Abstract
One of the most abundant flavonoids present in cacao is (−)-epicatechin (Epi) and this flavanol has been linked to the cardiovascular health promoting actions of cocoa products. We previously demonstrated that Epi reduces infarct size in rodent models of ischemia/reperfusion and permanent coronary occlusion. Reduced infarct size was associated with decreased left ventricular (LV) oxidative stress (OS) and indicators of inflammation factors, which foster myocardial fibrosis. In this study, we examine the antifibrotic actions of Epi in an aging female rat model of pre-heart failure with preserved ejection fraction (pre-HFpEF) as well as its potential to mitigate plasma levels of OS, proinflammatory/profibrotic cytokines, and improve passive and active LV function. Epi treatment [1 mg/(kg·d)] was provided daily by gavage from 21 to 22 months of age, whereas controls received water. A Millar catheter was used to assess hemodynamic function. Subsequently, hearts were arrested in diastole, a balloon inserted into the LV and passive pressure–volume curves generated. Fixed LV sections were processed for collagen area fraction quantification using Sirius Red staining. Treatment with Epi did not lead to detectable changes in LV contractile function. However, passive LV pressure volume curves were significantly right shifted with Epi. Collagen area fraction values indicated that Epi treatment significantly reduces LV fibrosis. Epi also significantly reduced plasma OS markers and levels of profibrotic and proinflammatory cytokines. In conclusion, Epi reduces cardiac fibrosis in an aged, female rat model of pre-HFpEF, which correlates with significant reductions in OS and cytokine levels in the absence of changes in LV contractile function.
Keywords: epicatechin, fibrosis, heart failure, HFpEF
INTRODUCTION
Heart failure (HF) afflicts 5+ million people in the United States and is one of the most concerning worldwide health problems. HF, is classified as either with reduced (HFrEF) or preserved (HFpEF) ejection fraction (EF).1 About 50% of HF patients suffer from HFpEF and this pathology is more prevalent in postmenopausal women (∼2/3).2 Patients with HFpEF usually present intolerance to exertion, shortness of breath, and edema that are further aggravated when performing physical activities.2
Common comorbidities present in female HFpEF patients include hypertension, insulin resistance, and overweight/obesity, which are associated with high levels of oxidative stress (OS) and circulating proinflammatory cytokines.2 HFpEF patients frequently develop left ventricular (LV) diastolic dysfunction that may be caused by alterations in either active relaxation and/or passive stiffness.1 It is postulated that increases in passive LV stiffness is partly related to the development of myocardial fibrosis. Unfortunately, conventional drugs used to treat HFrEF patients have failed to benefit those with HFpEF and no drugs are known to be effective in reducing tissue fibrosis.1
One of the most abundant flavonoids present in cacao beans is (−)-epicatechin (Epi) and this flavanol, has been linked to the cardiovascular health promoting actions of cacao products.3–5 We previously demonstrated that Epi is capable of reducing infarct size in rodent models of ischemia/reperfusion injury and permanent coronary occlusion.6–8 Reductions in infarct size were associated with decreased LV OS levels and indicators of inflammation factors, which promote the development of tissue fibrosis.
We recently developed an aging female rat model of pre-HFpEF driven by low estrogen levels, excess weight, and hypertension.9 Although EF remained normal, animals developed diastolic dysfunction, reduced cardiac output (CO), and myocardial fibrosis. These changes were associated with significant increases in blood levels of proinflammatory and profibrotic cytokines.10 Using a rodent model of muscular dystrophy (δ-sarcoglycan null mice) and severe cardiomyopathy, Ramirez-Sanchez et al., reported that 2 weeks of oral Epi treatment was able to enhance muscle and heart myocardial reactive oxygen species (ROS) buffering systems, decrease OS, and preserve mitochondrial proteins.11 Epi treatment also lead to significant reductions in skeletal muscle and myocardial fibrosis. Furthermore, Epi inhibits in endothelium and myocardium arginase 1 activity, an enzyme involved in the initial steps of collagen synthesis.12,13 However, no studies have evaluated the capacity of Epi to reduce myocardial fibrosis using an aged, female animal model of long-standing LV remodeling.
Therefore, the aim of this study was to evaluate the potential antifibrotic effect of Epi and its impact on LV structure and function in an aging female model of pre-HFpEF driven by inflammation, hypertension, and excess body weight (BW).
MATERIALS AND METHODS
In vivo studies
Eighteen-month-old Fischer F344 female rats were used for the study. Animals were obtained from the National Institutes of Health (NIH)/National Institutes of Aging colony and were housed in pairs and maintained in a 12-h light-dark cycle with ad libitum intake of regular rat chow and sterilized tap water. Animal care and use followed the NIH Guide for the Care and Use of Laboratory Animals guidelines and the Institutional Animal Care and Use Committee of the University of California San Diego School of Medicine reviewed and approved this protocol (S16070).
