Abstract
Sexual dimorphisms in humans and other species exist in visually evident features such as body size and less apparent characteristics, including disease prevalence. Current research is adding to a growing understanding of sex differences in stem cell function and response to external stimuli, including sex hormones such as estrogens. These differences are proving significant and directly impact both the understanding of stem cell processes in tissue repair and the clinical implementation of stem cell therapies. Adult stem cells of the musculoskeletal system, including those used for development and repair of muscle, bone, cartilage, fibrocartilage, ligaments, and tendons, are no exception. Both in vitro and in vivo studies have found differences in stem cell number, proliferative and differentiation capabilities, and response to estrogen treatment between males and females of many species. Maintaining the stemness and reducing senescence of adult stem cells is an important topic with implications in regenerative therapy and aging. As such, this review discusses the effect of estrogens on musculoskeletal system stem cell response in multiple species and highlights the research gaps that still need to be addressed. The following evidence from investigations of sex-related phenotypes in adult progenitor and stem cells are pieces to the big puzzle of sex-related effects on aging and disease and critical information for both fundamental tissue repair and regeneration studies and safe and effective clinical use of stem cells.
Impact Statement
This review summarizes current knowledge of sex differences in and the effects of estrogen treatment on musculoskeletal stem cells in the context of tissue engineering. Specifically, it highlights the impact of sex on musculoskeletal stem cell function and ability to regenerate tissue. Furthermore, it discusses the varying effects of estrogen on stem cell properties, including proliferation and differentiation, important to tissue engineering. This review aims to highlight the potential impact of estrogens and the importance of performing sex comparative studies in the field of tissue engineering.
Keywords: estrogen, sexual dimorphism, musculoskeletal stem cells, bone marrow mesenchymal stromal cells, adipose derived stem cells
Introduction
Studies of sex-based differences in humans have traditionally focused on visually evident features, including body size, anatomical differences, and life span. Before the encouragement of the National Institutes of Health (NIH) to include sex as a variable, most studies across mammalian species used solely male specimens. Reasons for this include concerns about complications due to the estrous cycle in females, the pressures of convention, and a lack of understanding of the potential effect of sex on results.1 These one-sided studies obscure important sex differences that could otherwise aid in future study design and discoveries. Furthermore, not including both sexes contributes to the lack of reproducibility in preclinical research,2 supported by the fact that women experience more adverse drug reactions than men.3
In a PubMed search of tissue engineering and regenerative medicine publications from 2019, only 28.4% of the 10,651 publications reported subject sex at all (Fig. 1). Of that subset of studies, only 38% reported using both male and female samples. Such issues highlight the need for including sex as a variable in preclinical studies, specifically those focused on regenerative therapies.
FIG. 1.
Percentage of tissue engineering and regenerative medicine publications with sex MeSH terms in 2019. A PubMed search was performed using: ““tissue engineering” OR “regenerative medicine” NOT review. PubMed's sex filters were used to determine the numbers of publications tagged with the MeSH terms “Male” and/or “Female” with results limited to 2019 using PubMed's year filter. MeSH, medical subject headings. Color images are available online.
Sexual dimorphism is seen in many diseases, including those of the musculoskeletal system. The reasons for such differences are manifold, complex, and not completely understood but include differences in joint and muscle anatomy, tissue mechanics, and both levels and signaling mechanisms of sex hormones.4 Sex-based differences are seen across a wide range of ages. Henschke et al. found a stepwise increase in the rate of musculoskeletal disorders in males and females from age 0 to 14 years, with differences in rates between sexes only appearing in the oldest groups.5 A study comparing adolescent athletes aged 12–17 to general population controls found that females in both groups had higher prevalence of symptoms in most body regions compared to age-matched males, while males in both groups had higher prevalence for elbow symptoms.6,7
Males and females experience aging in different ways, with sex hormones gradually decreasing as males age but rapidly declining in females during menopause.8 Epidemiological evidence illustrates the effect these natural changes in hormone reduction have on tissue homeostasis and function. Wolf et al. reviewed musculoskeletal disease rates in males and females4 and found differences in rates for many conditions, including joint injuries and osteoporosis. Females, especially after menopause, are more likely to develop osteoarthritis than males, and their disease is typically more severe.9,10 Although the reasons for these discrepancies are not fully understood, sex-based differences in cells' response to the microenvironment likely contribute.
Sex-based differences are found in stem cells from various tissues, including those of the musculoskeletal system, and have been shown to affect their therapeutic potential. The inherent ability to self-renew, produce trophic factors to stimulate and organize surrounding cells for repair, and differentiate into mature cell phenotypes makes stem cells a vital component of tissue engineering and regenerative therapies. Differences between sexes have been seen in musculoskeletal stem cell number, proliferation, and differentiation. Differences in patient relapse rates and nonrelapse mortality after allogenic hematopoietic stem cell transplants provide clinical evidence of the importance of stem cell donor sex to regenerative therapy.11
Animal studies have also found sex-based differences in the therapeutic potential of stem cells. For example, bone marrow-derived mesenchymal stem cells from female mice better aid in rat cardiac recovery after ischemia and endotoxemia than male cells,12,13 but male muscle-derived stem cells (MDSCs) have been found to heal defects in bone and cartilage more effectively.14–16 Many, although not all, of these differences have been linked to estrogens. For this reason, this review is focused on estrogen signaling and this hormone class' control of these stem cell processes.
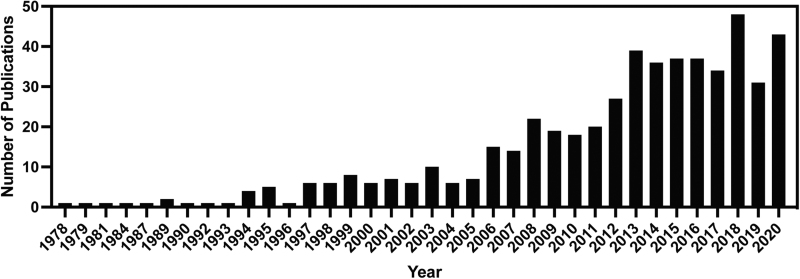
Maintaining the stemness and reducing senescence of stem cells is an important topic with implications in regenerative therapy and aging. The significance of the role sexual dimorphism plays in these processes is highlighted by the prevalence of this topic in previous reviews17–21 and the increasing number of publications on the topic (Fig. 2). The current review provides an updated and focused compilation of the effects of estrogens, most often 17β-estradiol (E2), on musculoskeletal stem cell processes critical for tissue engineering and stem cell therapies. The goal of this review is to compile what is known and highlight the research gaps that still need to be addressed to advance tissue engineering and regenerative therapies.
FIG. 2.
Publications on estrogen and musculoskeletal stem cells by year in PubMed. Search was performed on March 5, 2021 using the following search terms: estrogen AND stem cell AND (bone OR muscle OR adipose OR cartilage OR tendon OR Ligament) AND (proliferation OR apoptosis OR senescence OR viability OR differentiation) NOT (cardiovascular OR cancer OR urogenital system OR hematopoiesis). As of October 2021, there were already 44 publications that fit this search for 2021.
Sex-Based Differences in Musculoskeletal Stem Cells
Sex-based differences in cell number, proliferative ability, and differentiation potential occur in bone marrow-derived mesenchymal stromal cells (BM-MSCs), adipose-derived stem cells (ASCs), and MDSCs from multiple species. In general, males have more BM-MSCs with higher differentiation potential than females, although sex is typically not a factor in proliferation. Fewer BM-MSCs have been found in the bone marrow of female mice22 and rats23 than in males. It is hypothesized that this difference in progenitor cell number contributes to the ability of male rats to heal more efficiently after femoral bone defects than female rats.23 Katsara et al. found that male mouse BM-MSCs showed stronger osteogenic and adipogenic potential than female cells, although sex did not affect proliferative abilities.22 Similarly, no sex-related differences were seen in proliferation or senescence in BM-MSCs isolated from rats.23
Li et al., although, found that female smooth muscle progenitor cells derived from both embryonic stem cell lines and induced pluripotent stem cells were more proliferative than their male counterparts. Furthermore, female progenitor cells derived from induced pluripotent stem cells expressed more estrogen receptor β (ERβ) than male cells, but both sexes expressed equivalent levels of ERα.24 Depending on the tissue source and study design, nuclear ER expression levels have been shown to either be comparable or differentially expressed comparing ERα and ERβ between male and female cells. As such, it is not clear whether ER expression levels are a major factor in sex-related differences observed in stem cell behavior.
Sexual dimorphism in differentiation is seen in human ASCs. Aksu et al. found that male ASCs showed greater osteogenic differentiation compared to cells from female patients.25 Bianconi et al. used Transcriptome Mapper to analyze gene expression data for human ASCs from the Gene Expression Omnibus. Analysis of data from 12 males and 33 females between 18 and 71 years old revealed many chromosomal segments, and individual genes were differentially expressed between the sexes, including some related to differentiation.26
In general, male MDSCs have greater differentiation capabilities than female cells. In vitro studies show that MDSCs isolated from male mice have greater osteogenic27 and chondrogenic14 potential than those isolated from female mice. Similar differences were seen in human MDSCs, with male cells having greater chondrogenic and osteogenic potential than female cells.16 Deasy et al. found that male mouse MDSCs differentiate more after oxidative stress, potentially leading to a quicker depletion of the stem cell population than is seen for female cells.28
As seen in in vitro studies, in vivo studies reveal sex differences in differentiation potential, with male cells having the greater ability to regenerate tissue in most reports. Several studies have explored the regenerative capabilities of mouse MDSCs genetically engineered to express bone morphogenetic protein 4 (MDSC-BMP4). Male MDSC-BMP4 cells were better able to generate ectopic bone in sex-matched mice27 and articular cartilage in female rats14 than female MDSC-BMP4 cells. In a similar study, male mouse MDSC-BMP4 cells were implanted ectopically and into cranial defects in both unaltered and gonadectomized male and female mice. For both types of implants, male hosts showed greater bone formation than female hosts.15
Similar sex differences are seen in the bone-forming capabilities of human MDSCs genetically engineered to express bone morphogenetic protein 2 (hMDSC-BMP2) as were seen in mouse MDSCs. When hMDSC-BMP2 cells were implanted into calvarial bone defects in mice, cells of both sexes were able to regenerate bone, but male cells did so more efficiently.16 Conversely, Deasy et al. found that MDSCs from female mice regenerated skeletal muscle in mice more efficiently than cells from males.28 Overall, male musculoskeletal stem cells exhibit enhanced differentiation capacity compared to female cells.
Estrogens and Musculoskeletal Stem Cells
In the following sections, studies are divided first according to stem cell phenotype. Where applicable, they are then subdivided into in vitro and in vivo studies and again by species. Some studies are discussed in multiple sections. General trends are illustrated in Figure 3. Tables 1–4 contain summaries of all reviewed studies.
FIG. 3.
