Abstract
The pathogenesis of breast cancer is driven by multiple hormones and growth factors. One of these, prolactin (PRL), contributes to both mammary differentiation and oncogenesis, and yet the basis for these disparate effects has remained unclear. The focus of this review is to examine and place into context 2 recent studies that have provided insight into the roles of PRL receptors and PRL in tumorigenesis and tumor progression. One study provides novel evidence for opposing actions of PRL in the breast being mediated in part by differential PRL receptor (PRLr) isoform utilization. Briefly, homomeric complexes of the long isoform of the PRLr (PRLrL-PRLrL) promotes mammary differentiation, while heteromeric complexes of the intermediate and long PRLr (PRLrI-PRLrL) isoforms trigger mammary oncogenesis. Another study describes an immunodeficient, prolactin-humanized mouse model, NSG-Pro, that facilitates growth of PRL receptor-expressing patient-derived breast cancer xenografts. Evidence obtained with this model supports the interactions of physiological levels of PRL with estrogen and ERBB2 gene networks, the modulatory effects of PRL on drug responsiveness, and the pro-metastatic effects of PRL on breast cancer. This recent progress provides novel concepts, mechanisms and experimental models expected to renew interest in harnessing/exploiting PRLr signaling for therapeutic effects in breast cancer.
Keywords: Jak2/Stat5, mouse models, endocrine resistance, metastasis
Breast cancer arises from healthy mammary epithelium, a highly hormone-regulated tissue compartment. Hormonal cues are pertinent both for development and progression of breast cancer. For instance, estrogens are well established as tumor-promoters of breast cancer (1, 2), as further supported by the efficacy of estrogen receptor antagonists both for prevention and therapy (3). The importance of the pituitary hormone prolactin (PRL) and its receptors for human breast cancer development and progression has, despite evidence from rodent mammary tumor models (4-6), remained less clear due to several factors. These factors include a duality of PRL-induced pro-differentiation vs proliferative signals, inadequate experimental models, and a limited set of underpowered clinical trials testing PRL pathway suppression in breast cancer patients (7-10). This mini-review describes recent research progress that sheds important new light on PRL and PRL receptor (PRLr) biology directly relevant for human breast cancer. First, a report from the Clevenger laboratory documented oncogenic roles of an intermediate PRLr splice variant, PRLrI, of particular relevance for estrogen receptor (ER)-negative breast cancer (11). A second report from the Rui laboratory and collaborators introduced an immunodeficient prolactin-humanized mouse model that uncovered tumor-promoting effects of physiological levels of PRL on human ER-positive breast cancer xenografts (12). The 2 publications introduce new concepts and mechanisms and provide new experimental tools and models that will facilitate research to determine the impact of PRL-PRLr signaling in breast cancer. The new lines of progress are expected to revitalize interest in defining the tumor-promoting mechanisms of PRLr signaling and identify pharmacologic strategies that might benefit breast cancer patients with specific molecular phenotypes.
Prolactin Receptor in the Mammary Gland
PRL was originally identified as a neuroendocrine hormone of pituitary origin. PRL is required for the terminal growth and differentiation of the mammary gland; genetic deletion of PRL or PRLr in mice abrogates lactation (8, 13). Expressed on mammary epithelium, the PRLr is a member of the cytokine receptor superfamily. Ligand-induced dimerization of PRLr activates the Jak2 tyrosine kinase (14-16) with subsequent signaling via multiple downstream pathways, including ras-raf-MAPK, Akt, and Stat5a/b (hereafter Stat5) (17-22). Classically, Stat5, specifically the Stat5a isoform, has been thought of as a driver of pregnancy-associated alveolar differentiation required for lactation (23-25). These actions are associated with the tyrosine phosphorylation of this transcription factor. Stat5 tyrosine phosphorylation is mediated by the Jak2 tyrosine kinase at tyrosine (Y) residue 694 (26, 27). Phosphorylated Stat5 self-dimerizes and translocates to the nucleus, where it can be found in the nuclei of most lactating mammary epithelial cells, binding to many of the genes associated with mammary development and lactation (28, 29).
PRLr in Breast Cancer
In addition to its role in the normal differentiation and maturation of the human breast, substantial evidence for PRLr expression and action in malignant breast tissues exists. Both immunohistochemical and Oncomine analyses have revealed that the PRLr is highly overexpressed in breast cancer tissues (vs adjacent matched normal tissues) (30, 31). PRL stimulates the proliferation, survival, and motility of breast cancer cells in vitro (32). In breast cancer tissues, nuclear-localized tyrosine phosphorylated Stat5 (Nuc-pYStat5) has been detected in ~25% of tumors (with the lowest levels in metastases). The correlation that Nuc-pYStat5 is associated with a favorable prognosis and responsiveness to anti-estrogen therapy (33, 34) has further substantiated the hypothesis that Stat5 activity may oppose alternative Jak2-mediated signals in terms of breast cancer progression (7). An increased risk for metastatic progression in breast cancer patients with elevated prolactin levels has also been noted (8). Regarding PRL and risk for breast cancer development, in vivo studies revealed that transgenic mice overexpressing PRL develop ER+ or ER− mammary carcinomas within the first 12 to 18 months of life (4). Epidemiologic evidence from the National Nurses’ Health Study has further uncovered that elevated serum PRL levels are significantly associated with an increased relative risk of developing breast cancer (35, 36), particularly ER+ disease, and parallel the temporal risks noted in pregnancy-associated breast cancer (37, 38). While the association of clinical hyperprolactinemia with breast cancer risk is debated, the association is unlikely as most patients with hyperprolactinemia are hypoestrogenemic (39, 40).
Jak2-Stat5 and PRLr Signaling in Breast Cancer
How does the PRLr function in normal and malignant breast tissues? Ligand binding to the PRLr results in the rapid phosphorylation of the proximate Jak2 tyrosine kinase and the subsequent activation of multiple PRLr-associated signaling intermediaries such as Stat5, MAPK, AKT, Nek3, and Vav2 (8, 26, 41-43). These signals alter the expression of breast cancer–relevant PRL-target genes, including upregulation of cyclin D1, amphiregulin, epiregulin, PTHLH, CISH (44-46) and downregulation of BCL6 (47-49). Following PRL stimulation, Jak2 is activated within 30 to 60 seconds through autophosphorylation of the 2 Jak2 molecules that are constitutively associated with the PRLr dimer (14, 15). Loss of Jak2 activity results in an ablation of PRL-induced Stat5 phosphorylation, cyclin D1 gene expression, differentiation, and growth of mammary cells in vitro (50, 51). Loss of function studies have also revealed a requirement for Jak2 in the in vivo growth of mammary tissues and the initiation of oncogene-mediated tumorigenesis (52, 17).
A Truncated Mouse Prolactin Receptor Is Oncogenic
While the PRLr contributes to the pathogenesis of breast cancer (8), and may do so through the differential utilization of Jak2 and Stat5 as detailed above, the precise mechanism remains to be elucidated. Interestingly, while characterizing the phenotypic effects of a murine Stat1 knockout (53), it was observed that most of the females developed mammary cancer. Genomic sequencing of this cohort revealed that 100% of these tumors bore somatic truncation mutations in the PRLr which were all monoallelic, removing approximately 250 amino acids from the C-terminus of the mouse prolactin receptor (mPRLr) intracellular domain (ICD); these truncations occurred over a 90–base pair hot spot region. mPRLr truncations and altered Stat3/5 signaling were also noted in mammary preneoplastic lesions in the Stat1-/- mice (53). To assess the oncogenic potential of this mutational event, a truncated mPRLr (termed mPRLrT; which lacks approximately 50% of the PRLrL ICD, falling in the middle of the hot spot) was examined. Mouse embryonic fibroblasts were transfected with (1) the mPRLrT, or (2) the full length mPRLr (termed mPRLrL), or (3) both the mPRLrT and mPRLrL. Only when both the mPRLrL and mPRLrT were transfected was transformation of the mouse embryonic fibroblasts noted in vitro and in vivo (53). Based on these data, one would speculate that this shortened form the mPRLrT may be acting like erbB2, needing an erbB receptor (containing a tyrosine kinase) partner to achieve its full oncogenic potential (54). However, analysis of the TCGA genomic database revealed that only 0.4% of human breast cancers display comparable truncations/mutations, suggesting that truncation of the PRLr at the DNA level could only contribute to the pathogenesis of a minority of breast cancers (53); however, this analysis did not look for the alternatively spliced human PRLrI, first cloned and sequenced by the Clevenger laboratory (55).
