Abstract
Hospitals have demonstrated the benefits of both voluntary and mandatory bundled payments for joint replacement surgery. However, given generalizability and disparities concerns, it is critical to understand the availability of care through bundled payments to historically marginalized groups, such as racial and ethnic minorities and individuals with lower socioeconomic status (SES). This cross-sectional analysis of 3880 US communities evaluated the relationship between the proportion of Black and Hispanic individuals (minority share) or Medicare/Medicaid dual-eligible individuals (low SES share) and community-level participation in Bundled Payments for Care Improvement initiative (BPCI) (being a BPCI community) and Comprehensive Care for Joint Replacement (CJR) model (being a CJR community). An increase from the lowest to highest quartile of minority share was not associated with differences in the probability of being a BPCI community (3.5 percentage point [pp] difference, 95% confidence interval [CI] −1.2% to 8.3%, P = 0.15), but was associated with a 16.1 pp higher probability of being a CJR community (95% CI 10.3% to 22.0%, P < 0.0001). An increase from the lowest to highest quartile of low SES share was associated with a 6.0 pp lower probability of being a BPCI community (95% CI −10.9% to −1.2%, P = 0.02) and 19.0 pp lower probability of being a CJR community (95% CI −24.9% to −13.0%, P < 0.0001). These findings highlight that the greater the proportion of lower SES individuals in a community, the lower the likelihood that its hospitals participated in either voluntary or mandatory bundled payments. Policymakers should consider community socioeconomic characteristics when designing participation mechanisms for future bundled payment programs.
Keywords: Medicare, health equity, health disparities, bundled payments
Introduction
Over the past decade, the Centers for Medicare and Medicaid Services (CMS) has led a nationwide effort to test and scale up bundled payments. These arrangements hold providers accountable for the quality and costs of care for discrete care episodes, financially rewarding providers that contain costs below a benchmark target without compromising quality.
CMS has used 2 nationwide programs to engage hospitals in bundled payments for lower extremity joint replacement surgery (hereafter, “joint replacement”) bundles. In the voluntary Bundled Payments for Care Improvement initiative (BPCI), hospitals elected to accept bundled payment for episodes starting with admission for joint replacement and encompassing up to 90 days of postacute care. In the mandatory Comprehensive Care for Joint Replacement (CJR) model, hospitals in 67 metropolitan areas were selected by CMS and required to accept bundled payments for joint replacement episodes that were identical in design to episodes in BPCI.1,2 Hospitals in both programs have demonstrated 2%–4% cost savings with stable quality, with top performers in BPCI achieving savings of up to 20%.3–7
However, it is critical to understand whether these benefits, and care delivered through bundled payments, are available to historically marginalized patients, such as racial minorities and individuals with low socioeconomic status (SES). If not, results from BPCI and CJR may not be generalizable—a major limitation for policymakers amid a large ongoing voluntary bundled payment program and plans to consider future mandatory programs.8
In addition, if selective participation by hospitals prevents marginalized patients from receiving improved care under bundled payments, this dynamic could potentially exacerbate long-standing racial and SES disparities in joint replacement care.9–18 Similar issues of selective participation have been raised for mandatory bundled payments and other payment models such as accountable care organizations, but no data have evaluated these dynamics for voluntary bundled payments or comparing selective participation between voluntary and mandatory programs.19,20
Therefore, this study used Medicare claims and data from the BPCI and CJR programs to examine whether hospitals' bundled payment participation is related to the proportion of historically marginalized individuals in the communities they serve. The hypothesis was that hospital participation in both BPCI and CJR programs would be lower in communities with more marginalized individuals, with more pronounced differences for BPCI than for CJR.
Methods
Study overview
The 2010–2017 Medicare data were used to evaluate the association between the proportion of historically marginalized groups in geographic communities and hospital participation in joint replacement bundled payment programs. The University of Pennsylvania Institutional Review Board approved this study with a waiver of informed consent. The study followed guidelines from the Strengthening the Reporting of Observational Studies in Epidemiology statement.21
Data
The 2013–2017 BPCI and CJR hospital enrollment data were used to identify hospitals participating in each program. To avoid confounding from the effects of BPCI and CJR themselves, the analysis used Medicare Provider Analysis and Review data from a period before either program's start (2010–2012) to identify beneficiaries undergoing joint replacement (Medicare Severity-Disease Related Groups 469 and 470). Data were also incorporated from the Medicare Beneficiary Summary File and the American Community Survey.22
Hospitals and communities
The unit of analysis in this study was geographic community, as defined by hospital service area.23 Communities with at least 1 hospital in BPCI (BPCI hospitals) were defined as BPCI communities, whereas communities with at least 1 hospital in CJR (CJR hospitals) were defined as CJR communities. When communities contained both hospital types, communities were defined based on which program had greater representation in joint replacement procedures (eg, if a community had more joint replacement hospitalizations occurring under BPCI than CJR, it was categorized as a BPCI community). Because BPCI predated CJR, hospitals participating in the former could have subsequently become selected by CMS for the latter. In such cases, hospitals were assigned as CJR hospitals.