Summary of the effects of E2 on stem cells of the musculoskeletal system. The knee is used as a placeholder for other tissues due to the fact that it contains all tissue types of interest. Effects shown are the general trends for each cell type given the information presented in this review. Figure created using BioRender.com ASCs, adipose-derived stem cells; BM-MSCs, bone marrow-derived stromal cells; CPCs, chondrogenic progenitor cells; E2, 17β-estradiol; MDSCs, muscle-derived stem cells; PDLSCs, periodontal ligament stem cells; TDSCs, tendon-derived stem cells. Color images are available online.
Table 1.
Role of Estrogens on Bone Marrow-Derived Stromal Cell Stemness
| Cell type | Bone marrow derived stromal cells |
|||||
|---|---|---|---|---|---|---|
| Sex | Animal model, age | In vitro, in vivo | Hormone treatment | Response | Study | |
| Mouse cells | ||||||
| BM-MSCs | F and M | C57 mice, 8-week old | In vitro | None | After stress by LPS treatment or hypoxia: F: increased VEGF compared to M M: increased TNF and IL-6 compared to F; hypoxia induced more apoptosis compared to F |
Crisostomo et al.12 |
| BM-MSCs | F and M | BALB/c mice of different ages | In vitro | None | F and M: higher passages decreased adipogenic potential and increased osteogenic potential M: greater potential toward both adipogenic and osteogenic lineages compared to F |
Katsara et al.22 |
| BM-MSCs | F | C57/BL6 mice, 8-week old | In vitro | ovx | ovx: increased adipogenic markers; decreased osteogenic markers | Qi et al.31 |
| BM-MSCs | F | Swiss-Webster mice, 7-month old | In vitro | 10−10–10−6 M E2; ovx | E2 treatment: increased proliferation and differentiation to osteoblasts; decreased apoptosis; increased the expression of ERα; decreased the expression of ERβ ovx: proliferation and differentiation were lower than in cells from sham mice; apoptosis was higher. |
Zhou et al.29 |
| Bone marrow-derived stroma cell line ST2 stably overexpressing human ERα or ERβ | N/A | Mouse, age N/A | In vitro | 0–1 nM E2 | Cotreatment of cells with estrogen and (BMP)-2: increased osteogenesis compared to cells treated with just BMP-2 Treatment with E2: decreased adipogenesis |
Okazaki et al.33 |
| BM-MSCs | N/A | C57BL/6 mice, age N/A | In vitro | 10 nM E2 | E2 treatment: increased osteogenesis | Pang et al.32 |
| BM-MSCs | F | C57BL/6 mice, 8-week old | In vitro | 10−7 M E2; ovx | ovx mice: BM-MSCs more senescent, less proliferative, and lower osteogenic potential than those from sham animals; these deficiencies were alleviated by E2 treatment; effects linked to the JAK2/STAT3 pathway. | Wu et al.30 |
| Rat cells | ||||||
| BM-MSCs | F and M | Sprague-Dawley rats, 12-month old | In vitro | None | No sex related differences in proliferation, differentiation, or senescence F: fewer BM-MSCs compared to M M: MSCs showed superior healing compared to F |
Strube et al.23 |
| BM-MSCs | F | Sprague-Dawley rats, 12-week old | In vitro | ovx | ovx: reduced pluripotency and increased senescence through ERβ-SATB2 | Wu et al.34 |
| BM-MSCs | F and M | F-344 rats, 3-month old | In vitro | 10−6–10−12 M E2 | E2 treatment: F: lower concentrations increased proliferation rate and osteogenic potential M: no change in proliferation rate; increased osteogenic potential |
Hong et al.36 |
| BM-MSCs | F | Sprague-Dawley rats, 9-week old | In vitro | 10−7 M E2; ovx | E2 treatment: increased colony numbers and number of cells per colony of cells; no effect on osteogenic potential; decreased adipogenic potential; decreased apoptosis | Ayaloglu-Butun et al.35 |
| BM-MSCs | F | Sprague-Dawley rats, 8-week old | In vitro | 1 nM E2 | E2 treatment: increased number of cells in the S-phase; increased osteogenic differentiation; decreased chondrogenesis activated MAPK pathway | Zhao et al.37 |
| BM-MSCs | M | Sprague-Dawley rats, 4-week old | In vitro | 0, 1, 10, 100, 500, 1000 nM E2 | E2 treatment: no effect on viability; dose-dependent increases in osteogenesis. | Liu et al.38 |
| Larger animal cells | ||||||
| BM-MSCs | F and M | Mini-pig, 1-year old | In vitro | 0, 10−6, 10−8, 10−10, 10−10, 10−12, 10−14 M E2 | E2 treatment: reduced apoptosis-related gene expression and increased chondrogenesis in both sexes F: proliferation rates increased with decreasing concentration; increased osteogenic differentiation; decreased adipogenic differentiation M: lower concentrations increased proliferation while higher concentrations decreased proliferation; increased adipogenic differentiation |
Lee et al.39 |
| BM-MSCs | M | Beagle dogs, sexually mature | In vitro | 0, 10−7, 10−9, 10−11, 10−13, 10−15 M E2 | E2 treatment: Above 10−11 M: inhibited proliferation and increased apoptosis 10−11 M: increased proliferation; decreased apoptosis; no effect on osteogenesis or adipogenesis. |
Zhou et al.40 |
| Human cells | ||||||
| BM-MSCs | F | Human, 41–51-year old, perimenopausal | In vitro | 10 nM E2 | Osteogenic differentiation: ERα and ERβ expression increased Adipogenic differentiation: ERα expression increased; ERβ unchanged E2 treatment: increased osteogenesis; decreased adipogenesis |
Heim et al.48 |
| BM-MSCs | M | Human, 40–44-year old | In vitro | 1, 2, 4, 8, 10, 50, 100 nM E2 | E2 treatment: increased osteoblast proliferation in a dose dependent manner between 1 and 8 nM, with no further increase seen at higher concentrations; 1 and 2 nM E2 increased proliferation, but higher doses had no effect. | DiSilvio et al.41 |
| BM-MSCs | M | Human, adult | In vitro | 10 nM, 10 pM E2 | E2 treatment + osteogenic stimulation: enhanced osteogenic potential; no change in proliferation E2 treatment + adipogenic stimulation: increased adipogenic potential, decreased proliferation |
Hong et al.45 |
| BM-MSCs | M | Human, 18–45-year old | In vitro | 10−11–10−8 M E2 | E2 treatment: no effect on proliferation; inhibited chondrogenesis | Jenei-Lanzl et al.44 |
| BM-MSCs | M | Human, 31–62-year old | In vitro | 10−7, 10−9, 10−11 M E2 | E2 treatment: no effect on cell proliferation rate, time to senescence, or the expression of telomere and senescence-associated genes; decreased telomere shortening over time | Breu et al.43 |
| BM-MSCs | F and M | Human, 27.4 ± 6.1-year old | In vitro | 10−6–10−12 M E2 | E2 treatment: increased proliferation in both sexes; maintained proliferation rates through more passages than control cells; increased ERα expression; ERβ expression unchanged. | Hong et al.42 |
| BM-MSCs | F | Human, age N/A | In vitro | 100 pM–1 mM E2 | E2 treatment: increased osteogenic and adipogenic potential | Strong et al.47 |
| BM-MSCs | N/A (lot specific) | Human, age N/A (lot specific) | In vitro | 1 nM E2 | E2 treatment: upregulated expression of components of autophagosome genes; increased autophagic flux | Gavali et al.46 |
BM-MSC, bone marrow-derived mesenchymal stromal cell; BMP-2, bone morphogenetic protein 2; E2, 17β-estradiol; ERα, estrogen receptor α; ERβ, estrogen receptor β; F, female; IL-6, interleukin 6; LPS, lipopolysaccharide; M, male; MAPK, mitogen-activated protein kinase; N/A, not available; ovx, ovariectomized; TNF, tumor necrosis factor; SATB2, sequence binding protein 2; VEGF, vascular endothelial growth factor.
Table 4.
Role of Estrogens on Connective Tissue Derived Stem Cell Stemness
| Cell type | PDLSCs |
|||||
|---|---|---|---|---|---|---|
| Sex | Animal model, age | In vitro, in vivo | Hormone treatment | Response | Study | |
| Rat cells | ||||||
| PDLSCs | F | Sprague-Dawley rats, 3-month old | In vitro | 10−7 M E2 | E2 treatment: increased osteogenic potential through both ERα and ERβ. | Zhang et al.77 |
| PDLSCs | F | Sprague-Dawley rats, 3-month old | In vitro | 10−7 M E2; ovx | Cells from ovx rats: higher proliferation rates and lower osteogenic potential than cells from sham or ovx cells treated with E2; cells from all groups grew well on nHAC/PLA scaffold, although cells from ovx rats had lower osteogenic potential. | Ling-Ling et al.52 |
| Human cells | ||||||
| PDLSCs | F | Human, 18, 19, and 22-year old | In vitro | 10−7, 10−8, 10−9 M E2 | E2 treatment: increased osteogenic potential in a dose-dependent manner; both ERα and ERβ were important for osteogenic differentiation. | Pan et al.79 |
| PDLSCs | F and M | Human, 18–20-year old | In vitro | 10−6, 10−7, 10−8 M E2 | Treatment with 10−7 M E2: increased proliferation rates, proportion of cells in G2/M+S phase of the cell cycle, and expression of stemness-related genes; the PI3K/AKT pathway was involved E2 treatment in general: improved the proliferation, stemness, and differentiation potential of cells in long-term culture. |
Ou et al.78 |
| PDLSCs | N/A | Human, 12–16-year old | In vitro | 10−7 M E2 | E2 treatment: increased osteogenesis through activation of the Wnt/β-catenin pathway | Jiang et al.80 |
| Rats | ||||||
| PDLSCs | F | Sprague-Dawley rats, 3-month old | In vivo | ovx | ovx animals: contain more PDLSCs; proliferate faster but decrease sooner | Zhang et al.77 |
| PDLSCs | F | Sprague-Dawley rats, 3-month old | In vivo | 10−7 M E2; ovx | In seeded nHAC/PLA scaffolds implanted into SCID mice, all cell types led to new bone growth, with cells from ovx rats generating the least | Ling-Ling et al.52 |
| TDSCs | ||||||
| TDSCs | M | C57BL/6J mice, 6-month old | In vivo | None, but ERβ−/− mice compared to WT | Achilles tendon injury model: WT: more ERβ+ cells found in injured than noninjured animals ERβ−/−: lower cell density and higher adipocyte and blood vessel accumulation in scar site than WT |
Bian et al.81 |
| TDSCs | M | Sprague-Dawley rats, 6-week old | In vitro | 10−5, 10−7, 10−9 M LY3201 (ERβ agonist) | Treatment with 10−7 M LY3201: promoted cell proliferation; inhibited adipogenesis; other concentrations had no effect. | Bian et al.81 |
| CPCs | ||||||
| CPCs | F and M | Human, with OA | In vitro | 0.02 or 0.15 ng/mL E2 | F: greater percentage of cells expressed ERz and ERβ E2 treatment: F and M: increased ERα and decreased ERβ expression F: increased chondrogenesis |
Koelling and Miosge82 |
| FCSC | ||||||
| FCSCs | M | New Zealand White rabbits, 12-week old | In vivo | 0.1 mL of 100 ng/mL Sost once weekly for 7 weeks | Sclerostin (Wnt pathway inhibitor) treatment after post-traumatic OA induction: increased FCSC number in TMJ superficial zone; decreased joint damage; reduced joint swelling | Embree et al.83 |
| FCSCs | F | C57BL/6 mice, 3- or 13-week old | In vivo | 0.01 mg/60 days E2 pellet; ovx | E2 treatment in: Young mice: promoted chondrogenesis through ERα through upregulation of Sost Mature mice: promoted chondrogenesis and anabolic gene expression through ERα |
Robinson et al.84 |
CPC, chondrogenic progenitor cell; FCSC, fibrocartilage stem cell; nHAC/PLA, nano-hydroxyapatite/collagen/poly(L-lactide); OA, osteoarthritis; PDLSCs, periodontal ligament stem cells; SCID, severe combined immunodeficient; Sost, sclerostin; TDSC, tendon-derived stem cell; TMJ, temporomandibular joint.
