Abstract
The recent discovery of mesenchymal stem cells within periapical lesions (PL-MSC) has presented novel opportunities for managing periradicular diseases in adult teeth by way of enhancing tissue regeneration. This discovery coincides with the current paradigm shift toward biologically driven treatment strategies in endodontics, which have typically been reserved for non-vital immature permanent teeth. One such approach that shows promise is utilizing local endogenous non-collagenous dentine extracellular matrix components (dECM) to recruit and upregulate the intrinsic regenerative capacity of PL-MSCs in situ. At picogram levels, these morphogens have demonstrated tremendous ability to enhance the cellular activities in in vitro and in vivo animal studies that would otherwise be necessary for periradicular regeneration. Briefly, these include proliferation, viability, migration, differentiation, and mineralization. Therefore, topical application of dECMs during ortho- or retrograde root canal treatment could potentially enhance and sustain the regenerative mechanisms within diseased periapical tissues that are responsible for attaining favorable clinical and radiographic outcomes. This would provide many advantages when compared with conventional antimicrobial-only therapies for apical periodontitis (AP), which do not directly stimulate healing and have had stagnant success rates over the past five decades despite significant advances in operative techniques. The aim of this narrative review was to present the novel concept of exploiting endogenous dECMs as clinical tools for treating AP in mature permanent teeth. A large scope of literature was summarized to discuss the issues associated with conventional treatment modalities; current knowledge surrounding PL-MSCs; composition of the dECM; inductive potentials of dECM morphogens in other odontogenic stem cell niches; how treatment protocols can be adapted to take advantage of dECMs and PL-MSCs; and finally, the challenges currently impeding successful clinical translation alongside directions for future research.
Impact statement
Apical periodontitis (AP) is an inflammatory condition that is associated with a great degree of morbidity and ultimately leads to tooth loss. The purpose of this review was to summarize the current evidence pertaining to stem cell therapy in endodontics and present a novel clinical methodology through which they may be utilized to address AP. A comprehensive overview of the basic science, clinical translation, and potential challenges are presented in this review.
Keywords: dentine extracellular matrix components, endodontics, regenerative medicine, stem cells, tissue regeneration, wound healing
Introduction
Apical periodontitis (AP) is an inflammatory condition of the periodontium that exists when there is a dynamic equilibrium between putative endodontic microorganisms and host defense mechanisms.1 The ideal objective for treating this disease is to restore architecture and functions of the periradicular tissues that were lost to the immune response. Conventional therapies achieve these outcomes indirectly by reducing the microbial load within infected root canals to create a pro-healing environment.2 Although this approach may be enough to initiate periapical wound healing, which involves a highly co-ordinated sequence of hemostasis, inflammation, proliferation, and remodeling,3 it offers no additional stimulus for biological regeneration thereafter.4
Unaided, these endogenous processes are often insufficient to achieve complete tissue regeneration and will instead be compensated by reparative scar tissue.4 Persistent periapical radiolucencies may, therefore represent not only failure to eradicate intraradicular infection but also inadequate physiological regenerative processes, which could explain why larger lesions demonstrate higher treatment failure rates.5,6 It also suggests that to attain more predictable outcomes, it would be necessary to employ alternative strategies that simultaneously manage the microbial load and directly enhance intrinsic regenerative events within damaged periradicular tissues.
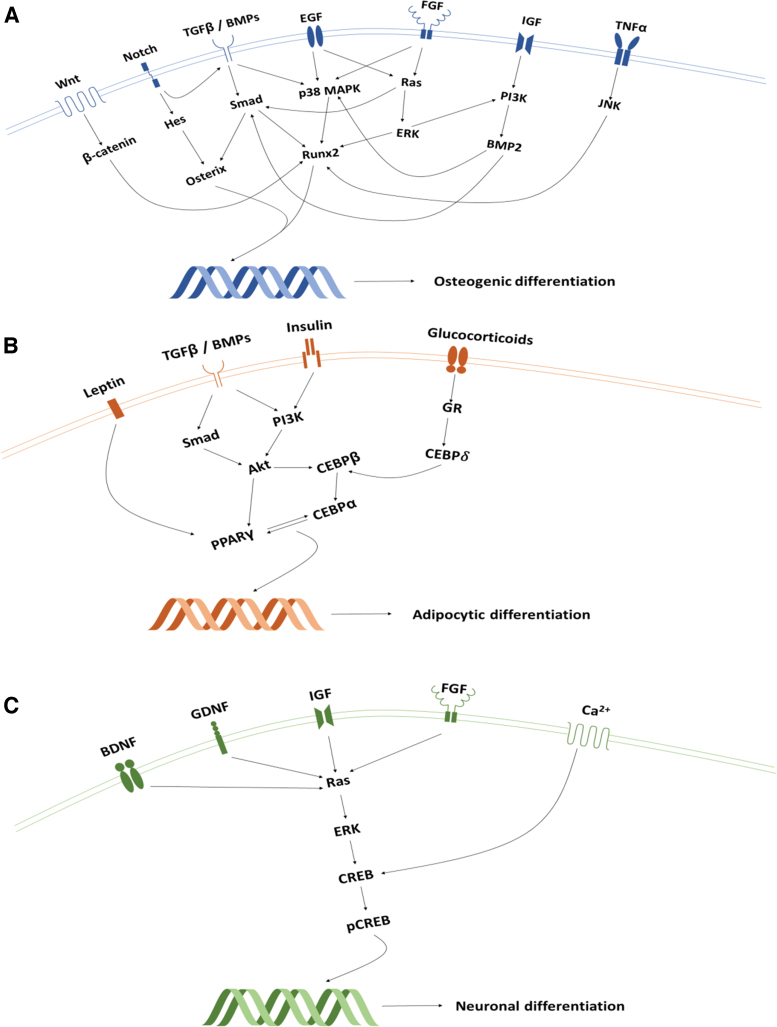
Stem cells are essential to wound-healing processes, as they possess high proliferation rates, self-renewal capabilities, and potential for multi-lineage differentiation.7,8 Embryonic stem cells are pluripotent, as they can develop into stromal cells from any of the three germinal layers whereas multipotent postnatal stem cells are more restricted to organ-specific lineages.9 The latter are more amenable to clinical translation due to their autologous nature and presence within almost all adult tissues.10 A subset of multipotent progenitors derived from the mesoderm germ layer, called “mesenchymal stem cells” (MSC), has attracted particular interest within regenerative endodontics as they can give rise to several mineral producing mesoderm lineages, including bone (Fig. 1).11
FIG. 1.
(A–C) A schematic illustration of osteogenic, adipocytic, and neuronal differentiation pathways in mesenchymal stem cells. Akt, protein kinase B; BDNF, brain-derived neurotrophic factor; BMP, bone matrix protein; Ca2+, calcium ions; CEBP, enhancer binding protein; CREB, cAMP response element-binding protein; EGF, epithelial growth factor; ERK, extracellular signal-regulated kinases; FGF, fibroblast growth factor; GDNF, glial cell line-derived neurotrophic factor; GR, glucocorticoid receptor; HES, hairy and enhancer of split-1; IGF, insulin-like growth factor; Jnk, c-Jun N-terminal kinases; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; PPAR, peroxisome proliferator-activated receptor; Runx2, runt-related transcription factor 2; TGFβ, transforming growth factor beta; TNF-α, tumour necrosis factor-alpha; Wnt, wingless/integrated. Color images are available online.
Moreover; although they are known to be harvested from bone marrow, other reservoirs have been isolated from within the pulp and associated periodontal tissues of permanent and deciduous teeth.9,12,13 Named according to their tissue of origin, these “dental MSC” niches include “dental pulp stem cells” (DPSC), “stem cells from human exfoliated deciduous teeth” (SHED), “periodontal ligament stem cells” (PDLSC), “dental follicle precursor cells” (DFPC), “stem cells of the apical papilla” (SCAP), “gingival MSCs,” “alveolar bone MSCs,” and “tooth germ progenitor cells.”9,12 When transplanted into in vivo human and animal models, these dental MSCs have demonstrated a potent capacity to regenerate pulp-like tissue in empty root canals,14–16 dentine-like tissues in endodontic perforation defects,17 and periodontal tissues in surgically created periodontal defects.18–20
Further, the positive outcomes revealed from their applications to other non dento-alveolar tissues, including the treatment of autoimmune, cardiovascular, endocrine, hepatic, musculoskeletal, neurodegenerative, ophthalmic, dermatological, and respiratory diseases, confirm their potential to be utilized as powerful therapeutic tools (Supplementary Table S1). Recent studies, however, have identified another clinically accessible dental MSC population directly within the inflamed periradicular tissues of infected mature permanent teeth.21,22 These periapical lesion-derived MSCs (PL-MSC) possess tremendous immunosuppressive and regenerative potential and could, therefore, provide exciting opportunities to develop therapies for AP that actively engage with the endogenous mechanisms of periradicular tissue regeneration.
The cellular events required for periradicular regeneration are co-ordinated by various growth factors, cytokines, chemokines, and angiogenic and neurotrophic signaling molecules.23 Noteworthy examples include members of the transforming growth factor-beta (TGF-β), bone morphogenetic protein (BMP), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and insulin growth factor (IGF) families, among many others.24 Although these polypeptides are endogenously secreted by host cells at the site of disease, they rapidly deplete due to their relatively short half-life within the extracellular environment.23 Fortunately, abundant reservoirs of these molecules are locally sequestered within the dentine's extracellular matrix.25 They are deposited by secreting odontoblasts during dentinogenesis and become fossilized during subsequent mineralization.
Thereafter, their bioactivity remains highly preserved through the formation of proteoglycan bonds but these can be immediately reinstated on release.26,27 This has previously been achieved on command through demineralizing irrigants,28–30 pulp capping agents,31–33 epigenetic modifiers,34 and dental adhesives.35 The resulting extracts, formally termed “dentine extracellular matrix components” (dECM), have demonstrated a potent capacity to upregulate regenerative events within various odontogenic MSC niches.33,36,37 It is, therefore, plausible to expose PL-MSCs in situ to this cocktail of bioactive molecules to enhance local tissue healing. This approach could overcome current limitations associated with conventional treatments for AP and provide clinicians with unique capabilities to actively apply a biologically driven therapy to the diseased periradicular tissues.
The aim of this narrative review was to explore the novel concept of exploiting endogenous dECMs to upregulate local MSC-mediated periradicular tissue regeneration in mature permanent teeth diagnosed with AP. All abbreviations used in this article are provided in Table 1.
Table 1.