Exposure and outcome variables
Two exposure variables were defined to represent historically marginalized patient groups. The first measure, low SES share, was defined by using Medicare claims to calculate the proportion of individuals in each geographic community who were eligible for both Medicare and Medicaid. To create the second, minority share, Medicare claims were used to calculate the average annual proportion of Black and Hispanic individuals in each geographic community. Communities were categorized into quartiles with respect to both exposure variables. The outcome variables were BPCI participation (communities with at least 1 BPCI hospital) and CJR participation (communities with at least 1 CJR hospital).
Covariates
Community-level patient measures used in this analysis included patient age, gender, clinical complexity (defined by Elixhauser mortality index), and prevalence of osteoarthritis and joint replacement surgery. Analysis also incorporated community size (number of Medicare fee-for-services beneficiaries) and community-level measures of provider capacity (hospital, skilled nursing facility [SNF], inpatient rehabilitation facility beds; home health staff), SNF concentration (defined by Herfindahl–Hirschman Index), and Medicare Advantage (MA) penetration.24
Statistical analysis
Characteristics were compared across community groups (BPCI vs. non-BPCI; CJR vs. non-CJR). Chi-squared tests were used to compare categorical variables and Wilcoxon rank sum tests were used to compare continuous variables.
Multivariable ordinary least squares regression was used to conduct cross-sectional analyses of the association between exposures (the proportion of low SES and racial or ethnic minority individuals in a community) and outcome (community participation in bundled payment programs) variables (Supplementary Methods). Two models, 1 for BPCI and 1 for CJR, were used to evaluate associations for each program separately.
Data used in base models were appended into a combined data set that included 2 observations per community—1 from the BPCI base model and 1 from the CJR base model. This approach enabled the use of a model that jointly considered community participation in either program as an outcome, and compared strengths of association for CJR versus BPCI using interaction terms between marginalized group exposure variables and an indicator of BPCI versus CJR base model. All models included both exposure variables.
All analyses were performed in SAS (version 9.4, SAS Institute). Statistical tests were 2 tailed and considered significant at α = 0.05.
Sensitivity analysis
Analyses was repeated by using (1) data from the American Community Survey, rather than Medicare data, to define minority share (area-level data of the proportion of Black and Hispanic individuals) and low SES share (area-level data of the proportion of individuals either under the federal poverty line or without a high school education); and (2) separate models for each exposure variable rather than models that included both.
Analyses were also replicated using (3) an alternative definition for BPCI hospitals, defining hospitals participating in BPCI as BPCI hospitals regardless of subsequent selection in a CJR area; and (4) multivariable logistic regression rather than ordinary least squares. Finally, to reflect the fact that CJR was designed to mandate hospital participation at level of metropolitan statistical area (MSA), CJR analyses were repeated using models that used clustering at MSA level.