At 18 months, and after 1 week of acclimatization, animals were subjected to ovariectomy as described by us.9 Fructose (10%) was provided in their drinking water beginning 5–7 days after ovariectomy. Fructose intake was used to induce changes that mimic metabolic syndrome features exhibited by HFpEF patients, such as hypertension, excess weight, and inflammation. At 21 months of age, animals were assigned into two groups: ovariectomy +10% fructose in drinking water (OVF; n = 22) and ovariectomy +10% fructose + Epi (OVFE; n = 8). For comparison purposes, data collected in our initial study from the OVF animal group9 was incorporated into this study database to increase the statistical analysis power. Epi [1 mg/(kg·d) by oral gavage] administration was performed daily, otherwise vehicle (water) was given for a period of 1 month. This dose was selected based on our previous work documenting its effectiveness in models of cardiovascular injury and fibrosis.11 At 22 months, animals were subjected to a terminal study evaluating arterial and LV hemodynamics and ex vivo passive LV pressure–volume curves. Hearts were fixed in formaldehyde at an LV pressure of ∼10 mmHg.
LV hemodynamics
Isoflurane-anesthetized animals with mechanical ventilation support had terminal LV hemodynamic evaluation at 22 months of age using a 2F conductance catheter (SPR-838 Millar Instruments). The pressure/conductance catheter was introduced in the carotid artery to record pressures and subsequently into the LV. LV pressure–volume loop measurements were acquired at a rate of 1000 Hz using AD Instruments Powerlab hardware and LabChart software. Data were acquired during baseline followed by partial inferior vena cava occlusions at diaphragm level to change filling volumes. After hemodynamic measurements, we performed saline injections for conductance/volume determination as previously described.9
LV pressure–volume curves
Following terminal hemodynamics, a diastolic arrest of the heart was performed using a slow infusion of 2.5 mL of cardioplegia (0.03 M 2,3-butanedione monoxime) and high potassium (50 mM) solution. The heart was excised and rinsed in ice-cold saline, remaining connective tissue was trimmed avoiding aortic tissue. Using a modified Langendorff system, the heart was cannulated through the aorta and was perfused in a retrograde manner with cardioplegic solution at room temperature and constant pressure (10–15 mmHg). Left atrium was removed and a balloon inserted into the LV.9 The balloon was coupled to a pressure transducer and an infusion pump. Passive LV pressure–volume curves were generated by inflating the balloon at a constant rate of 200 μL min−1. Pressure and volume data were recorded using DATAQ Software and Data Acquisition Systems (Akron, OH, USA) at a sampling rate of 10 Hz.
Following the mechanical analyses, LV were perfused with 10% formalin buffer through the aorta at a pressure ∼10–15 mmHg. Fixed tissue was stored for histology. Before fixation in a small subset of hearts (n = 3/group), a very small portion of the LV apex was flash frozen in liquid nitrogen and stored at −80°C for biochemical assays. PV curve slopes were calculated using physiological range values (5–20 mmHg) to assess for differences in slopes.
Histology
Collagen area fraction measurements were pursued using formalin-fixed, paraffin-embedded LV sections stained with Sirius Red as described by us.9 For transforming growth factor-beta 1 (TGF-β1) immunolabeling a boiled, citrate buffer at pH 6 was used for antigen retrieval. Slides were incubated overnight at 4°C with 1:100 dilution of the primary antibody (ab25121; Abcam) followed by incubation with horseradish peroxidase-conjugated secondary antibody (7074; Cell Signaling) for 1 h at room temperature. Brown color was generated using diaminobenzidine as a substrate.
Plasma cytokines
Blood was collected using LV puncture in vials containing ethylenediaminetetraacetic acid (EDTA) at the time of the terminal study. Plasma was separated by centrifugation for 10 min using a refrigerated centrifuge at 2000 g. Once separated, plasma samples were frozen for further analysis. Quantification of the proinflammatory markers; interleukin-1 beta (IL-1β), IL-2, tumor necrosis factor-alpha (TNF-α), and interferon-gamma was pursued. Samples were analyzed in a 96-well plate Milliplex Rat Cytokine/Chemokine Magnetic Bead Panel—Immunology Multiplex Assay (Cat. No. RECYTMAG-65K; EMD Millipore) following manufacturer instructions. Quantification was performed using LUMINEX software. The Milliplex assay kit sensitivities report minimum detectable concentrations (pg/mL) of IL-1β 0.8, IL-2 0.4, TNF-α 1.9, and IFN-γ 6.8. TGF-β1 levels were quantified using a Rat LAP Uncoated ELISA Kit (Cat. No. 88-50680-22; Thermo Fischer Scientific) following kit instructions. The minimum detectable sensitivity for the kit was 0.019 ng/mL.
Protein carbonylation
Protein carbonylation was used as a surrogate indicator of plasma and myocardial OS levels.11 Heart tissue samples were homogenized using a polytron tissue homogenizer in 500 μL of cold buffer (50 mM 4-morpholineethanesulfonic acid, pH 6.7, containing 1 mM EDTA) and centrifuged (10,000 g) for 15 min at 4°C. Supernatants were collected and incubated at room temperature for 15 min with streptomycin sulfate at a final concentration of 1%. Samples were centrifuged (6000 g) for 10 min at 4°C. Myocardial and plasma protein carbonylation was measured in samples using a colorimetric protein carbonyl assay kit in accordance to the instructions in the kit (Cat. No. 10005020; Cayman Chemical). The carbonyl protein assay kit coefficient of variation and sensitivity were 4.7% and 1 nmol, respectively. All samples were tested in duplicate at room temperature.