Definitions of Abbreviations Found in Text
| Abbreviation | Definition |
|---|---|
| AP | Apical periodontitis |
| BDNF | Brain-derived neurotrophic factor |
| BMP | Bone morphogenetic proteins |
| BSP | Bone sialoprotein |
| CD | Cluster of differentiation |
| CXCR4 | Chemokine receptor type 4 |
| dECM | Dentine extracellular matrix components |
| DFPC | Dental follicle precursor cells |
| DMP-1 | Dentine matrix protein 1 |
| DPP | Dentine phosphoprotein |
| DPSC | Dental pulp stem cells |
| DSPP | Dentin sialophosphoprotein |
| EDTA | Ethylenediaminetetraacetic acid |
| ESE | European Society of Endodontology |
| FGF | Fibroblast growth factors |
| HGF | Hepatocyte growth factor |
| IGF | Insulin growth factor |
| IL | Interleukin |
| MEPE | Matrix extracellular phosphoglycoprotein |
| MMP | Matrix metalloproteinases |
| MSC | Mesenchymal stem cells |
| NaOCl | Sodium hypochlorite |
| NGF | Nerve growth factor |
| NT3 | Neurotrophin 3 |
| NT4 | Neurotrophin 4 |
| OCN | Osteocalcin |
| ON | Osteonectin |
| OPN | Osteopontin |
| PDGF | Platelet-derived growth factor |
| PDLSC | Periodontal ligament stem cell |
| PlGF | Placental-derived growth factor |
| PL-MSC | Periapical lesion-derived mesenchymal stem cell |
| RUNX2/CBFA1 | Runt-related transcription factor 2 |
| SCAP | Stem cells of the apical papilla |
| SDF-1 | Stromal-derived factor 1 |
| SHED | Stem cells from human exfoliated deciduous teeth |
| TGF-β | Transforming growth factor-beta |
| TIMP | Tissue inhibitors of matrix metalloproteinases |
| TNF-α | Tumour necrosis factor alpha |
| VEGF | Vascular endothelial growth factors |
Current Issues Associated with Conventional Root Canal Therapy
Kakehashi et al. confirmed a direct causal relationship between putative endodontic microorganisms and periapical disease.38 Consequentially, therapeutic strategies for AP have focused exclusively on disinfecting necrotic root canals with the aim of relieving clinical signs and symptoms of inflammatory disease, preventing systemic bacterial spread, and ultimately retaining natural and functioning teeth.2 These outcomes are typically achieved through the use of antimicrobial solutions, primarily sodium hypochlorite (NaOCl), which possesses potent bactericidal and proteonacious properties, in conjunction with canal enlarging instruments.39
Significant advances in the chemo-mechanical debriding armamentarium have been made over the past 50 years, with some of the most revolutionary developments including highly flexible rotary/reciprocating file systems and machine-assisted irrigant agitation techniques. When compared with more conventional approaches, these now widely used practices facilitate deeper irrigant penetration into root dentine,40 greater intracanal debris and smear layer removal,41 and reductions in endodontic bacterial load and viability.42,43 It is, therefore, apparent that the operator's ability to disinfect root canals has significantly improved since the fundamental principles of endodontic therapy were first established.
Unfortunately, the aforementioned progress has not translated into improved clinical outcomes as success rates for root canal treatment have remained static for five decades. For instance, a systematic review by Ng et al. (2007) revealed that pooled success rates of all prior observational studies at 1 year follow-up ranged between 68% and 85% according to strict plain-film radiographic criteria.44 Thereafter, several prospective cohort studies reported comparable results of 83.0% (2–4 year follow-up) and 82.7% (5 year follow-up),5,6 with those reviewing patients over longer periods revealing even less favorable outcomes of 65.3% (20 year follow-up).45 Therefore, one out of five teeth with primary AP will in the short to medium term fail to heal after root canal treatment and eventually require more complicated and invasive remedial therapy.
Moreover, these figures likely underestimate the true incidence of treatment failure, as plain-film radiographs lack sensitivity for detecting periapical pathosis when compared with three-dimensional imaging techniques.46,47
Another issue is that microorganisms, and their by-products, cannot be completely eradicated from root canal systems due to complicated anatomy.48 Even root-filled teeth exhibiting no clinical or radiographic signs of AP harbor vital bacteria.49,50 Several inferences can be drawn from this finding. First, the ultimate objective of conventional approaches may be too idealistic, as residual bacteria are postoperatively unavoidable. Second, below a certain microbial load host mechanisms are capable of initiating, but not necessarily sustaining, regenerative events. Third, after surpassing this critical threshold, further disinfection provides no additional stimulus for endogenous periapical healing. These concepts are supported by several robust clinical investigations by Paredes-Vieyra et al., Liang et al., and Verma et al. who, respectively, demonstrated that intracanal medicaments, irrigant agitation techniques, and concentrated NaOCl solutions do not increase treatment success when compared with less aggressive disinfection protocols.51–53
It can be surmised that the effects of antimicrobial-only approaches on periradicular healing are finite and alternative methods, designed to initiate and sustain tissue healing, may yield more predictable outcomes. It must be stressed, however, that adequate endodontic disinfection still remains a fundamental prerequisite to provide an adequate micro-environment for any tissue repair strategy.
Periapical Lesion-Derived MSCs
In 2004, Maeda et al. successfully isolated “fibroblastic cells” from within the inflamed periradicular granulation tissues of mature infected teeth.54 Thereafter, Liao et al., Đokić et al., and Marrelli et al. in vitro all confirmed their highly proliferative, multipotent, and clonogenic properties.21,55,56 Further, mesenchymal surface markers, Cluster of Differentiation [CD]-13, -29, -44, -73, -90, -105, and -166 were highly expressed; whereas hematopoietic markers, namely CD-14, -19, -34, -45, and human leukocyte antigen-DR isotype, were not.21,55–61 These characteristics fulfilled the minimum criteria necessary for this population to be recognized as a distinct MSC niche. Although many terms have been used to refer to this group, “PL-MSCs” is considered most accurate in the absence of explicit histological diagnoses and thus is the preferred designation (Table 2).
Table 2.
Key Characteristics of Periapical Lesion-Derived Mesenchymal Stem Cells
| Aliases | Immunophenotype |
Proliferation |
Differentiation markers |
Mineralisation | Immunoregulatory effects | ||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Niche | Rate | Cell type | Genetic | Staining | |||
| Granulation tissue-derived stem cells Human fibroblastic cells Human periapical cyst-derived mesenchymal stem cells Inflamed periapical progenitor cells Periapical lesion-derived stem cells |
CD-13, CD-29, CD-44, CD-46, CD-73, CD-90, CD-105, CD-146, CD-166, Stro-1 | CD-14, CD-19, CD-34, CD-45, HLA-DR | DFPC DPSC PDLSC PL-MSC SCAP SHED |
++ ++ +++ + +++ ++ |
Odontoblast Osteoblast Cementoblast Chondrocyte Adipocyte Astrocyte |
DMP-1, DSSP ALP, BSP, MEPE, ON, OPN, RunX2/Cbfa1 BSP, OCN, OPN — ADIPOQ, GLUT-4, LPL, PPARɣ DAT, En1, Foxa2, GFAP, MAP2, MSX1, NF-H, NF-M, Nurr1, Pitx3, TH, β-III tubulin |
Alizarin Red S Alizarin Red S Alizarin Red S Alcian Blue Oil Red O — |
Calcific Tissues Fibrous Tissues |
Increases leukocytic production of TGF-β Inhibits differentiation of dendritic cells Reduces leukocytic production of IL-1β, -2, -5, -6, TNF-α, and IFN-ɣ Reduces leukocytic proliferation |
ADIPOQ, adiponectin; ALP, alkaline phosphatase; BSP, bone sialoprotein; CD, cluster of differentiation; DAT, dopamine transporter; DFPC, dental follicle precursor cells; DMP-1, dentine matrix protein 1; DPSC, dental pulp stem cell; DSSP, dentin sialophosphoprotein; En1, engrailed-1; Foxa2, forkhead box protein A2; GFAP, glial fibrillary acidic protein; GLUT-4, glucose transporter type 4; HLA-DR, human leukocyte antigen-DR isotype; IFN-ɣ, interferon gamma; IL, interleukin; LPL, lipoprotein lipase; MAP2, microtubule-associated protein 2; MEPE, matrix extracellular phosphoglycoprotein; MSX1, msh homeobox 1; NF-H, neurofilaments heavy; NF-M, neurofilaments medium; Nurr1, nuclear receptor related 1 protein; OCN, osteocalcin; ON, osteonectin; OPN, osteopontin; PDLSC, periodontal ligament stem cell; Pitx3, paired-like homeodomain transcription factor 3; PL-MSC, periapical lesion-derived stem cell; PPARɣ, peroxisome proliferator-activated receptor gamma; RunX2/Cbfa1, runt-related transcription factor 2; SCAP, stem cells of the apical papilla; SHED, stem cells from human exfoliated deciduous teeth; TGF-β, transforming growth factor-beta; TH, tyrosine hydroxylase; TNF-α, tumour necrosis factor alpha; +, low; ++, medium; +++, high.
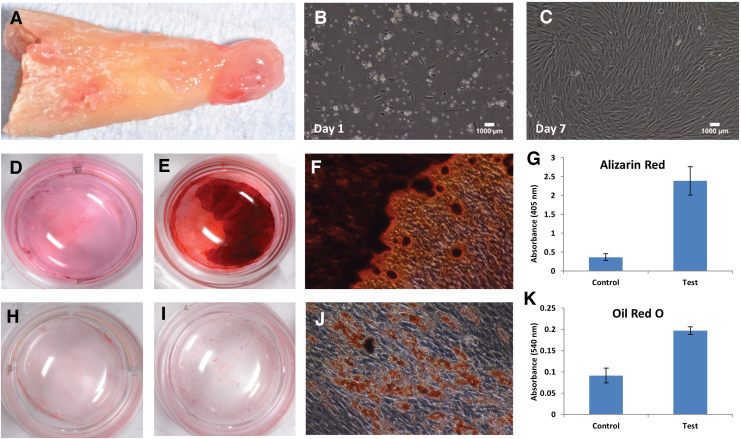
Figure 2 outlines preliminary data on the multipotent potential of primary PL-MSCs cultured from the apical granulomas of extracted teeth diagnosed with AP.
FIG. 2.
Multipotent potential of primary PL-MSCs. (A) PL-MSCs were isolated from the apical granuloma of extracted teeth via a collagenase type 1 enzyme digestion technique. Cells were cultured in a T25 flask with 20% fetal bovine serum supplemented α-MEM media, which was changed every 2 days. (B, C) Phase-contrast microscopy at 10 × magnification of PL-MSC cultures at day 1 (B) and day 7 (C). (D–G) Osteogenic differentiation after 21 days of culture with control or osteogenic induction media (α-MEM, 20% FBS, 1% penicillin/streptomycin, 2 mM glutamine, 0.2 mM ascorbic acid, 100 nm dexamethasone, 10 mM β-glycerophosphate). Staining with Alizarin Red S confirmed absence in control wells (D) and presence in test wells of mineral deposits (E, F). Staining was solubilised with 10% acetic acid, and subsequent intensity was quantified by using a microplate reader with an excitation wavelength set at 405 nm (G). (H–K) Adipogenic differentiation after 21 days of culture with control or adipogenic induction media (α-MEM, 20% FBS, 1% penicillin/streptomycin, 2 mM glutamine, 0.5 mM IBMX, 200 μM indomethacin, 10 μM insulin, 1 μM dexamethasone). Staining with Oil Red O confirmed absence in control wells (H) and presence in test wells of lipid droplets (I, J). Staining was solubilized with isopropanol, and subsequent intensity was quantified by using a microplate reader with an excitation wavelength set at 540 nm (K). All experiments were conducted up to passage 2 by using three biological replicates. Scale bars represent 1000 μm. Color images are available online.
