Abstract
Introduction:
Motor control requires a reciprocal volley between somatosensory and motor systems, with somatosensory feedback being essential for the online updating of motor commands to achieve behavioral outcomes. However, this dynamic interplay among sensorimotor brain systems serving motor control remains poorly understood.
Methods:
To address this, we designed a novel somatosensory entrainment-movement task, which 25 adults completed during magnetoencephalography (MEG). Specifically, participants completed a quasi-paced finger-tapping paradigm while subthreshold electrical stimulation was applied to the right median nerve at a sensorimotor-relevant frequency (15 Hz) and during a second condition where no electrical stimulation was applied. The MEG data were transformed into the time-frequency domain and imaged by using a beamformer to evaluate the effect of somatosensory feedback (i.e., entrainment) on movement-related oscillations and motor performance at the single trial level.
Results:
Our results indicated spectrally specific reductions in movement-related oscillatory power (i.e., theta, gamma) during 15 Hz stimulation in the contralateral motor cortex during motor execution. In addition, we observed robust cross-frequency coupling within the motor cortex and further, stronger theta-gamma coupling was predictive of faster reaction times, irrespective of condition (i.e., stim vs. no stim). Finally, in the presence of electrical stimulation, cross-frequency coupling of movement-related oscillations was reduced, and the stronger the entrained neuronal populations (i.e., increased oscillatory power) were before movement onset, the weaker the inherent theta-gamma coupling became in the motor cortex.
Discussion:
This novel exogenous manipulation paradigm provides key insights on how the somatosensory system modulates the motor cortical oscillations required for volitional movement in the normative sensorimotor system.
Impact statement
Functional coupling of neural oscillations has been proposed as a mechanism for neuronal communication both locally and across the cortex. Although the nested coupling of disparate rhythms governs higher-order cognitive processes, its role in the sensorimotor interactions serving motor control remains poorly understood. Herein, we provide evidence for a robust coupling of theta and gamma oscillations during motor execution in the presence and absence of continuous somatosensory feedback, with stronger functional coupling predictive of behavioral improvements. Further, stronger entrainment of neuronal populations led to substantially weakened motor cortical theta-gamma coupling, indicative of a dynamic interplay among sensorimotor cortices during movement.
Keywords: electrical stimulation, gamma, magnetoencephalography, sensory feedback, theta
Introduction
Volitional control of movement requires a dynamic interplay among somatosensory and motor systems, with reciprocal connections present along the ascending and descending pathways. In fact, somatosensory feedback to the motor system is critical for the online updating of motor commands to ultimately modify behavioral outcomes. Neurophysiological signatures of motor actions can be observed in the cortex as the coordinated recruitment of spatiotemporally precise neural oscillations across a distributed sensorimotor network.
For example, during planning of the performed movement, neural activity in the alpha (8–14 Hz) and beta range (15–30 Hz) exhibits strong decreases in bilateral primary motor cortices (M1), with additional responses often seen in the supplementary motor areas, superior parietal, and primary somatosensory cortices (Grent-‘t-Jong et al., 2014; Heinrichs-Graham and Wilson, 2015; Heinrichs-Graham et al., 2016; Tzagarakis et al., 2010). Such decreases in alpha and beta activity are thought to reflect the engagement of neuronal pools within these sensorimotor regions, as during rest somatosensory and motor cortices exhibit strong idling rhythms in the alpha and beta range, respectively (Engel and Fries, 2010). After motor execution, there is a robust increase in beta activity known as the post-movement beta rebound (PMBR). Although this increase in oscillatory power may simply reflect the motor cortex returning to idling beta levels, this response has also been linked to the active inhibition of motor cortical neurons and/or somatosensory reafference to the motor cortex (Gaetz et al., 2010; Heinrichs-Graham et al., 2017b; Jurkiewicz et al., 2006; Pfurtscheller and Lopes da Silva, 1999).
In addition to peri- and post-movement neural responses in the alpha and beta range, motor control is known to involve high-frequency gamma oscillations as well as lower frequency theta activity. Motor-related gamma oscillations (>30 Hz) are highly transient and coincide closely with movement onset, which has led the response to be frequently interpreted as a motor execution signal. The response is robustly modulated by different physical aspects of movement (e.g., force applied, frequency and number of movements being performed) (Gaetz et al., 2010, 2011; Muthukumaraswamy, 2010; Wilson et al., 2010, 2011b), and recent evidence suggests that it may also be modulated by higher-order cognitive demands, including response interference and the reorienting of attentional systems (Gaetz et al., 2013; Grent-‘t-Jong et al., 2013; Heinrichs-Graham et al., 2017a; Spooner et al., 2020b; Wiesman et al., 2020).
Finally, there is a short-lived neural response in the theta range (4–8 Hz) that is time-locked to movement onset and may be evoked or oscillatory in nature. Essentially, theta activity in the motor cortex may enhance the temporal coordination of the motor action (Igarashi et al., 2013; Tomassini et al., 2017).
Although there is abundant evidence supporting the role of multispectral oscillatory activity in motor control, far less is known about the dynamic volley between somatosensory and motor regions, and specific oscillatory responses, during active movement. Past studies addressing this gap have used a variety of experimental paradigms to probe the reciprocal interactions between sensorimotor systems along the peripheral–cortical pathway. For example, to evaluate the effect of voluntary movement on the somatosensory system, investigators have utilized simultaneous movement/electrical stimulation paradigms on a targeted limb (e.g., hand and median nerve). Broadly, these studies have suggested that active movement during single or paired-pulse electrical stimulation of the same limb attenuates the neural response to somatosensory stimulation, which may ultimately reflect efferent feedback along several levels of the ascending pathway (Gehringer et al., 2019; Kurz et al., 2018; Papakostopoulos et al., 1975; Seki and Fetz, 2012; Seki et al., 2003). In contrast, to evaluate the effect of somatosensory input on motor actions, investigators have implemented repetitive somatosensory stimulation protocols of the median nerve (e.g., trains of 10 pulses per trial).
These studies have shown that rhythmic trains of at least five pulses to the periphery (or cortex) at relevant sensorimotor frequencies entrain neuronal pools to oscillate synchronously at the applied rhythm (e.g., 12 Hz), as evidenced by an increase in local oscillatory power and/or phase synchrony (Morera Maiquez et al., 2020; Thut et al., 2011). Interestingly, recent evidence suggests that peripherally applied entrainment protocols may aid the functional recovery of fine and gross hand movements in patients with stroke and other brain injuries. These studies have shown that after long durations of repetitive somatosensory stimulation, motor performance (e.g., finger individuation, pinch strength, tapping, reach-to-grasp, activities of daily living) tends to improve in clinical populations (Celnik et al., 2007; Conforto et al., 2010; Klaiput and Kitisomprayoonkul, 2009; Koesler et al., 2009; Morera Maiquez et al., 2020; Tu-Chan et al., 2017; Wilson et al., 2011a; Wu et al., 2006).
Although the majority of this work suggests that peripheral somatosensory entrainment modulates neural and behavioral dynamics through changes in cortical excitability (Golaszewski et al., 2012; Kaelin-Lang et al., 2002; Ridding et al., 2000), the underlying mechanisms remain poorly understood, as entrainment of the periphery is generally administered in the absence of active motor performance and/or neuroimaging paradigms. Thus, although a handful of studies have filled critical gaps regarding the impact of movement on somatosensation and, conversely, somatosensory input on motor performance, the dynamic oscillatory interactions between somatosensory and motor cortices during volitional movement remain incompletely understood, especially in the context of sensory feedback to alter normative sensorimotor processing.
With these knowledge gaps in mind, we have developed a novel, simultaneous movement and somatosensory entrainment task to assess the evolving dynamic neural interactions serving voluntary movement during repetitive stimulation of the periphery. One potential parameter that could prove critical in identifying the dynamic interactions among motor and somatosensory neural systems involves the cross-frequency coupling of disparate oscillators during motor performance. Essentially, accumulating evidence suggests that the functional coupling of oscillations (e.g., power to power coupling, phase to power coupling) is important for information processing, as well as for short- and long-range neuronal communication during both higher-order cognitive tasks and motor performance (Bramson et al., 2018; Friese et al., 2013; Igarashi et al., 2013; Jensen and Colgin, 2007; Lisman and Jensen, 2013; Roux and Uhlhaas, 2014; Tort et al., 2009; von Nicolai et al., 2014; Voytek et al., 2015). Thus, we aimed at evaluating the presence of this phenomenon in the healthy sensorimotor system by using magnetoencephalography (MEG) and our novel motor-somatosensory entrainment paradigm. Briefly, 25 healthy adults performed a finger tapping task while subthreshold continuous electrical stimulation (CES) was applied to the right median nerve at a sensorimotor-relevant frequency (i.e., 15 Hz) and during a no-stimulation condition. The resulting MEG data were transformed in the time-frequency domain and imaged by using a beamformer.
