Abstract
Baicalin and berberine hydrochloride are the main chemical compositions of Scutellariae Radix and Coptidis Rhizoma, respectively. S. Radix and C. Rhizoma are two traditional Chinese herbs that are commonly used together in compounded formulations to treat colitis. Therefore, the combination of Baicalin and berberine hydrochloride (BBH) to treat colitis was studied. The results of pharmacological evaluations demonstrated the excellent protective effects of BBH on colitis induced by dextran sulfate sodium (DSS). BBH could improve the morphological condition of colitis in mice and maintain the balance of proinflammation cytokines (IL-6, IL-8, IL-1β, and TNF-α) and anti-inflammation cytokines (IL-4 and IL-10). The 16s rDNA sequencing revealed that BBH was able to modulate the composition of intestinal microflora, especially the abundances of Eubacterium_brachy_group, Holdemania, Erysipelotrichaceae_UCG_003, Christensenellaceae_R-7_group, and Sellimonas. The results of PICRUSt indicated that the therapeutic effects of BBH were tightly connected with DNA synthesis, replication and repair of gut microbiota. In summary, it was concluded that BBH could protect mice against DSS-induced colitis, and the protective effects were tightly correlated with gut microbiota.
Keywords: 16S rDNA, BBH, colitis, DSS, gut microbiota
INTRODUCTION
Two of the most common types of inflammatory bowel disease (IBD) are ulcerative colitis and Crohn's disease,1 both of which are characterized by mucosal or submucosal lesions of the colon.2 Abdominal discomfort, weight loss, diarrhea, hematochezia, and vomiting are some of the clinical manifestations of this chronic disease.3 It is generally believed that the incidence of IBD is closely associated with environmental factors, hereditary factors, and immune factors, as well as lifestyle habits.4
In recent years, the occurrence of colitis has increased in Asia.5 Although the pathogenesis of IBD remains elusive, it was recognized that imbalances inflammatory cytokines (IL-11, IL-10, IL-4, IL-1β, IL-6, IL-12 etc.) exerted significant roles in the pathogenesis and progression of IBD.6 The signaling pathways NF-κB and STAT3, which might control inflammation, immunological responses, and stress reactions, were revealed to be tightly associated with these cytokines. It was considered that the activation of NF-κB played an essential role in the occurrence of IBD, and the phosphorylation of STAT3 could aggravate the progression of this disease.
The gut microbiota's role in IBD is well acknowledged, despite the fact that the etiology and pathophysiology remain poorly understood.7 The human stomach is believed to contain >100 trillion microorganisms. Furthermore, the gut flora is a component of a complex network that keeps normal physiological processes running smoothly.8 Symbiotic intestinal microorganisms are beneficial to the host, and they can regulate the balance of gastrointestinal function and provide a stable and healthy intestinal environment.9 The breakdown of the balance leads to chronic inflammatory diseases.10
Emerging studies have shown that the occurrence of colitis is associated with disorders of intestinal flora, although the functions of some specific bacterial species are still controversial.11 Our previous work reported that the relative abundance of Turicibacter was greatly upregulated in colitis, whereas the abundance of Peptococcus, Gemella, Staphylococcus, and Jeotgalicoccus was all downregulated.12 Considering the importance of gut microbiota in human health and the progression of colitis, it has been a subject of intense interest to investigate the pathological mechanisms of some inflammation-related diseases. This strategy also helps us to seek effective drugs, which can modulate the ecology of gut microbiota to maintain the health conditions of human.
In clinical practice, aminosalicylic acid and immunosuppressive medications are often used to treat colitis, although its safety problems remain unsolved.13,14 Traditional Chinese medicine is well known for its notable efficacy as well as fewer side effects.15 There are many Chinese herbal medicines recorded to treat colitis in ancient literature. Also, many components of Chinese medicine and traditional Chinese patent medicines have been studied in clinical trials, such as Huanglian Jiedu decoction. Coptidis Rhizoma and Scutellariae Radix are often used together in Chinese herbal prescription, such as H. Jiedu decoction. Berberine and baicalin are the main active ingredients of C. Rhizoma and S. Radix, respectively.
It was reported that berberine treatment could induce the activation of mucosal CD4+ T cells and result in the improvement of colitis.16 By triggering the mechanistic target of rapamycin complex 1 (mTORC1) pathway, Li et al. showed that berberine may shield mice against the colitis caused by dextran sulfate sodium (DSS).17 In addition, berberine was said to affect intestinal flora and control the ratio of T regulatory cells (Treg) to T helper cell 17 (Th17).18 Baicalin is a flavone derivative and has been has been utilized extensively in clinical studies for its beneficial pharmacological effects, such as anti-inflammation, antibacterial, and antitumor.19–21 Feng reported that baicalin could downregulate the expression of Toll-like receptor 4 (TLR4) and NF-κB in DSS-induced colitis mice.22 In brief, both berberine and baicalin have the potential to improve the pathologies associated with experimental colitis in mice.
Taken together, because of the traditional use of C. Rhizoma and S. Radix, and the excellent protective effects of baicalin and berberine hydrochloride, the strategy of the combined use of baicalin and berberine hydrochloride (BBH) was investigated in this study. Moreover, to investigate the regulatory roles of baicalin and berberine on gut microbiota, we confirmed the protective effects of BBH on DSS-induced colitis and explored the relevance between the disease protection and gut microbiota in this work.
