Abstract
Context
In most patients presenting with hypoglycemia in emergency departments, the etiology of hypoglycemia is identified. However, it cannot be determined in approximately 10% of cases.
Objective
We aimed to identify the causes of unknown hypoglycemia, especially adrenal insufficiency.
Methods
In this cross-sectional study, we evaluated the etiology of hypoglycemia among patients in our emergency department with hypoglycemia (plasma glucose level < 70 mg/dL (3.9 mmol/L)] between April 1, 2016 and March 31, 2021 using a rapid adrenocorticotropic hormone (ACTH) test.
Results
There were 528 cases with hypoglycemia included [52.1% male; median age 62 years (range 19-92)]. The majority [389 (73.7%)] of patients were using antidiabetes drugs. Additionally, 33 (6.3%) consumed alcohol; 17 (3.2%) had malnutrition; 13 (2.5%), liver dysfunction; 12 (2.3%), severe infectious disease; 11 (2.1%), malignancy; 9 (1.7%), heart failure; 4 (0.8%), insulin autoimmune syndrome; 3 (0.6%), insulinoma; 2 (0.4%) were using hypoglycemia-relevant drugs; and 1 (0.2%) suffered from non-islet cell tumor. Rapid ACTH tests revealed adrenal insufficiency in 32 (6.1%). In those patients, serum sodium levels were lower (132 vs 139 mEq/L, P < 0.01), eosinophil counts were higher (14 vs 8%, P < 0.01), and systolic blood pressure was lower (120 vs 128 mmHg, P < 0.05) at baseline than in patients with the other etiologies.
Conclusion
The frequency of adrenal insufficiency as a cause of hypoglycemia was much higher than what we anticipated. When protracted hypoglycemia of unknown etiology is recognized, we recommend that the patient is checked for adrenal function using a rapid ACTH test.
Keywords: adrenal insufficiency, hypoglycemia, rapid ACTH test, emergency department
If one considers all patients with altered mentation presenting in the emergency department, hypoglycemia has been identified as the underlying process in approximately 7% of cases [1]. While the most common causes of hypoglycemia are antidiabetes drugs, there are many other causes of hypoglycemia, and in approximately 10% of cases, the etiology of hypoglycemia cannot be determined [2, 3], and glucose infusion is routinely used to maintain patients’ blood glucose levels.
Adrenal insufficiency (AI) is 1 cause of hypoglycemia. AI is the lack of cortisol (glucocorticoid) and/or aldosterone (mineralocorticoid) secretions from adrenal glands. AI is classified as primary (Addison disease), caused by diseases of the adrenal cortex; secondary, caused by impaired adrenocorticotropic hormone (ACTH) secretion due to pituitary abnormalities; and tertiary, caused by insufficient corticotropin-releasing hormone (CRH) secretion and function because of hypothalamic dysfunction [4-6]. The prevalence of primary AI is estimated at between 82 and 144 cases per million population in Western societies [7, 8] compared to an estimated 138 to 142 cases per million population in Japan [9, 10]. The currently estimated incidences of this disorder are 4.4 to 6.0 and 6.6 new cases per million population per year in Western societies [11] and in Japan [9], respectively, and it presents most often between 30 and 50 years of age [10, 12, 13]. Secondary AI occurs more frequently than primary AI [10, 14]. Its estimated prevalence is 150 to 280 per million [15, 16], and affected patients are often diagnosed in their 60s [10, 17]. The most common cause of tertiary AI is chronic exogenous administration of synthetic glucocorticoids, which causes prolonged suppression of hypothalamic CRH secretion through negative feedback mechanisms [18, 19]. Patients with chronic AI may develop acute AI (adrenal crisis) under stress such as infection, surgery, etc. Adrenal crisis is a fatal condition that causes circulatory disorders due to an absolute or relative deficiency of glucocorticoids. The estimated incidence is 6 to 8 and 6.3 per 100 chronic AI patients per year in Western societies and Japan, respectively [10, 16]. Therefore, detecting AI is critical in emergency medical care.
The objective of this study was to identify the cause of hypoglycemia of unknown etiology, especially AI, in emergency departments.
Methods
Study Population
This was a 5-year single-center cross-sectional study to investigate the causes of unexplained hypoglycemia. Inclusion criteria were male and female patients aged ≥18 years with hypoglycemia [plasma glucose level < 70 mg/dL (3.9 mmol/L)] with or without hypoglycemic symptoms who presented to the emergency department between April 1, 2016 and March 31, 2021. Furthermore, rapid ACTH test was performed on all patients who met inclusion/exclusion criteria except those whose cause of hypoglycemia was found to be antidiabetes drugs. Exclusion criteria were patients whose plasma glucose level was ≥70 mg/dL with hypoglycemic symptoms, patients who refused a rapid ACTH test, and patients who were pregnant. Also excluded were patients who have participated in other clinical trials, and patients who were judged by their physician to be ineligible for participation. This trial was approved by the institutional review board at Shin Komonji Hospital. Written informed consent was obtained from all participants before enrollment in the trial.