Protein oxidation
Myocardial oxidized protein levels were measured by Western blot analysis of dinitrophenylhydrazine (DNPH)-derived carbonyl groups on oxidized proteins using an Oxyblot kit (Sigma-Aldrich, USA). In brief, homogenized (see protein carbonylation section) protein samples (20 mg) were reacted with 2,4-DNPH for 15 min, followed by neutralization with a solution containing glycerol and β-mercaptoethanol. Samples were electrophoresed on a 4–14% polyacrylamide gel and electrotransferred to a polyvinylidene difluoride membrane. After blocking with 5% nonfat dry milk in Tris-buffered saline plus 0.1% Tween-20 for 1 h, membranes were incubated overnight in a cold room with a mouse anti-dinitrophenol antibody (1:250), followed by incubation in secondary horseradish-conjugated anti-rabbit (1:5000) for 1 h at room temperature.
After rinsing with wash buffer, the immunocomplexes were visualized using chemiluminescence kit (Amersham, Inc.) according to the manufacturer's instructions. Detected bands were normalized according to the values of glyceraldehyde-3-phosphate dehydrogenase used as a loading control. The bands from each group were analyzed using ImageJ NIH image software. OVF values were normalized to 100%.
Statistical analysis
Data are given as mean ± standard error of the mean. Statistical analysis used either a Student's unpaired t-test or analysis of variance. Analysis was carried out using SigmaPlot 10. Results were considered significant at P ≤ 0.05.
RESULTS
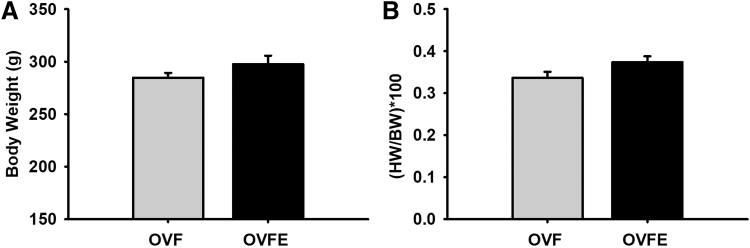
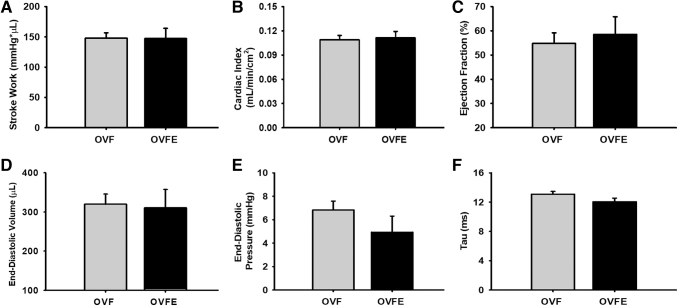
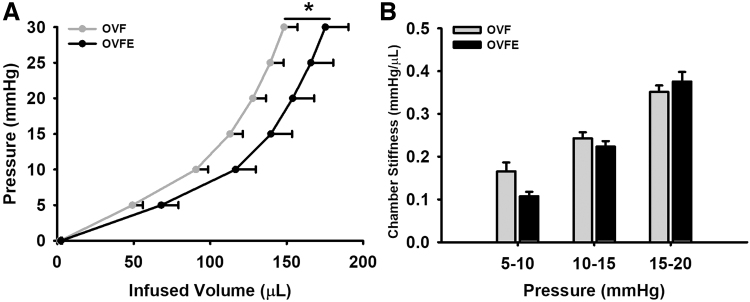
Total BW and normalized heart to BW values are given in Figure 1. No significant differences between both groups were noted in either of the parameters. LV hemodynamic data are summarized in Table 1 and select endpoints are given in Figure 2. As expected, high levels of systolic and diastolic blood pressure were detected in the OVF group (∼155/106 mmHg, respectively) and Epi did not reduce these levels. No differences emerged with Epi treatment in LV function parameters such as stroke work, cardiac index, ejection fraction, end diastolic volume/pressure, or isovolumic relaxation time constant (Tau, Fig. 2). LV passive pressure–volume results are given in Figure 3A. A significant right shifted curve was noted with Epi treatment versus the OVF group (P < .05). However, the analysis of chamber stiffness (Fig. 3B) by PV curve slope comparisons at three different pressure ranges (5–10, 10–15, and 15–20 mmHg) did not detect differences.
FIG. 1.
Average body weights and heart to body weight ratios of 22-month-old female rat groups. (A) Body weight and (B) heart weight/body weight ratio after a 30-day vehicle or (−)-epicatechin treatment. Values are given mean ± SEM. OVF (n = 22); OVFE (n = 8). OVF, ovariectomized +10% fructose; OVFE, ovariectomized +10% fructose + (−)-epicatechin; SEM, standard error of the mean.