Results
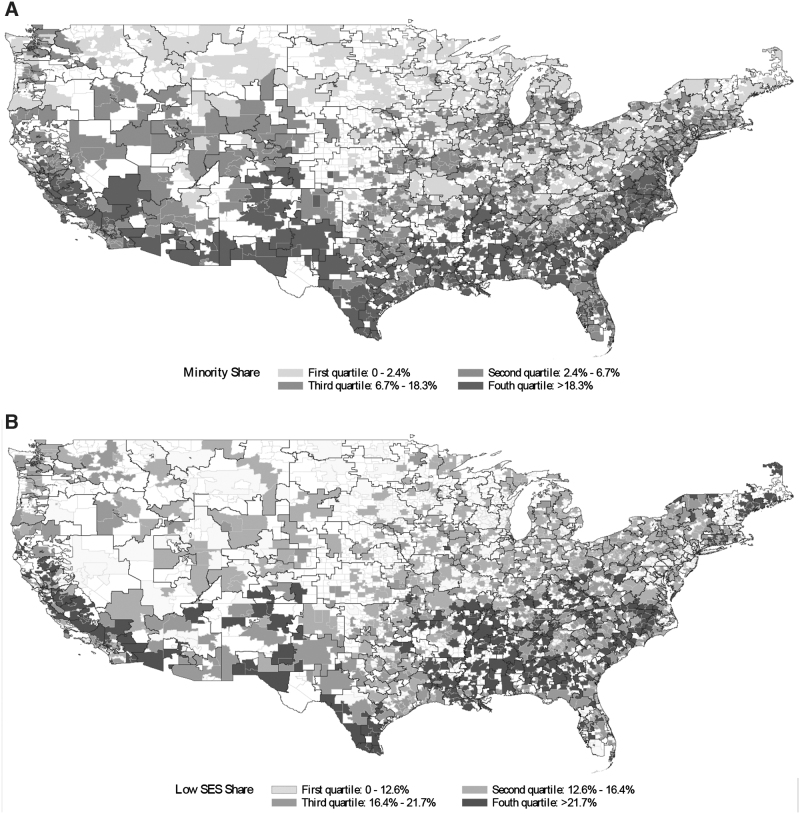
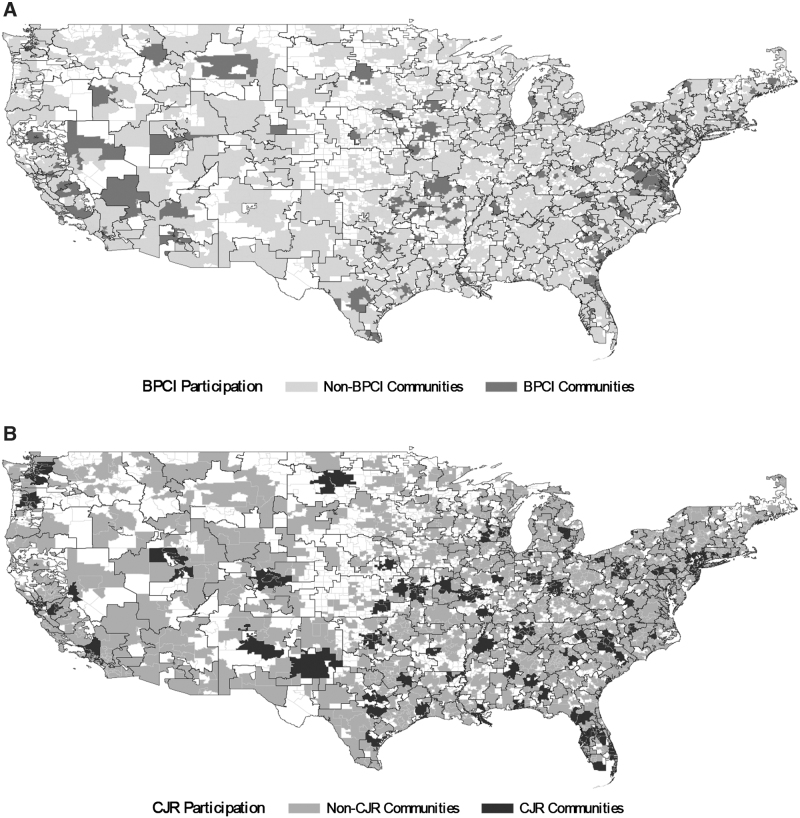
The study sample included 3880 geographic communities nationwide, which varied with respect to minority share and low SES share (Tables 1 and 2). Among these communities, there were 210 and 1730 BPCI and non-BPCI communities, respectively, as well as 389 and 1551 CJR and non-CJR communities, respectively. Both BPCI and CJR communities were distributed geographically across the country (Figs. 1 and 2).
Table 1.
