Abstract
To determine whether homologous recombination could be used to inactivate selected genes in Spiroplasma citri, plasmid constructs were designed to disrupt the motility gene scm1. An internal scm1 gene fragment was inserted into plasmid pKT1, which replicates in Escherichia coli but not in S. citri, and into the S. citri oriC plasmid pBOT1, which replicates in spiroplasma cells as well as in E. coli. Electrotransformation of S. citri with the nonreplicative, recombinant plasmid pKTM1 yielded no transformants. In contrast, spiroplasmal transformants were obtained with the replicative, pBOT1-derived plasmid pCJ32. During passaging of the transformants, the plasmid was found to integrate into the chromosome by homologous recombination either at the oriC region or at the scm1 gene. In the latter case, plasmid integration by a single crossover between the scm1 gene fragment carried by the plasmid and the full-length scm1 gene carried by the chromosome led to a nonmotile phenotype. Transmission of the scm1-disrupted mutant to periwinkle (Catharanthus roseus) plants through injection into the leafhopper vector (Circulifer haematoceps) showed that the motility mutant multiplied in the insects and was efficiently transmitted to plants, in which it induced symptoms similarly to the wild-type S. citri strain. These results suggest that the spiroplasmal motility may not be essential for pathogenicity and that, more broadly, the S. citri oriC plasmids can be considered promising tools for specific gene disruption by promoting homologous recombination in S. citri, a mollicute which probably lacks a functional RecA protein.
Spiroplasmas are eubacteria belonging to the class Mollicutes, a group of wall-less organisms which represents a branch in the phylogenetic tree of the gram-positive bacteria (41). In addition to the lack of cell wall, mollicutes are characterized by small genome size, low number of rRNA operons and tRNA genes, and limited metabolic activities. The molecular features of mollicutes have been extensively reviewed (1, 12, 31). Among mollicutes, spiroplasmas are characterized by helical morphology and motility (7–9, 21). Spiroplasmas are associated primarily with arthropods, mainly insects, and three of them, Spiroplasma citri, Spiroplasma kunkelii, and Spiroplasma phoeniceum, are pathogenic to plants (44). In plants, the organisms are located in, and restricted to, the phloem sieve tubes. They are transmitted from plant to plant by sap-feeding leafhopper vectors. S. citri was the first plant-pathogenic mollicute to have been cultured and characterized as the etiological agent of citrus stubborn disease (34). However, in spite of extensive characterization of S. citri, very little is known about the genetic determinants that govern the interactions between the spiroplasma and the two hosts in which it multiplies, the insect vector and the plant (2, 3, 13). In recent years, molecular tools have been developed for genetic analysis of S. citri. Plasmid vectors were constructed by combining the chromosomal replication origin of S. citri (oriC region) and the tetM gene of Tn916 to express cloned genes in S. citri (32, 33, 45). Also, transposon (Tn4001) mutagenesis was used to produce S. citri mutants affected in motility or in plant pathogenicity, and functional complementation of these mutants was achieved by transformation with oriC plasmids carrying the wild-type DNA (15–17, 19). However, in S. citri, specific gene disruption through homologous recombination has not yet been obtained.
In mollicutes, homologous recombination was first demonstrated to occur in Acholeplasma oculi (24, 25), then in Acholeplasma laidlawii (11) and Mycoplasma gallisepticum (6), and very recently in Mycoplasma genitalium (10). In previous studies (26, 27), we have shown that S. citri probably lacks a functional recA gene, suggesting that this organism is unable to undergo generalized homologous recombination dependent on the RecA protein. However, we have also shown that despite the absence of RecA protein, homologous recombination does occur in specific cases (26, 33). In particular, when S. citri ASP1 was transformed with the oriC plasmid pBOT1, it was shown that during passaging of spiroplasmal transformants, the plasmid integrated into the host chromosome by homologous recombination at the oriC region (33).
In the present study, we used a replicative, pBOT1-derived oriC plasmid to inactivate the S. citri motility gene scm1 through homologous recombination. The scm1 gene codes for a putative highly hydrophobic protein of 409 amino acids (19). This polypeptide was predicted to contain an N terminus characteristic of signal peptide sequences as well as 10 transmembrane α helices and a leucine zipper-like motif. However, the Scm1 protein does not show homology with known proteins, and its function is unknown. We chose to inactivate the motility gene scm1 for the following reasons: (i) the nucleotide sequence of the scm1 gene has been determined, (ii) it has been shown that disruption of scm1 by transposon insertion gives rise to a readily observable, nonmotile phenotype, and (iii) inactivation of the scm1 gene has no significant effect on the spiroplasma growth rate (19). In these early studies, an insertional mutant, G540, was obtained by transformation of a high-passage culture of S. citri GII-3 which had lost its ability to be transmitted by the leafhopper vector. In the present work, the isolation of a motility mutant by disruption of the scm1 gene in a low-passage, transmissible isolate of S. citri GII-3 was made to investigate the putative role of motility in the pathogenicity of S. citri. The scm1-disrupted mutant was experimentally transmitted to periwinkle plants through injection to the leafhopper vector, and it was found to be pathogenic.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli TG1 {supE hsdΔ5 thi Δ(lac-proAB) F′[traD36 proAB+ lacIq lacZΔM15]}, an EcoK− derivative of JM101, was used as the host strain for subcloning experiments and for propagation of plasmids pBOT1, pCJ3, pKT1, pKTM1, and pCJ32. Plasmids pBOT1 and pCJ3, carrying the S. citri oriC region and the scm1 gene, respectively, have been described elsewhere (19, 32, 33). Plasmid pKT1 was obtained by inserting the tetM gene, rescued from the pBOT1 plasmid as a 4.2-kbp BamHI-EcoRI fragment, into the pBluescript II KS+ vector (Stratagene Cloning Systems, La Jolla, Calif.). Constructions of plasmids pKTM1 and pCJ32 are described in Results. For transformation with plasmid DNA, E. coli competent cells were prepared according to the procedure described by Hanahan (18). S. citri GII-3 was originally isolated from its leafhopper vector, Circulifer haematoceps, captured in Morocco (40). From an early passage of this isolate, a triply cloned strain (38) was obtained and used in this study. This cloned strain was shown to be phytopathogenic by transmission to periwinkle (Catharanthus roseus) through injection to the leafhopper vector C. haematoceps. Spiroplasmas were grown at 32°C in SP4 medium (42) from which fresh yeast extract was omitted.