Table 1.
Arterial and Left Ventricular Catheter Hemodynamic Values Recorded in 22-Month-Old Female Rats Treated with Either Vehicle or (−)-Epicatechin
| Parameter | Group |
t-Test |
|
|---|---|---|---|
| OVF (n = 22) | OVFE (n = 8) | P | |
| Stroke work (mmHg × μL) | 14,801.41 ± 873.50 | 14,734.63 ± 1673.69 | .970 |
| CO (μL/min) | 46,067.27 ± 2184.42 | 48,312.50 ± 3339.60 | .593 |
| Stroke volume (μL) | 161.67 ± 7.04 | 163.66 ± 13.20 | .889 |
| Maximal LV volume (μL) | 351.08 ± 25.75 | 339.30 ± 48.54 | .821 |
| Minimum LV volume (μL) | 184.84 ± 23.86 | 175.60 ± 44.59 | .848 |
| End-systolic volume (μL) | 212.52 ± 25.37 | 204.28 ± 47.84 | .873 |
| End-diastolic volume (μL) | 319.66 ± 26.00 | 310.50 ± 47.36 | .861 |
| Maximal LV pressure (mmHg) | 143.49 ± 5.01 | 147.00 ± 8.92 | .726 |
| Minimum LV pressure (mmHg) | 1.63 ± 0.46 | −0.02 ± 0.88 | .086 |
| Mean LV pressure (mmHg) | 61.92 ± 3.01 | 63.68 ± 4.73 | .762 |
| Developed pressure (mmHg) | 141.85 ± 4.82 | 147.01 ± 8.40 | .589 |
| End-systolic pressure (mmHg) | 140.41 ± 4.89 | 141.6 ± 9.30 | .905 |
| End-diastolic pressure (mmHg) | 6.82 ± 0.76 | 4.93 ± 1.37 | .221 |
| Heart rate (bpm) | 286.38 ± 7.63 | 298.15 ± 6.86 | .388 |
| Ejection fraction (%) | 54.80 ± 4.34 | 58.49 ± 7.29 | .665 |
| Arterial elastance (mmHg/μL) | 0.91 ± 0.05 | 0.91 ± 0.09 | .989 |
| Maximal pressure change rate dP/dt+ (mmHg/sec) | 7031.59 ± 349.32 | 6989.50 ± 358.18 | .947 |
| Minimum pressure change rate dP/dt− (mmHg/sec) | −8189.27 ± 498.39 | −7948.88 ± 475.19 | .787 |
| Maximal volume change rate dV/dt + (μL/sec) | 6759.23 ± 405.15 | 6789.38 ± 1003.50 | .973 |
| Minimum volume change rate dV/dt − (μL/sec) | −9263.95 ± 1064.94 | −9746.38 ± 2440.25 | .834 |
| Tau (ms) | 13.06 ± 0.39 | 12.04 ± 0.49 | .165 |
| Diastolic aortic pressure (mmHg) | 106.23 ± 4.95 | 114.01 ± 4.26 | .378 |
| Systolic aortic pressure (mmHg) | 154.62 ± 9.61 | 165.95 ± 7.80 | .582 |
| Mean aortic pressure (mmHg) | 128.71 ± 6.66 | 135.73 ± 5.47 | .551 |
| Cardiac index (mL/min/cm2) | 0.108 ± 0.005 | 0.111 ± 0.007 | .814 |
| Stroke volume index (μL/cm2) | 0.381 ± 0.017 | 0.376 ± 0.030 | .895 |
| End-diastolic volume index (μL/cm2) | 0.756 ± 0.064 | 0.725 ± 0.120 | .813 |
| Preload recruitable stroke work | 78.57 ± 6.24 | 59.47 ± 5.98 | .089 |
| End-systolic pressure–volume relationship (mmHg/μL) | 0.658 ± 0.065 | 0.453 ± 0.056 | .081 |
| End-diastolic pressure–volume relationship (mmHg/μL) | 0.046 ± 0.004 | 0.040 ± 0.003 | .460 |
Data are given as mean ± standard error of the mean.
CO, cardiac output; LV, left ventricular; OVF, ovariectomy +10% fructose; OVFE, ovariectomy +10% fructose + epicatechin.
FIG. 2.
Carotid and LV hemodynamic values recorded using a conductance catheter in vehicle or in (−)-epicatechin treated rats. (A) Stroke work. (B) Cardiac index. (C) Ejection fraction. (D) End-diastolic volume. (E) End-diastolic pressure. (F) Isovolumic relaxation time constant (tau). Values are given as mean ± SEM. OVF (n = 22); OVFE (n = 8). LV, left ventricular.
FIG. 3.
Effects of (−)-epicatechin treatment on passive LV mechanical properties. (A) Pressure–volume curves; (B) LV slopes at pressure increments. Values are given as mean ± SEM, *P < .05 by analysis of variance. OVF (n = 22); OVFE (n = 8).