Importantly, we found spectrally specific modulation of movement-related neural dynamics as a function of CES (i.e., somatosensory entrainment). Specifically, we describe novel data that show dynamic coupling of oscillatory power outside of the entrained somatosensory frequency range in the precentral gyrus contralateral to movement, and further, the strength of this cross-frequency coupling was differentially moderated by the magnitude of the pre-movement oscillatory power entrained by the peripheral stimulation. Finally, cross-frequency coupling in the motor cortex strongly predicted motor performance irrespective of stimulation protocol. Our results provide novel insights on how exogenous manipulation of the somatosensory system may modulate the motor cortical oscillations that serve voluntary motor actions.
Materials and Methods
Participants
Twenty-five healthy young adults (12 females, Mage = 25.34 years, range 21–32 years old) participated in this study. All participants were right-handed based on self-report. Exclusionary criteria included any medical illness affecting the central nervous system (e.g., HIV/AIDS, chronic pain), neurological or psychiatric disorder, musculoskeletal disorders, history of head trauma, current substance abuse, and the MEG Laboratory's standard exclusion criteria (e.g., ferromagnetic implants). After a full description of the study was given to participants, written informed consent was obtained following the guidelines of the University of Nebraska Medical Center's Institutional Review Board, which approved the study protocol.
Experimental paradigm
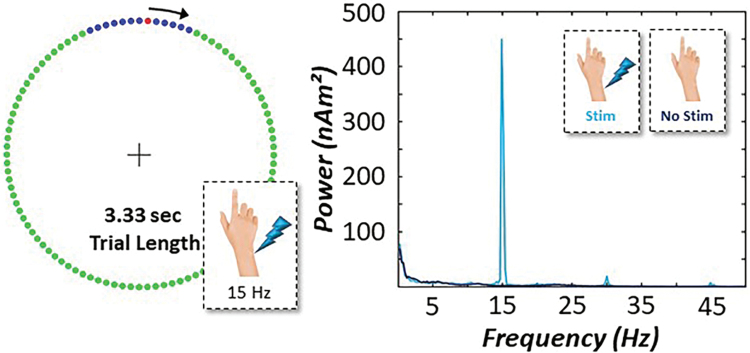
Participants were seated in a nonmagnetic chair with their head positioned within the MEG helmet-shaped sensor array. Participants were instructed to maintain fixation on a centrally located crosshair throughout the task and to perform a single flexion extension of the right index finger each time a red dot reached the target interval denoted in blue, which was always located around the 12 o'clock position. This dot completed a full rotation around the clock-like circle (without tick marks or numbers) every 3.33 sec, and it was meant to serve as a pacing mechanism. To evaluate the impact of somatosensory entrainment on movement-related oscillatory activity, subthreshold CES was applied to the right median nerve by using external cutaneous stimulators connected to a Digitimer DS7A constant-current stimulator system (Digitimer Ltd., Garden City, United Kingdom). Each participant underwent two separate runs. Briefly, to find the median nerve, 0.2 ms constant-current square waves were delivered at an amplitude that was strong enough to elicit a subtle twitch of the thumb. Once the median nerve was identified, the amplitude of the pulse was carefully lowered until the subtle twitch of the thumb was no longer visible. The pulse amplitude was then further reduced by 10% and remained constant across the entirety of the task and both runs. In one run, CES was applied at a relevant sensorimotor frequency (i.e., 15 Hz) to the right median nerve during completion of the clock-like motor task (i.e., stimulation condition). In another run, an identical CES (i.e., 15 Hz) protocol was applied, but the stimulation contacts were turned 180° away from the participant's right median nerve to ensure no contact with the participant's skin surface (Supplementary Fig. S1).
This approach of leaving the stimulation on throughout the duration of both conditions helped ensure that any stimulation-related electrical artifacts would be present in both neuroimaging data sets. Importantly, the conditions (i.e., with CES, without CES) were counterbalanced across all participants and each contained 120 trials. The total time to complete both runs was ∼14 min (Fig. 1). Pairwise statistical tests of task accuracy (i.e., response within blue target interval), reaction time (i.e., distance from onset of blue target), and coefficient of variation were conducted to evaluate behavior as a function of CES.
FIG. 1.
Simultaneous somatosensory entrainment-motor paradigm. (Left): Participants fixated on a centrally located crosshair as the red dot moved clockwise toward the blue target interval. Participants were instructed to respond with one tap of their right index finger any time the red dot was within the blue interval. CES at 15 Hz stimulation frequency was applied to the right median nerve during one run of the motor task, whereas in a separate run the same CES protocol was conducted but the stimulation contacts were turned away from the participant's wrist. Experimental conditions (i.e., stim vs. no stim) were counterbalanced across participants. (Right). Power spectral density of entrainment power in a representative subject shows an increase in power at 15 Hz in the motor cortex across the experimental epoch during CES (light blue) compared with no stimulation (dark blue). CES, continuous electrical stimulation. Color images are available online.
MEG data acquisition
All recordings were performed in a one-layer magnetically shielded room with active shielding engaged for environmental noise compensation. With an acquisition bandwidth of 0.1–330 Hz, neuromagnetic responses were sampled continuously at 1 kHz by using an Elekta/MEGIN MEG system (Helsinki, Finland) with 306 magnetic sensors, including 204 planar gradiometers and 102 magnetometers. Throughout data acquisition, participants were monitored by using a real-time audio–video feed from inside the magnetically shielded room. The MEG data from each participant were individually corrected for head motion and subjected to noise reduction by using the signal-space separation method with a temporal extension (Taulu and Simola, 2006; Taulu et al., 2005).
Structural magnetic resonance images processing and MEG coregistration
Before MEG measurement, four coils were attached to the participant's head and the locations of these coils, together with the three fiducial points and scalp surface, were determined with a 3D digitizer (Fastrak 3SF0002; Polhemus Navigator Sciences, Colchester, VT). Once the participant was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant's MEG data were coregistered with T1-weighted structural magnetic resonance images (sMRI) before source space analyses by using BESA MRI (version 2.0; BESA GmbH, Gräfelfing, Germany). Eight sMRI images were acquired with a Philips Achieva 3T X-series scanner by using an eight-channel head coil (TR: 8.09 ms; TE: 3.7 ms; field of view: 240 mm; slice thickness: 1 mm; no gap; in-plane resolution: 1.0 × 1.0 mm). The remaining 15 sMRI images were acquired by using a Siemens Skyra 3T scanner (Siemens Medical Solutions) with a 32-channel head coil and an MP-RAGE sequence with the following parameters: TR = 2400 ms; TE = 1.94 ms; flip angle = 8°; FOV = 256 mm; slice thickness = 1 mm (no gap); and voxel size = 1 × 1 × 1 mm. All sMRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space, along with the functional data, after beamforming (see MEG source imaging section below).
MEG preprocessing, time-frequency transformation, and sensor-level statistics
Cardiac and ocular artifacts were removed from the data by using signal-space projection, and the projection operator was accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). Epochs were of 3300 ms duration, with 0 ms defined as movement onset and the baseline being the −1300 to −800 ms window. Epochs containing artifacts were rejected based on a fixed threshold method supplemented with visual inspection, as were epochs containing incorrect responses (i.e., motor responses outside the blue target interval). On average, 94 trials per participant and condition remained after artifact rejection. Importantly, the number of trials used for final analyses did not significantly differ between conditions (p = 0.552).
Artifact-free epochs were further processed following two parallel pipelines. For the time domain (i.e., evoked) analyses, all epochs per condition and participant were averaged with respect to movement onset for each sensor in the array and normalized by using the baseline. For the oscillatory analysis, epochs were transformed into the time–frequency domain by using complex demodulation (Kovach and Gander, 2016), and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized by using the respective bin's baseline power, which was calculated as the mean power during the −1300 to −800 ms time period. The specific time–frequency windows used for source reconstruction were determined by statistical analysis of the sensor-level spectrograms across all participants' trials, task conditions, and gradiometers. Each data point in the spectrogram was initially evaluated by using a mass univariate approach based on the general linear model.