Transformation of S. citri.
Electrotransformation of S. citri with plasmid DNA was done as previously described (32, 36), with 1 to 30 μg of DNA in the case of the suicide plasmid pKTM1 and 1 to 5 μg in the case of the replicative plasmid pCJ32. The S. citri transformants were selected by plating on solid SP4 medium supplemented with 2 μg of tetracycline per ml. Individual colonies were picked and grown in broth medium containing 2 μg of tetracycline per ml. During propagation, the antibiotic concentration was progressively increased up to 15 μg/ml.
DNA isolation and Southern blot hybridization.
Large-scale and small-scale preparations of plasmid DNA amplified in E. coli were carried out according to standard procedures (35). Large-scale preparation of spiroplasmal genomic DNA has been described elsewhere (43). For small-scale preparation, spiroplasma cells from a 10-ml culture were collected by centrifugation and resuspended in 1.8 ml of STE buffer (100 mM NaCl, 10 mM Tris-HCl [pH 8], 1 mM EDTA). Cells were lysed by adding 200 μl of 10% sodium dodecyl sulfate. The lysate was heated at 65°C for 15 min and then treated with 100 μg of RNase for 30 min at 37°C. The DNA was further purified by phenol-chloroform deproteinization and ethanol-acetate precipitation and finally resuspended in 200 μl of sterile water or TE buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA). For Southern blot hybridization, restricted DNA was blotted to positively charged nylon membranes by the alkali transfer procedure and hybridized with the 32P-labeled probe under standard stringent conditions (35).
Primers and PCR amplification.
Primers CJ5 (5′-AATGACGGATCATCAACGG-3′), CJ6 (5′-CAATTACCAACCATGTTAGC-3′), CJ8 (5′-GGTTAGTAATGCTGATCGC-3′), CJ17 (5′-CTTTACAGGGAGATAGTGC-3′), CJ26 (5′-ATTGCTGGGGCAGTTGTTC-3′), and CJ27 (5′-CTAAATATTGTTCACATTAAAGTTTGTC-3′) correspond, respectively, to nucleotides 3928 to 3946, 3576 to 3595, 2328 to 2346, 2678 to 2696, 2572 to 2591, and 3604 to 3632 of the previously published sequence (19). Primers IS1 (5′-ATATTCTGTAAAGGATGACG-3′) and IS3 (5′-CTTTAACAGCTTCTCTG-3′) correspond, respectively, to nucleotides 155 to 174, and 923 to 939 of the IS256 nucleotide sequence (5). Primers Tet1 (5′-CTGCAAAAGATGGCGTAC-3′) and Tet2 (5′-CGTAAATGTAGTACTCCAC-3′) correspond, respectively, to nucleotides 521 to 538 and 1037 to 1055 of the tetM gene (4). Primer EV7 (5′-CAATAAGCAAGCATCTGTAATTAG-3′) corresponds to nucleotides 507 to 530 of the nucleotide sequence of the S. citri oriC region (45). Amplification was carried out in a 50-μl reaction mixture containing 5 to 20 ng of target DNA, 50 mM Tris-HCl (pH 8.8), 2 mM MgCl2, 200 μg of bovine serum albumin per ml, 0.05% W1 detergent, 0.2 mM deoxynucleoside triphosphates, 1 μM each primer, and 2.5 U of Taq DNA polymerase (GIBCO/BRL Life Technologies, Inc., Gaithersburg, Md.). The reaction was performed in a thermal cycler (Perkin-Elmer Cetus Corp., Norwalk, Conn.). Amplification was achieved in over 35 cycles, each of 45 s at 92°C, 45 s at the annealing temperature, and 1 min at 72°C, with an additional step of 10 min at 72°C. The annealing temperature was set to 52°C for primer pairs IS1-IS3, Tet1-Tet2, and Reverse-CJ5, 56°C for primer pairs CJ6-CJ8 and CJ8-EV7, and 58°C for primer pair CJ26-EV7. PCR products Reverse-CJ5 and CJ26-EV7 were cloned in E. coli by using the pGEM-T Easy vector (Promega Corporation, Madison, Wis.) and sequenced with a T7 sequencing kit (Pharmacia Biotech, Uppsala, Sweden).
Experimental transmission assay.
Microinjection of S. citri cultures into C. haematoceps leafhoppers and transmission to periwinkle (Catharanthus roseus) plants were carried out as previously described (14, 16). The insects were microinjected with about 0.2 μl of a 108-CFU/ml S. citri culture, i.e., approximately 2 × 104 spiroplasma cells per insect. For each transmission assay, 50 injected leafhoppers were caged on five separate young periwinkle plants (10 insects per plant) for a 2-week period. At the end of the transmission period, the insects were killed and the plants were kept at 30°C in the greenhouse. Under these conditions, the wild-type strain GII-3 produces severe symptoms within 2 weeks after the transmission period. Culture of S. citri from plants and insects as well as transmission through Parafilm membranes have also been described previously (14, 16). For determination of CFU per insect, groups of two to five leafhoppers were ground in 2 ml of SP4 medium. The extracts were passed through filter membranes (pore diameter, 0.45 μm), and 10-fold serial dilutions were plated. The number of CFU was determined as the average value of three independent extracts. In the case of plants, the extracts were prepared from 0.1 to 0.3 g of midribs that were minced with a razor blade in 2 ml of SP4 medium.
RESULTS
Construction of plasmids pKTM1 and pCJ32.
