Abstract
Background:
The etiology of necrotizing enterocolitis (NEC) is controversially discussed. One of the most recently proposed causes of NEC is an allergy to cow's milk protein. This study was designed to evaluate the effect of a maternal diet without bovine protein on the incidence of any NEC in very low birth weight (VLBW) infants.
Materials and Methods:
A pilot randomized controlled clinical trial was performed at Akbarabadi Hospital, Tehran, Iran, from December 2019 to July 2020, in women with VLBW infants. One hundred twenty mothers with VLBW neonates were randomly assigned to the intervention or the control group (60 in each). In the intervention group, mothers were given a dairy-free diet during the first 14 days after the newborn's onset of feeding. No special diet was given to the control group. The primary outcome of the study was the rate of any NEC in neonates, which was compared between groups. Any NEC was defined as Bell stage I or greater.
Results:
The minimum and maximum gestational ages were 26 and 33 weeks, respectively. The minimum birth weight of neonates was 700 g. The two groups did not differ significantly in terms of demographic and preinterventional clinical characteristics. Any NEC was reported in 0% and 10% (5/52) of neonates in the intervention and control groups, respectively; the difference was statistically significant (p = 0.028). The NEC symptoms began ∼34 days after birth. Four cases of NEC were classified as Bell stage I, and one was classified as Bell stage II. No statistical association was registered between sex, gestational age, birth weight, and the onset of feeding with the incidence of any NEC.
Conclusion:
The use of a cow's milk protein-free diet in mothers and exclusive breastfeeding in preterm VLBW infants may reduce the incidence of NEC. We recommend further studies with larger sample sizes in a multicenter setting. The study was registered at the Iranian Registry of Clinical Trials (IRCT20200415047086N1).
Keywords: necrotizing enterocolitis, bovine protein-free diet, very low birth weight, neonates, human milk
Introduction
Necrotizing enterocolitis (NEC) is a serious gastrointestinal condition that occurs most frequently in preterm infants.1 The incidence of NEC varies from 2% to 13% in preterm and very low birth weight (VLBW) infants.2–5 According to the research conducted so far, the best method of preventing NEC in human infants is to feed the infant with the mother's own milk (MOM).6,7 Ongoing research to identify risk factors for NEC is justified in view of the complications of the disease, long hospital stays, the consequent rise in health care costs, and the psychological burden of NEC for the family. A recent issue raised in this context was the intolerance to cow's milk protein.8–10
Allergic colitis usually appears within the first few months; the most commonly involved antigens are cow's milk protein and soy protein.11 About 5–30% of infants with allergic colitis are allergic to cow's milk proteins in human breast milk.12 This disorder is a non-immunoglobulin E (IgE)-mediated cellular delayed-type hypersensitivity and is limited to the gastrointestinal tract.12
Approximately 30–40% of patients with cow's milk protein allergy are unable to tolerate soy protein as well.13 The diagnosis is confirmed by medical history, removing cow's milk protein and soy protein from the diet, and observing the clinical response.13,14 The infants may have eosinophilia and, occasionally, elevated serum IgE levels.14 The removal of cow's milk protein and soy protein from the mother's diet is beneficial when the infant is given MOM, but the mother should also be given adequate quantities of calcium and vitamin D.15 When the infant is formula-fed, the formula should be changed to hydrolyzed proteins, which elicits a response rate of about 95%. Response rates of 100% have been noted for the amino acid formula.11,15,16
The aim of this study was to evaluate the effect of a maternal diet without bovine protein on the incidence of any NEC in VLBW neonates.
Materials and Methods
Study design and participants
A pilot randomized controlled clinical trial was performed at Akbarabadi Hospital affiliated to the Iran University of Medical Sciences, Tehran, Iran, from December 2019 to July 2020. The main aim of the study was to determine the effect of maternal diet without bovine protein on the incidence of any NEC in VLBW neonates. In view of the 50% reduction in the incidence of NEC (P2-P1 = 50%) reported by Nandhini et al.,17 the sample size was set to 52 neonates per group. An additional 20% were added to compensate for the anticipated loss to follow-up. The final estimated sample size was 60 patients in each group; the study power was 90% and the two-sided alpha error 0.05.