The Intermediate Human PRLr—A Splice Variant Isoform With Oncogenic Potential?
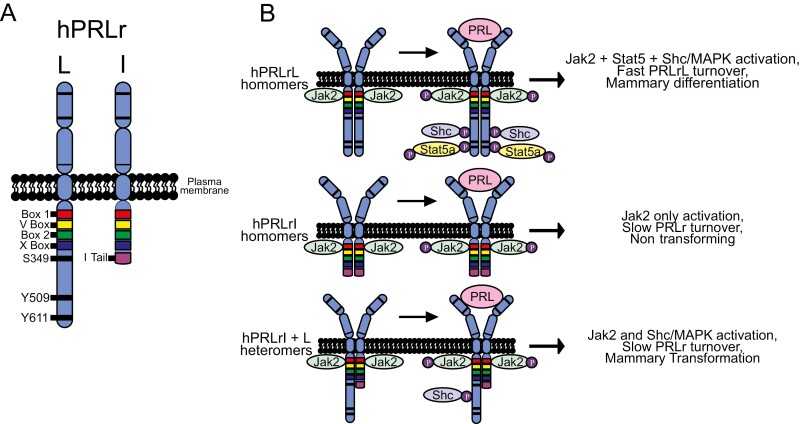
Seven different isoforms of the human PRLr are known to exist and are variably expressed on normal and malignant tissues, but the oncogenic properties of these isoforms have not been explored, in part because many of these isoforms are inactive or display altered ligand binding (8). However, the human PRLr intermediate (PRLrI) isoform is of particular interest. Alternative splicing of the PRLrL exon encompassing the PRLr ICD, results in the PRLrI which is truncated by an out-of-frame splice ending in a stop codon, resulting in a novel 13–amino acid tail (I-tail) (55). Aside from its novel tail, the PRLrI bears significant homology to the rat PRLrI isoform that was observed in the PRL-dependent, transformed rat lymphoma cell line Nb2 (56). Most significantly, the PRLrI has a considerable degree of similarity to the mPRLrT. The PRLrI is widely expressed in human organs, including mammary tissues and breast cancer cell lines (11). While lacking the S349 motif that directs ligand-induced PRLr phosphorylation and degradation, the PRLrI contains all of the Box 2 motif (thought to contribute to Jak2 activation (57, 58), and a conserved portion of the X-box that contains amino acid residue P334 that engages cyclophilin A (CypA) necessary for the full activation of the Jak2 kinase (59). However, the PRLrI also lacks residues Y509/Y587 and Y522 which are necessary for Stat5 and Shc docking to the PRLr (21, 60-63), respectively, raising the possibility that this receptor could allow for Jak2 activation in the absence of Stat5 binding/activation. Taken together, these data suggested that the PRLrI could function as an oncogenic driver through qualitative or quantitative alterations in receptor-associated signaling.
The actions of the PRLrI when introduced into the Ras transfected, but nontransformed, human mammary cell line MCF10AT were therefore examined (8). When both the PRLrI and PRLrL were co-transfected into MCF10AT, a 4-fold increase in proliferation, a 33% increased migration in the wound closure assay, and a 3-fold increase in anchorage-independent growth were observed (8). Comparable results were also obtained with the nontransformed non-Ras-transfected breast line, MCF10A. To prove the oncogenic function of the PRLrL/PRLrI couplet, definitive in vivo testing was performed (8). As a model, the immortalized human mammary line MCF10AT does not invade, nor do the cells metastasize when introduced intraductally into the mammary glands of immunosuppressed mice. Thus, to test the oncogenic function of the PRLrL/PRLrI couplet, combinations of PRLrI and/or PRLrL homodimers or heterodimers were stably transfected into MCF10AT. Pooled transfectants were teat-injected into the fourth mammary glands of immunocompromised NSG mice. Transfectants with PRLr homodimers demonstrated minimal growth, whereas the transfectants expression PRLr heterodimers (PRLrL + PRLrI) demonstrated progressive growth, requiring termination of the experiment within 30 weeks after initiation as the tumors of the PRLrL + PRLrI transfectants had reached the institutional animal care and use committee (IACUC) limit. Grossly, necropsy revealed bulky, fleshy tumors of diameter > 1.5 cm arising from the PRLrL + PRLrI transfectant MCF10AT cells, whereas the other transfectants only yielded 3- to 4-mm firm masses, consistent with residual fibrotic Matrigel. Furthermore, gross metastases were evident in the lymph nodes and lungs of heterodimeric PRLr transfectants but were not detected in the transfectants expression PRLrL homodimers. Histologically, scattered MCF10AT cells in a background of fibrosis and Matrigel were seen in the transfectants expression PRLr homodimers. In contrast, high-grade adenocarcinoma was present in the primary tumors of the transfectants expression PRLr heterodimers which demonstrated local architectural effacement and invasion. Histologic analysis of the lymph nodes and lungs verified the absence of metastases in homodimeric PRLr transfectant groups; in contrast, high-grade adenocarcinoma morphologically similar to that of the primary tumors was observed in > 75% of the lymph nodes and lungs of mice receiving PRLr heterodimers. Immunohistochemistry performed on these tumors revealed that they were negative for ER, progesterone receptor, and Her2, while demonstrating a high proliferative rate (Ki67 > 30%). Taken together, these data provide direct support for the notion that the PRLrI, in the presence of the PRLrL, may function as a dominant transformational driver and promote breast oncogenesis/tumorigenesis and tumor progression.
To corroborate and extend the results from the overexpression studies in untransformed MCF10AT cells with the PRLrI, knockdown of the PRLrI was performed on malignant breast cancer cell lines MCF7 and T47D, which both express the PRLrI and PRLrL, to determine if PRLrI also supports growth of established breast cancer (8). If the PRLrI remains an oncogenic driver in established breast cancer cells, reduction in its level should decrease its tumor-promoting potential. The data supported this hypothesis; reduction of the PRLrI in T47D resulted in significant reduction in cell proliferation, cell migration, and anchorage-independent growth. Comparable results were seen in MCF7, which displayed a lesser degree of knockdown (8).
The above observations with the mPRLrT (53) and the PRLrI (8) suggest that those structures within the C-terminal tail of the PRLrL provide key homeostatic signals and likely contribute to mammary cell differentiation. Loss of the C-terminal portion of the intracellular PRLrL domain, as supported by in our mechanistic studies with the PRLrI, results in an unleashing of the oncogenic potential of the PRLr following heterocomplex formation with the PRLrL. Examining the truncated ICD region that is missing from the PRLrI, 2 notable motifs are deleted. First, a phospho-degron that includes serine residue S349 is missing which recruits the ubiquitin ligase beta-TrCP, required for ligand-induced downregulation/internalization/degradation of the PRLr (31, 64-66). Decreased PRLr internalization is correlated with increased levels of PRLr observed in malignant breast cancer (31), presumptively increasing/prolonging ligand-induced signaling contributing to malignant transformation (67). Second, tyrosine motifs that include tyrosine residues Y509 and Y587 are missing, which are rapidly phosphorylated following Jak2 activation, and serve as docking sites required for Stat5 binding and activation (21).