Clinical observations of periradicular regeneration after endodontic therapy indicate that PL-MSCs primarily contribute to local intrinsic periapical wound-healing processes. This is supported by in vitro investigations confirming that these cells possess the necessary capabilities to restore such tissues. For instance, with appropriate cues PL-MSCs differentiate into osteoblasts, cementoblasts, adipocytes, astrocytes, and chondrocytes, all of which are relevant for regenerating the periodontium.54–56,58
In addition, when compared with other odontogenic MSCs, these multipotent properties are more directed toward osteogenesis.55,62 This was demonstrated through gene expression analyses where upon osteogenic induction, PL-MSCs exhibited transcriptional profiles more indicative of osteogenic differentiation than DPSCs (osteonectin [ON], bone sialoprotein [BSP], runt-related-transcription-factor 2 [RUNX2/CBFA1]), which instead greatly expressed odontogenic markers (dentin sialophosphoprotein [DSPP], dentine matrix protein [DMP]-1).62 The mineralization needed for these cells to be considered functional has also been confirmed through several in vitro differentiation assays,55,56,60 as well as in vivo subcutaneous implantation mouse models.21
Such stem-like characteristics, however, do vary with CD146-positive PL-MSC subpopulations exhibiting lower proliferative, clonogenic, and osteogenic potential than CD146-negative subpopulations.59 These properties may also be dampened by the inflammatory microenvironment, as indicated by weaker proliferation rates when compared with healthy DPSCs and PDLSCs.21,55,63
Stem cells from periradicular lesions also possess immunomodulatory properties. For instance, Đokić et al. initially demonstrated that PL-MSC co-cultures significantly reduced leukocytic proliferation, differentiation, and pro-osteoclastic cytokine production (Interleukin [IL]-1β, -2, -5, -6, tumor necrosis factor [TNF]-α, Interferon-ɣ), while simultaneously increasing anti-inflammatory growth factor secretion (TGF-β).55,57 These results were corroborated by Araujo-Pires et al., who in vivo detected a converse immunological profile in Chemokine Receptor Type [CXCR]4 knockout mice and higher expression of transcriptional markers for MSC mobilization (CD-29, -44, -73, CXCR4), differentiation (NANOG, Stro-1), and transmigration (CD-106, -166) within chronic, as opposed to acute, human periapical granulomas.22
More recently, Estrela et al. also observed a higher presence of MSCs within stable periradicular lesions. Collectively, these findings suggest that the immunosuppressive properties of PL-MSCs actively contribute to arresting progression of periapical diseases.64
Overall, the study investigations described earlier highlight the tremendous regenerative and immunomodulatory capabilities of PL-MSCs. They lay a strong foundation for preclinical in vivo studies, which should be performed, that explore their therapeutic potentials. Dentoalveolar, neurodegenerative, and skeletal diseases may particularly benefit from advances in this area due to the enhanced neurogenic and osteogenic commitment of this niche.58,61,62,65 Moreover, the immunomodulatory and mineralized regenerative properties demonstrated by PL-MSCs in vitro and in vivo, respectively, indicate that these cells are modulators of the periapical lesion healing process and thus making them ideal targets in novel tissue regeneration strategies for AP.21 One such approach would involve enhancing their regenerative capacity in situ by liberating endogenous signaling molecules from within the dentine's extracellular matrix.
dECM Components
More than 280 bioactive molecules have been identified within demineralized dentine.66,67 A vast majority of these are non-collagenous extracellular matrix proteins,66 which comprise ∼10% of the dentine's organic phase and are considered crucial for dentinogenesis.68 Growth factors constitute large proportions of this cohort and have been implicated in regulating dentine-pulp reparative and regenerative responses. Members of the TGF-β, BMP, VEGF, FGF, IGF, platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), placental-derived growth factor (PlGF), epidermal growth factor, and adrenomedullin families are frequently detected, with TGF-β1 often found in the greatest abundance.28,29,33,34,69–71
Several of these, namely VEGFs, FGFs, PDGFs, and PlGFs, are also known mediators of angiogenesis, which is a critical wound-healing process involving the formation of new blood vessels.72,73 Closely associated with these are neurotrophic factors that are responsible for developing intricate innervations within the dentin-pulp complex.74 Isolated examples include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 and 4 (NT3/NT4), and glial cell–line derived neurotrophic factor.75 Further, a broad range of pro- and anti-inflammatory cytokines, namely IL-1α, -1β, -4, -6, -8, -10, -12, and granulocyte-macrophage colony-stimulating factor, have also been detected within solubilized dECMs.32,76 These NF-ĸB signaling molecules likely contribute to immunoregulatory pulp mechanisms, as indicated by their capacity to induce a wide array of inflammatory events.77