To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for type 1 error. In the first stage, paired sample t-tests against baseline were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time–frequency bins containing potentially significant oscillatory deviations across all participants. In stage 2, time–frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also significant, and a cluster value was derived by summing all of the t-values of all data points in the cluster.
Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters (from stage 1) was tested directly by using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time–frequency windows that contained significant oscillatory events across all participants were subjected to the beamforming analysis.
MEG source imaging
Cortical oscillatory networks were imaged through the dynamic imaging of coherent sources (DICS) beamformer (Gross et al., 2001), which uses the cross-spectral density matrices to calculate source power for the entire brain volume. These images are typically referred to as pseudo-t maps, with units (pseudo-t) that reflect noise-normalized power differences (i.e., active vs. passive) per voxel. Following convention, we computed noise-normalized, source power per voxel in each participant by using baseline periods of equal duration and bandwidth (Hillebrand et al., 2005). The MEG preprocessing and imaging used the Brain Electrical Source Analysis (version 7.0; BESA GmbH) software. Further details of our analysis pipeline can be found in our recent publications (Spooner et al., 2018, 2019, 2020a,b; Wiesman and Wilson, 2020).
Normalized source power was computed over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution for the time–frequency periods identified through the sensor-level analyses. Before statistical analysis, each participant's MEG data, which were coregistered to native space structural MRI before beamforming, were transformed into standardized space by using the transform previously applied to the structural MRI volume and spatially resampled. The resulting 3D maps of brain activity were averaged across all participants and task conditions to assess the neuroanatomical basis of the significant oscillatory responses identified through the sensor-level analysis, and to allow identification of the peak voxels per oscillatory response.
Voxel time series data (i.e., “virtual sensors”) were extracted from each participant's data individually per condition by using the peak voxel from the grand-averaged beamformer images. To compute the virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded a time series for the specific coordinate in source space. Note that virtual sensor extraction was done per participant, once the coordinates of interest were known. Once the virtual sensor time series were extracted, we computed the envelope of the spectral power within the frequency range used in the beamforming analysis. From this time series, we computed the absolute (i.e., nonbaseline-corrected) response time series of each participant per task condition.
In regard to the time-domain (i.e., evoked) analysis, source images were computed by using standardized low-resolution brain electromagnetic tomography (sLORETA; regularization: Tikhonov 0.01%) (Pascual-Marqui, 2002). The resulting whole-brain maps were four-dimensional estimates of current density per voxel, per time sample across the experimental epoch. These data were normalized to the sum of the noise covariance and theoretical signal covariance, and thus the units are arbitrary. Using the temporal clusters identified in the sensor-level analysis for our transient oscillatory responses coinciding closely with movement onset (e.g., theta and gamma), these maps were averaged over the time surrounding movement (i.e., −125 to 125 ms) for both task conditions.
The resulting maps were then grand-averaged across the two conditions to determine the peak voxel of the time-domain neural response during movement onset across participants. From this peak, sLORETA units were extracted per task condition to derive estimates of the time-domain response for each participant.
To examine the effect of somatosensory entrainment (i.e., CES) on movement-related neural dynamics, we conducted paired sample t-tests between stim and no stim experimental conditions for each evoked and oscillatory response computed by using sLORETA and DICS reconstruction methods (i.e., pseudo-t values, sLORETA units), respectively. Further, absolute voxel time series data were extracted from peak oscillatory responses and pairwise comparisons were conducted between task conditions by using these data. All pairwise testing and Bayesian statistics were conducted by using JASP.
Theta-gamma cross-frequency coupling
Finally, we interrogated the predictive capacity of somatosensory entrainment power on the coupling of relevant oscillatory responses (i.e., theta and gamma oscillatory power). First, we extracted single-trial voxel time series data (i.e., baseline corrected) from the peak voxels per oscillatory response to conduct condition-wise multilevel models (MLM) in R. Specifically, we defined theta oscillatory power and task condition as fixed effects, with subject and trial number defined as a nested random effect to predict gamma oscillatory power on the single-trial level.
Next, we aimed at directly linking the strength of somatosensory entrainment (i.e., 15 Hz oscillatory power during the baseline) to theta-gamma cross-frequency coupling through a multilevel moderation analysis in R. Essentially, single-trial absolute time series data were extracted from the left M1 to derive an estimate of 15 Hz oscillatory power during the baseline period.
We hypothesized that increased 15 Hz power during the baseline period would be observed during CES compared with the no-stimulation condition and further, the magnitude of 15 Hz entrainment power in the motor cortex would significantly moderate theta-gamma cross-frequency coupling during movement differentially for stimulation and no-stimulation conditions. Finally, we evaluated whether the strength of cross-frequency coupling in the motor cortex was predictive of behavioral performance on our finger tapping task, regardless of stimulation condition. The MLM analyses were conducted by using the lme4 package in R and corrected for multiple comparisons by using Tukey's multiple-comparison test.
Results
There is considerable evidence supporting the use of peripheral somatosensory stimulation to enhance interactions between the peripheral–cortical somatosensory–motor pathways, thereby improving long-term motor performance in clinical populations (e.g., stroke). However, the impact of such stimulation on interactions between motor and somatosensory systems during concurrent behavior has yet to be examined, especially in the context of the healthy brain. To this end, 25 adults successfully completed our novel somatosensory–motor task during MEG.
Behavioral performance
Participants completed a quasi-paced finger tapping paradigm based on a clock-like pacing device concurrent with a continuous somatosensory entrainment protocol during MEG (Fig. 1). Irrespective of task manipulation (i.e., stimulation vs. no stimulation), participants performed well on the task with a mean overall accuracy of 94.8% (i.e., correctly responded when the red dot was within the blue target interval). We assessed measures of reaction time (i.e., response distance from target interval onset) and coefficient of variation to assess the variability in correct response times, given the quasi-paced nature of the task instructions (see the Experimental Paradigm section). Pairwise comparisons of reaction time (stim: M = 130.32 ms, SD = 42.62 ms; no stim: M = 129.31 ms, SD = 45.50 ms), coefficient of variation (stim: M = 45.57%, SD = 16.43%; no stim: M = 45.24%, SD = 13.86%), and task accuracy (stim: M = 93.26%, SD = 11.65%; no stim: M = 96.39%, SD = 5.60%) revealed no significant changes in behavioral performance during active somatosensory entrainment of the median nerve versus no such entrainment (ps > 0.205).
Sensor-level analysis
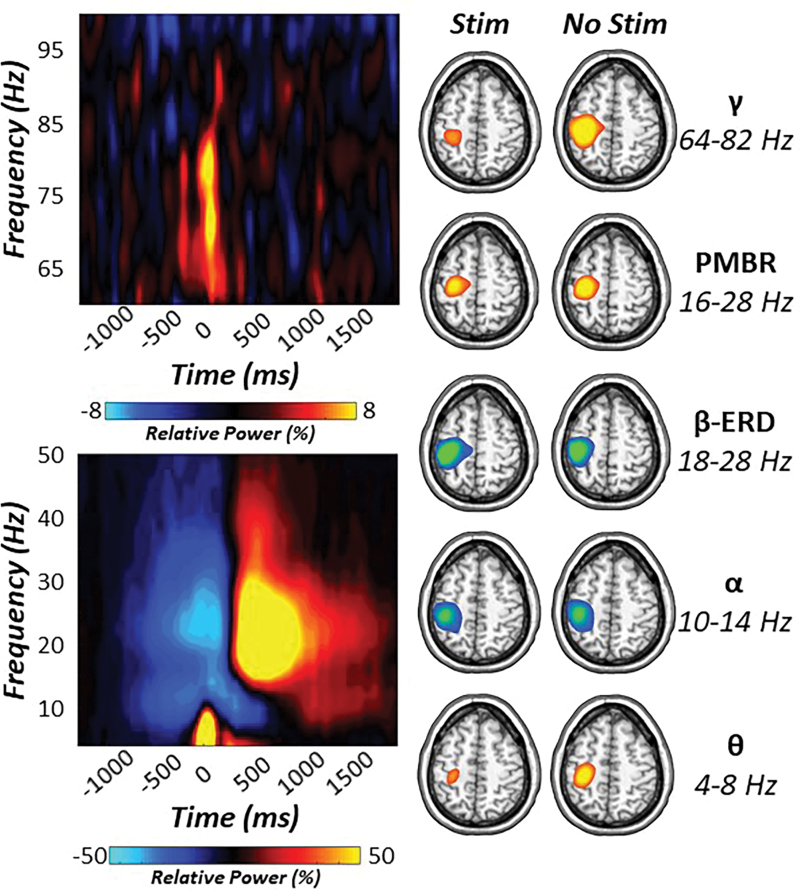
Statistical analysis of time–frequency spectrograms revealed significant movement-related oscillatory responses in the theta (4–8 Hz), alpha (10–14 Hz), beta (18–28 Hz), and gamma (64–82 Hz) bands in gradiometers near the sensorimotor strip across all participants and experimental conditions (p < 0.001, Fig. 2). Specifically, robust synchronizations in the theta band began about 125 ms before movement and tapered off about 250 ms later (i.e., −125 to 125 ms). Peri-movement neural responses in the alpha and beta range exhibited robust decreases (i.e., event-related desynchronization [ERD]) starting 300 ms before movement onset and lasting for about 200 ms after motor execution (i.e., −300 to 200 ms). In addition, a strong PMBR (16–28 Hz) was detected during the 350 to 850 ms window after movement onset. Finally, a robust movement-related gamma synchronization was observed in a subset of sensors over the contralateral sensorimotor cortices, and it began ∼125 ms before and lasted 125 ms after the movement was initiated (i.e., −125 to 125 ms).