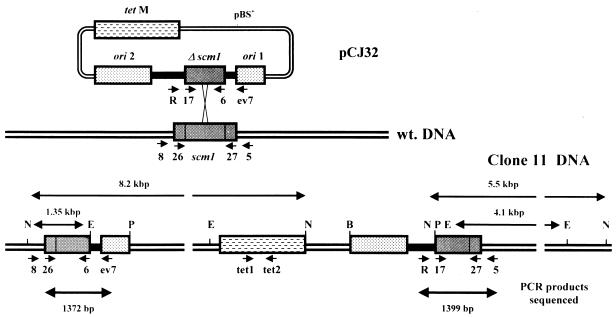
To demonstrate gene disruption by homologous recombination, an internal fragment of the scm1 gene was inserted into either a replicative or a nonreplicative plasmid vector and introduced into S. citri by electroporation. The scm1 gene fragment was obtained by PCR amplification of pCJ3 DNA with primers CJ6 and CJ17. The 918-bp amplification product was initially cloned in E. coli by using the pGEM-T Easy vector system and was then rescued from the recombinant plasmid either as an EcoRI fragment of 937 bp or as a Sau3AI fragment of 2,203 bp. The nonreplicative plasmid pKTM1 was obtained by inserting the EcoRI fragment containing the scm1 gene fragment into the EcoRI-linearized plasmid pKT1. The replicative plasmid pCJ32 was obtained by inserting the Sau3AI fragment at the BglII site of plasmid pBOT1, i.e., within the dnaA gene of the oriC fragment. As a result, the 1.95-kbp oriC region was divided into two separated fragments of 0.6 and 1.35 kbp that we named ori 1 and ori 2, respectively (see Fig. 2). In previous studies (33) we have shown that the presence of a functional dnaA gene on the plasmid is not required for plasmid replication and that during passaging of the spiroplasmal transformants, the pBOT1 plasmid integrates into the chromosome by homologous recombination at the oriC region. Hence, insertion of the scm1 gene fragment into the oriC region of pBOT1 was expected not to prevent plasmid replication and, in addition, to decrease the frequency of recombination in this region.
FIG. 2.
Schematic representation of pCJ32 integration by recombination at the oriC region. Regions ori 1 and ori 2 represent sequences of the oriC fragment upstream (ori 1) and downstream (ori 2) of the BglII site of plasmid pBOT1 (33). Δscm1, 0.9-kbp internal fragment of the scm1 gene obtained by PCR with primer pair CJ6-CJ17; tetM, tetracycline resistance gene of Tn916. The black regions represent pGEM-T Easy vector sequences flanking the scm1 gene fragment. Bg, BglII; E, EcoRI; P, PstI; wt. DNA, wild-type DNA. The maps are not to scale. The size of pCJ32 is 11.55 kbp. (A) Integration into the ori 1 region; (B) integration into the ori 2 region.
Transformation of S. citri with plasmids pKTM1 and pCJ32.
Electrotransformation of S. citri GII-3 with different amounts (1 to 30 μg) of the suicide plasmid pKTM1 repeatedly yielded no tetracycline-resistant transformants, indicating that in these experiments, integration of the plasmid into the host chromosome by a single crossover did not occur or occurred with a very low frequency, less than 10−9 transformants/CFU. Therefore, to enhance the opportunity for recombination between the plasmid and the chromosome, the scm1 gene fragment was introduced into spiroplasma cells by transformation with the replicative plasmid pCJ32. Transformation with this plasmid yielded spiroplasmal transformants with a transformation efficiency of 5 × 103 to 104 transformants/μg of plasmid DNA, corresponding to a frequency of 5 × 10−6 to 10−5 transformants/CFU. All of these tetracycline-resistant colonies showed a motile phenotype similar to that of the cells transformed with the scm1-free pBOT1 vector.
Behavior of plasmid pCJ32 during propagation of spiroplasmal transformants.
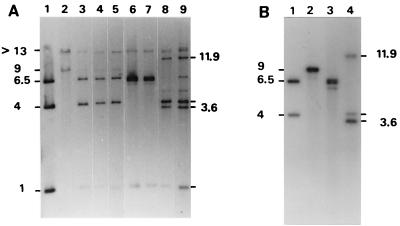
Thirty-one spiroplasmal transformants were grown in liquid SP4 medium containing tetracycline, and the presence of the pCJ32 plasmid in these transformants was demonstrated by PCR amplification with primer pair Tet1-Tet2 and Southern blot hybridization of total DNA with the pCJ32 probe (data not shown). To determine whether the plasmid was maintained extrachromosomally as a free plasmid or could be integrated into the spiroplasmal chromosome, the spiroplasmal transformants carrying pCJ32 were subcultured for 15 successive propagations in liquid medium, corresponding to approximately 50 generations. During in vitro propagations, the behavior of the plasmid was monitored by Southern blot hybridization of genomic DNA at passages 5, 10, and 15. At passage 5, all 31 spiroplasmal clones tested were found to contain pCJ32 as a free, extrachromosomal DNA. In the experiment represented in Fig. 1A, total DNA from spiroplasmal transformants was restricted by EcoRI and hybridized with the pCJ32 probe. All three EcoRI fragments of the purified pCJ32, with sizes of 1, 4, and 6.5 kbp (lane 1), were detected in the spiroplasmal transformants (clones 3, 11, and 30 [lanes 3, 4, and 5, respectively]). Two additional fragments larger than 13 and 9 kbp were faintly detected. These two fragments were identical to those in the wild-type strain (lane 2), which were previously shown to carry the scm1 gene and the oriC region, respectively (19). The detection of these two fragments suggested that no DNA insertion or recombination had occurred in these regions. At passage 10, some clones (8 of 31) still contained the free pCJ32 plasmid, but most of them presented new hybridization profiles (lanes 6 to 9). In clones 10 and 12 (lanes 8 and 9, respectively), the DNA band corresponding to the 6.5-kbp EcoRI fragment of the free plasmid (lane 1) was very faintly detected, whereas two additional fragments of 3.6 and 11.9 kbp were clearly seen. In clones 18 and 26 (lanes 6 and 7, respectively), the 4-kbp fragment of the free plasmid was no longer detected; instead, an additional fragment of approximately 6.5 kbp was found to be present. Interestingly, in all of clones 10, 12, 18, and 26, the 9-kbp chromosomal DNA fragment containing the oriC region was not detected, suggesting that in these clones, integration of the pCJ32 plasmid may have occurred in this region. Indeed, hybridization of genomic DNA with an oriC probe revealed that in clones 10, 12, 18, and 26, the pCJ32 plasmid had integrated into the spiroplasmal chromosome by homologous recombination at the oriC region, upstream of the BglII site (ori 1) in clones 18 and 26 and downstream of this site (ori 2) in clones 10 and 12. The results obtained with clones 10 and 18 are presented in Fig. 1B (lanes 3 and 4) and 2. In clone 18 (Fig. 1B, lane 3), the 9-kbp EcoRI fragment carrying the oriC region in the wild-type strain (lane 2) was not detected. Instead, the presence of three fragments of 6.2, 6.45, and 6.5 kbp hybridizing with the oriC probe indicates that plasmid integration did occur by recombination at the oriC region, upstream of the BglII site (Fig. 2A). In clone 10 (Fig. 1B, lane 4), the detection of three fragments of 3.6, 4, and 11.9 kbp indicates that in this clone also, plasmid integration occurred within the oriC region, downstream of the BglII site (Fig. 2B). These results were confirmed by additional Southern blot hybridizations of NsiI-plus-PstI-restricted DNA (data not shown).
