Abstract
The successful cytosolic delivery of nanoparticles is hampered by their endosomal entrapment and degradation. To push forward the smart development of nanoparticles we must reliably detect and quantify their endosomal escape process. However, the current methods employed are not quantitative enough at the nanoscale to achieve this. Nanoscopy is a rapidly evolving field that has developed a diverse set of powerful techniques in the last two decades, opening the door to explore nanomedicine with an unprecedented resolution and specificity. The understanding of key steps in the drug delivery process – such as endosomal escape – would benefit greatly from the implementation of the most recent advances in microscopy. In this review, we provide the latest insights into endosomal escape of nanoparticles obtained by nanoscopy, and we discuss the features that would allow these techniques to make a great impact in the field.
How nanoscopy can be applied towards the study and quantification of endosomal escape of nanoparticles.
1. Introduction
Using nanoparticles (NPs) to deliver drugs to cells (nanomedicine) was foreseen to be a true game-changer of the 21st century in improving the prevention, diagnosis and therapy of various diseases.1–6 The potential of these nanosized carriers in pharmaceutical applications has been envisioned since the 1970's to improve the delivery of therapeutic and imaging agents to specific target sites.7–11 The remarkable interest in NPs is attributed to the plethora of physical and biological advantages they offer in comparison to conventional medicines, such as improved efficacy and safety, enhanced solubility and pharmacokinetic profiles, and increased target selectivity.12–15
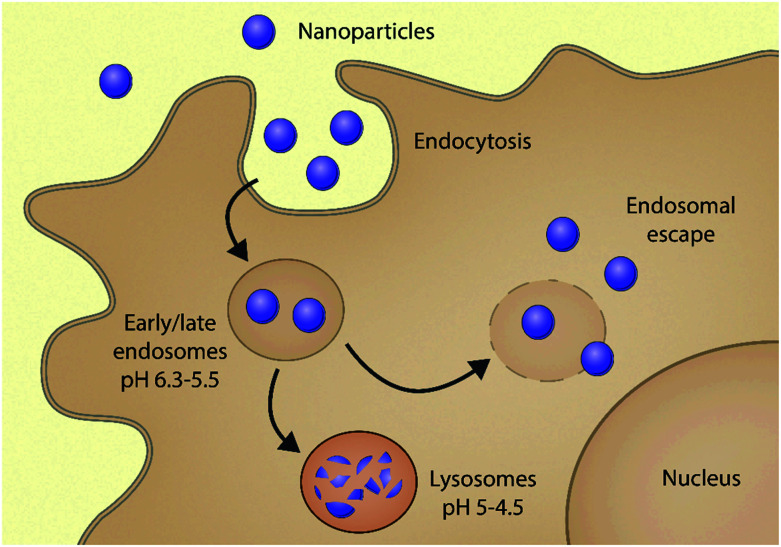
Although various NP formulations have been marketed,16,17 achieving efficient intracellular delivery still remains a significant challenge.18–22 One of the main culprits is that the majority of NPs – once taken up via endocytosis – are unavoidably distributed in endocytic vesicles. These acidic organelles can degrade the carrier-drug ensemble, reducing its bioavailability in the intracellular environment.23,24 Within these vesicles, pH gradually drops from neutral to acidic because of membrane-incorporated vacuolar-type ATPases. The cargo is first brought into the early endosome (pH ∼ 5.5); then finally the late endosome fuses with the lysosome and the cargo is degraded by hydrolytic enzymes present in the acidic milieu (pH 5–4.5). The recycling endosome may direct some cargo back to the cell surface, whilst the majority remain entrapped in the endo-lysosomal pathway, where they are degraded20,25 (Fig. 1). Endosomal entrapment thus represents one of the main bottlenecks in using NP systems for gene therapy20,26–28 and proteins or small molecular drugs for the treatment of a variety of diseases.20,29–31
Fig. 1. Scheme of the route followed by nanoparticles inside the cell. They are first internalized by endocytosis into early endosomes, where they are trafficked through the endolysosomal pathway and ultimately degraded in the lysosomes. Nanoparticles escaped from endosomes to avoid degradation and deliver their cargo into the cytoplasm.
Mechanisms through which NPs – and more importantly the therapeutic cargo – can escape these degrading vesicles have become the subject of intense research over the past few decades.19,20,24,32 Inspired by the innate ability of bacterial toxins and viruses to escape endosomal vesicles, various hypothetical endosomal escape mechanisms have been proposed and reviewed in the literature, such as the “proton sponge” effect, membrane fusion, pore formation, membrane disruption, and vesicle budding and collapse.24,27,32–36 Numerous strategies to enhance the escape of NPs have also been suggested, including endosomal buffering agents, membrane fusogenic peptides, lysosomotropic chemical agents,20,23,37–44 morphological-dependent changes45 or external stimuli such as photochemical internalization (PCI).46,47
It is crucial to note that the endosomal escape hypotheses suffer from many inconsistencies. For example, the “proton sponge” hypothesis – based on the buffering capacity of polycations, that are suggested to cause an increase in lysosomal pH – has been heavily disputed in the literature.48,49 As a result, the mechanism of the action of these formulation strategies is generally unknown. This is limiting the development of NPs with efficient endosomal escape, and it is further worsened by the absence of effective methods to detect – and more crucially to quantify – this process. Consequently, it is challenging to determine which strategies are efficient in improving the escape ability of NPs, hindering the development of successful formulations. Additionally, the lack of standardized methods leads to poor comparisons between different endosomal escape studies, leading to contradicting and inconclusive results.48–53
Standard methods used to assess endosomal escape commonly employ fluorescence microscopy, flow cytometry or mass spectrometry. However, fluorescence microscopy cannot be used alone to quantify the total number of particles inside cells, as this requires cumbersome calibration of the fluorescence signal, and lacks the resolution to quantify individual NPs below 250 nm. Flow cytometry measures relative fluorescence intensity rather than individual NPs, and mass spectrometry leads to the loss of spatial information.54 Readers are directed to other available reviews for information on these techniques and how they may compare with nanoscopy methods.24,35,55,56 Studying endosomal escape brings alongside certain challenges; it is a fast process, rare and occurs in the nanoscale. All these techniques have limited spatial resolution, are often poorly quantitative and fail to provide information on endosomal escape at the nanoscale and quantitative level, or with high molecular specificity within the cellular biological environment. Therefore, new, and improved techniques are necessary for the quantification of NP–cell interactions to allow comparison and integration of data and push forward the smart development of NPs.
Here we highlight the most prominent nanoscopy techniques and discuss the features that overcome the limitations of standard methods. We briefly emphasize on how they can be used for quantification of endosomal escape, and we provide a short perspective on how these techniques can help us gain more insight into the process of NP endosomal escape, leading to the development of more effective formulations.
2. Discussion
As previously highlighted, endosomal escape is a process that is fast and rare and occurs at the nanoscale. Here we briefly discuss the pros and cons of various nanoscopy techniques that can be used to quantitatively study this process. For a summary of the techniques discussed see Table 1, and for extra information on how quantification can be achieved using these techniques, see Table 2. We put emphasis on the power of electron microscopy (EM), super-resolution microscopy (SRM) and correlative imaging to answer sought-after questions regarding NP endosomal escape, and ultimately on improving the development of NPs with efficient therapeutic cytosolic delivery.
Comparison of the selected characteristics of the nanoscopy techniques and confocal microscopy discussed, where bold indicates the best and italics the worst in each category.
| Technique | Resolution XY | Resolution Z | Live-cell imaging | Multi-colour | Temporal resolution | Quantification | Overall simplicity of technique | References |
|---|---|---|---|---|---|---|---|---|
| Confocal microscopy | ∼200 nm | ∼400 nm | Yes | Yes (3 colours) | ms–s | Worst | Simple | 30,54,56,66 and 144–149 |
| EM | ∼1 nm | NA | No | No | ms | Good | Complex | 18,30,58,60–62,64,66–74,76–78 and 150–153 |
| SMLM | ∼20 nm | ∼80 nm | Noa | Yes (2–124 colours) | min | Good | Complex | 81–85 |
| STED | ∼50 nm | ∼150 nm | Yes | Yes (3 colours) | s | Good | Simple | 98–101 |
| SIM | ∼100 nm | ∼300 nm | Yes | Yes (3 colours) | ms–s | Bad | Simple | 107 and 112–115 |
| CLEM | ∼1 nm (EM) | Dependent on LM technique | No | Dependent on LM technique | Dependent on LM technique | Best | Very complex | 68,72 and 141–143 |
SMLM does not allow live cell imaging in most cases, but there are few examples.87,89
Overview of different types of quantification methods and how these can be achieved using nanoscopy and confocal microscopy, including information on throughput and disadvantages of the methods.