Other non-collagenous protein families released from the dentine matrix are those associated with regulating mineralization and maturation processes of human calcified tissues.78 Briefly, these include small integrin-binding ligand n-linked glycoproteins (DMP-1, BSP, osteopontin [OPN], dentine phosphoprotein [DPP], dentine sialoprotein, dentine glycoprotein, matrix-extracellular-phosphoglycoprotein [MEPE]); vitamin K-dependent glycoproteins (osteocalcin [OCN]); small leukine-rich proteoglycans (decorin, biglycan, fibromodulin, lumican, osteoadherin); secretory calcium-binding phosphoproteins (ON); and large aggregating proteoglycans (versican).
Many of these require enzymatic activation and therefore it is not unexpected that the dentine substrate also contains matrix metalloproteinases ([MMP]-2, -3, -8, -9, -20) and tissue inhibitors of MMPs ([TIMP]-1,-2),79–82 which also regulate extracellular matrix remodeling. Although serum proteins (albumin, Immunoglobulin-A, -M, Transferin, Fetuin-A) are also present, currently their functions are unknown.83,84
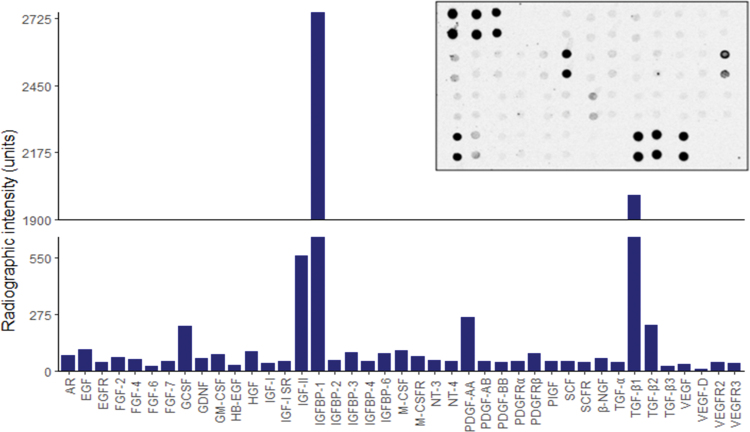
Given what has been cited earlier, dentine can no longer be considered an inert structural tissue but instead, a reservoir of potentially exploitable therapeutic auto- and paracrine cell-signaling molecules that resembles other connective tissues such as bone.85 Figure 3 represents the results of a broad human anti-body array conducted by our own research group on lyophilized dECM components extracted from dentine powder using ethylenediaminetetraacetic acid (EDTA). The endogenous nature of these morphogens overcomes many ethical issues associated with clinically using exogenous substitutes and the synergistic activity within solubilized dECMs; it exhibits a greater potency than single recombinant molecules.37,86,87 For these reasons, dECM extracts have been extensively studied for their ability to initiate regenerative events within various oral and dental MSC niches.
FIG. 3.
Human growth factor anti-body array of lyophilized dECM components extracted from dentine powder using 10% EDTA. A semi-quantitative autoradiographic image analysis technique was used to determine the relative radiographic intensity for a total of 41 different cytokines. A representative autoradiographic image is displayed in the top left corner. AR, amphiregulin; EGFR, epidermal growth factor receptor; GCSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; HB-EGF, heparin-binding epidermal growth factor; HGF, hepatocyte growth factor; IGFBP, insulin-like growth factor binding protein; M-CSF, macrophage colony-stimulating factor; M-CSFR, macrophage colony-stimulating factor receptor; NGF, nerve growth factor; NT, neurotrophin; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; PlGF, placental growth factor; SCF, stem cell factor; SCFR, stem cell factor receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor. Color images are available online.
Effects of dECM Components on Dental Stem Cells
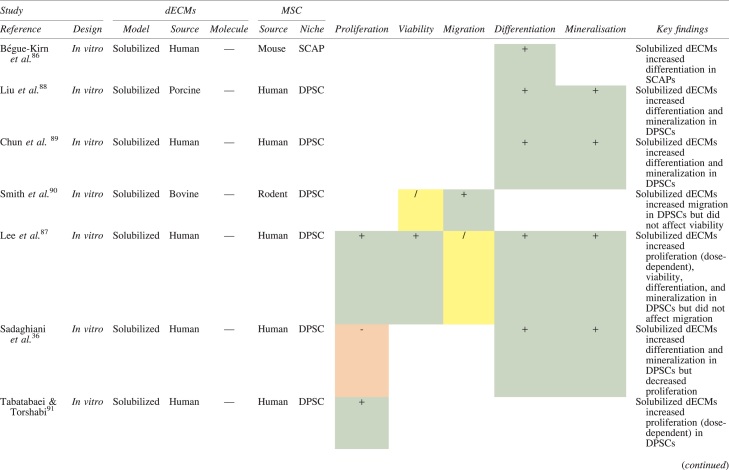
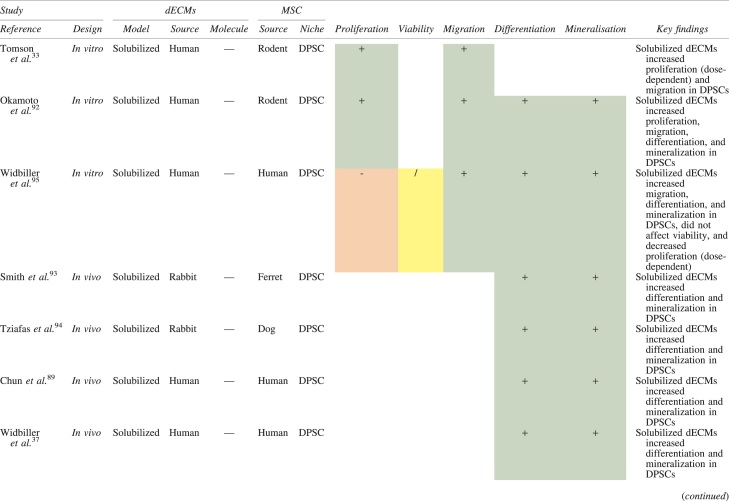
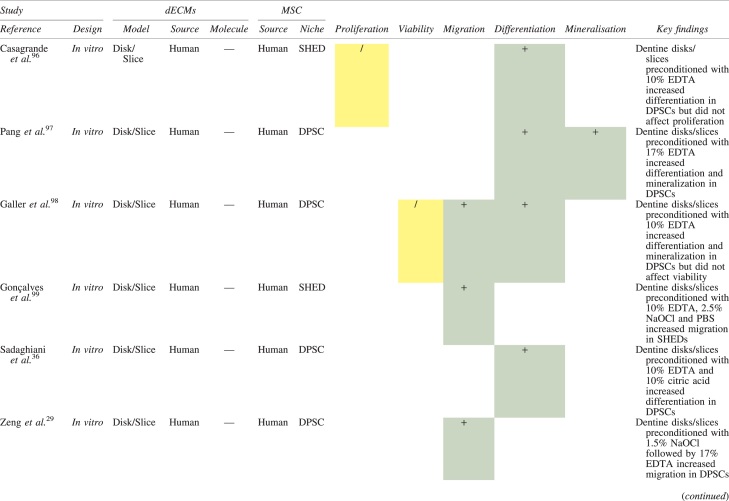
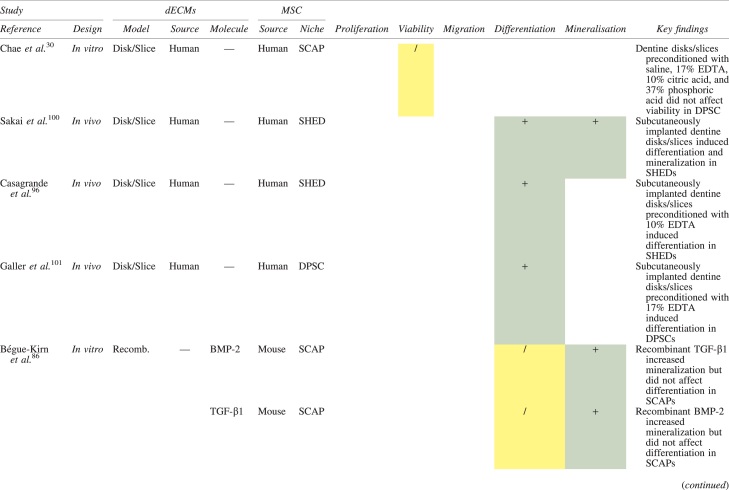
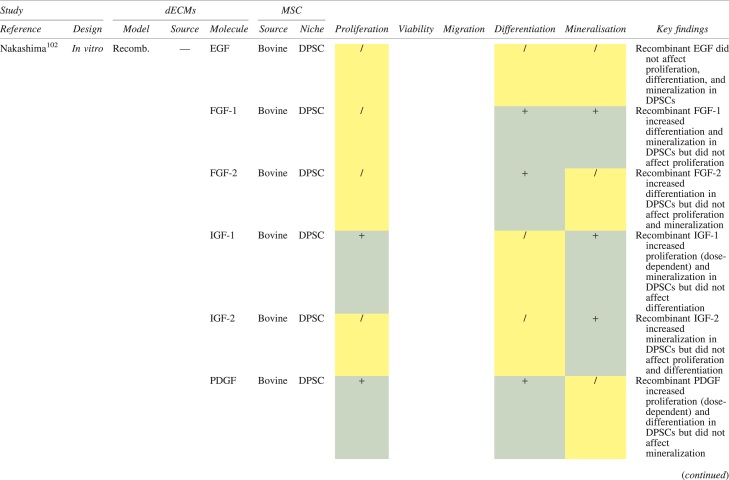
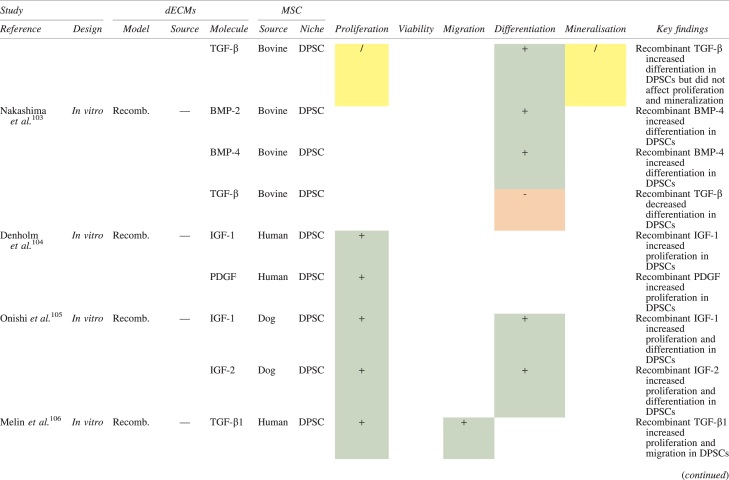
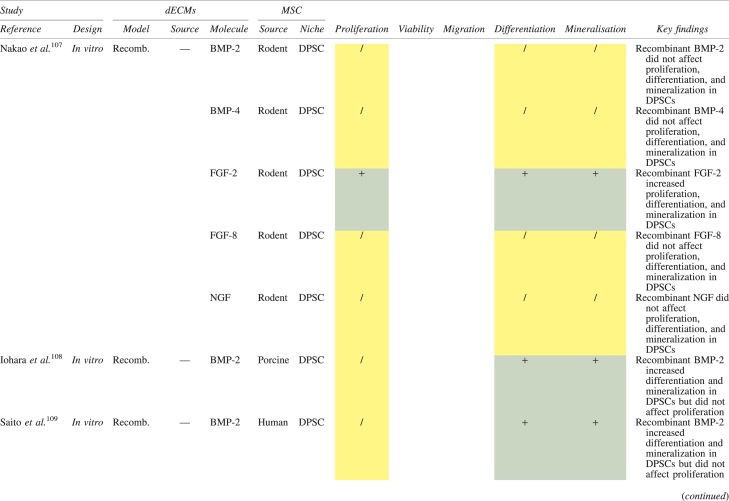
The effects of dECM application on dental MSC niches are summarized in Table 3.
Table 3.
Regenerative Effects of Dentine Extracellular Matrix Component on Odontogenic Stem Cell Niches
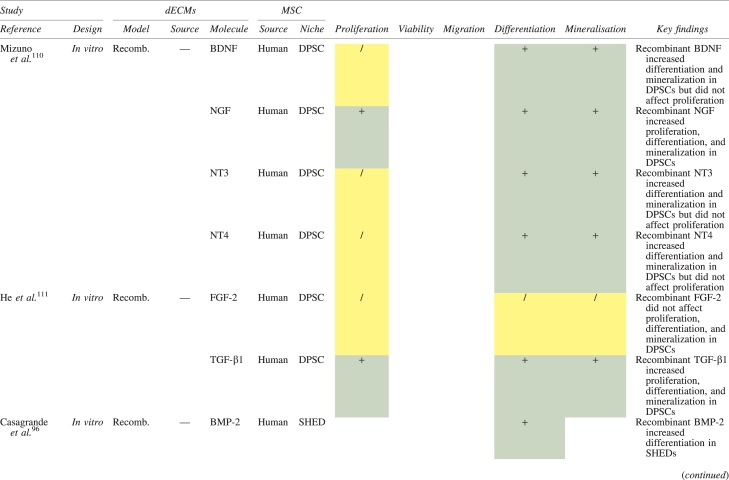
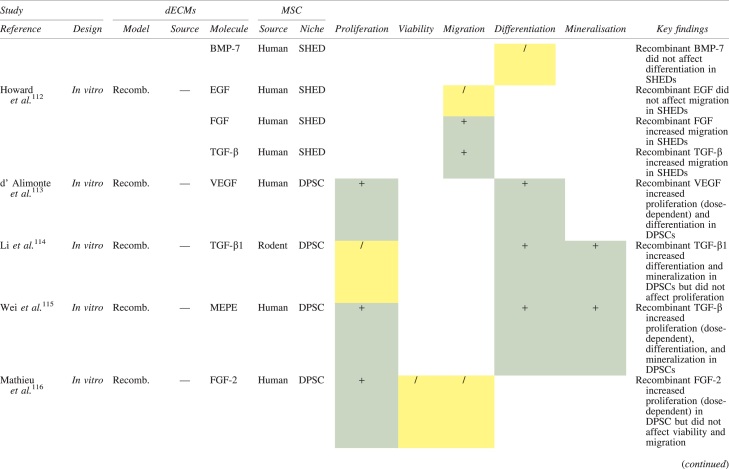
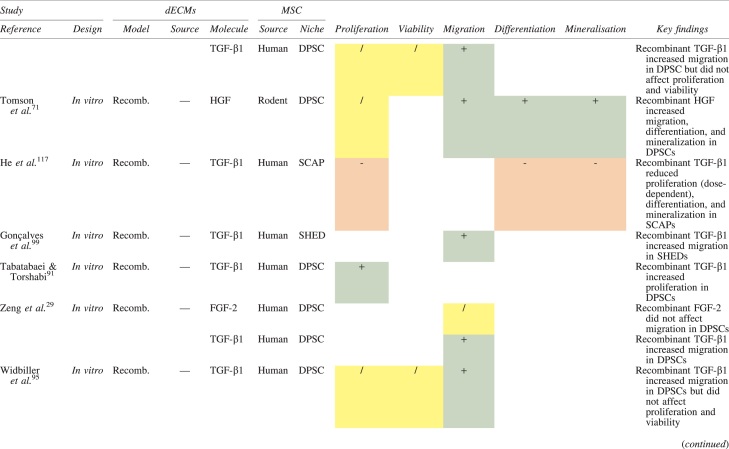
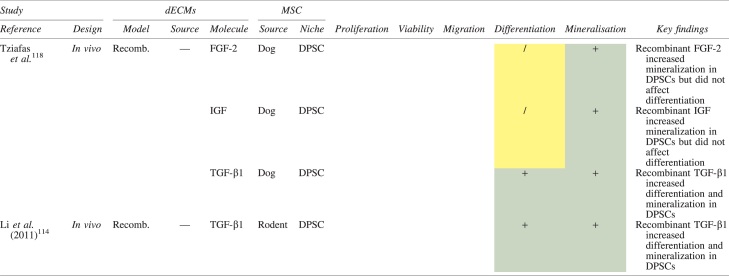
|
Studies have been arranged primarily on the dECM model used, followed by study design and then date.
BDNF, brain-derived neurotrophic factor; BMP, bone morphogenetic protein; dECM, dentin extracellular matrix component; DPSC, dental pulp stem cell; EGF, epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF, insulin growth factor; MEPE, matrix extracellular phosphoglycoprotein; MSC, mesenchymal stem cell; NGF, nerve growth factor; NT3, neurotrophin 3; NT4, neurotrophin 4; PDGF, platelet-derived growth factor; Recomb, recombinant; SCAP, stem cells of the apical papilla; SHED, stem cells from human exfoliated deciduous teeth; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor; +, increased; -,decreased; /, nil. Color images are available online.