FIG. 1.
Southern blot hybridization between EcoRI-restricted DNA extracted from S. citri transformants at various passages and the pCJ32 (A) and oriC (B) probes. (A) Lane 1, pCJ32 restricted by EcoRI; lane 2, DNA extracted from untransformed cells; lanes 3 to 5, DNA extracted at passage 5 from spiroplasmal clones 3, 11, and 30, respectively; lanes 6 to 9, DNA extracted at passage 10 from clones 18, 26, 10, and 12, respectively. (B) Lane 1, pCJ32 restricted by EcoRI; lane 2, DNA extracted from untransformed cells; lanes 3 and 4, DNA extracted at passage 15 from clones 18 and 10, respectively. Sizes are indicated in kilobase pairs.
Five spiroplasmal transformants (clones 11, 23, 24, 28, and 29) that still carried the pCJ32 plasmid as free extrachromosomal DNA at passage 10 were further subcultured for five additional passages and then tested by PCR for putative integration of the plasmid by recombination at the scm1 gene. According to the results in Fig. 3, PCR amplification of genomic DNA from spiroplasmal transformants with primer pairs CJ26-EV7 and Reverse-CJ5 would be obtained only if the pCJ32 plasmid had integrated into the scm1 gene, not if the plasmid was maintained extrachromosomally or had integrated into the oriC region. In addition, as a result of plasmid integration, the occurrence of two incomplete copies of the scm1 gene should lead to a nonmotile phenotype. The results presented in Fig. 4 show that no amplification was obtained with DNA from untransformed GII-3 cells (lanes 2 and 9) or with DNA from spiroplasmal clones 10 (lanes 4 and 11), 23 (lanes 5 and 12), 24 (lanes 6 and 13), and 28 and 29 (data not shown). In contrast, the expected amplification products of 1.4 kbp (1,372 bp) for primer pair CJ26-EV7 and 1.4 kbp (1,399 bp) for primer pair Reverse-CJ5 were obtained in the case of clone 11 (lanes 3 and 10). Therefore, a broth culture of this transformant was diluted and plated onto 0.8% agar SP4 plates to determine its motility behavior. Unexpectedly, most of the colonies harbored a motile phenotype similar to that of the wild-type strain GII-3, while only a few colonies (approximately 5%) showed a nonmotile phenotype. These observations indicated that at this stage, the culture was a mixture of spiroplasma cells carrying pCJ32 as a free plasmid (95%) and cells in which pCJ32 had integrated into the chromosome by recombination at the scm1 gene (5%). One of the nonmotile colonies was grown in liquid SP4, and the resulting culture was triply cloned (38) before being further analyzed by PCR and Southern blot hybridization. When plated on agar medium, the triply cloned culture yielded 100% colonies having a nonmotile phenotype. The appearance of these colonies (Fig. 5B) was identical to that of the insertional mutant G540 in which the scm1 gene was disrupted by Tn4001 insertion (19) (Fig. 5C). Dark-field microscopy observation of broth cultures revealed that this clone, like the insertional mutant G540, had retained its helical morphology but, due to the absence of rotational movement, was unable of translational motility in viscous medium. This spiroplasmal clone was named GII-3 m1.
FIG. 3.
Schematic representation of pCJ32 integration by recombination at the scm1 gene. The positions of primers (notation on figure in parentheses) Reverse (R), CJ5 (5), CJ6 (6), CJ8 (8), CJ17 (17), CJ26 (26), CJ27 (27), EV7 (ev7), Tet1 (tet1), and Tet2 (tet2) are indicated by short arrows. N, NsiI. For other abbreviations, see legend to Fig. 2. The maps are not to scale.
FIG. 4.
PCR amplification of genomic DNA from various spiroplasmal transformants with primer pairs CJ26-EV7 (lanes 1 to 6) and Reverse-CJ5 (lanes 8 to 13). Lane 7, 1-kbp DNA ladder; lanes 1 and 8, control without DNA. In lanes 2 to 6 and 8 to 13, target DNAs were from spiroplasmal clones 10, 11, 23, 24, and untransformed GII-3, respectively. Sizes are indicated in kilobase pairs.
FIG. 5.
Colonies of S. citri grown in 0.8% agar SP4 medium for 8 days. (A) Wild-type GII-3; (B) GII-3 m1; (C) Tn4001 insertional mutant G540.
Mapping the pCJ32 insertion site in spiroplasmal transformant GII-3 m1.
