Abstract
Voltage-gated calcium (CaV) channels form three subfamilies (CaV1–3). The CaV1 and CaV2 channels are heteromeric, consisting of an α1 pore-forming subunit, associated with auxiliary CaVβ and α2δ subunits. The α2δ subunits are encoded in mammals by four genes, CACNA2D1–4. They play important roles in trafficking and function of the CaV channel complexes. Here we report biallelic variants in CACNA2D1, encoding the α2δ-1 protein, in two unrelated individuals showing a developmental and epileptic encephalopathy. Patient 1 has a homozygous frameshift variant c.818_821dup/p.(Ser275Asnfs*13) resulting in nonsense-mediated mRNA decay of the CACNA2D1 transcripts, and absence of α2δ-1 protein detected in patient-derived fibroblasts. Patient 2 is compound heterozygous for an early frameshift variant c.13_23dup/p.(Leu9Alafs*5), highly probably representing a null allele and a missense variant c.626G>A/p.(Gly209Asp). Our functional studies show that this amino-acid change severely impairs the function of α2δ-1 as a calcium channel subunit, with strongly reduced trafficking of α2δ-1G209D to the cell surface and a complete inability of α2δ-1G209D to increase the trafficking and function of CaV2 channels. Thus, biallelic loss-of-function variants in CACNA2D1 underlie the severe neurodevelopmental disorder in these two patients. Our results demonstrate the critical importance and non-interchangeability of α2δ-1 and other α2δ proteins for normal human neuronal development.
Keywords: epileptic encephalopathy, calcium channel, loss-of-function, biallelic variants, CACNA2D1
Dahimene et al. report biallelic pathogenic variants in CACNA2D1, encoding a calcium channel subunit α2δ-1, in two unrelated individuals with developmental epileptic encephalopathy. One has a homozygous frameshift variant; the other is compound heterozygous for a frameshift and a missense variant rendering α2δ-1 non-functional.
Introduction
Voltage-gated calcium (CaV) channels are present in all excitable cells including neurons, and open following membrane depolarization, allowing Ca2+ entry.1 The α1 pore-forming subunits are encoded by a family of 10 mammalian genes, divided into three subfamilies (CaV1–3).1 In neurons, CaV2 channels are mainly presynaptic and involved in synaptic transmission.2
The CaV1 and CaV2 subfamilies are associated with auxiliary CaVβ and α2δ subunits.2 The α2δ proteins are encoded by four mammalian genes, CACNA2D1–4,2 encoding an α2δ pre-protein that is post-translationally cleaved into two polypeptides, α2 and δ.3 These extracellular glycoproteins remain disulphide-bonded together, linked into the plasma membrane by a glycosylphosphatidyl-inositol anchor.4 Human α2δ-1 pre-protein (P54289, UniProt) has 1103 amino acids. In conjunction with β, the α2δ subunits play important roles in trafficking and function of CaV1 and CaV2 channels.5
Evidence that α2δ proteins are involved in neurological disease has been reviewed recently.6 Initial evidence was for α2δ-2, and came from the spontaneous Cacna2d2 mouse mutant strains including ducky,7 which demonstrate absence epilepsy and severe cerebellar ataxia when both alleles are mutated. This reflects the strong expression of α2δ-2 in particular neurons, specifically cerebellar Purkinje cells.7 In humans, biallelic CACNA2D2 variants cause a phenotypic spectrum ranging from congenital ataxia with cerebellar vermian atrophy on brain imaging8 to cerebellar atrophy and developmental and epileptic encephalopathies (DEEs).9,10 DEEs are characterized by intractable seizures and developmental impairment or regression.11
Here we report two unrelated patients with biallelic CACNA2D1 variants, one with a homozygous frameshift variant and the other with compound heterozygosity for an early frameshift and a missense variant. Both individuals show a highly consistent phenotype, corresponding to DEE. We demonstrate that the homozygous frameshift variant causes α2δ-1 loss in patient fibroblasts, and investigate the effect of the α2δ-1 amino-acid change p.(Gly209Asp) on calcium channel function. Our data indicate that biallelic loss-of-function variants in CACNA2D1 underlie this DEE.