FIG. 2.
Sensor- and source-level oscillatory responses to movement. (Left): Movement-locked time–frequency spectrograms for two sensors near the contralateral sensorimotor cortex. The x-axis denotes time (in ms) with the onset of movement occurring at 0 ms, and the y-axis represents frequency (Hz). Relative power (i.e., percent change from baseline −1300 to −800 ms) is expressed in color scale bars below each spectrogram. (Right): Significant oscillatory responses were imaged with a beamformer. Strong decreases in beta (18–28 Hz) activity were observed before and after movement onset and localized to the contralateral M1 for both experimental conditions (stim and no stim). Similarly, decreases in alpha activity (10–14 Hz) were also observed in the contralateral M1. In contrast, robust increases in theta (4–8 Hz) and gamma (64–82 Hz) activity were seen during movement onset in the left M1, whereas increases in PMBR (16–28 Hz) were seen after movement execution in M1 contralateral to movement. PMBR, post-movement beta rebound. Color images are available online.
Importantly, to assess the contribution of the evoked, phase-locked signal, we re-ran the sensor-level analyses with the time-domain averaged signal regressed out of the single-trial data. This analysis indicated almost identical time–frequency windows, as described earlier, suggesting that all five movement-related responses comprised predominantly nonphase-locked oscillatory activity.
Somatosensory entrainment modulates evoked and oscillatory profiles of movement
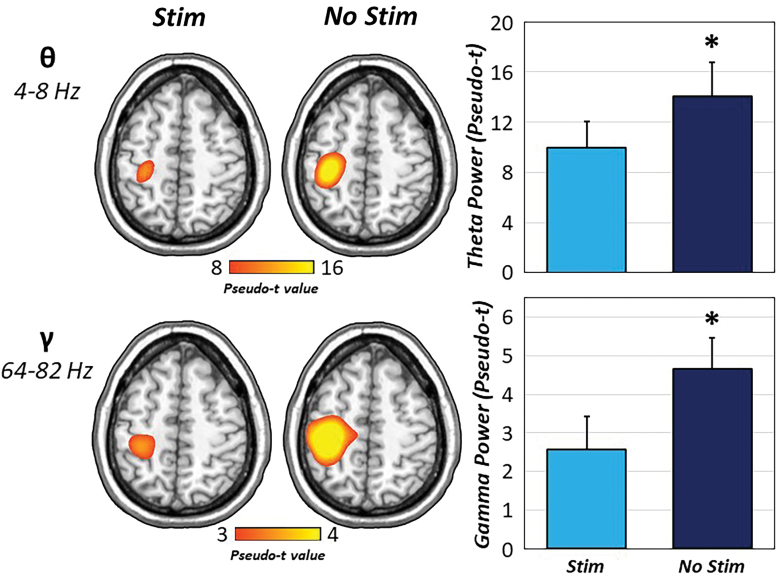
To identify the neural origin of the significant sensor-level time–frequency clusters, these windows were imaged by using a beamformer. The resulting maps indicated that all five oscillatory responses were centered on the contralateral M1, regardless of experimental condition (Fig. 2). As described in the methods, we examined the effect of somatosensory entrainment (i.e., stim vs. no stim) on each of these movement-related responses by using pairwise comparisons and Bayesian statistics. Interestingly, peri-movement theta and gamma activity revealed significant reductions in oscillatory power (pseudo-t) during CES compared with no stimulation [t(24) = −2.11, p = 0.046; t(24) = −2.06, p = 0.050; for theta and gamma, respectively; Fig. 3]. In contrast, oscillatory responses in the alpha and beta range were not affected by somatosensory entrainment, with Bayesian pairwise statistics indicating at least moderate evidence for the null hypothesis (alpha ERD: BF01 = 2.72, error % = 7.20 × 10−5; beta ERD: BF01 = 4.46, error % = 0.03; PMBR: BF01 = 2.50, error % = 7.09 × 10−5).
FIG. 3.
Somatosensory entrainment modulates theta and gamma oscillations. The results from pairwise t-tests of experimental condition (i.e., CES) on movement-related oscillatory activity revealed robust reductions in theta and gamma oscillatory power (pseudo-t) in the contralateral M1 when 15 Hz stimulation was applied to the right median nerve (light blue) relative to no peripheral stimulation (dark blue). *p < .05. Color images are available online.
To determine whether the effects of CES on movement-related oscillations were due to saturation of the somatosensory system by the electrical stimulation, absolute voxel time series were extracted from the left M1 in all participants and conditions for each frequency band to evaluate the effect of CES on spontaneous neural activity before movement. We hypothesized that if changes in peri-movement oscillatory power as a function of stimulation were due to saturation of the somatosensory system, then we should see differences in spontaneous neural activity before movement in relevant sensorimotor frequencies (Supplementary Fig. S2).
Pairwise t-tests revealed that spontaneous neural activity during the baseline was similar for both experimental conditions, and thus it was not affected by CES in any of the frequency bands relevant to our motor-related analyses (ps > 0.05). In fact, for the movement-related neural responses that exhibited significant alterations as a function of CES, pairwise Bayesian analyses revealed moderate evidence for the null hypothesis, suggesting that spontaneous neural activity during the baseline was similar across stimulation and no-stimulation conditions for theta and gamma oscillatory responses (theta: BF01 = 4.52, error % = 0.03; gamma: BF01 = 3.78, error % = 0.03; Supplementary Fig. S2).
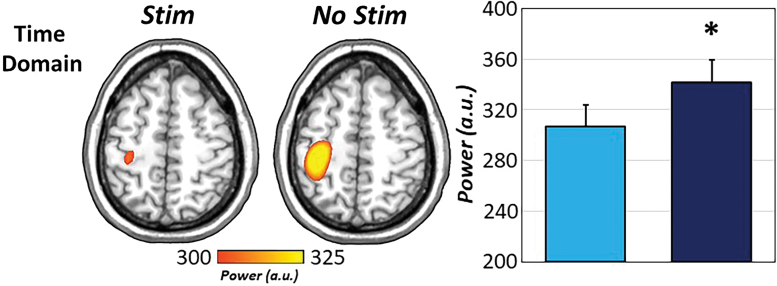
Finally, to interrogate the impact of CES on evoked motor responses, sLORETA source estimates were computed during motor execution (−125 to 125 ms) for all participants and task conditions and these were subjected to subsequent pairwise testing. Interestingly, evoked responses to movement were significantly reduced during CES compared with no stimulation, t(24) = −2.58, p = 0.017 (Fig. 4).
FIG. 4.
Movement-related evoked responses differ as a function of stimulation. Pairwise analysis of evoked movement-locked responses revealed significant reductions in sLORETA peak source estimates when 15 Hz CES (light blue) was applied to the right median nerve compared with when no stimulation was applied (dark blue). *p < .05. sLORETA, standardized low-resolution brain electromagnetic tomography. Color images are available online.