MATERIALS AND METHODS
Cell culture
RAW264.7, a mouse macrophage cell line (Cell Resource Center, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences), was cultured in RPMI-1640 (AD199522; HyClone, USA) containing 10% fetal bovine serum (1901571; Biological Industries, Israel) and Pen-Strep Solution (1831739; Biological Industries), at 37°C in a humidified incubator containing 5% CO2.
Nitric oxide production assay
RAW264.7 cells were plated in six-well plates at a density of 2.5 × 105 cells per well. Cells were then pretreated for 1 h after apposition with various ratios of BBH, and then lipopolysaccharide (LPS) at a final concentration of 1 g/mL was applied for 24 h. In a 96-well plate, 50 μL of cell culture medium and 50 μL of sulfonamide reagent were reacted. After incubation for 10 min, 50 μL of N-1-naphthylenediamine was added to each well, and then using enzyme labeling instrument at 560 nm, the absorbance value was measured.
DSS models
Male Kun Ming mice were obtained from Chengdu Dashuo Experimental Animal Co., Ltd., with a weight range of 20 ± 2 g. Mice were maintained under specific pathogen-free conditions and a 12-h light/12-h dark cycle. All animal experiments followed protocols confirmed by Institutional Ethical Committee of Shaanxi University of Chinese Medicine (SCXK [Sichuan] 2015-030). An experiment license was obtained with a specific approval signed by the Institutional Ethics Committee of Shaanxi University of Chinese Medicine.
DSS (36,000–50,000 DA) was acquired from MP Biomedicals, LLC. Baicalin and berberine hydrochloride were acquired from Chengdu PFID Biotechnology Co., Ltd. Sulfasalazine was purchased from Shanghai Xinyi Tianping Pharmaceutical Co., Ltd. All mice were randomly divided into control group, model group, low-dose group (20 mg/kg of BBH), medium-dose group (40 mg/kg of BBH), high-dose group (80 mg/kg of BBH), and positive control group (125 mg/kg of sulfasalazine).23 The control group drank distilled water. The ratio of baicalin to berberine hydrochloride in each dose group was 1:1.
Disease activity index
The disease activity index (DAI) score is widely accepted to evaluate the extent of colitis in animal models. During the experiment, the animals' weight alterations, stool viscosity, and hematochezia were recorded every day.24 DAI score was obtained by averaging the three values.
Cytokine assays
Mice were anesthetized with diethyl ether and eyeballs were removed to collect sanguis. The sanguis was left in Eppendorf tubes at room temperature for >1 h and then centrifuged at 3000 rpm for 5 min at 4°C (ThermoFisher Legend Micro 21R). The Elisa kits for Inflammatory factors (IL-1β, IL-4, IL-6, IL-8, IL-10, and TNF-α) were obtained from Neobio science, and the lot numbers were M190322-001b, M190322-003a, M190322-004a, M190322-104a, M190322-005a, and M190611-102a, respectively.
Histopathological evaluation
The tissues from the colon were removed, preserved for 48 h in 4% paraformaldehyde, and then embedded in paraffin. Hematoxylin-eosin (H&E) staining was used to gauge the level of inflammation. A pathologist performed the histologic score. Rare inflammatory cells were calculated at 0, low infiltration density of lamina propria cells as 1, expanded inflammatory cells in the submucosa as 2, and transmural inflammatory cell expansion as 3. No mucous membrane breakage was calculated at 0 for tissue damage, whereas intermittent mucosal damage was scored as 1, superficial mucosal erosion was calculated at 0, and substantial mucosal damage was counted as 3. From 0 to 6, the total histology score was possible.
DNA extraction
Each animal's feces was collected and stored at −80°C. Using the QIAamp DNA stool micro kit, the genomic DNA was extracted from stool samples. Agarose gel electrophoresis (agarose gel concentration: 1%; voltage: 170 V; electrophoresis time: 30 min) and measurements in nanometers were used to determine the integrity and amount of DNA (Thermo-Fisher-Scientific). For PCR testing and 16S rDNA sequencing, the isolated DNA could be applied directly.
16S rDNA Sequencing
The 16S rDNA gene was amplified using PCR. Using the forward 16S rDNA primer GTGCCAGCMGCCGGTAA and the reverse primer CCCCGYCAATTCMTTTRAGT, the v4-v5 (515-909) region was amplified. The QuantiFluor™-ST fluorescence quantification equipment (Promega, USA) was used to identify and quantify the PCR products, and the NEBNext Ultra II DNA Library Prep Kit was used to create the gene library (NEB, USA). Following the directions, sequencing was carried out using the Illumina MiSeq platform (Illumina, USA).
Paired read assembly of the raw sequencing data was performed by FLASH software (v 1.2.11; CBCB, Baltimore, MD, USA), and the quality was then checked using Trimmomatic, Pear, and USEARCH. Clean tags were then generated. UPARSE was used to cluster Operational Taxonomic Units at a similarity of 97% and a threshold of 0.005%. Then, OTUs were detected using Ribosomal Database Project classification with a confidence value of 70%. The ribosomal RNA database for 16S archaebacteria was downloaded from the Silva website.
