Abstract
Background
Predictive biomarkers are needed to identify oestrogen receptor-positive, human epidermal growth factor receptor 2-negative (ER + /HER2-) metastatic breast cancer (MBC) patients who would likely benefit from cyclin-dependent kinase 4 and 6 inhibitors combined with endocrine therapy. Therefore, we performed an exploratory study to evaluate the tumour heterogeneity parameters based on 16α-18F-fluoro-17β-oestradiol (18F-FES)-PET imaging as a potential marker to predict progression-free survival (PFS) in MBC patients receiving palbociclib combined with endocrine therapy.
Methods
Fifty-six ER + MBC patients underwent 18F-FES-PET/CT before the initiation of palbociclib. 18F-FES uptake was quantified and expressed as the standardized uptake value (SUV). Interlesional heterogeneity was qualitatively identified according to the presence or absence of 18F-FES-negative lesions. Intralesional heterogeneity was measured by the SUV-based heterogeneity index (HI = SUVmax/SUVmean). Association with survival was evaluated using the Cox proportional hazards model.
Results
A total of 551 metastatic lesions were found in 56 patients: 507 lesions were identified as 18F-FES-positive, 38 lesions were distributed across 10 patients without 18F-FES uptake, and the remaining 6 were liver lesions. Forty-three patients obtained a clinical benefit, and 13 developed progressive disease (PD) within 24 weeks. Nine out of 10 patients with an 18F-FES-negative site developed PD, and the median PFS was only 2.4 months. Among 46 patients with only 18F-FES-positive lesions, only four patients had PD, and the median PFS was 23.6 months. There were statistically significant differences between the two groups (P < 0.001). For the subgroup of patients with only 18F-FES-positive lesions, low FES-HI patients experienced substantially longer PFS times than those with high FES-HI (26.5 months vs. 16.5 months, P = 0.004).
Conclusions
18F-FES-PET may provide a promising method for identifying and selecting candidate ER + /HER2- MBC patients who would most likely benefit from palbociclib combined with endocrine treatment and could serve as a predictive marker for treatment response.
Trial registration NCT04992156, Date of registration: August 5, 2021 (retrospectively registered).
Keyword: 18F-FES, Tumour heterogeneity, Palbociclib, Endocrine therapy, Metastatic breast cancer
Background
Breast cancer is the most common malignant tumour in women and a leading cause of cancer-related deaths worldwide [1]. Hormone receptor-positive (HR +) breast cancer is the most common subtype and is a candidate for endocrine therapy [2]. Administering cyclin-dependent 4/6 kinase (CDK4/6) inhibitors in combination with endocrine therapy has become a standard of care for patients with HR + /human epidermal growth factor receptor 2-negative (HR + /HER2-) metastatic breast cancer (MBC) [3–5]. Palbociclib, a first-in-class, orally administered CDK4/6 inhibitor, has been shown to exhibit antitumour activity by causing cell cycle arrest and was approved in combination with endocrine therapy to treat HR + /HER2- MBC patients [6]. However, although this combination is highly effective, the majority of patients experience disease progression during treatment, and another cohort of patients is intrinsically resistant to the combination of CDK4/6 inhibitors and endocrine therapy [7–9]. As a result, predicting the patient response to CDK4/6 inhibitors plus endocrine therapy has become an area of major scientific interest so that both side effects, such as neutropenia, leukopenia, fatigue, and nausea, and high treatment costs can be avoided. Furthermore, depending on the predicted response, aggressive treatments could be commenced in those who may benefit from the combination of CDK4/6 inhibitors and endocrine therapy, whereas other more effective treatments could be introduced at an early time point for patients who are unlikely to respond.
At present, some potentially predictive biomarkers have been found for CDK4/6 inhibitors, such as cyclin E1 (CCNE1) [10], thymidine kinase 1 (TK1) mRNA expression [11], and circulating tumour DNA (ctDNA) [12]. However, these promising biomarkers have not been adopted in clinical practice because they are neither fully predictive nor routinely available. CDK4/6 enzymes are key promoters of tumour growth in HR + breast cancer and cooperate with oestrogen receptor (ER) pathway activation [13, 14]. ER status is essential in the selection of treatment protocols and is a well-known prognostic factor [5, 15, 16]. Moreover, higher ER expression is often associated with a better outcome for endocrine therapy [17]. Quite a few ER-positive primary breast cancer patients may eventually develop ER-negative metastatic lesions, and these patients are unlikely to benefit from ER-directed therapies [18, 19]. Collecting biopsy samples from metastatic tissue is not always feasible in daily practice due to the location of the metastatic lesion and the risks associated with biopsy [20]. In addition, a single biopsy may not be representative of the entire lesion, and tumour heterogeneity may limit the validity of the assessment of a single lesion [21].
Positron emission tomography (PET) with 16α-18F-fluoro-17β-oestradiol (18F-FES) is a noninvasive method that visualizes and quantifies the expression of ER in multiple tumours throughout the body (excluding lesions in the liver, where it is metabolized) [22, 23]. Other researchers and our previous studies have shown that 18F-FES uptake correlated well with ER expression measured by immunohistochemical staining, and 18F-FES PET played an important role in predicting the response to endocrine therapy [24–29]. Tumour heterogeneity has been shown to have a profound impact on malignant behaviour and treatment response, and molecular imaging provides an important noninvasive method for biologically characterizing tumour heterogeneity and predicting treatment results. Therefore, our study specifically aimed to assess the heterogeneity of intralesional and interlesional ER expression, as measured by 18F-FES-PET, to identify patients who may benefit from combination therapy and provide early predictive factors.
Methods
Patients
This retrospective study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The requirement for informed consent was waived owing to the retrospective nature of the study. This study was retrospectively registered with ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT04992156), and the other study ID number is YOUNGBC-15.
The subjects were patients with HR + /HER2- MBC who initiated palbociclib plus endocrine therapy between March 2017 and December 2020 in the Fudan University Shanghai Cancer Center. Patients who underwent an 18F-FES PET/computed tomography (CT) scan before the first regimen were enrolled in this study. Additional inclusion criteria were as follows: (1) female sex; (2) age ≥ 18 years; (3) histologically and cytologically confirmed MBC; (4) HR-positive and HER2-negative status, as defined according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines; and (5) complete medical records.
Assessment of treatment response
The patients underwent CT or magnetic resonance imaging (MRI) every 2–3 months during treatment until disease progression. Tumour response was assessed by the attending physicians according to the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1. Clinical data regarding baseline patient characteristics, treatment history, and efficacy of palbociclib plus endocrine therapy were retrospectively acquired from the electronic medical record system.