Procedures
The cause of hypoglycemia was investigated in those patients who qualified for the study. In the emergency department, a rapid ACTH loading test (250 µg synthetic 1-24 ACTH: tetracosactide acetate administered intravenously) was performed on all patients except those who were taking antidiabetes drugs. Blood specimens were collected by nurses before loading and at 30 and 60 minutes after ACTH administration.
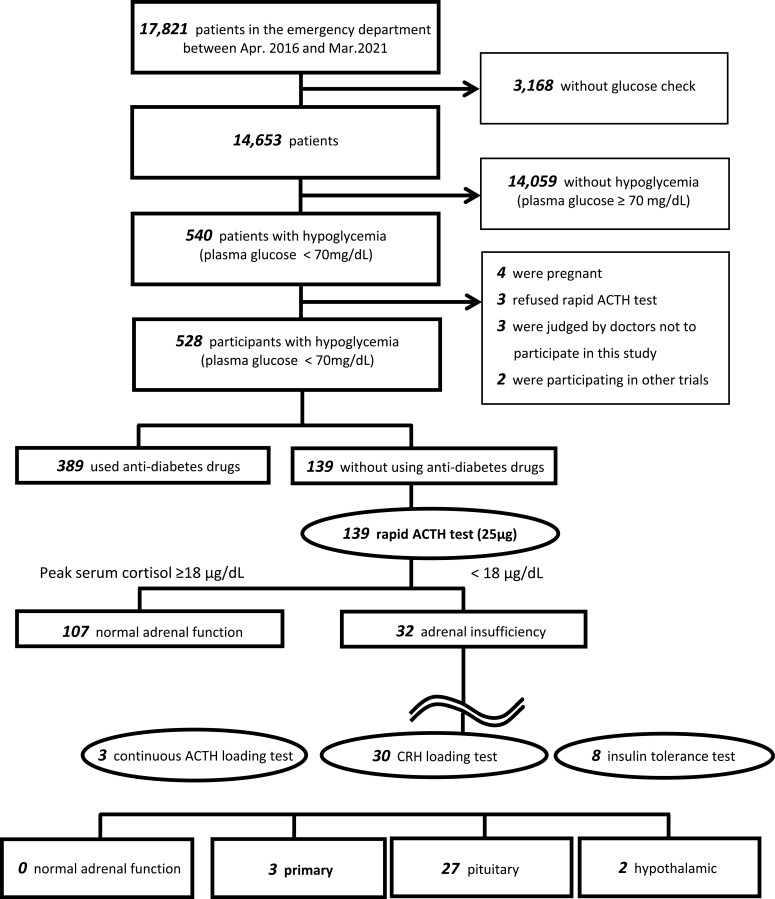
The diagnostic criterion for AI was peak serum cortisol levels of <18 μg/dL post rapid ACTH test [10, 20, 21]. Insulin tolerance test, CRH loading test, and/or continuous ACTH loading test were performed on patients who were diagnosed with AI to identify primary (adrenal), secondary (pituitary), or tertiary (hypothalamic) AI (Fig. 1).
Figure 1.
Study flow diagram.
Outcome Measures
The primary outcome was the rate of AI in patients with hypoglycemia presented to our emergency department. In addition, we investigated the clinical manifestations in each hypoglycemic etiology group.
Statistical Analyses
Analysis of variance and Dunnett’s test were performed to compare the AI-induced hypoglycemia group as a control group with other hypoglycemia groups. Statistical analyses were conducted using R-software, version 4.05 (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P-values < 0.05 were considered statistically significant.
Results
Characteristics of Study Subjects
Among the total of 17 821 patients who presented to our emergency department during the 5-year observation period, 528 patients were included in the study (Fig. 1). Of the 528 patients, 507 (96.0%) presented hypoglycemic symptoms, such as tremor, palpitations, sweating, hunger, paresthesia, dizziness, weakness, and confusion. Median age was 62 years (range 19-92), 52.1% were men, and mean plasma glucose levels were 48.5 mg/dL (95% CI 31.5-54.7 ng/dL) at the emergency department. Etiologies of hypoglycemia included 389 (73.7%) patients using antidiabetes drugs; 35 (6.6%) consumed alcohol; 19 (3.6%) had severe infections and/or sepsis; 18 (3.4%), malabsorption and/or undernutrition; 15 (2.8%), malignancies; 13 (2.5%), liver dysfunction; 9 (1.7%), heart failure; and 2 (0.4%) using hypoglycemia-relevant drugs. These were disopyramide phosphate and angiotensin-converting enzyme inhibitor. No checkpoint inhibitor was used. In addition, we found that 4 (0.8%) had insulin autoantibody syndrome, 3 (0.6%) had insulinoma, and 1 (0.2%) had non-islet cell tumor. The cause was unknown in 2 patients (0.4%), who were patients undergoing hemodialysis without diabetes (Table 1; Fig. 2).