Theta-gamma cross-frequency coupling is attenuated during somatosensory entrainment
Given our results demonstrating spectrally specific disruptions in oscillatory power as a function of CES, a linear mixed-effects model was used to evaluate the effects of task condition (i.e., stim and no stim) on the cross-frequency coupling of theta and gamma activity at the single-trial level. Importantly, accumulating evidence suggests that the functional coupling of low (i.e., theta) and high (i.e., gamma) frequency oscillations is important for information transfer and neuronal communication during both higher-order cognitive tasks and motor performance (Bramson et al., 2018; Friese et al., 2013; Igarashi et al., 2013; Jensen and Colgin, 2007; Lisman and Jensen, 2013; Roux and Uhlhaas, 2014; Tort et al., 2009; von Nicolai et al., 2014; Voytek et al., 2015). Thus, we assessed whether this phenomenon was present as a function of our motor task and experimental manipulation (i.e., somatosensory entrainment).
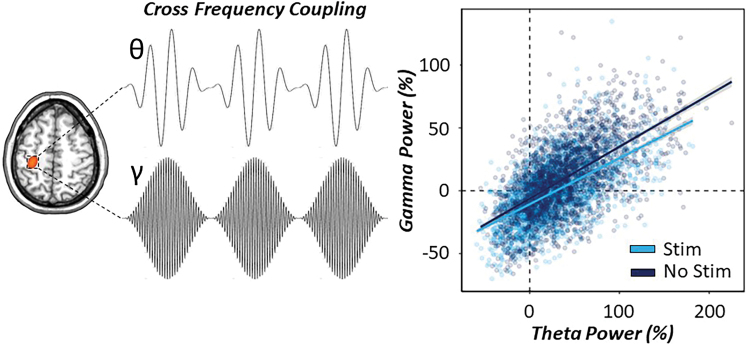
Briefly, as described in the methods, time series were extracted from the peak voxel in the left M1 and the spectral power envelope was computed for theta and gamma oscillatory responses at the single-trial level. Task condition (CES and no stim), theta power (continuous variable) and their interaction were treated as fixed effects, whereas subject and trial number were treated as a nested random effect to predict gamma oscillatory power. Our results indicated a significant main effect of theta power on gamma power, such that increases in peri-movement theta activity were significantly predictive of increases in gamma activity during movement onset [F(3838) = 310.10, p < 0.001]. As expected based on our previous analysis, there was also a main effect of condition, such that gamma power was significantly reduced during CES compared with no stimulation [F(3717) = 40.32, p < 0.001]. Finally, there was a significant theta by condition interaction on gamma oscillatory power, such that theta–gamma power to power coupling in the motor cortex was significantly reduced (i.e., shallower slope) during CES compared with no stimulation [F(3974) = 8.10, p = 0.004; Fig. 5].
FIG. 5.
Theta–gamma cross-frequency coupling during somatosensory entrainment. (Left): Conceptual image of power-to-power coupling of theta and gamma oscillations derived from peak activity in the contralateral M1 during movement onset. Significant cross-frequency coupling suggests that fluctuations in the power of slower (e.g., 4–8 Hz) oscillations are correlated with the fluctuations of faster (e.g., 70 Hz) gamma power oscillations. (Right): A single-trial linear mixed-effects model of theta oscillatory power and task condition on gamma oscillatory power during movement onset (−125 to 125 ms) was conducted to evaluate power-to-power coupling in our data. Relative theta power during movement onset was significantly predictive of MRGS power in the motor cortex and this was significantly modulated by task condition, such that application of 15 Hz CES (denoted in light blue) to the right median nerve significantly reduced the slope of theta–gamma power-to-power coupling compared with the no-stimulation condition (denoted in dark blue). Ninety-five percent confidence intervals are displayed in gray for each regression line. MRGS, movement-related gamma synchronization. Color images are available online.
The strength of somatosensory entrainment power moderates theta–gamma coupling
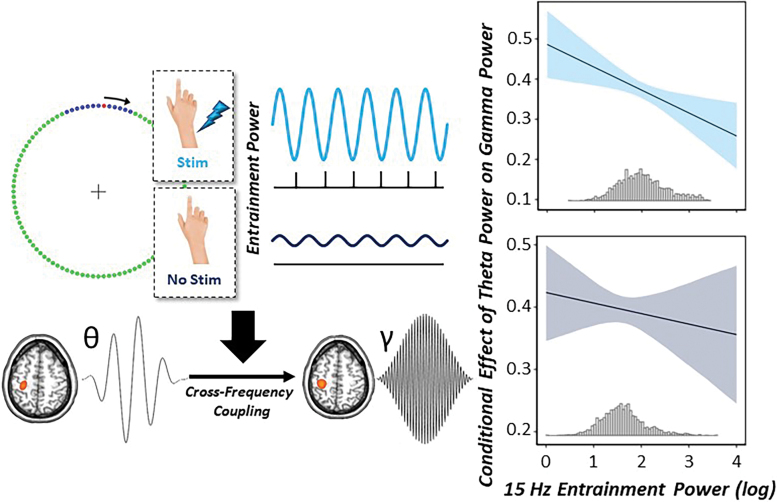
To directly interrogate whether the exogenous 15 Hz CES significantly modulated the strength of movement-related theta–gamma coupling, we next conducted a multilevel moderation analysis in R. For a conceptual model, see Figure 6. Briefly, we tested whether the magnitude of the neural response to somatosensory entrainment (i.e., 15 Hz power during the baseline) significantly moderated the relationship between theta and gamma oscillatory power in the motor cortex. We hypothesized that greater levels of 15 Hz spontaneous neural activity (i.e., somatosensory entrainment power: SE power) during the baseline would significantly reduce theta–gamma cross-frequency coupling in the motor cortex relative to lower levels of SE power at 15 Hz during the no-stimulation condition. The model included theta oscillatory power (continuous), condition (two levels), log-transformed 15 Hz spontaneous neural activity (SE power, continuous), theta by SE power (moderator), theta by condition, SE power by condition, and the three-way theta by SE power by condition interaction as fixed effects, with subject and trial number as a nested random effect.
FIG. 6.
Strength of entrainment power moderates movement-related cross-frequency coupling. (Left): Conceptual figure of the multilevel moderation analyses conducted using single-trial data. We hypothesized that the level of entrainment power that is modulated by CES (i.e., increased spontaneous power at 15 Hz with CES) would moderate the theta–gamma power to power coupling during movement onset in the left M1. (Right): Conditional effects of theta oscillatory power on gamma oscillatory power in the left M1 as a function of increasing 15 Hz entrainment power. The x-axis denotes log-transformed 15 Hz entrainment power during the baseline period in the left M1, whereas the y-axis denotes the conditional coefficients of theta power on gamma power in the left M1. Ninety-five percent confidence intervals are displayed in light and dark blue for stimulation and no-stimulation conditions, respectively. Below each regression line is a histogram displaying the distribution of the moderator (15 Hz entrainment power) for each condition. During 15 Hz CES, the conditional effect of theta–gamma power to power coupling significantly decreased with increasing levels of 15 Hz spontaneous neural activity during the baseline period. In contrast, in the absence of stimulation, there was no significant change in theta–gamma cross-frequency coupling as a function of increasing 15 Hz entrainment power. Color images are available online.
Interestingly, we observed significant main effects of theta oscillatory power [F(3731) = 287.63, p < 0.001], condition [F(3531) = 10.49, p = 0.001], and SE power [F(1802) = 166.20, p < 0.001] on gamma power in the motor cortex, such that increases in theta power were predictive of increased gamma power, CES significantly reduced gamma power compared with no stimulation, and increasing SE power during the baseline period was predictive of decreased gamma power in the motor cortex. In regard to our moderator variable (theta by SE power), we observed a significant interaction, such that with increased levels of 15 Hz somatosensory entrainment power in the motor cortex during the baseline, there were significant reductions in theta–gamma power to power coupling [F(3632) = 5.21, p < 0.001].
Finally, there was a significant three-way interaction (theta by SE power by condition), such that in the absence of stimulation, theta–gamma cross-frequency coupling was strong in the motor cortex and did not significantly change with increasing 15 Hz spontaneous neural activity (i.e., SE power). However, in the presence of CES, increased levels of 15 Hz SE power were associated with decreased theta–gamma oscillatory coupling in the left M1 contralateral to movement [F(3605) = 7.08, p = 0.008]. This suggests that there is a direct link between the strength of somatosensory entrainment induced by exogenous peripheral stimulation and the oscillatory dynamics of movement in the motor cortex (Fig. 6).