A relative decrease in Stat5 activity in relation to Jak2 activity has been speculated to contribute to mammary tumorigenesis (68). These 2 sub-hypotheses, namely that the PRLrI + PRLrL complex demonstrates increased stability and/or signaling (which are not mutually exclusive) have been initially examined. With regards to protein stability, cycloheximide treatment of CHO cells transfected with PRLrI, PRLrl, or both demonstrated that cells expressing only the PRLrL (i.e., as homodimers) demonstrated a half-life of approximately 3 hours; in contrast homodimers of the PRLrI or 1:1 heterodimers of PRLrL + PRLrI displayed a half-life of approximately 9 hours. Jak2/Stat5 signaling was also assessed to evaluate altered signaling from the PRLrl + PRLrI receptor heterodimers. MCF10AT homo- and heterodimeric PRLr transfectants were stimulated with ligand, and Jak2 and Stat5 phosphorylation assessed by immunoblot analysis. These studies revealed that a robust Stat5 phosphorylation was observed in the PRLrL homodimeric transfectants, but this was reduced 9-fold in PRLrI/PRLrL heterodimeric transfectants and was undetectable in PRLrI homodimeric transfectants. Correspondingly, Jak2 phosphorylation was increased by 2-fold in the PRLrI + PRLrL and PRLrI + PRLrI transfectants. Taken together with the data noted with the mPRLrT, these findings indicate that both altered PRLr stability and signaling (Fig. 1) may contribute to the oncogenic phenotype seen with PRLrL + PRLrI transfectants, and further suggest that enhanced Jak2 activity unopposed by Stat5 tyrosine phosphorylation/activity may contribute to malignant transformation (8). Indeed, recent unpublished data from the Clevenger lab (not shown) would suggest that some of the oncogenic activity of the hPRLrI may reside in the I-tail. The absence of the I-tail in other species may explain their lack of oncogenicity.
Figure 1.
Working model of PRLrI-driven breast cancer, PRLrI expression in human breast cancer, and the PRLrI is co-transforming with the PRLrL. A, PRLrI is generated by an out-of-frame alternative splicing event, losing both the phospho-degron serine 349 (S349) as well as both putative Stat5A docking sites, tyrosines 509 and 611 (Y509 and Y611, respectively), while gaining a novel 13–amino acid tail (I-Tail). B, Altered stability and/or signaling of the PRLrL + I heterodimer, distinct from that of either homodimer, contributes to mammary transformation.
These and precedent (30) data show that the PRLrI is expressed in the majority of breast cancer cells lines. To determine the frequency of expression of the PRLrI transcript and protein and its functional significance in human breast cancer, a 2-prong strategy was employed. First, the transcriptome (with splice variants) was analyzed from the TCGA in 1100 patients with breast cancer. Second, a tissue microarray including 250 distinct primary human breast tumors (160 ER−/PR− and 90 ER+/PR+) was assessed. Preliminary analysis of the TCGA data revealed that the PRLrI RNA was 2-fold overexpressed (P < 0.01) in malignant tissues compared with normal adjacent tissues. Although > 85% of all breast cancers were found to have some level of expression of both the PRLrI and PRLrL (n = 250 patients; consistent with RNA and immunohistochemistry data (8)), overexpression of the PRLrI was seen in 20% of breast cancers when examined at the RNA level. In tumors where PRLrI overexpression (< 3-fold) was observed, a highly significant correlation with triple-negative breast cancer (P < 10-60) and brain metastasis (P < 10-20) was observed. This association of human PRLrI expression with triple-negative disease is of note, as the PRLr has been associated with luminal disease; however, such association has been classically associated with the PRLrL, and not considered the PRLrI. Conversely, breast cancers that demonstrated PRLrL overexpression (20% of breast cancers) were significantly associated with the luminal phenotype, cell differentiation and vesicular trafficking, and FOXA 2/3 activation. These transcriptomic data were confirmed at the protein level using a specific anti-PRLrI antibody (8). Greater than 85% of clinical human breast cancer tissues were PRLrI-positive at the protein level as well (with overexpression occurring in 20%). Interestingly, a significant positive correlation between PRLrI expression and tumor grade and Ki67 status (both mRNA and protein) was noted. Collectively, these provocative data are consistent with the PRLrl functioning as a cooperative oncogenic driver in breast cancer (69), working in concert with the PRLrL and perhaps other oncogenic drivers in the breast.
Prolactin-Humanized Mice for More Accurate in Vivo Modeling of Human Breast Cancer
It has been well established that PRL is a mammary tumor promoter in rodents through experimental hyperprolactinemia studies (70, 71) or more recently through genetic approaches (72, 73). Our understanding of the biological roles of PRL on growth, metastasis, and drug responsiveness of human breast cancer xenograft models have been complicated by the realization that mouse PRL is not only a poor agonist for human PRLr (74) but is in fact an antagonist for human PRLr (75). Mouse hosts therefore deprive PRLr-positive human breast cancer xenografts of an essential hormone expected to govern tumor engraftment, growth, metastasis, and drug responsiveness (75, 76). The realization that mice lack a physiological ligand for human PRLr, and that mouse PRL is instead an antagonist, complicates the interpretation of previously reported inhibitory effects of PRLr antagonists on human breast cancer xenograft tumors (77-79). For instance, the importance of the antagonist activity of mouse PRL toward human PRL receptors on human breast xenograft tumors that are activated by putative autocrine/paracrine human prolactin (80-83), remains to be determined. Pharmacological administration to standard mice of PRLr antagonists that are selective for human and not mouse PRLr may inhibit growth of PRLr-positive human xenograft tumors by suppressing basal PRLr activity or interfering with other ligand-independent PRLr signaling, for example, via crosstalk or collateral activation of PRLr by ERBB2 in breast cancer cells (reviewed in (84)). The humanized PRLr antagonist antibody LFA102 inhibited growth of human breast cancer xenograft tumors in nude mice reportedly without antagonist activity toward mouse PRLr (77). In contrast, pharmacological PRLr antagonists that act on both human and mouse PRLr, including the human PRL analogs, G129R and Δ1-9-G129R (85-88), may additionally exert indirect effects on human tumors by interfering with mouse PRLr in one or more of the many endocrine organs regulated by PRL.
A recent publication describes immunodeficient PRL-humanized mice that overcome these experimental limitations and provides new information about how physiological levels of human PRL modulates biology, growth, metastasis, and drug responsiveness of xenografted human breast tumors in vivo (12). Human but not mouse PRL activated human PRLr and effectively stimulated proliferation of human T47D breast cancer cells in 3D culture in vitro (12). Importantly, human PRL was equipotent with mouse PRL as an activator of mouse PRLr (12), allowing the team to genetically engineer mice that express physiological levels of human PRL in the absence of mouse PRL under the control of the mouse PRL gene promoter. Specifically, human PRL cDNA was introduced into the first coding exon of the mouse PRL gene. After backcrossing the mice for more than 10 generations into the immunodeficient NSG strain to generate the prolactin-humanized NSG-Pro strain, circulating hPRL levels in female NSG-Pro mice averaged 10 ng/mL, which corresponds to physiological levels in women (89). There was no evidence of hyperprolactinemia (human PRL > 30 ng/mL). Circulating levels of human PRL increased 2-fold in response to estrogen, consistent with intact physiological estrogen-driven pituitary PRL gene expression (89).
NSG-Pro Mice Provide Evidence of Tumor-Promoting Effects of Human PRL in Human Breast Cancer Xenografts
NSG-Pro mice greatly enhanced take rates and tumor growth of implanted ER-positive human breast cancers. The first series of 65 consecutive xenografts from patients with therapy-naïve, invasive primary ER+ breast cancer, an engraftment rate of 43% (28 of 65 ER-positive tumors) was achieved in the NSG-Pro mouse (12). This series was performed by a single trained operator, with ample tumor tissue, and rapid transition from surgery to implantation. Successful engraftment of a patient-derived xenograft (PDX) was defined as doubling in size or viable growth for more than 3 serial passages. The engraftment rate of 43% is markedly higher than the 2.5% previously reported in the largest study using standard immunodeficient mice (90). High engraftment rates were also observed for ERBB2-enriched (40%; 2/5 tumors) and triple-negative breast cancer (TNBC; 57%; 4/7 tumors) subtypes, which both frequently express PRLr transcripts according to the TCGA data set (12). A growing panel of serially transplantable breast cancer PDX tumor models has been established, which generally recapitulate the histological characteristics, Ki67 levels as well as hormone receptor expression status (ER, PR, ERBB2) of the patient tumors.