To further confirm that pCJ32 had integrated into the spiroplasmal chromosome by homologous recombination at the scm1 gene, genomic DNA from GII-3 m1 was restricted with various enzymes and hybridized with the scm1 probe. In Fig. 6, the hybridization patterns of GII-3 m1 DNA are compared to those of the DNA extracted from untransformed cells. Regardless of the enzymes used to restrict the DNA (EcoRI, HindIII, NsiI, or SpeI), two fragments were found to hybridize with the scm1 probe in the case of GII-3 m1 (Fig. 6, lanes 2, 4, 6, 9, 12, and 15), whereas only one fragment was found to contain scm1 sequences in the untransformed cells (lanes 1, 3, 5, 8, 11, 14, and 17). Such a duplication of the scm1 sequences is in agreement with the integration of pCJ32 at the scm1 gene via a single crossover. In addition, sizes of the restriction fragments hybridizing with the probe fully match those of the map in Fig. 3, predicted on the basis of recombination between the scm1 gene fragment carried by the plasmid and the full-length scm1 gene carried by the chromosome. In particular, the two EcoRI fragments larger than 12 and 4.1 kbp (Fig. 6, lane 12) were detected neither in the purified plasmid (lane 13) nor in the untransformed cells (lane 11). Also, as expected from the restriction map of Fig. 3, the 4.1-kbp EcoRI fragment (Fig. 6, lane 12) was also found in the EcoRI-plus-NsiI double digest of GII-3 m1 DNA (Fig. 6, lane 9). To further characterize the site of plasmid integration in GII-3 m1, the two regions containing scm1 sequences were amplified by PCR with primer pairs CJ26-EV7 and Reverse-CJ5, and the amplification products were cloned and sequenced. Sequence analyses showed that the CJ26-EV7-amplified DNA fragment contained the scm1 N-terminal sequences (upstream of CJ17) that are not present in pCJ32 and did not contain the C-terminal end (downstream of CJ6). In turn, the Reverse-CJ5 fragment was found to contain the C-terminal end of the scm1 gene but not the N terminus. The occurrence of these two truncated copies of the scm1 gene in the chromosomal DNA of GII-3 m1 indicates that in this transformant, the pCJ32 plasmid has integrated into the spiroplasmal chromosome by homologous recombination at the scm1 gene, via a single crossover. To assess the stability of plasmid integration in vitro, the scm1-disrupted mutant was propagated in liquid medium with or without tetracycline for more than 100 generations and then plated on 0.8% agar plates. No reversion to the motile phenotype was noted, regardless of the presence or absence of tetracycline as the selection pressure. Of the more than 1,000 colonies observed, none had spiroplasmas with a motile phenotype.
FIG. 6.
Southern blot hybridization of genomic DNA from S. citri GII-3 and GII-3 m1 with the scm1 probe. DNAs from GII-3 (lanes 1, 3, 5, 8, 11, 14, and 17) and GII-3 m1 (lanes 2, 4, 6, 9, 12, 15, and 18) were restricted by HindIII (lanes 1 and 2), HindIII plus NsiI (lanes 3 and 4), NsiI (lanes 5 and 6), NsiI plus EcoRI (lanes 8 and 9), EcoRI (lanes 11 and 12), SpeI (lanes 14 and 15), and EcoRV (lanes 17 and 18). Plasmid pCJ32 (lanes 7, 10, and 13) was restricted by NsiI (lane 7), NsiI plus EcoRI (lane 10), and EcoRI (lane 13). Sizes are indicated in kilobase pairs.
Experimental transmission of GII-3 m1 to periwinkle plants.
The motility mutant GII-3 m1 was tested for its ability to be transmitted by the leafhopper vector and to induce symptoms in the host plant. A culture of GII-3 m1 was microinjected into 50 leafhoppers as described in Materials and Methods, and the injected insects were caged on five separate periwinkle plants (10 insects per plant). After a 2-week period, the insects were removed and tested for transmission through a Parafilm membrane. Plant symptoms were monitored for 6 weeks after insect removal. Multiplication of S. citri in the injected leafhoppers and in the plants was followed by determining the number of CFU per insect or per milligram of midribs. For each experiment, wild-type S. citri GII-3 and S. citri-free SP4 medium were used as positive and negative controls, respectively. Spiroplasmal transformant clone 18, in which the pCJ32 plasmid has integrated into the oriC region, was also tested. The results are summarized in Table 1. As expected, in the case of the wild-type strain GII-3 as well as with clone 18, severe symptoms were produced within 2 weeks after insect removal; in agreement with these results, transmission assays through Parafilm membranes were all positive. Interestingly, similar results were obtained with the scm1-disrupted mutant GII-3 m1. Four of five periwinkle plants (P1, P2, P4, and P5) showed symptoms characteristic of the disease; only one (P3) was symptomless. This absence of symptoms could be explained by the fact that plant P3 was uninfected; most (7 of 10) of the insects fed on this plant died 1 day after injection. Also, Table 1 shows that the three surviving leafhoppers failed to transmit spiroplasmas through the Parafilm membrane, suggesting that these three insects were probably poorly infected. Despite this particular case, the results clearly show that the motility mutant GII-3 m1 was transmitted by the leafhopper vector to the other four periwinkle plants, in which it induced symptoms within 2 weeks after transmission. Determinations of CFU showed that in the injected leafhoppers, the motility mutant GII-3 m1 multiplied to approximately the same titer (1.4 × 106 CFU/insect) as did the wild-type strain GII-3 (1.5 × 106 CFU/insect) and clone 18 (1.7 × 106 CFU/insect). In the plants, spiroplasma titers in the symptomatic leaves were also found to have similar values: 5.7 × 103, 9.1 × 103, and 6.8 × 103 CFU/mg of midribs for GII-3 m1, GII-3, and clone 18, respectively. Symptoms in these plants (asymmetric and chlorotic young leaves, chlorosis on older leaves, stunting, and eventually lethal yellowing) were identical to those shown by plants infected with clone 18 or with the wild-type strain GII-3. To further confirm that symptom expression was due to the multiplication of mutant GII-3 m1, spiroplasmas were cultured from GII-3 m1-infected plants and dilutions of the culture were plated on 0.8% agar medium. All colonies expressed a nonmotile phenotype, indicating that no contaminant or no revertant spiroplasma was present. In addition, Southern blot hybridization of genomic DNA with the scm1 probe proved the gene organization around the scm1 gene to be identical to that of the injected spiroplasma (data not shown).
TABLE 1.
Experimental transmission of S. citri GII-3, GII-3 m1, and GII-3 ori (clone 18) to periwinkle (Catharanthus roseus) by the leafhopper vector C. haematoceps
| Inoculant | Plant | Living insects at indicated day after injectiona
|
Transmission through Parafilm membranes | Symptoms | ||
|---|---|---|---|---|---|---|
| 1 | 15 | |||||
| S. citri GII-3 | P1 | 8 | 8 | + | + | |
| P2 | 8 | 8 | + | + | ||
| P3 | 8 | 7 | + | + | ||
| P4 | 9 | 9 | + | + | ||
| P5 | 5 | 4 | + | + | ||
| SP4 medium | P1 | 7 | 4 | − | − | |
| P2 | 4 | 4 | − | − | ||
| GII-3 m1 | P1 | 9 | 5 | + | + | |
| P2 | 9 | 5 | + | + | ||
| P3 | 3 | 3 | − | − | ||
| P4 | 7 | 4 | + | + | ||
| P5 | 8 | 4 | + | + | ||
| GII-3 ori (clone 18) | P1 | 9 | 6 | + | + | |
| P2 | 9 | 8 | + | + | ||
| P3 | 10 | 6 | + | + | ||
| P4 | 9 | 6 | + | + | ||
| P5 | 9 | 8 | + | + | ||
All plants were initially submitted to infection by 10 injected insects.