Migration
Dentine matrix components have demonstrated chemotactic properties in vitro via transwell migration, matrigel invasion, and scratch wound assays.33,90,92 When solubilized, these extracts exhibit considerable potency with DPSC recruitment occurring at just picogram levels.37 Moreover, root segments pre-conditioned with demineralizing agents induce similar migratory effects in DPSCs and SHEDs, which contrasts the relatively inert properties of their deproteinized counterparts.29,98,99 Other in vitro studies using single recombinant growth factors indicate that these properties can be attributed to the presence of known chemoattractants such as TGF-β1, HGF, and FGF.29,71,106,112,116
Proliferation
Solubilized dECMs induce time- and dose-dependent MSC proliferation. These properties, however, are observed only up to a critical threshold, after which anti-mitogenic events become apparent. For example, dECM applications less than 100 μgmL−1 enhance DPSC proliferation in vitro,33,87,91,92 whereas greater concentrations inhibit further growth.36,37 This observation, which is also witnessed in endothelial cell cultures and angiogenic tube formation assays,73 could be explained as being the net outcome induced by various molecules within dECM extracts. Some constituents, namely TGF-β1,117,119 inhibit proliferation in several cell types but may also attenuate effects of other stimulatory growth factors such as MEPE, PDGF, VEGF, IGF, and FGF.102,104,105,107,113,115,116 Moreover, dECM-induced terminal differentiation may further contribute to reducing cell numbers over time.36
Apoptosis
Solubilized extracts induce limited apoptotic effects in MSCs.37,87,90,98 Higher dECM concentrations have even been found to aid DPSC viability, as indicated by reduced caspase-3 activity and increased serine threonine kinase gene expression.87 This could be accredited to dentinal morphogens that possess anti-apoptotic potential such as DPPs and PDGF, which activate downstream signaling cascades for cell survival.120,121
Differentiation
Numerous in vitro studies using DPSCs, SCAPs, and SHEDs indicate that dECM extracts are powerful inducers of osteo- and odontoblastic differentiation. For instance, topical applications stimulate organization and formation of elongated cellular processes that extend into tubules of pre-conditioned dentine disks.88,89,97,98 This is accompanied by significant increases in mRNA expression for genes characteristic of odonto- and osteogenic commitment. These include DSPP, DMP-1, OPN, OCN, BSP, RUNX2/CBFA1, MEPE, type 1 collagen, alkaline phosphatase, distal-less homeobox 5, and msh homeobox 2.36,37,87,92,96,97
In addition, when DPSCs and SHEDs are implanted subcutaneously alongside dECMs, differentiation events still transpire.93–95,100,101,118 Dentine-derived BMP-2, in particular, is essential in this process, as is demonstrated when blockade of BMP-2 signals, which are otherwise transduced down osteogenic smad-1/5/8 and p38 mitogen-activated-protein-kinase pathways, inhibited odontoblastic gene expression in SHEDs.96 Further, many studies using recombinant growth factors continue to display the potent differentiating activity of BMP-2.103,107–109 Nevertheless, other dentine morphogens that may act concomitantly include TGF-β1, although it exhibits suppressive effects via smad-3 dependent mechanisms in SCAPs; PDGF; FGF; BMP-4; IGF; HGF; VEGF; NGF; BDNF; NT3; NT4; MEPE; and TNF-α.71,102,103,105,107,110,111,113–115,122
Mineralization
Colorimetric methods for calcium quantification demonstrate that dECMs significantly accelerate mineralised matrix production within MSCs.37,84,87,88,92 Calcified nodules indicating functioning osteo- and odontoblasts can be visually observed as early as 5 days post-exposure and become more prominent thereafter.87 When tested in vivo, using subcutaneous implantation models, this deposition leads to osseous, dentinal, and collagenous-like tissue formation.93–95,100,114 This feature can be ascribed to the ability of dentinal morphogens to upregulate genes that code for extracellular matrix protein production in teeth and bone.
Overall, dECMs possess bioactive properties that, if applied to PL-MSCs, could be of clinical utility for periradicular tissue regeneration.
Potential Therapeutic Approach
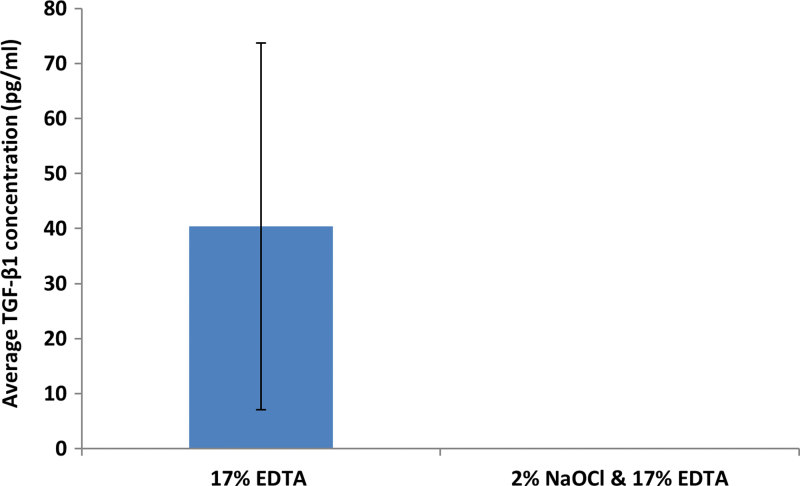
The principles underlying cell-free homing techniques, where MSCs are recruited and stimulated in situ by supplying damaged tissues with signaling molecules,13 could be utilized to exploit endogenous dECMs for the treatment of AP in mature permanent teeth. Conceptually speaking, common chelating agents, namely, 17% EDTA, can be used as the primary irrigant throughout chemo-mechanical debridement to preserve and maximize the release of dentine matrix proteins into root canals. These would otherwise be negatively impacted by the proteonacious properties exhibited by even low concentrations of NaOCl.28,99 This observation is consistent with pilot data obtained by our own research group (Fig. 4).
FIG. 4.
Data obtained from our research group demonstrating the deleterious effects of sodium hypochlorite on the solubilization of dentine extracellular matrix components when delivered into prepared root canals of extracted mature permanent human teeth (n = 10) via conventional needle irrigation. A sandwich ELISA technique was used to detect TGF-β1 concentration (pg/mL) from 100 μL of EDTA after irrigation with or without 2% sodium hypochlorite. Error bars represent standard deviation. Color images are available online.
Ultrasonic agitation has been found to significantly assist dECM release and, thus, is an essential irrigant adjunct after instrumentation.123 These solubilized morphogens could then be encouraged to egress into periapical tissues, by way of manual dynamic activation and patency filing. Subsequently, they would act as chemoattractants to local tissue PL-MSCs present within the peripheral capsular region of granulomas, and subsequently enhance their regenerative potential.124 This interaction will likely require precise pre-enlargement of the apical foramen after accurately determining its position.13
Moreover; antimicrobial inter-appointment medicaments, namely calcium hydroxide, may further prolong dECM exposure due to their ability to liberate bioactive dentine molecules.31,33 This two-stage approach provides additional disinfection, potentially compensating for the absence of NaOCl, and helps confirm resolution of active disease before obturation. Thereafter, routine clinical and radiographic examination would be required to monitor periapical healing (Fig. 5).
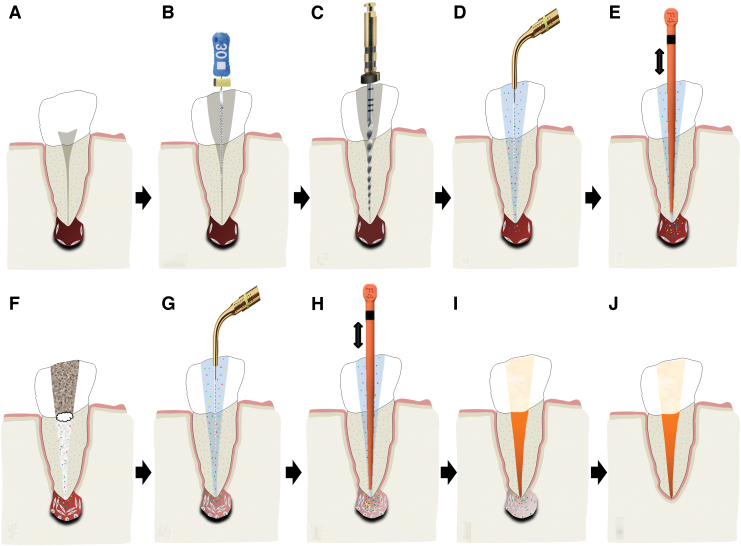
FIG. 5.
A schematic illustration of the proposed protocol for enhancing periradicular tissue regeneration in mature permanent teeth by using endogenous dECM components. (A) Single-rooted mature permanent tooth diagnosed with apical periodontitis; (B) accessing pulp chamber and conservative pre-enlargement of apical foramen; (C) chemomechanical preparation of root canal using a chelating agent; (D) passive ultrasonic activation of irrigant to stimulate release of dECMs into the root canal; (E) manual dynamic activation to encourage periapical bioavailability of dECMs; (F) interappointment calcium hydroxide medicament; (G) irrigation and passive ultrasonic activation to release dECMs; (H) manual dynamic activation to encourage periapical bioavailability of dECMs; (I) obturation; (J) annual clinical and radiographic review. Color images are available online.
The theoretical basis of the proposed approach is derived from preclinical animal studies that have utilized recombinant components of the dentine matrix to regenerate dentoalveolar tissues. For instance; Kim et al. reported that BMP-7 and stromal-derived factor-1 (SDF-1), delivered subcutaneously into rats via 200 μm micro-channels in bioprinted human molar scaffolds, increased both recruitment of endogenous MSCs and angiogenesis and ultimately led to regeneration of an anatomically shaped tooth like-structure.125
Remarkably, in this model a de novo periodontal ligament and alveolar bone was also observed as integrating with the native bone at the scaffold interface after 9 weeks. When the same molecules, plus FGF, were used to coat the roots of intentionally avulsed mandibular premolars in beagle dogs, they were found to contribute to the re-establishment of highly organized periodontal ligament tissues after delayed re-implantation.126,127 These neo-fibers inserted deeply into the adjacent cementum and alveolar bone and prevented the onset of external replacement or inflammatory root resorption.
Kim et al. were also able to demonstrate that without the use of stem cell transplantation, re-cellularized and re-vascularized dental pulp-like tissue was regenerated across the entire length of endodontically treated human-sized root canals after 3 weeks of exposure to FGF, VEGF, PDGF, NGF, and BMP7 in a subcutaneous implantation mouse model.128 Similar observations were reported by Suzuki et al.129 These studies, in particular, provide the strongest support for the proposed protocol as they demonstrate in vivo regeneration of the very tissues necessary for a de novo periodontium using a cell-free approach.
Further support for the homing potential of dECMs, however, comes from the applications of other prevalent dentine matrix proteins, such as DPP and DMP-1, in rat models. For example, exposure to DPP induced odontoblastic differentiation and subsequent reparative dentine bridge formation in inflamed pulp tissue and DMP-1 impregnated scaffolds exhibited marked extracellular matrix deposition and neovascularization in endodontic perforation defects.17,130
In other areas of medicine, stem cell homing techniques utilizing TGF-β3 molecules have successfully contributed to regenerating entire humeral condyles in rabbits after radical resection.131 Collectively, these findings have to date been clinically translated into novel pulp preservation and regeneration protocols and provide proof of concept for the therapeutic potentials of dECMs when used in stem cell homing techniques as described earlier.132–134 Nevertheless, although the proposed approach circumvents many ethical issues related to cell-based transplantation strategies, it is at present only speculative. Numerous hurdles are still required to be overcome before successful clinical translation.
