Dear Editor,
We read with great interest the article reported treating the patients diagnosed with COVID-19 by tixagevimab-cilgavimab.1 With the evolution of the SARS-CoV-2 and the continued increase in cases and transmission efficiency due to mutations, additional drug treatment is necessary for patients with high-risk diseases in addition to the vaccine of COVID-19 through convalescent plasma. The development of monoclonal antibodies represents a new option with potential therapeutic and prophylactic applications.
Tixagevimab-cilgavimab is one type of long-acting monoclonal antibody combination produced from B cells donated by patients recovered from COVID-19, which is given via intramuscular injection to prevent and treat COVID-19. Tixagevimab-cilgavimab can bind to several different sites on the SARS-CoV-2 spike protein and may help overcome immune escape and maintain susceptibility to SARS-CoV-2 variants.2
In December 2021, tixagevimab-cilgavimab received emergency use authorization from the United States FDA for preventing COVID-19 in currently uninfected adults and children, and was subsequently approved in countries including Canada, United Kingdom, et al. In February 2022, the United States FDA increased the drug injection dose from 150mg to 300mg due to the in vitro neutralization effect of tixagevimab-cilgavimab on the omicron variant. At present, there are several studies reporting the impact of tixagevimab-cilgavima applying on patient clinical outcomes, the conclusions of which are not consistent, however. Hence, we performed a meta-analysis to evaluate the therapeutic effect of tixagevimab/cilgavimab in treating COVID-19 patients.
Articles were searched in the electronic libraries, databases, and websites including (PubMed, Cochrane Library databases, Google Scholar, and medRxiv) from December 1, 2019 to July 30, 2022. The retrieve strategy consisted of combination of following terms, without language, year, and publication restrictions: ("SARS-CoV-2 or "covid-19" or "nCoV-2019" or "novel corona virus" or "2019-nCoV") and ("tixagevimab cilgavimab" or "tixagevimab" or "cilgavimab" or "AZD7442").
The inclusion and exclusion criterion of this meta-analysis were as follows: Studies compared of clinical outcomes between tixagevimab-cilgavimab and control (placebo) treated groups were included. Original studies associated with subsequent reviews, letters, editorials, conference abstracts, case reports, and duplicate publications were excluded. Information on studies and participants' baseline characteristics was also extracted,including the first author's name, year of publication, study design, country of origin, number of participants, mortality of patients, side effects and the number of cases diagnosed COVID-19.
The statistical analysis of this study was conducted using RevMan (version 5.4, (Cochran Collaboration, University of Oxford) and Stata 16.0. Dichotomous variables were analyzed using odds ratios (ORs) with 95% (95% CI) intervals of evidence. Heterogeneity was assessed using Cochran's Q test and i2 statistic. The P value < 0.05 was considered statistically significant.
After a literature search, five studies were included, including 14489 adult COVID-19 patients, with 7295 in the tixagevimab-cilgavimab treated group and 7194 in control group3, 4, 5, 6, 7. Changes in patients’ population and study characteristics included in this study are shown in Table 1 . Two studies were from the United States, and the remaining were multicenter studies from several countries. Three studies were randomized controlled trials and two studies were retrospective cohort study. In the included studies, intramuscular drug therapy was used. The five eligible studies were published in 2022 and patient sample sizes of which ranged from 903 to 5197.
Table 1.
The basic information of the included literature.
| Study | Population | Study type | Country | Intervention | All person(No.) | Intervention(No.) | Control(No.) |
|---|---|---|---|---|---|---|---|
| Holland 2022 | ≥18 years | Randomized controlled trial | United States | Tixagevimab/cilgavimab vs Placebo | 1417 | 710 | 707 |
| Xu 2022 | ≥18 years | cohort | United States | Tixagevimab/cilgavimab | 1848 | 1848 | cohort |
| Levin A 2022 | ≥18 years | Randomized controlled trial | multicenter study | Tixagevimab/cilgavimab vs Placebo | 5197 | 3460 | 1737 |
| Montgomery 2022 | ≥18 years | Randomized controlled trial | multicenter study | Tixagevimab/cilgavimab vs Placebo | 903 | 452 | 451 |
