Abstract
Context
Iatrogenic hypoglycemia remains one of the leading hindrances of optimal glycemic management in insulin-treated diabetes. Recurring hypoglycemia leads to a condition of hypoglycemia-associated autonomic failure (HAAF). HAAF refers to a combination of (i) impaired hormonal counterregulatory responses and (ii) hypoglycemia unawareness to subsequent hypoglycemia, substantially increasing the risk of severe hypoglycemia. Several studies since the 1990s have experimentally induced HAAF, yielding variable results.
Objective
The aim of this review was to assess the varying designs, clinical outcomes, potential assets, and drawbacks related to these studies.
Method
A systemic literature search was conducted on PubMed and Embase in winter 2021 to include all human studies attempting to experimentally induce HAAF. In different combinations, the search terms used were “hypoglycemia-associated autonomic failure,” “HAAF,” “hypoglycemia,” “recurring,” “recurrent,” “repeated,” “consecutive,” and “unawareness,” yielding 1565 publications. Inclusion criteria were studies that had aimed at experimentally inducing HAAF and measuring outcomes of hormonal counterregulation and awareness of hypoglycemia.
Results
The literature search yielded 27 eligible publications, of which 20 were successful in inducing HAAF while statistical significantly impairing both hormonal counterregulation and impairing awareness of hypoglycemia to subsequent hypoglycemia. Several factors were of significance as regards inducing HAAF: Foremost, the duration of antecedent hypoglycemia should be at least 90 minutes and blood glucose should be maintained below 3.4 mmol/L. Other important factors to consider are the type of participants, insulin dosage, and the risk of unintended hypoglycemia prior to the study.
Conclusion
Here we have outlined the most important factors to take into consideration when designing a study aimed at inducing HAAF, including to take into consideration other disease states susceptible to hypoglycemia, thus hopefully clarifying the field and allowing qualified studies in the future.
Keywords: hypoglycemia, hypoglycemia-associated autonomic failure, type 1 diabetes, type 2 diabetes
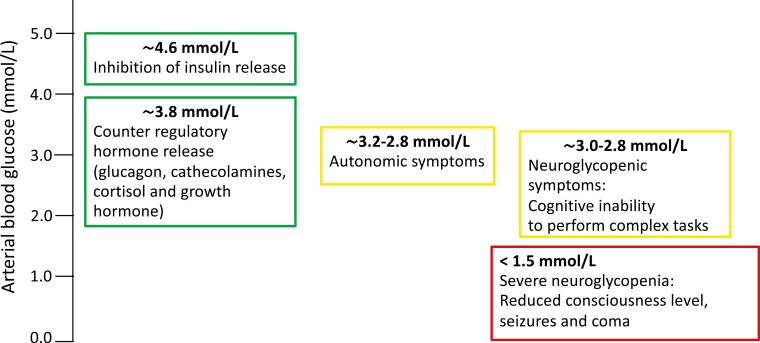
Presently, iatrogenic hypoglycemia is a common and dreaded side effect to insulin therapy and some oral antidiabetic drugs, and “still remains one of the leading hindrances for optimal glycemic management of insulin-treated diabetes” [1]. In individuals without diabetes, hypoglycemia leads to a timely well-organized sequence of hormonal counterregulatory responses comprising decreased secretion of insulin from the β cells at a glycemic blood glucose threshold of approximately 4.5 mmol/L and increased secretion of glucagon by the α cells, increased release of epinephrine and norepinephrine (catecholamines) together with cortisol from the adrenal glands, and growth hormone (GH) from the pituitary gland at a blood glucose concentration of approximately 3.8 mmol/L. The combined metabolic response to these hormones includes an increased endogenous glucose production primarily from the liver and to a lesser extent from the kidneys, enabling a restoration of blood glucose to a normal concentration. Furthermore, the hormones increase the rate of lipolysis and of ketogenesis, which relieve glucose needs in virtually all tissues, augmenting proteolysis and ureagenesis and decreasing peripheral glucose disposal [2-4]. Likewise, autonomic symptoms of hypoglycemia arise allowing protective behavioral actions of approximately 3.2 mmol/L, whereas neuroglycopenia impairs it and inhibits protective behavior actions [5] (Fig. 1).
Figure 1.
Normal glycemic thresholds and counterregulation during hypoglycemia is characterized by inhibition of insulin secretion, counterregulatory hormone release in the hierarchical order of glucagon, catecholamines (epinephrine and norepinephrine), cortisol, and growth hormone followed by autonomic symptom onset. If hypoglycemia prevails, cognitive inability to perform complex tasks and later severe neuroglycopenia develop.