The primary endpoint of this study was progression-free survival (PFS), which was defined as the time from treatment initiation to disease progression or death from any cause. The second objective of this study was to evaluate the clinical benefit rate (CBR), which was defined as the percentage of patients experiencing complete response (CR), partial response (PR), and stable disease (SD) for at least 24 weeks according to the RECIST 1.1 criteria.
18F-FES PET/CT procedure
The synthesis and quality control of 18F-FES were conducted as described previously [27, 30]. The patients received approximately 222 MBq of 18F-FES intravenously over 1–2 min. Whole-body (head to mid-thigh) PET/CT was performed 60 min after tracer injection using a Siemens Biograph 16 HR PET/CT scanner or mCT Flow PET/CT scanner (Knoxville, Tennessee, USA) according to European Association of Nuclear Medicine (EANM) guidelines [31]. Low-dose CT was acquired for attenuation and scatter correction. A PET emission scan covering the same spatial range was performed immediately after the low-dose CT scan. To analyse the images, Gaussian filter iteration was used to reconstruct the emission images. Patients who had been administered ER antagonists were required to discontinue them for at least 6 weeks to avoid false-negative 18F-FES results [29].
Image analysis
We used a multimodality computer platform (Syngo, Siemens, Knoxville, TN, USA) for image viewing and tracer uptake quantification. Two board-certified nuclear medicine physicians (> 5 years of working experience) evaluated the images independently and were blinded to the clinical outcomes. In the case of a discrepancy between the two physicians, a consensus was reached on a final reading for the statistical analyses. Volumes of interest (VOIs) were manually drawn around the area of avid tumour uptake visible on PET (higher than adjacent normal tissue background) with the corresponding low-dose CT serving as a guide. The lesions outlined on the 18F-FES PET image have to be identified and located by 18F-FDG PET/CT and diagnostic CT or MRI. To reduce the partial volume effect and the limitation of resolution, 18F-FES uptake was quantitated in measurable lesions with diameters greater than 1.0 cm. In patients with numerous metastatic lesions, up to an arbitrary maximum of 20 lesions were selected for analysis according to the guidelines of the EANM [31].
The 18F-FES uptake of a lesion was semiquantitatively expressed as the maximum standardized uptake value (SUVmax) [31]. The mean standardized uptake value (SUVmean) was quantified using a 50% threshold of the SUVmax of the lesion. In line with previous studies in our centre, those with SUVmax ≥ 1.8 were defined as FES-positive lesions [27]. FES-Hot5 was defined as the geometric mean FES SUVmax of the 5 hottest lesions (up to 5 lesions per patient). Interlesional heterogeneity was qualitatively identified according to whether the patient had or did not have FES-negative lesions. A quantitative measure of intralesional heterogeneity, the heterogeneity index (HI), which has been previously used in breast cancer patients, was obtained by dividing the SUVmax by the SUVmean (HI = SUVmax/SUVmean) [27, 32].
Statistical analysis
Continuous variables are displayed as the median value and range. Survival analyses were performed using the Kaplan‒Meier method, and survival was compared by the log-rank test. Each parameter was dichotomized using the median as a threshold. The Cox proportional hazards model was used for univariate and multivariate analyses, and the results are expressed as the hazard ratio with its corresponding 95% confidence interval [CI] and P value. Multivariate analysis with forward stepwise selection was performed with the variables that were proven to be significant in univariate analysis to explore independent significant factors. The association between pretreatment 18F-FES PET image parameters and patients with a clinical benefit from palbociclib combined with endocrine therapy was calculated by the Pearson’s chi-square test or Mann‒Whitney U test. All data analyses were performed using SPSS Statistics version 20.0 (IBM Corporation, Armonk, NY, USA). All statistical tests were two-sided, and a P value less than 0.05 denoted a statistically significant difference.
Results
Patient characteristics
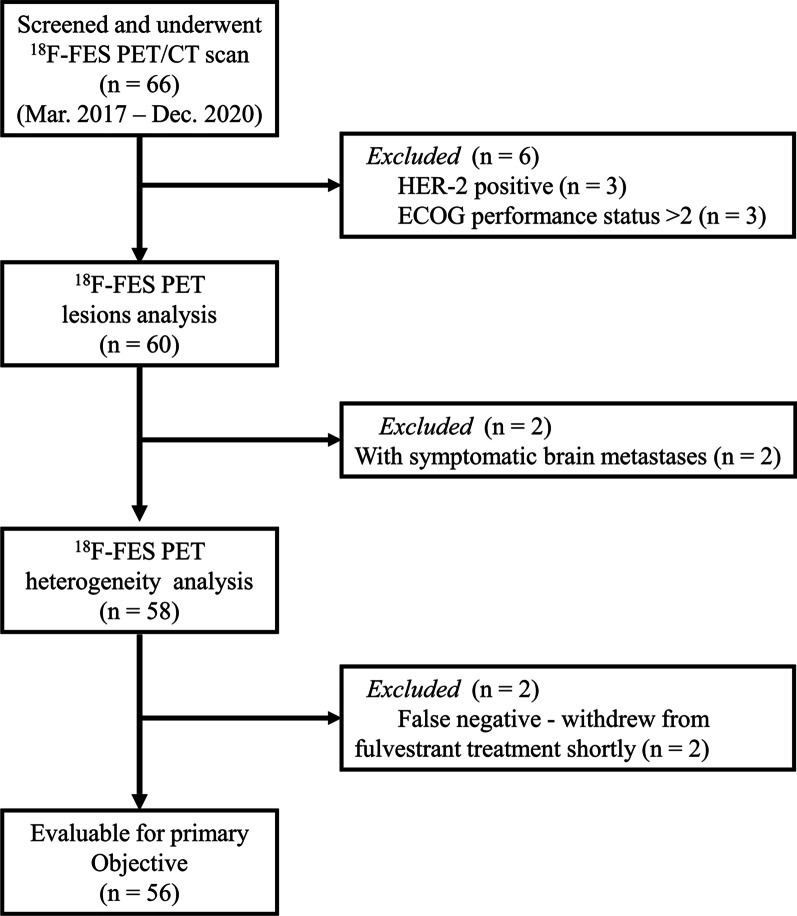
From March 2017 to December 2020, 66 patients with MBC underwent an 18F-FES PET/CT scan before starting treatment with palbociclib. A total of 10 patients were excluded from this analysis: three had HER2 + disease, three had an Eastern Cooperative Oncology Group (ECOG) performance status score of > 2, and two had symptomatic brain metastases. Furthermore, in two patients, the treatment response to fulvestrant was evaluated by 18F-FES PET/CT, and the drug was not discontinued before the examination. The lesions of both patients were 18F-FES negative, which we considered to be false negatives [33], so they were excluded from the final analysis. After excluding 10 patients who were not eligible, a total of 56 patients were eventually included in our analysis, as illustrated in Fig. 1.