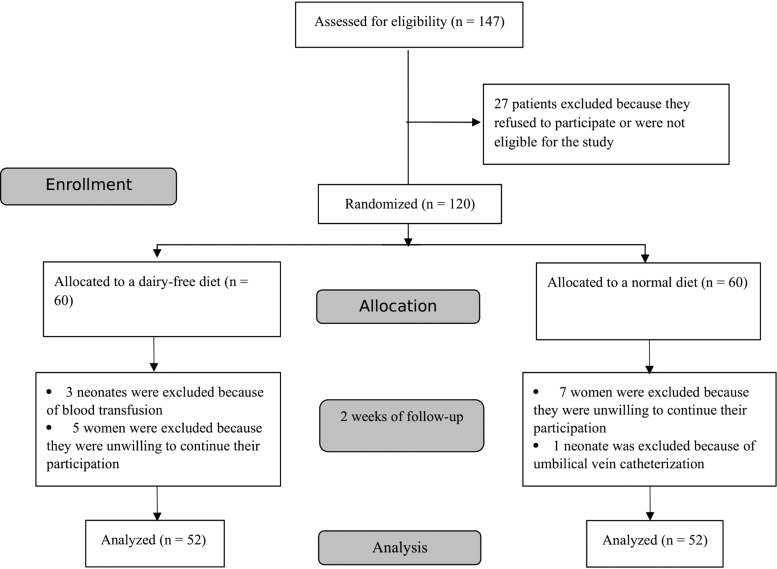
Mothers and neonates who fulfilled the following inclusion criteria were eligible for the study: (1) Iranian nationality, (2) VLBW neonates (birth weight <1,500 g), (3) no intrauterine growth restriction, and (4) no congenital disease. Neonates who needed a blood transfusion or umbilical vein catheterization were excluded from the study. Mothers who did not fully adhere to the protocol in terms of the dairy-free diet or were unwilling to continue their participation were excluded from the study (Fig. 1).
FIG. 1.
Flowchart of the study.
Intervention, randomization, and blinding of study participants
The main investigator, pediatrician, nurse, radiologist, statistician, and outcome assessor were blinded to the group allocation. The enrolled mothers (n = 120) were randomly assigned to one of the two groups through a computer-generated random table using a block size of 4 (i.e., AABB, ABAB, BAAB, BABA, BBAA, and ABBA). A nurse who was not involved in the research project was asked to prepare the coded envelopes using sequential numbers, and the researcher allocated the patients to one of the two groups on the basis of the envelopes. Mothers in the intervention group had a dairy-free diet for 14 days (n = 60) (the 14 days started from the infant's birth or from the first day of feeding), whereas the control group ingested a normal diet (n = 60).
The diet for the intervention group met the mother's daily requirement of protein without dairy protein, and was prepared by a nutritionist from the beginning of the infant's feeding to the time of discharge. Mothers of the second group had a normal and unrestricted diet. For the first 14 days, neonates in the intervention group were exclusively given MOM. Infants in the second group, whose mothers were on an unrestricted diet, were given pasteurized donor milk. To prevent nonadherence to the dairy-free diet, mothers were given the necessary instructions as well as prescriptions for calcium supplements. After the first 14 days, neonates in both groups were exclusively given human milk (MOM or pasteurized donor milk that none of the milk was fortified) until discharge from the hospital.
Outcomes, measurements, and follow up
The primary endpoint of the study was the rate of any NEC in neonates in the two groups. Based on the study by Bell et al., NEC is divided into three stages to provide appropriate treatment.18 For the purposes of this study, any NEC was defined as Bell stage I or higher.
General parameters, including sex, gestational age (week), birth weight in grams, Apgar score at 1 and 5 minutes after birth, age at the onset of feeding (days), mechanical ventilation, duration of mechanical ventilation (days), duration of continuous positive airway pressure and noninvasive ventilation (days), resuscitation, the occurrence of any NEC, and the stage of NEC, were entered in a questionnaire specifically designed for the study. The data were either obtained directly from the parents or extracted from their medical records.