All (5 out of 5) of the ER+/PRLr+ breast cancer xenograft tumors tested grew significantly faster in NSG-Pro mice compared with standard NSG mice, documenting evidence for human PRL-stimulated growth of human breast cancer (12). These xenograft models included 3 cell line-derived (MCF7, T47D, and ZR75.1) and 2 PDX models (BCX1 and BCX2) that had been established in the NSG-Pro mice. In contrast, a TNBC PDX tumor line that lacked detectable human PRLr expression grew equally well in NSG-Pro and NSG animals (12). Follow-on molecular analyses of the initial PDX tumors shed new light on interactions between PRL and other central growth pathways in ER-positive breast cancer. RNA-Seq analyses of the 2 estrogen-dependent PDX tumor models BCX1 and BCX2 grown in NSG-Pro vs conventional NSG mice, revealed that PRL collaborates with estrogen receptor 1(ESR1)- and Her2 (ERBB2)-modulated gene networks (12). These observations provide further evidence for cooperative crosstalk between PRLR-ESR1 (45, 91-96) and PRLR-ERBB2 (84, 86, 97) pathways in breast cancer. Estrogen receptors and ERBB2 represent well-established breast cancer-promoting pathways and are clinically successful drug targets (8, 98). Collectively, the new data provided direct evidence for control of key breast cancer–relevant pathways by physiological PRL levels that are likely critical for the improved engraftment, growth, and maintenance of patient-derived ER+ breast cancer in NSG-Pro mice.
The NSG-Pro Model Facilitates the Metastatic Progression of ER+ Breast Cancer
Another critical impediment to studying the biology and therapy responses of ER+ human breast cancer is the lack of orthotopic models that spontaneously metastasize to vital organs and recapitulate progression to macrometastatic and fatal disease (99). In support of emerging evidence for pro-metastatic roles for PRL (100-102), several therapy-naïve, estrogen-dependent primary ER+ tumors grown in the NSG-Pro model recapitulated the progression to systemic disease. Necropsies and histological examination of mice bearing ER+ PDX tumors lines, BCX1, BCX2, or BCX3, revealed that 100% of the animals exhibited lung metastases within 50 days for BCX1 (50 out of 50) and within 80 days for BCX2 and BCX3 (20 out of 20).
A key question is whether prolactin promotes continued growth of residual lung metastases after surgical removal of the primary tumors. To assess whether established spontaneous metastatic lesions in NSG-Pro mice remained dependent on human PRL, BCX1 tumors were orthotopically implanted, grown for 55 days and then surgically resected. Sixty days later, the mice were treated with either vehicle or bromocriptine for 14 days, which suppresses the release of PRL from the pituitary gland (103), or with the potent LFA102 antibody antagonizing the human PRLr (104) for 10 days. Subsequent analysis of metastatic burden in the lungs revealed that blockade of prolactin signaling significantly decreased the BCX1 metastatic burden in the lungs in the absence of the primary tumors, which had been excised. These results revealed for the first time that physiological levels of circulating human PRL can support the growth of latent distant metastases of a human ER+ breast cancer model, and they provide the first preclinical evidence of efficacy of PRL pathway targeting against disseminated disease. In addition to spontaneous lung metastases, ER+ PDX tumors grown in these mice also metastasized to other common sites of clinical metastases of luminal breast cancers (105), including bone and liver (12). While studies of additional tumor models are needed, the results raise the prospect that PRL-targeted drugs may benefit some patients with metastatic ER-positive and possibly ER-negative breast cancers that express PRLrI. Future studies will also investigate which forms of PRLr are predominant in these new tumor models.
Utility of PRL-Humanized Mice for Studies of Breast Cancer Drug Response and Resistance Beyond PRLr Pathway Targeting
NSG-Pro mice represent the first in vivo preclinical model in which appropriate targeting of PRLr signaling in human cancer cells can be tested. Intriguingly, the NSG-Pro model also provided evidence that physiological levels of human PRL in mice affect tumor responsiveness to drugs that do not directly target PRLr signaling. Tamoxifen frequently regresses ER+ breast tumors in premenopausal patients (106), but it has been a conundrum that the drug historically was not able to regress ER+ human breast cancer xenografts in mice despite decelerating tumor growth (1, 107, 108). However, tamoxifen significantly regressed ER+ T47D tumors grown in NSG-Pro mice but did not regress size-matched tumors in NSG mice (12). The tamoxifen-induced reduction in tumor burden significantly prolonged survival of NSG-Pro subjects. In addition to the T47D xenograft model, 3 out of 3 therapy-naïve ER+ PDX tumor models tested (BCX1, BCX2 and BCX3), also consistently regressed in response to tamoxifen in NSG-Pro mice. The findings suggest that physiological levels of human PRL in the NSG-Pro mouse model are critical for accurately recapitulating the therapeutic effects of anti-estrogens, and possibly other drugs, as they are being observed in patients with ER+ breast cancer. The reasons for the selective tamoxifen-induced regression of T47D tumors grown in estrogenized NSG-Pro mice and not in NSG mice remain to be determined. Since T47D tumors grown in NSG-Pro mice benefit from both PRL and E2, the tumors may expand a more differentiated and estrogen-dependent luminal-like cancer cell population, while in NSG mice the cancer cells remain less differentiated and hence less responsive to anti-estrogens. Roles for PRL in suppression of stem/progenitor features of breast cancer cells have been reported in models of both Luminal and Her2-enriched breast cancer (46-48, 109).
Stat5 phosphorylation was generally low in BCX1, BCX2, and BCX3, with nuclear-localized pY-Stat5 most readily detected in BCX2 tumors grown in NSG-Pro mice, which are also the slowest growing and least metastatic tumors of the 3 PDX models. We have previously reported poor prognosis and increased risk of endocrine resistance associated with loss of nuclear-localized pYStat5 (34, 110). It will be of great interest to determine the levels of pYStat5, nuclear Stat5a, and PRLr isoforms in the growing panel of ER-positive PDX models to determine correlation with metastasis and responsiveness to anti-estrogens. After prolonged tamoxifen exposure of BCX1 and BCX2 PDX models in NSG-Pro mice, resistant tumors emerged in each PDX model from a minor pool of residual cancer cells (12). BCX1 tumors with acquired tamoxifen-resistance were interrogated and consistently showed ERBB2 protein upregulation without gene amplification and became sensitive to trastuzumab. Crosstalk between PRLr and ERBB2 in breast cancer cells (reviewed in (84)) may underpin development ERBB2-driven endocrine therapy resistance in breast cancer patients. The NSG-Pro mouse is expected to be useful as a precision medicine tool to identify PDX tumors that will default to the ERBB2 escape pathway in response to anti-estrogen therapy. Patients with such tumors may be tested for benefit of combined targeting of ERBB2 and ER signaling to overcome or prevent endocrine resistance. Based on the initial characterization of breast cancer PDX tumors grown in NSG-Pro mice, physiological levels of human PRL restores key elements of the endocrine environment needed for growth as well as adequate drug responsiveness of PRLr-expressing breast cancer, making NSG-Pro mice more relevant for testing of pharmacological agents against breast cancer than conventional mouse models.
Working Model of How PRLrL-PRLrL Homodimers and PRLrL-PRLrI Heterodimers Influence Breast Cancer Biology and Tumor Phenotype
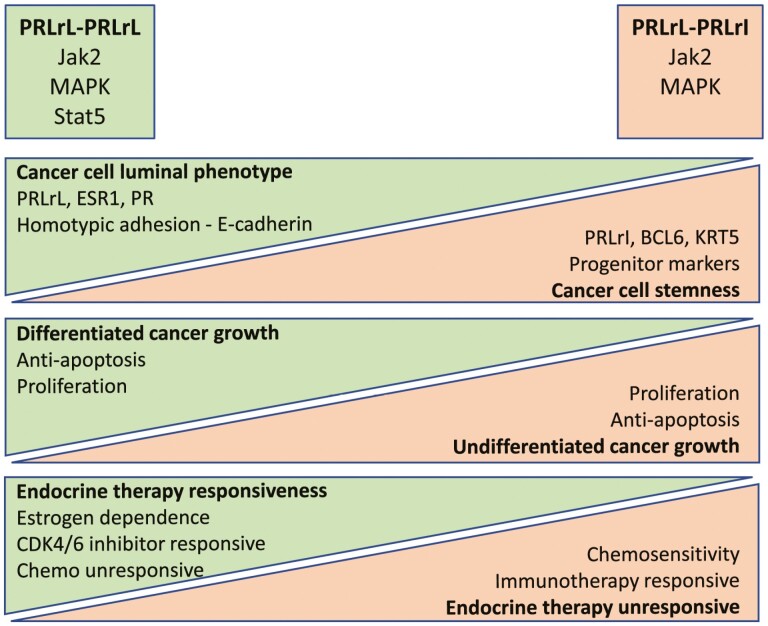
Based on the recent progress summarized here, and other progress in the field, we propose a working model of the relative impact of PRLrL-PRLrL homodimers and PRLrL-PRLrI heterodimers on breast cancer biology (Fig. 2). Activation of Stat5 is a key feature of signaling by PRLrL homodimers that is absent from signaling by PRLrL-PRLrI-heterodimers. Stat5 activation is associated with a well-differentiated luminal cancer cell phenotype that is hormone receptor positive and maintains the epithelial feature of homotypic adhesion, as well as responsiveness to endocrine therapy. In accordance, cancer cells with predominant PRLrL-PRLrI heterodimer signaling feature Jak2-MAPK activation unopposed by Stat5, and these cells take on more undifferentiated stem/progenitor characteristics and are preferentially sensitive to chemotherapy.