Fig. 1.
Patient flowchart for inclusion and exclusion. Abbreviations: HER-2, human epidermal growth receptor 2; ECOG, Eastern Cooperative Oncology Group
Of these 56 patients, the median age was 55.5 years (range, 23–74 years), 42 patients had a natural postmenopausal status, 14 patients achieved postmenopausal status by the use of luteinizing hormone releasing hormone (LHRH) agonists, 37 patients received palbociclib combined with fulvestrant, and 19 patients received palbociclib plus letrozole. The numbers of patients receiving palbociclib in the first-, second-, third-, and later-line settings were 38 (67.8%), 9 (16.1%), 4 (7.2%), and 5 (8.9%), respectively. Nearly one-third of the patients (32.1%) had at least three metastatic sites, with bone being the most common metastatic site (66.1%), and nearly one-half of the patients (46.4%) had visceral metastasis. The clinical characteristics of the 56 MBC patients are listed in Table 1.
Table 1.
Patient demographics and disease characteristics at time of FES PET scan
| Characteristics | N = 56 | % |
|---|---|---|
| Age, years | ||
| Median | 55.5 | |
| Range | 23–74 | |
| < 55 years | 25 | 44.6 |
| ≥ 55 years | 31 | 55.4 |
| Menopausal status | ||
| Premenopausal a | 14 | 25.0 |
| Postmenopausal | 42 | 75.0 |
| Disease-free interval b | ||
| > 5 y | 31 | 55.4 |
| ≤ 5 y | 22 | 39.3 |
| Histology of primary breast cancer | ||
| IDC | 49 | 87.5 |
| ILC | 7 | 12.5 |
| Hormone-receptor status | ||
| ER-positive and PR-positive | 46 | 82.1 |
| ER-positive and PR-negative | 10 | 17.9 |
| Metastatic sites | ||
| Nonvisceral | 30 | 53.6 |
| Bone | 37 | 66.1 |
| Bone-only | 12 | 21.4 |
| Visceral disease | 26 | 46.4 |
| Any lung | 13 | 23.2 |
| Pleural | 7 | 12.5 |
| Peritoneum | 1 | 1.8 |
| Ovarian | 2 | 3.6 |
| Liver | 6 | 7.0 |
| No. of disease sites | ||
| 1 | 18 | 32.1 |
| 2 | 20 | 35.8 |
| ≥ 3 | 18 | 32.1 |
| De novo metastatic disease | 3 | 5.4 |
| Lines of therapy prior to palbociclib | ||
| 0 | 38 | 67.9 |
| 1 | 9 | 16.1 |
| 2 | 4 | 7.1 |
| ≥ 3 | 5 | 8.9 |
| Prior ET for metastatic disease | ||
| None | 43 | 76.8 |
| Yes | 13 | 23.2 |
| Prior ET type for metastatic disease | ||
| Antiestrogen ± LH-RH analog | 8 | 14.3 |
| Aromatase inhibitor ± LH-RH analog | 9 | 16.1 |
| Prior chemotherapy for metastatic disease | ||
| None | 43 | 76.8 |
| Yes | 13 | 23.2 |
| Endocrine therapy following FES PET | ||
| palbociclib + Aromatase inhibitor | 19 | 33.9 |
| palbociclib + fulvestrant | 37 | 66.1 |
| Outcome | ||
| CR | 3 | 5.4 |
| PR | 6 | 10.7 |
| SD | 34 | 60.7 |
| PD | 13 | 23.2 |
| Clinical benefit | ||
| None | 13 | 23.2 |
| Yes | 43 | 76.8 |
| PFS | ||
| Events | 34 | 60.7 |
| Censored | 22 | 39.3 |
| With negative 18F-FES lesions | ||
| None | 46 | 82.1 |
| Yes | 10 | 17.9 |
a For premenopausal women, palbociclib combination with endocrine therapy was given upon the administration of gonadotropin-releasing hormone agonist
b Patients with stage IV breast cancer at initial diagnosis were excluded (N = 3)
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor; PR, progesterone receptor; ET, endocrine therapy; CR, complete responses; PR, partial responses; SD, stable disease; PD, progressive disease; PFS, progression-free survival
Qualitative and quantitative results of 18F-FES PET/CT
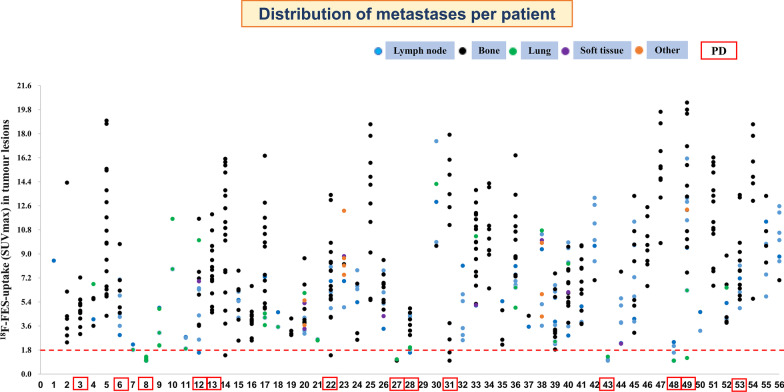
A total of 551 lesions were identified and localized in 56 patients using 18F-FES PET/CT, 18F-FDG (n = 22), or other conventional imaging techniques. The number of lesions per patient ranged from 1 to 20, with a median of 9 lesions. Lesions were present in the bones (n = 361, 65.5%), lymph nodes (n = 127, 23.0%), lung (n = 26, 4.7%), pleura (n = 13, 2.4%), peritoneum (n = 1, 0.2%), ovaries (n = 3, 0.5%), soft tissue (n = 8, 1.5%), breast (n = 6, 1.1%), and liver (n = 6, 1.1%). Among 551 lesions, 507 with 18F-FES PET SUVmax ≥ 1.8 were identified as 18F-FES-positive lesions. Thirty-eight lesions (22 bone, six lymph nodes, seven lung, two soft tissue, and one breast) were considered 18F-FES-negative lesions in 10 patients (17.9%), out of which seven (12.5%) had both 18F-FES-positive and 18F-FES-negative lesions, and three had only 18F-FES-negative lesions. The other six were liver metastases, and the status of 18F-FES could not be quantified or qualified. The 18F-FES PET SUVmax varied greatly among lesions (median, 6.7; range, 1.0–20.3) and patients (median, 6.5; range, 1.1–15.4), as depicted in Fig. 2.