Estrogens and BM-MSCs
Mouse cells
E2 treatment has effects on apoptosis rates, proliferation, and ER expression of BM-MSCs from mice. BM-MSCs isolated from ovariectomized (ovx) mice were more apoptotic,29 senescent,30 and less proliferative29,30 than sham controls, and E2 treatment improved these characteristics.29,30 E2 treatment also increased the expression of ERα and decreased the expression of ERβ.29 Wu et al. found that E2 control of proliferation and senescence was linked to the JAK2/STAT3 pathway.30
Estrogens are important for BM-MSC differentiation in mice. BM-MSCs isolated from ovx mice showed decreased osteogenic29–31 and increased adipogenic31 differentiation compared to sham controls. Multiple studies have found that E2 treatment of mouse BM-MSCs increased their osteogenic potential.29,30,32 Similarly, Okazaki et al. found that E2 treatment of mouse BM-MSCs that overexpress human ERα or ERβ promoted osteogenic differentiation, although it decreased adipogenic potential. In both cell lines, these effects were blocked by the nonspecific ER antagonist ICI 182,780. These data suggest similar roles for both receptors in these processes.33
Rat cells
E2 treatment also affects many cell cycle-related characteristics of rat BM-MSCs. Wu et al. found that BM-MSCs from ovx Sprague-Dawley rats exhibited increased senescence compared to sham controls.34 Ayaloglu-Butun et al. reported that apoptosis rates were similar between BM-MSCs from intact and ovx female Sprague-Dawley rats and decreased with E2 treatment. They also explored the effects of E2 treatment on BM-MSCs from both groups and found that it caused an increase in the colony numbers and number of cells per colony isolated from both groups.35 Hong et al. reported that E2 treatment increased proliferation in BM-MSCs from female rats but not from males.36 A study by Zhao et al. revealed that E2 treatment of BM-MSCs isolated from female Sprague-Dawley rats increased the number of cells in the S-phase of the cell cycle compared to controls.37
Hormone status and E2 treatment have more complicated effects on differentiation in rat BM-MSCs than in mouse cells. Wu et al. found that BM-MSCs from ovx Sprague-Dawley rats exhibited reduced stemness and osteogenic differentiation compared to sham controls. These phenotypes were rescued by E2 treatment through ERβ and special AT-rich sequence binding protein 2 (SATB2) signaling.34 Conversely, Ayaloglu-Butun et al. saw no differences in the osteogenic or adipogenic potential of BM-MSCs isolated from intact and ovx female Sprague-Dawley rats. Furthermore, E2 treatment had no effect on osteogenic potential but decreased adipogenic potential in this study.35
Differences between the two studies are potentially explained by differing differentiation protocols and assay readouts. Several groups report that E2 treatment increases the osteogenic potential of BM-MSCs isolated from male36,38 and female36,37 rats. Furthermore, one of these studies revealed that E2 treatment decreased chondrogenic potential in female rat BM-MSCs and activated the MAPK pathway.37
Larger animal cells
Lee et al. studied the effect of estrogen treatment on BM-MSCs collected from mature mini-pigs of both sexes. The study revealed that 1 pM E2 was the optimum concentration to increase proliferation of cells of both sexes, while 1 and 0.01 μM E2 decreased proliferation in male cells. In addition, E2 treatment reduced apoptosis-related gene expression in both sexes. It also decreased adipogenic differentiation in females but increased it in males, increased osteogenic differentiation in females but had no effect in males, and increased chondrogenic potential, although not significantly, in both sexes.39 Zhou et al. found that E2 treatment of BM-MSCs isolated from male beagles at concentrations above 10 pM inhibited proliferation and increased apoptosis. Ten pico molar E2 showed some signs of increasing proliferation and decreasing apoptosis, but had no effect on osteogenesis or adipogenesis.40
Human cells
In most studies, treatment with E2 increased proliferation of human BM-MSCs, although the effect was at times concentration or sex dependent. DiSilvio et al. observed that 1 and 2 nM of E2 increased proliferation of male human BM-MSCs, but that higher concentrations had no effect.41 Hong et al. found that 0.1–10 nM E2 increased the proliferation of BM-MSCs from both sexes, but that 0.001 nM E2 only increased male cell proliferation. In addition, E2 supplementation maintained proliferation rates through more passages than control.42
Conversely, other studies have found E2 treatment to have no effect on male BM-MSC proliferation in 2D43 (0.01–100 nM) or 3D44 (0.01–10 nM) culture. Hong et al. found that stimulation of male BM-MSCs with 10 pM or 10 nM E2 had no effect on cell proliferation during osteogenesis, but inhibited proliferation during adipogenesis.45 Due to the fact that concentrations of E2 used in these studies generally overlap, other explanations such as differences in cell source and experimental conditions must be considered.
Effects are seen in other aspects of human BM-MSC cycle regulation after treatment with E2 as well. Gavali et al. found that E2 treatment of a human BM-MSC line (ATCC PCS-500-012) upregulated expression of two subunits of RAB3 GTPase Activating Protein Complex, which modulates autophagy.46 Breu et al. studied the effects of E2 treatment on male BM-MSCs and found that it had no effect on time to senescence or the expression of telomere and senescence-associated genes. E2 treatment did decrease telomere shortening over time in a dose-dependent manner, though.43
E2 treatment also increases the differentiation potential of human BM-MSCs, although not universally. It was reported to increase both the adipogenic and osteogenic potential of human BM-MSCs isolated from females47 and males.45 Heim et al. reported that E2 increased osteogenesis but decreased adipogenesis in BM-MSCs isolated from perimenopausal females.48 Jenei-Lanzl et al. found that E2 treatment inhibited chondrogenesis in male human BM-MSCs in 3D culture. This effect was linked to membrane-associated ERs rather than classical intracellular ERs.44
Multiple studies have explored the expression of ERs in BM-MSCs. Heim et al. found that ERα expression increased during both adipogenic and osteogenic differentiation, but that ERβ expression only increased during osteogenic differentiation in BM-MSCs from perimenopausal females.48 Furthermore, Hong et al. found that ERα expression in BM-MSCs was increased by E2 supplementation while ERβ expression was unchanged.42
E2's effects on BM-MSCs have been investigated by many and cover several species, as reviewed in Table 1. Generally, E2 treatments increased proliferation and decreased apoptosis and senescence of cells from both females and males of the species considered. In most cases, osteogenesis increased and adipogenesis decreased when treated with E2.
Estrogens and ASCs
In vitro
Mouse cells
Zhang et al. used the ERα agonist propyl pyrazole triol (PPT), the ERβ agonist diarylpropionitrile (DPN), and the ERα/β nonspecific antagonist ICI 182,780 to study the roles each ER plays in male mouse ASCs. The study revealed that both ER agonists increased cell proliferation in a dose dependent manner that was blocked by coincubation with the antagonist, but that the effect of the ERα agonist PPT was greater. In addition, only PPT caused a statistically significant improvement in wound healing and migration. When the ASCs were grown in brown adipogenic differentiation media, PPT stimulated brown adipogenesis, while DPN inhibited it.49
Rat cells
In general, E2 treatment increases rat ASC proliferation. Feng et al. found that E2 increased proliferation and myogenic differentiation of Sprague-Dawley ASCs from both sexes grown both in 2D and on an electrospun poly(lactide)/poly(caprolactone) nanofibrous scaffold, suggesting E2 as a promising tool in tissue engineering.50 Interestingly, a wider range of E2 concentrations was found to increase cell proliferation in female cells (0.01 nM–0.1 μM E2) than in male cells (0.1 nM–0.1 μM E2),50 an effect opposite to that seen in human BM-MSCs.42
Studies of preadipocytes from femoral, epididymal, and parametrial tissue of intact male and intact and ovx female Sprague Dawley rats by Dieudonne et al. revealed that 10 nM E2 treatment had no effect on the proliferation of male cells from any studied tissue but did increase proliferation in femoral cells from both ovx and intact females.51 The lack of E2 response in male cells in this study could be explained by the single concentration of E2 used, although this concentration was seen to have effects in the study by Feng et al.,52 or differences in assay conditions.
Human cells
In most reported cases, E2 treatment increased proliferation and survival in human ASCs. Roncari and Van found that E2 treatment increased the cell count and proliferation rate of adipocyte precursors but did not alter cell size.53 Furthermore, E2 treatment of female ASCs increased cell proliferation and decreased apoptosis of cells cultured in serum-free media.54 Hong et al. reported that E2 stimulation of female ASCs increased proliferation during osteogenesis but not during adipogenesis.55 Anderson et al. explored the effects of multiple concentrations of E2 on preadipocytes isolated from subcutaneous and omental tissue of male and female humans. They found that E2 treatment increased proliferation in all tissues. Time to maximal proliferation varied by sex and tissue type, occurring later in men and omental tissue.56
While exploring the effects of ASCs isolated from subcutaneous adipose tissue from the femoral and abdominal regions of postmenopausal women treated with E2 and placebo, Cox-York et al. found that E2 treatment significantly increased the differentiation potential of femoral stem cells but not abdominal stem cells.57 Ng et al. found that there was no difference in the chondrogenic potential of ASCs collected from female humans that were pregnant, premenopause, or menopausal.58
Most studies report that E2 treatment improves the differentiation potential of ASCs from humans. E2 treatment of ASCs isolated from females has been found to increase osteogenic47,55 and adipogenic47,54,55 potential. Sadeghi et al., although, found that E2 treatment of human ASCs (sex not specified) inhibited chondrogenesis.59
In vivo
Mouse
Lapid et al. found that adipose progenitor cell number and proliferation were increased in ovx mice compared to sham controls. In this study, the authors also generated adipose-lineage ERα-mutant male and female mice and found that they displayed significant reductions in adipose progenitor cell number compared to control mice. The adipogenic potential of adipose progenitor cells was found to be reduced in mutant animals compared to controls, while the ability to differentiate into smooth muscle was increased. These results indicate that ERα is important for adipogenesis and for maintaining an adipose progenitor cell population.60 ERβ-mutant animals were not investigated.