| Technique | Quantification | Quantification process | Throughput | Disadvantages |
|---|---|---|---|---|
| aConfocal | Co-localization | NP endosomal vesicles are tagged with different fluorophores and average fluorescence intensity is calculated (e.g. using ImageJ – Color2/JACoP). Co-localization calculated using Mander's/Pearson's correlation coefficients56,148 | Fairly good throughput – (i.e. a few cells, tens of lysosomes and tens of NP clusters) | -Limited resolution |
| -Cannot resolve individual NPs | ||||
| -Localization precision is affected by resolution | ||||
| Particle tracking | The total number of particles and endosome co-localized NPs is tracked and counted with particle tracking software. As NPs cannot be individually detected, particle events are calculated instead, whereby a single NP event likely corresponds to one vesicle containing NPs75,154 | -Invisible particles (i.e. due to bleaching of fluorophores or de-coupling of fluorescent dyes) | ||
| -Number of particles is underestimated | ||||
| -Choice of fluorophore can influence results (e.g. pH sensitive dyes can have reduced signals in acidic vesicles) | ||||
| -Fluorescence must be quantified in relation to a control to account for fluorophore instability | ||||
| EM | Direct visualization and quantification | The ratio between NPs found in the cytosol and endosomes is calculated148,155,156 | Low throughput – (i.e. one cell, tens of NPs and a few endosomes per field of view) | -Complicated sample preparation |
| Can distinguish between intracellular/extracellular/intramembranous nanoparticles | -Generally, samples are fixed and sectioned (i.e. no living cells) | |||
| Serial sectioning or electron tomography | Imaging in 3D of sequential sample sections. Location, size, and the number of vesicles as well as NPs can be calculated in whole 3D cells74,156 | -Difficulty in distinguishing different intracellular vesicles | ||
| Stereological image analysis | Using the relative particle distribution within cells (RDI). Tests if NPs are localizing randomly or specifically within cellular compartments. The particle density of each compartment is calculated by relating the number of particle events in the specific compartment to the fractional volume of the compartment157 | -Particles must be smaller than the section thickness (∼150 nm) | ||
| Correlating the total number of intracellular particles of a sample with the total cell number of that sample. Using the fractionator principle54,158 | -Quantification from 3D reconstructions is difficult | |||
| The density of intracellular particles is multiplied by the average cell volume to calculate the average number of NPs per cell75 | -Restricted to samples with adequate atomic contrast | |||
| SMLM | Spatial analysis and clustering | Single-molecule localization microscopy techniques produce point cloud data as a result of multiple localizations in time. These data can be analyzed to identify objects, and determine densities or spatial correlations93 | Intermediate – low throughput. The field of view may vary from one to few cells. The imaging time would greatly depend on the specific technique used (seconds to minutes) | -In some cases, there are undesired non-specific interactions or background noise |
| Molecule counting | Single-molecule localization microscopy techniques are based on the identification of individual molecules. Therefore, it is possible to quantify the exact number of molecules on a specific area.159,160 For example, the ligands or proteins on the surface of a nanoparticle161,162 | -High amount of data that can make the quantification process slow | ||
| Stability of NPs and vesicles | The increased resolution and precise molecule counting of super-resolution microscopy allow the determination of the stability of small objects such as nanoparticles and vesicles. It is possible to establish their shape96 and observe the degradation in time99 | |||
| STED/SIM | Size and shape of NPs | The improved resolution of these techniques allows the measurements of the size and shape of smaller objects compared to confocal microscopy109,113,114 | Good throughput – imaging times in the millisecond-second range | -No single-molecule quantification |
| Co-localization | Standard colocalization coefficient calculations are also applied for these techniques, although better resolution yields more precise results109 | |||
| CLEM | Combination of FM and EM techniques | Generally, fluorescence microscopy is carried out prior to EM. Images can be manually aligned using plugins such as eC-CLEM. Quantification can be achieved either via EM or FM, or both.74,163 Detection of ‘invisible particles’ in light microscopy is possible with CLEM, as well as compartment-specific quantification | Low throughput – still limited by EM | -Complex and time-consuming sample preparation |
| -NPs must be detectable using both light and electron microscopes | ||||
| -Alignment mismatch can affect correlation |
Quantification is not absolute (not at a single particle level) due to the resolution of the microscope.
2.1. Electron microscopy and cryo-electron microscopy
With a near atomic resolution,57 EM is an irreplaceable tool in studying the physio-chemical properties of NPs and quantifying their voyage through the endo-lysosomal pathway.30,37,58–74 EM can even detect a low number (few hundreds) of single nanoparticles escaping endosomal structures, and since it is a label-free method, it will localise and quantify NPs generally untraceable by standard light microscopy methods. TEM was demonstrated to quantify approximately 150-times more NP/cell compared to NP events/cell using a confocal laser scanning microscope.75 EM allows direct visualisation and quantification of NPs and endosomal compartments and can distinguish between intracellular/extracellular/intramembranous NPs, both in 2D and 3D (Table 2).
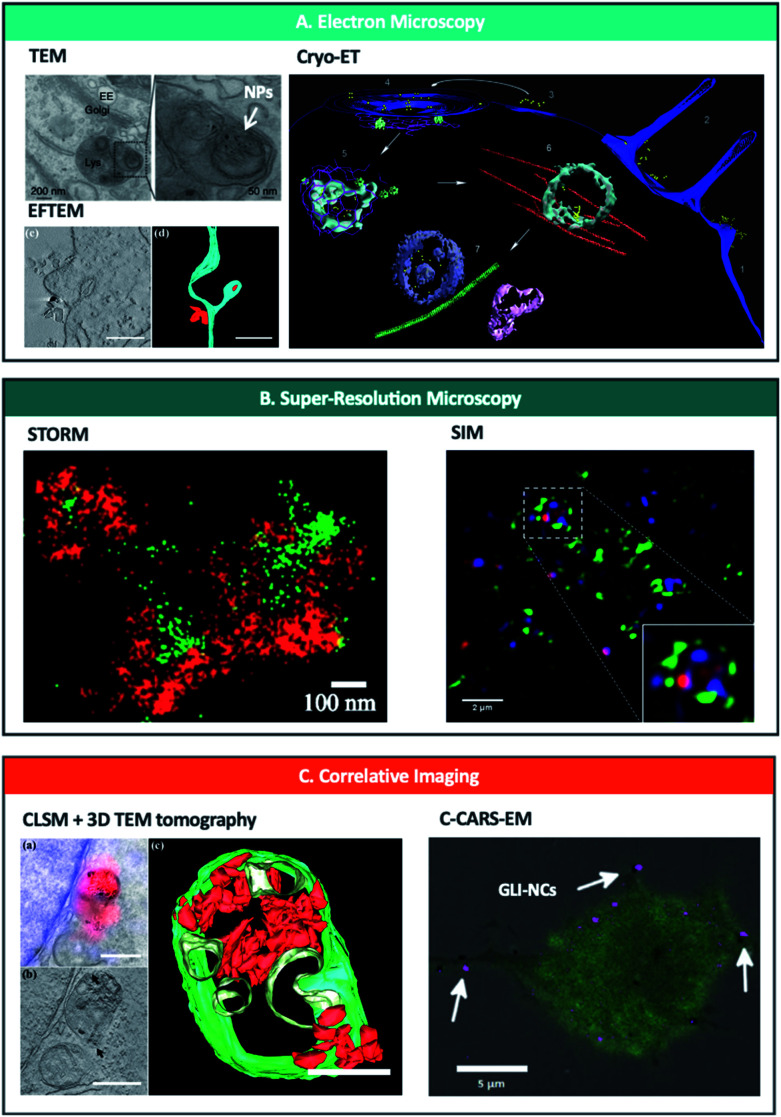
As a pioneering example, Gilleron et al.37 developed one of the most promising semi-automatic approaches using TEM, quantifying the amount of siRNA-conjugated colloidal gold NPs escaping from various endo-lysosomal compartments (Fig. 2A, top left). The authors developed a gold detection software that automatically detects and quantifies the total number of gold NPs in each image, based on the threshold intensity of gold. Using this, they quantified the ratio of siRNA-gold within the endosomes and in the cytosol to calculate endosomal escape and found that only <2% of siRNA-gold escaped the endosomes in HeLa cells. Furthermore, using distinct mathematical models in combination with a pharmacological blockade of endosomal progression, they observed that release occurs mainly from the early endosome. Additionally, developments in staining methods such as photoconversion of diaminobenzene (DAB) – that allows the conversion of a fluorescent dye into an electron-dense signal – in combination with immunoelectron microscopy demonstrate that EM can be used to examine the interactions of NPs with cellular organelles and to detect if they are intact or degraded after endo-lysosomal breakdown.76,77
Fig. 2. Nanoscopy techniques used to study and/or quantify endo-lysosomal trafficking of nanoparticles. (A) Electron microscopy techniques including TEM (upper left),37 EFTEM (lower left)74 and Cryo-ET (right)69 can be used to track and quantify nanoparticles in intracellular vesicles. Reprinted (adapted) with permission from ref. 37 Copyright © 2013 Nature America, Inc., from ref. 74 © 2019 American Chemical Society and from ref. 69 Copyright © Azubel et al. eLife. (B) Super-resolution microscopy has been used to image nanoparticles bursting out of endosomes with STORM (left)96 and SIM (right).117 Reprinted (adapted) with permission from ref. 96 and 117 Copyright 2018 American Chemical Society. (C) Correlative imaging combines different microscopic techniques such as CLSM and 3D TEM tomography (left)74 or C-CARS and EM (right)70 and offers spatiotemporal localization of labelled NPs and biomolecules with high specificity and sensitivity at a highly subcellular level; quantification possible with CLSM + 3D TEM tomography. Reprinted (adapted) with permission from ref. 74 Copyright © 2019 American Chemical Society and from ref. 70 Copyright © 2018 The Authors. Biotechnology Journal Published by Wiley-VCH Verlag GmbH & Co. KGaA.
One of the limitations of conventional EM is that the image acquired corresponds to a distorted, dehydrated form of the natural specimen, due to the need for drying, staining or plastic embedding the sample. Using cryo-EM, the specimen exists in a near-native frozen-hydrated state, maintaining the structures of interest as they would be in solution.78,79 However, to date, the only paper exploiting cryo-EM to study the trafficking of NPs within the endosomal pathway (albeit indirectly) is by Azubel et al.69 who employed cryo-electron tomography (cryo-ET) to study the endosomal trafficking of fibroblast growth factor 21 tagged to gold NPs (AuNP-FGF21) (Fig. 2A, right). By using 3D tomographic reconstruction, they were able to unequivocally identify gold NPs inside/outside various cellular structures including endosomes. Although the authors did not focus on quantifying the gold NPs, this technique has great potential to quantify the endosomal escape of various inorganic NPs, as well as that of different proteinaceous ligands/protein-based cargo at a single-particle level and with great localisation precision.