Fig. 2.
Distribution of metastases per patient and 18F-FES uptake (SUVmax) of all metastases in individual patients. The red dashed line indicates the SUVmax threshold of 1.8. Abbreviation: PD, progressive disease
Correlation between tumour response and 18F-FES PET
Twenty patients had measurable lesions on baseline CT or MRI according to RECIST, 25 patients had non-measurable visceral lesions or lymph nodes, and 12 patients had only bone metastases. At the time of analysis, 34 (60.7%) patients had documented disease progression: 33 patients developed radiological PD, and one patient developed deterioration of symptoms and a fourfold increase in the tumour marker CA153, which was defined as clinical PD. An overall CBR of 76.8% was observed, with three patients with CR (5.4%), six patients with PR (10.7%) and 34 patients with SD (60.7%) at ≥ 24 weeks. A total of 13 patients (23.2%) had PD within 6 months from palbociclib combined with endocrine therapy.
The baseline tumour 18F-FES uptake in metastatic patients with clinical benefit from palbociclib was similar to that in patients with PD (median SUV max, 6.5 vs. 5.0; P > 0.05). However, it is interesting that nine of 10 patients with at least one 18F-FES-negative site developed PD, and among 46 patients with 100% 18F-FES-positive disease, only four patients had PD within 6 months (Fig. 2, P < 0.001). Using the presence of any 18F-FES-negative metastatic lesion to distinguish between patients with clinical benefit and PD leads to a positive predictive value (PPV) and negative predictive value (NPV) of 91.3% and 90.0%, respectively. This underlined that patients with any lesion lacking 18F-FES uptake above background were unlikely to benefit from palbociclib-based therapy.
Predictive value of 18F-FES-PET for PFS
At the time of analysis, the median PFS in the whole population was 16.1 months (range 1.9–35.6 + ; 95% CI 6.8–25.4). Before analysing 18F-FES PET parameters to predict PFS, we evaluated the patients’ disease characteristics before palbociclib treatment. The median PFS was 21.6 months in patients treated with palbociclib as the first line of treatment, 23.6 months as the second line of treatment, and 4.2 months as the third or further line of treatment (log-rank test P = 0.005). Patients treated with palbociclib as first-line and second-line treatment showed HRs of 0.33 (95% CI 0.12–0.93) and 0.29 (95% CI 0.13–0.65) for PFS compared to those treated with palbociclib as third-line or subsequent-line treatment. Nevertheless, the disease-free interval (DFI) from adjuvant treatment, number of disease sites, presence of visceral disease and types of endocrine therapy were not found to be prognostic factors of PFS in the whole cohort (Table 2).
Table 2.
Univariate and multivariate Cox regression analyses for prediction of PFS for the entire patients
| Parameters | No | Event | Median PFS | Log-rank | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|---|
| (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Disease-free interval | ||||||||
| ≤ 5 y | 22 | 11 | 23.9(1.6–46.3) | 0.366 | 1.40(0.67–2.89) | 0.369 | NA | |
| > 5 y | 31 | 22 | 15.6(10.1–21.1) | |||||
| No. of disease sites | ||||||||
| 1 | 18 | 13 | 12.0(0.9–23.1) | 0.690 | ||||
| 2 | 20 | 12 | 12.1(0.6–23.6) | 0.89(0.41–1.97) | 0.789 | NA | ||
| ≥ 3 | 18 | 9 | 23.9(14.6–33.2) | 0.69(0.29–1.63) | 0.397 | |||
| Visceral disease | ||||||||
| No | 30 | 20 | 12.0(6.4–17.6) | 0.191 | 0.64(0.32–1.26) | 0.196 | NA | |
| Yes | 26 | 14 | 23.9(14.4–33.3) | |||||
| Lines of therapy prior to palbociclib | ||||||||
| 0 | 38 | 19 | 21.6(12.6–30.6) | 0.33(0.12–0.93) | 0.036* | |||
| 1 | 9 | 6 | 23.6(7.8–39.4) | 0.005* | 0.29(0.13–0.65) | 0.003* | / | 0.170 |
| ≥ 2 | 9 | 9 | 4.2(3.8–4.7) | |||||
| Types of endocrine therapy | ||||||||
| palbociclib + fulvestrant | 37 | 25 | 12.8(7.0–18.7) | 0.186 | 0.60(0.28–1.29) | 0.192 | NA | |
| palbociclib + letrozole | 19 | 9 | 26.5(4.5.7–48.5) | |||||
| Presence of FES-negative lesions | ||||||||
| Yes | 10 | 10 | 2.4(1.1–3.7) | < 0.001* | 0.04(0.01–0.13) | < 0.001* | 0.04(0.01–0.13) | < 0.001* |
| No | 46 | 24 | 23.6(15.8–31.4) | |||||
PFS Progression-free survival; CI Confidence interval; HR Hazard ratio; MBC Metastatic breast cancer; SUVmax Maximum standard uptake value
* Indicates statistically significant differences (P < 0.05); N/A: Analysis not performed as univariate analysis not significant
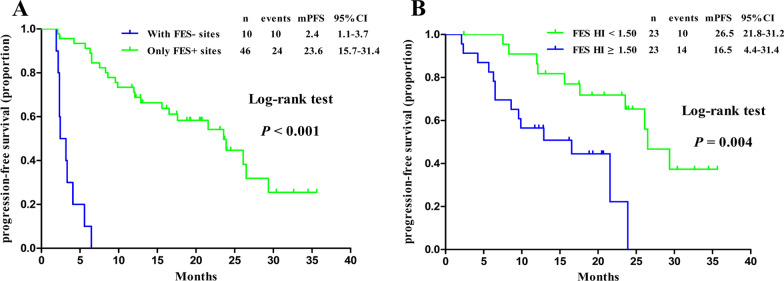
Next, we evaluated the predictive value of 18F-FES PET parameters for survival in patients receiving palbociclib-based therapy. A total of 46 patients had only 18F-FES-positive sites, and their median PFS was 23.6 months (95% CI 15.8–31.4); 10 patients (seven had both positive and negative 18F-FES metastases, and three had no 18F-FES-positive metastases) had at least one 18F-FES-negative metastatic site, and their median PFS was only 2.4 months (95% CI 1.1–3.7) (log-rank P < 0.001, Fig. 3A and Fig. 4). Using univariate analysis, we found lines of advanced systemic therapy with palbociclib and presence of 18F-FES-negative lesions to be significantly correlated with PFS. However, in multivariate analysis using Cox proportional hazards models, only presence of 18F-FES-negative lesions was found to be a single determinant of PFS (HR = 0.04, 95% CI 0.01–0.13, P < 0.001, Table 2).