DISCUSSION
In this study, a motility mutant of S. citri was generated by disruption of the scm1 gene through homologous recombination. Production of mutants by gene disruption or allelic exchange, both of which are dependent on homologous recombination, is crucial to assess the role of individual genes in various processes such as pathogenesis. It has been extensively used in many bacteria, including the mollicutes A. laidlawii (11) and M. genitalium (10). However, the homologous recombination process usually requires the RecA protein (22, 28), which is probably absent from S. citri (26, 27). Therefore, in the absence of a functional RecA protein, the opportunity for homologous recombination was expected to be severely limited when nonreplicating DNA was used. Indeed, when spiroplasma cells were transformed with the suicide plasmid pKTM1, no tetracycline-resistant transformants were obtained, indicating that no recombination had occurred between the plasmid carrying the scm1 gene fragment and the spiroplasmal chromosome. Similarly, it was shown that the recA mutant strain 8195 of A. laidlawii could not be transformed with the nonreplicative plasmid pKA8195, whereas the wild-type strain JA1 could be (11). In S. citri, plasmid integration by homologous recombination at the scm1 gene could be obtained only when spiroplasma cells were transformed with the replicative plasmid pCJ32. This plasmid construct was based on the oriC plasmid pBOT1, which was shown to replicate in S. citri as a free plasmid before it integrates into the spiroplasmal chromosome by recombination at the oriC region (33). Plasmid integration was thought to be due to the incompatibility of oriC plasmids. It is known that in gram-positive bacteria, the presence of plasmids containing dnaA boxes strongly inhibits bacterial growth. As a result, one copy of oriC plasmid can barely coexist with the chromosome; the plasmid either is lost or integrates into the bacterial chromosome (29). Because general recombination is most efficient when it operates on long stretches of highly homologous DNA, inserting the scm1 gene fragment within the oriC region was expected to decrease the frequency of recombination in this region. In spite of this cloning strategy, the results showed that most of the recombination events leading to pCJ32 integration still occurred at the oriC region, not only within the 1.3-kbp ori 1 but also within the 0.6-kbp ori 2 fragment (Fig. 2), the size of which was still much greater than the minimal extent of homology (estimated to 70 bp) necessary to promote homologous recombination in Bacillus subtilis (20). Interestingly, in transformant GII-3 m1, plasmid integration did not occur at the oriC region but instead occurred within the scm1 gene, leading to the structure expected for the accurate product of homologous recombination between the scm1 gene fragment carried by pCJ32 and the full-length scm1 gene of the spiroplasmal chromosome. Indeed, disruption of the scm1 gene led to a nonmotile phenotype, indicating that neither of the two truncated copies of the scm1 gene, resulting from plasmid integration, was translated to a functional polypeptide. The phenotype of the scm1-disrupted mutant was identical to that of the scm1 mutant G540 obtained by Tn4001 mutagenesis (19). Due to the absence of rotational movement, these two mutants are incapable of translational motility in viscous medium and form compact, sharp-edged colonies, even when plated in low-agar medium.
This study and others from our laboratory (17) demonstrate gene disruption through homologous recombination in S. citri. These results highlight the great potential of replicative oriC plasmids to promote homologous recombination in S. citri, an organism which probably lacks the RecA protein. The use of replicative oriC plasmids increases the time over which recombination can occur (compared to nonreplicative plasmids); in addition, the incompatibility of oriC plasmids is used as a selection pressure for plasmid integration. However, our results showed that in most of the transformants, plasmid integration occurred at the oriC region rather than at the scm1 gene. In other words, recombination at oriC was much more frequent than recombination at the scm1 gene. In the plasmid vector pBOT1, the oriC fragment comprises the dnaA gene flanked by two dnaA box regions. However, we have shown that the dnaA gene and the dnaA box region upstream of it are not required for plasmid replication, suggesting that in this plasmid, the replication origin could be reduced to the dnaA box region located downstream of the dnaA gene (33). Assuming that recombination frequency is, in part, a function of the length of homologous sequences, the use of oriC plasmids with a replication origin reduced to the shortest fragment still containing the dnaA boxes should decrease the frequency of plasmid integration at the oriC region and, in turn, increase the frequency of recombination within the gene of interest. In addition, it would be useful to study systematically the effect of length of homology on recombination in S. citri and determine whether the frequency of recombination is gene dependent. Whether allelic exchange via a double crossover could be obtained by using extremely long regions of homology also must be determined. For most bacteria, an understanding of gene function is dependent on the availability of relevant mutants. The ability to construct site-specific mutations in S. citri will provide novel insights into the biology of this plant-pathogenic mollicute.
