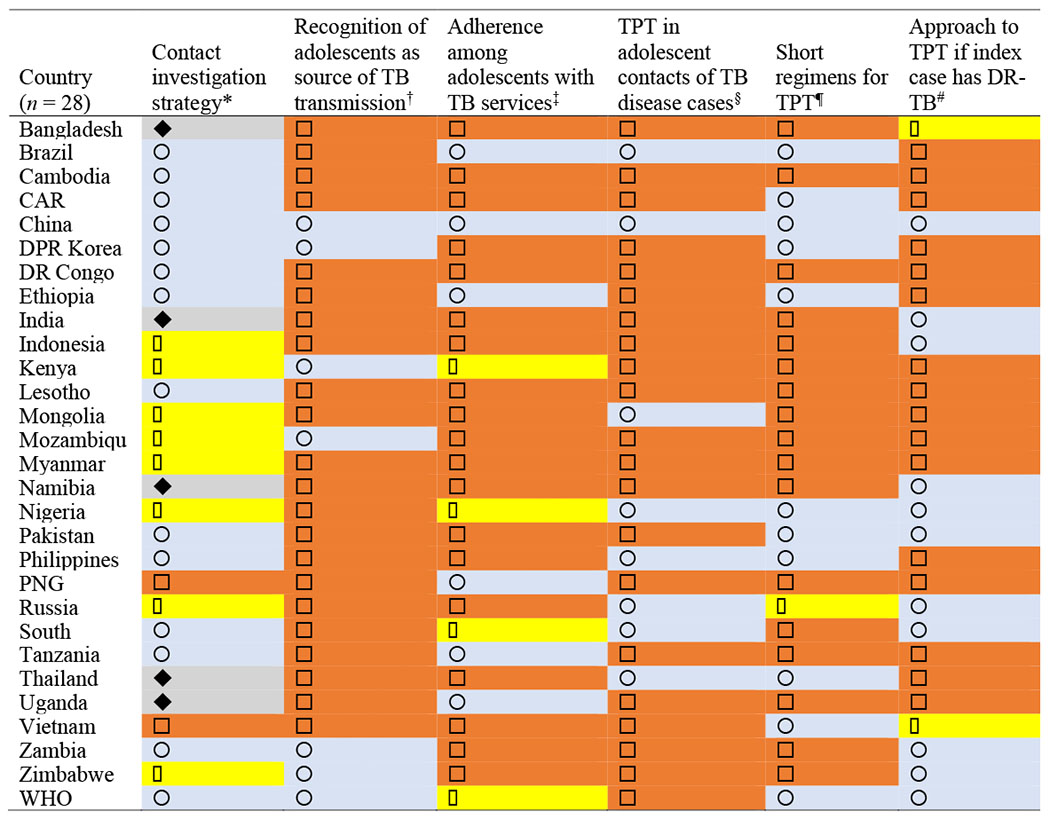
|
 = contact screening is recommended for all household and close contacts;
= contact screening is recommended for all household and close contacts;  = No mention of contact screening;
= No mention of contact screening;  = contact screening prioritizes all children;
= contact screening prioritizes all children;  = contact screening prioritizes children<5.
= contact screening prioritizes children<5.
 = adolescents are recognized as an important source of TB transmission;
= adolescents are recognized as an important source of TB transmission;  = No mention of adolescents’ role in TB transmission.
= No mention of adolescents’ role in TB transmission.
 = specified approaches to improve adherence with TB care in adolescents;
= specified approaches to improve adherence with TB care in adolescents;  = did not address adherence among adolescents;
= did not address adherence among adolescents;  = acknowledged low adherence but didn’t specify approaches to address it.
= acknowledged low adherence but didn’t specify approaches to address it.
TPT = TB preventive therapy; DR-TB = drug-resistant TB; CAR = Central African Republic; DPR = Democratic People’s Republic; DR = Democratic Republic; PNG = Papua New Guinea.