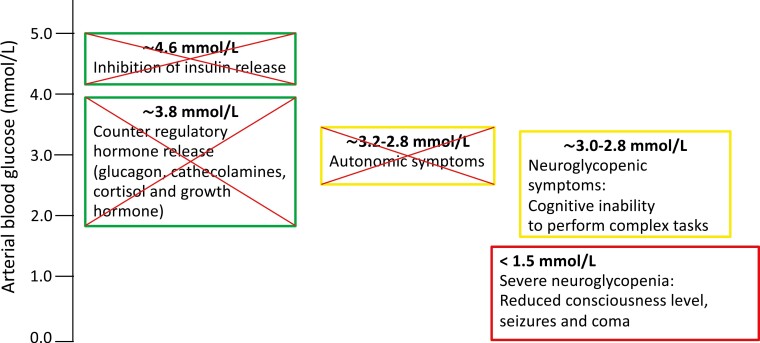
Hypoglycemia-associated Autonomic Failure
After the introduction of insulin therapy in 1922 [6], clinicians soon recognized that some insulin-treated individuals did not experience symptoms during hypoglycemia [7]. Presumably, this early observation reflects hypoglycemia unawareness—an inherent component of hypoglycemia-associated autonomic failure (HAAF) [8]. HAAF is induced by a recent antecedent episode of hypoglycemia causing a (mal-)adaptive condition with defective hormonal counterregulatory responses and hypoglycemia unawareness during a subsequent hypoglycemic episode (Fig. 2). Inherent to repeated hypoglycemia is a lowering of the glycemic threshold at which the counterregulatory responses occur. These phenomena can result in a vicious cycle of recurring hypoglycemia, significantly increasing the risk of severe hypoglycemia, rendering the individual unaware until blood glucose concentrations become too low to maintain adequate brain function and impairment of cognition and consciousness supervenes [9, 10] (see Fig. 2). It is estimated that HAAF is present in 25% of individuals with type 1 diabetes [11], and in some individuals with longer duration of type 2 diabetes [12]. The mechanism behind HAAF remains to be fully clarified though several hypotheses exist [13]. The aim of this review is to assess the varying design in studies aimed at experimentally inducing HAAF in individuals with and without diabetes. Furthermore, we aimed at describing the clinical outcomes and potential drawbacks based on these studies in the hope of encouraging future qualified studies aiming at understanding and preventing HAAF and severe hypoglycemia.
Figure 2.
Compromised hypoglycemic counterregulation. Individuals treated with insulin therapy lack the ability to decrease insulin levels. Furthermore, hypoglycemia-associated autonomic failure (HAAF) is induced by recent antecedent hypoglycemia causing impaired hormonal counterregulatory responses and hypoglycemia unawareness during a subsequent hypoglycemic episode, making the individual susceptible to recurrent hypoglycemia severalfold, increasing the risk of severe hypoglycemia.
Materials and Methods
To identify studies attempting to describe and induce HAAF in humans, the databases PubMed and EMBASE were searched from their inception until January 2022. Inclusion criteria were studies that had aimed at experimentally inducing HAAF measuring outcomes of hormonal counterregulation and awareness of hypoglycemia (studies measuring only hormonal counterregulation and not awareness of hypoglycemia were also included). A search string was composed after guidance from a trained librarian. In different combinations, the search terms used were “hypoglycemia-associated autonomic failure,” “HAAF,” “hypoglycemia,” “recurring,” “recurrent,” “repeated,” “consecutive,” and “unawareness,” yielding 1565 publications. The search was limited to English language manuscripts (excluding 174 publications) and human studies (excluding 339 publications), yielding 1052 potentially eligible publications. Following the screening of titles and scrutinizing abstracts, we found 27 papers eligible for this review. In addition, we also searched the reference list of the 27 eligible papers for additional publications. Data extraction was performed using a standard data-extraction sheet. Though the term impaired awareness of hypoglycemia (IAH) [14] is related to HAAF, studies merely investigating IAH were excluded, as we opted for the HAAF definition coined by Philip Cryer predicating the coexistence of defective hormonal counterregulatory response and hypoglycemia unawareness [15]. Studies combing exercise and hypoglycemia were also omitted.
Results
A total of 27 studies have attempted to experimentally induce HAAF during the period 1991 to September 2020 (Table 1). To summarize, 20 studies succeeded in inducing HAAF (both statistically significantly impaired hormonal counterregulation and IAH to subsequent hypoglycemia [including 6 studies not assessing symptoms]); 5 studies used 1 antecedent hypoglycemic episode and 15 studies used 2 or more antecedent hypoglycemic episodes. Five studies partially induced HAAF (either statistically significant impaired hormonal counterregulation or IAH; here is also included 1 study inducing HAAF in 13 of the 24 participants); 1 study used 1 antecedent hypoglycemic episode and 4 studies used 2 or more antecedent hypoglycemic episodes. Seventeen studies assed hypoglycemic symptoms; 12 recorded IAH to subsequent hypoglycemia, 5 studies recorded no changes in hypoglycemic symptoms to subsequent hypoglycemia. Seven studies investigated cognitive function or brain function. Two studies did not induce HAAF (neither statistically significant impaired hormonal counterregulation nor IAH); both studies used a single, short antecedent hypoglycemia episode.
Table 1.