In many pathogenic bacteria, motility and chemotaxis are major factors of virulence, allowing the bacterial cells to reach the site of infection and invade the host tissues (30, 39). In this respect, it was hypothesized that the motility of S. citri might play a role in the spiroplasma-host interactions. In particular, it was proposed that motility, by facilitating dispersal in the host, would be one of the pathogenicity factors developed by spiroplasmas (21). To test this hypothesis, the scm1-disrupted motility mutant was experimentally transmitted to periwinkle plants through leafhopper vectors injected with the mutant. We found that the motility mutant multiplied in the insects and was efficiently transmitted to periwinkles, in which it induced symptoms similar to those obtained with control spiroplasma strains: the wild-type strain GII-3 and clone 18 in which pCJ32 has integrated into the oriC region. These results suggest that S. citri motility, and particularly the rotational movement responsible for translational motility in viscous medium that is lost in the scm1 mutant, is not required for the spiroplasma to reach and invade the salivary glands. In addition, the occurrence of severe symptoms in the GII-3 m1-infected periwinkles suggests that the scm1 gene product, and hence motility, is not essential for pathogenicity. This is in good agreement with the fact that the nonhelical, nonmotile S. citri ASP1 was isolated from a symptomatic sweet orange tree and found to cause symptoms when experimentally transmitted to broad bean seedlings (37). It is noteworthy that no motile revertants were isolated, either from GII-3 m1-injected insects or from GII-3 m1-infected periwinkles, suggesting that motility might not be an advantage for S. citri propagation, not only in vitro but also in the insect vector and in the host plant. Furthermore, both in insects and in plants, the titer of GII-3 m1 was found to be not significantly lower than the titers of the motile, control strains. However, the possibility that the motility behavior of the scm1-disrupted mutant in its hosts might be different from that observed in vitro cannot be excluded. According to the life cycle of S. citri, spiroplasmas ingested from plant phloem face two barriers in the insect vector, the gut epithelium and the salivary gland membrane (13, 23). Comparison of transmissible and nontransmissible lines of S. citri BR3 led to the characterization of a gene encoding an adhesin-like protein. However, the involvement of this protein in spiroplasma-insect vector interactions remains to be demonstrated (46). In our studies, transmission assays in which spiroplasmas were injected directly into the insect hemolymph showed that the scm1-disrupted mutant was not affected in its ability to cross the salivary gland membrane to reach the saliva duct. Whether this mutant is able to cross the gut epithelium barrier when the leafhopper vector is fed on scm1 mutant-infected plants is currently under investigation.
ACKNOWLEDGMENTS
We are grateful to our colleagues J. M. Bové for critical reading of the manuscript and P. Gaurivaud for helpful discussions. We also thank P. Bonnet and J. B. Reynaud for growing plants and insects.
This work was financially supported in part by an AIP Microbiologie grant from INRA.
REFERENCES
- 1.Bové J M. Molecular features of mollicutes. Clin Infect Dis. 1993;17(Suppl. 1):S10–S31. doi: 10.1093/clinids/17.supplement_1.s10. [DOI] [PubMed] [Google Scholar]
- 2.Bové J M. Spiroplasmas: infectious agents of plants, arthropods, and vertebrates. Wien Klin Wochenschr. 1997;109:604–612. [PubMed] [Google Scholar]
- 3.Bové J M, Carle P, Garnier M, Laigret F, Renaudin J, Saillard C. Molecular and cellular biology of spiroplasmas. In: Whitcomb R F, Tully J G, editors. The mycoplasmas. V. New York, N.Y: Academic Press, Inc.; 1989. pp. 243–364. [Google Scholar]
- 4.Burdett V. Nucleotide sequence of the tet(M) gene of Tn916. Nucleic Acids Res. 1982;18:6137. doi: 10.1093/nar/18.20.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne M E, Rouch D A, Skurray R A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamycin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989;81:361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- 6.Cao J, Kapke P A, Minion F C. Transformation of Mycoplasma gallisepticum with Tn916, Tn4001, and integrative plasmid vectors. J Bacteriol. 1994;176:4459–4462. doi: 10.1128/jb.176.14.4459-4462.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole R M, Tully J G, Popkin T G, Bové J M. Morphology, ultrastructure, and bacteriophage infection of the helical mycoplasma-like organism (Spiroplasma citri gen. nov., sp. nov.) cultured from stubborn disease of citrus. J Bacteriol. 1973;115:367–386. doi: 10.1128/jb.115.1.367-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels M J, Longland J M, Gilbart J. Aspects of motility and chemotaxis in spiroplasmas. J Gen Microbiol. 1980;118:429–436. [Google Scholar]
- 9.Davis R E, Worley J F. Spiroplasma: motile, helical microorganism associated with corn stunt disease. Phytopathology. 1973;63:403–408. [Google Scholar]
- 10.Dhandayuthapani S, Rasmussen W G, Baseman J B. Disruption of gene mg218 of Mycoplasma genitalium through homologous recombination leads to an adherence-deficient phenotype. Proc Natl Acad Sci USA. 1999;96:5227–5232. doi: 10.1073/pnas.96.9.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dybvig K, Woodward A. Construction of recA mutants of Acholeplasma laidlawii by insertional inactivation with a homologous DNA fragment. Plasmid. 1992;28:262–266. doi: 10.1016/0147-619x(92)90058-i. [DOI] [PubMed] [Google Scholar]
- 12.Dybvig K, Voelker L L. Molecular biology of mycoplasmas. Annu Rev Microbiol. 1996;50:25–57. doi: 10.1146/annurev.micro.50.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher J, Wayadande A, Melcher U, Ye F. The phytopathogenic mollicute-insect vector interface: a closer look. Phytopathology. 1998;88:1351–1358. doi: 10.1094/PHYTO.1998.88.12.1351. [DOI] [PubMed] [Google Scholar]
- 14.Foissac X, Danet J L, Saillard C, Whitcomb R F, Bové J M. Experimental infection of plants by spiroplasmas. In: Razin S, Tully J G, editors. Molecular and diagnostic procedures in mycoplasmology. Vol. 2. New York, N.Y: Academic Press, Inc.; 1995. pp. 385–389. [Google Scholar]
- 15.Foissac X, Saillard C, Bové J M. Random insertion of Tn4001 in the genome of Spiroplasma citri strain GII3. Plasmid. 1997;37:80–86. doi: 10.1006/plas.1996.1271. [DOI] [PubMed] [Google Scholar]
- 16.Foissac X, Saillard C, Danet J L, Gaurivaud P, Paré C, Laigret F, Bové J M. Mutagenesis by insertion of transposon Tn4001 into the genome of Spiroplasma citri: characterization of mutants affected in plant pathogenicity and transmission to the plant by the leafhopper vector Circulifer haematoceps. Mol Plant-Microbe Interact. 1997;10:454–461. [Google Scholar]
- 17.Gaurivaud, P., J. L. Danet, F. Laigret, and J. M. Bové. Submitted for publication.