Challenges to Successful Clinical Translation and Directions for Future Research
The greatest challenge associated with implementing the protocol cited earlier is developing chemo-mechanical debridement regimes that sufficiently disinfect root canals while preserving dECMs and PL-MSCs. This would particularly affect NaOCl use, which has proven detrimental to stem cell viability,135,136 dentine matrix growth factor bioavailability,28,99 and induction of key tissue regeneration events.96,101 Although lower concentrations and contact times of 1.5% and 5 min, respectively, have been advocated for regenerative endodontic treatments, these parameters are derived from studies only investigating MSC viability.98,135,136 Therefore, it is currently unknown how they influence dECM release. Further, the ideal strength for NaOCl's antimicrobial efficacy is reported as 2.5%,137 which is otherwise cytotoxic to MSCs and significantly reduces the bioavailability of dECMs.99,136
What has been cited earlier suggests that if NaOCl were to be administered even in a limited capacity, its deleterious effects on dECMs would need mitigating, which is supported by pilot data (Fig. 4). This could perhaps be achieved by enhancing the activity of demineralizing agents or mechanically removing the affected dentinal substrate, the latter of which requires a prerequisite understanding of NaOCl's penetrative capabilities. However, should these methods lead to no avail, NaOCl will need to be substituted for alternative antimicrobial strategies. For instance, the thicker and less fragile root canal walls in mature permanent teeth allow for more emphasis on conventional instrumentation and intracanal medicaments, which have in vivo shown greater contribution to endodontic disinfection than lower NaOCl concentrations.138
Moreover; EDTA, which is currently considered a weak antimicrobial agent, destabilizes the outer cell membranes of gram-negative bacteria and deteriorates the macrostructures of established biofilms.139 Although these effects alone may not always induce cell death, they could potentially be enhanced enough to do so when combined with mechanical instrumentation and irrigant agitation techniques. The reductions in microbial load achieved through these mechanisms may equate to that of NaOCl treatment and exceed the threshold necessary to control infection while preserving the biological components within dentine.2 Further investigations are required to test these hypotheses.
Another challenge is that the effectiveness of the proposed strategy has yet to be proven in concept. Although the regenerative potentials of dECMs have been demonstrated in DPSCs, SCAPs, and SHEDs; it is currently unknown whether similar effects are observed in cultures of PL-MSCs. This niche has already demonstrated different stem-like characteristics and thus may yield results at variance to that of other MSCs.55 Animal studies, utilizing the intentional pulp exposure model of AP, could be employed to further support or challenge the aforementioned hypothesis. They would provide valuable histological and radiographic insight into the periradicular healing process at key time points after dECM exposure, which is data that ethically cannot be attained in vivo using human participants.
Rodents such as rats and mice provide researchers endodontic anatomy (i.e., molar teeth), infected root canal microflora, and wound-healing physiology comparable to that of humans and they conform to the public opposition of using larger animals, thus making them the species of choice.140–142 Overall, these preliminary studies are necessary to justify more time-consuming, labor-intensive, expensive, and appropriately powered prospective randomized controlled trials, which would be the ultimate means of demonstrating the clinical effectiveness of the proposed intervention. Such investigations would also benefit from more sensitive outcome measures that could longitudinally detect biological changes within the periradicular tissues.
Conclusion
The discovery of multipotent stem cells within periapical lesions presents novel opportunities for managing AP by way of harnessing local tissue regeneration. Multiple in vitro studies have confirmed their immunomodulatory and stem cell-like characteristics, which implicates them as being key determiners of the periapical healing process and provides the foundations for subsequent in vivo investigation. Further, there is extensive evidence demonstrating that components within the dentine's extracellular matrix are capable of upregulating the very regenerative responses within dental MSCs that would otherwise be necessary for periradicular regeneration. This includes the enhancement of cellular proliferation, migration, viability, differentiation, and mineralization.
It is well established that these bioactive properties can be harnessed by clinicians on command with common chelating agents such as EDTA, which provides the theoretical and clinical basis of the proposed protocol. Further in vitro and in vivo studies, however, are still required to determine the regenerative effects of dECMs in PL-MSC cultures, optimal irrigant regimes for liberating dECMs, and their effects on the clinical success rates of root canal treatment. Such investigations at the very least would improve understanding of the biological mechanisms associated with periradicular healing, which could in future lead to the development of regenerative endodontic treatment strategies for AP.
Supplementary Material
Authors' Contributions
S.S. contributed to conception, design, data acquisition and interpretation, drafted, and critically revised the article; N.B. contributed to data acquisition and interpretation, drafted, and critically revised the article; J.C. contributed to conception, drafted, and critically revised the article; P.R.C. contributed to conception, drafted, and critically revised the article; P.L.T. contributed to conception, drafted, and critically revised the article.
All authors gave their final approval and agree to be accountable for all aspects of the work.
Disclosure Statement
All authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Funding Information
This project has been supported by a research grant from the British Endodontic Society (RDGW20229).
Supplementary Material
References
- 1. Nair, P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med 15, 348, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Siqueira, J.F.Jr., and Rôças, I.N.. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 34, 1291.e3, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Gurtner, G.C., Werner, S., Barrandon, Y., and Longaker, M.T.. Wound repair and regeneration. Nature 453, 314, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Lin, L.M., and Rosenberg, P.A.. Repair and regeneration in endodontics. Int Endod J 44, 889, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Ng, Y.L., Mann, V., and Gulabivala, K.. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: part 1: periapical health. Int Endod J 44, 583, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Ricucci, D., Russo, J., Rutberg, M., Burleson, J.A., and Spångberg, L.S.. A prospective cohort study of endodontic treatments of 1,369 root canals: results after 5years. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112, 825, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Wu, Y., Chen, L., Scott, P.G., and Tredget, E.E.. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25, 2648, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Stappenbeck, T.S., and Miyoshi, H.. The role of stromal stem cells in tissue regeneration and wound repair. Science 324, 1666, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Huang, G.T., Gronthos, S., and Shi, S.. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88, 792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo, B., and Parham, L.. Ethical issues in stem cell research. Endocr Rev 30, 204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pittenger, M.F., Mackay, A.M., Beck, S.C., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999. [DOI] [PubMed] [Google Scholar]
- 12. and Sharpe, P.T.. Dental mesenchymal stem cells. Development (Cambridge, England) 143, 2273, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Kim, S.G., Malek, M., Sigurdsson, A., Lin, L.M., and Kahler, B.. Regenerative endodontics: a comprehensive review. Int Endod J 51, 1367, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Cordeiro, M.M., Dong, Z., Kaneko, T., et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34, 962, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Huang, G.T., Yamaza, T., Shea, L.D., et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 16, 605, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosa, V., Zhang, Z., Grande, R.H., and Nör, J.E.. Dental pulp tissue engineering in full-length human root canals. J Dent Res 92, 970, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alsanea, R., Ravindran, S., Fayad, M.I., et al. Biomimetic approach to perforation repair using dental pulp stem cells and dentin matrix protein 1. J Endod 37, 1092, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seo, B.M., Miura, M., Gronthos, S., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Liu, Y., Zheng, Y., Ding, G., et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26, 1065, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding, G., Liu, Y., Wang, W., et al. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 28, 1829, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao, J., Al Shahrani, M., Al-Habib, M., Tanaka, T., and Huang, G.T.. Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J Endod 37, 1217, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Araujo-Pires, A.C., Biguetti, C.C., Repeke, C.E., et al. Mesenchymal stem cells as active prohealing and immunosuppressive agents in periapical environment: evidence from human and experimental periapical lesions. J Endod 40, 1560, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Barrientos, S., Stojadinovic, O., Golinko, M.S., Brem, H., and Tomic-Canic, M.. Growth factors and cytokines in wound healing. Wound Repair Regen 16, 585, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Kim, S.G., Zhou, J., Solomon, C., et al. Effects of growth factors on dental stem/progenitor cells. Dent Clin North Am 56, 563, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith, A.J., Scheven, B.A., Takahashi, Y., Ferracane, J.L., Shelton, R.M., and Cooper, P.R.. Dentine as a bioactive extracellular matrix. Arch Oral Biol 57, 109, 2012. [DOI] [PubMed] [Google Scholar]