Summary of key elements in the design of the 27 studies previously attempting to induce hypoglycemia-associated autonomic failure
| Reference | Year | Participants | Sex | No. of antecedent hypoglycemia episodes | Nadir blood glucose, mmol/L | Duration of antecedent hypoglycemia, min | Impaired hormonal counterregulation?a | Impaired symptoms?a |
|---|---|---|---|---|---|---|---|---|
| [16] | 1991 | 9 Healthy | Both | 1 (afternoon) | 2.8 | 90 | Yes | Yes |
| [17] | 1991 | 8 Healthy | Both | 1 (afternoon) | 3.0 | 90 | Yes | Not assessed |
| [18] | 1992 | 10 Healthy | Both | 3: 1 (afternoon) day 1; 1 (afternoon) day 2; and 1 (afternoon) day 3 | 3.3, 2.8, and 2.2 | 90 × 3 | Partially (no reduction in gluc, nore, or GH but reduction in epi and cort) | Yes |
| [19] | 1992 | 13 T1D and 7 controls | Both | 1 (afternoon) | 3.0 | 120 | Partially (no reduction in gluc, nore, or epi but reduction in GH and cort) | Not assessed |
| [20] | 1993 | 26 T1D and 12 controls | Both | 1 (afternoon) | 2.8 | 90 | Yes | Yes |
| [21] | 1993 | 18 T1D | Both | 3: 1 (afternoon) day 1; 1 (afternoon) day 2; and 1 (afternoon) day 3 | 3.3, 2.2, and 1.7 | 30, 30, and 20 | Yes | Yes |
| [22] | 1994 | 8 T1D and 7 controls | Both | One (afternoon) | 2.8 | 90 | Yes | No |
| [23] | 1994 | 12 Healthy | Both | 4: 1 (afternoon) day 1; 1 (afternoon) day 2; 1 (afternoon) day 3; and 1 (afternoon) day 4 | 3.6, 3.1, and 2.5 | 120 × 4 | Yes | Yes |
| [24] | 1995 | 10 Healthy | Male | 4: 1 (afternoon) day 1; 1 (afternoon) day 2; 1 (afternoon) day 3; and 1 (afternoon) day 4 | 2.8 | 15 × 4 | No | No |
| [25] | 1996 | 16 Healthy | Male | 2 (morning and afternoon) | 2.9 | 120 × 2 | Yes | Not assessed |
| [26] | 1997 | 8 Healthy | Both | 3 protocols; I) 2 prolonged episodes day 1 (morning and afternoon); II) 2 intermediate episodes day 1 (morning and afternoon); III) 2 short episodes (morning and afternoon) | 2.9 | I) 120 × 2, II) 5 × 2, and III) 30a 2 | Yes (all 3 protocols) | Yes (protocol II and III) |
| [27] | 2002 | 13 T2D and 15 controls | Both | 2 (morning and afternoon) | 2.8 | 120 × 2 | No (not in participants with T2D) | Yes |
| [28] | 2005 | 16 Healthy | Both | 2 (morning and afternoon) | 2.7 | 30 × 2 | Partially (no reduction in gluc and epi but reduction in GH, nore, and cort) | Not assessed |
| [29] | 2006 | 8 Healthy | Both | 2 (morning and afternoon) | 2.8 | 180 × 2 | Yes | No |
| [30] | 2008 | 9 Healthy | Both | 1 (afternoon) | 3.0 | 120 | Yes | Yes |
| [31] | 2009 | 8 Healthy | Both | 2 (morning and afternoon) | 3.3 | 90a 2 | Yes | Not assessed |
| [32] | 2011 | 17 Healthy | Both | 2 (morning and afternoon) | 3.0 | 120 × 2 | Yes | Not assessed |
| [33] | 2011 | 8 T1D | Both | 2 (morning and afternoon) | 3.3 | 120 × 2 | Yes | Not assessed |
| [34] | 2013 | 16 Healthy | Male | 2 (morning and afternoon) | 2.3 | 40 × 2 | Yes | Yes |
| [35] | 2014 | 14 Healthy | Male | 2 (morning and afternoon) | 2.2 | 40 × 2 | Partially (no reduction in gluc or epi but reduction in GH and cort) | Yes |
| [36] | 2014 | 16 Healthy | Both | 3: 2 episodes day 1 (morning and afternoon); 1 (afternoon) episode day 2 (morning) | 2.8 | 120 × 3 | Yes | Yes |
| [37] | 2015 | 12 Healthy | Both | 2 (morning and afternoon) | 2.9 | 120 × 2 | Yes | Not assessed |
| [38] | 2016 | 27 Healthy | Both | 2 (morning and afternoon) | 2.9 | 120 × 2 | Yes | Yes |
| [39] | 2017 | 13 Healthy | Both | 3: 2 episodes day 1 (morning and afternoon); 1 (afternoon) episode day 2 (morning) | 2.8 | 120 × 3 | Yes | No |
| [40] | 2017 | 5 Healthy | Male | 3: 2 episodes day 1 (morning and afternoon); 1 (afternoon) episode day 2 (morning) | 2.8 | 120 × 3 | Yes | No |
| [41] | 2020 | 9 T1D and 9 controls | Male | 1 (afternoon) | 2.5 | 30 | No | Not assessed |
| [42] | 2020 | 24 Healthy | Both | 2 episodes | 3.0 | 120 × 2 | Partially (in 13/24) | Not assessed |
Abbreviations: cort, cortisol; GH, growth hormone; gluc, glucagon; epi, epinephrine; nore, norepinephrine; T1D, type 1 diabetes; T2D, type 2 diabetes.
a Subsequent hypoglycemia.
Baseline Demographics
All studies were conducted with participants with median age younger than 35 years apart from 4 studies; 1 study included participants with type 2 diabetes (median age 50 years) and 3 studies had healthy participants with a median age younger than 42 years. The number of participants within each group ranged from 5 to 27 as further outlined in Table 1. Overall, 6 studies investigated 2 study groups consisting of participants with diabetes and healthy controls, and 21 studies had 1 study group consisting of either participants without diabetes (20 studies) or participants with type 1 diabetes (1 study). Twenty studies were conducted both with female and male participants and 7 studies with only male participants.
Discussion
Since the 1990s, HAAF has been experimentally induced in humans in order to improve the understanding of the condition; Heller and Cryer were the first in 1991 to successfully induce HAAF in individuals without diabetes [16]. Since then, several studies have followed including studies in individuals with type 1 and type 2 diabetes [17-42]. The fact that it can be reproduced both in individuals with and without diabetes underlines that HAAF is not specific for diabetes but rather for hypoglycemia, sleep, and exercise [8]. This concept is further supported by several case reports of hypoglycemia unawareness in spontaneous hypoglycemia, including individuals with insulinoma [43]. Most studies (21 of 27) examined participants of both sexes, thus increasing representativeness and transferability.