Table 1.
Etiologies of hypoglycemia
| Etiologies | Antidiabetes drugs | Alcohol abuse | Malformation | Liver dysfunction | Severe infection | Malignancy | Heart failure | Insulin-autoimmune | Insulinoma | hypoglycemia-related drugs | Unknown | Non-islet cell tumor | Adrenal insufficiency |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjectsa | 389 (73.7) | 35 (6.6) | 18 (3.4) | 13 (2.5) | 19 (3.6) | 15 (2.8) | 9 (1.7) | 4 (0.8) | 3 (0.6) | 2 (0.4) | 2 (0.4) | 1 (0.2) | 32 (6.1) |
| Cooccurrence of AIb | — | 2 (5.7) | 1 (5.6) | 0 (0) | 7 (36.8) | 4 (26.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (43.8) |
| Without AI | 389 (73.7) | 33 (6.3) | 17 (3.2) | 13 (2.5) | 12 (2.3) | 11 (2.1) | 9 (1.7) | 4 (0.8) | 3 (0.6) | 2 (0.4) | 2 (0.4) | 1 (0.2) | — |
Data are given as n (%).
Abbreviation: AI, adrenal insufficiency.
aPercentage of the total 528 patients.
bPercentage of concomitant AI of each etiology.
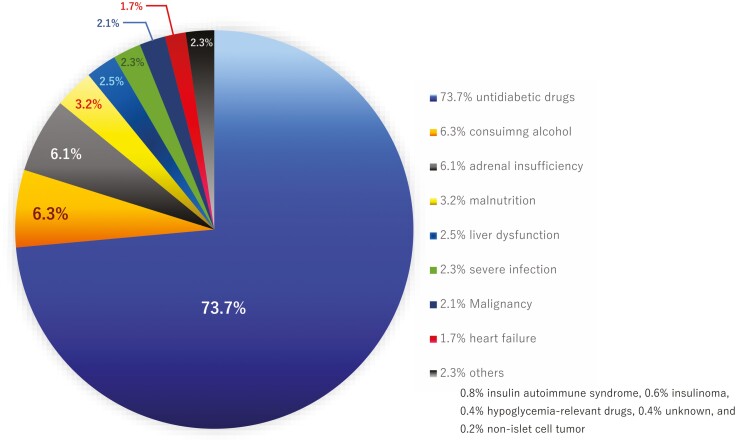
Figure 2.
Percentage of each etiology in patients with hypoglycemia. Out of 528 patients with hypoglycemia transported to our emergency department, 389 (73.7%) were on antidiabetes drugs; 35 (6.6%) had alcohol abuse problem; 32 (6.1%), adrenal insufficiency; 18 (3.4%), malnutrition; 15 (2.8%), liver dysfunction; 11 (2.1%), malignancy; 9 (1.7%), heart failure; 7 (1.3%), severe infection; 4 (0.8%), insulin antibody syndrome; 3 (0.6%), insulinoma; 2 (0.4%) were receiving hypoglycemia-related drugs; 2 (0.4%) had unknown etiology; and 1 (0.2%), non-islet cell tumor.
One hundred thirty-nine patients whose etiology could not be diagnosed in the emergency department or who did not take any antidiabetes drugs underwent rapid ACTH tests as the previously noted etiologies may not be the actual cause of hypoglycemia. Testing revealed AI in 32 (6.1%) of 528 patients. The differences between the non-AI and AI regarding the response to rapid ACTH test is shown in Table 2. Subsequent tests revealed the following: 3 were primary, 27 were secondary (pituitary), and 2 were tertiary (hypothalamic) (Fig. 1). Of the 3 patients with primary AI, 1 had 21-hydeoxylase deficiency, 1 suffered from a fungal infection, and 1 had a bilateral adrenal metastasis of lung cancer. Of the 27 patients with secondary AI, all had an isolated ACTH deficiency. Both patients with tertiary AI were chronic steroid users.
Table 2.