Inclusion of adolescents in TB preventive services
Table 2 shows the range of approaches to TB prevention and how they compare with WHO guidelines. Two countries (Papua New Guinea and Vietnam, 7%) did not specify a contact tracing strategy in their guidelines, five countries (Bangladesh, India, Namibia, Thailand, and Uganda, 18%) prioritized young children under the age of 5, eight countries (29%) recommended screening all pediatric contacts of TB cases and 13 countries (46%) concurred with WHO recommendations to screen all household or close contacts of people with pulmonary TB. Adolescents were mentioned as an important source of TB transmission among children in the guidelines of six countries (China, DPR Korea, Kenya, Mozambique, Zambia, and Zimbabwe, 21%), while the remaining 22 countries (79%) had no specific approach to prevention concerning adolescents in particular. All countries recommended BCG vaccination for all healthy newborns, while the Russian Federation was the only country on either list to recommend a booster at age 6–7 years.
Table 2 also shows that very few countries specified an approach to TB care in adolescents. The WHO guidelines acknowledged the low adherence characteristic of this age group, also noted in the guidelines of three countries (Kenya, Nigeria, and South Africa, 11%). Six countries (21%) incorporated adolescent-specific approaches that ranged from separate guidelines specifically addressing TB screening and management among school children and directly observed therapy (DOT) facilitated by schools (China); using education and peer counselling (Brazil, Ethiopia, Tanzania, and Uganda); and recommending hospitalization for the intensive phase of TB disease therapy (Papua New Guinea). The remaining 19 countries (68%) did not include a tailored approach to adherence among adolescents.
Most countries (71%) followed the WHO lead on focusing on TPT by treating asymptomatic people exposed to confirmed cases of pulmonary TB disease, especially children under the age of 5 and people living with HIV, with TPT provided without the need for testing. Eight countries (Brazil, China, Mongolia, Nigeria, the Philippines, the Russian Federation, South Africa and Thailand, 29%) recommend a formal diagnosis of LTBI in contacts of TB index cases with either TST or IGRA prior to LTBI treatment. Among these countries, the Philippines incorporated a caveat for LTBI diagnosis and treatment for contacts under the age of 5 of clinically diagnosed TB index cases and age 5–14 contacts of bacteriologically confirmed TB index cases.
Figure 2 shows that recent guidelines were more likely to endorse shorter regimens similar to WHO recommendations for TPT/LTBI treatment, with 10 countries (36%) including 3 months of weekly rifapentine plus isoniazid (3HP), 4 months of daily rifampin (4R) or 3 months of daily rifampin plus isoniazid (3HR) for TPT/LTBI treatment. All of these countries’ guidelines were published in 2018 or thereafter, except for Nigeria (2015) and Ethiopia (2017). Russian guidelines did not specify regimens, and the remaining 17 countries (61%) endorsed daily isoniazid for 6 or 9 months. Sixteen (57%) of the 17 countries that did not endorse shorter-course preventive regimens had guidelines that were published before 2018.
Figure 2.