Indisputably, EM is an irreplaceable asset in the tracking and quantification of NP endosomal escape. However, it can only be used on fixed or frozen samples and it is inappropriate for studying dynamic changes. Cellular samples must be cut into <200 nm thin sections and exposed to various staining and washing steps that can lead to the loss of NPs.80 Also, at the expense of high resolution, only a small field of view (a few endosomes and a few tens of NPs) can be analysed at one time, making this a low-throughput technique. Lastly, EM has reduced molecular specificity, thus making it difficult to distinguish between different types of vesicles within the endosomal pathway.60
2.2. Super-resolution microscopy or optical nanoscopy
In the history of light microscopy, better lenses were used to improve resolution by focusing more light onto the sample, such as the pinhole in confocal microscopy.81 However, Abbe's diffraction's law82 determines that the ultimate resolution of any light microscope is limited to 200–350 nm due to light diffraction. The advent of SRM83 allows overcoming this limitation combining the advantages of fluorescence microscopy with nanometric resolutions. The specific labelling of proteins, multicolour ability and live-cell imaging at subcellular resolutions transformed this method into a new powerful tool to study endosome escaping.
2.2.1. Single-molecule localization microscopy (STORM, PALM and PAINT)
Single-molecule localization microscopy (SMLM) is a group of fluorescence SRM techniques based on the localization of single molecules with resolutions down to tens of nanometres. It was in 2006 when SMLM was introduced bringing in the idea of stochastically having only a sparse subset of the fluorophores ‘on’ at a time and repeating the process until the whole sample is analysed.84–88 By superimposing those sparse single-molecule images we can reconstruct the initial image at a higher resolution. The difference between the various techniques relies on how they cause the fluorophores to switch between ‘on’ and ‘off’ states. Stochastic optical reconstruction microscopy (STORM)86,89 and photoactivated localization microscopy (PALM)85,87 are based on the photoswitching and photoactivation of organic dyes and fluorescent proteins respectively, and meanwhile point accumulation for imaging in nanoscale topography (PAINT)88,90–92 is based on the binding and unbinding of free diffusing fluorescent labelled probes to the target molecule.
The main advantages of these techniques are that they have an excellent resolution (5–25 nm) to visualize NPs and intracellular vesicles, and they can offer a powerful quantitative tool with single-molecule precision,93i.e. molecular counting. Moreover, they offer multicolour imaging, bringing in the possibility of labelling multiple subcellular structures as well as delivery carriers at the same time. In particular PAINT, by multiple rounds of imaging with different target probes91,94 or by kinetic fingerprinting the binding interaction, has recently achieved 124 colour super-resolution imaging.95 The main disadvantage of SMLM techniques is that they also require long imaging times to reconstruct the final image (few minutes to an hour), making them generally not suitable for live cell imaging.
Recently, STORM has been applied to observe endosomal escape of siRNA polyplexes96 (Fig. 2B). In this study, they imaged polyplexes carrying siRNA in early and late endosomes with 2-colour STORM to directly visualize the rupture of endosomes and the release of polyplexes. They first measured the size of polyplexes in biological environments from 2D STORM images. Then, they observed the shape of individual endosomes and polyplexes inside cells to establish how the endosomal escape process was occurring. Finally, they combined 2-colour STORM images to determine the level of colocalization of polyplexes and endosomes by counting individual polyplexes. In fact, STORM has also been used recently to study the trafficking and stability of NPs in cells.97–99 This and other techniques have shown the capability to image in 3D at the nanometric-scale resolution subcellular structures, such as endosomes and lysosomes, opening the door to a deeper understanding of endosomal escape.100
2.2.2. Stimulated emission depletion (STED)
Stimulated emission depletion (STED) is a SRM technique initially proposed by Stefan W. Hell in the 90s101 and firstly applied on biological samples in 2000.102 It works by shrinking the excitation laser beam using a second doughnut-shape laser. This second beam depletes fluorescence and as a result only fluorescence from the centre of the doughnut is collected. The main advantage of STED is that it offers diffraction unlimited resolution at imaging speeds similar to a confocal – time resolution of seconds – as well as 3D and tissue imaging, as recently demonstrated by the imaging of NP internalization in 3D103 and the crossing of the blood–brain barrier in brain tissue samples.104 However, to effectively deplete fluorophore emission with a circular shaped beam, it requires a high intensity laser that may cause photodamage to cells,105 although live-cell imaging can be carried out to some extent.106
STED nanoscopy has been applied in internalization and trafficking of NPs.107–109 Specifically, Li Shang and co-workers investigated the internalization of transferrin NPs and measured the size of NP-loaded early endosomes with STED in live cells to conclude that particles were clustered inside the vesicles.109 STED has not been used to date to study endosomal escape of NPs; however, due to the multiple advantages of this technique, we can foresee the potential of STED to contribute to this field.
2.2.3. Structured illumination microscopy (SIM)
Structured illumination microscopy (SIM) is a SRM technique based on the Moiré effect, in which the sample is illuminated with a known pattern in different orientations and the resulting images can be deconvoluted into a higher resolution image.110 SIM can achieve a resolution half of Abbe's theoretical limit, around 100–150 nm, as well as a fast imaging speed – below 1 second – and low light exposure to the sample compared to other SRM methods. This makes it the ideal SRM method for live-cell imaging.
SIM has had a great impact in studying cell–NP interactions due to its fast imaging speed, live-cell capabilities, and low restrictions on fluorophore selection. It has been applied to investigate NP internalization,111 and trafficking112 – as well as shape113 and degradation114 inside cells – and subcellular dynamic processes at few milliseconds time resolution.115,116 Focusing on endosomal escape, SIM has been recently used to image the rupture of endosomes and the delivery of siRNA into the cytoplasm in breast cancer cells117 (Fig. 2B). Moreover, SIM has revealed that PEI polyplexes are found close to the internal side of the membrane of lysosomes/late endosomes, rather than at a central position in the vesicle.118 These findings prove the potential of SIM to investigate endosome–NP interactions in live cells, where an intermediate resolution is sufficient.
2.3. Frontiers in fluorescence micro/nanoscopy
The field of microscopy is constantly evolving and releasing new tools to tackle the challenges at the micro and nanoscopic scales. Recent developments have proven to be powerful techniques to study NPs in the biological environment offering better resolution and live-cell imaging features. Specifically, AiryScan119 and RESOLFT120 came into play to reduce photobleaching in confocal and STED microscopy respectively, for improved live-cell imaging, dynamic studies and higher throughputs. Moreover, recently developed MINFLUX (minimum photon fluxes) has achieved an outstanding resolution of 1–3 nm with low laser exposure in 3 dimensions.121,122
2.4. Dynamic imaging
Endosomal trafficking and escape of NPs not only occur at the nanoscale, but are also a dynamic process. Some microscopy techniques can be combined with other tools to further investigate dynamic processes, such as fluorescence resonance energy transfer (FRET),123–125 fluorescence correlation spectroscopy (FCS)126,127 and single-particle tracking (SPT).128–132 Interestingly, SPT has been extensively used to study the co-localization and quantification of NPs within endocytic vesicles.128,133–139 For instance, Zahid et al.133 used live-cell SPT in combination with multidimensional analysis to characterize the intracellular distributions of quantum dot (QD) properties and to quantify their endosomal escape. The knowledge provided by SPT data analysis – especially when combined with other techniques – can be used to understand the underlying biological mechanisms of what discriminates formulations that achieve endosomal escape from those that cannot.
2.5. Correlative imaging
Various papers report the endosomal escape of NPs using several independent microscopic techniques.30,58,68,140,141 However, a correlative approach is more desirable, as it bridges the advantages of two distinct techniques by imaging the same region of interest and overlapping important information from the two methods. Despite a much greater image interpretation confidence – that would not be possible with either of the methods individually142 – there is a very low number of publications in the area. This is probably related to the complex and cumbersome procedures required for sample handling and image aligning.143
Correlative light and electron microscopy (CLEM) are perhaps the most explored group of correlated techniques. This combination allows spatiotemporal localization of labelled biomolecules with high specificity and sensitivity (FM), and with (sub)-nanometer resolution and precise subcellular localization of NPs within the cell (EM). In practice, quantification precision can be greatly improved using CLEM, as ‘invisible particles’ (i.e. not labelled with a fluorescent dye/not electron dense enough) can be detected. Also, since it can be difficult to distinguish different endosomal compartments based just on the TEM morphology, correlation with fluorescently labelled compartments in light microscopy can also improve NP localization precision.
To date, the only CLEM approach used to quantitatively study endo-lysosomal tracking of NPs has been developed by Han et al.74 (Fig. 2c, left). Using confocal laser scanning microscopy (CLSM) and 3D TEM tomography, they were able to demonstrate the localization of fNDs within endosomes, lysosomes and autophagosomes. Using the high-resolution TEM tomography results, they precisely quantified single fNDs found in clusters within the endosomal vesicles. However, quantification of single fNDs (not within clusters) was only possible at the single-particle level by using EFTEM (energy filtered TEM) as an additional method (Fig. 2A, bottom left). Furthermore, EFTEM permitted autonomous TEM screening of the whole sample, demonstrating the potential of this technique to precisely identify and quantify intracellular NPs.
Haruta et al.144 used the local surface plasmon resonance (LSPR) of gold NPs as a tag for biological samples in CLEM. To alleviate the problem of the resolution mismatch of several orders of magnitude between the two techniques, EM has also been correlated with SRM. Fluorescent nanodiamonds (FNDs) have been studied at nanometer resolution using STED-TEM145 and integrated light and scanning EM.146 However, in these examples NPs have been used for correlative purposes rather than to quantify or answer specific questions regarding intracellular trafficking. SRM-EM in fact offers a powerful tool to quantify and track endosomal escape and research in this area would benefit greatly the nanomedicine community.