Figure 2.
Working model of the relative impact of predominant PRLrL-PRLrL homodimers vs increased proportion of PRLrL-PRLrI heterodimers on breast cancer biology and tumor phenotype.
Therapeutic Implications
The above findings reveal tantalizing roles for PRL in the oncogenesis and progression of breast cancer and suggest that therapies that target PRLr signaling have potential to be effective. Indeed, a phase I clinical trial with the anti-PRLr monoclonal antibody LFA102 directed against the extracellular domain of human PRLr has been carried out (104, 111). Such therapy was safe in breast cancer patients, but little evidence of a therapeutic signal was seen. Why? One explanation for the lack of clinical effectiveness of this monoclonal is that it was directed to the PRLr extracellular domain common to both the PRLrL and PRLrI. In theory, this antibody should target both isoforms and lead to a degradation of receptor and/or a blockade in signaling; however, this could be counterproductive as it could interfere with both pro-oncogenic and pro-differentiative signals. Another explanation again lies with the extracellular epitope recognized by this antibody. To be effective, most or all of the PRLr should be expressed on the cell surface enabling monoclonal antibody recognition; certainly, this is the case with Her2. However, microscopy studies have demonstrated that most of the PRLr protein is not expressed on the cell surface, but instead the majority of the protein is found in intracellular reserves within the endoplasmic reticulum/Golgi (112, 113). Thus, much of the PRLr present in breast cancer cells may not be recognized by the monoclonal antibodies generated to date. Other possible reasons for clinical trial failure include limited tumor selection criteria or inclusion of patients with too advanced cancers.
Could the interruption of PRLr-associated signaling pathways be the means to a therapeutic end in breast cancer? Although considerable attention has been paid to the Jak2/Stat5 pathway during PRLr signaling, several other pathways play into PRLr transduction, as is seen with most other receptor complexes. One evident PRLr-associated pathway is the Shc-Grb2-Vav-Ras-Mek-MAPK pathway. Recognized as a PRLr-associated pathway for some time, recent transcriptomic analysis has reinforced the role of this pathway in breast cancer oncogenesis (17, 20, 72, 114, 115). It is interesting to note that while the PRLrL-PRLrI heterodimers and PRLrL-PRLrL homodimers are capable of ligand-induced MAPK activation, PRLrI-PRLrI homodimers are incapable of MAPK activation (and also appear not to have oncogenic activity). This may be due to the fact that the recognized binding site for Shc occurs at Tyr522, a residue which is missing from PRLrI-PRLrI homodimers. Other PRLr-associated signaling pathways that are also of potential therapeutic utility in breast cancer include GSK3 (116) and Nek3 (117-119). However, full discussion of these pathways is beyond the scope of this mini-review. Given the interplay of multiple PRLr-associated signaling pathways, and the potential for therapeutic rebound and escape frequently encountered by interruption of a single pathway (120), consideration should be given to the combined use of 2 or more small molecular inhibitors of these pathways in breast cancer.
The new progress highlighted here provides direct support for a role of PRLr in breast tumorigenesis, tumor growth, and tumor progression. Distortions in the balance between the PRLrL and PRLrI splice variants represent a novel mechanism that may markedly affect the tumor-promoting effects of PRL in breast cancer. A more in-depth understanding of how the stoichiometry of PRLr-L homodimer and PRLr-L-PRLrI heterodimers affects breast tumor biology may uncover the proliferative and tumor-promoting PRLr signals, which in turn may be used as markers to identify breast cancer patients with increased likelihood to benefit from PRLr pathway–targeted therapies. The new prolactin-humanized NSG-Pro mice are available from Jackson laboratory and hold potential to facilitate key preclinical testing and analysis of PRLr pathway–targeted agents in patient-derived xenograft models of breast cancer. Promising results from such preclinical studies will in turn provide rationale for clinical trials of likely responders.
Glossary
Abbreviations
- ER
estrogen receptor
- ICD
intracellular domain
- mPRLr
mouse prolactin receptor
- mPRLrL
mouse prolactin receptor full-length form
- mPRLrT
mouse prolactin receptor truncated form
- PDX
patient-derived xenograft
- PRL
prolactin
- PRLr
prolactin receptor
- PRLrI
prolactin receptor intermediate form
- PRLrL
prolactin receptor long form
- Stat5
signal transducer and activator of transcription 5a/b
Contributor Information
Charles V Clevenger, Department of Pathology, Virginia Commonwealth University, Richmond, VA 23298-06629, USA.
Hallgeir Rui, Department of Pathology, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Funding
C.C., NIH R01173305; H.R. NIH R01 CA267549
Author Contributions
C.C. and H.R. devised the project, wrote the manuscript, created the figures, provided support and critical review, and discussed the review and provided editorial feedback of the manuscript.
Disclosures
The authors have nothing to declare.
Data Availability
Data sharing is not applicable to this review as no datasets were generated or analyzed during the current study.