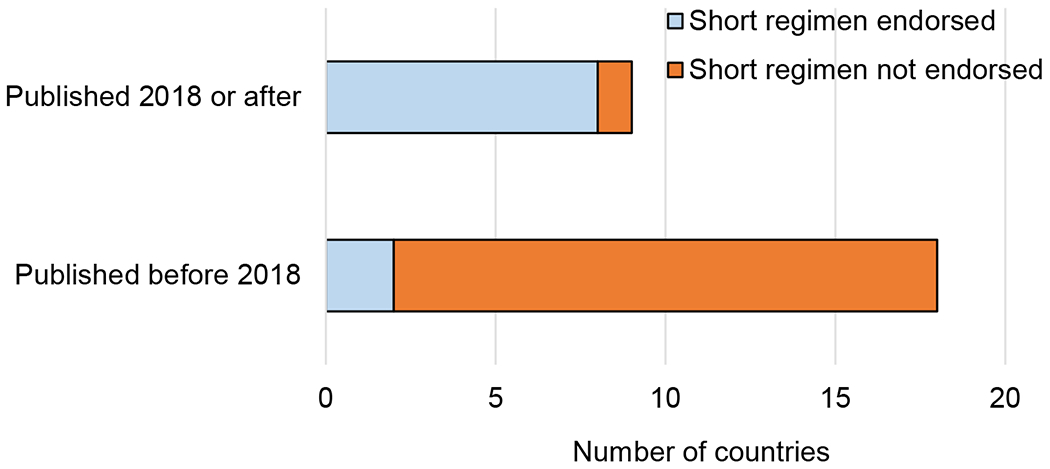
Endorsement of short regimens for TPT: guidelines of countries with high TB burden which recommend short-course TPT regimens by year of publication. TPT = TB preventive therapy.
Regarding practices for contacts of people with drug-resistant TB, 10 countries (36%) mirrored WHO guidelines which recommends referral to specialized TB centers for follow up and individualized consideration for preventive treatment with second-line anti-TB medication.
DISCUSSION
To our knowledge, this the first scoping review to attempt to differentiate the national approaches of high-burden countries to TB prevention and management in children and adolescents. We found that most countries do not universally agree on definitions for adolescents, do not explicitly define and address the challenges faced by adolescents with TB care, and in many cases, do not align with the current WHO recommendations. Recently updated country guidelines were more likely to follow WHO recommendations on shorter-course regimens for TPT/LTBI treatment.
Useful lessons can be learned from each country’s approach. For example, Russian guidelines (Supplementary Table S1, reference 21), which emphasize individualized regimens tailored to drug susceptibility, recommend screening for all school children using TST if aged ≤14 years and CXR if aged 15–17 years on enrollment in school and on an annual basis afterwards. If either test is positive, the individual should then be evaluated for TB disease and treated for TB or LTBI based on the outcome of TB disease evaluation. China’s guidelines (Supplementary Table S1, reference 5) are notable for having a dedicated document addressing TB in school children. This document recommends the use of CXR to screen all school age children with concern for TB and use the school infrastructure to administer TB therapy which allows children to continue their education, thereby minimizing potential barriers of transportation and DOT adherence. Such a program appears to support the growing evidence that TB transmission among adolescents is not restricted to household activities.17 While studies that specifically evaluate school-based TB screening are sparse, one recent study by Dorjee et al. found that a school-based program for active TB case-finding and TB prevention was highly successful in a high-burden setting when community acceptance was achieved.18 In alignment with the WHO End TB Strategy, novel methods to extend the reach of TPT should be considered in an adolescent-friendly manner.14,16
It is also important to note that resources differ among countries and funding can be applied to other aspects of care, a point explicitly acknowledged in the guidelines of some low-income countries. For example, the 2016 guidelines from Bangladesh (Supplementary Table S1, reference 1) note that only 39 GeneXpert (Cepheid, Sunnyvale, CA, USA) machines had been installed in hospitals across the country at the time of publication, which may limit the ability to make a microbiological confirmation of TB disease. While access to resources for TB diagnosis and management may have considerably improved from the time of guidelines publishing, and Bangladesh’s scale up of Xpert testing capacity is an example,19 country-level income differences appeared to influence recommendations on bacteriologic confirmation of TB or to routinely employ the use of other diagnostic modalities such as CXR.20
Our review also found multiple approaches to the dosing of TB medications for active TB disease among adolescents, which did not appear to associate with country-level income, but rather more likely reflected the paucity of updated pharmacokinetic studies to inform dosing in older children and adolescents in particular.21 It is well understood that the initiation of adequate anti-TB treatment is essential to reduce morbidity and mortality.22 However, additional investigations are needed to understand optimized doses, shorter combination regimens, and user-friendly formulations, which can be tailored to the needs of adolescents to ensure maximum treatment success.23 It is important to highlight that the shorter TPT, while approved and recommended for adolescents, could present some challenges: for example, the 3HP regimen currently carries a large pill burden, and ongoing studies examining dispersible formulations are eagerly anticipated to maximize acceptability.24,25