Differences between non-AI and AI regarding the response to rapid ACTH test
| AI (n = 32) | |||||
|---|---|---|---|---|---|
| Serum cortisol levels, µg/dL (reference range 4.5-21.1) |
Non-AI (n = 107) |
Primary (n = 3) |
Secondary (n = 27) | Tertiary (n = 2) | P-values |
| Baseline | 8.5 ± 1.3 | 4.3 ± 2.0 | 4.5 ± 1.5 | 3.9 ± 1.3 | 0.035* |
| After 30 minutes | 19.7 ± 4.1 | 8.1 ± 3.6 | 7.3 ± 2.3 | 5.6 ± 2.8 | 0.007** |
| After 60 minutes | 23.9 ± 5.7 | 9.2 ± 4.0 | 9.8 ± 3.4 | 8.1 ± 3.5 | 0.004** |
Data are given as mean ± SD.
Abbreviation: AI, adrenal insufficiency.
*P < 0.05.
** P < 0.01.
AI was found in 2 (5.7%) of the 35 patients with alcohol abuse, 7 (36.8%) of the 19 with severe infection and/or sepsis, 1 (5.6%) of the 18 with malnutrition, and 4 (26.7%) of the 15 with malignancies (Table 1). Of the 4 patients with malignancies and AI, 1 had a bilateral metastasis of lung cancer who suffered from a primary AI. The others had neither metastasis nor adrenal cancer, but 2 had breast cancer and 1 had colorectal cancer, and they suffered from secondary AI.
In comparing hypoglycemic patients induced by AI with those induced by other etiologies, the former had significantly lower serum potassium levels (132 vs 140 mEq/L, P = 0.007), lower systolic blood pressure (120 vs 128 mmHg, P = 0.039), and higher eosinophil counts (14 vs 8%, P = 0.002). There were no significant differences in blood glucose levels (P = 0.31) (Table 3).
Table 3.
Clinical manifestation of hypoglycemia
| Antidiabetes drugs | Alcohol abuse | Malformation | Liver dysfunction | Severe infection | Malignancy | Heart failure | Insulin autoimmune | Insulinoma | hypoglycemia-related drugs | Unknown | Non-islet cell tumor | Adrenal insufficiency | P-values | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects, n (%) | 389 (73.7) | 33 (6.3) | 17 (3.2) | 13 (2.5) | 12 (2.3) | 11 (2.1) | 9 (1.7) | 4 (0.8) | 3 (0.6) | 2 (0.4) | 2 (0.4) | 1 (0.2) | 32 (6.1) | |
| Age, years | 61 | 65 | 67 | 59 | 71 | 68 | 70 | 65 | 58 | 69 | 71 | 68 | 63 | ns |
| Male, % | 49 | 71 | 60 | 50 | 54 | 56 | 50 | 50 | 67 | 50 | 50 | 100 | 52 | ns |
| BMI kg/m2 | 23.6 | 20.5 | 19.8 | 22.7 | 21.7 | 18.2 | 20.7 | 22.5 | 27.1 | 24.6 | 21.3 | 19.7 | 21.5 | ns |
| Systolic BP, mmHg | 137 | 129 | 128 | 134 | 129 | 128 | 125 | 134 | 132 | 130 | 128 | 129 | 120 | 0.039* |
| Plasma glucose, mg/dL | 47 | 51 | 56 | 52 | 51 | 53 | 59 | 46 | 50 | 62 | 45 | 50 | 48 | ns |
| Hemoglobin, mg/dL | 14.6 | 13.7 | 11.9 | 13.4 | 13.7 | 11.6 | 12.9 | 14.7 | 15.9 | 13.8 | 13.5 | 11.6 | 12.4 | ns |
| White blood cells, μL | 5200 | 4600 | 3900 | 4100 | 12800 | 4800 | 6200 | 3500 | 4800 | 6700 | 5100 | 8500 | 3900 | ns |
| Lymphocyte, % | 36 | 40 | 32 | 31 | 40 | 27 | 35 | 31 | 42 | 39 | 46 | 28 | 39 | ns |
| Eosinocyte, % | 4 | 8 | 5 | 10 | 8 | 5 | 6 | 11 | 8 | 6 | 7 | 9 | 14 | 0.002** |
| Total cholesterol, mg/dL | 239 | 211 | 166 | 223 | 206 | 178 | 219 | 221 | 243 | 207 | 175 | 178 | 184 | ns |
| Serum Na, mEq/L | 143 | 137 | 135 | 140 | 147 | 139 | 141 | 140 | 139 | 141 | 138 | 136 | 132 | 0.007** |
| Serum K, mEq/L | 4 | 3.0 | 3.4 | 4.1 | 3.8 | 3.5 | 3.9 | 4.1 | 3.6 | 3.7 | 5.6 | 3.8 | 3.9 | ns |
| CRP, mg/dL | 0.58 | 0.84 | 0.72 | 1.12 | 18.6 | 3.25 | 1.70 | 1.12 | 0.75 | 1.03 | 2.37 | 8.13 | 2.06 | ns |
Abbreviations: BMI, body mass index; systolic BP, systolic blood pressure; CRP, C-reactive protein; na, not significant; serum K, serum kalium; serum Na, serum natrium.