Community Characteristics
| Non-BPCI communities | BPCI communities | P | Non-CJR communities | CJR communities | P | |
|---|---|---|---|---|---|---|
| Communities (no.) | 1730 | 210 | 1551 | 389 | ||
| Population | ||||||
| Female, mean %, (SD) | 50.6 (1.7) | 51.0 (1.0) | 0.0004 | 50.5 (1.7) | 51.0 (1.3) | <0.0001 |
| Age, mean % (SD) | ||||||
| 0–64 | 85.2 (4.2) | 86.4 (3.7) | <0.0001 | 85.3 (3.9) | 85.8 (4.8) | <0.0001 |
| 65–74 | 8.0 (2.2) | 7.3 (1.8) | <0.0001 | 8.0 (2.1) | 7.5 (2.2) | <0.0001 |
| 75–84 | 4.8 (1.5) | 4.4 (1.4) | <0.0001 | 4.8 (1.4) | 4.7 (1.9) | 0.0001 |
| ≥85 | 2.0 (0.8) | 1.9 (0.7) | 0.1637 | 1.9 (0.7) | 2.0 (1.0) | 0.5050 |
| Medicare population | ||||||
| Medicare beneficiaries, mean no. (SD) | 20670 (28567) | 47623 (58620) | <0.0001 | 20906 (30645) | 34280 (44083) | <0.0001 |
| Clinical complexity, mean score (SD)a | 8.3 (1.0) | 8.5 (0.9) | 0.0358 | 8.3 (1.0) | 8.3 (0.9) | 0.7159 |
| MA participation, mean % (SD) | 22.6 (14) | 23.3 (12.4) | 0.1863 | 20.7 (12.8) | 30.5 (14.8) | <0.0001 |
| Osteoarthritis prevalence, mean % (SD) | 2.9 (0.9) | 2.8 (0.9) | 0.0708 | 2.9 (0.9) | 2.6 (0.8) | <0.0001 |
| LEJR prevalence, mean % (SD) | 1.1 (0.3) | 1.1 (0.2) | 0.2771 | 1.1 (0.2) | 1.0 (0.3) | <0.0001 |
| Provider capacity | ||||||
| Total hospital beds, mean no. (SD) | 426 (881) | 1141 (2053) | <0.0001 | 421 (938) | 831 (1526) | <0.0001 |
| Total IRF bed, mean no. (SD) | 24 (60) | 72 (153) | <0.0001 | 24 (70) | 47 (99) | <0.0001 |
| Total SNF bed count, mean no. (SD) | 50 (119) | 153 (404) | <0.0001 | 55 (173) | 83 (190) | <0.0001 |
| Total HHA staff, mean no. (SD) | 237 (723) | 641 (1455) | <0.0001 | 238 (752) | 450 (1121) | <0.0001 |
| Concentration of SNFs, mean HHI (SD) | 3746.2 (2436.8) | 2524.1 (2132.5) | <0.0001 | 3721.8 (2414.1) | 3169.7 (2472.3) | <0.0001 |
Wilcoxon rank sum tests were used for comparisons.
Clinical complexity was defined by the Elixhauser Comorbidity Index.
BPCI, Bundled Payments for Care Improvement; CJR, Comprehensive Care for Joint Replacement; HHA, Home Health Agency; HHI, Herfindahl–Hirschman Index; IRF, Institutional Rehabilitation Facility; LEJR, lower extremity joint replacements; MA, Medicare Advantage; SD, standard deviation; SNF, skilled nursing facility.
Table 2.
Minority Share and Low Socioeconomic Status Share by Communities
| Non-BPCI communities | BPCI communities | P | Non-CJR communities | CJR communities | P | |
|---|---|---|---|---|---|---|
| Minority share, no. (%) | <0.0001 | <0.0001 | ||||
| Lowest quartile | 462 (26.71) | 23 (10.95) | 445 (28.69) | 40 (10.28) | ||
| Second quartile | 437 (25.26) | 48 (22.86) | 379 (24.44) | 106 (27.25) | ||
| Third quartile | 414 (23.93) | 71 (33.81) | 364 (23.47) | 121 (31.11) | ||
| Highest quartile | 417 (24.10) | 68 (32.38) | 363 (23.40) | 122 (31.36) | ||
| Low SES share, no. (%) | 0.2108 | 0.0007 | ||||
| Lowest quartile | 421 (24.34) | 64 (30.48) | 361 (23.28) | 124 (31.88) | ||
| Second quartile | 436 (25.20) | 50 (23.81) | 382 (24.63) | 104 (26.74) | ||
| Third quartile | 432 (24.97) | 52 (24.76) | 403 (25.98) | 81 (20.82) | ||
| Highest quartile | 441 (25.49) | 44 (20.95) | 405 (26.11) | 80 (20.57) |
Chi-squared tests were used for comparisons. Minority share and low SES share information was derived from the Master Beneficiary Summary File.