Human
Studies comparing ASCs collected from females of different reproductive status have shown that cells from pregnant donors proliferate more rapidly than cells from premenopausal or menopausal women,58 but that there is not a significant difference in rates between premenopausal and menopausal women.56,58 Conversely, Cox-York et al. compared ASCs isolated from subcutaneous adipose tissue from postmenopausal women treated with E2 and placebo and found that E2 treatment did not alter proliferation, susceptibility to apoptosis, or expression of ERα or ERβ mRNA.57
Studies of the effects of estrogens on ASCs are summarized in Table 2. In most cases, E2-treated ASCs showed increased differentiation potential and proliferation and decreased apoptosis; these are similar trends compared to BM-MSCs.
Table 2.
Role of Estrogens on Adipose Derived Stem Cell Stemness
| Cell type | ASCs |
|||||
|---|---|---|---|---|---|---|
| Sex | Animal model, age | In vitro, in vivo | Hormone treatment | Response | Study | |
| Mouse cells | ||||||
| ASCs | M | C57BL/6 mice, 8-week old | In vitro | 0, 50, 100, 200 nM PPT (ERα agonist), DPN (ERβ agonist), or 182,780 (ER antagonist) | Both agonists increased stem cell proliferation. ERα agonist encouraged wound healing and cell migration. ERα agonist stimulates brown adipogenesis, while ERβ agonist inhibits it. | Zhang et al.49 |
| Rat cells | ||||||
| ASCs | F and M | Sprague-Dawley rats, 1-month old | In vitro | 10−7–10−11 E2 | E2 treatment: increased cell proliferation and myogenic differentiation; treated cells formed a more solid cell layer on electrospun mesh than control cells | Feng et al.50 |
| Preadipocytes from femoral, epididymal, and parametrial tissue | F and M | Sprague-Dawley rats, age N/A | In vitro | 10, 100, 1000 nM E2; ovx | E2 treatment: increased preadipocyte growth rate from both ovx and intact females, but not from males; increased GPDH activity in cells from females, but not in males | Dieudonne et al.51 |
| Human cells | ||||||
| ASCs | F and M | Human, 35–54-year old | In vitro | None | M showed greater osteogenesis compared to F | Aksu et al.25 |
| ASCs | F and M | Human, 18–71-year old | In vitro | None | Many chromosomal segments and individual genes were found to be differentially expressed between the sexes, including some related to immunomodulation, differentiation, and cell-cell or cell-ECM adhesion. | Bianconi et al.26 |
| Omental adipose-derived precursor cells | F and M | Human, 20–60-year old | In vitro | 0.5–500 ng/mL E2 | E2 treatment: increased adipose-derived precursor cell count and replication; did not alter cell size | Roncari and Van53 |
| Preadipocytes from subcutaneous and omental tissue | F and M | Human, pre-and postmenopausal for F | In vitro | 10−7, 10−8, 10−9 M E2 | E2 treatment: increased proliferation in preadipocytes from all sources; time to maximal proliferation varied by sex and tissue type, occurring later in men and omental tissue | Anderson et al.56 |
| ASCs | F | Human, 45-year old | In vitro | 10−8–10−11 M E2 | E2 treatment + adipogenic stimulation: enhanced adipogenic potential; did not alter proliferation during adipogenesis E2 treatment + osteogenic stimulation: enhanced osteogenic potential: increased proliferation |
Hong et al.55 |
| ASCs | F | Human, pregnant, premenopause, menopause | In vitro | 10−8 M E2 | Hormone status: no difference in chondrogenic potential between groups E2 treatment: no effect on proliferation or ER expression |
Ng et al.58 |
| ASCs | F | Human, 22–30-year old | In vitro | 10−6–10−10 M E2 | E2 treatment: increased cell proliferation, VEGF production, and adipogenic potential; decreased apoptosis in serum-free media | Luo et al.54 |
| ASCs | F | Human, age N/A | In vitro | 100 pM-1 mM E2 | E2 treatment: increased osteogenic and adipogenic potential | Strong et al.47 |
| ASCs | N/A | Human, 25–55-year old | In vitro | 10−8 M E2 | E2 treatment: decreased chondrogenesis | Sadeghi et al.59 |
| ASCs | F | Human, 45–60-year old (postmenopausal) | In vivo | 3 × 0.005 mg/14 days E2 patches | E2 treatment: increased differentiation in stem cells obtained from the femoral region but not from the abdominal region | Cox-York et al.57 |
| Mouse | ||||||
| White adipose progenitor cells | F and M | C57 mice, age N/A | In vivo | None | ERα promotes adipogenic lineage commitment, ERα-mutant mice experience characteristic metabolic symptoms consistent with brown phenotype | Lapid et al60 |
| Human | ||||||
| ASCs | F | Human, pregnant, premenopause, menopause | In vivo | None | Hormone status: cells from pregnant donors showed a higher proliferation rate than the other groups | Ng et al.58 |
| ASCs | F | Human, 45–60-year old (postmenopausal) | In vivo | 3 × 0.005 mg/14 days E2 patches | E2 treatment: did not alter proliferation, susceptibility to TNF-α, or mRNA expression of ERα or β. | Cox-York et al.57 |
ASC, adipose-derived stem cell; DPN, diarylpropionitrile; ECM, extracellular matrix; GPDH, glycerol-3-phosphate dehydrogenase; PPT, propyl pyrazole triol.
Estrogens and MDSCs
In vitro
Mouse cells
Intact hormone status and the presence of functioning ERβ are important for mouse satellite cell function. Satellite cells of ovx mice were found to be impaired in self-renewal and differentiation abilities compared to those collected from control mice.61,62 In addition, work with satellite cells isolated from satellite cell-specific conditional ERβ knockout (ERβKO) mice revealed that both male and female ERβKO cells failed to proliferate compared to wild type (WT) control, indicating the importance of ERβ signaling for proliferation of both sexes.
In addition, when single myofibers were isolated from mice and cultured in floating conditions, a common method for studying the activation and function of the associated satellite cells,63 myofibers isolated from KO animals generated less satellite cells than WT, but the proportion of proliferative, self-renewing, and differentiation-committed satellite cells was the same. This indicates that ERβ regulates satellite cell proliferation rate but not fate decision.64 This study did not investigate the effects of ERαKO; therefore, comparisons between the roles of ERs in these processes cannot be made.
E2 treatment impairs myogenic differentiation in cells isolated from mice. E2 treatment was found to impair mouse myoblast differentiation in both the C2C12 immortalized cell line (sex not specified)65,66 and myoblasts isolated from the hind limb muscle of C57BL/6 mice (sex not specified).65 In addition, Ogawa et al. found that E2 inhibited myogenesis in satellite cells isolated from the hind-limb muscles of neonatal and young female mice.66
Cow cells
Kamanga-Sollo et al. reported that treatment of proliferating satellite cells isolated from steers with 10 nM E2 increased proliferation rate, but only when cells were cultured in 1% insulin-like growth factor (IGF) binding protein 3-free swine serum. No E2-related effect on proliferation was seen in cells cultured in standard swine serum or fetal bovine serum.67 This increase in proliferation rate was tied to signaling through ER, insulin-like growth factor receptor, MEK1, and PI3K/protein kinase B (AKT) pathways.68
In another study, Kamanga-Sollo et al. reported that the effects of E2 on IGF-1 mRNA levels and proliferation are mediated through different mechanisms, with IGF-1 mRNA levels, which they had previously shown to increase with E2 treatment,67 likely being controlled through G protein-coupled receptor 30 (GPR30).69 Further studies of fused bovine satellite cells isolated from steers revealed that treatment with E2 caused a concentration-dependent increase in protein synthesis and decrease in protein degradation. Both of these effects were linked to ERα and/or ERβ.70
Human cells
Li et al. found that E2 treatment stimulated proliferation in human male smooth muscle progenitor cells derived from both embryonic stem cell lines and induced pluripotent stem cells but not in female cells of either population. In addition, E2 treatment increased myogenic gene markers and suppressed extracellular matrix (ECM) degradation in female cells but not in male cells from both sources.24
In vivo
Mouse
Collins et al. found that ovx mice had fewer satellite cells in multiple types of muscle samples compared to control mice and that E2 treatment rescued satellite cell numbers.61 Conversely, Kitajima and Ono did not observe a decrease in the number of satellite cells in the extensor digitorum longus of ovx mice compared to control mice, but they did find an increase in the number of myonuclei per fiber.62
In agreement with in vitro studies, E2 treatment has been found to impair myogenesis in vivo. Ogawa et al. report that E2 treatment of ovx mice decreased the ratio of skeletal muscle mass to body weight and increased ubiquitin-specific peptidase 19 expression, indicating that E2 inhibits myogenesis in vivo.66 A study of satellite cell-specific conditional ERβKO mice by Seko et al. revealed that ERβ is important for regulation of postnatal muscle growth but not muscle maintenance in females but not males.64 ERαKO animals were not investigated in this study.
Rat
E2 treatment affects the number of satellite cells found in rat muscles after exercise. Tiidus et al. found that the total number of satellite cells in the soleus and white vastus of Sprague-Dawley rats increased after exercise and that this effect was enhanced by E2 treatment. E2 treatment had no effect on the number of satellite cells in nonexercised rats.71 Further work showed that this increase was seen in numbers of total, activated, and proliferating satellite cells.72 It was found that ERα likely plays a primary role in this increase.73,74 Mangan et al. also found that the PI3K/AKT pathway was implicated in a similar increase in satellite cell numbers in the soleus and white gastrocnemius muscles of Sprague-Dawley rats.75
E2 treatment can also stimulate muscle regeneration after injury. Greater satellite cell activation and proliferation plus muscle regeneration compared to ovx control was seen in ovx Wistar rats treated with E2 and the ERβ-selective agonist 8β-VE2 but not with the ERα agonist 16α-LE2, indicating a role for ERβ in muscle regeneration.76
Human
Collins et al. studied needle muscle biopsies taken from the vastus lateralis of the same women when they were perimenopausal and again after they were postmenopausal and found that the number of satellite cells collected from each patient decreased with change in menopausal state.61
Overall, MDSCs from both males and females are affected by E2 treatments. Cells treated with E2 saw an increase in proliferation and cell numbers. Osteogenic differentiation potential was increased with E2 treatment, while myogenic differentiation potential was decreased. Studies of MDSCs are summarized in Table 3.
Table 3.