Materials and methods
Patients
Informed consent for genetic analyses was obtained for the two patients. Genetic studies were performed clinically or as approved by the Institutional Review Boards of the relevant institutions (Ethics Committee, Hamburg Medical Chamber; PV3802). The patients’ parents provided written informed consent for study participation, clinical data and specimen collection, genetic analysis and publication of relevant findings.
Exome sequencing, analysis and variant validation
Genomic DNA was extracted from peripheral blood samples using standard procedures. We performed trio exome sequencing with DNA samples of Patient 1 and both healthy parents (Supplementary material). Primer sequences are in Supplementary Table 1. For Patient 2, trio exome sequencing was undertaken with DNA samples of the proband and both healthy parents at Baylor Genetics (Supplementary material).
RNA isolation and transcript analysis
RNA isolation from fibroblasts, complementary DNA synthesis, polymerase chain reaction (PCR) and Sanger sequencing of amplicons to analyse CACNA2D1 transcripts were performed as described.12 Quantitative PCR to determine the relative mRNA levels of CACNA2D1 and CACNA2D3 was performed as described.12 Primer sequences are in Supplementary Table 1.
Bioinformatic analysis of CACNA2D1 homologues
Details of bioinformatic analysis are described in the Supplementary material.
Cell culture and antibodies
Primary fibroblasts, tsA-201 cells and hippocampal neurons were cultured as described (Supplementary material). The antibodies used are described in Supplementary material.
Immunoblot analysis of fibroblasts
Whole-cell lysates from patient and control fibroblasts were prepared, and immunoblotting performed as described12 (Supplementary material).
Expression constructs, mutagenesis and cell transfection
Details of expression constructs, transfection markers and procedures are detailed in Supplementary material. The tsA-201 cells were transfected with PolyJet or Fugene6 (as stated) according to manufacturers’ protocols. Hippocampal neurons were transfected with Lipofectamine 2000 (Life Technologies).
Electrophysiology
CaV2.1 and CaV2.2 currents in transfected tsA-201 cells were investigated by whole-cell patch-clamp recording (Supplementary material).
Cell surface biotinylation, co-immunoprecipitation and immunocytochemistry
Cell surface biotinylation, co-immunoprecipitation, immunoblotting and immunocytochemistry was performed as described in Kadurin et al.13 (Supplementary material).
Data analysis and availability
Data analysis and availability are described in the Supplementary material.
Results
Biallelic variants in CACNA2D1 in two unrelated individuals with DEE
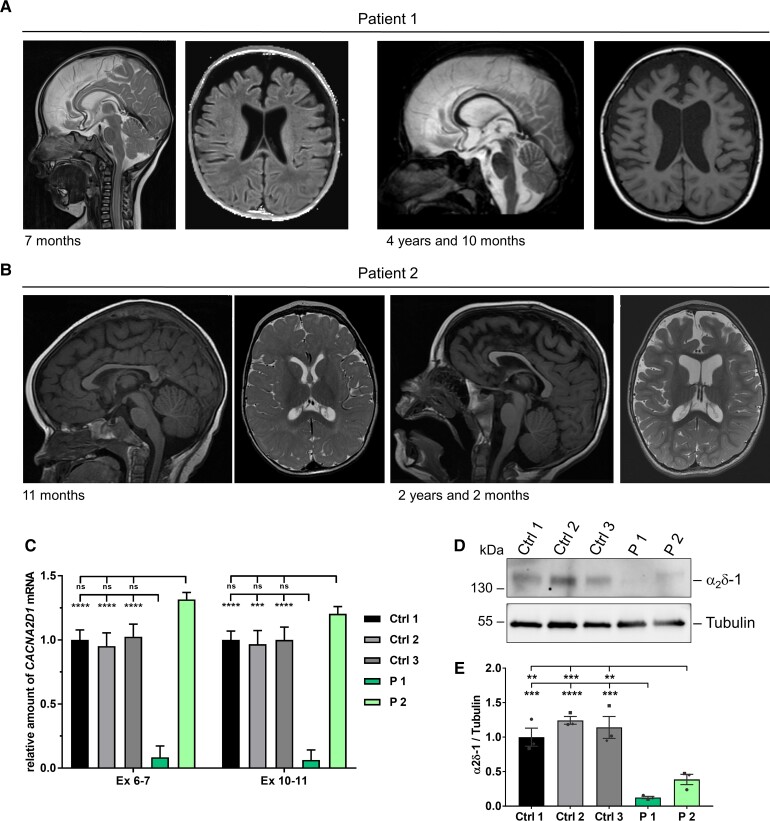