BPCI, Bundled Payments for Care Improvement; CJR, Comprehensive Care for Joint Replacement; SES, socioeconomic status.
FIG. 1.
Minority share and low SES share in communities nationwide, 2010–2012. (A) Minority share. (B) Low SES share. SES, socioeconomic status.
FIG. 2.
BPCI and CJR participation in communities nationwide, 2010–2012. (A) BPCI participation. (B) CJR participation. BPCI, Bundled Payments for Care Improvement initiative; CJR, Comprehensive Care for Joint Replacement.
BPCI versus non-BPCI communities
Several characteristics differed between the 2 community groups (Table 1). BPCI communities tended to be bigger (average of 47623 beneficiaries per community vs. average of 20,670 beneficiaries per non-BPCI community, P < 0.0001) with fewer individuals over the age of 65 years (13.6% vs. 14.8% in non-BPCI communities, P < 0.0001). Compared with non-BPCI communities, BPCI communities also had greater patient clinical complexity, MA penetration, and measures of health care provider capacity.
More BPCI communities were in the highest quartile of minority share (32.4% vs. 24.1% of non-BPCI communities, P < 0.0001) (Table 2). In contrast, BPCI and non-BPCI communities were similar with respect to low SES share (21.0% of BPCI communities vs. 25.5% of non-BPCI communities in the highest quartile, P = 0.21).
In adjusted multivariable analysis (Table 3), an increase from the lowest to highest quartile of minority share was not associated with differences in the probability of being a BPCI community (3.5% difference in probability, 95% confidence interval (CI) −1.2% to 8.3%, P = 0.15). Conversely, an increase from the lowest to highest quartile of low SES share was associated with a 6.0 percentage point (pp) lower probability of being a BPCI community (95% CI −10.9% to −1.2%, P = 0.02).
Table 3.
Adjusted Analysis of the Association Between Minority and Low Socioeconomic Status Share and the Likelihood of Being a Bundled Payment Community
| Being a BPCI community |
Being a CJR community |
Being a CJR vs. BPCI community |
||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| Minority share, % | ||||||
| Lowest quartile (referent) | ||||||
| Second quartile | 2.2 (−1.7 to 6.1) | 0.2651 | 13.7 (9.0 to 18.5) | <0.0001 | 8.0 (1.9 to 14.1) | 0.0106 |
| Third quartile | 4.8 (0.8 to 8.9) | 0.0185 | 16.6 (11.7 to 21.5) | <0.0001 | 6.9 (0.8 to 13) | 0.0278 |
| Highest quartile | 3.5 (−1.2 to 8.3) | 0.1451 | 16.1 (10.3 to 22) | <0.0001 | 10.0 (3.3 to 16.7) | 0.0034 |
| Low SES share, % | ||||||
| Lowest quartile (referent) | ||||||
| Second quartile | −4.0 (−7.9 to −0.1) | 0.0437 | −6.6 (−11.3 to −1.8) | 0.0068 | −0.9 (−7.0 to 5.3) | 0.7775 |
| Third quartile | −3.4 (−7.5 to 0.7) | 0.1084 | −14.2 (−19.3 to −9.2) | <0.0001 | −7.8 (−14.1 to −1.5) | 0.0156 |
| Highest quartile | −6.0 (−10.9 to −1.2) | 0.0151 | −19.0 (−24.9 to −13.0) | <0.0001 | −7.1 (−14 to −0.2) | 0.0450 |
Regression models were adjusted for the following community-level variables: population gender, age, clinical severity, prevalence of osteoarthritis, and joint replacement surgery; Medicare beneficiary population, Medicare Advantage penetration, and measures of provider capacity.
BPCI, Bundled Payments for Care Improvement; CI, confidence interval; CJR, Comprehensive Care for Joint Replacement; SES, socioeconomic status.
CJR versus non-CJR communities
CJR and non-CJR communities also differed with respect to a number of characteristics (Table 1). Compared with non-CJR communities, CJR communities were bigger (average of 34,280 beneficiaries per community versus average of 20906 beneficiaries per community) with greater MA penetration (30.5% vs. 20.7%) (P < 0.0001 for both). CJR communities also had greater provider capacity than non-CJR communities.