Diversity analysis and differential analysis
Principal component analysis (PCA) based on OTUs of all samples was used to identify the potential principal components through dimensionality reduction. The unweighted pair group method with arithmetic mean was applied for hierarchical clustering based on beta diversity distance matrix. Beta diversity distance matrix was computed with Bray-Curtis by Qiime. The online tool Linear discriminant analysis Effect Size (LefSe) is an algorithm for seeking high-dimensional biomarker, which was applied for finding microbiota species with significant differences in abundance among different groups. The linear discriminant analysis (LDA) score was set as 3 for the effect size threshold, and other values were default.
Metagenome prediction analysis
PICRUSt was adopted to predict the subgenomic functionality of microbial communities based on the 16S rDNA genes in this work strictly following the instructions in the manual.
Statistical analysis
The statistical analysis of data in this study was represented as mean ± SEM. Statistical differences between groups were examined using GraphPad Prism 8 software with Student's t-test. P < .05 was considered statistically significant.
RESULTS
Effect of different ratios of BBH on nitric oxide production in LPS-induced RAW264.7 macrophages
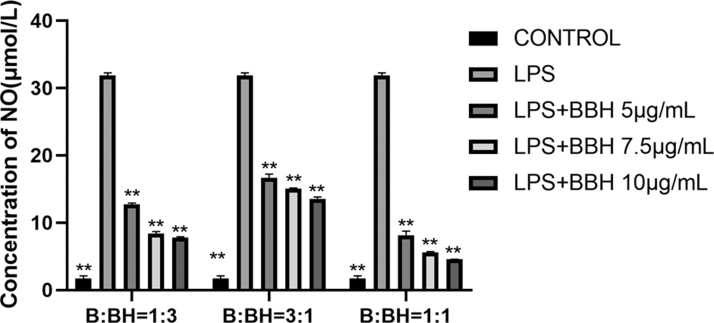
LPS was utilized to increase the production of nitric oxide (NO) in the alveolar macrophages to imitate the chronic inflammatory state for analyzing the anti-inflammatory effects of BBH. The results in Figure 1 indicated that after 24 h of LPS stimulation, NO secretion in the homogenates massively increased. Pretreatment with various ratios of the three BBH concentrations (5, 7.5, and 10 g/mL) before LPS stimulation reduced the augmentation of NO. In comparison to the control group, the LPS group produced more NO. It was discovered that BBH, when the ratio of baicalin to berberine hydrochloride is 1:1, has the greatest inhibitory effect on LPS-induced NO production. These findings demonstrated that BBH inhibits NO generation in LPS-induced inflammation, which is a mechanism through which BBH exerts anti-inflammatory effects.
FIG. 1.
Effect of different ratios of BBH on NO Production in LPS-Induced RAW264.7 Macrophages. RAW264.7 cells were pretreated with different ratios of BBH for 1 h after apposition, and then stimulated with LPS for 24 h. The NO production was higher in the LPS group than in the control group. The level of NO was lower in different ratios of BBH groups than in the LPS group. Treatment with a BBH preparation with a baicalin to berberine hydrochloride ratio of 1:1 had a greater effect on basal level of NO secretion in RAW 264.7 cells. n = 3, **P < .01 compared with LPS. BBH, baicalin and berberine hydrochloride; LPS, lipopolysaccharide; NO, nitric oxide.
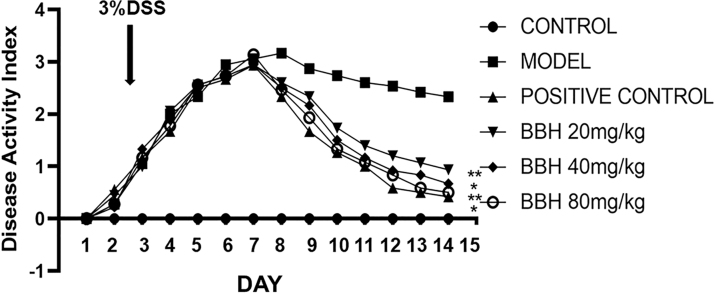
BBH decreased the DAI score of mice with DSS-induced colitis
The DAI score was adopted to evaluate the health conditions of experimental animals. The results in Figure 2 showed the DAI scores for all groups over 14 days. It was observed that after freely drinking the 3% DSS, the colitis model was successfully established with a DAI score of almost 3. By treating with Sulfasalazine Tablets (SASP) and three doses of BBH, the DAI scores of each group were all decreased significantly, indicating the protective effects of SASP and BBH. In conclusion, the outcomes of DAI indicated that BBH could inhibit the weight reduction and ameliorate the hematochezia of mice with induced colitis. The efficacy of three concentrations of BBH was almost equal to that of SASP.
FIG. 2.
DAI of mice. After colitis induction, the animals were treated for 7 days with distilled water, 3% of DSS, or 125 mg/kg of SASP or three groups with different doses of BBH. The model group showed no significant improvement after 14 days, while the SASP and three doses of BBH groups exhibited visibly improved DAI of disease mice. n = 6, **P < .01 compared with model. DAI, disease activity index; DSS, dextran sulfate sodium; SASP, Sulfasalazine Tablets.