Varying Designs
It has been demonstrated that a single episode of hypoglycemia in the afternoon is enough to significantly impair hormonal counterregulatory responses and hypoglycemic symptoms to subsequent hypoglycemia the following morning. For this to occur, the duration of the single antecedent hypoglycemic episode should be at least 90 minutes [16, 17, 20, 22, 30] as we [41] and others [31] have shown unaltered counterregulation after single antecedent hypoglycemia of shorter duration (30 minutes and 15 minutes) in participants with type 1 diabetes and participants without diabetes. Furthermore, HAAF can be induced by 2 episodes of antecedent hypoglycemia the same day each lasting 40 minutes with blood glucose maintained around 2.2 to 2.3 mmol/L in participants without diabetes [34, 35]. Likewise, other studies have investigated the effects of repeated antecedent hypoglycemia, and it is apparent that 2 or more shorter antecedent hypoglycemia episodes effectively impair counterregulation to subsequent hypoglycemia; Davis et al [26] showed that 2 episodes of antecedent hypoglycemia in individuals without diabetes with blood glucose maintained at 2.9 mmol/L for only 5 minutes the same day was sufficient to reduce hormonal counterregulatory responses in subsequent hypoglycemia the following morning, although hypoglycemic symptoms were not statistically significantly reduced. On the other hand, Peters et al [24] were not able to induce HAAF in individuals without diabetes using 1 episode of hypoglycemia with nadir blood glucose of 2.8 mmol/L lasting 15 minutes every day for 4 consecutive days. More recently, frequently used models include an episode of 90 to 120 minutes antecedent hypoglycemia with blood glucose maintained at 2.8 to 3.3 mmol/L in the morning followed by a similar hypoglycemic episode in the afternoon the day before the actual study day [25-27, 29, 31-33, 37, 38]; some studies have additionally added a third 120-minute antecedent hypoglycemia the morning of the actual study day [18, 36, 39, 40].
In general, the first antecedent hypoglycemic episodes have been induced in the afternoon 18 to 24 hours before the actual hypoglycemic event of interest [16-42]. The subsequent hypoglycemic episode of interest was in most studies induced in a manner identical to the antecedent hypoglycemia. Overall, the blood glucose nadirs of antecedent hypoglycemia have varied in the range between 1.7 mmol/L and 3.9 mmol/L [16-42]. One study [26] explored the effects of varying depths of antecedent hypoglycemia with regard to hormonal counterregulatory responses in participants without diabetes; they found that when antecedent hypoglycemic blood glucose concentrations were reduced and maintained at 3.9 mmol/L, only adrenaline and glucagon responses to subsequent hypoglycemia were significantly reduced while blood glucose concentrations of 3.3 mmol/L during antecedent hypoglycemia also led to significant reductions in noradrenaline and GH concentrations to subsequent hypoglycemia. These findings to some extent support the proposed hierarchy of the glycemic threshold levels at which the designated hormones are secreted [2, 5] (see Fig. 1). Another important factor related to recurring hypoglycemia is the lowering of glycemic thresholds in individuals with diabetes due to previous recurring hypoglycemia [44], meaning that a lower nadir blood glucose is required to obtain useful data on counterregulation. The 2 studies unable to induce HAAF using a single antecedent hypoglycemic episode had blood glucose nadirs of 2.5 mmol/L [41] and 2.8 mmol/L [22] examining individuals with and without type 1 diabetes. Therefore, the most important factor to successfully induce HAAF by means of a single antecedent hypoglycemic episode appears to be a duration of at least 90 minutes, whereas it is apparent that the depth of the antecedent hypoglycemic episode is of lesser importance as long as it is below 3.4 mmol/L blood glucose.
Methodology
The most frequently used method for the induction of hypoglycemia is the hyperinsulinemic clamp technique [45]. The technique enables investigators to reduce the blood glucose in a safe and stepwise manner, and blood glucose can be maintained at any desired level. The most commonly used dosage of insulin is 1 to 2 mU/kg/min leading to supraphysiological plasma insulin concentrations, which overcomes the presence of insulin resistance both in type 1 and type 2 diabetes patients [46]. It is clearly important to ensure similar insulin concentrations when comparing several groups. Individuals with diabetes may have higher basal insulin concentrations compared with healthy participants [47], potentially affecting α-cell glucagon secretion and endogenous glucose production. Usually the participants eventually are given intravenous glucose to restore euglycemia, a procedure that obviously affects the natural course of hormonal and metabolic counterregulation. Peters et al [24] used an insulin tolerance test (ITT) [48] to induce hypoglycemia in individuals with and without type 1 diabetes. During an ITT, an insulin bolus (usually 0.1 IU/kg body weight) is intravenously injected and nadir blood glucose is reached after 30 to 40 minutes. The gradually progressing hypoglycemia mimics to some extent more faithfully the real-life hypoglycemia often induced by subcutaneously injected insulin compared with the stepwise hypoglycemic clamp, though hypoglycemia cannot be maintained at any desired level. Furthermore, during an ITT there is a certain risk of not achieving hypoglycemia, especially in insulin-resistant individuals, and variable nadirs are often encountered making comparisons between participants problematic.
Over time, there have been certain discrepancies as regards successful induction of HAAF; one study reported impaired hormonal counterregulation to subsequent hypoglycemia in healthy participants but failed in matched participants with type 2 diabetes [27]. This is probably because it may be more difficult to further attenuate the counterregulation in individuals who per se already have an impaired counterregulation [19]. Several studies have been discrepant as regards impairing both hormonal counterregulation and hypoglycemic symptoms [21, 28, 38, 39]. This is most likely related to the design and methods used for obtaining and analyzing data, or could reflect a dissociation between the 2 inherent components of HAAF.