*P < 0.05.
** P < 0.01.
Discussion
In our study, AI was found to be the third-highest etiology [32/528 (6.1%)] after antidiabetes drugs [389/528 (73.7%)] and alcohol abuse [35/528 (6.6%)], among the hypoglycemic patients transported to the emergency department. Previous reports showed that the etiologies of hypoglycemia transported to emergency departments were antidiabetes drugs in 54% to 88% [1], unrelated to diabetes in 12% to 46% [22], and unknown in 1% to 6% [2, 3].
The primary causes of hypoglycemia are insulin and oral hypoglycemic drugs. Patients with type 1 diabetes who used insulin had 1 to 3.2 hypoglycemic attacks per year [23, 24], and type 2 diabetic patients with ≥5 years of insulin treatment experienced an average of 0.7 hypoglycemic attacks per year [24]. Patients prescribed oral hypoglycemic drugs, especially sulfonylureas, were also at high risk of hypoglycemia [25]. The incidence of hypoglycemia in patients with type 2 diabetes treated with oral hypoglycemic drugs was reportedly 0.35× per year [26].
Alcohol inhibits gluconeogenesis in the body but does not affect glycogenolysis [27]. Thus, hypoglycemia occurs after several days of alcohol consumption with limited ingestion of food and after hepatic glycogen stores are depleted [28]. Alcohol ingestion is often the cause of, or a contributing factor to, hypoglycemia encountered in patients coming to emergency departments.
In our study, we found that the ratio of secondary and tertiary AIs, rather than primary AI, was as high as 29/32 (90.1%) in all types of AI. It is rare for adult primary AI to cause hypoglycemia in the absence of infection, fever, or alcohol ingestion [29]. In contrast, hypoglycemia is more common in secondary AI caused by isolated ACTH deficiency [30-32]. As a result, we concluded that Addison’s disease would be diagnosed quickly and treated; however, ACTH insufficiency was often overlooked, possibly because the absence of dehydration and hypotension permits patients to tolerate their illness longer. In accordance with the guidelines [10, 21, 33], administration of hydrocortisone to our AI patients promptly improved their hypoglycemia, and glucose administration was no longer necessary. It is beneficial to make an early diagnosis of AI because it is not necessary to continue to administer glucose wastefully. In addition, in septic shock, patients with AI showed the depressed pressor sensitivity to noradrenaline, which may be substantially improved by physiological doses of hydrocortisone [34]. Eventually, of the 32 patients with AI, 21 (65.6%) improved their hypothalamic-pituitary-adrenal axis and no longer needed hydrocortisone to maintain their blood glucose.
In addition to hypoglycemia, clinical manifestations of AI included hyponatremia (serum natrium < 135 mEq/L), normocytic normochromic anemia (male, <13 g/dL; female, <12 g/dL), low serum total cholesterol level (<150 mg/dL), eosinophilia (>8%), relative leukopenia, hypotension, and hyperkalemia (serum potassium > 4.5 mEq/L) [10, 21, 35]. However, in our study, 3 symptoms—hyponatremia, hypotension, and eosinophilia—were more frequently observed in AI as compared to other etiologies of hypoglycemia.
Hyponatremia can occur in both primary and secondary AIs and is found in 70% to 80% of patients with AI [35].
The underlying etiology is different in each case. In primary AI, hyponatremia and hypovolemia are caused by aldosterone deficiency, whereas in secondary AI, hyponatremia is due to the lack of cortisol, which leads to increased vasopressin secretion and dilutional or hypervolemic hyponatremia [19, 35, 36]. Hyponatremia can occur early in the disease and maybe the initial manifestation [37]; in contrast, the number of patients with hyperkalemia was not significantly large in our study. Hyperkalemia often occurs due to aldosterone deficiency. Therefore, hyperkalemia is observed in patients with primary AI who have both aldosterone and cortisol secretory deficiency. In contrast, patients with secondary or tertiary AI usually have normal mineralocorticoid function due to compensation for the intact renin-angiotensin system. In our study, hyperkalemia did not occur in the AI group because the proportion of secondary and tertiary AI was overwhelmingly higher than that of primary.