More CJR communities were in the highest quartile of minority share (31.4% vs. 23.4% of non-CJR communities, P < 0.0001) (Table 2). Conversely, more non-CJR communities were in the highest quartile of low SES share (26.1% vs. 20.6% of CJR communities, P = 0.0007).
In multivariable analysis (Table 3), an increase from the lowest to highest quartile of minority share was associated with a 16.1 pp higher probability of being a CJR community (95% CI 10.3% to 22.0%, P < 0.0001). In contrast, an increase from the lowest to highest quartile of low SES share was associated with a 19.0 pp lower probability of being a CJR community (95% CI −24.9% to −13.0%, P < 0.0001).
BPCI versus CJR
The relationship between both of the exposure variables and bundled payment participation was stronger for CJR than for BPCI (Table 3). Moving from the lowest to highest quartile of minority share was associated with a higher probability of being a CJR community that was 10.0 pps (95% CI 3.3% to 16.7%, P = 0.0034) greater than the increase in probability of being a BPCI community. Conversely, moving from the lowest to highest quartile of low SES share was associated with 7.1 pp lower probability (95% CI −14.0% to −0.2%, P = 0.045) of being a CJR community, as compared with being a BPCI community.
Sensitivity analysis
Results from sensitivity analyses were qualitatively similar to those from the primary analyses (Supplementary Tables S1–S5). Overall, increases from the lowest to highest quartile of low SES share were associated with a lower probability of being a bundled payment community for both BPCI and CJR, whereas increases between the lowest and highest quartile of minority share were associated with a higher probability of being a bundled payment community for both programs.
Discussion
To our knowledge, this is the first study evaluating the relationship between the prevalence of historically marginalized groups and hospital participation in bundled payments in US communities. It demonstrates that hospitals were less likely to participate in bundled payments in communities with more low SES individuals than with more affluent communities. In contrast, hospitals in communities with more racial and ethnic minorities were more likely to participate than communities with fewer minorities. These findings, which were stronger for mandatory versus voluntary programs, pose 3 important policy implications.
First, these results highlight the need to distinguish between different historically marginalized groups when evaluating the presence of bundled payment hospitals in communities. Although study findings for low SES share were consistent with prior hypotheses, findings about minority share were not. These directionally opposite results highlight how selective hospital participation may limit generalizability of bundled payment program findings for some groups (eg, SES) but not necessarily others (eg, race or ethnicity). With respect to potential disparities, these results suggest that if bundled payment hospitals try to improve performance by cherry-picking and avoiding high-risk patients, they likely perceive forms of risk (eg, racial and ethnic vs. SES) differently. Future work should elucidate these distinctions.
Second, this study underscores the need to evaluate how bundled payments affect disparities among low SES individuals. Presently, that is a critical knowledge gap: few bundled payment analyses have directly studied outcomes in these patients, and none have explicitly evaluated how the payment model impacts disparities in outcomes—that is, how differences between marginalized and nonmarginalized patients change over time.25 Such insight is urgently needed to ensure that payment models promote rather than impede equity.26
By demonstrating that hospitals in lower income communities are less likely to join BPCI or be included in CJR, this study provides a foundation and impetus for future work. Policymakers should ensure that any care improvements prompted by new payment models reach all beneficiaries. In this case, if care through bundled payment participants is not even available to low SES individuals, overall results from BPCI and CJR cannot be easily generalized to those communities: positive results may not apply and observed lack of harms should be interpreted cautiously and caveated.
Third, findings from this study also demonstrate the importance of program participation mechanism. Voluntary programs allow hospitals to make participation decisions based on organizational readiness, but 1 potential concern is that such freedom may result in selection that reduces the possibility that marginalized patients could experience care and improved outcomes under bundled payments (eg, if hospitals located in certain communities elect not to participate in bundled payments).27 Comparatively speaking, policymaker-mandated participation could better mitigate such selection and increase generalizability of bundled payment program findings by requiring participation from all hospitals across large, highly populated areas.
These results provide new insight into these dynamics. Although not definitive, observed associations were stronger for CJR than for BPCI: a comparatively greater increase in likelihood of participation based on minority share and greater decrease based on low SES share. Not only do these findings suggest that mandatory participation is not a panacea for ensuring participation and addressing potential access issues, but our results also spotlight the importance of the criteria used to mandate participation. Although mandates can theoretically support greater equity by reducing selective hospital participation, they may not actually accomplish that goal if implemented without directly considering factors such as SES.