- 26. Schönherr, E., and Hausser, H.J.. Extracellular matrix and cytokines: a functional unit. Dev Immunol 7, 89, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baker, S.M., Sugars, R.V., Wendel, M., et al. TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcif Tissue Int 85, 66, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Galler, K.M., Buchalla, W., Hiller, K.A., et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod 41, 363, 2015. [DOI] [PubMed] [Google Scholar]
- 29. Zeng, Q., Nguyen, S., Zhang, H., et al. Release of growth factors into root canal by irrigations in regenerative endodontics. J Endod 42, 1760, 2016. [DOI] [PubMed] [Google Scholar]
- 30. Chae, Y., Yang, M., and Kim, J.. Release of TGF-β1 into root canals with various final irrigants in regenerative endodontics: an in vitro analysis. Int Endod J 51, 1389, 2018. [DOI] [PubMed] [Google Scholar]
- 31. Graham, L., Cooper, P.R., Cassidy, N., Nor, J.E., Sloan, A.J., and Smith, A.J.. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 27, 2865, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Cooper, P.R., Takahashi, Y., Graham, L.W., Simon, S., Imazato, S., and Smith, A.J.. Inflammation-regeneration interplay in the dentine-pulp complex. J Dent 38, 687, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Tomson, P.L., Lumley, P.J., Smith, A.J., and Cooper, P.R.. Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int Endod J 50, 281, 2017. [DOI] [PubMed] [Google Scholar]
- 34. Duncan, H.F., Smith, A.J., Fleming, G.J., Reid, C., Smith, G., and Cooper, P.R.. Release of bio-active dentine extracellular matrix components by histone deacetylase inhibitors (HDACi). Int Endod J 50, 24, 2017. [DOI] [PubMed] [Google Scholar]
- 35. Ferracane, J.L., Cooper, P.R., and Smith, A.J.. Dentin matrix component solubilization by solutions of pH relevant to self-etching dental adhesives. J Adhes Dent 15, 407, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Sadaghiani, L., Gleeson, H.B., Youde, S., Waddington, R.J., Lynch, C.D., and Sloan, A.J.. Growth factor liberation and DPSC response following dentine conditioning. J Dent Res 95, 1298, 2016. [DOI] [PubMed] [Google Scholar]
- 37. Widbiller, M., Eidt, A., Lindner, S.R., et al. Dentine matrix proteins: isolation and effects on human pulp cells. Int Endod J 51(Suppl 4), e278, 2018. [DOI] [PubMed] [Google Scholar]
- 38. Kakehashi, S., Stanley, H.R., and Fitzgerald, R.J.. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 20, 340, 1965. [DOI] [PubMed] [Google Scholar]
- 39. Schilder, H. Cleaning and shaping the root canal. Dent Clin North Am 18, 269, 1974. [PubMed] [Google Scholar]
- 40. Virdee, S.S., Farnell DJJ, Silva, M.A., Camilleri, J., Cooper, P.R., and Tomson, P.L.. The influence of irrigant activation, concentration and contact time on sodium hypochlorite penetration into root dentine: an ex vivo experiment. Int Endod J 53, 986, 2020. [DOI] [PubMed] [Google Scholar]
- 41. Virdee, S.S., Seymour, D.W., Farnell, D., Bhamra, G., and Bhakta, S.. Efficacy of irrigant activation techniques in removing intracanal smear layer and debris from mature permanent teeth: a systematic review and meta-analysis. Int Endod J 51, 605, 2018. [DOI] [PubMed] [Google Scholar]
- 42. Azim, A.A., Aksel, H., Zhuang, T., Mashtare, T., Babu, J.P., and Huang, G.T.. Efficacy of 4 irrigation protocols in killing bacteria colonized in dentinal tubules examined by a novel confocal laser scanning microscope analysis. J Endod 42, 928, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakamura, V.C., Pinheiro, E.T., Prado, L.C., et al. Effect of ultrasonic activation on the reduction of bacteria and endotoxins in root canals: a randomized clinical trial. Int Endod J 51(Suppl 1), e12, 2018. [DOI] [PubMed] [Google Scholar]
- 44. Ng, Y.L., Mann, V., Rahbaran, S., Lewsey, J., and Gulabivala, K.. Outcome of primary root canal treatment: systematic review of the literature - part 1. Effects of study characteristics on probability of success. Int Endod J 40, 921, 2007. [DOI] [PubMed] [Google Scholar]
- 45. Prati, C., Pirani, C., Zamparini, F., Gatto, M.R., and Gandolfi, M.G.. A 20-year historical prospective cohort study of root canal treatments. A Multilevel analysis. Int Endod J 51, 955, 2018. [DOI] [PubMed] [Google Scholar]
- 46. Barthel, C.R., Zimmer, S., and Trope, M.. Relationship of radiologic and histologic signs of inflammation in human root-filled teeth. J Endod 30, 75, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Patel, S., Wilson, R., Dawood, A., Foschi, F., and Mannocci, F.. The detection of periapical pathosis using digital periapical radiography and cone beam computed tomography - part 2: a 1-year post-treatment follow-up. Int Endod J 45, 711, 2012. [DOI] [PubMed] [Google Scholar]
- 48. Siqueira Junior, J.F., Rôças IDN, Marceliano-Alves, M.F., Pérez, A.R., and Ricucci, D.. Unprepared root canal surface areas: causes, clinical implications, and therapeutic strategies. Braz Oral Res 32(Suppl 1), e65, 2018. [DOI] [PubMed] [Google Scholar]
- 49. Molander, A., Reit, C., Dahlén, G., and Kvist, T.. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J 31, 1, 1998. [PubMed] [Google Scholar]
- 50. Becerra, P., Ricucci, D., Loghin, S., Gibbs, J.L., and Lin, L.M.. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod 40, 133, 2014. [DOI] [PubMed] [Google Scholar]
- 51. Paredes-Vieyra, J., and Enriquez, F.J.. Success rate of single- versus two-visit root canal treatment of teeth with apical periodontitis: a randomized controlled trial. J Endod 38, 1164, 2012. [DOI] [PubMed] [Google Scholar]
- 52. Liang, Y.H., Jiang, L.M., Jiang, L., et al. Radiographic healing after a root canal treatment performed in single-rooted teeth with and without ultrasonic activation of the irrigant: a randomized controlled trial. J Endod 39, 1218, 2013. [DOI] [PubMed] [Google Scholar]
- 53. Verma, N., Sangwan, P., Tewari, S., and Duhan, J.. Effect of different concentrations of sodium hypochlorite on outcome of primary root canal treatment: a randomized controlled trial. J Endod 45, 357, 2019. [DOI] [PubMed] [Google Scholar]
- 54. Maeda, H., Wada, N., Nakamuta, H., and Akamine, A.. Human periapical granulation tissue contains osteogenic cells. Cell Tissue Res 315, 203, 2004. [DOI] [PubMed] [Google Scholar]
- 55. Đokić, J., Tomić, S., Cerović, S., Todorović, V., Rudolf, R., and Čolić, M.. Characterization and immunosuppressive properties of mesenchymal stem cells from periapical lesions. J Clin Periodontol 39, 807, 2012. [DOI] [PubMed] [Google Scholar]
- 56. Marrelli, M., Paduano, F., and Tatullo, M.. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int J Biol Sci 9, 1070, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dokić, J., Tomić, S., Marković, M., Milosavljević, P., and Colić, M.. Mesenchymal stem cells from periapical lesions modulate differentiation and functional properties of monocyte-derived dendritic cells. Eur J Immunol 43, 1862, 2013. [DOI] [PubMed] [Google Scholar]
- 58. Marrelli, M., Paduano, F., and Tatullo, M.. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J Dent Res 94, 843, 2015. [DOI] [PubMed] [Google Scholar]
- 59. Paduano, F., Marrelli, M., Palmieri, F., and Tatullo, M.. CD146 expression influences periapical cyst mesenchymal stem cell properties. Stem Cell Rev Reports 12, 592, 2016. [DOI] [PubMed] [Google Scholar]
- 60. Tatullo, M., Marrelli, M., Palmieri, F., Rengo, C., Paduano, F., and Spagnuolo, G.. Human Periapical Cysts-Mesenchymal Stem Cells Cultured with Allogenic Human Serum are a “clinical-grade” construct alternative to bovine fetal serum and indicated in the regeneration of endo-periodontal tissues. G Ital Endod 32, 36, 2018. [Google Scholar]
- 61. Tatullo, M., Spagnuolo, G., Codispoti, B., et al. PLA-based mineral-doped scaffolds seeded with human periapical cyst-derived MSCs: a promising tool for regenerative healing in dentistry. Materials (Basel) 12, 597, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tatullo, M., Falisi, G., Amantea, M., Rastelli, C., Paduano, F., and Marrelli, M.. Dental pulp stem cells and human periapical cyst mesenchymal stem cells in bone tissue regeneration: comparison of basal and osteogenic differentiated gene expression of a newly discovered mesenchymal stem cell lineage. J Biol Regul Homeost Agents 29, 713, 2015. [PubMed] [Google Scholar]
- 63. Alongi, D.J., Yamaza, T., Song, Y., et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 5, 617, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Estrela, C., Freitas Silva, B.S., Silva, J.A., Yamamoto-Silva, F.P., Pinto-Júnior, D.D., and Gomez, R.S.. Stem cell marker expression in persistent apical periodontitis. J Endod 43, 63, 2017. [DOI] [PubMed] [Google Scholar]
- 65. Tatullo, M., Codispoti, B., Spagnuolo, G., and Zavan, B.. Human Periapical Cyst-Derived Stem Cells Can Be A Smart “Lab-on-A-Cell” to investigate neurodegenerative diseases and the related alteration of the Exosomes' Content. Brain Sci 9, 358, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Park, E.S., Cho, H.S., Kwon, T.G., et al. Proteomics analysis of human dentin reveals distinct protein expression profiles. J Proteome Res 8, 1338, 2009. [DOI] [PubMed] [Google Scholar]
- 67. Jágr, M., Eckhardt, A., Pataridis, S., and Mikšík, I.. Comprehensive proteomic analysis of human dentin. Eur J Oral Sci 120, 259, 2012. [DOI] [PubMed] [Google Scholar]
- 68. Butler, W.T., and Ritchie, H.. The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol 39, 169, 1995. [PubMed] [Google Scholar]
- 69. Finkelman, R.D., Mohan, S., Jennings, J.C., Taylor, A.K., Jepsen, S., and Baylink, D.J.. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res 5, 717, 1990. [DOI] [PubMed] [Google Scholar]
- 70. Tomson, P.L., Grover, L.M., Lumley, P.J., Sloan, A.J., Smith, A.J., and Cooper, P.R.. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent 35, 636, 2007. [DOI] [PubMed] [Google Scholar]
- 71. Tomson, P.L., Lumley, P.J., Alexander, M.Y., Smith, A.J., and Cooper, P.R.. Hepatocyte growth factor is sequestered in dentine matrix and promotes regeneration-associated events in dental pulp cells. Cytokine 61, 622, 2013. [DOI] [PubMed] [Google Scholar]
- 72. Roberts-Clark, D.J., and Smith, A.J.. Angiogenic growth factors in human dentine matrix. Arch Oral Biol 45, 1013, 2000. [DOI] [PubMed] [Google Scholar]
- 73. Zhang, R., Cooper, P.R., Smith, G., Nör, J.E., and Smith, A.J.. Angiogenic activity of dentin matrix components. J Endod 37, 26, 2011. [DOI] [PubMed] [Google Scholar]
- 74. Nosrat, C.A., Fried, K., Ebendal, T., and Olson, L.. NGF, BDNF, NT3, NT4 and GDNF in tooth development. Eur J Oral Sci 106(S1), 94, 1998. [DOI] [PubMed] [Google Scholar]
- 75. Austah, O., Widbiller, M., Tomson, P.L., and Diogenes, A.. Expression of neurotrophic factors in human dentin and their regulation of trigeminal neurite outgrowth. J Endod 45, 414, 2019. [DOI] [PubMed] [Google Scholar]
- 76. Graham, L., Smith, A.J., Sloan, A., and Cooper, P.R.. Cytokine release from human dentine. J Dent Res 86, 2007. Available at: https://iadr.abstractarchives.com/abstract/bsdr07-95046/cytokine-release-from-human-dentine (accessed June 1, 2021).