Heart rate and systolic blood pressure in hypoglycemia without AI are slightly raised. In secondary and tertiary AI, hypotension is less prominent [6, 30]. However, in most patients with primary AI, the blood pressure is low, and some have postural hypotension. These symptoms are primarily due to volume depletion resulting from aldosterone deficiency [38]. Glucocorticoids are necessary for adrenal medullary epinephrine synthesis, and patients with AI have decreased serum epinephrine and a compensatory increase in serum norepinephrine concentrations [39]. This may cause slightly lower basal systolic blood pressure and an exaggerated increase in pulse rate in response to upright posture.
Relative eosinophilia was reported to be a marker of AI [10, 40]. One study found that the combination of a history of glucocorticoid withdrawal, nausea, hyperkalemia, and eosinophilia was a useful predictor of AI in an inpatient population [41]. In contrast, subsequent small series suggested that the eosinophil count is >500/µL in <20% of patients with AI [42]. Therefore, the presence of eosinophilia was found incidentally; other causes such as allergy or infection should also be investigated [43].
In our study, there were 2 patients with hypoglycemia whose etiology was undetermined; however, both were undergoing hemodialysis. Chronic kidney disease likely involves impaired gluconeogenesis, reduced renal clearance of insulin, and reduced renal glucose production [44], which may increase the risk of hypoglycemia. However, previous data on chronic kidney disease as a risk factor for hypoglycemia has been conflicting. Some [45, 46] but not all [47] previous studies have noted such an association.
This was a single-center prospective observational study where the results were obtained at a facility with an endocrinology and emergency medicine specialist in the Kitakyushu area of Japan. Thus, the generalizability of the proportion of hypoglycemic patients and the proportion of patients with diabetes among all patients in emergency departments is unknown. An ACTH test may not be available in all facilities and the turnaround time on the test if available may preclude this diagnosis being made in the emergency department. In addition, because the rapid ACTH test was not performed in the emergency department on the hypoglycemic patients who used antidiabetes drugs due to the study protocol, the proportion of AI may have been higher if testing had been performed for all patients. However, after admission, rapid ACTH tests were performed on the 4 antidiabetes drug users who did not improve their plasma glucose levels despite an intravenous drip infusion of glucose for ≥2 days, but no AI was found in any of them. Therefore, we thought that AI might not be the cause of hypoglycemia in patients using antidiabetes drugs.
In conclusion, the probability of AI was much greater than we anticipated as a cause of hypoglycemia. When protracted hypoglycemia of unknown etiology with hyponatremia, hypotension, and/or eosinophilia is recognized, we recommend that the patient be checked for adrenal function using the rapid ACTH loading test. There are no untoward side effects, and allergic reactions are extraordinarily rare. ACTH tests can be performed without monitoring by an advanced healthcare provider and are less expensive.
Contributor Information
Tetsuya Kawahara, Email: k-tetsuy@med.uoeh-u.ac.jp, Department of Endocrinology and Diabetes, Shinkomonji Hospital, Kitakyushu, Japan.
Maiko Tsuji, Department of Emergency Medicine, Shinkomonji Hospital, Kitakyushu, Japan.
Naoki Tominaga, Department of Emergency Medicine, Shinkomonji Hospital, Kitakyushu, Japan.
Nagahiro Toyama, Department of Emergency Medicine, Shinkomonji Hospital, Kitakyushu, Japan.
Mikio Toda, Department of Internal Medicine, Shinkomonji Hospital, Kitakyushu, Japan.
Funding
This study was supported by a grant from the Kitakyushu Medical Association. The sponsor did not contribute to the design, collection, management, analysis, interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication.
Disclosures
The authors have nothing to disclose.
Author Contributions
T.K. wrote the first draft of the manuscript and was mainly responsible for the designation of the methodology. All authors were responsible for the critical revision of the article for important intellectual content. T.K., M.T., N.T., and T.N. were responsible for the collection and assembly of data. M.T. was responsible for data management. T.K. completed the main part of the data analyses, and all authors discussed the analysis plan and results and provided input to the manuscript. All authors had access to the final study results and were responsible for the final approval of the manuscript.