| Kertes 2022 | ≥12 years | cohort | Israel | Tixagevimab/cilgavimab | 5124 | 825 | 4299 |
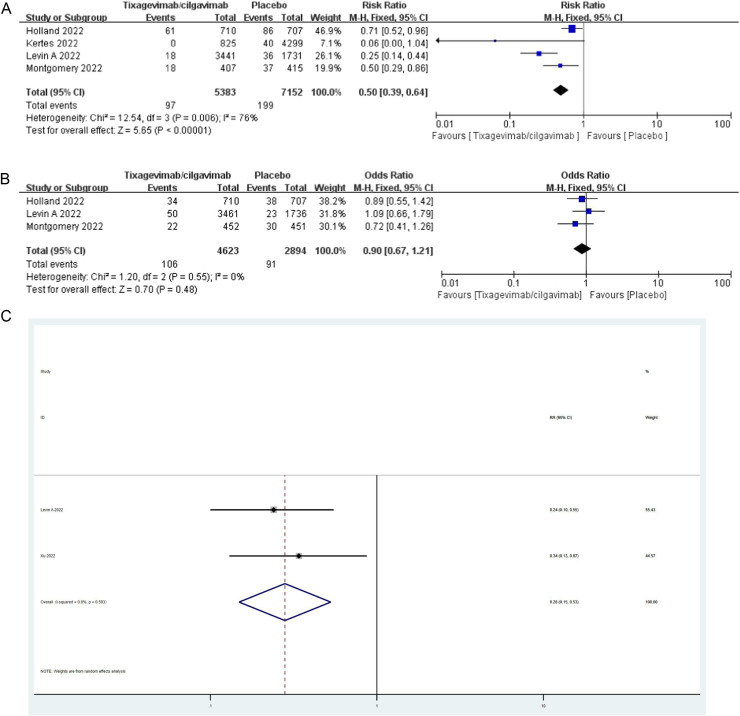
Our meta-analysis showed that the overall mortality in the tixagevimab-cilgavimab treated group was significantly lower than that in the control group (RR=0.50,95%CI: 0.39, 0.64, P<0.01; I2=76%)(Fig. 1A ). Furthermore, tixagevimab-cilgavimab treatment was not associated with the development of serious adevents in patients (OR=0.90,95%CI: 0.67, 1.21, P=0.48; I2=0%)(Fig. 1B). In addition, the protection against COVID-19 was significantly improved in the tixagevimab-cilgavimab group compared with the control group(RR=0.28,95%CI: 0.15, 0.53, P<0.01; I2=0%) (Fig. 1C). Hence,the results provide the evidence that treatment with tixagevimab-cilgavimab in COVID-19 patients had a significant benefit in terms of mortality and SARS-COVID-2 infection.
Fig.1. A.
Association between tixagevimab-cilgavimab treatment and mortality.
B Association between tixagevimab-cilgavimab treatment and serious adevents.
C Association between tixagevimab-cilgavimab treatment and COVID-19 infection.
In addition, one study data from the AstraZeneca showed a protective effect against symptomatic COVID-19 infection in RT-PCR-negative patients treated with tixagevimab/cilgavimab compared with placebo-treated control recipients (RR 73%; 95% CI 27-90), which is also consistent with our findings.2Furthermore, it deserves our attention that several studies conclusions were drawn in settings where the major proportion of omicron mutations was present. In the context of the reduced neutralizing activity of several Monoclonal antibodies due to the omicron mutations, tixagevimab-cilgavimab may still serve as an effective and targeted therapeutic strategy.1
Our study has several limitations to be aware of. The number of articles included in this study is five, which is small for a meta-analysis. There was also a significant heterogeneity (I2>50%) in mortality and serious side effects. In addition, there may be differences in the treatment regimens and vaccination status of the patient populations in these studies. Despite these limitations, our study provides considerable value as the first meta-analysis to investigate the effects of tixagevimab-cilgavimab therapy in COVID-19 infected patients.
In conclusion, the use of tixagevimab-cilgavimab to treat COVID-19 patients has significant benefits in terms of protection against COVID-19 infection and mortality, while having no effect on the occurrence of serious side effects in patients. Further studies are required to confirm these findings.
Funding information
None declared.
Declaration of Competing Interest
The authors declare that they have no competing interest
Acknowledgements
None
References
- 1.Vellas C, Kamar N, Izopet J. Resistance mutations in SARS-CoV-2 omicron variant after tixagevimab-cilgavimab treatment. J Infect. 2022 Jul 22 doi: 10.1016/j.jinf.2022.07.014. S0163-4453(22)00422-4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keam SJ. Tixagevimab + cilgavimab: first approval. Drugs. 2022 Jun 21:1–10. doi: 10.1007/s40265-022-01731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yinong Young-Xu, Lauren Epstein, Vincent C Marconi, et al. Tixagevimab/Cilgavimab for prevention of COVID-19 during the omicron surge: retrospective analysis of national VA electronic data medRxiv2022.05.28.22275716; doi:https://doi.org/10.1101/2022.05.28.22275716 [DOI] [PMC free article] [PubMed]
- 4.Holland Thomas L, Ginde Adit A, Paredes Roger, et al. Tixagevimab/Cilgavimab for treatment of hospitalised COVID-19 patients: a randomised, double-blind, Phase 3 Trial. Available at SSRN: https://doi.org/10.2139/ssrn.4087355 [DOI] [PMC free article] [PubMed]
- 5.Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022 Jun 7 doi: 10.1016/S2213-2600(22)00180-1. S2213-2600(22)00180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin MJ, Ustianowski A, De Wit S, et al. PROVENT study group. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022 Jun 9;386(23):2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kertes Jennifer, David Shirley Shapiro Ben, Engel-Zohar Noya, et al. Association between AZD7442 (tixagevimab-cilgavimab) administration and SARS-CoV-2 infection, hospitalization and mortality. Clin Infect Dis. 2022:ciac625. doi: 10.1093/cid/ciac625. [DOI] [PMC free article] [PubMed] [Google Scholar]