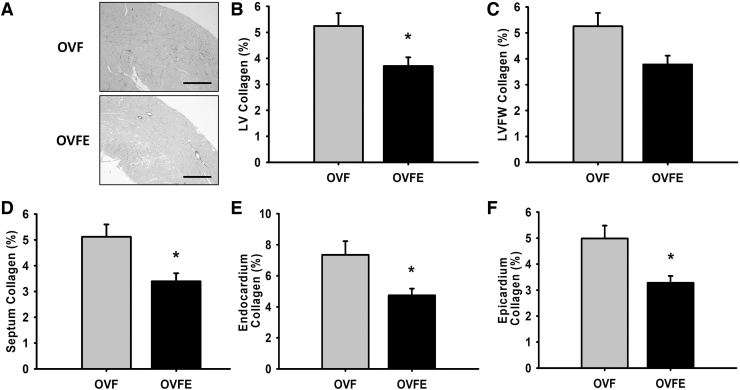
As given in Figure 4, LV collagen quantification yielded significantly higher levels of myocardial fibrosis in total LV, septum, free wall, endocardium, and epicardium regions of the OVF animals versus those treated with Epi (P < .05). Epi-induced reductions in collagen area averaged from ∼5.5 to 3.5% (i.e., a 2% reduction in collagen area). The analysis of papillary muscle fibrosis also indicated that Epi was able to mitigate collagen deposition in this muscle structure (data not shown).
FIG. 4.
Effects of (−)-epicatechin treatment on myocardial fibrosis. (A) Representative sections from LV. (B–F) Morphometric and histological analysis of collagen area fraction (%) in LV tissue sections in female rats. OVF (n = 22); OVFE (n = 8). Values are given as mean ± SEM, *P < .05 by t-test. LVFW, LV free wall.
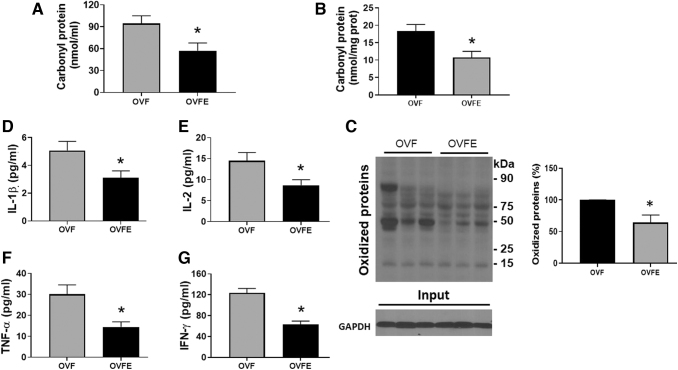
Values of plasma protein carbonyls detected a significant ∼39.2% decrease (P = .02) with Epi treatment (Fig. 5A). The analysis of myocardial protein carbonylation levels (Fig. 5B) also yielded ∼41.4% reduction (18.34 ± 3.2 nmol/mg protein for OVF and 10.75 ± 2.6 with Epi treatment, P = .04) as well as with myocardial total protein oxidation (Fig. 5C; 98 ± 3.1 arbitrary units for OVF and 64.3 ± 8.3 or ∼34.4% reduction with Epi treatment, P = .039).
FIG. 5.
Effects of (−)-epicatechin treatment on oxidative stress and pro-inflammatory cytokines levels. (A) Carbonyl protein levels in plasma and (B) myocardium. (C) Myocardial oxidized protein levels as detected by Western blots (left image) and their normalized values (right panel). Plasma levels of IL-1β (D), IL-2 (E), TNF-α (F) and, IFN-γ (G). Values are given as mean ± SEM. Student's t-test. *, P < .05 versus OVF. GADPH, glyceraldehyde 3-phosphate dehydrogenase; IFN-γ, interferon-gamma; IL-1β, interleukin 1-beta; IL-2, interleukin 2; TNF-α, tumor necrosis factor-alpha.
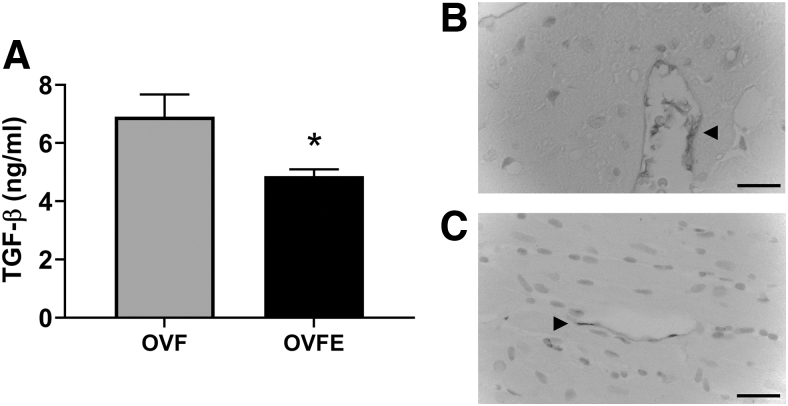
Results from plasma cytokines are given in Figure 5D–G. As can be observed, Epi treatment significantly decreased (P < .05) the levels of the proinflammatory cytokines IL-1β, IL-2, IFN-γ, and TNF-α. Epi-induced decreases were also noted in plasma samples for the profibrotic factor TGF-β1 (P < .001 vs. OVF), which were also detected by immunostaining (Fig. 6).