Cross-frequency coupling in the motor cortex predicts motor performance
Finally, we aimed at addressing whether the strength of movement-related theta–gamma power to power coupling in the motor cortex predicted behavioral performance on our simple finger-tapping paradigm. Briefly, given the lack of condition-wise differences in motor performance between our experimental manipulations (i.e., stim vs. no stim), we estimated the predictive capacity of cross-frequency coupling on reaction time, irrespective of task condition.
Specifically, we conducted a linear mixed-effects model of theta power (continuous), gamma power (continuous) and their interaction as fixed effects, with subject and trial number as a nested random effect to predict single-trial reaction times (i.e., distance from the onset of the blue target). Our results indicated a robust prediction of behavior by neuronal coupling, such that increased theta–gamma coupling in the motor cortex during movement onset was significantly predictive of faster reaction times, irrespective of task condition [F(3536) = 4.62, p = 0.032; Fig. 7].
FIG. 7.
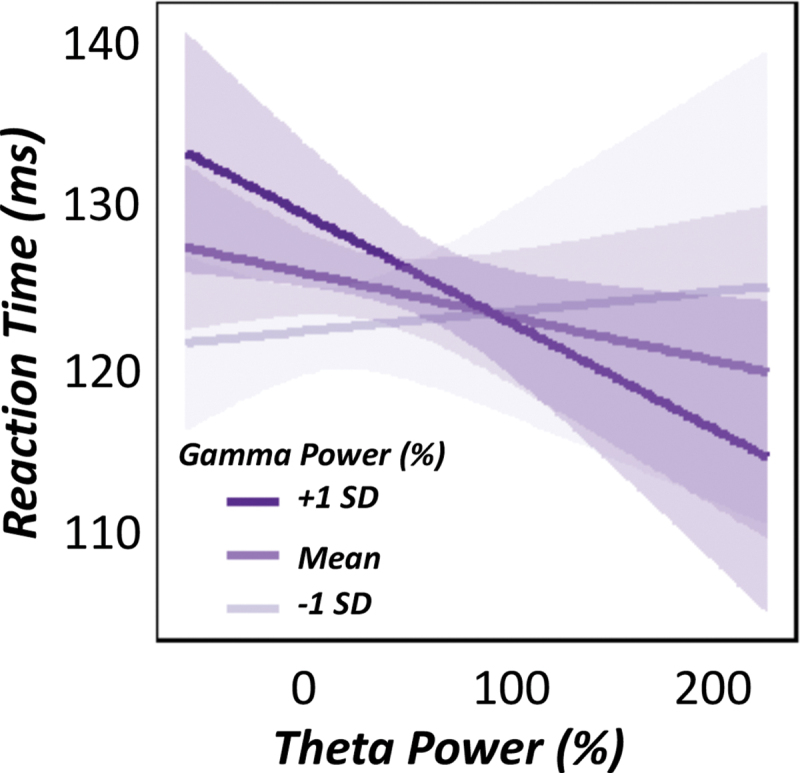
Theta–gamma coupling predicts motor performance. Linear mixed-effects model of theta power (continuous), gamma power (continuous) and their interaction on reaction time. Graphic denotes the relationship between relative theta power (%) on the x-axis and reaction time (ms) on the y-axis. Regression lines depicting the relationships for lower and higher gamma power are based on ±1 SD cutoffs of the mean relative gamma power (also displayed). These cutoffs were used for visualization purposes only, as relative gamma power was treated as a continuous variable for each model. Ninety-five percent confidence intervals are shown for each regression fit line. There was a significant theta x gamma power interaction, such that during instances of greater gamma power (e.g., +1 SD), greater theta responses in the contralateral M1 were predictive of faster reaction times, irrespective of task condition. SD, standard deviation. Color images are available online.
Discussion
Using a novel, simultaneous somatosensory entrainment-movement paradigm and advanced oscillatory and time-domain analysis of MEG data, we interrogated the dynamic interactions among somatosensory and motor systems during voluntary movement in a sample of healthy young adults. We observed significant reductions in the evoked time-domain response, as well as theta and gamma oscillations during motor execution, whereas oscillatory activity in the alpha and beta range were not affected by peripheral entrainment.
Interestingly, our novel paradigm elicited robust theta–gamma power to power cross-frequency coupling in the motor cortex contralateral to movement and we observed that 15 Hz entrainment of the somatosensory system (i.e., increased 15 Hz spontaneous power) significantly reduced this inherent theta–gamma coupling during finger tapping. Finally, our data connect stronger theta–gamma cross-frequency coupling within the motor cortex with faster reaction times, regardless of the presence of stimulation. The implications of these novel findings are discussed later.
Somatosensory entrainment of the periphery has long been used therapeutically to augment behavioral approaches (e.g., physical therapy) for improving motor function in those with substantial brain injuries (e.g., stroke) and/or neurological disorders. Collectively, previous work suggests that entrainment of the somatosensory system improves motor performance in clinical populations (Celnik et al., 2007; Klaiput and Kitisomprayoonkul, 2009; Koesler et al., 2009; Morera Maiquez et al., 2020; Tu-Chan et al., 2017; Wu et al., 2006), while hindering performance in healthy adults (e.g., pinch strength, reaction time, reach to grasp kinematics, etc.) (Koesler et al., 2008). Although paradigms such as these have been informative regarding the net effect of somatosensory input on motor outcomes, the underlying neuronal dynamics and coupling remain poorly understood. In the current study, we found significant reductions in movement-related oscillatory power in both low (i.e., theta) and high (i.e., gamma) frequencies in the contralateral primary motor cortex during somatosensory entrainment, with oscillatory power being relatively unchanged in the alpha and beta bands. Interestingly, the evoked response time-locked to movement onset was also significantly reduced during somatosensory stimulation.
To date, only a few studies have evaluated the impact of peripheral somatosensory stimulation on the spectrotemporal dynamics serving motor performance. In one study, Tu-Chan and colleagues (2017) evaluated resting-state neurophysiological activity before and after a 2-h somatosensory entrainment protocol (pulse trains applied at 10 Hz) in patients with stroke. Interestingly, the authors observed significant ipsilesional reductions in low-frequency spontaneous power (i.e., delta and theta) in the absence of movement and further, such changes were associated with improvements in finger fractionation outside the neuroimaging protocol (Tu-Chan et al., 2017). Although a comparison to our study is difficult given the vast differences in experimental design (e.g., stimulation duration/frequency, online vs. offline neurophysiological recording, clinical population studied), our results reasonably align with this previous work, suggesting that both low-frequency oscillations and evoked neural activity are attenuated during (or following) continuous somatosensory stimulation, and take this work in important new directions.
Our most important finding was likely the presence of theta–gamma power to power coupling in the left primary motor cortex during movement execution. Specifically, we observed robust movement-related theta–gamma cross-frequency coupling at the single trial level and further, this coupling was significantly reduced during somatosensory entrainment compared with when no stimulation was applied.
Importantly, our results also indicated that the stronger the 15 Hz entrainment power was during the baseline (i.e., before movement), the weaker the inherent theta–gamma coupling was during movement execution, thus directly linking movement-related neural dynamics with peripherally entrained spontaneous neural activity. Finally, this study is the first to link cross-frequency coupling in the motor cortex to concurrent behavior in humans, and our findings clearly indicated that stronger theta–gamma coupling in the primary motor cortex contralateral to movement was predictive of faster reaction times (i.e., response distance from target window onset), irrespective of stimulation protocol.
Invasive and noninvasive neurophysiological studies have broadly implicated the functional coupling of oscillations as a mechanism for information transfer and neuronal communication both locally and across the cortex, with the coupling of theta and gamma oscillations being particularly critical for cognitive processing (Jensen and Colgin, 2007; Lisman and Jensen, 2013). Specifically, theta and gamma oscillations are thought to be nested within one another, such that the phase and/or amplitude of the faster oscillation (i.e., gamma) is temporally coordinated with and modulated by the phase and/or amplitude of the slower moving oscillation (i.e., theta) to govern neural processing and subsequent behavior.