This review focused on identifying the manners in which the unique needs of adolescents, who experience TB differently than younger children,3 were highlighted throughout national guidelines. The findings of this review supports the recent calls by the WHO and other researchers to report disaggregated age group data (0–4, 5–9, 10–14, and 15–19 years) for children and adolescents.26,27 A promising shift in this direction is manifested in the most recent global TB report with 10 high-burden countries reporting TB notification data that are disaggregated based on these 5-year age groups.2 This shift will enable improved understanding of the burden experienced by adolescents, thereby enabling the development and evaluation of a tailored approach to healthcare in adolescents in the light of requests from the WHO and others to redesign health services that are more friendly to adolescents.28–30
Strengths of this review include the focus on approaches of individual countries to TB in adolescents and children, the scoping review methodology, which allowed the exploration of different themes among varying levels of detail of the primary sources, and the comparison of country-specific approaches to WHO guidance. However, the review was constrained by the reliance on open sources to obtain the relevant guidelines, in addition to contacting the national TB programs directly, which was unsuccessful for five target countries. We acknowledge the possible existence of desk guides and other working documents that might be utilized in countries where we did not obtain guidelines or supplement those that we were able to obtain. For older guidelines, it is possible that practice has been adapted to other international or regional norms that are no longer reflected in an older guideline. In addition, we only reviewed the guidelines documents and did not employ study team members in the individual countries to verify the penetration of these national recommendations into everyday practice in representative settings. Instead, we view this scoping review as a starting point for further targeted analyses.
In conclusion, many guidelines from high-burden countries exclude discussions on the specific needs of adolescents when it comes to TB care. The level of income of a country was associated with some, but not all recommendations, and countries with more recently updated guidelines were more likely to reflect WHO guidance. This scoping review identified areas for adolescent-specific attention across the TB care cascade, and further supports the calls to disaggregate data for the adolescent age span.
Supplementary Material
Acknowledgements
This work was funded by a National Institute of Health (Bethesda, MD, USA) training grant 5T32AI007046. SH and TT are supported by NIH R01 AI137080. The authors would like to thank J Liu for help with translating the guidelines from China.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Health for the world’s adolescents. A second chance in the second decade. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 2.World Health Organization. Global tuberculosis report, 2020. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 3.Marais BJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8(4):392–402. [PubMed] [Google Scholar]
- 4.Snow KJ, et al. The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J 2018; 51(2):1702352. [DOI] [PubMed] [Google Scholar]
- 5.Kim B, White K. How can health professionals enhance interpersonal communication with adolescents and young adults to improve health care outcomes?: systematic literature review. Int J Adolesc Youth 2018; 23(2): 198–218. [Google Scholar]
- 6.Snow KJ, et al. Adolescent tuberculosis. Lancet Child Adolesc Health 2020; 4(1): 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second edition. Geneva, Switzerland: WHO, 2014. [PubMed] [Google Scholar]
- 8.White PH, Cooley WC. Supporting the health care transition from adolescence to adulthood in the medical home from the American Academy of Pediatrics guidance for the clinician in rendering pediatric care. Pediatrics 2018; 142(5):e20182587. [DOI] [PubMed] [Google Scholar]
- 9.Enane LA, et al. Loss to follow-up among adolescents with tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis 2016; 20(10): 1320–1325. [DOI] [PubMed] [Google Scholar]