Data Availability
The data that support the findings of this study are not publicly available because they were used under license for the current study only. However, the corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Malouf R, Brust JC. Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol. 1985;17(5):421-430. doi: 10.1002/ana.410170502 [DOI] [PubMed] [Google Scholar]
- 2. Sako A, Yasunaga H, Matsui H, et al. Hospitalization with hypoglycemia in patients without diabetes mellitus: a retrospective study using a national inpatient database in Japan, 2008-2012. Medicine (Baltim). 2017;96(25):e7271. doi:10.1097/MD.0000000000007271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nirantharakumar K, Marshall T, Hodson J, et al. Hypoglycemia in non-diabetic in-patients: clinical or criminal? PLoS One. 2012;7(7):e40384. doi: 10.1371/journal.pone.0040384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(4):1059-1067. doi: 10.1210/jc.2009-0032 [DOI] [PubMed] [Google Scholar]
- 5. Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335(16):1206-1212. doi: 10.1056/NEJM199610173351607 [DOI] [PubMed] [Google Scholar]
- 6. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152-2167. doi: 10.1016/S0140-6736(13)61684-0 [DOI] [PubMed] [Google Scholar]
- 7. Erichsen MM, Lovas K, Skinningsrud B, et al. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab. 2009;94(12):4882-4890. doi:10.1210/jc.2009-1368 [DOI] [PubMed] [Google Scholar]
- 8. Chakera AJ, Vaidya B. Addison disease in adults: diagnosis and management. Am J Med. 2010;123(5):409-413. doi: 10.1016/j.amjmed.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 9. Takayanagi R. 3. Epidemiology of adrenal insufficiency. Nippon Naika Gakkai Zasshi. 2008;97(4):711-715. doi: 10.2169/naika.97.711 [DOI] [Google Scholar]
- 10. Yanase T, Tajima T, Katabami T, et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: a Japan Endocrine Society clinical practice guideline [opinion]. Endocr J. 2016;63(9):765-784. doi: 10.1507/endocrj.EJ16-0242 [DOI] [PubMed] [Google Scholar]
- 11. Lovas K, Husebye ES. High prevalence and increasing incidence of Addison’s disease in western Norway. Clin Endocrinol (Oxf). 2002;56(6):787-791. doi:10.1046/j.1365-2265.2002.t01-1-01552.x [DOI] [PubMed] [Google Scholar]
- 12. Meyer G, Neumann K, Badenhoop K, Linder R. Increasing prevalence of Addison’s disease in German females: health insurance data 2008-2012. Eur J Endocrinol. 2014;170(3):367-373. doi:10.1530/EJE-13-0756 [DOI] [PubMed] [Google Scholar]
- 13. Kong MF, Jeffcoate W. Eighty-six cases of Addison’s disease. Clin Endocrinol (Oxf). 1994;41(6):757-761. doi: 10.1111/j.1365-2265.1994.tb02790.x [DOI] [PubMed] [Google Scholar]
- 14. Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361(9372):1881-1893. doi: 10.1016/S0140-6736(03)13492-7 [DOI] [PubMed] [Google Scholar]
- 15. Tomlinson JW, Holden N, Hills RK, et al. ; West Midlands Prospective Hypopituitary Study Group. Association between premature mortality and hypopituitarism. Lancet. 2001;357(9254):425-431. doi: 10.1016/s0140-6736(00)04006-x [DOI] [PubMed] [Google Scholar]
- 16. Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162(3):597-602. doi:10.1530/EJE-09-0884 [DOI] [PubMed] [Google Scholar]
- 17. Regal M, Paramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf). 2001;55(6):735-740. doi: 10.1046/j.1365-2265.2001.01406.x [DOI] [PubMed] [Google Scholar]
- 18. Woods CP, Argese N, Chapman M, et al. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur J Endocrinol. 2015;173(5):633-642. doi:10.1530/EJE-15-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar R, Wassif WS. Adrenal insufficiency. J Clin Pathol. 2022;75(7):435-442. doi:10.1136/jclinpath-2021-207895 [DOI] [PubMed] [Google Scholar]
- 20. Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017;45(12):2078-2088. doi: 10.1097/CCM.0000000000002737 [DOI] [PubMed] [Google Scholar]
- 21. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. doi: 10.1210/jc.2015-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jang CW, Kim YK, Kim KH, Chiara A, Lee MS, Bae JH. Predictors for high-risk carotid plaque in asymptomatic Korean population. Cardiovasc Ther. 2020;2020:6617506. doi: 10.1155/2020/6617506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2010;39(3):641-654. doi: 10.1016/j.ecl.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140-1147. doi:10.1007/s00125-007-0599-y [DOI] [PubMed] [Google Scholar]
- 25. Holstein A, Hammer C, Hahn M, Kulamadayil NS, Kovacs P. Severe sulfonylurea-induced hypoglycemia: a problem of uncritical prescription and deficiencies of diabetes care in geriatric patients. Expert Opin Drug Saf. 2010;9(5):675-681. doi: 10.1517/14740338.2010.492777 [DOI] [PubMed] [Google Scholar]