A more distinct approach was achieved by Saarinen et al.70 who used correlative coherent anti-Stokes Raman scattering and TEM (C-CARS-EM) to image glibenclamide-nanocrystals (GLI-NCs) in macrophages (Fig. 2c, right). The combination of a label-free and chemically specific C-CARS technique with the excellent resolution and precision of TEM, allowed precise localization of GLI-NCs within endosomal vesicles. Although not achieved in this work, this technique also has the potential to be quantitative. For example, using 3D information from C-CARS together with precise localization of nanocrystals from EM, one can calculate the ratio between NPs found in the cytosol and in the endosomes (endosomal escape).
3. Conclusions and perspectives
Whilst significant progress has been made on developing a rich formulation databank of NPs for cytosolic delivery,16,17 our understanding of the physiochemical and biological requisites for achieving endosomal escape has been hampered.20,26–31 Our grasp of these mechanisms is hampered by the limitations of the standard techniques used to localise and quantify them. As discussed in this review, nanoscopy techniques – independently, or in correlation – hold the promise of answering some essential questions regarding endosomal escape. Some of these questions include how and which physiochemical properties of NPs influence endosomal escape? Which of the proposed endosomal escape mechanisms stand true and how can we improve the formulation of NPs to exploit them? Can we relate endosomal escape to the time and location at which it occurs intracellularly?
Here we have highlighted the relevance of nanoscopy and some of the most recent discoveries in endosomal escape possible only using these methods. With a plethora of advanced microscopic techniques available, it is essential that we weigh the pros and cons of each technique to best suit the scientific question proposed (Tables 1 and 2). For a process such as endosomal escape – that is fast and rare and occurs at the nanoscale – it may seem challenging to answer the various questions projected using individual methods. But as we have seen in this review, we are no longer restricted to a ‘one method at a time’ approach.70,74,142,144–146 The benefits of correlative imaging – especially of SRM-EM – are of tremendous relevance in obtaining quantitative information on NP endosomal trafficking. Furthermore, as the amount of imaging data is increasing, automated quantification is becoming crucial in reducing manual analysis of images (and increasing throughput) and extracting more valuable data found in microscopic images, as well as making these techniques available to a broader research community.
Overall, these new developments in the field of imaging will lead to exciting times ahead for the study of endosomal escape. We prompt the nanomedicine community to adopt the newest techniques available to achieve a better understanding of NP trafficking as well as to facilitate the rational design of NPs with the ability to overcome endosomal barriers.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The project that gave rise to this work received the support of a fellowship from “la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/DI18/11660039. This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 713673.
Biographies
Biography
Teodora Andrian.

Teodora qualified with a Master of Pharmacy (MPharm) from King's College London (KCL) in 2016. During this period, she also worked with AstraZeneca on the validation of an ADMET predictor. In 2016 she did her MPharm research project at Utrecht University (The Netherlands) working with thermosensitive liposomes. She completed her pre-registration training and qualified as a pharmacist (UK). In 2017 worked as a research assistant at KCL on albumin nanoparticles for delivery to the lungs. In 2018 she started her La Caixa Foundation INPhINIT PhD Fellowship at IBEC, working on combining super-resolution microscopy and electron microscopy to study nanoparticles.
Biography
Roger Riera.

Roger obtained his BSc in Biotechnology at the University of Barcelona in 2015, completing the final project at the University of Stuttgart. In 2017, he obtained his MSc in Bioengineering at Institut Químic de Sarrià (IQS). He joined the Nanoscopy for Nanomedicine group in IBEC for his MSc project on STORM imaging of polyplexes and then moved to TU/e in 2018 where he started his PhD. His research aim is to quantitatively study the interaction of polyplexes with cells and how they are transported inside with super resolution microscopy for immunotherapy applications.
Biography
Silvia Pujals.

Dr Pujals obtained her BSc in Chemistry and IQS Chemical Engineer degree at Institut Químic de Sarrià (Universitat Ramon Llull). Her MSc and PhD in Organic Chemistry were from Universitat de Barcelona (UB) in the field of cell-penetrating peptides. She then moved to Kyoto University for her postdoc and focused on biophysics and electron microscopy. With expertise in drug delivery, peptide synthesis and optical and electron microscopy her research aims to combine a rational design of nanomaterials with advanced optical techniques for targeted drug delivery.
Biography
Lorenzo Albertazzi.

Dr Albertazzi obtained his PhD in Biophysics (2011) from Scuola Normale Superiore (Pisa, Italy). He then joined the Eindhoven University of Technology (TU/e, The Netherlands) as a postdoctoral researcher. In 2013, he was awarded a fellowship at the Institute for Complex Molecular Systems (ICMS) at TU/e, and in 2014 he became a NWO/VENI fellow. In 2015, he moved to IBEC to start the ‘Nanoscopy for Nanomedicine’ group. In 2018 he was appointed Associate Professor at the TU/e department of Biomedical Engineering. He is aiming to achieve a molecular understanding of synthetic materials in the biological environment, using optical microscopy and nanoscopy.
References
- Tran S. DeGiovanni P.-J. Piel B. Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin. Transl. Med. 2017;6(1):44. doi: 10.1186/s40169-017-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Tan S. Li S. Shen Q. Wang K. Cancer drug delivery in the nano era: an overview and perspectives. Oncol. Rep. 2017;38(2):611–624. doi: 10.3892/or.2017.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018;9:16. doi: 10.3389/fimmu.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafisco M. Alogna A. Miragoli M. Catalucci D. Cardiovascular nanomedicine: the route ahead. Nanomedicine. 2019;14(18):2391–2394. doi: 10.2217/nnm-2019-0228. [DOI] [PubMed] [Google Scholar]
- Martín Giménez V. M. Kassuha D. E. Manucha W. Nanomedicine applied to cardiovascular diseases: latest developments. Ther. Adv. Cardiovasc. Dis. 2017;11(4):133–142. doi: 10.1177/1753944717692293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerkova K. Dolezelikova K. Bozdechova L. Heger Z. Zurek L. Adam V. Nanomaterials with active targeting as advanced antimicrobials. WIRES Nanomed. Nanobi. 2020:e1636. doi: 10.1002/wnan.1636. [DOI] [PubMed] [Google Scholar]; , available from https://onlinelibrary.wiley.com/doi/abs/10.1002/wnan.1636
- El-Readi M. Z. Althubiti M. A. Cancer Nanomedicine: A New Era of Successful Targeted Therapy. J. Nanomater. 2019;2019:1–13. doi: 10.1155/2019/4927312. [DOI] [Google Scholar]
- Wang Y. Yang P. Zhao X. Gao D. Sun N. Tian Z. et al., Multifunctional Cargo-Free Nanomedicine for Cancer Therapy. Int. J. Mol. Sci. 2018;19(10):2963. doi: 10.3390/ijms19102963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S. Matoba T. Koga J. Nakano K. Egashira K. Anti-inflammatory Nanomedicine for Cardiovascular Disease. Front. Cardiovasc. Med. 2017;4:87. doi: 10.3389/fcvm.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J. K. Das G. Fraceto L. F. Campos E. V. R. Rodriguez-Torres M. del P. Acosta-Torres L. S. et al., Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M. Mirshekari H. Aliakbari M. Sahandi-Zangabad P. Hamblin M. R. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnol. Rev. 2016;5(2):195–207. [Google Scholar]; , available from https://www.degruyter.com/view/j/ntrev.2016.5.issue-2/ntrev-2015-0057/ntrev-2015-0057.xml
- Caster J. M. Patel A. N. Zhang T. Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials: investigational nanomedicines in 2016. WIRES Nanomed. Nanobi. 2017;9(1):e1416. doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- Havel H. Finch G. Strode P. Wolfgang M. Zale S. Bobe I. et al., Nanomedicines: From Bench to Bedside and Beyond. AAPS J. 2016;18(6):1373–1378. doi: 10.1208/s12248-016-9961-7. [DOI] [PubMed] [Google Scholar]
- Eskandari Z. Bahadori F. Celik B. Onyuksel H. Targeted Nanomedicines for Cancer Therapy, From Basics to Clinical Trials. J. Pharm. Pharm. Sci. 2020;23:132–157. doi: 10.18433/jpps30583. [DOI] [PubMed] [Google Scholar]
- Garbayo E. Pascual-Gil S. Rodríguez-Nogales C. Saludas L. Estella-Hermoso de Mendoza A. Blanco-Prieto M. J. Nanomedicine and drug delivery systems in cancer and regenerative medicine. WIRES Nanomed. Nanobi. 2020:e1367. doi: 10.1002/wnan.1637. [DOI] [PubMed] [Google Scholar]; , available from https://onlinelibrary.wiley.com/doi/abs/10.1002/wnan.1637
- Ventola C. L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. 2017;42(12):742–755. [PMC free article] [PubMed] [Google Scholar]