In the case of CJR, the program was designed to select regions based on historical volume and spending for joint replacement surgery, but not based on SES. To the extent that CJR was meant to include and yield benefits to a range of different communities, results from this study build on earlier work and suggest that this approach to selecting regions may have inadvertently excluded poorer communities—even to a greater extent than voluntary programs that can suffer from issues of selective participation, such as BPCI.19 Well-intentioned CJR mandates may have inadvertently exacerbated access issues for low-income communities by failing to address the existing relationship between procedural volume, spending, and SES (low-SES individuals being less likely than others in a given region to undergo joint replacement).28
This study has limitations. First, as with all observational studies, it was susceptible to residual confounding. However, analyses accounted for a range of covariates and involved a series of sensitivity analyses to test the robustness of our results. Second, although this study evaluated the presence of bundled payment hospitals in communities, future work is needed to assess the related implications (eg, whether marginalized groups experience outcomes disparities under bundled payments).
Third, study findings may not generalize to nonjoint replacement bundled payment episodes, such as those for medical conditions. Fourth, results from this analysis yield policy-relevant insights for bundled payment policy, but future work is needed to evaluate how hospitals choose to engage in other programs (eg, community partnerships, initiatives to address social determinants of health) and payment models in communities across the country.
Nonetheless, these results are highly salient given that a potential benefit of mandatory versus voluntary participation is generalizability: the ability to generate results that better reflect what would happen if payment models were implemented more broadly nationwide. To leverage these benefits of mandatory participation without unintentionally limiting their generalizability, policymakers may need to directly consider SES and other social determinants when selecting communities in which to mandate participation.
Conclusion
Hospitals in low-income communities were less likely to participate in voluntary—and to an even greater extent, mandatory—bundled payment programs. These findings raise concerns about generalizability of overall program results and potential disparities, and suggest that policymakers should consider communities' social factors and participation type in the design of future bundled payment programs.
Supplementary Material
Disclaimer
This article does not necessarily represent the views of the US government or the Department of Veterans Affairs.
Author Disclosure Statement
Dr. Liao reports personal fees from Kaiser Permanente Washington Health Research Institute, textbook royalties from Wolters Kluwer, and honoraria from Wolters Kluwer, the Journal of Clinical Pathways, and the American College of Physicians, all outside the submitted work. Dr. Navathe reports grants from Hawaii Medical Service Association, grants from Anthem Public Policy Institute, grants from Commonwealth Fund, grants from Oscar Health, grants from Cigna Corporation, grants from Robert Wood Johnson Foundation, grants from Donaghue Foundation, grants from Pennsylvania Department of Health, grants from Ochsner Health System, grants from United Healthcare, grants from Blue Cross Blue Shield of NC, grants from Blue Shield of CA, grants from Humana.
Dr. Navathe also reports personal fees from Navvis Healthcare, personal fees from Agathos, Inc., personal fees and equity from Navahealth, personal fees from YNHHSC/CORE, personal fees from Maine Health Accountable Care Organization, personal fees from Maine Department of Health and Human Services, personal fees from National University Health System—Singapore, personal fees from Ministry of Health—Singapore, personal fees from Elsevier Press, personal fees from Medicare Payment Advisory Commission, personal fees from Cleveland Clinic, personal fees from Analysis Group, personal fees from VBID Health, personal fees from Advocate Physician Partners, personal fees from the Federal Trade Commission, and equity from Embedded Healthcare, and noncompensated board membership from Integrated Services, Inc., outside the submitted work.
Funding Information
This study was supported in part by the National Institute on Minority Health and Health Disparities and the Agency for Healthcare Research and Quality. Funders did not have any role in the research.
Supplementary Material
References
- 1. Centers for Medicare and Medicaid Services. Bundled payments for care improvement (BPCI) initiative: https://innovation.cms.gov/initiatives/bundled-payments/ Accessed October 27, 2021.
- 2. Centers for Medicare and Medicaid Services. Comprehensive care for joint replacement model. https://innovation.cms.gov/initiatives/CJR Accessed October 27, 2021.