Neonates were monitored in regard of NEC from the onset of feeding until discharge from the hospital. The diagnosis of NEC and its severity were established according to Bell's staging criteria.19 NEC was diagnosed by a clinical team at the hospital, whose members were not involved in the research project.
Ethical statement
All steps of the study were performed in accordance with the Declaration of Helsinki (ethical principles for medical research involving human subjects), and the regulations of the ethics committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1398.504). Informed consent was obtained from parents after explaining the study protocol. The members were free to discontinue their participation at any time. All personal data were treated confidentially and only reported in collective form.
Statistical analysis
The data were computerized and analyzed using the Statistical Package of Social Sciences (SPSS), version 21.0 (SPSS, Inc., Chicago, IL, USA). Qualitative variables were described by frequency (percentage), and comparison between groups was performed with the chi-square test. Quantitative variables were expressed as mean (standard deviation) and median (interquartile range [IQR]). The one-sample Kolmogorov–Smirnov test was used to test for normal distribution of quantitative data. Student's t-test was used for data comparison between groups. The relationship between variables and the incidence of any NEC was investigated by logistic regression. The level of significance was set to 0.05.
Results
Of 147 VLBW neonates, 120 were deemed eligible for the study (60 each in the intervention and the control group). Of the eligible cases, eight each were excluded from the intervention and control groups. Finally, 52 cases were analyzed in each group (Fig. 1).
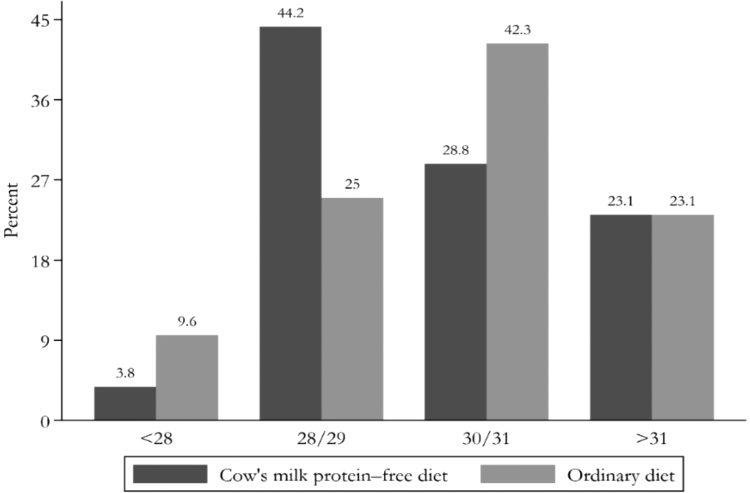
The minimum and maximum gestational age of the neonates was 26 and 33 weeks, respectively. The comparison of gestational age in the two groups is shown in Figure 2.
FIG. 2.
Comparison of gestational age in the intervention and control groups.
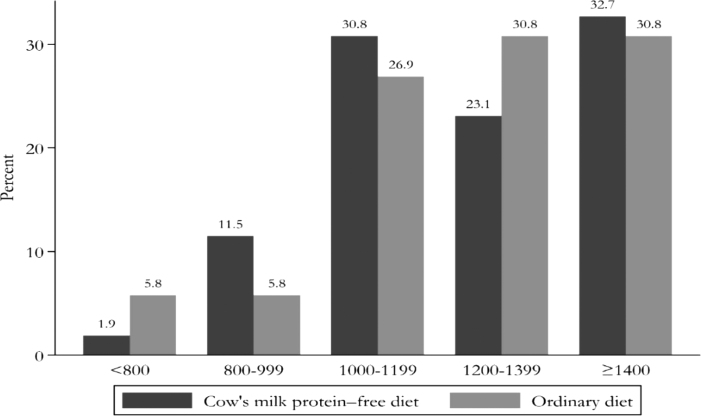
The minimum and maximum neonatal birth weight was 700 and 1,500 g, respectively. Nearly 32% of the neonates included in the study weighed >1,400 g. A comparison of neonatal birth weight in two groups is shown in Figure 3.