Some studies have induced HAAF with statistically significant reductions in cortisol and/or GH and/or epinephrine and/or norepinephrine but without statistically significant reductions in glucagon during subsequent hypoglycemia [22, 29, 39, 40]. This could either reflect lack of analytical performance with earlier glucagon kits [49] or indicate that defective glucagon responses are already prevailing regardless of glycemic intervention. One study [42] aimed at inducing HAAF applying a robust model of 2 antecedent episodes of hypoglycemia lasting 120 minutes but reported successful induction of HAAF in merely 13 of 24 participants without diabetes. The authors [42] contemplate that interindividual variability in hormonal responses may explain an individual’s susceptibility to HAAF.
All studies measuring symptoms used a standardized questionnaire to asses autonomic and neuroglycopenic symptoms. Some studies included more complex methods of assessing cognitive function including brain function [20, 21, 23, 30, 34, 39, 40]. Limitations of cognitive testing include incorrect administration and scoring increases the risk of skewed results. Furthermore, the lack of consensus concerning appropriate tests for measurement of cognitive function makes it difficult to compare between studies. Lastly, it is important to exert caution before extrapolating the results of neurophysiological measurements to cerebral function as a whole.
Unintended Hypoglycemia
One of the major difficulties in investigating HAAF in individuals with diabetes is the potential interference from unintended hypoglycemia before the study day. Most studies seek to overcome this by instructing participants to frequently monitor blood glucose and avoid hypoglycemia 1 to 5 days [21, 22, 41] or 14 days [33] before the study days. Some studies have hospitalized the participants to control outside interference the night preceding and during the study [19, 22, 30, 37, 38, 41]. It should be noted that once HAAF is present only scrupulous avoidance of hypoglycemia for 1 to 3 weeks seems to reverse the condition [50-52], implying that avoidance of hypoglycemia for a few days preceding the study is insufficient. Furthermore, if participants only measure blood glucose during the daytime, there is a risk of unrecognized, long-lasting nocturnal hypoglycemic episodes [53]. The increased availability of novel technological devices including continuous glucose monitoring may offer a way to overcome these challenges [54]. Lastly, it has been demonstrated that HAAF also occurs with hypoglycemia associated with exercise and sleep [8]. This emphasizes the importance of appropriate guidance and precautions prior to experimental days, including strict absence of exercise.
Perspectives
Based on the studies we have scrutinized, it is clear that the hormonal counterregulatory responses to hypoglycemia are derailed in individuals with type 1 diabetes and long-lasting type 2 diabetes, but whether the actual metabolic responses following hypoglycemia, for example, stimulated gluconeogenesis, proteolysis, and lipolysis, are afflicted is more uncertain. Some studies reported augmented endogenous glucose production and decreased rates of lipolysis post hypoglycemia in individuals with type 1 diabetes compared with individuals without diabetes [55, 56], whereas others have found comparable metabolic responses between individuals with and without type 1 diabetes [41]. Furthermore, few studies have investigated the relationship between hypoglycemia and the posthypoglycemic insulin-resistance state in type 1 diabetes. One potential mechanism protecting participants with type 1 diabetes against hypoglycemia could be insulin resistance evidently present both in skeletal muscle and adipose tissue during euglycemia [46] and following hypoglycemia [41], allowing for adequate metabolic responses despite derailed hormonal responses in a compensatory manner. Some studies have assessed cognitive function during subsequent hypoglycemia with more complex methods [20, 23, 34] and a few studies have assessed cerebral function including using middle latency auditory evoked potentials as a measure of neurophysiological function [21], measuring cerebral alterations in brain glucose uptake [23], cerebral blood flow by positron emission topography [30], hypothalamic glucose transport [39], and cerebral glycogen levels [40] using magnetic resonance imaging. Functional magnetic resonance imaging and utilization of different positron emission topography tracers are potential future directions to test cognitive function during hypoglycemia and could be ways of revealing the unknown mechanisms behind HAAF.
Of note, other hypoglycemia-prone disease states are less well characterized metabolically, to some extent because they are much less prevalent than diabetes. There is evidence that people with spontaneous hypoglycemia caused by insulinoma display defective counterregulation and features of HAAF [5,57], and it has been reported that people with non–islet cell tumor hypoglycemia associated with insulin-like growth factor-2 have low levels of cortisol and GH [58, 59]. In addition, it is becoming increasingly clear that a substantial proportion of individuals with diabetes exhibit exocrine pancreatic dysfunction and reduced pancreatic volume [60]; conversely, “pancreatic/type 3c” diabetes secondary to exocrine pancreatic disease is relatively common in adults and often misdiagnosed as type 2 diabetes [61, 62]. Clearly such exocrine pancreatic abnormalities, together with gastrointestinal transit disturbances [63], have implications for absorption and glucagon responses during hypoglycemia. Finally, type 1 diabetes increases the risk of associated autoimmune disease, including adrenocortical failure, and people with concomitant Addison disease and type 1 diabetes have increased risk both of hypoglycemia and adrenal crisis [64, 65]. It would appear advisable to incorporate the existence of additional organ dysfunction including HAAF in the setting of therapeutic targets, perhaps aiming at less-strict glycemic windows in closed-loop systems likely to dominate the future; adding adjustable glucagon infusion to such systems could be considered in selected patients with defective counterregulation [66].
In our review, we chose not to perform further statistical analyses to compare results from the varying studies, based on the fact that the number of individuals studied in general is limited and that a vast number of heterogeneous design issues determine the outcome. In general, systematic reviews and meta-analyses will tend to be biased toward evidence of inefficacy of interventions if the statistical power of studies is not taken into consideration.