- 26. Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749-755. doi:10.1111/j.1464-5491.2005.01501.x [DOI] [PubMed] [Google Scholar]
- 27. Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410 [DOI] [PubMed] [Google Scholar]
- 28. Marks V, Teale JD. Drug-induced hypoglycemia. Endocrinol Metab Clin North Am. 1999;28(3):555-577. doi: 10.1016/s0889-8529(05)70088-8 [DOI] [PubMed] [Google Scholar]
- 29. Yamamoto T, Fukuyama J, Hasegawa K, Sugiura M. Isolated corticotropin deficiency in adults: report of 10 cases and review of literature. Arch Intern Med. 1992;152(8):1705-1712. [PubMed] [Google Scholar]
- 30. Burke CW. Adrenocortical insufficiency. Clin Endocrinol Metab. 1985;14(4):947-976. doi: 10.1016/s0300-595x(85)80084-0. [DOI] [PubMed] [Google Scholar]
- 31. Stacpoole PW, Interlandi JW, Nicholson WE, Rabin D. Isolated ACTH deficiency: a heterogeneous disorder: critical review and report of four new cases. Medicine (Baltim). 1982;61(1):13-24. [PubMed] [Google Scholar]
- 32. Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87(6):457-461. doi: 10.1136/adc.87.6.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mah PM, Jenkins RC, Rostami-Hodjegan A, et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2004;61(3):367-375. doi: 10.1111/j.1365-2265.2004.02106.x [DOI] [PubMed] [Google Scholar]
- 34. Annane D, Bellissant E, Sebille V, et al. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenal function reserve. Br J Clin Pharmacol. 1998;46(6):589-597. doi: 10.1046/j.1365-2125.1998.00833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216-226. doi: 10.1016/S2213-8587(14)70142-1 [DOI] [PubMed] [Google Scholar]
- 36. Feldman RD, Gros R. Vascular effects of aldosterone: sorting out the receptors and the ligands. Clin Exp Pharmacol Physiol. 2013;40(12):916-921. doi: 10.1111/1440-1681.12157 [DOI] [PubMed] [Google Scholar]
- 37. Cuesta M, Garrahy A, Slattery D, et al. The contribution of undiagnosed adrenal insufficiency to euvolaemic hyponatraemia: results of a large prospective single-centre study. Clin Endocrinol (Oxf). 2016;85(6):836-844. doi: 10.1111/cen.13128 [DOI] [PubMed] [Google Scholar]
- 38. Letizia C, Cerci S, Centanni M, et al. Circulating levels of adrenomedullin in patients with Addison’s disease before and after corticosteroid treatment. Clin Endocrinol (Oxf). 1998;48(2):145-148. doi: 10.1046/j.1365-2265.1998.3531170.x [DOI] [PubMed] [Google Scholar]
- 39. Zuckerman-Levin N, Tiosano D, Eisenhofer G, Bornstein S, Hochberg Z. The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. J Clin Endocrinol Metab. 2001;86(12):5920-5924. doi: 10.1210/jcem.86.12.8106 [DOI] [PubMed] [Google Scholar]
- 40. Thorn GW, Forsham PH, et al. A test for adrenal cortical insufficiency; the response to pituitary andrenocorticotropic hormone. J Am Med Assoc. 1948;137(12):1005-1009. doi: 10.1001/jama.1948.02890460001001 [DOI] [PubMed] [Google Scholar]
- 41. Oboni JB, Marques-Vidal P, Pralong F, Waeber G. Predictive factors of adrenal insufficiency in patients admitted to acute medical wards: a case control study. BMC Endocr Disord. 2013;13:3. doi: 10.1186/1472-6823-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spry C. Eosinophilia in Addison’s disease. Yale J Biol Med. 1976;49(4):411-413. [PMC free article] [PubMed] [Google Scholar]
- 43. Skiest DJ, Keiser P. Clinical significance of eosinophilia in HIV-infected individuals. Am J Med. 1997;102(5):449-453. doi: 10.1016/S0002-9343(97)00048-X [DOI] [PubMed] [Google Scholar]
- 44. Alsahli M, Gerich JE. Hypoglycemia, chronic kidney disease, and diabetes mellitus. Mayo Clin Proc. 2014;89(11):1564-1571. doi: 10.1016/j.mayocp.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 45. Busch M, Lehmann T, Wolf G, Gunster C, Muller UA, Muller N. Antidiabetic therapy and rate of severe hypoglycaemia in patients with type 2 diabetes and chronic kidney disease of different stages: a follow-up analysis of health insurance data from Germany. Exp Clin Endocrinol Diabetes. 2021;129(11):821-830. doi: 10.1055/a-1129-6699 [DOI] [PubMed] [Google Scholar]
- 46. Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121-1127. doi: 10.2215/CJN.00800209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ahmad I, Zelnick LR, Batacchi Z, et al. Hypoglycemia in people with type 2 diabetes and CKD. Clin J Am Soc Nephrol. 2019;14(6):844-853. doi: 10.2215/CJN.11650918 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available because they were used under license for the current study only. However, the corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.