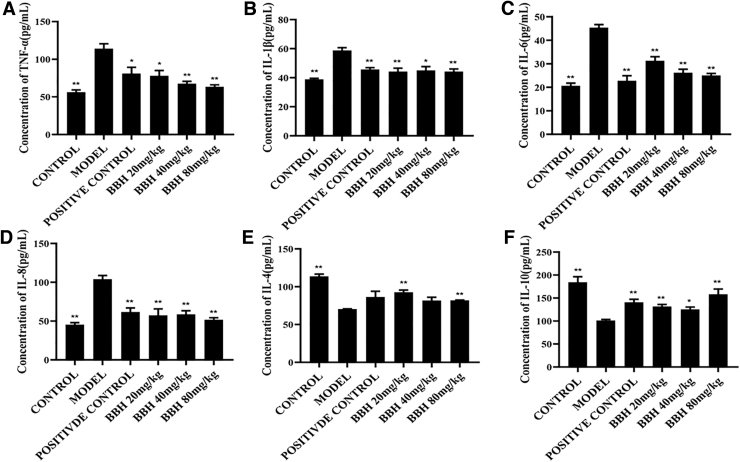
BBH regulated the levels of inflammatory cytokines
In our experiment, six inflammatory cytokines, including four proinflammatory cytokines IL-6, IL-8, IL-1β, and TNF-α, and two anti-inflammatory cytokines IL-4 and IL-10, were investigated. The results in Figure 3 show that the levels of proinflammatory cytokines IL-6, IL-8, IL-1β, and TNF-α were significantly increased, while the levels of anti-inflammatory cytokines IL-4 and IL-10 were greatly decreased after DSS-induced colitis, compared with the control group. After administration of SASP and three concentrations of BBH, the column heights of all proinflammatory cytokines were dramatically decreased. The column heights of IL-4 and IL-10 were increased compared with that in the model group. Collectively, these results demonstrated that the protective action of BBH was related with the balance of inflammatory cytokines.
FIG. 3.
Changes in inflammatory cytokines in each group. (A) Comparison of TNF-α levels in different groups. (B) Comparison of IL-1β levels in different groups. (C) Comparison of IL-6 levels in different groups. (D) Comparison of IL-8 levels in different groups. (E) Comparison of IL-4 levels in different groups. (F) Comparison of IL-10 levels in different groups. After administration with SASP and three doses of BBH, the levels of IL-1β, IL-6, IL-8, and TNF-α were noticeably decreased, whereas the levels of IL-10 and IL-4 increased clearly. Error lines indicate mean ± SEM; n = 6; *P < .05 compared with model, **P < .01 compared with model.
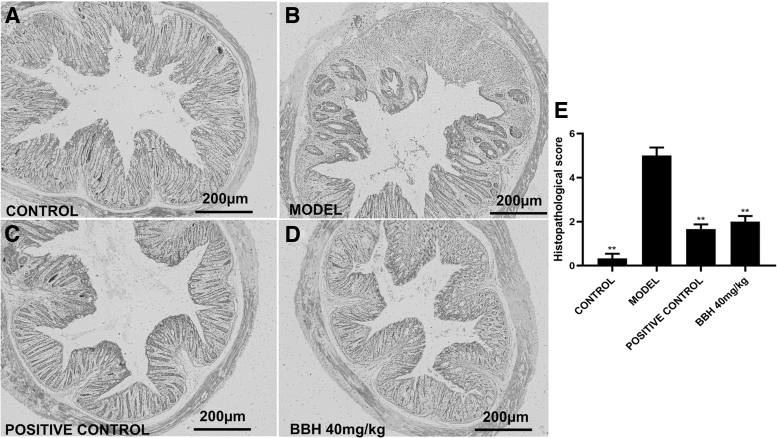
BBH improved the pathological structure of colitis mice with DSS-induced colitis
The results of H&E staining in Figure 4 revealed the histological characteristics of all groups in this work. The colon structure was clear and complete in the control group, including mucosal and submucous layer, internal-circular muscle, and external-longitudinal muscle, with the normal structure of crypts and many goblet cells (Fig. 4A). After freely drinking 3% DSS for 7 days, there was noticeable infiltration of multiple inflammatory cell types, the distortion and atrophy of the crypts; even the crypts and goblet cells had almost disappeared in some parts of the colon of the model group. These all-suggested serious colonic tissue damage and multiple erosive lesions, indicating the successful colitis model (Fig. 4B). Figure 4C showed the functions of sulfasalazine with a dose of 125 mg/kg.
FIG. 4.
Histological features at 100 × magnification. (A) Colonic mucosa in control group with regular arranged crypts and goblet cells. (B) The histologic features in the colitis group. (C) The histologic characteristics in the SASP group. (D) The histologic characteristics in the BBH group with a dose of 40 mg/kg. (E) Histological score obtained by blind histopathological analysis. Error lines indicate means ± SEM; n = 6, **P < .01 compared with model.
Although the colonic structure was fine, cellular structures were still won. The outcome demonstrated that SASP could improve the colitis condition induced by DSS. The action of 40 mg/kg of BBH was shown in Figure 4D. The structure of the colon was intact, and inflammatory cells had almost disappeared, implying that 40 mg/kg of BBH could improve the symptoms of DSS-induced colitis compared with the model group. The bar charts in Figure 4E showed the histological lesion scores for all groups. The column heights of each group were significantly changed, which suggested the curative effect of SASP and BBH on colitis.