- Farjadian F. Ghasemi A. Gohari O. Roointan A. Karimi M. Hamblin M. R. Nanopharmaceuticalsand nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2019;14(1):93–126. doi: 10.2217/nnm-2018-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. Kim J. Herrera M. Mukherjee A. Kabanov A. V. Sahay G. Brief update on endocytosis of nanomedicines. Adv. Drug Delivery Rev. 2019;144:90–111. doi: 10.1016/j.addr.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degors I. M. S. Wang C. Rehman Z. U. Zuhorn I. S. Carriers Break Barriers in Drug Delivery: Endocytosis and Endosomal Escape of Gene Delivery Vectors. Acc. Chem. Res. 2019;52(7):1750–1760. doi: 10.1021/acs.accounts.9b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby L. I. Cortez-Jugo C. M. Such G. K. Johnston A. P. R. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles: endosomal escape of polymeric nanoparticles. WIRES Nanomed. Nanobi. 2017;9(5):e1452. doi: 10.1002/wnan.1452. [DOI] [PubMed] [Google Scholar]
- Wilhelm S. Tavares A. J. Dai Q. Ohta S. Audet J. Dvorak H. F. et al., Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1(5):16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- Rosenblum D. Joshi N. Tao W. Karp J. M. Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9(1):1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis S. Kumarasinghe E. S. Salerno T. Mihai C. Ketova T. Senn J. J. et al., A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018;26(6):1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A. Selby L. I. Johnston A. P. R. Such G. K. The Endosomal Escape of Nanoparticles: Towards More Efficient Cellular Delivery. Bioconjugate Chem. 2018;30(2):263–272. doi: 10.1021/acs.bioconjchem.8b00732. [DOI] [PubMed] [Google Scholar]
- Stewart M. P. Lorenz A. Dahlman J. Sahay G. Challenges in carrier-mediated intracellular delivery: moving beyond endosomal barriers: challenges in carrier-mediated intracellular delivery. WIRES Nanomed. Nanobi. 2016;8(3):465–478. doi: 10.1002/wnan.1377. [DOI] [PubMed] [Google Scholar]
- Durymanov M. Reineke J. Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front. Pharmacol. 2018;9:971. doi: 10.3389/fphar.2018.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D. Enhancing endosomal escape for nanoparticle mediated siRNA delivery. Nanoscale. 2014;6(12):6415. doi: 10.1039/C4NR00018H. [DOI] [PubMed] [Google Scholar]
- Kanasty R. Dorkin J. R. Vegas A. Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- Yang Y. Wang Z. Peng Y. Ding J. Zhou W. A Smart pH-Sensitive Delivery System for Enhanced Anticancer Efficacy via Paclitaxel Endosomal Escape. Front. Pharmacol. 2019;10:10. doi: 10.3389/fphar.2019.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-W. Luther D. C. Kretzmann J. A. Burden A. Jeon T. Zhai S. et al., Protein Delivery into the Cell Cytosol using Non-Viral Nanocarriers. Theranostics. 2019;9(11):3280–3292. doi: 10.7150/thno.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella C. Klok H.-A. Controlling and Monitoring Intracellular Delivery of Anticancer Polymer Nanomedicines. Macromol. Biosci. 2017;17(10):1700022. doi: 10.1002/mabi.201700022. [DOI] [PubMed] [Google Scholar]
- Bus T. Traeger A. Schubert U. S. The great escape: how cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B. 2018;6(43):6904–6918. doi: 10.1039/C8TB00967H. [DOI] [PubMed] [Google Scholar]
- Ahmad A. Khan J. M. Haque S. Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie. 2019;160:61–75. doi: 10.1016/j.biochi.2019.02.012. [DOI] [PubMed] [Google Scholar]
- Pei D. Buyanova M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjugate Chem. 2019;30(2):273–283. doi: 10.1021/acs.bioconjchem.8b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens T. F. Remaut K. Demeester J. De Smedt S. C. Braeckmans K. Intracellular delivery of nanomaterials: how to catch endosomal escape in the act. Nano Today. 2014;9(3):344–364. doi: 10.1016/j.nantod.2014.04.011. [DOI] [Google Scholar]
- Shete H. K. Prabhu R. H. Patravale V. B. Endosomal Escape: A Bottleneck in Intracellular Delivery. J. Nanosci. Nanotechnol. 2014;14(1):460–474. doi: 10.1166/jnn.2014.9082. [DOI] [PubMed] [Google Scholar]
- Gilleron J. Querbes W. Zeigerer A. Borodovsky A. Marsico G. Schubert U. et al., Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013;31(7):638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- Hou K. K. Pan H. Schlesinger P. H. Wickline S. A. A role for peptides in overcoming endosomal entrapment in siRNA delivery — A focus on melittin. Biotechnol. Adv. 2015;33(6):931–940. doi: 10.1016/j.biotechadv.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. Lee R. J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Delivery Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- Tai W. Gao X. Functional peptides for siRNA delivery. Adv. Drug Delivery Rev. 2017;110–111:157–168. doi: 10.1016/j.addr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Tam Y. Y. C. Chen S. Zaifman J. van der Meel R. Ciufolini M. A. et al., The Niemann-Pick C1 Inhibitor NP3.47 Enhances Gene Silencing Potency of Lipid Nanoparticles Containing siRNA. Mol. Ther. 2016;24(12):2100–2108. doi: 10.1038/mt.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Djemaa S. David S. Hervé-Aubert K. Falanga A. Galdiero S. Allard-Vannier E. et al., Formulation and in vitro evaluation of a siRNA delivery nanosystem decorated with gH625 peptide for triple negative breast cancer theranosis. Eur. J. Pharm. Biopharm. 2018;131:99–108. doi: 10.1016/j.ejpb.2018.07.024. [DOI] [PubMed] [Google Scholar]
- dos Santos Rodrigues B. Banerjee A. Kanekiyo T. Singh J. Functionalized liposomal nanoparticles for efficient gene delivery system to neuronal cell transfection. Int. J. Pharm. 2019;566:717–730. doi: 10.1016/j.ijpharm.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shae D. Becker K. W. Christov P. Yun D. S. Lytton-Jean A. K. R. Sevimli S. et al., Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019;14(3):269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y. T. Ge Z. Zhang B. Xiu P. Li Q. Wang Y. Pore formation induced by nanoparticles binding to a lipid membrane. Nanoscale. 2020;12(14):7902–7913. doi: 10.1039/C9NR10534D. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T. Miki S. Kobayashi S. Haraguchi T. Nakata E. Hirakawa K. et al., The molecular mechanism of photochemical internalization of cell penetrating peptide-cargo-photosensitizer conjugates. Sci. Rep. 2016;5(1):18577. doi: 10.1038/srep18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Jothar L. Beztsinna N. van Nostrum C. F. Hennink W. E. Oliveira S. Selective Cytotoxicity to HER2 Positive Breast Cancer Cells by Saporin-Loaded Nanobody-Targeted Polymeric Nanoparticles in Combination with Photochemical Internalization. Mol. Pharmaceutics. 2019;16(4):1633–1647. doi: 10.1021/acs.molpharmaceut.8b01318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjaminsen R. V. Mattebjerg M. A. Henriksen J. R. Moghimi S. M. Andresen T. L. The Possible “Proton Sponge ” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Mol. Ther. 2013;21(1):149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Z. ur. Hoekstra D. Zuhorn I. S. Mechanism of Polyplex- and Lipoplex-Mediated Delivery of Nucleic Acids: Real-Time Visualization of Transient Membrane Destabilization without Endosomal Lysis. ACS Nano. 2013;7(5):3767–3777. doi: 10.1021/nn3049494. [DOI] [PubMed] [Google Scholar]
- Ngwa V. M. Axford D. S. Healey A. N. Nowak S. J. Chrestensen C. A. McMurry J. L. A versatile cell-penetrating peptide-adaptor system for efficient delivery of molecular cargos to subcellular destinations. PLoS One. 2017;12(5):e0178648. doi: 10.1371/journal.pone.0178648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Whittaker G. R. Fusion of Enveloped Viruses in Endosomes: Virus Fusion in Endosomes. Traffic. 2016;17(6):593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Wang Y. Li Y. Bodemann B. Zhao T. Ma X. et al., A nanobuffer reporter library for fine-scale imaging and perturbation of endocytic organelles. Nat. Commun. 2015;6(1):8524. doi: 10.1038/ncomms9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L. M. P. De Smedt S. C. Remaut K. Braeckmans K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018;129:184–190. doi: 10.1016/j.ejpb.2018.05.034. [DOI] [PubMed] [Google Scholar]
- Elsaesser A. Barnes C. A. McKerr G. Salvati A. Lynch I. Dawson K. A. et al., Quantification of nanoparticle uptake by cells using an unbiased sampling method and electron microscopy. Nanomedicine. 2011;6(7):1189–1198. doi: 10.2217/nnm.11.70. [DOI] [PubMed] [Google Scholar]
- Vermeulen L. M. P. Brans T. De Smedt S. C. Remaut K. Braeckmans K. Methodologies to investigate intracellular barriers for nucleic acid delivery in non-viral gene therapy. Nano Today. 2018;21:74–90. doi: 10.1016/j.nantod.2018.06.007. [DOI] [Google Scholar]
- Méndez-Ardoy A. Lostalé-Seijo I. Montenegro J. Where in the Cell Is our Cargo? Methods Currently Used To Study Intracellular Cytosolic Localisation. ChemBioChem. 2019;20(4):488–498. doi: 10.1002/cbic.201800390. [DOI] [PubMed] [Google Scholar]
- Erni R. Rossell M. D. Kisielowski C. Dahmen U. Atomic-Resolution Imaging with a Sub-50-pm Electron Probe. Phys. Rev. Lett. 2009;102(9):096101. doi: 10.1103/PhysRevLett.102.096101. [DOI] [PubMed] [Google Scholar]