Role of Estrogens on Muscle Derived Stem Cell Stemness
| Cell type | MDSCs |
|||||
|---|---|---|---|---|---|---|
| Sex | Animal model, age | In vitro, in vivo | Hormone treatment | Response | Study | |
| Mouse cells | ||||||
| MDSCs | F and M | C57BL/6J mice, 3-week old | In vitro | None | M: more rapid and greater extent of osteogenesis | Corsi et al.27 |
| MDSCs | F and M | C57.BL10 mice, 3-week old | In vitro | None | M: undergo chondrogenesis more effectively and produce larger pellets with richer ECM; chondrogenic potential maintained in long term culture | Matsumoto et al.14 |
| ERβKO satellite cells from the extensor digitorum longus | F and M | Mice with ERβKO satellite cells, 6- and 20-week old | In vitro | None | M and F ERβKO satellite cells: failed to proliferate compared to WT cells; proportion of proliferative, self-renewing, and differentiation-committed cells not effected | Seko et al.64 |
| C2C12 cell line (immortalized mouse myoblasts) | N/A | Mouse, age N/A | In vitro | 0, 0.1, 1, 10, 100, 1000 nM E2; 10 nM PPT; 10 nM DPN; 1 μM ICI 182,780 | E2 treatment: inhibited myogenesis; increased USP19 mRNA PPT (ERα agonist) treatment: inhibited myogenesis DPN (ERβ agonist) treatment: no change in myogenesis E2 and ICI 182,780 (ER antagonist) cotreatment: E2 inhibitory effects blocked |
Ogawa et al.66 |
| Satellite cells from hind limb muscles | F | Kwl:ddY mice, 3–5-day old (neonatal) or 7–8-week old (young) | In vitro | 0, 0.1, 1, 10, 100, 1000 nM E2 | E2 treatment: inhibited myogenesis; increased USP19 mRNA and protein levels in a dose-dependent manner | Ogawa et al.66 |
| C2C12 cell line (immortalized mouse myoblasts) | N/A | Mouse, age N/A | In vitro | 0, 0.01, 0.1, 0.5, 1 μM E2 | E2 treatment: impaired myoblast differentiation | Go et al.65 |
| Myoblasts isolated from the hind limb muscle | N/A | C57BL/6 mice, 1-month old | In vitro | 1 μM E2 | E2 treatment: impaired myoblast differentiation | Go et al.65 |
| Cow cells | ||||||
| Proliferating satellite cells from semimembranous muscle | M | Castrated cattle (Steer) | In vitro | 0.001, 0.01, 0.1, 1, 10 nM E2 | Treatment with: 0.001 nM E2: increase in ERα and IGFBP-3 mRNA 0.01–10 nM E2: increase in IGF-1 mRNA 10 nM E2: increase in proliferation rate |
Kamanga-Sollo et al.67 |
| Proliferating satellite cells from semimembranous muscle | M | Castrated cattle (Steer) | In vitro | 10 nM E2; 10 nM ICI 182,780; 10 μg/mL JB1; 0, 20, 100, 500 μM PD98059; 0, 100, 500, 1000 nM wortmannin | E2 treatment: increased IGF-1 mRNA in the presence of FBS not SS; increased proliferation rate in the presence of SS not FBS; proliferation increase blocked by ICI 182,780 (ER antagonist), JB1 (competitive inhibitor of IGFR-1), PD980059 (MEK1 inhibitor), and wortmannin (PI3K/Akt pathway inhibitor) | Kamanga-Sollo et al.68 |
| Proliferating satellite cells from semimembranous muscle | M | Castrated cattle (Steer) | In vitro | 10 nM E2; 10, 100 nM ICI 182,780; 10, 100 nM G1; 100, 1000 nM BSA-E2 | ICI 182,780 (ER antagonist) treatment: increase in IGF-1 mRNA G1 (GPR30 agonist) or BSA-E2 (cell impermeable E2) treatment: increase in IGF-1 mRNA, no change in proliferation rate |
Kamanga-Sollo et al.69 |
| Fused satellite cells from semimembranous muscle | M | Castrated cattle (Steer) | In vitro | 0.1, 1, 10 nM E2, 100 nM ICI 182,780, 100 nM G1 (GPR30 agonist) | E2 treatment: concentration-dependent increase in protein synthesis; decrease in protein degradation; blocked by ICI 182,780 cotreatment G1 treatment: no change in protein synthesis or degradation |
Kamanga-Sollo et al.70 |
| Human cells | ||||||
| MDSCs | F and M | Human, 12–92-year old | In vitro | None | M: undergo chondrogenesis and osteogenesis more than F | Scibetta et al.16 |
| Smooth muscle progenitor cells from embryonic stem cell line | F and M | Human, blastocyst stage embryo | In vitro | 0, 0.1, 1.0, 10 nM E2 | E2 treatment: F: increased myogenesis and reduced ECM degradation M: increased proliferation |
Li et al.24 |
| Smooth muscle progenitor cells from induced pluripotent stem cells | F and M | Human, 28–45-year old | In vitro | 0, 0.1, 1.0, 10 nM E2 | F express more ERβ; F and M express equivalent ERα E2 treatment: F: increased myogenesis and decreased ECM degradation M: increased proliferation |
Li et al.24 |
| Mouse | ||||||
| MDSCs | F and M cells and hosts | C57BL/6J mice, age N/A | In vivo | None | M hosts: greater bone formation area and density regardless of sex of implanted cells | Corsi et al.27 |
| MDSCs | F and M | C57 mice, 3-week old | In vivo | None | F: regenerated skeletal muscle more efficiently M: faster MDSC pool depletion |
Deasy et al.28 |
| MDSCs | F and M cells, F hosts | C57.BL10 mice, 3-week old (cells); nude rats, 12-week old (hosts) | In vivo | None | M cells: greater cartilage regeneration in osteochondral defect | Matsumoto el al.14 |
| MDSCs isolated from lower limbs | M cells; M and F hosts | C57BL/6J mice (cells); C57BL/6J mice, 12-week old (hosts) | In vivo | ovx/castrated | Ectopic bone formation: M hosts (unaltered and castrated) formed more bone than both F (unaltered and ovx) Cranial defect healing: M hosts formed more bone than F |
Meszaros et al.15 |
| ERβKO satellite cells | F and M | Mice with ERβKO satellite cells, 6- and 20-week old | In vivo | None | F ERβKO mice: reduction in muscle weight and regeneration after injury compared to control; not exacerbated by ovx M ERβKO mice: no change from control |
Seko et al.64 |
| Satellite cells from gastrocnemius and soleus muscles | F | Kwl:ddY mice, 7-week old | In vivo | 0.1 mg/kg estradiol valerate; ovx | E2 treatment of ovx animals: decreased ratio of skeletal muscle mass to body weight; increased USP19 expression | Ogawa et al.66 |
| Satellite cells from extensor digitorum longus | F | C57BL/6 mice, 6-week old | In vivo, in vitro | 0.01 mg/60 days slow-release E2 pellet; ovx | ovx: change in number of myonuclei per fiber, not number of satellite cells per fiber; muscles did not regenerate well after injury; satellite cells deficient in self-renewal and differentiation | Kitajima and Ono62 |
| Satellite cells from diverse muscles | F | C57/BL6 and Pax7-ZsGreen mice, 3–4 month old | In vivo | 0.18 mg/60 days slow release E2 pellet; ovx | ovx: fewer satellite cells; satellite cells impaired in self-renewal and differentiation, higher apoptosis E2 treatment: restored satellite cell number in ovx |
Collins et al.61 |
| Rat | ||||||
| Satellite cells from the soleus and white vastus | M | Sprague-Dawley, 11-week old | In vivo | 25 mg/21 days E2 pellet | E2 treatment with exercise: increase in satellite cell number compared to exercise alone | Tiidus et al.71 |
| Satellite cells from the soleus and white vastus | F | Sprague-Dawley rats, 11-week old | In vivo | 0.25 mg/21 days E2 pellet; ovx | ovx animals with E2 treatment and exercise: increase in total, activated, and proliferating satellite cells compared to exercise alone | Enns and Tiidus72 |
| Satellite cells from the soleus and white vastus | F | Sprague-Dawley rats, 11-week old | In vivo | 0.25 mg/21 days E2 pellet; 5 mg/kg ICI 182,780 (ER antagonist); ovx | ovx animals with E2 treatment and exercise: increase in total, activated, and proliferating satellite cells compared to exercise alone; results blocked by ER antagonist | Enns et al.73 |
| Satellite cells from the soleus and white vastus | F | Sprague-Dawley rats, 11-week old | In vivo | 0.25 mg/21 days E2 pellet; 0.5 mg/day PPT (ERα agonist); ovx | ovx animals with exercise and E2 or PPT treatment: increase in total, activated, and proliferating satellite cells compared to exercise alone | Thomas et al.74 |
| Satellite cells from gastrocnemius | F | Wistar rats, 8-week old | In vivo | 40 μg/kg bw/d E2; 10 μg/kg bw/d 16α-LE2; 100 μg/kg bw/d 8β-VE2; ovx | E2 and 8β-VE2 (ERβ agonist) treatment in ovx animals: greater satellite cell activation and proliferation and muscle regeneration seen after injury compared to ovx control | Velders et al.76 |
| Satellite cells from the soleus and white gastrocnemius | F | Sprague-Dawley rats, 8-week old | In vivo | 0.25 mg/21 days slow release E2 pellet; ovx | ovx animals with E2 treatment and exercise: increase in total, activated, and proliferating satellite cells compared to exercise alone; results linked to PI3K/Akt pathway | Mangan et al.75 |
| Human | ||||||
| Satellite cells from the vastus lateralis | F | Human, peri- to postmenopause | In vivo | None | Samples were taken from the same women at peri- and postmenopause. Satellite cell number decreased. | Collins et al.61 |
| MDSCs | F and M cells, male hosts | Human, 12–92-year old (cells), ICR-SCID mice, 8-week old (host) | In vivo | None | M cells: better able to regenerate bone | Scibetta et al.16 |
AKT, protein kinase B; ERβKO, ERβ knockout; FBS, fetal bovine serum; GPR30, G protein-coupled receptor 30; ICI, Imperial Chemical Industries; IGF, insulin-like growth factor; MDSC, muscle-derived stem cell, SS, swine serum; WT, wild type.