Fig. 3.
Kaplan‒Meier curve of progression-free survival (PFS) according to heterogeneity determined by 18F-FES PET. A PFS predicted by interlesional heterogeneity, with patients stratified by the presence or absence of 18F-FES-negative lesions in the whole cohort. B PFS predicted by intralesional heterogeneity, with patients stratified by the median FES-HI in the subgroup cohort with only 18F-FES-positive lesions
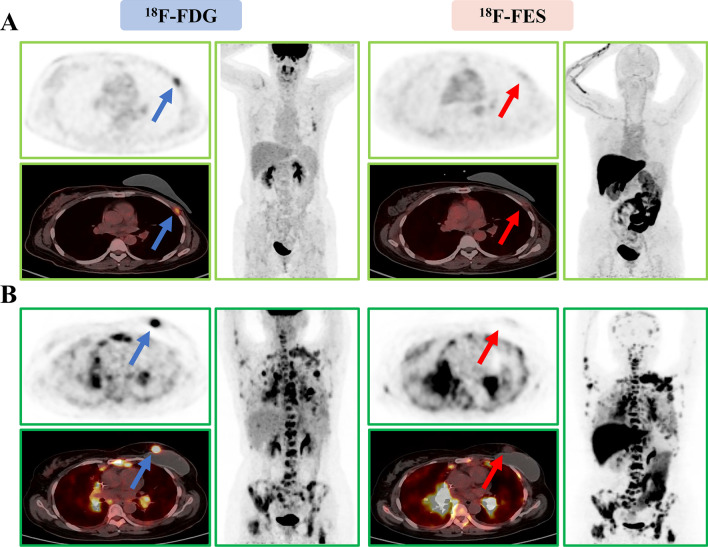
Fig. 4.
Representative imaging of patients with at least one 18F-FES-negative lesion. A Only 18F-FES-negative lesions (Fig. 2, #43). This 50-year-old woman had 4 18F-FDG-positive lesions in her chest wall and lymph nodes, but all were negative on 18F-FES PET. She was on palbociclib combined with letrozole as first-line treatment for 2.3 months until progression occurred. B Presence of 18F-FES-positive and 18F-FES-negative lesions (Fig. 2, #49). This 53-year-old woman had innumerable 18F-FDG-positive and 18F-FES-positive lesions in the pleura, lung, lymph nodes, and bone, but the left chest wall metastasis showed outstanding uptake of 18F-FDG but not of 18F-FES. She was on palbociclib combined with fulvestrant as third-line treatment for 5.6 months until progression occurred
The presence or absence of 18F-FES-negative lesions represents only interlesional heterogeneity and not intralesional heterogeneity. Hence, we conducted an exploratory analysis using identified PET biomarkers to predict survival in patients with only 18F-FES-positive lesions. Intralesional heterogeneity was measured by dividing the SUVmax by the SUVmean across the 18F-FES-positive metastatic lesions, which is the HI. In the 18F-FES-positive subgroup analysis, the median values of SUVmax, FES-Hot5 and HI were used as the cut-off values, which were 6.5 (range 1.9–15.4), 8.1 (range 2.0–17.2) and 1.50 (range 1.33–1.57), respectively. Regrettably, SUVmax and FES-Hot5 were not predictive of PFS in this subgroup (log-rank P = 0.258 and 0.575, respectively, Table 3). A total of 23 patients with high HI-FES had obviously shorter PFS times than those with low HI (HI ≥ 1.50, median PFS 16.5 months, 95% CI 4.4–28.6 vs. HI < 1.5, median PFS 26.5 months, 95% CI 21.8–32.2; log-rank P = 0.004, Fig. 3B and Fig. 5). Patients with low HI-FES showed an HR of 0.27 for PFS (95% CI 0.10–0.70; P = 0.007) compared to those with high HI-FES (Table 3).
Table 3.
Univariate and multivariate Cox regression analyses for prediction of PFS in the subgroup of patients with only FES-positive sites
| Parameters | No | Event | Median PFS | Log-rank | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|---|
| (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| FES SUVmax | ||||||||
| ≥ 6.5 | 23 | 14 | 21.6(13.5–29.6) | 0.258 | 0.63(0.28–1.42) | 0.262 | NA | |
| < 6.5 | 23 | 10 | 29.4(13.4–45.4) | |||||
| FES Hot5 lesions | ||||||||
| ≥ 8.1 | 23 | 13 | 23.6(14.3–32.8) | 0.575 | 0.79(0.35–1.79) | 0.576 | NA | |
| < 8.1 | 23 | 11 | 23.9(9.3–38.5) | |||||
| FES HI a | ||||||||
| ≥ 1.50 | 23 | 14 | 16.5(4.4–28.6) | 0.004* | 0.27(0.10–0.70) | 0.007* | NA | |
| < 1.50 | 23 | 10 | 26.5(21.8–32.2) | |||||
PFS Progression-free survival; CI Confidence interval; HR Hazard ratio; SUVmax Maximum standard uptake value; SUVmean Mean standard uptake value; HI Heterogeneity index
a HI = SUVmax/SUVmean; * Indicates statistically significant differences (P < 0.05)
Fig. 5.
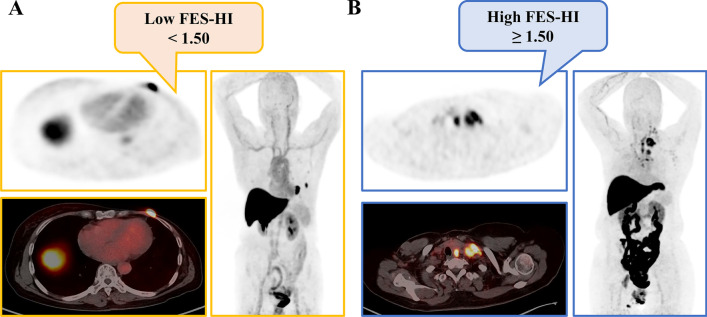
Representative imaging of patients with only 18F-FES-positive lesions. A Low FES-HI (Fig. 2, #10). This 67-year-old woman had 2 18F-FES-positive lesions in the chest wall and lung, with no 18F-FES-negative lesions. The median FES-HI for the 2 avid lesions was 1.38. She was on palbociclib combined with letrozole as first-line treatment for 30.4 months without disease progression. B High FES-HI (Fig. 2, #32). This 56-year-old woman had 6 18F-FES-positive lesions in the lymph nodes, with no 18F-FES-negative lesions. The median FES-HI for the 6 avid lesions was 1.52. She was on palbociclib combined with fulvestrant as first-line treatment for 16.5 months until progression
Discussion
Currently, there are no clinically available biomarkers for prescribing CDK4/6 inhibitors except for ER expression mainly from primary tumour tissues [9, 15]. However, the expression status of ER in breast cancer may change during the course of disease progression or treatment [34]. ER status discordance rates between primary and metastatic breast cancer sites may reach approximately 32%, which might change the therapeutic strategy and sensitivity for breast cancer patients [35]. Although biopsy is the gold standard for assessing ER status, it is sometimes unreliable due to interlesional and intralesional heterogeneities in ER expression. Furthermore, biopsy is an invasive procedure with the risk of serious complications and poor manoeuvrability and patient compliance. 18F-FES PET/CT has been shown to have the potential to assess ER expression in all tumour lesions, supporting individualized treatment strategy choices, and could aid physicians in making therapeutic decisions [30, 36].