BBH ameliorated the intestinal microecology of colitis mice induced by DSS
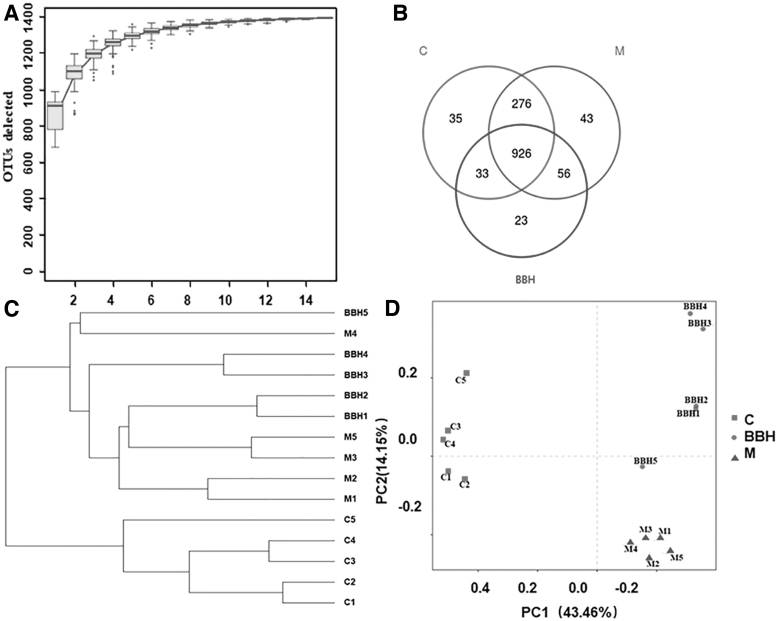
In this work, 16S rDNA genetically augmenting (V4-V5 region) and sequencing was adopted to explore the internal microbial community of each group. As shown in Figure 5A, species accumulation curves gradually increased and finally reached saturation, demonstrating that 15 samples were enough for sequencing. A total of 1392 OTUs were detected, and 926 OTUs coexisted in all three groups (Fig. 5B). The beta diversity of different groups was calculated by PCA and cluster analysis based on the Unifrac Distance Matrix.
FIG. 5.
Evaluation of Illumina MiSeq sequencing data and the beta diversity. (A) Specaccum species accumulation curves. (B) Venn diagram displaying different amounts of OTUs in the three groups. (C represents control group; M represents model group; BBH group with 80 mg/kg). (C) The clustering tree of intestinal microflora showing the β diversity of intestinal flora in different groups. (D) Multiple sample PCA. n = 5 for each group. OTUs, Operational Taxonomic Units; PCA, principal component analysis.
The cluster tree (Fig. 5C) showed that the diversities of the BBH group and model group were considerably different from that of the control group. The results of PCA (Fig. 5D) revealed that the microbial communities of the model group and control group were separated greatly, indicating that the colitis model was successful. The large distance between the model group and BBH group indicated that BBH could modulate the structure and abundance of the microbial community in colitis mice.
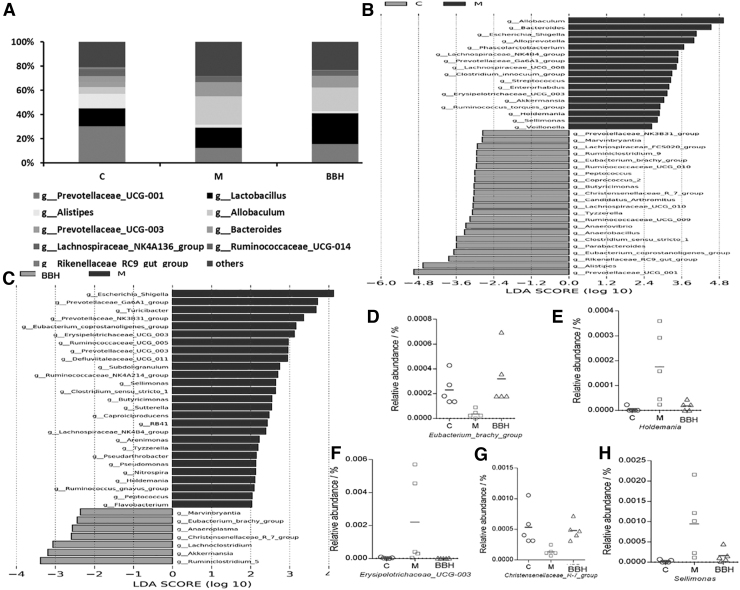
The species and relative abundance of intestinal microbiota in all groups were also analyzed by LefSe with a threshold value of 2. The column of the relative abundance revealed that the most prominent species were Prevotellaceae, Lactobacillus, Alistipes, Allobaculum, and Bacteroides (Fig. 6A). It was obviously observed that the abundance of Prevotellaceae was decreased in the model group compared with that of the control. After treatment with BBH, the abundance of Prevotellaceae increased. The tendency of Lactobacillus and Allobaculum in three groups gave a contrary result compared with that of Prevotellaceae.
FIG. 6.
Relative genus abundance and difference in dominant microorganisms among groups in LefSe analysis. (A) Relative genus abundance in each group. (B) Dark gray color means upregulated genera and gray color means downregulated in each group compared with the control group. Control group and model group. (C) Model groups and BBH treatment groups. (D) Relative abundance of Eubacterium_brachy_group. (E) Relative abundance of Holdemania. (F) Relative abundance of Erysipelotrichaceae_UCG-003. (G) Relative abundance of Christensenellaceae_R-7_group. (H) Relative abundance of Sellimonas. n = 5 for each group. LDA, linear discriminant analysis; LefSe, LDA effect size.