FIG. 3.
Comparison of birth weight in the intervention and control groups.
Baseline variables, including sex, gestational age, birth weight, birth Apgar, and 5-minute Apgar scores, did not differ significantly between the two groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Two Groups
| Variables | Intervention group (n = 52) | Control group (n = 52) | p a |
|---|---|---|---|
| Sex (male) | 21 (40.4%) | 28 (53.9%) | 0.169 |
| Gestational age, weeks | 29.9 ± 1.5 | 30.05 ± 1.7 | 0.736 |
| Birth weight, grams | 1210 ± 221 | 1,234 ± 199 | 0.574 |
| Birth Apgar | 6.2 ± 1.8 | 5.9 ± 1.9 | 0.371 |
| 5-Minute Apgar | 8.4 ± 0.93 | 8.1 ± 1.07 | 0.116 |
| Mechanical ventilation (yes) | 33 (63.5) | 34 (65.4) | 0.51 |
| Duration of mechanical ventilation, days | 3.84 ± 3.85 | 6.05 ± 9.05 | 0.201 |
| CPAP/NIV duration, days | 3.04 ± 1.7 | 4.3 ± 5.05 | 0.113 |
| Day of onset of feeding | 4.9 ± 2.7 | 6.7 ± 8.5 | 0.143 |
| Resuscitation (yes) | 33 (63.5%) | 38 (73.1%) | 0.292 |
| Discharge from hospital | 55.5 ± 31.6 | 67.5 ± 32.2 | 0.057 |
| Any NEC (yes) | 0 | 5 (10.0%) | 0.028 |
Continuous values are presented as mean ± SD, and categorical variables as numbers (%).
The p-value was calculated by the chi-square test (for categorical variables) or the Student's t-test (for continuous variables) to determine differences between groups.
CPAP, continuous positive airway pressure; NEC, necrotizing enterocolitis; NIV, noninvasive ventilation; SD, standard deviation.
The duration of mechanical ventilation was 2.21 days longer in the control group than in the intervention group, but the difference was not statistically significant. Feeding was started 4.9 ± 2.7 and 6.7 ± 8.5 days after birth in the intervention and control groups, respectively. Overall, 68.3% (71/104) of the neonates included in this study needed resuscitation. The resuscitation rate did not differ significantly between groups. By day 90, 36.54% (19/52) and 65.38% (34/52) of neonates were discharged in the intervention and control groups, respectively.
Any NEC was reported in 0% and 10% (5/52) of patients in the intervention and control groups, respectively; the difference was significant (p = 0.028). Symptoms of any NEC started nearly 34 days after birth. Four cases of NEC were classified as Bell stage I, and one was classified as Bell stage II. Three cases of any NEC were male (60%). The median (IQR) gestational age was 29 (29, 30) weeks, the median birth weight 1,250 g, and the median Apgar score at birth 5. The chi-square test revealed no statistical relationship between sex and the incidence of any NEC (p = 0.777). The logistic regression test showed that gestational age, birth weight, and the onset of feeding did not affect the incidence of any NEC (p = 0.362, p = 0.827, and p = 0.974, respectively). Clinical characteristics of the two groups are shown in Table 1.
Discussion
Despite numerous publications on the etiology of NEC, the role of cow's milk protein in the incidence of NEC has been scarcely investigated.6,10,20 This randomized double-blind study was designed to evaluate the effect of maternal diet without bovine protein on the incidence of NEC in VLBW neonates.