FIG. 6.
Effects of (−)-epicatechin treatment on plasma TGF-β1 levels (A). TGF-β1 immunostaining of representative sections of the LV (B). OVF (n = 10); (C) OVFE (n = 7). Arrowhead highlights areas of positive staining. Values are given as mean ± SEM, *P < .05 by t-test. TGF-β1, transforming growth factor-beta 1.
DISCUSSION
The treatment of HFpEF continues to represent a challenge as no effective therapeutic agents have been developed to improve the capacity of the LV to accommodate greater filling volumes and thus, increase CO upon greater demand. As fibrosis has emerged as one of the possible causes for increased stiffness, novel agents are needed to be identified that have the capacity to mitigate or reverse the enhanced deposition of collagens as so far, no agents have been proven as effective.
Factors commonly associated with the development of HFpEF in women include age, low estrogen levels (as per menopause), obesity, and hypertension. Although the real cause of LV stiffening as seen in patients with HFpEF has not been identified, a significant number of patients develop myocardial fibrosis.1 It is well recognized that with aging greater levels of OS develop that foster an inflammatory milieu.14,15 Higher levels of OS are also detected in women upon menopause as estrogens are known to enhance ROS buffering systems and mitigate inflammation.16–18 Obesity and hypertension are also associated with increases in OS and inflammation.19 Cytokines such as TNF-α, IFN-γ, IL-1β, and IL-2 are recognized as important mediators of inflammation and their circulating levels are known to increase with aging.14,15 Proinflammatory cytokines can activate cardiac fibroblasts to increase production of collagens leading to fibrosis a process, that is critically dependent on enhanced TGF-β1 activity.20,21
As two-third of HFpEF patients are older women, animal models need to be developed that can be add to our understanding of the pathophysiology of the disease and evaluate potential therapies. Recently, we developed a female rat model (used in this study) that incorporated the intersecting roles of aging, low estrogen, and excess weight on driving alterations on cardiac structure/function.9 Ovariectomized aged female rats provided 10% fructose in water increased BW (∼20% vs. aged alone) and developed hypertension (mean pressure of 134 mmHg).
As reported in our study, echocardiography detected very modest LV remodeling (including wall thickness), whereas catheter hemodynamics demonstrated significant (P < .05) decreases versus intact (control) aged rats in stroke volume (from 194.8 in controls to 161.8 μL or a 15% reduction), stroke volume index (from 0.627 to 0.376 or 52%), CO (from 63.5 to 46.3 mL/min or 27%), and increases in the time constant of isovolumic relaxation or tau that reflects altered diastolic function (from 10 to 13.4 ms or 34%) while demonstrating preserved EF of 55%. Histology indicated papillary and interstitial fibrosis. Thus, OVF rats recapitulate many cardiovascular features present in female HFpEF patients and can be used to evaluate potential therapies.
Studies have demonstrated that the consumption of cacao (i.e., cocoa) products reduces blood pressure and improves vascular function in smokers, patients with diabetes, postmenopausal women, and patients with atherosclerosis.3–5 Population studies have reported the beneficial effects of consuming small amounts of cocoa products yielding reductions in overall risk for cardiovascular disease of ∼40%.3 An association was identified in 32,000 Swedish women aged 48–83 years between the modest habitual consumption of chocolate and reduced incidence of HF hospitalization or death.4 The health effects of cocoa products are attributed to the actions of Epi, the most abundant cacao flavanol. In preclinical and clinical studies, we have extensively characterized a unique and potent ability of Epi to exert cardiac and vascular protective effects.6–8,19
Most recently, Ramirez-Sanchez et al11 reported on the effects of Epi treatment on 2.5-month-old δ-sarcoglycan null mice that developed a very severe form of muscular dystrophy characterized by extensive skeletal and cardiac muscle cell death and fibrosis. Epi treatment reduced muscle protein carbonylation, equilibrated reduced/oxidized glutathione ratio, and improved ROS buffering systems. Most interestingly, Epi treatment also demonstrated a notable reduction in myocardial and skeletal muscle fibrosis, leading to improved muscle performance. In a proof of concept clinical trial,22 multiple markers of mitochondrial and skeletal muscle health improved in patients with Becker muscular dystrophy treated for 8 weeks with Epi, thus supporting the potential of the flavanol to exert beneficial effects on muscle. We also reported on the capacity of Epi to reduce arginase activity thus potentially reducing the synthesis of collagen.12,13
Various classes of antifibrotic candidate drugs have been evaluated in the setting of adverse cardiac remodeling and fibrosis with limited success and include those that target the renin–angiotensin–aldosterone system, β-blockers, crosslink breaker compounds, modulators of inflammation. and agents such as relaxin.23 Drugs tested in the preclinical and clinical setting include angiotensin-converting enzyme inhibitors, angiotensin receptor type 1 blockers such as losartan and aldosterone blockers such as spironolactone or eplerenone.23 Crucial to the initial validation of these agents are studies using chronic animal models of cardiac remodeling. In a study using aged spontaneously hypertensive rats with severe cardiac remodeling and fibrosis, treatment with lisinopril reduced chamber hypertrophy, fibrosis, and ventricular stiffness.24 In an animal model of cardiac remodeling and fibrosis secondary to a Duchenne-like muscular dystrophy, the use of losartan for 2 years led to a reduction in cardiac fibrosis.25 Small clinical studies have historically reported encouraging results.