Conclusion
The most important factors to successfully induce experimentally HAAF with a single antecedent hypoglycemic episode is a duration of at least 90 minutes with a glucose nadir below 3.4 mmol/L blood glucose. Combining shorter repeated antecedent hypoglycemia the same day is effective in impairing counterregulation to subsequent hypoglycemia. The risk of unintended hypoglycemia in participants with diabetes before the study is an important confounder and needs to be considered. Furthermore, other disease states susceptible to hypoglycemia are less well characterized metabolically and should be taken into account when designing future studies. Clarification of these issues are of importance for the design and interpretation of future studies of HAAF, insulin resistance, and hormonal and metabolic responses, and eventually our understanding and potential prevention of severe hypoglycemia.
Acknowledgments
We would like to thank colleagues at the Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Denmark, for providing valuable suggestion in the process of preparing this manuscript.
Glossary
Abbreviations
- GH
growth hormone
- HAAF
hypoglycemia-associated autonomic failure
- IAH
impaired awareness of hypoglycemia
- ITT
insulin tolerance test
Contributor Information
Mads Bisgaard Bengtsen, Email: madsbengtsen@clin.au.dk, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, 8200 Aarhus N, Denmark.
Niels Møller, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, 8200 Aarhus N, Denmark.
This study has not been presented previously.
Financial Support
This work was supported by Aarhus University in Denmark.
Author Contributions
M.B.B. conducted the initial search for eligible papers and collected data and wrote the manuscript; N.M. reviewed and edited the manuscript, is the guarantor of this work, and takes responsibility for the integrity of the data and written manuscript.
Disclosures
The authors have nothing to disclose.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
- 1. Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14(6):750-756. doi: 10.4158/EP.14.6.750 [DOI] [PubMed] [Google Scholar]
- 2. Frier BM, Heller S, McCrimmon R, eds. Hypoglycaemia in Clinical Diabetes. 3rd ed. Wiley, Wiley Blackwell;2014. [Google Scholar]
- 3. Santiago JV, Clarke WL, Shah SD, Cryer PE. Epinephrine, norepinephrine, glucagon, and growth hormone release in association with physiological decrements in the plasma glucose concentration in normal and diabetic man. J Clin Endocrinol Metab. 1980;51(4):877-883. doi: 10.1210/jcem-51-4-877 [DOI] [PubMed] [Google Scholar]
- 4. Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9(1):463-473; quiz 474. [PMC free article] [PubMed] [Google Scholar]
- 5. Cryer PE. Symptoms of hypoglycemia, thresholds for their occurrence, and hypoglycemia unawareness. Endocrinol Metab Clin North Am. 1999;28(3):495-500, v. doi: 10.1016/s0889-8529(05)70084-0 [DOI] [PubMed] [Google Scholar]
- 6. Karamitsos DT. The story of insulin discovery. Diabetes Res Clin Pract. 2011;93(Suppl 1):S2-S8. doi: 10.1016/S0168-8227(11)70007-9 [DOI] [PubMed] [Google Scholar]
- 7. Fletcher AA, Campbell WR. The blood sugar following insulin administration and the symptom complex: hypoglycemia. J Metab Res. 1922;2:637-649. [Google Scholar]
- 8. Cryer PE. Chapter 23—Hypoglycemia-associated autonomic failure. Handb Clin Neurol. 2013;117:295-307. doi: 10.1016/B978-0-444-53491-0.00023-7 [DOI] [PubMed] [Google Scholar]
- 9. Boyle PJ, Schwartz NS, Shah SD, Clutter WE, Cryer PE. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med. 1988;318(23):1487-1492. doi: 10.1056/NEJM198806093182302 [DOI] [PubMed] [Google Scholar]
- 10. White NH, Skor DA, Cryer PE, Levandoski LA, Bier DM, Santiago JV. Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med. 1983;308(9):485-491. doi: 10.1056/NEJM198303033080903 [DOI] [PubMed] [Google Scholar]
- 11. Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2008;25(4):501-504. doi: 10.1111/j.1464-5491.2008.02413.x [DOI] [PubMed] [Google Scholar]
- 12. Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87(1):64-68. doi: 10.1016/j.diabres.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 13. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54(12):3592-3601. doi: 10.2337/diabetes.54.12.3592 [DOI] [PubMed] [Google Scholar]
- 14. Graveling AJ, Frier BM. Impaired awareness of hypoglycaemia: a review. Diabetes Metab. 2010;36(Suppl 3):S64-S74. doi: 10.1016/S1262-3636(10)70470-5 [DOI] [PubMed] [Google Scholar]
- 15. Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metab. 2001;281(6):E1115-E1121. doi: 10.1152/ajpendo.2001.281.6.E1115 [DOI] [PubMed] [Google Scholar]
- 16. Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40(2):223-226. doi: 10.2337/diab.40.2.223 [DOI] [PubMed] [Google Scholar]
- 17. Davis MR, Shamoon H. Counterregulatory adaptation to recurrent hypoglycemia in normal humans. J Clin Endocrinol Metab. 1991;73(5):995-1001. doi: 10.1210/jcem-73-5-995 [DOI] [PubMed] [Google Scholar]
- 18. Widom B, Simonson DC. Intermittent hypoglycemia impairs glucose counterregulation. Diabetes. 1992;41(12):1597-1602. doi: 10.2337/diab.41.12.1597 [DOI] [PubMed] [Google Scholar]
- 19. Davis MR, Mellman M, Shamoon H. Further defects in counterregulatory responses induced by recurrent hypoglycemia in IDDM. Diabetes. 1992;41(10):1335-1340. doi: 10.2337/diab.41.10.1335 [DOI] [PubMed] [Google Scholar]