Estrogens and Periodontal Ligament Stem Cells
In vitro
Rat cells
Periodontal ligament stem cells (PDLSCs) isolated from ovx rats have decreased osteogenic potential compared to sham controls, and E2 treatment is able to improve this.52,77 Zhang et al. linked both ERα and ERβ to the E2-dependent increase in osteogenic potential of PDLSCs from rats.77
Human cells
E2 treatment is generally beneficial for human PDLSCs. Ou et al. found that treatment with 0.1 μM E2 increased proliferation rates compared to control and improved the proliferation, stemness, and both osteogenic and adipogenic potential of PDLSCs in long-term culture. Treatment with E2 also increased the proportion of cells in the G2/M + S phase of the cell cycle and increased expression of stemness-related genes through the PI3K/AKT pathway.78 Similarly, Pan et al. found that E2 treatment of PDLSCs isolated from female humans increased osteogenic potential of PDLSCs in a dose-dependent manner. This effect was linked to both ERα and ERβ.79 In addition, E2 treatment increased osteogenesis in PDLSCs from adolescents through activation of the Wnt/β-catenin pathway.80
In vivo
The hormone status of rats affects the properties of their PDLSCs. Zhang et al. reported that periodontal ligaments from ovx Sprague-Dawley rats contained more PDLSCs than those from control animals. Furthermore, cells collected from the different groups showed different metabolic kinetics, with ovx cells being more metabolically active at early time points but the two groups showing equivalent levels by day 11.77 Similarly, E et al. found that PDLSCs isolated from ovx rats and grown in 2D culture had higher proliferation rates than cells from sham animals or ovx cells treated with E2. They additionally studied PDLSCs isolated from ovx and sham rats and grown on 3D nano-hydroxyapatite/collagen/poly(L-lactide) scaffolds with and without E2 treatment. When the seeded scaffolds were implanted in SCID mice, all cell types led to new bone growth after 12 weeks, with cells from ovx rats generating the least.52
The effect of E2 treatment on the proliferation of PDLSCs is dependent on the environment, as proliferation increased in vitro, but decreased in vivo. E2 treatment's effects on differentiation capabilities are similar to what has been seen in cell types discussed previously. Osteogenic and adipogenic differentiation improved with the treatments. Studies of connective tissue-derived stem cells, including PDLSCs, are summarized in Table 4.
Estrogens and Tendon-Derived Stem Cells
Bian et al. studied tendon-derived stem cells (TDSCs) isolated from male Sprague-Dawley rats. Treatment of TDSCs with 0.1 μM of the ERβ agonist LY2301 promoted cell proliferation and inhibited adipogenesis, but no effect was seen at other concentrations. Bian et al. also explored the importance of ERβ on Achilles tendon healing in mice using an ERβ−/− male mouse wound model. The authors reported that more ERβ+ cells were present in the scar of injured WT mice 8 days after injury than in noninjured control mice. ERβ−/− mice had lower cell density and higher adipocyte and blood vessel accumulation in the scar site compared to WT. The low cell density was the result of higher levels of apoptosis and lower levels of cell proliferation. These results indicate that the absence of ERβ results in inferior wound healing.81 ERα−/− mice were not included in this study, so direct comparisons between the roles of these two receptors cannot be made.
Estrogens and Chondrogenic Progenitor Cells
Koelling and Miosge found that E2 treatment of chondrogenic progenitor cells isolated from the cartilage of male and female humans with osteoarthritis (OA) had differing effects depending on subject sex and E2 concentration. An increase in chondrogenesis was seen in females after treatment with 0.07 nM E2, but the effects were less clear in males and in both sexes at 0.55 nM E2. ERα and ERβ were both expressed in a greater percentage of female cells than male cells, and E2 treatment caused an increase in the expression of ERα in both sexes. Treatment with 0.07 nM E2 caused no effect in female cells and an increase in ERβ expression in males, while 0.55 nM E2 caused ERβ expression to decrease in both sexes.82
Estrogens and Fibrocartilage Stem Cells
Embree et al. have characterized fibrocartilage stem cells (FCSCs), located in the superficial zone of the temporomandibular joint (TMJ) articular fibrocartilage, that have been shown to have differentiation capabilities similar to other mesenchymal stromal cell sources.83 This population of cells requires inhibition of the canonical Wnt signaling pathway, such as through sclerostin (Sost), to maintain the stem cell pool and promote tissue repair after injury. E2 signaling through ERα has been shown to promote early evidence of new tissue formation in an ovx model with the effects being mediated by upregulation of Sost.84 As such, it is possible that E2 promotes FCSC self-renewal and promotes tissue repair after injury in the TMJ by inhibiting canonical Wnt signaling.
Summary of Findings and Potential Mechanisms
The hope for the use of stem cells as therapies for many diseases and illnesses has driven the scientific community to study their safety and efficacy. Understanding their innate properties—proliferative abilities, differentiation potential, etc.—is vital to their potential future use in the realm of tissue engineering and regenerative medicine. Also key to our understanding: the role that sex plays in cell behavior and response. As shown in this review, there are inherent differences between cells from male and female donors across multiple species. Male cells were found to have stronger osteogenic, chondrogenic, and adipogenic differentiation capabilities than their female counterparts. Although proliferation was typically not found to be different between male and female cells, more cells were found in male hosts than in females.
With some sex-based differences tied to estrogens, treatment of stem cells with estrogens like E2 has been investigated by many, with the hope of improving stem cell capabilities. A generalized summary of their findings can be seen in Figure 3. Across multiple species and cell types, cells treated with E2 were found to increase proliferation and decrease apoptosis. Differentiation of the stem cells was also affected, with osteogenic potential often increasing and adipogenic, chondrogenic, and myogenic potentials varying depending on the cell type. With a better understanding of the differences between male and female cells, and the differences in their responses to E2 treatment, stem cells can be better utilized in the fields of tissue engineering and regenerative medicine.
Many of these studies also worked to identify the receptors and pathways involved in the observed responses to E2 treatment. Some common themes emerged, which are summarized in Figure 4 and below with further context. Much of this effort focused on identifying which estrogen receptor is responsible for the various changes caused by E2 treatment. Estrogens modulate transcription through both classical and nonclassical pathways. In the classical pathway, an estrogen binds to ERα or ERβ resulting in a receptor conformational change, receptor dimerization, and translocation into the nucleus.85 The receptor complex then typically binds to DNA sequences termed estrogen response elements and acts as an enhancer, recruiting coregulators to promote gene transcription.86,87 In the nonclassical pathway, estrogens bind to membrane-associated ERs such as GPR30 or the classical ERs interact with other transcriptional pathways through protein–protein interactions.88,89
FIG. 4.
Summary of pathways linked to effects of E2 on stem cells of the musculoskeletal system. (A) Many effects have been linked to canonical estrogen receptors α and/or β. The canonical estrogen signaling pathway is pictured featuring homodimerization, although not all the listed effects have been linked exclusively to this pathway and the types of dimers formed have not been investigated. Effects seen in studies that did not investigate both receptors were omitted. (B) Other cell responses have been linked to membrane-bound estrogen receptors such as GPR30 rather than canonical estrogen receptors. (C) The PI3K/AKT pathway has been linked to several cell responses to estrogen treatment. This pathway can be activated by estrogen signaling through routes, including estrogen-stimulated promotion of transcription of PI3K/AKT pathway components and the direct binding of the estrogen/estrogen receptor complex to the p85 subunit of PI3K/AKT, as previously reviewed.92 Figure created using BioRender.com AKT, protein kinase B; EGFR, epidermal growth factor receptor; ER, estrogen receptor; GPR30, G protein-coupled receptor 30; IGF-1, insulin-like growth factor 1; IGFR-1, insulin-like growth factor receptor-1; PDK1, phosphoinositide-dependent kinase-1; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase. Color images are available online.
ERα and ERβ have similar structures and are both composed of multiple domains (A–F). The amino acid sequence homology between the two receptors varies across the domains and is highest for the DNA binding domain (C domain) and the ligand binding domain (E domain). The two receptors have overlapping yet distinct and often opposite downstream effects. These differences are not fully understood, but are due, in part, to differences in their F domains90 and preferential use of different coregulators.91
Both ERα and ERβ have been implicated in increases in osteogenic potential of BM-MSCs33 and PDLSCs,77,79 so it would appear that genes involved in this process are regulated by both receptors. Other responses were found to be ER specific or were only investigated in terms of a single receptor.
In the reviewed articles, ERα was frequently seen to play important roles in ASCs, while ERβ signaling was studied in the context of muscle satellite cells. ERα has been linked to ASC adipogenesis and population maintenance,60 as well as E2-stimulated increases in satellite cell number, although a similar role for ERβ was not fully excluded in these studies.73,74 Furthermore, the ERα agonist PPT increased ASC proliferation, wound healing, migration, and brown adipogenesis, while the ERβ agonist DPN had lesser effects on proliferation, no effect on wound healing or migration, and inhibited brown adipogenesis.49
ERβ serves important roles in muscle satellite cells. Treatment of injured ovx Wistar rats with the ERβ-selective agonist 8β-VE2 but not with the ERα agonist 16α-LE2 led to greater satellite cell activation and proliferation plus muscle regeneration compared to ovx control.76 In addition, satellite cells isolated from satellite cell-specific ERβKO mice revealed that both male and female ERβKO cells failed to proliferate compared to WT control. In addition, isolated myofibers from KO animals cultured in floating conditions generated less satellite cells than WT, but the proportion of proliferative, self-renewing, and differentiation-committed cells was the same. This indicates that ERβ regulates satellite cell proliferation but not fate decision.64 Furthermore, Seko et al. used KO studies to show that ERβ is important for regulation of postnatal muscle growth but not muscle maintenance in female but not male mice.64 ERαKO animals were not investigated in these two studies, limiting the conclusions that can be drawn.
Some of the effects of E2 treatment have also been linked to membrane-associated ERs. For example, the inhibition of chondrogenesis in male human BM-MSCs in 3D culture by E2 was tied to membrane-associated ERs such as GPR30 by Jenei-Lanzal et al.44 In addition, Kamanga-Sollo et al. found that although E2-stimulated increases in bovine satellite cell proliferation were mediated through classical ERs, increases in IGF-1 mRNA levels were controlled by GPR30.69
The PI3K/AKT pathway has been tied to E2 treatment-induced increases in proliferation rate68 and cell number75 of satellite cells and proliferation rate and expression of stemness-related genes in PDLSCs.78 These results are not surprising given that PI3K/AKT activation leads to cell proliferation and that this pathway is closely linked to and can be activated by ER signaling.92 Furthermore, this pathway has been linked to the maintenance of the undifferentiated state of human embryonic stem cells and to differentiation of many types of stem cells, including ADSCs and PDLSCs, as discussed in a review by Ramazzotti et al.93
Other pathways have been linked to the effects of estrogen treatment as well. E2 treatment increased osteogenesis in PDLSCs from adolescents through activation of the Wnt/β-catenin pathway.80 Another study revealed that E2 treatment decreased chondrogenic potential in female rat BM-MSCs and activated the MAPK pathway.37 In addition, Wu et al. found that E2 control of proliferation and senescence of BM-MSCs were linked to the JAK2/STAT3 pathway.30
Although the studies summarized above have contributed much to the understanding of estrogen signaling in stem cells, there is still work to be done. Many of the studies into the roles of ERα and ERβ investigated only one of the receptors, not both. Future studies of these mechanisms should focus on both receptors to allow for full elucidation of the differing roles of the canonical ERs. In addition, more data are needed to understand the mechanisms behind these differing roles. Differences in receptor expression levels dependent on tissue source and donor sex, differences in ER homo- versus hetero-dimerization, and differences in downstream gene targets are possible explanations that could be further pursued. In addition, little information is available on the roots of the sex differences seen in stem cells. It is possible that these differences are based on sexual dimorphisms in ER receptor expression levels or preferred signaling modalities, but studies must be performed to test these hypotheses.