- 77. Lara, V.S., Figueiredo, F., da Silva, T.A., and Cunha, F.Q.. Dentin-induced in vivo inflammatory response and in vitro activation of murine macrophages. J Dent Res 82, 460, 2003. [DOI] [PubMed] [Google Scholar]
- 78. Orsini, G., Ruggeri Jr, A., Mazzoni, A., et al. A review of the nature, role, and function of dentin non-collagenous proteins. Part 1: proteoglycans and glycoproteins. Endod Topics 21, 1, 2009 [Google Scholar]
- 79. Chaussain, C., Eapen, A.S., Huet, E., et al. MMP2-cleavage of DMP1 generates a bioactive peptide promoting differentiation of dental pulp stem/progenitor cell. Eur Cell Mater 18, 84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Santos, J., Carrilho, M., Tervahartiala, T., et al. Determination of matrix metalloproteinases in human radicular dentin. J Endod 35, 686, 2009. [DOI] [PubMed] [Google Scholar]
- 81. Mazzoni, A., Papa, V., Nato, F., et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent 39, 231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Niu, L.N., Zhang, L., Jiao, K., et al. Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. J Dent 39, 536, 2011. [DOI] [PubMed] [Google Scholar]
- 83. Thomas, M., and Leaver, A.G.. Identification and estimation of plasma proteins in human dentine. Arch Oral Biol 20, 217, 1975. [DOI] [PubMed] [Google Scholar]
- 84. Okamura, K., Tsubakimoto, K., Uobe, K., Nishida, K., and Tsutsui, M.. Serum proteins and secretory component in human carious dentin. J Dent Res 58, 1127, 1979. [DOI] [PubMed] [Google Scholar]
- 85. Sampath, T.K., and Reddi, A.H.. Discovery of bone morphogenetic proteins - A historical perspective. Bone 140, 115548, 2020. [DOI] [PubMed] [Google Scholar]
- 86. Bègue-Kirn, C., Smith, A.J., Ruch, J.V., et al. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol 36, 491, 1992. [PubMed] [Google Scholar]
- 87. Lee, C.P., Colombo, J.S., Ayre, W.N., Sloan, A.J., and Waddington, R.J.. Elucidating the cellular actions of demineralised dentine matrix extract on a clonal dental pulp stem cell population in orchestrating dental tissue repair. J Tissue Eng 6, 2041731415586318, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu, J., Taocong, J., Ritchie, H.H., Smith, A.J., and Brian, H.C.. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim 41, 232, 2005. [DOI] [PubMed] [Google Scholar]
- 89. Chun, S.Y., Lee, H.J., Choi, Y.A., et al. Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration. Tissue Eng Part A 17, 181, 2011. [DOI] [PubMed] [Google Scholar]
- 90. Smith, J.G., Smith, A.J., Shelton, R.M., and Cooper, P.R.. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp Cell Res 318, 2397, 2012. [DOI] [PubMed] [Google Scholar]
- 91. Tabatabaei, F.S., and Torshabi, M.. Effects of Non-Collagenous Proteins, TGF-β1, and PDGF-BB on viability and proliferation of dental pulp stem cells. J Oral Maxillofac Res 7, e4, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Okamoto, M., Takahashi, Y., Komichi, S., Cooper, P.R., and Hayashi, M.. Dentinogenic effects of extracted dentin matrix components digested with matrix metalloproteinases. Sci Reports 8, 10690, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Smith, A.J., Tobias, R.S., Plant, C.G., Browne, R.M., Lesot, H., and Ruch, J.V.. In vivo morphogenetic activity of dentine matrix proteins. J Biol Buccale 18, 123, 1990. [PubMed] [Google Scholar]
- 94. Tziafas, D., Alvanou, A., Panagiotakopoulos, N., et al. Induction of odontoblast-like cell differentiation in dog dental pulps after in vivo implantation of dentine matrix components. Arch Oral Biol 40, 883, 1995. [DOI] [PubMed] [Google Scholar]
- 95. Widbiller, M., Driesen, R.B., Eidt, A., et al. Cell homing for pulp tissue engineering with endogenous dentin matrix proteins. J Endod 44, 956.e2, 2018. [DOI] [PubMed] [Google Scholar]
- 96. Casagrande, L., Demarco, F.F., Zhang, Z., Araujo, F.B., Shi, S., and Nör, J.E.. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res 89, 603, 2010. [DOI] [PubMed] [Google Scholar]
- 97. Pang, N.S., Lee, S.J., Kim, E., et al. Effect of EDTA on attachment and differentiation of dental pulp stem cells. J Endod 40, 811, 2014. [DOI] [PubMed] [Google Scholar]
- 98. Galler, K.M., Widbiller, M., Buchalla, W., et al. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int Endod J 49, 581, 2016. [DOI] [PubMed] [Google Scholar]
- 99. Gonçalves, L.F., Fernandes, A.P., Cosme-Silva, L., et al. Effect of EDTA on TGF-β1 released from the dentin matrix and its influence on dental pulp stem cell migration. Braz Oral Res 30, e131, 2016. [DOI] [PubMed] [Google Scholar]
- 100. Sakai, V.T., Zhang, Z., Dong, Z., et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89, 791, 2010. [DOI] [PubMed] [Google Scholar]
- 101. Galler, K.M., D'Souza, R.N., Federlin, M., et al. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod 37, 1536, 2011. [DOI] [PubMed] [Google Scholar]
- 102. Nakashima, M. The effects of growth factors on DNA synthesis, proteoglycan synthesis and alkaline phosphatase activity in bovine dental pulp cells. Arch Oral Biol 37, 231, 1992. [DOI] [PubMed] [Google Scholar]
- 103. Nakashima, M., Nagasawa, H., Yamada, Y., and Reddi, A.H.. Regulatory role of transforming growth factor-beta, bone morphogenetic protein-2, and protein-4 on gene expression of extracellular matrix proteins and differentiation of dental pulp cells. Dev Biol 162, 18, 1994. [DOI] [PubMed] [Google Scholar]
- 104. Denholm, I.A., Moule, A.J., and Bartold, B.M.. The behaviour and proliferation of human dental pulp cell strains in vitro, and their response to the application of platelet-derived growth factor-BB and insulin-like growth factor-1. Int Endod J 31, 251, 1998. [DOI] [PubMed] [Google Scholar]
- 105. Onishi, T., Kinoshita, S., Shintani, S., Sobue, S., and Ooshima, T.. Stimulation of proliferation and differentiation of dog dental pulp cells in serum-free culture medium by insulin-like growth factor. Arch Oral Biol 44, 361, 1999. [DOI] [PubMed] [Google Scholar]
- 106. Melin, M., Joffre-Romeas, A., Farges, J.C., Couble, M.L., Magloire, H., and Bleicher, F.. Effects of TGFbeta1 on dental pulp cells in cultured human tooth slices. J Dent Res 79, 1689, 2000. [DOI] [PubMed] [Google Scholar]
- 107. Nakao, K., Itoh, M., Tomita, Y., Tomooka, Y., and Tsuji, T.. FGF-2 potently induces both proliferation and DSP expression in collagen type I gel cultures of adult incisor immature pulp cells. Biochem Biophys Res Commun 325, 1052, 2004. [DOI] [PubMed] [Google Scholar]
- 108. Iohara, K., Nakashima, M., Ito, M., Ishikawa, M., Nakasima, A., and Akamine, A.. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 83, 590, 2004. [DOI] [PubMed] [Google Scholar]
- 109. Saito, T., Ogawa, M., Hata, Y., and Bessho, K.. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod 30, 205, 2004. [DOI] [PubMed] [Google Scholar]
- 110. Mizuno, N., Shiba, H., Xu W-p, et al. Effect of neurotrophins on differentiation, calcification and proliferation in cultures of human pulp cells. Cell Biol Int 31, 1462, 2007. [DOI] [PubMed] [Google Scholar]
- 111. He, H., Yu, J., Liu, Y., et al. Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int 32, 827, 2008. [DOI] [PubMed] [Google Scholar]
- 112. Howard, C., Murray, P.E., and Namerow, K.N.. Dental pulp stem cell migration. J Endod 36, 1963, 2010. [DOI] [PubMed] [Google Scholar]
- 113. d'Alimonte, I., Nargi, E., Mastrangelo, F., et al. Vascular endothelial growth factor enhances in vitro proliferation and osteogenic differentiation of human dental pulp stem cells. J Biol Regul Homeost Agents 25, 57, 2011. [PubMed] [Google Scholar]
- 114. Li, Y., Lü, X., Sun, X., Bai, S., Li, S., and Shi, J.. Odontoblast-like cell differentiation and dentin formation induced with TGF-β1. Arch Oral Biol 56, 1221, 2011. [DOI] [PubMed] [Google Scholar]
- 115. Wei, X., Liu, L., Zhou, X., Zhang, F., and Ling, J.. The effect of matrix extracellular phosphoglycoprotein and its downstream osteogenesis-related gene expression on the proliferation and differentiation of human dental pulp cells. J Endod 38, 330, 2012. [DOI] [PubMed] [Google Scholar]
- 116. Mathieu, S., Jeanneau, C., Sheibat-Othman, N., Kalaji, N., Fessi, H., and About, I.. Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J Endod 39, 228, 2013. [DOI] [PubMed] [Google Scholar]
- 117. He, W., Zhang, J., Niu, Z., et al. Regulatory Interplay between NFIC and TGF-β1 in apical papilla-derived stem cells. J Dent Res 93, 496, 2014. [DOI] [PubMed] [Google Scholar]
- 118. Tziafas, D., Alvanou, A., Papadimitriou, S., Gasic, J., and Komnenou, A.. Effects of recombinant basic fibroblast growth factor, insulin-like growth factor-II and transforming growth factor-beta 1 on dog dental pulp cells in vivo. Arch Oral Biol 43, 431, 1998. [DOI] [PubMed] [Google Scholar]
- 119. Massagué, J., Blain, S.W., and Lo, R.S.. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103, 295, 2000. [DOI] [PubMed] [Google Scholar]
- 120. Romashkova, J.A., and Makarov, S.S.. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401, 86, 1999. [DOI] [PubMed] [Google Scholar]
- 121. Fujisawa, R., Mizuno, M., and Tamura, M.. Effect of dentin phosphoprotein on phosphate-induced apoptosis of odontoblast-like cells. Cells Tissues Organs 189, 60, 2009. [DOI] [PubMed] [Google Scholar]
- 122. Paula-Silva, F.W., Ghosh, A., Silva, L.A., and Kapila, Y.L.. TNF-alpha promotes an odontoblastic phenotype in dental pulp cells. J Dent Res 88, 339, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Widbiller, M., Eidt, A., Hiller, K.A., Buchalla, W., Schmalz, G., and Galler, K.M.. Ultrasonic activation of irrigants increases growth factor release from human dentine. Clin Oral Invest 21, 879, 2017. [DOI] [PubMed] [Google Scholar]
- 124. Estrela, C., Carmo Souza, P.O., Barbosa, M.G., et al. Mesenchymal stem cell marker expression in periapical abscess. J Endod 45, 716, 2019. [DOI] [PubMed] [Google Scholar]
- 125. Kim, K., Lee, C.H., Kim, B.K., and Mao, J.J.. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res 89, 842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhu, W., Zhang, Q., Zhang, Y., Cen, L., and Wang, J.. PDL regeneration via cell homing in delayed replantation of avulsed teeth. J Transl Med 13, 357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xu, M., Wei, X., Fang, J., and Xiao, L.. Combination of SDF-1 and bFGF promotes bone marrow stem cell-mediated periodontal ligament regeneration. Biosci Reports 39, BSR20190785, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kim, J.Y., Xin, X., Moioli, E.K., et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A 16, 3023, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Suzuki, T., Lee, C.H., Chen, M., et al. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res 90, 1013, 2011. [DOI] [PubMed] [Google Scholar]
- 130. Altankhishig, B., Polan MAA, Qiu, Y., Hasan, M.R., and Saito, T.. Dentin phosphophoryn-derived peptide promotes odontoblast differentiation in vitro and dentin regeneration in vivo. Materials (Basel) 14, 874, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lee, C.H., Cook, J.L., Mendelson, A., Moioli, E.K., Yao, H., and Mao, J.J.. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376, 440, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Galler, K.M., Eidt, A., and Schmalz, G.. Cell-free approaches for dental pulp tissue engineering. J Endod 40(4 Suppl), S41, 2014. [DOI] [PubMed] [Google Scholar]
- 133. Galler, K.M., Krastl, G., Simon, S., et al. European Society of Endodontology position statement: revitalization procedures. Int Endod J 49, 717, 2016a. [DOI] [PubMed] [Google Scholar]
- 134. Duncan, H.F., Galler, K.M., Tomson, P.L., et al. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J 52, 923, 2019. [DOI] [PubMed] [Google Scholar]
- 135. Trevino, E.G., Patwardhan, A.N., Henry, M.A., et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod 37, 11095, 2011. [DOI] [PubMed] [Google Scholar]
- 136. Martin, D.E., De Almeida, J.F., Henry, M.A., et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod 40, 51, 2014. [DOI] [PubMed] [Google Scholar]
- 137. Marion, J., Manhães, F., Bajo, H., and Duque, T.. Efficiency of different concentrations of sodium hypochlorite during endodontic treatment. Literature review. Dent Press Endod 2, 32, 2012. [Google Scholar]
- 138. Windley, W., 3rd, Teixeira, F., Levin, L., Sigurdsson, A., and Trope, M.. Disinfection of immature teeth with a triple antibiotic paste. J Endod 31, 439, 2005. [DOI] [PubMed] [Google Scholar]
- 139. de Almeida, J., Hoogenkamp, M., Felippe, W.T., Crielaard, W., van der and Waal, S.V.. Effectiveness of EDTA and Modified Salt Solution to Detach and Kill Cells from Enterococcus faecalis Biofilm. J Endod 42, 320, 2016. [DOI] [PubMed] [Google Scholar]
- 140. Tani-Ishii, N., Wang, C.Y., Tanner, A., and Stashenko, P.. Changes in root canal microbiota during the development of rat periapical lesions. Oral Microbiol Immunol 9, 129, 1994. [DOI] [PubMed] [Google Scholar]
- 141. Dammaschke, T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab Anim 44, 1, 2010. [DOI] [PubMed] [Google Scholar]
- 142. Nagendrababu, V., Kishen, A., Murray, P.E., et al. Preferred Reporting Items for Animal Studies in Endodontology: a development protocol. Int Endod J 52, 1290, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.