- 18.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 19.Jacob C, Nouzières F, Duret S, Bové J M, Renaudin J. Isolation, characterization, and complementation of a motility mutant of Spiroplasma citri. J Bacteriol. 1997;179:4802–4810. doi: 10.1128/jb.179.15.4802-4810.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khasanov F K, Zvingila D J, Zainullin A A, Prozorov A A, Bashkirov V I. Homologous recombination between plasmid and chromosomal DNA in Bacillus subtilis requires approximately 70 bp of homology. Mol Gen Genet. 1992;234:494–497. doi: 10.1007/BF00538711. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff H. Motility. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 289–306. [Google Scholar]
- 22.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H Y, Gumpf D J, Oldfield G N, Calavan E C. The relationship of Spiroplasma citri and Circulifer tenellus. Phytopathology. 1983;73:585–590. [Google Scholar]
- 24.Mahairas G G, Jian C, Minion F C. Transformation of Mycoplasma pulmonis: demonstration of homologous recombination, introduction of cloned genes, and preliminary description of an integrating shuttle system. Author's correction. J Bacteriol. 1993;175:3692. doi: 10.1128/jb.171.4.1775-1780.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahairas G G, Minion F C. Transformation of Mycoplasma pulmonis: demonstration of homologous recombination, introduction of cloned genes, and preliminary description of an integrating shuttle system. J Bacteriol. 1989;171:1775–1780. doi: 10.1128/jb.171.4.1775-1780.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marais A, Bové J M, Renaudin J. Spiroplasma citri virus SpV1-derived cloning vector: deletion formation by illegitimate and homologous recombination in a spiroplasmal host strain which probably lacks a functional recA gene. J Bacteriol. 1996;178:862–870. doi: 10.1128/jb.178.3.862-870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marais A, Bové J M, Renaudin J. Characterization of the recA gene regions of Spiroplasma citri and Spiroplasma melliferum. J Bacteriol. 1996;178:7003–7009. doi: 10.1128/jb.178.23.7003-7009.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller R V, Kokjohn T A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- 29.Ogasawara N, Moriya S, Yoshikawa H. Initiation of chromosome replication: structure and function of oriC and DnaA protein in eubacteria. Res Microbiol. 1991;142:851–859. doi: 10.1016/0923-2508(91)90065-i. [DOI] [PubMed] [Google Scholar]
- 30.Ottemann K M, Miller J F. Roles for motility in bacterial-host interactions. Mol Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- 31.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Rev. 1999;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renaudin J, Bové J M. Plasmid and viral vectors for gene cloning and expression in Spiroplasma citri. In: Razin S, Tully J G, editors. Molecular and diagnostic procedures in mycoplasmology. San Diego, Calif: Academic Press; 1995. pp. 167–178. [Google Scholar]
- 33.Renaudin J, Marais A, Verdin E, Duret S, Foissac X, Laigret F, Bové J M. Integrative and free Spiroplasma citri oriC plasmids: expression of the Spiroplasma phoeniceum spiralin in Spiroplasma citri. J Bacteriol. 1995;177:2800–2877. doi: 10.1128/jb.177.10.2870-2877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saglio P, Lhospital M, Laflèche D, Dupont G, Bové J M, Tully J G, Freundt E A. Spiroplasma citri gen. and sp. n.: a mycoplasma-like organism associated with “stubborn” disease of citrus. Int J Syst Bacteriol. 1973;23:191–204. [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 36.Stamburski C, Renaudin J, Bové J M. First step toward a virus-derived vector for gene cloning and expression in spiroplasmas, organisms which read UGA as a tryptophan codon: synthesis of chloramphenicol acetyltransferase in Spiroplasma citri. J Bacteriol. 1991;173:2225–2230. doi: 10.1128/jb.173.7.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsend R, Markham P G, Plaskitt K A, Daniels M J. Isolation and characterization of a non-helical strain of Spiroplasma citri. J Gen Microbiol. 1977;100:15–21. [Google Scholar]
- 38.Tully J G. Cloning and filtration techniques for mycoplasmas. Methods Mycoplasmol. 1983;1:173–177. [Google Scholar]
- 39.Vande Broek A, Vanderleyden J. The role of bacterial motility, chemotaxis, and attachment in bacteria-plant interactions. Mol Plant-Microbe Interact. 1995;8:800–810. [Google Scholar]
- 40.Vignault J C, Bové J M, Saillard C, Vogel R, Faro A, Venegas L, Stemmer W, Aoki S, McCoy R E, Al-beldawi A S, Larue M, Tuzcu O, Ozsan M, Nhami A, Abassi M, Bonfils J, Moutous G, Fos A, Poutiers F, Viennot-Bourgin G. Mise en culture de spiroplasmes à partir de matériel végétal et d'insectes provenant de pays circum méditerranéens et du Proche Orient. C R Acad Sci Ser III. 1980;290:775–780. [Google Scholar]
- 41.Weisburg W G, Tully J G, Rose D L, Petzel J P, Oyaizu H, Yang D, Mandelco L, Sechrest J, Lawrence T G, Van Etten J, Maniloff J, Woese C R. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989;171:6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitcomb R F. Culture media for spiroplasmas. Methods Mycoplasmol. 1983;1:147–159. [Google Scholar]
- 43.Williamson D L, Renaudin J, Bové J M. Nucleotide sequence of the Spiroplasma citri fibril protein gene. J Bacteriol. 1991;173:4353–4362. doi: 10.1128/jb.173.14.4353-4362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williamson D L, Whitcomb R F, Tully J G, Gasparich G E, Rose D L, Carle P, Bové J M, Hackett K J, Adams J R, Henegar R B, Konai M, Chastel C, French F E. Revised group classification of the genus Spiroplasma. Int J Syst Bacteriol. 1998;48:1–12. doi: 10.1099/00207713-48-1-1. [DOI] [PubMed] [Google Scholar]
- 45.Ye F, Renaudin J, Bové J M, Laigret F. Cloning and sequencing of the replication origin (oriC) of the Spiroplasma citri chromosome and construction of autonomously replicating artificial plasmids. Curr Microbiol. 1994;29:23–29. doi: 10.1007/BF01570187. [DOI] [PubMed] [Google Scholar]
- 46.Ye F, Melcher U, Fletcher J. Molecular characterization of a gene encoding a membrane protein of Spiroplasma citri. Gene. 1997;189:95–100. doi: 10.1016/s0378-1119(96)00840-2. [DOI] [PubMed] [Google Scholar]