- Guglielmi V. Carton F. Vattemi G. Arpicco S. Stella B. Berlier G. et al., Uptake and intracellular distribution of different types of nanoparticles in primary human myoblasts and myotubes. Int. J. Pharm. 2019;560:347–356. doi: 10.1016/j.ijpharm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Chu Z. Miu K. Lung P. Zhang S. Zhao S. Chang H.-C. et al., Rapid endosomal escape of prickly nanodiamonds: implications for gene delivery. Sci. Rep. 2015;5(1):11661. doi: 10.1038/srep11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-GA I. Manzaneda-González V. Kisovec M. Almendro-Vedia V. Muñoz-Úbeda M. Anderluh G. et al., pH-triggered endosomal escape of pore-forming Listeriolysin O toxin-coated gold nanoparticles. J. Nanobiotechnol. 2019;17(1):108. doi: 10.1186/s12951-019-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. Carton F. Marengo A. Berlier G. Stella B. Arpicco S. et al., Fluorescence and electron microscopy to visualize the intracellular fate of nanoparticles for drug delivery. Eur. J. Histochem. 2016;60(2):2640. doi: 10.4081/ejh.2016.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]; , available from http://ejh.it/index.php/ejh/article/view/2640
- Hondow N. Brown M. R. Starborg T. Monteith A. G. Brydson R. Summers H. D. et al., Quantifying the cellular uptake of semiconductor quantum dot nanoparticles by analytical electron microscopy: quantifying the cellular uptake of semiconductor quantum dot nanoparticles. J. Microsc. 2016;261(2):167–176. doi: 10.1111/jmi.12239. [DOI] [PubMed] [Google Scholar]
- Cabezón I. Manich G. Martín-Venegas R. Camins A. Pelegrí C. Vilaplana J. Trafficking of Gold Nanoparticles Coated with the 8D3 Anti-Transferrin Receptor Antibody at the Mouse Blood–Brain Barrier. Mol. Pharmaceutics. 2015;12(11):4137–4145. doi: 10.1021/acs.molpharmaceut.5b00597. [DOI] [PubMed] [Google Scholar]
- Iacovita C. Florea A. Dudric R. Pall E. Moldovan A. Tetean R. et al., Small versus Large Iron Oxide Magnetic Nanoparticles: Hyperthermia and Cell Uptake Properties. Molecules. 2016;21(10):1357. doi: 10.3390/molecules21101357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N. Liu Y. He M. Niu M. Zhao Y. Zhu Y. et al., Delivery of vincristine sulfate-conjugated gold nanoparticles using liposomes: a light-responsive nanocarrier with enhanced antitumor efficiency. Indian J. Nephrol. 2015:3081. doi: 10.2147/IJN.S79550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilchrist K. V. Evans B. C. Brophy C. M. Duvall C. L. Mechanism of Enhanced Cellular Uptake and Cytosolic Retention of MK2 Inhibitory Peptide Nano-polyplexes. Cell. Mol. Bioeng. 2016;9(3):368–381. doi: 10.1007/s12195-016-0446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraire J. C. Houthaeve G. Liu J. Raes L. Vermeulen L. Stremersch S. et al., Vapor nanobubble is the more reliable photothermal mechanism for inducing endosomal escape of siRNA without disturbing cell homeostasis. J. Controlled Release. 2020;319:262–275. doi: 10.1016/j.jconrel.2019.12.050. [DOI] [PubMed] [Google Scholar]
- Patel S. Ashwanikumar N. Robinson E. Xia Y. Mihai C. Griffith J. P. et al., Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020;11(1):983. doi: 10.1038/s41467-020-14527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azubel M. Carter S. D. Weiszmann J. Zhang J. Jensen G. J. Li Y. et al., FGF21 trafficking in intact human cells revealed by cryo-electron tomography with gold nanoparticles. eLife. 2019;8:e43146. doi: 10.7554/eLife.43146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen J. Gütter F. Lindman M. Agopov M. Fraser-Miller S. J. Scherließ R. et al., Cell-Nanoparticle Interactions at (Sub)–Nanometer Resolution Analyzed by Electron Microscopy and Correlative Coherent Anti-Stokes Raman Scattering. Biotechnol. J. 2019;14(4):1800413. doi: 10.1002/biot.201800413. [DOI] [PubMed] [Google Scholar]
- Cristofolini T. Dalmina M. Sierra J. A. Silva A. H. Pasa A. A. Pittella F. et al., Multifunctional hybrid nanoparticles as magnetic delivery systems for siRNA targeting the HER2 gene in breast cancer cells. Mater. Sci. Eng. C. 2020;109:110555. doi: 10.1016/j.msec.2019.110555. [DOI] [PubMed] [Google Scholar]
- Deshayes S. Konate K. Dussot M. Chavey B. Vaissière A. Van T. N. N. et al., Deciphering the internalization mechanism of WRAP:siRNA nanoparticles. Biochim. Biophys. Acta, Biomembr. 2020;1862(6):183252. doi: 10.1016/j.bbamem.2020.183252. [DOI] [PubMed] [Google Scholar]
- Malatesta M. Transmission electron microscopy for nanomedicine: novel applications for long-established techniques. Eur. J. Histochem. 2016;60(4):2751. doi: 10.4081/ejh.2016.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]; , available from http://www.ejh.it/index.php/ejh/article/view/2751
- Han S. Raabe M. Hodgson L. Mantell J. Verkade P. Lasser T. et al., High-Contrast Imaging of Nanodiamonds in Cells by Energy Filtered and Correlative Light-Electron Microscopy: Toward a Quantitative Nanoparticle-Cell Analysis. Nano Lett. 2019;19(3):2178–2185. doi: 10.1021/acs.nanolett.9b00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothen-Rutishauser B. Kuhn D. A. Ali Z. Gasser M. Amin F. Parak W. J. et al., Quantification of gold nanoparticle cell uptake under controlled biological conditions and adequate resolution. Nanomedicine. 2014;9(5):607–621. doi: 10.2217/nnm.13.24. [DOI] [PubMed] [Google Scholar]
- Grecchi S. Malatesta M. Visualizing endocytotic pathways at transmission electron microscopy via diaminobenzidine photo-oxidation by a fluorescent cell-membrane dye. Eur. J. Histochem. 2014;58(4):2449. doi: 10.4081/ejh.2014.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]; , available from http://ejh.it/index.php/ejh/article/view/2449
- Pellicciari C., Biggiogera M. and Malatesta M., DAB Photo-Oxidation as a Tool for Detecting Low Amounts of Free and Membrane-Bounded Fluorescent Molecules at Transmission Electron Microscopy, 2015, vol. 9 [Google Scholar]
- Stewart P. L. Cryo-electron microscopy and cryo-electron tomography of nanoparticles: cryo-electron microscopy and cryo-electron tomography of nanoparticles. WIRES Nanomed. Nanobi. 2017;9(2):e1417. doi: 10.1002/wnan.1417. [DOI] [PubMed] [Google Scholar]
- Cheng Y. Single-Particle Cryo-EM at Crystallographic Resolution. Cell. 2015;161(3):450–457. doi: 10.1016/j.cell.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut K. Oorschot V. Braeckmans K. Klumperman J. De Smedt S. C. Lysosomal capturing of cytoplasmic injected nanoparticles by autophagy: an additional barrier to non viral gene delivery. J. Controlled Release. 2014;195:29–36. doi: 10.1016/j.jconrel.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Basic Confocal Microscopy, ed. W. G. Jerome and R. L. Price, Cham, Springer International Publishing, 2018, available from http://link.springer.com/10.1007/978-3-319-97454-5 [Google Scholar]
- Abbe E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Archiv für mikroskopische Anatomie. 1873;9(1):413–468. doi: 10.1007/BF02956173. [DOI] [Google Scholar]
- The Nobel Prize in Chemistry, 2014, NobelPrize.org, available from https://www.nobelprize.org/prizes/chemistry/2014/advanced-information/ [Google Scholar]
- Bretschneider S. Eggeling C. Hell S. W. Breaking the Diffraction Barrier in Fluorescence Microscopy by Optical Shelving. Phys. Rev. Lett. 2007;98(21):218103. doi: 10.1103/PhysRevLett.98.218103. [DOI] [PubMed] [Google Scholar]
- Betzig E. Patterson G. H. Sougrat R. Lindwasser O. W. Olenych S. Bonifacino J. S. et al., Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science. 2006;313(5793):1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- Rust M. J. Bates M. Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat. Methods. 2006;3(10):793–796. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S. T. Girirajan T. P. K. Mason M. D. Ultra-High Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys. J. 2006;91(11):4258–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharonov A. Hochstrasser R. M. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. U. S. A. 2006;103(50):18911–18916. doi: 10.1073/pnas.0609643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilemann M. van de Linde S. Schüttpelz M. Kasper R. Seefeldt B. Mukherjee A. et al., Subdiffraction-Resolution Fluorescence Imaging with Conventional Fluorescent Probes. Angew. Chem., Int. Ed. 2008;47(33):6172–6176. doi: 10.1002/anie.200802376. [DOI] [PubMed] [Google Scholar]
- Giannone G. Hosy E. Levet F. Constals A. Schulze K. Sobolevsky A. I. et al., Dynamic Superresolution Imaging of Endogenous Proteins on Living Cells at Ultra-High Density. Biophys. J. 2010;99(4):1303–1310. doi: 10.1016/j.bpj.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann R. Avendaño M. S. Woehrstein J. B. Dai M. Shih W. M. Yin P. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and Exchange-PAINT. Nat. Methods. 2014;11(3):313–318. doi: 10.1038/nmeth.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew M. D. Lee S. F. Ptacin J. L. Lee M. K. Twieg R. J. Shapiro L. et al., Three-dimensional superresolution colocalization of intracellular protein superstructures and the cell surface in live Caulobacter crescentus. Proc. Natl. Acad. Sci. U. S. A. 2011;108(46):E1102–E1110. doi: 10.1073/pnas.1114444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicovich P. R. Owen D. M. Gaus K. Turning single-molecule localization microscopy into a quantitative bioanalytical tool. Nat. Protoc. 2017;12(3):453–460. doi: 10.1038/nprot.2016.166. [DOI] [PubMed] [Google Scholar]