- 20. Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91(3):819-828. doi: 10.1172/JCI116302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lingenfelser T, Renn W, Sommerwerck U, et al. Compromised hormonal counterregulation, symptom awareness, and neurophysiological function after recurrent short-term episodes of insulin-induced hypoglycemia in IDDM patients. Diabetes. 1993;42(4):610-618. doi: 10.2337/diab.42.4.610 [DOI] [PubMed] [Google Scholar]
- 22. Rattarasarn C, Dagogo-Jack S, Zachwieja JJ, Cryer PE. Hypoglycemia-induced autonomic failure in IDDM is specific for stimulus of hypoglycemia and is not attributable to prior autonomic activation. Diabetes. 1994;43(6):809-818. doi: 10.2337/diab.43.6.809 [DOI] [PubMed] [Google Scholar]
- 23. Boyle PJ, Nagy RJ, O’Connor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A. 1994;91(20):9352-9356. doi: 10.1073/pnas.91.20.9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peters A, Rohloff F, Kerner W. Preserved counterregulatory hormone release and symptoms after short term hypoglycemic episodes in normal men. J Clin Endocrinol Metab. 1995;80(10):2894-2898. doi: 10.1210/jcem.80.10.7559871 [DOI] [PubMed] [Google Scholar]
- 25. Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest. 1996;98(3):680-691. doi: 10.1172/JCI118839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis SN, Shavers C, Mosqueda-Garcia R, Costa F. Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes. 1997;46(8):1328-1335. doi: 10.2337/diab.46.8.1328 [DOI] [PubMed] [Google Scholar]
- 27. Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724-733. doi: 10.2337/diabetes.51.3.724 [DOI] [PubMed] [Google Scholar]
- 28. Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in healthy humans subjected to recurrent hypoglycemia. J Neurosci Res. 2005;82(4):525-530. doi: 10.1002/jnr.20654 [DOI] [PubMed] [Google Scholar]
- 29. Goldberg PA, Weiss R, McCrimmon RJ, Hintz EV, Dziura JD, Sherwin RS. Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure. Diabetes. 2006;55(4):1121-1126. doi: 10.2337/diabetes.55.04.06.db05-1169 [DOI] [PubMed] [Google Scholar]
- 30. Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57(2):470-475. doi: 10.2337/db07-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leu J, Cui MH, Shamoon H, Gabriely I. Hypoglycemia-associated autonomic failure is prevented by opioid receptor blockade. J Clin Endocrinol Metab. 2009;94(9):3372-3380. doi: 10.1210/jc.2009-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramanathan R, Cryer PE. Adrenergic mediation of hypoglycemia-associated autonomic failure. Diabetes. 2011;60(2):602-606. doi: 10.2337/db10-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vele S, Milman S, Shamoon H, Gabriely I. Opioid receptor blockade improves hypoglycemia-associated autonomic failure in type 1 diabetes mellitus. J Clin Endocrinol Metab. 2011;96(11):3424-3431. doi: 10.1210/jc.2011-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klement J, Pais I, Strube J, et al. NMDA receptor blockade by memantine does not prevent adaptation to recurrent hypoglycaemia in healthy men. Diabetes Obes Metab. 2013;15(4):310-315. doi: 10.1111/dom.12027 [DOI] [PubMed] [Google Scholar]
- 35. Klement J, Mergelkuhl B, Born J, Lehnert H, Hallschmid M. Role of γ-aminobutyric acid signalling in the attenuation of counter-regulatory hormonal responses after antecedent hypoglycaemia in healthy men. Diabetes Obes Metab. 2014;16(12):1274-1278. doi: 10.1111/dom.12358 [DOI] [PubMed] [Google Scholar]
- 36. Moheet A, Kumar A, Eberly LE, Kim J, Roberts R, Seaquist ER. Hypoglycemia-associated autonomic failure in healthy humans: comparison of two vs three periods of hypoglycemia on hypoglycemia-induced counterregulatory and symptom response 5 days later. J Clin Endocrinol Metab. 2014;99(2):664-670. doi: 10.1210/jc.2013-3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joy NG, Tate DB, Younk LM, Davis SN. Effects of acute and antecedent hypoglycemia on endothelial function and markers of atherothrombotic balance in healthy humans. Diabetes. 2015;64(7):2571-2580. doi: 10.2337/db14-1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mikeladze M, Hedrington MS, Joy N, et al. Acute effects of oral dehydroepiandrosterone on counterregulatory responses during repeated hypoglycemia in healthy humans. Diabetes. 2016;65(10):3161-3170. doi: 10.2337/db16-0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seaquist ER, Moheet A, Kumar A, et al. Hypothalamic glucose transport in humans during experimentally induced hypoglycemia-associated autonomic failure. J Clin Endocrinol Metab. 2017;102(9):3571-3580. doi: 10.1210/jc.2017-00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Öz G, DiNuzzo M, Kumar A, et al. Cerebral glycogen in humans following acute and recurrent hypoglycemia: implications on a role in hypoglycemia unawareness. J Cereb Blood Flow Metab. 2017;37(8):2883-2893. doi: 10.1177/0271678X16678240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bengtsen MB, Støy J, Rittig NF, et al. A human randomized controlled trial comparing metabolic responses to single and repeated hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab. 2020;105(12):dgaa645. doi: 10.1210/clinem/dgaa645 [DOI] [PubMed] [Google Scholar]