For example, there is a substantial amount of literature implicating theta–gamma cross-frequency coupling in associative learning (Lisman and Jensen, 2013; Tort et al., 2009), memory (Friese et al., 2013; Roux and Uhlhaas, 2014), and response interference processes in humans and animals (Bramson et al., 2018), with particular emphasis on the phase of theta oscillations modulating the amplitude of gamma activity. However, the functional coupling of these oscillations within the motor cortex, especially during movement, is less well understood. In animals, studies have shown that during movement (e.g., treadmill running, lever pull) and at rest, the rat motor cortex exhibits substantial theta–gamma coupling, and further, this coupling is often related to distinct phases of the movement being performed (e.g., lever pull vs. hold) (Igarashi et al., 2013; Johnson et al., 2017; von Nicolai et al., 2014).
In humans, noninvasive neuromodulatory and neuroimaging techniques have been used to probe similar interactions, although the results require careful interpretation, as they may not solely reflect sensorimotor processing in isolation from higher-order cognitive demands (Glim et al., 2019; Voytek et al., 2015). Importantly, the current study was the first to robustly link theta and gamma oscillations within the human motor cortex during a sensorimotor paradigm and further relate the strength of this functional coupling to changes in behavioral performance. Our results also provide new insights into a pathway by which entrainment of the somatosensory system via peripheral stimulation modulates neural dynamics in the motor cortex by altering neuronal communication among the sensorimotor oscillations that are important for the execution of voluntary movements.
Mechanistically, such changes in oscillatory power and cross-frequency theta–gamma coupling induced by 15 Hz peripheral somatosensory entrainment may reflect local alterations in cortical excitability and/or GABAergic inhibitory drive. For example, using transcranial magnetic stimulation (TMS), studies have shown that after long bouts of peripheral stimulation, excitability of the motor cortices tends to increase, as evidenced by increases in the motor-evoked potentials (MEP) elicited by TMS (Celnik et al., 2007; Kaelin-Lang et al., 2002; Ridding et al., 2000; Schabrun et al., 2012). Similarly, repetitive peripheral electrical stimulation has been associated with robust increases in the somatosensory evoked potential, measured outside of the entrainment protocol (Schabrun et al., 2012; Veldman et al., 2018).
Importantly, these changes in sensorimotor cortical excitability seem to be consistent regardless of health or disease states, suggesting that the directional changes in behavioral outcomes (i.e., behavioral decrements or improvements) may be dependent on the baseline state of the participant. In other words, if a disease is associated with hypo-excitability, then the stimulation can lead to improvement, but in other cases (e.g., normo-excitability) the stimulation may lead to decrements in performance.
Such changes in cortical excitability may involve alterations in GABAergic interneuronal pools (Celnik et al., 2007; Kaelin-Lang et al., 2002). Specifically, a host of electrophysiological studies have long implicated both endogenous and exogenous GABA-mediated neuronal drive as essential for the modulation of local pyramidal cells, giving rise to specific oscillatory activity that is thought to underlie cognitive and sensorimotor processes (Bartos et al., 2007; Buzsáki and Wang, 2012; Edden et al., 2009; Fries, 2009, 2015; Fries et al., 2007; Gaetz et al., 2011; Johnson et al., 2017; Muthukumaraswamy et al., 2009; Singer, 1999; Uhlhaas and Singer, 2012; Uhlhaas et al., 2009; Vinck et al., 2013).
In regard to the current study, we found significant reductions in theta and gamma response power, concomitant with changes in the inherent functional coupling of these sensorimotor oscillations. These results may reflect a direct effect of altered GABA activity, as previous studies have effectively linked activity in local GABA-mediated circuits to the generation and modulation of theta and/or gamma oscillatory activity (Gaetz et al., 2011; Gao et al., 2013; Johnson et al., 2017). Critically, such modulation has been shown through pharmacological manipulations of GABA (e.g., GABA antagonist administration increases theta power and decreases gamma power) (Johnson et al., 2017), as well as by studying naturally occurring differences in healthy and aging adults (Gaetz et al., 2011; Gao et al., 2013). The TMS studies have also demonstrated the same link between GABAergic activity and such neural oscillations (Nowak et al., 2017).
In regard to somatosensory entrainment, we propose that changes in movement-related theta and gamma dynamics may be the result of changes in GABAergic inhibitory function induced by the peripheral entrainment itself (i.e., at 15 Hz beta frequency). For example, numerous studies have implicated increased GABA activity in the motor cortex with increased spontaneous beta power (e.g., 15–30 Hz) and subsequent beta oscillations during movement (Gaetz et al., 2011; Hall et al., 2011; Muthukumaraswamy et al., 2013). As a result of our somatosensory entrainment protocol, we effectively increased the power of 15 Hz oscillatory activity during the baseline period when stimulation was applied and further, the greater the 15 Hz oscillatory power in the motor cortex induced by CES, the weaker the theta–gamma cross-frequency coupling was during movement execution. Thus, peripheral entrainment of neural oscillations at 15 Hz may exogenously alter GABA levels, giving rise to the decreased oscillatory power in the theta and gamma range and subsequent alterations in movement-related coupling outside of the entrained frequency.
The idea that local GABAergic circuits play a role in the peripheral–cortical pathway during (or after) electrical stimulation is also corroborated by previous work administering GABAA receptor agonists (i.e., lorazepam), effectively blocking the increased MEP (i.e., cortical excitability) induced by repetitive somatosensory stimulation (Kaelin-Lang et al., 2002). Taken together, these findings suggest that the neural mechanisms governing sensorimotor interactions along the peripheral–cortical pathway may be GABA-mediated, and although we did not explicitly measure GABA concentrations in our participants, future work in this area would be incredibly informative to fully unravel the sensorimotor milieu during movement.
Conclusions
To conclude, we developed a novel simultaneous somatosensory–motor paradigm using CES of the periphery, which induced reductions in low- and high-frequency oscillatory activity and further, led to substantial decreases in the functional coupling of theta and gamma oscillatory power in the motor cortex. We also demonstrated a groundbreaking link between cross-frequency coupling in the motor cortex, regardless of stimulation protocol, and strong increases in theta–gamma coupling, which were predictive of faster reaction times on our motor task.
Although previous studies have established the static changes in neurophysiology associated with peripheral stimulation (e.g., increased cortical excitability) and subsequent motor action (e.g., improved performance), the spectral and temporal interplay among sensorimotor brain regions during active movement was largely unknown, as the interrogation of these systems has primarily been conducted in isolation before this study. Ultimately, our data provide critical insights on how continuous input to the somatosensory system modulates cortical oscillations serving motor control and subsequent performance, and implicates the dynamic coupling of multispectral oscillatory responses in the performance of simple finger-tapping paradigms.
Although our goal was to understand the neural population-level dynamics underlying motor control, the current data may also be incredibly informative for neurorehabilitation. Based on our findings, targeting the multispectral coupling of cortical oscillations pertinent to the execution of voluntary movements may especially impact clinical outcomes.
Supplementary Material
Acknowledgments
The authors would like to thank all their volunteers for participating in the study, as well as their staff and collaborators for their contributions.
Authors' Contributions
Conceptualization (R.K.S. and T.W.W.), Methodology (R.K.S. and A.I.W.), Formal analysis (R.K.S. and A.I.W.), Investigation (R.K.S.), Writing—Original draft (R.K.S.), Writing—Review and editing (R.K.S., A.I.W. and T.W.W.), Resources (T.W.W.), Supervision (T.W.W.), Project administration (R.K.S. and T.W.W.), and Funding Acquisition (R.K.S., A.I.W. and T.W.W.).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was supported by grants R01-MH116782 (Tony W. Wilson), R01-MH118013 (Tony W. Wilson), R01-DA047828 (Tony W. Wilson), RF1-MH117032 (Tony W. Wilson), T32-NS105594 (Rachel K. Spooner), and F31-AG055332 (Alex I. Wiesman) from the National Institutes of Health, grant #1539067 from the National Science Foundation (Tony W. Wilson), and the NASA Nebraska Space Grant Fellowship (Rachel K. Spooner).