- Agasti S. Wang Y. Schueder F. Sukumar A. Jungmann R. Yin P. DNA-barcoded labeling probes for highly multiplexed Exchange-PAINT imaging. Chem. Sci. 2017;8(4):3080–3091. doi: 10.1039/C6SC05420J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade O. K. Woehrstein J. B. Nickels P. C. Strauss S. Stehr F. Stein J. et al., 124-Color Super-resolution Imaging by Engineering DNA-PAINT Blinking Kinetics. Nano Lett. 2019;19(4):2641–2646. doi: 10.1021/acs.nanolett.9b00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnilowicz M. Glab A. Bertucci A. Caruso F. Cavalieri F. Super-resolution Imaging of Proton Sponge-Triggered Rupture of Endosomes and Cytosolic Release of Small Interfering RNA. ACS Nano. 2019;13(1):187–202. doi: 10.1021/acsnano.8b05151. [DOI] [PubMed] [Google Scholar]
- van der Zwaag D. Vanparijs N. Wijnands S. De Rycke R. De Geest B. G. Albertazzi L. Super Resolution Imaging of Nanoparticles Cellular Uptake and Trafficking. ACS Appl. Mater. Interfaces. 2016;8(10):6391–6399. doi: 10.1021/acsami.6b00811. [DOI] [PubMed] [Google Scholar]
- Feiner-Gracia N. Olea R. A. Fitzner R. El Boujnouni N. van Asbeck A. H. Brock R. et al., Super-resolution Imaging of Structure, Molecular Composition, and Stability of Single Oligonucleotide Polyplexes. Nano Lett. 2019;19(5):2784–2792. doi: 10.1021/acs.nanolett.8b04407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera R. Feiner-Gracia N. Fornaguera C. Cascante A. Borrós S. Albertazzi L. Tracking the DNA complexation state of pBAE polyplexes in cells with super resolution microscopy. Nanoscale. 2019;11(38):17869–17877. doi: 10.1039/C9NR02858G. [DOI] [PubMed] [Google Scholar]
- Kiuchi T. Higuchi M. Takamura A. Maruoka M. Watanabe N. Multitarget super-resolution microscopy with high-density labeling by exchangeable probes. Nat. Methods. 2015;12(8):743–746. doi: 10.1038/nmeth.3466. [DOI] [PubMed] [Google Scholar]
- Hell S. W. Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994;19(11):780–782. doi: 10.1364/OL.19.000780. [DOI] [PubMed] [Google Scholar]
- Klar T. A. Jakobs S. Dyba M. Egner A. Hell S. W. Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. Proc. Natl. Acad. Sci. U. S. A. 2000;97(15):8206–8210. doi: 10.1073/pnas.97.15.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R. Rossi S. Pinelli S. Alinovi R. Sciancalepore C. Delmonte N. et al., In-vivo vascular application via ultra-fast bioprinting for future 5D personalised nanomedicine. Sci. Rep. 2020;10(1):3205. doi: 10.1038/s41598-020-60196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J.-N. Golombek S. K. Baues M. Dasgupta A. Drude N. Rix A. et al., Multimodal and multiscale optical imaging of nanomedicine delivery across the blood-brain barrier upon sonopermeation. Theranostics. 2020;10(4):1948–1959. doi: 10.7150/thno.41161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäldchen S. Lehmann J. Klein T. van de Linde S. Sauer M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 2015;5(1):15348. doi: 10.1038/srep15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian N. Goryaynov A. Lessard M. D. Hooker G. Toomre D. Rothman J. E. et al., Assessing photodamage in live-cell STED microscopy. Nat. Methods. 2018;15(10):755–756. doi: 10.1038/s41592-018-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuschel H., Ruckelshausen T., Cavelius C. and Kraegeloh A., Quantification of Internalized Silica Nanoparticles via STED Microscopy, BioMed Res. Int., Hindawi, 2015, 2015, e961208. available from https://www.hindawi.com/journals/bmri/2015/961208/ [DOI] [PMC free article] [PubMed]
- Hanne J. Falk H. J. Görlitz F. Hoyer P. Engelhardt J. Sahl S. J. et al., STED nanoscopy with fluorescent quantum dots. Nat. Commun. 2015;6(1):7127. doi: 10.1038/ncomms8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L. Gao P. Wang H. Popescu R. Gerthsen D. Nienhaus G. U. Protein-based fluorescent nanoparticles for super-resolution STED imaging of live cells. Chem. Sci. 2017;8(3):2396–2400. doi: 10.1039/C6SC04664A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M. G. L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000;198(2):82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- Chen X. Cui J. Ping Y. Suma T. Cavalieri F. Besford Q. A. et al., Probing cell internalisation mechanics with polymer capsules. Nanoscale. 2016;8(39):17096–17101. doi: 10.1039/C6NR06657G. [DOI] [PubMed] [Google Scholar]
- Teplensky M. H. Fantham M. Li P. Wang T. C. Mehta J. P. Young L. J. et al., Temperature Treatment of Highly Porous Zirconium-Containing Metal–Organic Frameworks Extends Drug Delivery Release. J. Am. Chem. Soc. 2017;139(22):7522–7532. doi: 10.1021/jacs.7b01451. [DOI] [PubMed] [Google Scholar]
- Chen X. Cui J. Sun H. Müllner M. Yan Y. Fung Noi K. et al., Analysing intracellular deformation of polymer capsules using structured illumination microscopy. Nanoscale. 2016;8(23):11924–11931. doi: 10.1039/C6NR02151D. [DOI] [PubMed] [Google Scholar]
- Tolstik E. Osminkina L. A. Matthäus C. Burkhardt M. Tsurikov K. E. Natashina U. A. et al., Studies of silicon nanoparticles uptake and biodegradation in cancer cells by Raman spectroscopy. Nanomedicine. 2016;12(7):1931–1940. doi: 10.1016/j.nano.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Li D. Shao L. Chen B.-C. Zhang X. Zhang M. Moses B. et al., Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science. 2015;349(6251):aab3500. doi: 10.1126/science.aab3500. [DOI] [PMC free article] [PubMed] [Google Scholar]; , available from http://science.sciencemag.org/content/349/6251/aab3500
- Guo Y. Li D. Zhang S. Yang Y. Liu J.-J. Wang X. et al., Visualizing Intracellular Organelle and Cytoskeletal Interactions at Nanoscale Resolution on Millisecond Timescales. Cell. 2018;175(5):1430–1442.e17. doi: 10.1016/j.cell.2018.09.057. [DOI] [PubMed] [Google Scholar]
- Ben Djemaa S. Hervé-Aubert K. Lajoie L. Falanga A. Galdiero S. Nedellec S. et al., gH625 Cell-Penetrating Peptide Promotes the Endosomal Escape of Nanovectorized siRNA in a Triple-Negative Breast Cancer Cell Line. Biomacromolecules. 2019;20(8):3076–3086. doi: 10.1021/acs.biomac.9b00637. [DOI] [PubMed] [Google Scholar]
- Bus T. Englert C. Reifarth M. Borchers P. Hartlieb M. Vollrath A. et al., 3rd generation poly(ethylene imine)s for gene delivery. J. Mater. Chem. B. 2017;5(6):1258–1274. doi: 10.1039/C6TB02592G. [DOI] [PubMed] [Google Scholar]
- Huff J. The Airyscan detector from ZEISS: confocal imaging with improved signal-to-noise ratio and super-resolution. Nat. Methods. 2015;12(12):i–ii. doi: 10.1038/nmeth.f.388. [DOI] [Google Scholar]
- Hofmann M. Eggeling C. Jakobs S. Hell S. W. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl. Acad. Sci. U. S. A. 2005;102(49):17565–17569. doi: 10.1073/pnas.0506010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarotti F. Eilers Y. Gwosch K. C. Gynnå A. H. Westphal V. Stefani F. D. et al., Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science. 2017;355(6325):606–612. doi: 10.1126/science.aak9913. [DOI] [PubMed] [Google Scholar]
- Gwosch K. C. Pape J. K. Balzarotti F. Hoess P. Ellenberg J. Ries J. et al., MINFLUX nanoscopy delivers 3D multicolor nanometer resolution in cells. Nat. Methods. 2020;17(2):217–224. doi: 10.1038/s41592-019-0688-0. [DOI] [PubMed] [Google Scholar]
- Wang Z. Luo M. Mao C. Wei Q. Zhao T. Li Y. et al., A Redox-Activatable Fluorescent Sensor for the High-Throughput Quantification of Cytosolic Delivery of Macromolecules. Angew. Chem. 2017;129(5):1339–1343. doi: 10.1002/ange.201610302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. Wang L. Duval K. Fan J. Zhou S. Chen Z. Dimeric Drug Polymeric Micelles with Acid-Active Tumor Targeting and FRET-Traceable Drug Release. Adv. Mater. 2018;30(3):1705436. doi: 10.1002/adma.201705436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayamajhi S. Marchitto J. Nguyen T. D. T. Marasini R. Celia C. Aryal S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf., B. 2020;188:110804. doi: 10.1016/j.colsurfb.2020.110804. [DOI] [PubMed] [Google Scholar]
- Lanzanò L. Scipioni L. Di Bona M. Bianchini P. Bizzarri R. Cardarelli F. et al., Measurement of nanoscale three-dimensional diffusion in the interior of living cells by STED-FCS. Nat. Commun. 2017;8(1):65. doi: 10.1038/s41467-017-00117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E. Schneider F. Galiani S. Urbančič I. Waithe D. Lagerholm B. C. et al., Measuring nanoscale diffusion dynamics in cellular membranes with super-resolution STED–FCS. Nat. Protoc. 2019;14(4):1054–1083. doi: 10.1038/s41596-019-0127-9. [DOI] [PubMed] [Google Scholar]
- Shin K. Song Y. Goh Y. Lee K. Two-Dimensional and Three-Dimensional Single Particle Tracking of Upconverting Nanoparticles in Living Cells. Int. J. Mol. Sci. 2019;20(6):1424. doi: 10.3390/ijms20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. Xi P. Wang B. Zhang L. Enderlein J. van Oijen A. M. Nanoparticles for super-resolution microscopy and single-molecule tracking. Nat. Methods. 2018;15(6):415–423. doi: 10.1038/s41592-018-0012-4. [DOI] [PubMed] [Google Scholar]