- 42. Lontchi-Yimagou E, Aleksic S, Hulkower R, et al. Plasma epinephrine contributes to the development of experimental hypoglycemia-associated autonomic failure. J Clin Endocrinol Metab. 2020;105(11):3416-3427. doi: 10.1210/clinem/dgaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sugawa T, Murakami T, Yabe D, et al. Hypoglycemia unawareness in insulinoma revealed with flash glucose monitoring systems. Intern Med. 2018;57(23):3407-3412. doi: 10.2169/internalmedicine.1173-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes. 1988;37(7):901-907. doi: 10.2337/diab.37.7.901 [DOI] [PubMed] [Google Scholar]
- 45. Defronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:214-223. [DOI] [PubMed] [Google Scholar]
- 46. Donga E, Dekkers OM, Corssmit EPM, Romijn JA. Insulin resistance in patients with type 1 diabetes assessed by glucose clamp studies: systematic review and meta-analysis. Eur J Endocrinol. 2015;173(1):101-109. doi: 10.1530/EJE-14-0911 [DOI] [PubMed] [Google Scholar]
- 47. Gregory JM, Smith TJ, Slaughter JC, et al. Iatrogenic hyperinsulinemia, not hyperglycemia, drives insulin resistance in type 1 diabetes as revealed by comparison with GCK-MODY (MODY2). Diabetes. 2019;68(8):1565-1576. doi: 10.2337/db19-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. doi: 10.1210/jc.2015-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wewer Albrechtsen NJ, Kuhre RE, Hornburg D, et al. Circulating glucagon 1-61 regulates blood glucose by increasing insulin secretion and hepatic glucose production. Cell Rep. 2017;21(6):1452-1460. doi: 10.1016/j.celrep.2017.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344(8918):283-287. doi: 10.1016/s0140-6736(94)91336-6 [DOI] [PubMed] [Google Scholar]
- 51. Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes. 1993;42(11):1683-1689. doi: 10.2337/diab.42.11.1683 [DOI] [PubMed] [Google Scholar]
- 52. Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43(12):1426-1434. doi: 10.2337/diab.43.12.1426 [DOI] [PubMed] [Google Scholar]
- 53. Novodvorsky P, Bernjak A, Chow E, et al. Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care. 2017;40(5):655-662. doi: 10.2337/dc16-2177 [DOI] [PubMed] [Google Scholar]
- 54. Choudhary P, Amiel SA. Hypoglycaemia in type 1 diabetes: technological treatments, their limitations and the place of psychology. Diabetologia. 2018;61(4):761-769. doi: 10.1007/s00125-018-4566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Enoksson S, Caprio SK, Rife F, Shulman GI, Tamborlane WV, Sherwin RS. Defective activation of skeletal muscle and adipose tissue lipolysis in type 1 diabetes mellitus during hypoglycemia. J Clin Endocrinol Metab. 2003;88(4):1503-1511. doi: 10.1210/jc.2002-021013 [DOI] [PubMed] [Google Scholar]
- 56. Bernroider E, Brehm A, Krssak M, et al. The role of intramyocellular lipids during hypoglycemia in patients with intensively treated type 1 diabetes. J Clin Endocrinol Metab. 2005;90(10):5559-5565. doi: 10.1210/jc.2004-1756 [DOI] [PubMed] [Google Scholar]
- 57. Mitrakou A, Fanelli C, Veneman T, et al. Reversibility of unawareness of hypoglycemia in patients with insulinomas. N Engl J Med. 1993;329(12):834-839. doi: 10.1056/NEJM199309163291203 [DOI] [PubMed] [Google Scholar]
- 58. Fukuda I, Asai A, Nagamine T, et al. Levels of glucose-regulatory hormones in patients with non-islet cell tumor hypoglycemia: including a review of the literature. Endocr J. 2017;64(7):719-726. doi: 10.1507/endocrj.EJ17-0072 [DOI] [PubMed] [Google Scholar]
- 59. Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab. 2014;99(3):713-722. doi: 10.1210/jc.2013-3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Foster TP, Bruggeman B, Campbell-Thompson M, Atkinson MA, Haller MJ, Schatz DA. Exocrine pancreas dysfunction in type 1 diabetes. Endocr Pract. 2020;26(12):1505-1513. doi: 10.4158/EP-2020-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Woodmansey C, McGovern AP, McCullough KA, et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care. 2017;40(11):1486-1493. doi: 10.2337/dc17-0542 [DOI] [PubMed] [Google Scholar]
- 62. Hart PA, Bradley D, Conwell DL, et al. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol. 2021;6(8):668-675. doi: 10.1016/S2468-1253(21)00019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goyal RK. Gastric emptying abnormalities in diabetes mellitus. N Engl J Med. 2021;384(18):1742-1751. doi: 10.1056/NEJMra2020927 [DOI] [PubMed] [Google Scholar]
- 64. Nederstigt C, Uitbeijerse BS, Janssen LGM, Corssmit EPM, de Koning EJP, Dekkers OM. Associated auto-immune disease in type 1 diabetes patients: a systematic review and meta-analysis. Eur J Endocrinol. 2019;180(2):135-144. doi: 10.1530/EJE-18-0515 [DOI] [PubMed] [Google Scholar]
- 65. Mortimer B, Naganur VD, Satouris P, Greenfield JR, Torpy DJ, Rushworth RL. Acute illness in patients with concomitant Addison’s disease and type 1 diabetes mellitus: increased incidence of hypoglycaemia and adrenal crises. Clin Endocrinol (Oxf). 2020;93(2):104-110. doi: 10.1111/cen.14219 [DOI] [PubMed] [Google Scholar]
- 66. Bisgaard Bengtsen M, Møller N. Mini-review: glucagon responses in type 1 diabetes—a matter of complexity. Physiol Rep. 2021;9(16):e15009. doi: 10.14814/phy2.15009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.