Brilla et al demonstrated that in hypertensive patients treated with lisinopril for 6 months, significant reductions in myocardial fibrosis occurred accompanied by improved diastolic function.26 In another study, patients were randomized to conventional treatment with or without eplerenone. In epleronone patients, LV relaxation recorded by echocardiography improved more than those on conventional treatment. Diastolic function improvement was associated with slower increases in plasma procollagen metabolites.27 Edwards et al reported similar data in patients with HFpEF where the effects of spironolactone on LV function and circulating markers of collagen turnover were compared versus placebo.28 After 40 weeks of treatment, spironolactone improved LV relaxation and attenuated increases in type III procollagen peptides. However, large clinical studies in HFpEF patients using these classes of agents have failed and thus for this type of HF no effective therapies have been identified and novel candidate agents with potential antifibrotic actions need to be explored.
Results from this study indicate that treatment with Epi for a period of 1 month late in the development of adverse remodeling and fibrosis was effective in reducing plasma OS levels to ∼45% versus controls. Significant reductions of ∼50% were also noted in plasma levels of the four cytokines tested. Epi also reduced circulating levels of TGF-β1 by ∼25%, which were also confirmed by immunostaining of tissue samples. These observations are in line with published reports on the capacity of flavonoids to reduce inflammation.29,30 A detailed assessment of LV fibrosis yielded a significant decrease in % collagen area of ∼1/3 in the Epi-treated group versus controls thus reducing levels to close to normal for aged animals. Despite reducing fibrosis, hemodynamic results failed to detect improvements in diastolic or systolic LV function. Passive pressure–volume curves indicated a right-shifted curve for the Epi-treated group, suggesting a more compliant LV chamber. However, the analysis of LV slopes showed only modest decreases with Epi.
These results suggest that despite beneficial shifts in factors recognized to promote adverse changes in LV structure and function (including fibrosis), these did not translate into functional gains. It is possible that earlier and longer treatment with Epi would be required to trigger detectable improvements in function. Alternatively, higher degrees of fibrosis may be required to develop so as to detect the possible impact of Epi.
In conclusion, using a rodent model of pre-HFpEF late treatment with Epi demonstrated its capacity to reduce fibrosis possibly through reductions in OS and cytokine levels. However, these effects did not translate into structural and functional improvements suggesting that such changes may possibly require earlier interventions that merit further investigations.
ACKNOWLEDGMENTS
The authors the technical support of Diane Huang with all the animal and physiological studies.
AUTHORs' CONTRIBUTIONS
M.B.-P.: Investigation, data curation, writing—reviewing and editing. I.R.-S.: Investigation, data curation, writing—original draft preparation, reviewing, and editing. A.G.-C.: Investigation, data curation, writing—reviewing and editing. B.I.: Investigation, data curation, writing—original draft preparation, reviewing, and editing. V.N.: Investigation, data curation. M.H.: Investigation, data curation. R.G.: Methodology, investigation, data curation, writing—reviewing and editing. N.C.: Methodology, investigation, data curation, writing—reviewing and editing. G.C.: Supervision, data curation, formal analysis, writing—reviewing and editing. F.V.: Conceptualization, funding acquisition, project administration, supervision, data curation, writing—reviewing and editing.
AUTHOR DISCLOSURE STATEMENT
Drs. Villarreal is a co-founder and stockholder (Dr. Ceballos) of Epirium Bio, Inc.
FUNDING INFORMATION
Funding was provided by the Department of Defense PR150090 and National Institutes of Health DK98717, AG47326 to F.V. and Consejo Nacional de Ciencia y Tecnologia, Mexico 253769 to G.C.