The results of LefSe are shown in Figure 6B and C. There were 22 genera that were increased in the control group and 7 genera increased in the BBH group compared with the model group. It was also found that 17 genera decreased in the colitis group compared with the control group, and 27 genera decreased in model group compared with the BBH group. Among them, the relative abundance of the Eubacterium_brachy_group and Christensenellaceae_R-7_group was downregulated in DSS-induced colitis and upregulated after BBH treatment (Fig. 6D, G). In contrast, the relative abundances of Holdemania, Erysipelotrichaceae_UCG_003, and Sellimonas were increased in DSS-induced colitis and declined after BBH treatment (Fig. 6E, F, H). In conclusion, the effects of BBH were connected to the abundance of microbiota and tightly connected with the relative abundances of Eubacterium_brachy_group, Holdemania, Erysipelotrichaceae_UCG_003, Christensenellaceae_R-7_group, and Sellimonas.
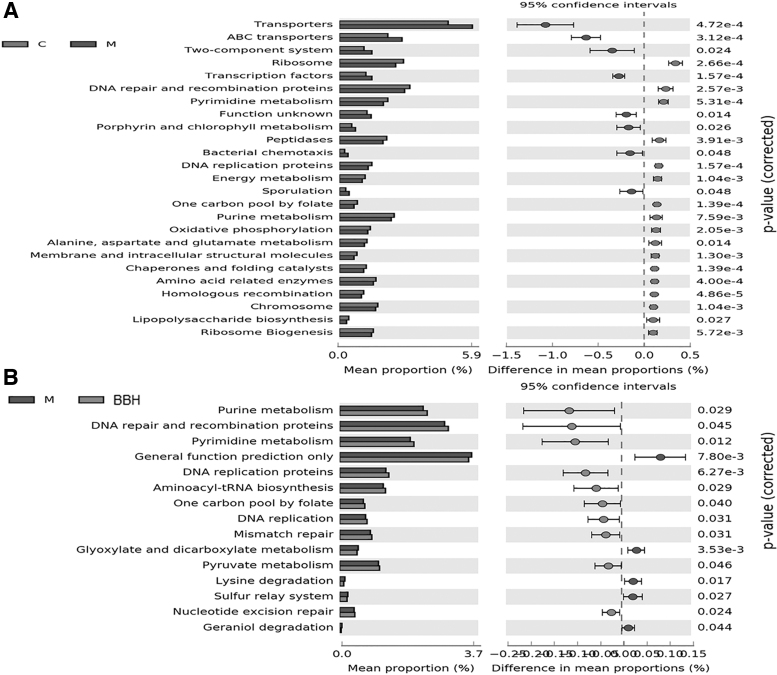
Gut microbiota structure is stable and abundant, which plays an important role in maintaining human health. In addition, the function of microbiota was also very significant. Therefore, metagenomic technology and the prediction tool of PICRUSt were used to explore the influence of BBH on microbiota function. As shown in Figure 7A, eight metabolism or biosynthesis pathways were upregulated in the model group, and 17 pathways were upregulated in the control group. After treatment with BBH, a total of 10 metabolism or biosynthesis pathways were upregulated and 5 pathways were downregulated (Fig. 7B). Among these results, the functions of purine metabolism, pyrimidine metabolism, DNA repair and recombination proteins, DNA replication proteins, and one carbon pool by folate were all decreased in model group and upregulated after administration of BBH. These results indicate that BBH can affect the synthesis and replication of DNA.
FIG. 7.
Functional changes in the microbiome following DSS and BBH treatment. (A) Control and Model group. (B) Model and BBH group. Extended error bar plot of genes drawn by STAMP software, significant value among groups were calculated by Wilcoxon test.
DISCUSSION
C. Rhizoma and S. Radix are two traditional Chinese herbs commonly used in combination in Chinese medicine formulae to exert their heat-clearing and detoxifying effects, especially in colitis treatment. Berberine and baicalin are the main chemical compositions in C. Rhizoma and S. Radix, respectively. These two compounds have been used to treat colitis, such as berberine hydrochloride tablets. Moreover, it has been reported that berberine can modulate the gut microbiota in the colon to regulate the balance of Treg/Th17 for colitis treatment.18 Moreover, the therapeutic actions of baicalin were related to TLR4 and NF-κB.22 To evaluate traditional use of C. Rhizoma and S. Radix and the therapeutic effects of berberine hydrochloride and baicalin, the combination of berberine and baicalin with a one to one ratio was studied for its protective effects against colitis.
The results of DAI showed that BBH exhibited excellent therapeutic effects, which were comparable to that of SASP (Positive Control). The concentrations of proinflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α were all sharply increased, and the concentrations of anti-inflammatory cytokines IL-10 and IL-4 were all notably decreased in the model group. These results demonstrated that our colitis model was successfully established. By treating with SASP and BBH, the column heights of IL-1β, IL-6, IL-8, and TNF-α were all decreased, and the column heights of IL-10 and IL-4 were all increased. These results further demonstrated the significant roles of inflammation-associated cytokines in the occurrence and development of colitis, and that BBH could modulate the balance of positive and negative cytokines associated with inflammation in colitis.