- 10.Osman M, et al. Mortality in South African children and adolescents routinely treated for tuberculosis. Pediatrics 2021; 147(4): e2020032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nduba V, et al. Prevalence of tuberculosis in adolescents, western Kenya: implications for control programs. Int J Infect Dis 2015; 35(C): 11–17. [DOI] [PubMed] [Google Scholar]
- 12.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8(1): 19–32. [Google Scholar]
- 13.World Health Organization. WHO releases new global lists of high-burden countries for TB, HIV-associated TB and drug-resistant TB. Geneva, Switzerland: WHO, 2021; https://www.who.int/news/item/17-06-2021-who-releases-new-global-lists-of-high-burden-countries-for-tb-hiv-associated-tb-and-drug-resistant-tb. Accessed July 2020. [Google Scholar]
- 14.World Health Organization. WHO consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. Geneva, Switzerland: WHO, 2020. https://apps.who.int/iris/bitstream/handle/10665/331170/9789240001503-eng.pdf. Accessed July 2021. [Google Scholar]
- 15.Zwerling A, et al. BCG World Atlas. 3rd ed. 2020; http://www.bcgatlas.org/index.php. Accessed July 2021. (check—please provide name and place of publication)
- 16.Stop TB Partnership. Estimates for total number of people who died from any form of TB (including DR-TB or TB-HIV co-infection). Geneva, Switzerland: WHO, 2020. http://stoptb.org/resources/cd/MappingTool_Main.html. Accessed July 2021. [Google Scholar]
- 17.Martinez L, et al. Paediatric tuberculosis transmission outside the household: challenging historical paradigms to inform future public health strategies. Lancet Respir Med 2019; 7(6): 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorjee K, et al. High Prevalence of active and latent tuberculosis in children and adolescents in Tibetan schools in India: the Zero TB Kids Initiative in Tibetan refugee children. Clin Infect Dis 2019; 69(5): 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banu S, et al. Social Enterprise Model (SEM) for private sector tuberculosis screening and care in Bangladesh. PLoS One 2020; 15(11): e0241437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedrazzoli D, et al. Can tuberculosis patients in resource-constrained settings afford chest radiography? Eur Respir J 2017; 49(3): 1601877. [DOI] [PubMed] [Google Scholar]
- 21.Hibma JE, et al. Rifapentine population pharmacokinetics and dosing recommendations for latent tuberculosis infection. Am J Respir Crit Care Med 2020; 202(6): 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins HE, et al. Mortality among children diagnosed with tuberculosis: Systematic review and meta-analysis. Lancet Infect Dis 2016; 17(3): 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Roadmap towards ending TB in children and adolescents. Geneva, Switzerland: WHO, 2018. https://www.euro.who.int/en/publications/abstracts/roadmap-towards-ending-tb-in-children-and-adolescents,-2nd-ed-2018 Accessed September 2021. [Google Scholar]
- 24.Stop TB Partnership. Global Drug Facility Medicines Catalog. Geneva, Switzerland: WHO, 2021. http://www.stoptb.org/gdf/drugsupply/pc3.asp?PID=1131 Accessed September 2021. [Google Scholar]
- 25.Treatment Action Group. An activist’s guide to rifapentine for the treatment of TB infection. New York, NY USA: TAG, 2020. https://www.treatmentactiongroup.org/wp-content/uploads/2020/04/rifapentine_guide_2020.pdf. Accessed September 2021. [Google Scholar]
- 26.Diaz T, et al. A call for standardised age-disaggregated health data. Lancet Healthy Longev 2021; 2(7): e436–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Rapid communication on updated guidance on the management of tuberculosis in children and adolescents. Geneva, Switzerland: WHO, 2021. https://www.who.int/publications/i/item/9789240033450. Accessed September 2021. [Google Scholar]
- 28.Requejo J, Strong K. Redesigning health programmes for all children and adolescents. BMJ 2021; 372: n533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Global accelerated action for the health of adolescents (AA-HA!): guidance to support country implementation. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 30.World Health Organization. Adolescent friendly health services for adolescents living with HIV: from theory to practice. Geneva, Switzerland: WHO, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