Key Challenges, Critical Issues, and Unanswered Questions
Stem cell therapies were initially expected to revolutionize the treatment of musculoskeletal disorders. This optimism has been tempered over the years by the lack of convincing preclinical and clinical trial data and unsupported claims made by groups targeting uninformed consumers. This led the Food and Drug Administration (FDA) to publish a cautionary article in 2017 calling for a reliance on sound science in the field of stem cell therapy94 in addition to multiple statements on the FDA website warning consumers about the existence and risk of unapproved stem cell therapies.
The enthusiasm and promise of stem cell therapies have not been completely cast aside, though. Hematopoietic progenitor cells are FDA approved to treat disorders that affect the production of blood. As of July 2021, there are 124 active studies listed on clinicaltrials.gov investigating stem cells for the treatment of musculoskeletal diseases. Multiple reviews have been published highlighting the state of the art in stem cell therapies, including some focused on musculoskeletal applications.95–98 However, as highlighted in this review, the role of stem cell sex must be considered.
There are several critical issues and key unanswered questions that must guide the future studies in this area. While the role of estrogen signaling is complex and not well understood, estrogens are critical as stem cells from ovx animals tend to be less robust and functional. Given the importance of stem cell sex to their properties and the outcomes of regenerative therapy, it is imperative that more studies state the sex of the subjects used and perform sex comparative studies.
Furthermore, the challenges in making comparisons between studies for this review highlight the need for standardization. Media components such as phenol red, an estrogen mimetic compound, growth factors, and endogenous estrogens in fetal bovine serum can mask the effects of E2 treatment and cause inconsistent results between studies. In addition, differences are seen in the effects of estrogens based on the concentrations used, as well as variabilities between tissue sources, donors within a species, and donors of separate species. This greatly complicates the application of knowledge gained from one model onto another. The standardization of conditions and determination of an ideal model for studying the effects of E2 on musculoskeletal tissue are needed to produce results relevant to human disease. Furthermore, there is a need for more physiological tissue models for in vitro and ex vivo testing to parse out E2 effects in a more controlled manner with the ability to include mechanical effects, which do not affect males and females equally.
The above data highlight the importance of sex and estrogens for many key stem cell properties and emphasize the potential for improving tissue engineering and regenerative therapy. It is possible that more work in this area, beginning with a conscious effort by researchers to state the sex of research subjects and perform more comparative studies, could allow stem cell therapies to revolutionize tissue engineering as originally hoped. Furthermore, properly controlled studies of the effects of E2, other estrogens, and selective estrogen receptor modulators should be carried out to establish a deeper understanding of their potential roles in regenerative therapy.
Authors' Contributions
All listed authors contributed significantly to this work.
Disclaimer
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (no. UL1TR002366) and by NIH NIGMS (P20GM103638).
References
- 1. Clayton, J.A., and Collins, F.S.. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins, F.S., and Tabak, L.A.. Policy: NIH plans to enhance reproducibility. Nature 505, 612, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franconi, F., Brunelleschi, S., Steardo, L., and Cuomo, V.. Gender differences in drug responses. Pharmacol Res 55, 81, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Wolf, J.M., Cannada, L., Van Heest, A.E., O'Connor, M.I., and Ladd, A.L.. Male and female differences in musculoskeletal disease. J Am Acad Orthop Surg 23, 339, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Henschke, N., Harrison, C., McKay, D., et al. . Musculoskeletal conditions in children and adolescents managed in Australian primary care. BMC Musculoskelet Disord 15, 164, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Legault É., Descarreaux, M., and Cantin, V.. Musculoskeletal symptoms in an adolescent athlete population: a comparative study. BMC Musculoskelet Disord 16, 210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel, N.M., Mundluru, S.N., Beck, N.A., and Ganley, T.J.. Which factors increase the risk of reoperation after meniscal surgery in children? Orthop J Sports Med 7, 2325967119842885, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sohn, J., and Huard, J.. Sexual dimorphism in stem cell–based therapies for the musculoskeletal system. Oper Tech Orthop 26, 166, 2016. [Google Scholar]
- 9. Srikanth, V.K., Fryer, J.L., Zhai, G., et al. . A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil 13, 769, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Safiri, S., Kolahi, A.A., Smith, E., et al. . Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis 79, 819, 2020. [DOI] [PubMed] [Google Scholar]
- 11. Kim, H.T., Zhang, M.J., Woolfrey, A.E., et al. . Donor and recipient sex in allogeneic stem cell transplantation: what really matters. Haematologica 101, 1260, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crisostomo, P.R., Markel, T.A., Wang, M., et al. . In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery 142, 215, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Manukyan, M.C., Weil, B.R., Wang, Y., et al. . Female stem cells are superior to males in preserving myocardial function following endotoxemia. Am J Physiol Regul Integr Comp Physiol 300, R1506-14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsumoto, T., Kubo, S., Meszaros, L.B., et al. . The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair. Arthritis Rheum 58, 3809, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Meszaros, L.B., Usas, A., Cooper, G.M., and Huard, J.. Effect of host sex and sex hormones on muscle-derived stem cell-mediated bone formation and defect healing. Tissue Eng Part A 18, 1751, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scibetta, A.C., Morris, E.R., Liebowitz, A.B., et al. . Characterization of the chondrogenic and osteogenic potential of male and female human muscle-derived stem cells: implication for stem cell therapy. J Orthop Res 37, 1339, 2019. [DOI] [PubMed] [Google Scholar]
- 17. Dulken, B., and Brunet, A.. Stem cell aging and sex: are we missing something? Cell Stem Cell 16, 588, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ardakani, A.H.G., and Khan, W.S.. The influence of ageing and gender in musculoskeletal stem cell. Curr Stem Cell Res Ther 13, 432, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Ray, R., Novotny, N.M., Crisostomo, P.R., et al. . Sex steroids and stem cell function. Mol Med 14, 493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tajiri, N., Duncan, K., Borlongan, M.C., et al. . Adult stem cell transplantation: is gender a factor in stemness? Int J Mol Sci 15, 15225, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun, H., Wang, H., and Hu, S.. Effects of estrogen on diverse stem cells and relevant intracellular mechanisms. Sci China Life Sci 53, 542, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Katsara, O., Mahaira, L.G., Iliopoulou, E.G., et al. . Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev 20, 1549, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Strube, P., Mehta, M., Baerenwaldt, A., et al. . Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone 45, 1065, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Li, Y., Wen, Y., Green, M., et al. . Cell sex affects extracellular matrix protein expression and proliferation of smooth muscle progenitor cells derived from human pluripotent stem cells. Stem Cell Res Ther 8, 156, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aksu, A.E., Rubin, J.P., Dudas, J.R., and Marra, K.G.. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg 60, 306, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Bianconi, E., Casadei, R., Frabetti, F., et al. . Sex-specific transcriptome differences in human adipose mesenchymal stem cells. Genes (Basel) 11, 909, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corsi, K.A., Pollett, J.B., Phillippi, J.A., et al. . Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex. J Bone Miner Res 22, 1592, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Deasy, B.M., Lu, A., Tebbets, J.C., et al. . A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol 177, 73, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou, S., Zilberman, Y., Wassermann, K., et al. . Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem Suppl 36, 144, 2001. [DOI] [PubMed] [Google Scholar]
- 30. Wu, W., Fu, J., Gu, Y., et al. . JAK2/STAT3 regulates estrogen-related senescence of bone marrow stem cells. J Endocrinol 245, 141, 2020. [DOI] [PubMed] [Google Scholar]
- 31. Qi, M., Zhang, L., Ma, Y., et al. . Autophagy maintains the function of bone marrow mesenchymal stem cells to prevent estrogen deficiency-induced osteoporosis. Theranostics 7, 4498, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pang, X.G., Cong, Y., Bao, N.R., Li, Y.G., and Zhao, J.N.. Quercetin stimulates bone marrow mesenchymal stem cell differentiation through an estrogen receptor-mediated pathway. Biomed Res Int 2018, 4178021, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okazaki, R., Inoue, D., Shibata, M., et al. . Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 143, 2349, 2002. [DOI] [PubMed] [Google Scholar]
- 34. Wu, G., Xu, R., Zhang, P., et al. . Estrogen regulates stemness and senescence of bone marrow stromal cells to prevent osteoporosis via ERbeta-SATB2 pathway. J Cell Physiol 233, 4194, 2018. [DOI] [PubMed] [Google Scholar]
- 35. Ayaloglu-Butun, F., Terzioglu-Kara, E., Tokcaer-Keskin, Z., and Akcali, K.C.. The effect of estrogen on bone marrow-derived rat mesenchymal stem cell maintenance: inhibiting apoptosis through the expression of Bcl-xL and Bcl-2. Stem Cell Rev Rep 8, 393, 2012. [DOI] [PubMed] [Google Scholar]
- 36. Hong, L., Sultana, H., Paulius, K., and Zhang, G.. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: a gender difference. J Steroid Biochem Mol Biol 114, 180, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao, Y., Yi, F.Z., Zhao, Y.H., et al. . The distinct effects of estrogen and hydrostatic pressure on mesenchymal stem cells differentiation: involvement of estrogen receptor signaling. Ann Biomed Eng 44, 2971, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Liu, G., Lu, Y., Mai, Z., et al. . Suppressing MicroRNA-30b by estrogen promotes osteogenesis in bone marrow mesenchymal stem cells. Stem Cells Int 2019, 7547506, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee, W.J., Lee, S.C., Lee, J.H., Rho, G.J., and Lee, S.L.. Differential regulation of senescence and in vitro differentiation by 17beta-estradiol between mesenchymal stem cells derived from male and female mini-pigs. J Vet Sci 17, 159, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou, Z.H., Gu, C.W., Li, J., et al. . 17 beta-estradiol affects proliferation and apoptosis of canine bone marrow mesenchymal stem cells in vitro. Pol J Vet Sci 23, 235, 2020. [DOI] [PubMed] [Google Scholar]