It was reported that IL-6 and TNF-α are two predominant inflammatory cytokines in the entire duration of IBD. These two cytokines were closely related to the activation of NF-κB and STAT3, respectively.25 For the modulatory effects of BBH on the levels of IL-6 and TNF-α, it was predicted that BBH could regulate the protein activation of NF-κB and STAT3. In addition, it was clearly observed the intact colon structure, the regular arranged crypts, and goblet cells were restored after the administration of BBH. These results demonstrated that BBH could significantly reduce histological injury markers in DSS-induced colitis in mice.
It is well known that gut microbiota are closely related with human health. Increasing evidence has demonstrated that the occurrence and progression of colitis are tightly associated with changes in the gut microbiota.26 Our previous work also confirmed that the balance of microecology was crucial to the health conditions of mice with experimental colitis. The results clearly demonstrated a good beta diversity of different groups, which implied that BBH could improve the diversity and abundance of gut microbiota.
Relative abundances of bacterial genera in each group demonstrated that BBH could modulate the microbial community structure, especially the abundance of Prevotellaceae, Lactobacillus, Alistipes, Allobaculum, and Bacteroides. The results of LefSe analysis found Eubacterium_brachy_group, Holdemania, Erysipelotrichaceae_UCG_003, Christensenellaceae_R-7_group, and Sellimonas were associated with the protective effects of BBH. In addition, it has been reported that Eubacterium was negatively correlated with DSS-induced colitis, but positively correlated with the control.27
The Christensenellaceae_R-7_group was correlated with the healthy status of the host.28 Our results also confirmed the important function of the Christensenellaceae_R-7_group in maintaining the health of experimental mice. It was reported that Erysipelotrichi belonged to Firmicutes, and the increased abundance of Erysipelotrichi was associated with colorectal cancer and TNF-α related to gastrointestinal inflammation.29 The abundance of Erysipelotrichaceae_UCG_003 was also increased in the model group. Erysipelotrichi also appeared to affect cholesterol and lipid metabolism in the gastrointestinal tract. Our results showed that the upregulated abundance of Holdemania was closely related with colitis induced by DSS. Moreover, the abundance of Holdemania was linked to impaired lipid and glucose metabolisms.30
The results of PICRUSt showed that BBH could change the functions of the microbiome, especially the functions of purine metabolism, pyrimidine metabolism, DNA repair and recombination proteins, DNA replication proteins, and one carbon pool by folate. It was also concluded that downregulation of these functions was positively correlated with colitis induced by DSS. It is well known that one carbon pool by folate, such as N10-formyltetrahydrofolate, plays a significant role in the denovo synthesis of nucleotides, and the downregulation of one carbon pool metabolism by folate depletion could lead to the inhibition of purine and pyrimidine metabolism. The decreased expression level genes related to DNA repair, recombination proteins, and DNA replication proteins could result in the inhibition of bacterial reproduction. After treatment with BBH, all these functions of the microbiome were upregulated. Therefore, it was predicted that the therapeutic effects of BBH were tightly connected with DNA synthesis, replication, and repair of gut microbiota.
In summary, this study demonstrates that BBH can significantly ameliorate the pathologies associated with DSS-induced colitis in mice. BBH was shown to reverse the imbalance between inflammatory and anti-inflammatory cytokines, and thus restore the structure of colon tissue to a healthy state. In addition, BBH regulated the intestinal microorganisms and biological metabolism pathways to improve the health status of mice with DS-induced colitis.
AUTHORs' CONTRIBUTIONS
Z.W., L.L., Y.Y., and J.Y. designed and conducted the study and analyzed the data. Y.Y., L.Q., D.Z., Y.L., Z.Z., K.W., and G.Z., L.P.: technical support, acquisition of data, and analysis and interpretation of data; Z.W., Y.L., and Z.T.: obtained funding and revision of the article. All authors read and approved the final article.
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
FUNDING INFORMATION
Funding for this work was provided by the Youth Innovation Team of Shaanxi Universities (Education Department of Shaanxi Provincial Government [2019], No. 90), the National Natural Science Foundation of China (Nos. 81803951, 81973687), the Key Research and Development Program of Shaanxi province (20JY012, 2018ZDCXL-SF-01-02-02), Science and Technology Young Nova Program of Shaanxi province (2019KJXX-025), Shaanxi Provincial Science and Technology Agency Project (2016KTTSSF01-01-01), the Major Increase and Reduction Project at the Central Level “the Ability Establishment for Sustainable Utilization of Valuable Chinese Medicine Resources” (No. 2060302), and Subject Innovation Team of Quality Control and Resources Development of “Qin Medicine” of Shaanxi University of Chinese Medicine (2019-QN01).