In the present study, we investigated the 18F-FES-PET imaging characteristics and tumour responses of patients with MBC who received a CDK4/6 inhibitor combined with endocrine therapy. Boers et al. found that patients with 100% 18F-FES positivity benefitted most from palbociclib plus letrozole compared to those with heterogeneous or absent 18F-FES uptake (HR 2.1) [37]. However, the limitation of this study is that it only evaluated the interlesional heterogeneity between MBC patients with and without 18F-FES-negative lesions and failed to investigate the intralesional heterogeneity among patients with 100% 18F-FES-positive metastatic lesions. Recently, a feasible quantitative method for measuring heterogeneity, HI, has been investigated. Our previous series of studies successfully used pretreatment 18F-FDG HI to predict survival in MBC patients [32, 38, 39], and 18F-FES HI can better reflect the heterogeneity of ER expression, especially in patients with metastases after treatment [27]. In this context, we designed this exploratory study to demonstrate how interlesional heterogeneity in the presence or absence of ER-positive lesions and intralesional heterogeneity in all ER-positive patients could predict the efficacy of palbociclib plus letrozole or fulvestrant based on 18F-FES-PET imaging.
Our study demonstrated that pretreatment 18F-FES PET has value in predicting whether patients treated with palbociclib will respond to therapy. From our data, patients (9/10) who had any 18F-FES-negative lesions were more likely to develop PD within 24 weeks of therapy initiation (with no clinical benefit). In contrast, almost all patients (42/46) who had only 18F-FES-positive lesions obtained a clinical benefit. This is different from the results of Boers et al., who found that a considerable number of 18F-FES-negative lesions also showed a response [37]. One explanation may be that some of their 18F-FES-negative lesions may still exhibit mild ER expression because they used an 18F-FES SUVmax cut-off of 2.0, which is higher than the value of 1.8 we used. Another explanation could be the differences in treatment patterns and patient characteristics, and the efficacy of palbociclib in the real-world setting differed. However, based on their research, it seems that physicians will still be puzzled by whether the presence of 18F-FES-negative lesions means that patients can benefit from palbociclib plus endocrine therapy. In comparison, our imaging indicators provided a better-stratified method to identify who could benefit from palbociclib plus endocrine therapy and who is not likely to benefit, which is more in conformity with the concept of precision medicine.
In the survival analysis, patients with 18F-FES-negative lesions exhibited a poorer prognosis with an obviously shorter median PFS than those who had only 18F-FES-positive lesions (2.4 months vs. 23.6 months, log-rank P < 0.001). Moreover, patients with additional lines of advanced systemic therapy with palbociclib (P = 0.005) had a worse prognosis. However, due to the strong interplay between ER pathways and CDK4/6 signalling, only the presence or absence of 18F-FES uptake significantly and independently correlated with the outcome in the multivariate analysis. Moreover, one specific difference between our report and previous reports is that we further analysed the subgroup of patients with only 18F-FES-positive lesions. This was undertaken because, even if this cohort has a good response to palbociclib treatment, considering the heterogeneity of ER expression in tumours, the final efficacy in each patient may be different. As expected, patients with a low FES-HI had significantly longer PFS times than those with a high FES-HI (median PFS, 26.5 months vs. 16.5 months, log-rank P = 0.004).
Thus, our results suggested that for ER-positive (primary) MBC, patients with any ER-negative lesion by 18F-FES PET are unlikely to benefit from palbociclib plus endocrine therapy, and it might be better to make changes to the treatment protocol. Patients with only 18F-FES-positive lesions are potential candidates for combination therapy, but the efficacy in patients with high FES-HI is unsatisfactory. This group of patients should choose chemotherapy or other endocrine therapy options, such as chidamide in combination with endocrine therapy or another CDK4/6 inhibitor in combination with endocrine therapy [40].
There were some limitations to this study. First, given the retrospective design of the study, the disease characteristics of the patients in the cohort were heterogeneous, which may include patients with inherently different prognostic factors independent of 18F-FES uptake. However, we eliminated some known factors that can affect 18F-FES uptake, such as discontinuation of drugs known to bind ER less than 6 weeks before 18F-FES PET imaging. In addition, the study was conducted in a single centre and was based on a small cohort of Asian patients, so the optimal cut-off values identified in this study might not be applicable to all patients, and external validation is needed. Given that the patients in our study underwent many pretreatments, but only some patients had an 18F-FDG PET scan concurrently, CT or MRI scans may have shown bone lesions that were no longer active, leading to an overestimation of the number of 18F-FES-negative lesions. Moreover, our study did not analyse the relationship between 18F-FES uptake and ESR1 gene amplification and mutation in the tumour biopsy, as these assays provide insufficient data at present to guide therapy for HR + /HER2- MBC[41] and are not performed routinely in our centre. Although ER expression is required for a response to palbociclib plus endocrine therapy, other pathways may also affect the efficacy of palbociclib, such as the androgen receptor (AR) signalling pathway [42]. A single 18F-FES PET scan failed to show crosstalk with other pathways; therefore, it would be of interest in future studies to add multiple molecular imaging probes to improve the predicted response to palbociclib-based treatment, such as 18F-fluoro-5α-dihydrotestosterone (18F-FDHT)-PET for imaging AR [43] and 18F-FDG PET for imaging the glycolytic metabolism tumour burden [44]. Finally, we were unable to obtain serial tumour biopsies to assess ER status, especially liver metastases (ER status cannot be reliably measured in liver metastases due to high background 18F-FES avidity), thus limiting information on the accuracy of the 18F-FES PET imaging of ER status.
Conclusion
Our study showed that interlesional and intralesional heterogeneity demonstrated by 18F-FES-PET/CT provided a promising way to predict palbociclib plus endocrine therapy efficacy and provided a novel method for better stratifying and selecting candidate MBC patients who would most likely benefit from palbociclib plus endocrine therapy.