- 3. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA 2016;316:1267–1278. [DOI] [PubMed] [Google Scholar]
- 4. Navathe AS, Emanuel EJ, Venkataramani AS, et al. Spending and quality after three years of Medicare's voluntary bundled payment for joint replacement surgery. Health Aff (Millwood) 2020;39:58–66. [DOI] [PubMed] [Google Scholar]
- 5. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med 2017;177:214–222. [DOI] [PubMed] [Google Scholar]
- 6. Finkelstein A, Ji Y, Mahoney N, Skinner J. Mandatory Medicare bundled payment program for lower extremity joint replacement and discharge to institutional postacute care: interim analysis of the first year of a 5-year randomized trial. JAMA 2018;320:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnett ML, Wilcock A, McWilliams JM, et al. Two-year evaluation of mandatory bundled payments for joint replacement. N Engl J Med 2019;380:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Medicare and Medicaid Services. BPCI Advanced. https://innovation.cms.gov/initiatives/bpci-advanced Accessed October 27, 2021.
- 9. Ibrahim SA, Kim H, McConnell KJ. The CMS comprehensive care model and racial disparity in joint replacement. JAMA 2016;316:1258–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson MG, May DS, Kelly JJ. Racial differences in the use of total knee arthroplasty for osteoarthritis among older Americans. Ethn Dis 1994;4:57–67. [PubMed] [Google Scholar]
- 11. Escarce JJ, Epstein KR, Colby DC, Schwartz JS. Racial differences in the elderly's use of medical procedures and diagnostic tests. Am J Public Health 1993;83:948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health 1996;86:70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz BP, Freund DA, Heck DA, et al. Demographic variation in the rate of knee replacement: a multi-year analysis. Health Serv Res 1996;31:125–140. [PMC free article] [PubMed] [Google Scholar]
- 14. Giacomini MK. Gender and ethnic differences in hospital-based procedure utilization in California. Arch Intern Med 1996;156:1217–1224. [PubMed] [Google Scholar]
- 15. Barrack RL, Ruh EL, Chen J, et al. Impact of socioeconomic factors on outcome of total knee arthroplasty. Clin Orthop Relat Res 2014;472:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Escalante A, Espinosa-Morales R, del Rincón I, Arroyo RA, Older SA. Recipients of hip replacement for arthritis are less likely to be Hispanic, independent of access to health care and socioeconomic status. Arthritis Rheum 2000;43:390–399. [DOI] [PubMed] [Google Scholar]
- 17. Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Understanding ethnic differences in the utilization of joint replacement for osteoarthritis: the role of patient-level factors. Med Care 2002;40(1 Suppl):I44–I51. [DOI] [PubMed] [Google Scholar]
- 18. Ibrahim SA, Burant CJ, Siminoff LA, Stoller EP, Kwoh CK. Self-assessed global quality of life: a comparison between African-American and White older patients with arthritis. J Clin Epidemiol 2002;55:512–517. [DOI] [PubMed] [Google Scholar]
- 19. Yasaitis LC, Pajerowski W, Polsky D, Werner RM. Physicians' participation in ACOs is lower in places with vulnerable populations than in more affluent communities. Health Aff (Millwood) 2016;35:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liao JM, Huang Q, Ibrahim SA, et al. Between-community low-income status and inclusion in mandatory bundled payments in Medicare's comprehensive care for joint replacement model. JAMA Netw Open 2021;4:e211016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elm Ev, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Census Bureau. American Community Survey. https://www.census.gov/programs-surveys/acs/data.html Accessed August 27, 2021.
- 23. Dartmouth Atlas of Health Care. Dartmouth Atlas Project. https://www.dartmouthatlas.org Accessed October 28, 2021.
- 24. Laine CR. The Herfindahl-Hirschman index: a concentration measure taking the consumer's point of view. Antitrust Bull 1995;40:423–432. [Google Scholar]
- 25. Maughan B, Kahvecioglu DC, Marrufo G, et al. Medicare's bundled payments for care improvement initiative maintained quality of care for vulnerable patients. Health Aff (Millwood) 2019;38:561–568. [DOI] [PubMed] [Google Scholar]
- 26. Liao JM, Lavizzo-Mourey RJ, Navathe AS. A national goal to advance health equity through value-based payment. JAMA 2021;325:2439–2440. [DOI] [PubMed] [Google Scholar]
- 27. Liao JM, Pauly MV, Navathe AS. When should Medicare mandate participation in alternative payment models? Health Aff (Millwood). 2020;39:3059. [DOI] [PubMed] [Google Scholar]
- 28. Skinner J, Zhou W, Weinstein J. The influence of income and race on total knee arthroplasty in the United States. J Bone Joint Surg Am 2006;88:2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.