REFERENCES
- 1. Murakami S, Tasaka Y, Takatori S, et al. : Effect of Eucommia ulmoides leaf extract on chronic dextran sodium sulfate-induced colitis in mice. Biol Pharm Bull 2018;41:864–868. [DOI] [PubMed] [Google Scholar]
- 2. Sedghi S, Barreau F, Morilla I, et al. : Increased proliferation of the ileal epithelium as a remote effect of ulcerative colitis. Inflamm Bowel Dis 2016;22:2369–2381. [DOI] [PubMed] [Google Scholar]
- 3. Xu X, Xu C, Saud S, et al. : Effect of Kuijie granule on the expression of TGF-β/Smads signaling pathway in patients with ulcerative colitis. Evid Based Complement Alternat Med 2016;2016:2601830–2601838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dulai PS, Jairath V: Acute severe ulcerative colitis: Latest evidence and therapeutic implications. Ther Adv Chronic Dis 2018;9:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang YZ, Li YY: Inflammatory bowel disease: Pathogenesis. World J Gastroenterol 2014;20:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z, Wang L, Wu X, et al. : Polysaccharide extracted from Portulacae oleracea L. exerts protective effects against dextran sulfate sodium-induced colitis through inhibition of NF-κB. Am j transl re 2018;10:2502–2510. [PMC free article] [PubMed] [Google Scholar]
- 7. Singh V, Kumar M, San Yeoh B, et al. : Inhibition of interleukin-10 signaling induces microbiota-dependent chronic colitis in apolipoprote in E deficient mice. Inflamm Bowel Dis 2016;22:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Chen G, Yang Q, et al. : Gut microbiota drives the attenuation of dextran sulphate sodium-induced colitis by Huangqin decoction. Oncotarget 2017;8:48863–48874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank DN, St Amand AL, Feldman RA, et al. : Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007;104:13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamada N, Seo SU, Chen GY, et al. : Role of the gut microbiota in immunity and inflammatory disease. Nat\ rev Immunol 2013;13:321–335. [DOI] [PubMed] [Google Scholar]
- 11. Schreiner P, Neurath MF, Ng SC, et al. : Mechanism-based treatment strategies for IBD: Cytokines, cell adhesion molecules, JAK inhibitors, gut flora, and more. Inflamm Bowel Dis 2019;4:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang YN, Yu JG, Zhang DB, et al. : Indigo Naturalis Ameliorates dextran sulfate sodium-induced colitis in mice by modulating the intestinal microbiota community. Molecules 2019;24:4086–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukuda T, Naganuma M, Sugimoto S, et al. : The risk factor of clinical relapse in ulcerative colitis patients with low dose 5-aminosalicylic acid as maintenance therapy: A report from the IBD registry. PLoS One 2017;12:0187737–0187748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xi M, Wang X, Ge J, et al. : N′-[(3-[benzyloxy]benzylidene]-3,4,5-trihydroxybenzohydrazide (1) protects mice against colitis induced by dextran sulfate sodium through inhibiting NFκB/IL-6/STAT3 pathway. Biochem Biophys Res Commun 2016;477:290–296. [DOI] [PubMed] [Google Scholar]
- 15. Xu Z, Liu T, Zhou Q, et al. : Roles of Chinese Medicine and Gut Microbiota in Chronic Constipation. Evid Based Complement Alternat Med 2019;2019:9372563–9372573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahara M, Takaki A, Hiraoka S, et al. : Berberine improved experimental chronic colitis by regulating interferon-γ- and IL-17A-producing lamina propria CD4(+) T cells through AMPK activation. Sci Rep-UK 2019;9:11934–11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Q, Qu X, Pang X, et al. : Berberine protects mice against dextran sulfate sodium-induced colitis by activating mTORC1 pathway. Front Pharmacol 2019;10:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui H, Cai Y, Wang L, et al. : Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front Pharmacol 2018;9:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong SJ, Zhong YQ, Lu WT, et al. : Baicalin inhibits lipopolysaccharide-induced inflammation through signaling NF-κB pathway in HBE16 airway epithelial cells. Inflammation 2015;38:1493–1501. [DOI] [PubMed] [Google Scholar]
- 20. Xiao JR, Do CW, To CH: Potential therapeutic effects of baicalein, baicalin, and wogonin in ocular disorders. J Ocul Pharmacol Ther 2014;30:605–614. [DOI] [PubMed] [Google Scholar]
- 21. Lee D, Ko WK, Hwang DS, et al. : Use of baicalin-conjugated gold nanoparticles for apoptotic induction of breast cancer cells. Nanoscale Res Lett 2016;11:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feng J, Guo C, Zhu Y, et al. : Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int J Clin Exp Med 2014;7:4063–4072. [PMC free article] [PubMed] [Google Scholar]
- 23. Oz HS, Chen T, de Villiers WJ: Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front Pharmacol 2013;4:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fitzpatrick LR, Wang J, Le T: In vitro and in vivo effects of gliotoxin, a fungal metabolite: Efficacy against dextran sodium sulfate-induced colitis in rats. Digest Dis Sci 2000;45:2327–2336. [DOI] [PubMed] [Google Scholar]
- 25. Wang Z, Wu X, Wang CL, et al. : Tryptanthrin protects mice against dextran sulfate sodium-induced colitis through inhibition of TNF-α/NF-κB and IL-6/STAT3 pathways. Molecules 2018;23:1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishida A, Inoue R, Inatomi O, et al. : Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11:1–10. [DOI] [PubMed] [Google Scholar]
- 27. Sila S, Jelić M, Trivić I, et al. : Altered gut microbiota is present in newly diagnosed pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2020;70:497–502. [DOI] [PubMed] [Google Scholar]
- 28. Santos-Marcos JA, Haro C, Vega-Rojas A, et al. : Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol Nutr Food Res 2019;63:1800870–1800880. [DOI] [PubMed] [Google Scholar]
- 29. Seo B, Yoo JE, Lee YM, et al. : Sellimonas intestinalis gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 2016;66:951–956. [DOI] [PubMed] [Google Scholar]
- 30. Mancabelli L, Milani C, Lugli GA, et al. : Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol Ecol 2017;93:153–161. [DOI] [PubMed] [Google Scholar]