- Gabriel M. Moya-Díaz J. Gallo L. I. Marengo F. D. Estrada L. C. Single particle tracking of internalized metallic nanoparticles reveals heterogeneous directed motion after clathrin dependent endocytosis in mouse chromaffin cells. Methods Appl. Fluoresc. 2017;6(1):014003. doi: 10.1088/2050-6120/aa8c64. [DOI] [PubMed] [Google Scholar]
- Hou S. Lang X. Welsher K. Robust real-time 3D single-particle tracking using a dynamically moving laser spot. Opt. Lett. 2017;42(12):2390. doi: 10.1364/OL.42.002390. [DOI] [PubMed] [Google Scholar]
- Zagato E. Forier K. Martens T. Neyts K. Demeester J. Smedt S. D. et al., Single-particle tracking for studying nanomaterial dynamics: applications and fundamentals in drug delivery. Nanomedicine. 2014;9(6):913–927. doi: 10.2217/nnm.14.43. [DOI] [PubMed] [Google Scholar]
- Zahid M. U. Ma L. Lim S. J. Smith A. M. Single quantum dot tracking reveals the impact of nanoparticle surface on intracellular state. Nat. Commun. 2018;9(1):1830. doi: 10.1038/s41467-018-04185-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. Li Q. Liang L. Li J. Wang K. Li J. et al., Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nat. Commun. 2017;8(1):15646. doi: 10.1038/ncomms15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Li W. Yin W. Guo J. Zhang Z.-P. Zeng D. et al., Single-Particle Tracking of Human Immunodeficiency Virus Type 1 Productive Entry into Human Primary Macrophages. ACS Nano. 2017;11(4):3890–3903. doi: 10.1021/acsnano.7b00275. [DOI] [PubMed] [Google Scholar]
- Bhatia D. Arumugam S. Nasilowski M. Joshi H. Wunder C. Chambon V. et al., Quantum dot-loaded monofunctionalized DNA icosahedra for single-particle tracking of endocytic pathways. Nat. Nanotechnol. 2016;11(12):1112–1119. doi: 10.1038/nnano.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany M. Szoka F. C. Co-localization of fluorescent labeled lipid nanoparticles with specifically tagged subcellular compartments by single particle tracking at low nanoparticle to cell ratios. J. Drug Targeting. 2016;24(9):857–864. doi: 10.1080/1061186X.2016.1233976. [DOI] [PubMed] [Google Scholar]
- Deville S. Penjweini R. Smisdom N. Notelaers K. Nelissen I. Hooyberghs J. et al., Intracellular dynamics and fate of polystyrene nanoparticles in A549 Lung epithelial cells monitored by image (cross-) correlation spectroscopy and single particle tracking. Biochim. Biophys. Acta, Mol. Cell Res. 2015;1853(10):2411–2419. doi: 10.1016/j.bbamcr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Deschout H. Martens T. Vercauteren D. Remaut K. Demeester J. De Smedt S. et al., Correlation of Dual Colour Single Particle Trajectories for Improved Detection and Analysis of Interactions in Living Cells. Int. J. Mol. Sci. 2013;14(8):16485–16514. doi: 10.3390/ijms140816485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawinkel J. Richter U. Torres-Mapa M. L. Westermann M. Gamrad L. Rehbock C. et al., Optical and electron microscopy study of laser-based intracellular molecule delivery using peptide-conjugated photodispersible gold nanoparticle agglomerates. J. Nanobiotechnol. 2016;14(1):2. doi: 10.1186/s12951-015-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. Naydenov B. Chakrabortty S. Wuensch B. Hübner K. Ritz S. et al., Fluorescent Nanodiamond–Gold Hybrid Particles for Multimodal Optical and Electron Microscopy Cellular Imaging. Nano Lett. 2016;16(10):6236–6244. doi: 10.1021/acs.nanolett.6b02456. [DOI] [PubMed] [Google Scholar]
- Behzadi S. Serpooshan V. Tao W. Hamaly M. A. Alkawareek M. Y. Dreaden E. C. et al., Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017;46(14):4218–4244. doi: 10.1039/C6CS00636A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K., Nilsson T. and Fernandez-Rodriguez J., Challenges for CLEM from a Light Microscopy Perspective, in Correlative Imaging, ed. P. Verkade and L. Collinson, Wiley, 1st edn, 2019, pp. 23–35, available from https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119086420.ch2 [Google Scholar]
- Haruta T. Hasumi K. Ikeda Y. Konyuba Y. Fukuda T. Nishioka H. Local surface plasmon resonance of gold nanoparticles as a correlative light and electron microscopy (CLEM) tag for biological samples. Microscopy. 2019:dfz031. doi: 10.1093/jmicro/dfz031. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. Peurla M. Koho S. Deguchi T. Näreoja T. Chang H.-C. et al., STED-TEM Correlative Microscopy Leveraging Nanodiamonds as Intracellular Dual-Contrast Markers. Small. 2018;14(5):1701807. doi: 10.1002/smll.201701807. [DOI] [PubMed] [Google Scholar]
- Hemelaar S. R. de Boer P. Chipaux M. Zuidema W. Hamoh T. Martinez F. P. et al., Nanodiamonds as multi-purpose labels for microscopy. Sci. Rep. 2017;7(1):720. doi: 10.1038/s41598-017-00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuni B. Inose T. Ricci M. Fujita Y. Van Zundert I. Masuhara A. et al., Polymeric Engineering of Nanoparticles for Highly Efficient Multifunctional Drug Delivery Systems. Sci. Rep. 2019;9(1):2666. doi: 10.1038/s41598-019-39107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliani M. Tremolanti C. Signore G. Nanocarriers for Protein Delivery to the Cytosol: Assessing the Endosomal Escape of Poly(Lactide-co-Glycolide)-Poly(Ethylene Imine) Nanoparticles. Nanomaterials. 2019;9(4):652. doi: 10.3390/nano9040652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs C. A., Fluorescence Microscopy: A Concise Guide to Current Imaging Methods, Curr. Protoc. Neurosci., 2010, 50(1), Ch. 2, Unit2.1, available from https://onlinelibrary.wiley.com/doi/abs/10.1002/0471142301.ns0201s50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J. A. Darjuan M. M. Mercer J. E. Chen S. van der Meel R. Thewalt J. L. et al., On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA. ACS Nano. 2018;12(5):4787–4795. doi: 10.1021/acsnano.8b01516. [DOI] [PubMed] [Google Scholar]
- Yanez Arteta M. Kjellman T. Bartesaghi S. Wallin S. Wu X. Kvist A. J. et al., Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2018;115(15):E3351–E3360. doi: 10.1073/pnas.1720542115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. Gennis R. B. Single-particle cryo-EM studies of transmembrane proteins in SMA copolymer nanodiscs. Chem. Phys. Lipids. 2019;221:114–119. doi: 10.1016/j.chemphyslip.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi J. Ferguson S. Raja H. Hacker C. Marius P. Ward R. et al., Enhanced imaging of lipid rich nanoparticles embedded in methylcellulose films for transmission electron microscopy using mixtures of heavy metals. Micron. 2017;99:40–48. doi: 10.1016/j.micron.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. D. Parak W. J. Zhang F. Ali Z. Röcker C. Nienhaus G. U. et al., Fluorescent-Magnetic Hybrid Nanoparticles Induce a Dose-Dependent Increase in Proinflammatory Response in Lung Cells in vitro Correlated with Intracellular Localization. Small. 2010;6(6):753–762. doi: 10.1002/smll.200901770. [DOI] [PubMed] [Google Scholar]
- Hondow N. Brown A. Summers H. D. Rowan Brown M. Rees P. Holton M. D. et al., Quantifying Nanoparticle–Cell Interactions. Microsc. Microanal. 2014;20(S3):1300–1301. doi: 10.1017/S143192761400823X. [DOI] [Google Scholar]
- Summers H. D. Brown M. R. Holton M. D. Tonkin J. A. Hondow N. Brown A. P. et al., Quantification of Nanoparticle Dose and Vesicular Inheritance in Proliferating Cells. ACS Nano. 2013;7(7):6129–6137. doi: 10.1021/nn4019619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger C. Mühlfeld C. Ali Z. Lenz A.-G. Schmid O. Parak W. J. et al., Quantitative Evaluation of Cellular Uptake and Trafficking of Plain and Polyethylene Glycol-Coated Gold Nanoparticles. Small. 2010;6(15):1669–1678. doi: 10.1002/smll.201000528. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. G. The smooth fractionator BlackwellScience, Ltd. J. Microsc. 2002;207:191–210. doi: 10.1046/j.1365-2818.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- Jungmann R. Avendaño M. S. Dai M. Woehrstein J. B. Agasti S. S. Feiger Z. et al., Quantitative super-resolution imaging with qPAINT. Nat. Methods. 2016;13(5):439–442. doi: 10.1038/nmeth.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchner E. M. Walter J. M. Kasper R. Huang B. Lim W. A. Counting molecules in single organelles with superresolution microscopy allows tracking of the endosome maturation trajectory. Proc. Natl. Acad. Sci. U. S. A. 2013;110(40):16015–16020. doi: 10.1073/pnas.1309676110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcanale P. Miret-Ontiveros B. Arista-Romero M. Pujals S. Albertazzi L. Nanoscale Mapping Functional Sites on Nanoparticles by Points Accumulation for Imaging in Nanoscale Topography (PAINT) ACS Nano. 2018;12(8):7629–7637. doi: 10.1021/acsnano.7b09063. [DOI] [PubMed] [Google Scholar]
- Feiner-Gracia N. Beck M. Pujals S. Tosi S. Mandal T. Buske C. et al., Super-Resolution Microscopy Unveils Dynamic Heterogeneities in Nanoparticle Protein Corona. Small. 2017;13(41):1701631. doi: 10.1002/smll.201701631. [DOI] [PubMed] [Google Scholar]
- Le Trequesser Q. Devès G. Saez G. Daudin L. Barberet P. Michelet C. et al., Single Cell In Situ Detection and Quantification of Metal Oxide Nanoparticles Using Multimodal Correlative Microscopy. Anal. Chem. 2014;86(15):7311–7319. doi: 10.1021/ac501318c. [DOI] [PubMed] [Google Scholar]