Supplementary Material
References
- Bartos M, Vida I, Jonas P. 2007. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56. [DOI] [PubMed] [Google Scholar]
- Bramson B, Jensen O, Toni I, et al. 2018. Cortical oscillatory mechanisms supporting the control of human social-emotional actions. J Neurosci 38:5739–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ. 2012. Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Hummel F, Harris-Love M, et al. 2007. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil 88:1369–1376. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Ferreiro KN, Tomasi C, et al. 2010. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair 24:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Muthukumaraswamy SD, Freeman TC, et al. 2009. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 29:15721–15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. 2010. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 20:156–165. [DOI] [PubMed] [Google Scholar]
- Ernst M. 2004. Permutation methods: a basis for exact inference. Stat Sci 19:10. [Google Scholar]
- Fries P. 2009. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32:209–224. [DOI] [PubMed] [Google Scholar]
- Fries P. 2015. Rhythms for cognition: communication through coherence. Neuron 88:220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Nikolić D, Singer W. 2007. The gamma cycle. Trends Neurosci 30:309–316. [DOI] [PubMed] [Google Scholar]
- Friese U, Köster M, Hassler U, et al. 2013. Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage 66:642–647. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, et al. 2011. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage 55:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Liu C, Zhu H, et al. 2013. Evidence for a motor gamma-band network governing response interference. Neuroimage 74:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, et al. 2010. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage 51:792–807. [DOI] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, et al. 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehringer JE, Arpin DJ, VerMaas JR, et al. 2019. The strength of the movement-related somatosensory cortical oscillations differ between adolescents and adults. Sci Rep 9:18520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glim S, Okazaki YO, Nakagawa Y, et al. 2019. Phase-amplitude coupling of neural oscillations can be effectively probed with concurrent TMS-EEG. Neural Plast 2019:6263907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golaszewski SM, Bergmann J, Christova M, et al. 2012. Modulation of motor cortex excitability by different levels of whole-hand afferent electrical stimulation. Clin Neurophysiol 123:193–199. [DOI] [PubMed] [Google Scholar]
- Grent-‘t-Jong T, Oostenveld R, Jensen O, et al. 2013. Oscillatory dynamics of response competition in human sensorimotor cortex. Neuroimage 83:27–34. [DOI] [PubMed] [Google Scholar]
- Grent-‘t-Jong T, Oostenveld R, Jensen O, et al. 2014. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J Neurophysiol 112:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, et al. 2001. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SD, Stanford IM, Yamawaki N, et al. 2011. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage 56:1506–1510. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Arpin DJ, Wilson TW. 2016. Cue-related temporal factors modulate movement-related beta oscillatory activity in the human motor circuit. J Cogn Neurosci 28:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Hoburg JM, Wilson TW. 2017a. The peak frequency of motor-related gamma oscillations is modulated by response competition. Neuroimage 165:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Kurz MJ, Gehringer JE, et al. 2017b. The functional role of post-movement beta oscillations in motor termination. Brain Struct Funct 222:3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. 2015. Coding complexity in the human motor circuit. Hum Brain Mapp 36:5155–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, et al. 2005. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J, Isomura Y, Arai K, et al. 2013. A θ–γ oscillation code for neuronal coordination during motor behavior. J Neurosci 33:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Colgin LL. 2007. Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci 11:267–269. [DOI] [PubMed] [Google Scholar]
- Johnson NW, Özkan M, Burgess AP, et al. 2017. Phase-amplitude coupled persistent theta and gamma oscillations in rat primary motor cortex in vitro. Neuropharmacology 119:141–156. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, et al. 2006. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage 32:1281–1289. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, et al. 2002. Modulation of human corticomotor excitability by somatosensory input. J Physiol 540:623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiput A, Kitisomprayoonkul W. 2009. Increased pinch strength in acute and subacute stroke patients after simultaneous median and ulnar sensory stimulation. Neurorehabil Neural Repair 23:351–356. [DOI] [PubMed] [Google Scholar]
- Koesler IB, Dafotakis M, Ameli M, et al. 2008. Electrical somatosensory stimulation modulates hand motor function in healthy humans. J Neurol 255:1567–1573. [DOI] [PubMed] [Google Scholar]
- Koesler IB, Dafotakis M, Ameli M, et al. 2009. Electrical somatosensory stimulation improves movement kinematics of the affected hand following stroke. J Neurol Neurosurg Psychiatry 80:614–619. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Gander PE. 2016. The demodulated band transform. J Neurosci Methods 261:135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM, et al. 2018. Haptic exploration attenuates and alters somatosensory cortical oscillations. J Physiol 596:5051–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Jensen O. 2013. The θ-γ neural code. Neuron 77:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Morera Maiquez B, Sigurdsson HP, Dyke K, et al. 2020. Entraining movement-related brain oscillations to suppress tics in Tourette syndrome. Curr Biol 30:2334–2342.e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. 2010. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol 104:2873–2885. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, et al. 2009. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A 106:8356–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JF, Wilson SJ, et al. 2013. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage 66:36–41. [DOI] [PubMed] [Google Scholar]
- Nowak M, Hinson E, van Ede F, et al. 2017. Driving human motor cortical oscillations leads to behaviorally relevant changes in local GABA. J Neurosci 37:4481–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostopoulos D, Cooper R, Crow HJ. 1975. Inhibition of cortical evoked potentials and sensation by self-initiated movement in man. Nature 258:321–324. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. 2002. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110:1842–1857. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Miles TS, et al. 2000. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res 131:135–143. [DOI] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. 2014. Working memory and neural oscillations: α-γ versus θ-γ codes for distinct WM information? Trends Cogn Sci 18:16–25. [DOI] [PubMed] [Google Scholar]
- Schabrun SM, Ridding MC, Galea MP, et al. 2012. Primary sensory and motor cortex excitability are co-modulated in response to peripheral electrical nerve stimulation. PLoS One 7:e51298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Fetz EE. 2012. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci 32:890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz EE. 2003. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6:1309–1316. [DOI] [PubMed] [Google Scholar]
- Singer W. 1999. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24:49–65, 111–125. [DOI] [PubMed] [Google Scholar]
- Spooner RK, Eastman JA, Rezich MT, et al. 2020a. High-definition transcranial direct current stimulation dissociates fronto-visual theta lateralization during visual selective attention. J Physiol 598:987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Mills MS, et al. 2018. Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. Neuroimage Clin 20:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Proskovec AL, et al. 2019. Rhythmic spontaneous activity mediates the age-related decline in somatosensory function. Cereb Cortex 29:680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Proskovec AL, et al. 2020b. Prefrontal theta modulates sensorimotor gamma networks during the reorienting of attention. Hum Brain Mapp 41:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J. 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51:1759–1768. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. 2005. Applications of the signal space separation method. IEEE Trans Signal Process 53:3359–3372. [Google Scholar]
- Thut G, Veniero D, Romei V, et al. 2011. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol 21:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini A, Ambrogioni L, Medendorp WP, et al. 2017. Theta oscillations locked to intended actions rhythmically modulate perception. Elife 6:e25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, et al. 2009. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A 106:20942–20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu-Chan AP, Natraj N, Godlove J, et al. 2017. Effects of somatosensory electrical stimulation on motor function and cortical oscillations. J Neuroeng Rehabil 14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, et al. 2010. Beta-band activity during motor planning reflects response uncertainty. J Neurosci 30:11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, et al. 2009. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2012. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron 75:963–980. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. 1997. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35:135–140. [DOI] [PubMed] [Google Scholar]
- Veldman MP, Maurits NM, Zijdewind I, et al. 2018. Somatosensory electrical stimulation improves skill acquisition, consolidation, and transfer by increasing sensorimotor activity and connectivity. J Neurophysiol 120:281–290. [DOI] [PubMed] [Google Scholar]
- Vinck M, Womelsdorf T, Buffalo EA, et al. 2013. Attentional modulation of cell-class-specific gamma-band synchronization in awake monkey area v4. Neuron 80:1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Nicolai C, Engler G, Sharott A, et al. 2014. Corticostriatal coordination through coherent phase-amplitude coupling. J Neurosci 34:5938–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Kayser AS, Badre D, et al. 2015. Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat Neurosci 18:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Koshy SM, Heinrichs-Graham E, et al. 2020. Beta and gamma oscillations index cognitive interference effects across a distributed motor network. Neuroimage 213:116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW. 2020. Attention modulates the gating of primary somatosensory oscillations. Neuroimage 211:116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Fleischer A, Archer D, et al. 2011a. Oscillatory MEG motor activity reflects therapy-related plasticity in stroke patients. Neurorehabil Neural Repair 25:188–193. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, et al. 2010. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn 73:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, et al. 2011b. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol 36:596–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Seo HJ, Cohen LG. 2006. Influence of electric somatosensory stimulation on paretic-hand function in chronic stroke. Arch Phys Med Rehabil 87:351–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.