REFERENCES
- 1. Gevaert AB, Boen JRA, Segers VF, et al. Heart failure with preserved ejection fraction: A review of cardiac and noncardiac pathophysiology. Front Physiol 2019;10(1):638; doi: 10.3389/fphys.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bozkurt B, Khalaf S. Heart failure in women. Methodist Debakey Cardiovasc J 2017;13(4):216–223; doi: 10.14797/mdcj-13-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corti R, Flammer AJ, Hollenberg NK, et al. Cocoa and cardiovascular health. Circulation 2009;119(10):1433–1441; doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 4. Lamuela-Raventós RM, Romero-Pérez AI, Andrés-Lacueva C. Review: Health effects of cocoa flavonoids. Food Sci Technol Int 2005;11(3):159–176; doi: 10.1177/1082013205054498. [DOI] [Google Scholar]
- 5. Ellam S, Williamson G. Cocoa and human health. Annu Rev Nutr 2013;33(1):105–128; doi: 10.1146/annurev-nutr-071811-150642. [DOI] [PubMed] [Google Scholar]
- 6. Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, et al. Short- and long-term effects of (−)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Hear Circ Physiol 2008;295(2):H761–H767; doi: 10.1152/ajpheart.00413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamazaki KG, Taub PR, Barraza-Hidalgo M, et al. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. J Am Coll Cardiol 2010;55(25):2869–2876; doi: 10.1016/j.jacc.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamazaki KG, Andreyev AY, Ortiz-Vilchis P, et al. Intravenous (−)-epicatechin reduces myocardial ischemic injury by protecting mitochondrial function. Int J Cardiol 2014;175(2):297–306; doi: 10.1016/j.ijcard.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bustamante M, Garate-Carrillo A, Ito BR, et al. Unmasking of oestrogen-dependent changes in left ventricular structure and function in aged female rats: A potential model for pre-heart failure with preserved ejection fraction. J Physiol 2019;597(7):1805–1817; doi: 10.1113/JP277479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loredo-Mendoza ML, Ramirez-Sanchez I, Bustamante-Pozo MM, et al. The role of inflammation in driving left ventricular remodeling in a pre-HFpEF model. Exp Biol Med 2020;245(8):748–757; doi: 10.1177/1535370220912699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramirez-Sanchez I, De Los Santos S, Gonzalez-Basurto S, et al. (−)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic δ-sarcoglycan null mouse striated muscle. FEBS J 2014;281(24):5567–5580; doi: 10.1111/febs.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ortiz-vilchis P, Ortiz-flores M, Pacheco M, et al. The cardioprotective effects of (-)-Epicatechin are mediated through arginase activity inhibition in a murine model of ischemia/reperfusion 2018;818(May 2017):335–342; doi: 10.1016/j.ejphar.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 13. Garate-Carrillo A, Navarrete-Yañez V, Ortiz-Vilchis P, et al. Arginase inhibition by (−)-Epicatechin reverses endothelial cell aging. Eur J Pharmacol 2020;885:173442; doi: 10.1016/j.ejphar.2020.173442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis Int Soc Aging Dis 2011;2(2):158–173. [PMC free article] [PubMed] [Google Scholar]
- 15. Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett 2006;236(1):13–23; doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 16. Chakrabarti S, Lekontseva O, Davidge ST. Estrogen is a modulator of vascular inflammation. IUBMB Life 2008;60(6):376–382; doi: 10.1002/iub.48. [DOI] [PubMed] [Google Scholar]
- 17. Monteiro R, Teixeira D, Calhau C.. Estrogen signaling in metabolic inflammation. Mediators Inflamm 2014;2014(1):615917; doi: 10.1155/2014/615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Störk S, Van Der Schouw YT, Grobbee DE, et al. Estrogen, inflammation and cardiovascular risk in women: A critical appraisal. Trends Endocrinol Metab 2004;15(2):66–72; doi: 10.1016/j.tem.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19. McDermott MM, Criqui MH, Domanchuk K, et al. Cocoa to improve walking performance in older people with peripheral artery disease: The COCOA-PAD pilot randomized clinical trial. Circ Res 2020;126(5):589–599; doi: 10.1161/CIRCRESAHA.119.315600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 2011;51(4):600–606; doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-β1. Mol Genet Metab 2000;71(1–2):418–435; doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 22. McDonald CM, Ramirez-Sanchez I, Oskarsson B, et al. (−)-Epicatechin induces mitochondrial biogenesis and markers of muscle regeneration in adults with Becker muscular dystrophy. Muscle and Nerve 2021;63(2):239:249; doi: 10.1002/mus.27108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li AH, Liu PP, Villarreal FJ, et al. Dynamic changes in myocardial matrix and relevance to disease: Translational perspectives. Circ Res 2014;114(5):916–927; doi: 10.1161/CIRCRESAHA.114.302819. [DOI] [PubMed] [Google Scholar]
- 24. Brilla CG, Matsubara L, Weber KT. Advanced hypertensive heart disease in spontaneously hypertensive rats: Lisinopril-mediated regression of myocardial fibrosis. Hypertension 1996;28(2):268–275; doi: 10.1161/01.HYP.28.2.269. [DOI] [PubMed] [Google Scholar]
- 25. Bish LT, Mark Y, Meg MS, et al. Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One 2011;6(6):e20856; doi: 10.1371/journal.pone.0020856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 2000;102(12):1388–1393; doi: 10.1161/01.CIR.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 27. Deswal A, Richardson P, Bozkurt B, et al. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (RAAM-PEF). J Card Fail 2011;17(8):634–642; doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 28. Edwards NC, Ferro CJ, Kirkwood H, et al. Effect of spironolactone on left ventricular systolic and diastolic function in patients with early stage chronic kidney disease. Am J Cardiol 2010;106(10):1505–1511; doi: 10.1016/j.amjcard.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 29. Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc 2010;69:273–278; doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 30. Nijveldt RJ, Van Nood E, Van Hoorn DEC, et al. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr 2001;74(4):418–425; doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]