- 41. DiSilvio, L., Jameson, J., Gamie, Z., Giannoudis, P.V., and Tsiridis, E.. In vitro evaluation of the direct effect of estradiol on human osteoblasts (HOB) and human mesenchymal stem cells (h-MSCs). Injury 37(Suppl. 3), S33–S42, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Hong, L., Zhang, G., Sultana, H., Yu, Y., and Wei, Z.. The effects of 17-β estradiol on enhancing proliferation of human bone marrow mesenchymal stromal cells in vitro. Stem Cells Dev 20, 925, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Breu, A., Sprinzing, B., Merkl, K., et al. . Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. J Orthop Res 29, 1563, 2011. [DOI] [PubMed] [Google Scholar]
- 44. Jenei-Lanzl, Z., Straub, R.H., Dienstknecht, T., et al. . Estradiol inhibits chondrogenic differentiation of mesenchymal stem cells via nonclassic signaling. Arthritis Rheum 62, 1088, 2010. [DOI] [PubMed] [Google Scholar]
- 45. Hong, L., Colpan, A., and Peptan, I.A.. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng 12, 2747, 2006. [DOI] [PubMed] [Google Scholar]
- 46. Gavali, S., Gupta, M.K., Daswani, B., et al. . Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim Biophys Acta Mol Cell Res 1866, 1498, 2019. [DOI] [PubMed] [Google Scholar]
- 47. Strong, A.L., Ohlstein, J.F., Jiang, Q., et al. . Novel daidzein analogs enhance osteogenic activity of bone marrow-derived mesenchymal stem cells and adipose-derived stromal/stem cells through estrogen receptor dependent and independent mechanisms. Stem Cell Res Ther 5, 105, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heim, M., Frank, O., Kampmann, G., et al. . The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 145, 848, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Zhang, W., Schmull, S., Du, M., et al. . Estrogen receptor alpha and beta in mouse: adipose-derived stem cell proliferation, migration, and brown adipogenesis in vitro. Cell Physiol Biochem 38, 2285, 2016. [DOI] [PubMed] [Google Scholar]
- 50. Feng, C., Hu, J., Liu, C., et al. . Association of 17-beta estradiol with adipose-derived stem cells: new strategy to produce functional myogenic differentiated cells with a nano-scaffold for tissue engineering. PLoS One 11, e0164918, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dieudonne, M.N., Pecquery, R., Leneveu, M.C., and Giudicelli, Y.. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology 141, 649, 2000. [DOI] [PubMed] [Google Scholar]
- 52. Ling-Ling, E., Xu, W.H., Feng, L., et al. . Estrogen enhances the bone regeneration potential of periodontal ligament stem cells derived from osteoporotic rats and seeded on nano-hydroxyapatite/collagen/poly(L-lactide). Int J Mol Med 37, 1475, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roncari, D.A., and Van, R.L.. Promotion of human adipocyte precursor replication by 17beta-estradiol in culture. J Clin Invest 62, 503, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luo, S., Hao, L., Li, X., et al. . Adipose tissue-derived stem cells treated with estradiol enhance survival of autologous fat transplants. Tohoku J Exp Med 231, 101, 2013. [DOI] [PubMed] [Google Scholar]
- 55. Hong, L., Colpan, A., Peptan, I.A., et al. . 17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng 13, 1197, 2007. [DOI] [PubMed] [Google Scholar]
- 56. Anderson, L.A., McTernan, P.G., Barnett, A.H., and Kumar, S.. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab 86, 5045, 2001. [DOI] [PubMed] [Google Scholar]
- 57. Cox-York, K.A., Erickson, C.B., Pereira, R.I., Bessesen, D.H., and Van Pelt, R.E.. Region-specific effects of oestradiol on adipose-derived stem cell differentiation in post-menopausal women. J Cell Mol Med 21, 677, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ng, L.W., Yip, S.K., Wong, H.K., et al. . Adipose-derived stem cells from pregnant women show higher proliferation rate unrelated to estrogen. Hum Reprod 24, 1164, 2009. [DOI] [PubMed] [Google Scholar]
- 59. Sadeghi, F., Esfandiari, E., Hashemibeni, B., et al. . The effect of estrogen on the expression of cartilage-specific genes in the chondrogenesis process of adipose-derived stem cells. Adv Biomed Res 4, 43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lapid, K., Lim, A., Clegg, D.J., Zeve, D., and Graff, J.M.. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat Commun 5, 5196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Collins, B.C., Arpke, R.W., Larson, A.A., et al. . Estrogen regulates the satellite cell compartment in females. Cell Rep 28, 368.e6, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kitajima, Y., and Ono, Y.. Estrogens maintain skeletal muscle and satellite cell functions. J Endocrinol 229, 267, 2016. [DOI] [PubMed] [Google Scholar]
- 63. Renzini, A., Benedetti, A., Bouchè, M., et al. . Culture conditions influence satellite cell activation and survival of single myofibers. Eur J Transl Myol 28, 7567, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seko, D., Fujita, R., Kitajima, Y., et al. . Estrogen receptor β controls muscle growth and regeneration in young female mice. Stem Cell Rep 15, 577, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Go, G.Y., Lee, S.J., Jo, A., et al. . Bisphenol A and estradiol impede myoblast differentiation through down-regulating Akt signaling pathway. Toxicol Lett 292, 12, 2018. [DOI] [PubMed] [Google Scholar]
- 66. Ogawa, M., Yamaji, R., Higashimura, Y., et al. . 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J Biol Chem 286, 41455, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kamanga-Sollo, E., Pampusch, M.S., Xi, G., et al. . IGF-I mRNA levels in bovine satellite cell cultures: effects of fusion and anabolic steroid treatment. J Cell Physiol 201, 181, 2004. [DOI] [PubMed] [Google Scholar]
- 68. Kamanga-Sollo, E., White, M.E., Hathaway, M.R., et al. . Roles of IGF-I and the estrogen, androgen and IGF-I receptors in estradiol-17beta- and trenbolone acetate-stimulated proliferation of cultured bovine satellite cells. Domest Anim Endocrinol 35, 88, 2008. [DOI] [PubMed] [Google Scholar]
- 69. Kamanga-Sollo, E., White, M.E., Chung, K.Y., Johnson, B.J., and Dayton, W.R.. Potential role of G-protein-coupled receptor 30 (GPR30) in estradiol-17beta-stimulated IGF-I mRNA expression in bovine satellite cell cultures. Domest Anim Endocrinol 35, 254, 2008. [DOI] [PubMed] [Google Scholar]
- 70. Kamanga-Sollo, E., White, M.E., Hathaway, M.R., Weber, W.J., and Dayton, W.R.. Effect of Estradiol-17beta on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol 39, 54, 2010. [DOI] [PubMed] [Google Scholar]
- 71. Tiidus, P.M., Deller, M., and Liu, X.L.. Oestrogen influence on myogenic satellite cells following downhill running in male rats: a preliminary study. Acta Physiol Scand 184, 67, 2005. [DOI] [PubMed] [Google Scholar]
- 72. Enns, D.L., and Tiidus, P.M.. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol (1985) 104, 347, 2008. [DOI] [PubMed] [Google Scholar]
- 73. Enns, D.L., Iqbal, S., and Tiidus, P.M.. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf) 194, 81, 2008. [DOI] [PubMed] [Google Scholar]
- 74. Thomas, A., Bunyan, K., and Tiidus, P.M.. Oestrogen receptor-alpha activation augments post-exercise myoblast proliferation. Acta Physiol (Oxf) 198, 81, 2010. [DOI] [PubMed] [Google Scholar]
- 75. Mangan, G., Bombardier, E., Mitchell, A.S., Quadrilatero, J., and Tiidus, P.M.. Oestrogen-dependent satellite cell activation and proliferation following a running exercise occurs via the PI3K signalling pathway and not IGF-1. Acta Physiol (Oxf) 212, 75, 2014. [DOI] [PubMed] [Google Scholar]
- 76. Velders, M., Schleipen, B., Fritzemeier, K.H., Zierau, O., and Diel, P.. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J 26, 1909, 2012. [DOI] [PubMed] [Google Scholar]
- 77. Zhang, B., Li, Y., Zhou, Q., and Ding, Y.. Estrogen deficiency leads to impaired osteogenic differentiation of periodontal ligament stem cells in rats. Tohoku J Exp Med 223, 177, 2011. [DOI] [PubMed] [Google Scholar]
- 78. Ou, Q., Wang, X., Wang, Y., Wang, Y., and Lin, X.. Oestrogen retains human periodontal ligament stem cells stemness in long-term culture. Cell Prolif 51, e12396, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pan, F., Zhang, R., Wang, G., and Ding, Y.. Oestrogen receptors are involved in the osteogenic differentiation of periodontal ligament stem cells. Biosci Rep 31, 117, 2011. [DOI] [PubMed] [Google Scholar]
- 80. Jiang, B., Xu, J., Zhou, Y., et al. . Estrogen enhances osteogenic differentiation of human periodontal ligament stem cells by activating the Wnt/β-catenin signaling pathway. J Craniofac Surg 31, 583, 2020. [DOI] [PubMed] [Google Scholar]
- 81. Bian, X., Liu, T., Zhou, M., et al. . Absence of estrogen receptor beta leads to abnormal adipogenesis during early tendon healing by an up-regulation of PPARgamma signalling. J Cell Mol Med 23, 7406, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Koelling, S., and Miosge, N.. Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthritis Rheum 62, 1077, 2010. [DOI] [PubMed] [Google Scholar]
- 83. Embree, M.C., Chen, M., Pylawka, S., et al. . Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun 7, 13073, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Robinson, J.L., Soria, P., Xu, M., et al. . Estrogen promotes mandibular condylar fibrocartilage chondrogenesis and inhibits degeneration via estrogen receptor alpha in female mice. Sci Rep 8, 8527, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beato, M., Herrlich, P., and Schutz, G.. Steroid hormone receptors: many actors in search of a plot. Cell 83, 851, 1995. [DOI] [PubMed] [Google Scholar]
- 86. Carroll, J.S., Meyer, C.A., Song, J., et al. . Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38, 1289, 2006. [DOI] [PubMed] [Google Scholar]
- 87. Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29, 2905, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Levin, E.R. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol (Baltimore, MD) 19, 1951, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Manolagas, S.C., and Kousteni, S.. Perspective: nonreproductive sites of action of reproductive hormones. Endocrinology 142, 2200, 2001. [DOI] [PubMed] [Google Scholar]
- 90. Skafar, D.F., and Zhao, C.. The multifunctional estrogen receptor-alpha F domain. Endocrine 33, 1, 2008. [DOI] [PubMed] [Google Scholar]
- 91. Madak-Erdogan, Z., Charn, T.H., Jiang, Y., et al. . Integrative genomics of gene and metabolic regulation by estrogen receptors α and β, and their coregulators. Mol Syst Biol 9, 676, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ciruelos Gil, E.M. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev 40, 862, 2014. [DOI] [PubMed] [Google Scholar]
- 93. Ramazzotti, G., Ratti, S., Fiume, R., et al. Phosphoinositide 3 kinase signaling in human stem cells from reprogramming to differentiation: a tale in cytoplasmic and nuclear compartments. Int J Mol Sci 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marks, P.W., Witten, C.M., and Califf, R.M.. Clarifying stem-cell therapy's benefits and risks. N Engl J Med 376, 1007, 2017. [DOI] [PubMed] [Google Scholar]
- 95. Labusca, L., Zugun-Eloae, F., and Mashayekhi, K.. Stem cells for the treatment of musculoskeletal pain. World J Stem Cells 7, 96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Judson, R.N., and Rossi, F.M.V.. Towards stem cell therapies for skeletal muscle repair. NPJ Regen Med 5, 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kimbrel, E.A., and Lanza, R.. Next-generation stem cells—ushering in a new era of cell-based therapies. Nat Rev Drug Discov 19, 463, 2020. [DOI] [PubMed] [Google Scholar]
- 98. Zumwalt, M., and Reddy, A.P.. Stem cells for treatment of musculoskeletal conditions—orthopaedic/sports medicine applications. Biochim Biophys Acta Mol Basis Dis 1866, 165624, 2020. [DOI] [PubMed] [Google Scholar]