Acknowledgements
The authors would like to thank my colleagues in the Multiple Disciplinary Team of Breast Cancer and the Nuclear Medicine team at Fudan University Shanghai Cancer Center.
Abbreviations
- ER + /HER2-
Estrogen receptor positive, human epidermal growth factor receptor 2 negative
- MBC
Metastatic breast cancer
- CDK4/6
Cyclin-dependent kinase 4 and 6
- 18F-FES
16α-18F-fluoro-17β-oestradiol
- PET
Positron emission tomography
- HI
Heterogeneity index
- PFS
Progression-free survival
- SUV
Standardized uptake value
- HI
Heterogeneity index
- PD
Progressive disease
- HR +
Hormone receptor-positive
- ECOG
Eastern cooperative oncology group
- AI
Aromatase inhibitor
- SERD
Selective estrogen receptor degrader
- LHRHa
Luteinizing hormone-releasing hormone analogue
- PPV
Positive predictive value
- NPV
Negative predictive value
- CBR
Clinical benefit rate
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- VOIs
Volumes of interest
- HR
Hazard ratio
- CI
Confidence interval
- 18F-FDG
18F-fuorodeoxyglucose
- AR
Androgen receptor
- 18F-FDHT
18F-fluoro-5α-dihydrotestosterone
Author contributions
C-L, ZY-Y, BY-W conceived and designed the experiments, C-L, SH-H, XP-X, and YP-Z performed the experiments, C-L and SH-H analysed the data and wrote the paper, ZY-Y, BY-W and SL-S contributed to manuscript revision. All authors read and approved the final manuscript.
Funding
This research is sponsored by National Natural Science Foundation of China (81874114), Shanghai Sailing Program (20YF1408500), Shanghai Committee of Science and Technology Fund (22DZ2204500), Shanghai Municipal Health Commission (202040269) Science and Technology Development Fund of Shanghai Pudong New Area (PKJ2020-Y54).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study has been approved by the Fudan University Shanghai Cancer Center Ethic Committee and Institutional Review Boards for clinical investigation, and the need for informed consent was waived as it’s a retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cheng Liu and Shihui Hu contributed equally to this work
Contributor Information
Biyun Wang, Email: wangbiyun0107@hotmail.com.
Shaoli Song, Email: shaoli-song@163.com.
Zhongyi Yang, Email: yangzhongyi21@163.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA: US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106(5). [DOI] [PMC free article] [PubMed]
- 3.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, et al. Endocrine Therapy for hormone receptor-positive metastatic breast cancer: american society of clinical oncology guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(25):3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet (London, England) 2020;395(10226):817–827. doi: 10.1016/S0140-6736(20)30165-3. [DOI] [PubMed] [Google Scholar]
- 6.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Li W, Gong C, Zheng Y, Ouyang Q, Xie N, Qu Q, Ge R, Wang B. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol. 2021;13:17588359211022890. doi: 10.1177/17588359211022890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, Pearson A, Guzman M, Rodriguez O, Grueso J, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Can Res. 2016;76(8):2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey K, An HJ, Kim SK, Lee SA, Kim S, Lim SM, Kim GM, Sohn J, Moon YW. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int J Cancer. 2019;145(5):1179–1188. doi: 10.1002/ijc.32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, DeMichele A, Harbeck N, André F, Bayar MA, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(14):1169–1178. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Re M, Bertolini I, Crucitta S, Fontanelli L, Rofi E, De Angelis C, Diodati L, Cavallero D, Gianfilippo G, Salvadori B, et al. Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat. 2019;178(1):57–62. doi: 10.1007/s10549-019-05365-y. [DOI] [PubMed] [Google Scholar]
- 12.Darrigues L, Pierga JY, Bernard-Tessier A, Bièche I, Silveira AB, Michel M, Loirat D, Cottu P, Cabel L, Dubot C, et al. Circulating tumor DNA as a dynamic biomarker of response to palbociclib and fulvestrant in metastatic breast cancer patients. Breast Cancer Res BCR. 2021;23(1):31. doi: 10.1186/s13058-021-01411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 14.Miller TW, Balko JM, Fox EM, Ghazoui Z, Dunbier A, Anderson H, Dowsett M, Jiang A, Smith RA, Maira SM, et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1(4):338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang H, Huang D, Yang F, Guan X. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat. 2018;168(2):287–297. doi: 10.1007/s10549-017-4612-y. [DOI] [PubMed] [Google Scholar]
- 16.Patel HK, Tao N, Lee KM, Huerta M, Arlt H, Mullarkey T, Troy S, Arteaga CL, Bihani T. Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors. Breast Cancer Res BCR. 2019;21(1):146. doi: 10.1186/s13058-019-1230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieuwerts AM, Inda MA, Smid M, van Ooijen H, van de Stolpe A, Martens JWM, Verhaegh WFJ. ER and PI3K pathway activity in primary ER positive breast cancer is associated with progression-free survival of metastatic patients under first-line tamoxifen. Cancers. 2020;12(4):802. doi: 10.3390/cancers12040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sighoko D, Liu J, Hou N, Gustafson P, Huo D. Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: Biological difference or misclassification? Oncologist. 2014;19(6):592–601. doi: 10.1634/theoncologist.2013-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrijver W, Suijkerbuijk KPM, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(6):568–580. doi: 10.1093/jnci/djx273. [DOI] [PubMed] [Google Scholar]
- 20.van Es SC, van der Vegt B, Bensch F, Gerritse S, van Helden EJ, Boon E, Angus L, Overbosch J, der Menke-van Houven VO, Verheul HM, et al. Decalcification of breast cancer bone metastases with EDTA does not affect ER PR and HER2 results. Am J Surg Pathol. 2019;43(10):1355–1360. doi: 10.1097/PAS.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 21.Lindström LS, Yau C, Czene K, Thompson CK, Hoadley KA, Van't Veer LJ, Balassanian R, Bishop JW, Carpenter PM, Chen YY, et al. Intratumor heterogeneity of the estrogen receptor and the long-term risk of fatal breast cancer. J Natl Cancer Inst. 2018;110(7):726–733. doi: 10.1093/jnci/djx270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao GJ, Clark AS, Schubert EK, Mankoff DA. 18F-fluoroestradiol PET: current status and potential future clinical applications. J Nucl Med. 2016;57(8):1269–1275. doi: 10.2967/jnumed.116.175596. [DOI] [PubMed] [Google Scholar]