References
- 1. Osborne CK, Hobbs K, Clark GM. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985;45(2):584-590. [PubMed] [Google Scholar]
- 2. Eden JA. Breast cancer, stem cells and sex hormones. Part 2: the impact of the reproductive years and pregnancy. Maturitas. 2010;67(3):215-218. doi: 10.1016/j.maturitas.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 3. Fischer B, Redmond C, Fischer ER. Systemic adjuvant therapy in the treatment of primary operable breast cancer: National surgical adjuvant breast and bowel project experiment. NCI Monogr. 1986;1:35-43. [PubMed] [Google Scholar]
- 4. Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22(30):4664-4674. doi: 10.1038/sj.onc.1206619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Welsch CW. Interaction of Estrogen and Prolactin in Spontaneous Mammary Tumorgenesis of the Mouse. J Toxicol Environ Health. 1976;1:161-175. [PubMed] [Google Scholar]
- 6. Welsch CW. Prophylaxis of Spontaneously Developing Mammary Carcinoma in C3H/HeJ Female Mice by Suppression of Prolactin. Cancer Res. 1973;33:2939-2946. [PubMed] [Google Scholar]
- 7. Wagner KU, Rui H. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia. 2008;13(1):93-103. doi: 10.1007/s10911-008-9062-z [DOI] [PubMed] [Google Scholar]
- 8. Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24(1):1-27. doi: 10.1210/er.2001-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fritze D, Queisser W, Schmid H, et al. Prospective randomized trial concerning hyper- and normoprolactinemia and the use of bromoergocryptine in patients with metastatic breast cancer. Onkologie. 1986;9(6):305-312. doi: 10.1159/000216041 [DOI] [PubMed] [Google Scholar]
- 10. Holtkamp W, Nagel GA. [Bromocriptine in chemotherapy-resistant, metastatic breast cancer. Results of the GO-MC-BROMO 2/82 AIO Study]. Onkologie. 1988;11(3):121-127. doi: 10.1159/000216502 [DOI] [PubMed] [Google Scholar]
- 11. Grible JM, Zot P, Olex AL, et al. The human intermediate prolactin receptor is a mammary proto-oncogene. NPJ Breast Cancer. 2021;7(1):37. doi: 10.1038/s41523-021-00243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun Y, Yang N, Utama FE, et al. NSG-Pro mouse model for uncovering resistance mechanisms and unique vulnerabilities in human luminal breast cancers. Sci Adv. 2021;7(38):eabc8145. doi: 10.1126/sciadv.abc8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ormandy CJ, Camus A, Barra J, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11(2):167-178. doi: 10.1101/gad.11.2.167 [DOI] [PubMed] [Google Scholar]
- 14. Rui H, Kirken RA, Farrar WL. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994;269(7):5364-5368. [PubMed] [Google Scholar]
- 15. Rui H, Lebrun JJ, Kirken RA, Kelly PA, Farrar WL. JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology. 1994;135(4):1299-1306. doi: 10.1210/endo.135.4.7925093 [DOI] [PubMed] [Google Scholar]
- 16. Wagner KU, Krempler A, Triplett AA, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004;24(12):5510-5520. doi: 10.1128/MCB.24.12.5510-5520.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erwin RA, Kirken RA, Malabarba MG, Farrar WL, Rui H. Prolactin activates Ras via signaling proteins SHC, growth factor receptor bound 2, and son of sevenless. Endocrinology. 1995;136(8):3512-3518. doi: 10.1210/endo.136.8.7628388 [DOI] [PubMed] [Google Scholar]
- 18. Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, et al. Prolactin induces c-Myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene. 2004;23(44):7378-7390. doi: 10.1038/sj.onc.1208002 [DOI] [PubMed] [Google Scholar]
- 19. Krishnan N, Pan H, Buckley DJ, Buckley A. Prolactin-regulated pim-1 transcription: identification of critical promoter elements and Akt signaling. Endocrine. 2003;20(1-2):123-130. doi: 10.1385/endo:20:1-2:123 [DOI] [PubMed] [Google Scholar]
- 20. Clevenger CV, Torigoe T, Reed JC. Prolactin induces rapid phosphorylation and activation of prolactin receptor-associated RAF-1 kinase in a T-cell line. J Biol Chem. 1994;269(8):5559-5565. [PubMed] [Google Scholar]
- 21. DaSilva L, Rui H, Erwin RA, et al. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol. 1996;117(2):131-140. doi: 10.1016/0303-7207(95)03738-1 [DOI] [PubMed] [Google Scholar]
- 22. Kirken RA, Malabarba MG, Xu J, et al. Prolactin stimulates serine/tyrosine phosphorylation and formation of heterocomplexes of multiple Stat5 isoforms in Nb2 lymphocytes. J Biol Chem. 1997;272(22):14098-14103. doi: 10.1074/jbc.272.22.14098 [DOI] [PubMed] [Google Scholar]
- 23. Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7(1):49-66. doi: 10.1023/a:1015770423167 [DOI] [PubMed] [Google Scholar]
- 24. Hennighausen L, Robinson GW, Wagner KU, Liu W. Prolactin signaling in mammary gland development. J Biol Chem. 1997;272(12):7567-7569. doi: 10.1074/jbc.272.12.7567 [DOI] [PubMed] [Google Scholar]
- 25. Hennighausen L, Robinson GW, Wagner KU, Liu X. Developing a mammary gland is a stat affair. J Mammary Gland Biol Neoplasia. 1997;2(4):365-372. doi: 10.1023/a:1026347313096 [DOI] [PubMed] [Google Scholar]
- 26. Clevenger CV. Role of Stat family transcription factors in human breast cancer. Am J Pathol. 2004;165(5):1449-1460. doi: 10.1016/S0002-9440(10)63403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grimley PM, Dong F, Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999;10(2):131-157. doi: 10.1016/s1359-6101(99)00011-8 [DOI] [PubMed] [Google Scholar]
- 28. Gallego MI, Binart N, Robinson GW, et al. Prolactin, growth hormone, and epidermal growth factor activate Stat 5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229:163-175. doi: 10.1006/dbio.2000.9961 [DOI] [PubMed] [Google Scholar]
- 29. Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1(4):467-475. doi: 10.1016/s1534-5807(01)00064-8 [DOI] [PubMed] [Google Scholar]
- 30. Clevenger CV, Chang W-P, Ngo W, Pasha TLM, Montone KT, Tomaszewski JE. Expression of prolactin and prolactin receptor in human breast carcinoma: Evidence for an autocrine/paracrine loop. Am J Pathol. 1995;146(3):695-705. [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Clevenger CV, Minkovsky N, et al. Stabilization of prolactin receptor in breast cancer cells. Oncogene. 2006;25(13):1896-1902. doi: 10.1038/sj.onc.1209214 [DOI] [PubMed] [Google Scholar]
- 32. Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoattractant for human breast carcinoma. Endocrinology. 1999;140(11):5447-5450. doi: 10.1210/endo.140.11.7245 [DOI] [PubMed] [Google Scholar]
- 33. Nevalainen MT, Xie J, Torhorst J, et al. Signal transducer and activator of transcription-5 (Stat5) activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053-2060. doi: 10.1200/jco.2004.11.046 [DOI] [PubMed] [Google Scholar]
- 34. Peck AR, Witkiewicz AK, Liu C, et al. Loss of nuclear localized and tyrosine phosphorylated Stat5 in breast cancer predicts poor clinical outcome and increased risk of antiestrogen therapy failure. J Clin Oncol. 2011;29(18):2448-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(7):629-634. [DOI] [PubMed] [Google Scholar]
- 36. Tworoger SS, Sluss P, Hankinson SE. Association between Plasma Prolactin Concentrations and Risk of Breast Cancer among Predominately Premenopausal Women. Cancer Res. 2006;66(4):2476-2482. doi: 10.1158/0008-5472.CAN-05-3369 [DOI] [PubMed] [Google Scholar]
- 37. Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia 2009;14:87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281-291. doi: 10.1038/nrc1839 [DOI] [PubMed] [Google Scholar]
- 39. Dekkers O, Romijn J, de Boer A, Vandenbroucke J, Dekkers OM. The risk for breast cancer is not evidently increased in women with hyperprolactinemia. Pituitary. 2010;13:195-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng Y, Mo W, Yu Y, et al. Breast carcinoma associated with prolactinemia. Cancer Biol Ther. 2017;18:132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marotta LL, Almendro V, Marusyk A, Hahn WC, Frank DA, Polyak K. The Jak2/Stat3 signaling pathway is required for growth of CD44+/CD42- stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC. Rac1 mediates STAT3 activation by autocrine IL-6. PNAS. 2001;98(16):9014-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee I-S, Liu Y, Narazaki M, Hibi M, Kishimoto T, Taga T. Vav is associated with signal transducing molecules gp130,Grb2, and Erk2, and is tyrosine phosphorylated in response to interleukin-6. FEBS Lett. 1997;401:133-137. doi: 10.1016/s0014-5793(96)01456-1 [DOI] [PubMed] [Google Scholar]