The incidence of NEC in this study in general, without considering the study arms, was 4.8%. The incidence of NEC has been reported to vary from 2% to 13% in preterm and VLBW infants.2–5 A maternal diet devoid of bovine protein for 14 days was shown to affect the incidence of NEC (0% versus 10% in the intervention and control groups, respectively). Our data support previous studies which reported that bovine protein has an effect on NEC in VLBW neonates.21,22 Abrams et al. divided preterm infants into the two groups of MOM and formula feeding with cow's milk protein, and reported an NEC incidence of 5% in the MOM group and 17% in the cow's milk group; the between-group difference was significant.23
In a study performed by Cordova et al., 9 of 14 infants with an intolerance to cow's milk developed NEC after an average of 22 days and the mothers had a history of consuming a bovine protein diet,10 which is consistent with our data. In a multicenter study comparing MOM and cow's milk in regard of neonatal complications in 1,587 neonates, significantly fewer cases of NEC were observed in infants fed on human milk (6.9%) than in those fed on cow's milk protein (16.7%). Mortality rates, sepsis, retinopathy of prematurity, bronchopulmonary dysplasia,6 nutritional intolerance, the incidence of NEC, and hospitalization costs per infant were lower in infants who were fed with MOM.7,24
In 2020, a retrospective study was performed on 319 infants, of whom one group was fed with breast milk plus formula and another with breast milk and donated breast milk. The NEC rate was lower in exclusively breastfed infants (8% versus 6%), although the breastfed/formula-fed group was less expensive in terms of hospitalization and feeding costs.25
In this study, the symptoms of NEC started nearly 34 days after birth, and the length of the hospital stay was shorter in the intervention group than in the control group (67.5 ± 32.2 days versus 55.5 ± 31.6 days). The data of this study revealed that an exclusively human milk-based diet in preterm infants is associated with better recovery of body composition through the promotion of fat-free mass deposition, which may ultimately lead to better metabolic and neurodevelopmental outcomes.26,27
In this study, logistic regression showed no statistical relationship between sex, gestational age, birth weight, and the onset of feeding with the incidence of NEC. Contrary to our data, a previous study reported an inverse relationship between gestational age, birth weight and the incidence of NEC, and a slightly higher prevalence of NEC in male neonates.28 Preterm birth has been described as the most important risk factor for NEC, since >90% of neonates with NEC are preterm infants.19,29 An allergy to cow's milk protein may cause milk intolerance and play a role in the pathogenesis of the disease.30,31
Strengths and limitations
The main strength of this study was the randomized blind assessment of patients while considering several criteria to reduce the effect of confounders on the study outcomes. Although the present investigations yielded important data, its limitations are worthy of note. One of these was the small sample size. A second limitation was the short follow-up period until discharge. A third limitation was that we included any stage of NEC. Stage 1, or suspected NEC, includes patients who present with the mildest of symptoms. It has been proven that the majority of patients with stage 1 NEC never develop intestinal necrosis. Thus, these may have been cases of nonspecific feeding intolerance and not NEC. The preliminary data obtained in this study should be confirmed in high-quality clinical trials with large sample sizes and a long follow-up period, including the various stages of NEC.
Conclusion
This study showed that a 2-week bovine protein-free diet for mothers of VLBW infants with similar demographics and health conditions is able to reduce the incidence of NEC in neonates. The data need to be confirmed in multicenter studies with larger sample sizes and staging of NEC.
Acknowledgment
The authors would like to thank the Aliasghar Clinical Research Development Center (AACRDC) for editorial work/search/assistance.
Disclosure Statement
No competing financial interests exist.
Funding Information
All authors confirm that the study was supported by the Iran University of Medical Sciences (Grant number: 98-4-4-13377).