- 23.Kurland BF, Wiggins JR, Coche A, Fontan C, Bouvet Y, Webner P, Divgi C, Linden HM. Whole-body characterization of estrogen receptor status in metastatic breast cancer with 16α-18f-fluoro-17β-estradiol positron emission tomography: meta-analysis and recommendations for integration into clinical applications. Oncologist. 2020;25(10):835–844. doi: 10.1634/theoncologist.2019-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Xu X, Yuan H, Zhang Y, Zhang Y, Song S, Yang Z. Dual Tracers of 16α-[18F]fluoro-17β-Estradiol and [18F]fluorodeoxyglucose for prediction of progression-free survival after fulvestrant therapy in patients with HR+/HER2- metastatic breast cancer. Front Oncol. 2020;10:580277. doi: 10.3389/fonc.2020.580277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He M, Liu C, Shi Q, Sun Y, Zhang Y, Xu X, Yuan H, Zhang Y, Liu Y, Liu G, et al. The predictive value of early changes in (18) F-fluoroestradiol positron emission tomography/computed tomography during fulvestrant 500 mg therapy in patients with estrogen receptor-positive metastatic breast cancer. Oncologist. 2020;25(11):927–936. doi: 10.1634/theoncologist.2019-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurland BF, Peterson LM, Lee JH, Schubert EK, Currin ER, Link JM, Krohn KA, Mankoff DA, Linden HM. Estrogen receptor binding (18F-FES PET) and glycolytic activity (18F-FDG PET) predict progression-free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res. 2017;23(2):407–415. doi: 10.1158/1078-0432.CCR-16-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z, Sun Y, Xu X, Zhang Y, Zhang J, Xue J, Wang M, Yuan H, Hu S, Shi W, et al. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18F-fluoroestradiol PET/CT. Clin Nucl Med. 2017;42(6):421–427. doi: 10.1097/RLU.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 28.van Kruchten M, Glaudemans A, de Vries EFJ, Schröder CP, de Vries EGE, Hospers GAP. Positron emission tomography of tumour [(18)F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42(11):1674–1681. doi: 10.1007/s00259-015-3107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chae SY, Ahn SH, Kim SB, Han S, Lee SH, Oh SJ, Lee SJ, Kim HJ, Ko BS, Lee JW, et al. Diagnostic accuracy and safety of 16α-[(18)F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: a prospective cohort study. Lancet Oncol. 2019;20(4):546–555. doi: 10.1016/S1470-2045(18)30936-7. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Gong C, Liu S, Zhang Y, Zhang Y, Xu X, Yuan H, Wang B, Yang Z. (18)F-FES PET/CT influences the staging and management of patients with newly diagnosed estrogen receptor-positive breast cancer: a retrospective comparative study with (18)F-FDG PET/CT. Oncologist. 2019;24(12):e1277–e1285. doi: 10.1634/theoncologist.2019-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Liu C, Wang B, Hu X, Gong C, Zhao Y, Xie Y, Zhang Y, Song S, Yang Z, et al. Prediction of pretreatment 18F-FDG-PET/CT parameters on the outcome of first-line therapy in patients with metastatic breast cancer. Int J Gen Med. 2021;14:1797–1809. doi: 10.2147/IJGM.S293998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Ayres KL, Goldman DA, Dickler MN, Bardia A, Mayer IA, Winer E, Fredrickson J, Arteaga CL, Baselga J, et al. (18)F-Fluoroestradiol PET/CT measurement of estrogen receptor suppression during a Phase I trial of the novel estrogen receptor-targeted therapeutic GDC-0810: using an imaging biomarker to guide drug dosage in subsequent trials. Clin Cancer Res. 2017;23(12):3053–3060. doi: 10.1158/1078-0432.CCR-16-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, Tong Y, Chen X, Shen K. Association of biomarker discrepancy and treatment decision, disease outcome in recurrent/metastatic breast cancer patients. Front Oncol. 2021;11:638619. doi: 10.3389/fonc.2021.638619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, Nesbit E, Kruser TJ, Chan J, Braunstein S, et al. Estrogen/progesterone receptor and HER2 discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival. Neuro Oncol. 2020;22(9):1359–1367. doi: 10.1093/neuonc/noaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Kruchten M, Glaudemans AW, de Vries EF, Beets-Tan RG, Schröder CP, Dierckx RA, de Vries EG, Hospers GA. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53(2):182–190. doi: 10.2967/jnumed.111.092734. [DOI] [PubMed] [Google Scholar]
- 37.Boers J, Venema CM, de Vries EFJ, Glaudemans A, Kwee TC, Schuuring E, Martens JWM, Elias SG, Hospers GAP, Schröder CP. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur J Cancer (Oxford England: 1990) 2020;126:11–20. doi: 10.1016/j.ejca.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Gong C, Ma G, Hu X, Zhang Y, Wang Z, Zhang J, Zhao Y, Li Y, Xie Y, Yang Z, et al. Pretreatment (18)F-FDG uptake heterogeneity predicts treatment outcome of first-line chemotherapy in patients with metastatic triple-negative breast cancer. Oncologist. 2018;23(10):1144–1152. doi: 10.1634/theoncologist.2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Liu C, Zhang Y, Gong C, Li Y, Xie Y, Wu B, Yang Z, Wang B. Prognostic value of tumor heterogeneity on 18F-FDG PET/CT in HR+HER2- metastatic breast cancer patients receiving 500 mg fulvestrant: a retrospective study. Sci Rep. 2018;8(1):14458. doi: 10.1038/s41598-018-32745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et al. Breast Cancer version 3202, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 41.Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, Johnston SRD, Korde LA, Litton JK, Macrae ER et al: Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol Off J Am Soc Clin Oncol 2021;Jco2101392. [DOI] [PMC free article] [PubMed]
- 42.Hickey TE, Selth LA, Chia KM, Laven-Law G, Milioli HH, Roden D, Jindal S, Hui M, Finlay-Schultz J, Ebrahimie E, et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat Med. 2021;27(2):310–320. doi: 10.1038/s41591-020-01168-7. [DOI] [PubMed] [Google Scholar]
- 43.Jacene H, Liu M, Cheng SC, Abbott A, Dubey S, McCall K, Young D, Johnston M, Van den Abbeele AD, Overmoyer B. Imaging androgen receptors in breast cancer with (18)F-Fluoro-5α-dihydrotestosterone PET: a pilot study. J Nucl Med. 2022;63(1):22–28. doi: 10.2967/jnumed.121.262068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonelli M, Terenziani R, Zoppi S, Fumarola C, La Monica S, Cretella D, Alfieri R, Cavazzoni A, Digiacomo G, Galetti M, et al. Dual inhibition of CDK4/6 and PI3K/AKT/mTOR signaling impairs energy metabolism in MPM cancer cells. Int J Mol Sci. 2020;21(14):5165. doi: 10.3390/ijms21145165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.