- 44. Sato T, Tran TH, Peck AR, et al. Global profiling of prolactin-modulated transcripts in breast cancer in vivo. Mol Cancer. 2013;12:59. doi: 10.1186/1476-4598-12-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rasmussen LM, Frederiksen KS, Din N, et al. Prolactin and oestrogen synergistically regulate gene expression and proliferation of breast cancer cells. Endocr Relat Cancer. 2010;17(3):809-822. doi: 10.1677/ERC-09-0326 [DOI] [PubMed] [Google Scholar]
- 46. Fang F, Rycyzyn MA, Clevenger CV. Role of c-Myb during prolactin-induced signal transducer and activator of transcription 5a signaling in breast cancer cells. Endocrinology. 2009;150(4):1597-1606. doi: 10.1210/en.2008-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goodman CR, Sato T, Peck AR, et al. Steroid induction of therapy-resistant cytokeratin-5-positive cells in estrogen receptor-positive breast cancer through a BCL6-dependent mechanism. Oncogene. 2016;35(11):1373-1385. doi: 10.1038/onc.2015.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato T, Tran TH, Peck AR, et al. Prolactin suppresses a progestin-induced CK5-positive cell population in luminal breast cancer through inhibition of progestin-driven BCL6 expression. Oncogene. 2014;33(17):2215-2224. doi: 10.1038/onc.2013.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tran TH, Utama FE, Lin J, et al. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res. 2010;70(4):1711-1721. doi: 10.1158/0008-5472.CAN-09-2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xie J, LeBaron MJ, Nevalainen MT, Rui H. Role of tyrosine kinase Jak2 in prolactin-induced differentiation and growth of mammary epithelial cells. J Biol Chem. 2002;277(16):14020-14030. doi: 10.1074/jbc.M112399200 [DOI] [PubMed] [Google Scholar]
- 51. Sakamoto K, Creamer BA, Triplett AA, Wagner KU. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol. 2007;21(8):1877-1892. doi: 10.1210/me.2006-0316 [DOI] [PubMed] [Google Scholar]
- 52. Sakamoto K, Creamer BA, Triplett AA, Wagner K-U. The Janus kinase 2 (Jak2) is required for expression and nuclear accumulation of Cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol. 2007;21:2218-2232. [DOI] [PubMed] [Google Scholar]
- 53. Griffith OL, Chan SR, Griffith M, et al. Truncating Prolactin Receptor Mutations Promote Tumor Growth in Murine Estrogen Receptor-Alpha Mammary Carcinomas. Cell Rep. 2016;17(1):249-260. doi: 10.1016/j.celrep.2016.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19(53):6102-6114. doi: 10.1038/sj.onc.1203973 [DOI] [PubMed] [Google Scholar]
- 55. Kline JB, Roehrs H, Clevenger CV. Functional characterization of the intermediate isoform of the human prolactin receptor. J Biol Chem. 1999;274(50):35461-35468. doi: 10.1074/jbc.274.50.35461 [DOI] [PubMed] [Google Scholar]
- 56. Gout PW, Beer CT, Noble RL. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980;40(7):2433-2436. [PubMed] [Google Scholar]
- 57. Domanksi P, Fish E, Nadeau OW, et al. A region of the á subunit of the interferon à receptor different from Box 1 interacts with Jak1 and is sufficient to actovated the Jak-Stat pathway and induce an antiviral state. J Biol Chem. 1997;272:26388-26393. [DOI] [PubMed] [Google Scholar]
- 58. Wang Y-D, Wood WI. Amino acids of the human growth hormone receptor that are required for proliferation and Jak-STAT signaling. Mol Endocrinol. 1995;9:303-311. doi: 10.1210/mend.9.3.7539888 [DOI] [PubMed] [Google Scholar]
- 59. Zheng J, Koblinski J, Dutson LB, Feeney YB, Clevenger CV. Peptidyl-prolyl isomerase regulation of Jak2 activation and the progression of human breast cancer. Cancer Res. 2008;68:7769-7778. [DOI] [PubMed] [Google Scholar]
- 60. Das R, Vonderhaar BK. Involvement of SHC, GRB2, SOS, and RAS in prolactin signal transduction in mammary epithelial cells. Oncogene. 1996;13(6):1139-1145. [PubMed] [Google Scholar]
- 61. Batzer AG, Blaikie P, Nelson K, Schlessinger J, Margolis B. The phosphotyrosine interaction domain of Shc binds an LXNPXY motif on the epidermal growth factor receptor. Mol Cell Biol. 1995;15(8):4403-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Songyang Z, Shoelson SE, McGlade J, et al. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14(4):2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goupille O, Daniel N, Bignon C, Jolivet J, Djiane J. Prolactin signal transduction to milk protein genes: carboxy-terminal part of the prolactin receptor and its tyrosine phosphorylation are not obligatory for JAK2 and STAT5 activation. Mol Endocrinol. 1997;127:155-169. [DOI] [PubMed] [Google Scholar]
- 64. Chang W-P, Clevenger CV. Modulation of growth factor receptor function by isoform heterodimerization. Proc Natl Acad Sci USA. 1996;93(12):5947-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang W-P, Ye Y, Clevenger CV. Stoichiometric structure/function analysis of the prolactin receptor signaling domains by receptor chimeras. Mol Cell Biol. 1998;18(2):896-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Swaminathan G, Varghese B, Thangavel C, et al. Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J Endocrinol. 2008;196(2):R1-R7. [DOI] [PubMed] [Google Scholar]
- 67. Plotnikov A, Varghese B, Tran TH, Liu C, Rui H, Fuchs SY. Impaired turnover of prolactin receptor contributes to transformation of human breast cells. Cancer Res. 2009;69(7):3165-3172. doi: 10.1158/0008-5472.CAN-08-4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Raccurt M, Tam SP, Lau P, et al. Suppressor of cytokine signaling gene expression is elevated in breast carcinoma. Br J Cancer. 2003;89(3):524-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV. Expression of Prolactin and its receptor in human breast carcinoma. Endocrinology. 1997;138(12):5555-5560. doi: 10.1210/endo.138.12.5605 [DOI] [PubMed] [Google Scholar]
- 70. Pearson OH, Llerena O, Llerena L, Molina A, Butler T. Prolactin-dependent rat mammary cancer: a model for man? Trans Assoc Am Physicians. 1969;82:225-238. [PubMed] [Google Scholar]
- 71. Welsch CW, Nagasawa H. Prolactin and murine mammary tumorigenesis: a review. Cancer Res. 1977;37(4):951-963. [PubMed] [Google Scholar]
- 72. Shea MP, O’Leary KA, Fakhraldeen SA, et al. Antiestrogen Therapy Increases Plasticity and Cancer Stemness of Prolactin-Induced ERalpha(+) Mammary Carcinomas. Cancer Res. 2018;78(7):1672-1684. doi: 10.1158/0008-5472.CAN-17-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. O’Leary KA, Shea MP, Schuler LA. Modeling prolactin actions in breast cancer in vivo: insights from the NRL-PRL mouse. Adv Exp Med Biol. 2015;846:201-220. doi: 10.1007/978-3-319-12114-7_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Utama FE, LeBaron MJ, Neilson LM, et al. Human prolactin receptors are insensitive to mouse prolactin: implications for xenotransplant modeling of human breast cancer in mice. J Endocrinol. 2006;188(3):589-601. doi: 10.1677/joe.1.06560 [DOI] [PubMed] [Google Scholar]
- 75. Utama FE, Tran TH, Ryder A, LeBaron MJ, Parlow AF, Rui H. Insensitivity of human prolactin receptors to nonhuman prolactins: relevance for experimental modeling of prolactin receptor-expressing human cells. Endocrinology. 2009;150(4):1782-1790. doi: 10.1210/en.2008-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wagner KU. Models of breast cancer: quo vadis, animal modeling? Breast Cancer Res. 2004;6(1):31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Damiano JS, Rendahl KG, Karim C, et al. Neutralization of prolactin receptor function by monoclonal antibody LFA102, a novel potential therapeutic for the treatment of breast cancer. Mol Cancer Ther. 2013;12(3):295-305. doi: 10.1158/1535-7163.MCT-12-0886 [DOI] [PubMed] [Google Scholar]
- 78. Chen NY, Holle L, Li W, Peirce SK, Beck MT, Chen WY. In vivo studies of the anti-tumor effects of a human prolactin antagonist, hPRL-G129R. Int J Oncol. 2002;20(4):813-818. doi: 10.3892/ijo.20.4.813 [DOI] [PubMed] [Google Scholar]
- 79. Borcherding DC, Hugo ER, Fox SR, et al. Suppression of Breast Cancer by Small Molecules That Block the Prolactin Receptor. Cancers (Basel). 2021;13(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Clevenger CV, Plank TL. Prolactin as an autocrine/paracrine factor in breast tissue. J Mammary Gland Biol Neoplasia. 1997;2(1):59-68. doi: 10.1023/a:1026325630359 [DOI] [PubMed] [Google Scholar]