References
- 1. Henry MC, Moss R. Necrotizing enterocolitis. In: Ashcraft's Pediatric Surgery, 5th ed. Holcomb III GW, Murphy J, eds. Philadelphia: Saunders, 2010, pp. 439–455. [Google Scholar]
- 2. Guthrie SO, Gordon PV, Thomas V, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003;23:278–285. [DOI] [PubMed] [Google Scholar]
- 3. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: A systematic review and meta-analysis. BMC Pediatr 2020;20:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med 2009;60:111–124. [DOI] [PubMed] [Google Scholar]
- 6. Hair AB, Peluso AM, Hawthorne KM, et al. Beyond necrotizing enterocolitis prevention: Improving outcomes with an exclusive human milk-based diet. Breastfeed Med 2016;11:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Assad M, Elliott M, Abraham J. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol 2016;36:216–220. [DOI] [PubMed] [Google Scholar]
- 8. Lenfestey MW, de la Cruz D, Neu J. Food protein-induced enterocolitis instead of necrotizing enterocolitis? A neonatal intensive care unit case series. J Pediatr 2018;200:270–273. [DOI] [PubMed] [Google Scholar]
- 9. Neu J, Modi N, Caplan M. Necrotizing enterocolitis comes in different forms: Historical perspectives and defining the disease. Semin Fetal Neonatal Med 2018;23:370–373. [DOI] [PubMed] [Google Scholar]
- 10. Cordova J, Sriram S, Patton T, et al. Manifestations of cow's-milk protein intolerance in preterm infants. J Pediatr Gastroenterol Nutr 2016;62:140–144. [DOI] [PubMed] [Google Scholar]
- 11. Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy Asthma Proc 2015;36:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nowak-Wegrzyn A, Muraro A. Food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol 2009;9:371–377. [DOI] [PubMed] [Google Scholar]
- 13. Järvinen KM, Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome (FPIES): Current management strategies and review of the literature. J Allergy Clin Immunol Pract 2013;1:317–322.e4. [DOI] [PubMed] [Google Scholar]
- 14. Caminiti L, Salzano G, Crisafulli G, et al. Food protein induced enterocolitis syndrome caused by rice beverage. Ital J Pediatr 2013;39:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cunningham K, Scanlan B, Coghlan D, et al. Infants with FPIES to solid food proteins—Chicken, rice and oats. Ir Med J 2014;107:151. [PubMed] [Google Scholar]
- 16. Mane SK, Hollister ME, Bahna SL. Food protein-induced enterocolitis syndrome to trivial oral mucosal contact. Eur J Pediatr 2014;173:1545–1547. [DOI] [PubMed] [Google Scholar]
- 17. Nandhini LP, Biswal N, Adhisivam B, et al. Synbiotics for decreasing incidence of necrotizing enterocolitis among preterm neonates—A randomized controlled trial. J Matern Fetal Neonatal Med 2016;29:821–825. [DOI] [PubMed] [Google Scholar]
- 18. Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368:1271–1283. [DOI] [PubMed] [Google Scholar]
- 20. Agyemang A, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome: A comprehensive review. Clin Rev Allergy Immunol 2019;57:261–271. [DOI] [PubMed] [Google Scholar]
- 21. Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156:562–567.e1. [DOI] [PubMed] [Google Scholar]
- 22. Herrmann K, Carroll K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med 2014;9:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abrams SA, Schanler RJ, Lee ML, et al. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed Med 2014;9:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huston RK, Markell AM, McCulley EA, et al. Improving growth for infants ≤1250 grams receiving an exclusive human milk diet. Nutr Clin Pract 2018;33:671–678. [DOI] [PubMed] [Google Scholar]
- 25. Johnson TJ, Berenz A, Wicks J, et al. The economic impact of donor milk in the Neonatal Intensive Care Unit. J Pediatr 2020;224:57–65.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cerasani J, Ceroni F, De Cosmi V, et al. Human milk feeding and preterm infants' growth and body composition: A literature review. Nutrients 2020;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boquien CY. Human milk: An ideal food for nutrition of preterm newborn. Front Pediatr 2018;6:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel BK, Shah JS. Necrotizing enterocolitis in very low birth weight infants: A systemic review. ISRN Gastroenterol 2012;2012:562594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gephart SM, McGrath JM, Effken JA, et al. Necrotizing enterocolitis risk: State of the science. Adv Neonatal Care 2012;12:77–87; quiz 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pensabene L, Salvatore S, D'Auria E, et al. Cow's milk protein allergy in infancy: A risk factor for functional gastrointestinal disorders in children? Nutrients 2018;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brill H. Approach to milk protein allergy in infants. Can Fam Physician 2008;54:1258–1264. [PMC free article] [PubMed] [Google Scholar]