- 81. Nitze LM, Galsgaard ED, Din N, et al. Reevaluation of the proposed autocrine proliferative function of prolactin in breast cancer. Breast Cancer Res Treat. 2013;142(1):31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Manni A, Pontari M, Wright C. Autocrine stimulation by prolactin of hormone-responsive breast cancer growth in culture. Endocrinology. 1985;117(5):2040-2043. [DOI] [PubMed] [Google Scholar]
- 83. Manhes C, Goffin V, Kelly PA, Touraine P. Autocrine prolactin as a promotor of mammary tumour growth. J Dairy Res. 2005;72 Spec No: 58-65. [DOI] [PubMed] [Google Scholar]
- 84. Kavarthapu R, Anbazhagan R, Dufau ML. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers (Basel). 2021;13:4685-4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Goffin V, Bernichtein S, Touraine P, Kelly PA. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev. 2005;26(3):400-422. [DOI] [PubMed] [Google Scholar]
- 86. Scotti ML, Langenheim JF, Tomblyn S, Springs AE, Chen WY. Additive effects of a prolactin receptor antagonist, G129R, and herceptin on inhibition of HER2-overexpressing breast cancer cells. Breast Cancer Res Treat. 2008;111(2):241-250. [DOI] [PubMed] [Google Scholar]
- 87. Chen WY, Ramamoorthy P, Chen N, Sticca R, Wagner TE. A human prolactin antagonist, hPRL-G129R, inhibits breast cancer cell proliferation through induction of apoptosis. Clin Cancer Res. 1999;5(11):3583-3593. [PubMed] [Google Scholar]
- 88. Bernichtein S, Kayser C, Dillner K, et al. Development of pure prolactin receptor antagonists. J Biol Chem. 2003;278(38): 35988-35999. [DOI] [PubMed] [Google Scholar]
- 89. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523-1631. [DOI] [PubMed] [Google Scholar]
- 90. Cottu P, Marangoni E, Assayag F, et al. Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res Treat. 2012;133(2):595-606. [DOI] [PubMed] [Google Scholar]
- 91. Gonzalez L, Zambrano A, Lazaro-Trueba I, et al. Activation of the unliganded estrogen receptor by prolactin in breast cancer cells. Oncogene. 2009;28(10):1298-1308. [DOI] [PubMed] [Google Scholar]
- 92. Arendt LM, Evans LC, Rugowski DE, Garcia-Barchino MJ, Rui H, Schuler LA. Ovarian hormones are not required for PRL-induced mammary tumorigenesis, but estrogen enhances neoplastic processes. J Endocrinol. 2009;203(1):99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gutzman JH, Nikolai SE, Rugowski DE, Watters JJ, Schuler LA. Prolactin and estrogen enhance the activity of activating protein 1 in breast cancer cells: role of extracellularly regulated kinase 1/2-mediated signals to c-fos. Mol Endocrinol. 2005;19(7):1765-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kavarthapu R, Tsai Morris CH, Dufau ML. Prolactin induces up-regulation of its cognate receptor in breast cancer cells via transcriptional activation of its generic promoter by cross-talk between ERalpha and STAT5. Oncotarget. 2014;5(19):9079-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Barcus CE, Holt EC, Keely PJ, Eliceiri KW, Schuler LA. Dense collagen-I matrices enhance pro-tumorigenic estrogen-prolactin crosstalk in MCF-7 and T47D breast cancer cells. PLoS One. 2015;10(1):e0116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. O’Leary KA, Jallow F, Rugowski DE, et al. Prolactin activates Eralpha in the absence of ligand in female mammary development and carcinogenesis in vivo. Endocrinology. 2013;154(12):4483-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kavarthapu R, Dufau ML. Role of EGF/ERBB1 in the transcriptional regulation of the prolactin receptor independent of estrogen and prolactin in breast cancer cells. Oncotarget. 2016;7(40):65602-65613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Leehy KA, Truong TH, Mauro LJ, Lange CA. Progesterone receptors (PR) mediate STAT actions: PR and prolactin receptor signaling crosstalk in breast cancer models. J Steroid Biochem Mol Biol. 2018;176:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Matthews SB, Sartorius CA. Steroid Hormone Receptor Positive Breast Cancer Patient-Derived Xenografts. Horm Cancer. 2017;8(1):4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yonezawa T, Chen KH, Ghosh MK, et al. Anti-metastatic outcome of isoform-specific prolactin receptor targeting in breast cancer. Cancer Lett. 2015;366(1):84-92. [DOI] [PubMed] [Google Scholar]
- 101. Sutherland A, Forsyth A, Cong Y, et al. The Role of Prolactin in Bone Metastasis and Breast Cancer Cell-Mediated Osteoclast Differentiation. J Natl Cancer Inst. 2016;108(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zheng Y, Comaills V, Burr R, et al. COX-2 mediates tumor-stromal prolactin signaling to initiate tumorigenesis. Proc Natl Acad Sci USA. 2019;116(12):5223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Utian WH, Begg G, Vinik AI, Paul M, Shuman L. Effect of bromocriptine and chlorotrianisene on inhibition of lactation and serum prolactin. A comparative double-blind study. Br J Obstet Gynaecol. 1975;82(9):755-759. [DOI] [PubMed] [Google Scholar]
- 104. Agarwal N, Machiels JP, Suarez C, et al. Phase I Study of the Prolactin Receptor Antagonist LFA102 in Metastatic Breast and Castration-Resistant Prostate Cancer. Oncologist. 2016;21(5):535-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cummings MC, Simpson PT, Reid LE, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Henderson IC, Canellos GP. Cancer of the breast: the past decade (second of two parts). N Engl J Med. 1980;302:78-90. [DOI] [PubMed] [Google Scholar]
- 107. Osborne CK, Coronado EB, Robinson JP. Human breast cancer in the athymic nude mouse: cytostatic effects of long-term antiestrogen therapy. Eur J Cancer Clin Oncol. 1987;23(8):1189-1196. [DOI] [PubMed] [Google Scholar]
- 108. Fagan DH, Uselman RR, Sachdev D, Yee D. Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment. Cancer Res. 2012;72(13):3372-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hachim IY, Lopez-Ozuna VM, Hachim MY, Lebrun JJ, Ali S. Prolactin hormone exerts anti-tumorigenic effects in HER-2 overexpressing breast cancer cells through regulation of stemness. Stem Cell Res. 2019;40:101538. [DOI] [PubMed] [Google Scholar]
- 110. Nevalainen MT, Xie J, Torhorst J, et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053-2060. [DOI] [PubMed] [Google Scholar]
- 111. Minami H, Ando Y, Tamura K, Tajima T, Isaacs R. Phase I Study of LFA102 in Patients With Advanced Breast Cancer or Castration-resistant Prostate Cancer. Anticancer Res. 2020;40(9):5229-5235. [DOI] [PubMed] [Google Scholar]
- 112. Clevenger CV, Altmann SW, Prystowsky MB. Requirement of nuclear prolactin for interleukin-2-stimulated proliferation of T lymphocytes. Science. 1991;253(5015):77-79. [DOI] [PubMed] [Google Scholar]
- 113. Clevenger CV, Russell DH, Appasamy PM, Prystowsky MB. Regulation of IL2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci USA. 1990;87: 6460-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Goupille O, Barnier J-V, Guibert B, Paly J, Djiane J. Effect of PRL on MAPK activation: negative regulatory role of the C-terminal part of the PRL receptor. Mol Cell Endocrinol. 2000;159(1-2):133-146. [DOI] [PubMed] [Google Scholar]
- 115. Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Prolactin signaling through focal adhesion complexes is amplified by stiff extracellular matrices in breast cancer cells. Oncotarget. 2016;7(30):48093-48106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Plotnikov A, Li Y, Tran TH, et al. Oncogene-mediated inhibition of GSK-3B impairs degradation of the prolactin receptor. Cancer Res. 2008;2008:1354-1361. [DOI] [PubMed] [Google Scholar]
- 117. Harrington KM, Clevenger CV. Identification of NEK3 Kinase Threonine 165 as a Novel Regulatory Phosphorylation Site That Modulates Focal Adhesion Remodeling Necessary for Breast Cancer Cell Migration. J Biol Chem. 2016;291(41):21388-21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Miller SL, Antico G, Raghunath PN, Tomaszewski JE, Clevenger CV. Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells. Oncogene. 2007;26(32):4668-4678. [DOI] [PubMed] [Google Scholar]
- 119. Miller SL, DeMaria JE, Freier D, Riegal AM, Clevenger CV. Novel assocation of Vav2 and Nek3 modulates signaling through the human prolactin receptor. Mol Endocrinol. 2004;19:939-949. [DOI] [PubMed] [Google Scholar]
- 120. Li Y, Kong X, Xuan L, Wang Z, Huang YH. Prolactin and endocrine therapy resistance in breast cancer: The next potential hope for breast cancer treatment. J Cell Mol Med. 2021;25(22):10327-10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review as no datasets were generated or analyzed during the current study.