Through GeneMatcher,14 we identified the two unrelated male individuals, Patients 1 and 2, with a highly consistent phenotype corresponding to DEE. The two affected individuals carried biallelic pathogenic variants in CACNA2D1 (Tables 1 and 2). Patient 1 developed generalized seizures at age 19 months, while onset of epilepsy was at age 11.5 months in Patient 2. At last examination, Patient 1 was 4 years 11 months and Patient 2 was 4 years old. Both were microcephalic, had severe hypotonia, absent speech, spasticity, choreiform movements, orofacial dyskinesia and cortical visual impairment (Tables 1 and 2, and case reports in the Supplementary material). Brain imaging revealed corpus callosum hypoplasia and progressive volume loss in both (Fig. 1A and B). Patient 1 had no cardiac anomalies, while a tiny patent foramen ovale was found in Patient 2 (Tables 1 and 2).
Table 1.
Family history, growth parameters and manifestation of first symptoms in patients with biallelic CACNA2D1 variants
| Patient 1 | Patient 2 | |
|---|---|---|
| Ethnicity | Afghan | Caucasian, Native American |
| Parental consanguinity | First cousins | No |
| Family history | Epileptic encephalopathy in paternal uncle | No |
| Sex | Male | Male |
| CACNA2D1 variants (NM_000722.3) | c.818_821dup/p.(Ser275Asnfs*13) homozygous | c.13_23dup/p.(Leu9Alafs*5) and c.626G>A/p.(Gly209Asp) compound heterozygous |
| Pregnancy | Uncomplicated | Uncomplicated |
| Birth at | 37 weeks, uncomplicated | 40 weeks |
| Birth weight, g/z-score | 2960/−0.5 | 3345/−0.73 |
| Birth length, cm/z-score | 50/−0.2 | Unknown |
| OFC at birth, cm/z-score | 33/−1.0 | Unknown |
| Age at last examination | 4 years 11 months | 4 years |
| Weight at last examination, kg/z-score | 18/−0.4 | 15.5/−0.52 |
| Length at last examination, cm/z-score | 110/−0.2 | 97.8/−0.68 |
| OFC at last examination, cm/z-score | 48.9/−2.1 | 47.5/−2.02 |
| Manifestation | ||
| First symptoms at age of | 3 months | <2 months |
| First clinical signs | Severe hypotonia with poor head control, no visual attention | Hypotonia with poor head control, decreased visual attention |
Table 2.
Neurological and other findings in patients with biallelic CACNA2D1 variants
| Neurological signs | ||
|---|---|---|
| Global developmental delay | Profound | Profound |
| Motor skills | No achievement of motor milestones | Rolls to one side, reaches for toys intermittently |
| Muscular hypotonia | Severe axial, insufficient head control | Markedly low axial tone, appendicular tone is also low, increased with activation |
| Spasticity | In all extremities, starting at 2 years | Hands often fisted since birth, spastic catch at elbows and knees more prominent over time, back arching episodes beginning <12 months |
| Dystonic movements | Choreiform movements of upper extremities, orofacial dyskinesia, onset <2 years | Distal choreiform movements of all extremities, orofacial dyskinesia and generalized dystonic episodes, onset <10 months |
| Intellectual disability | Profound | Profound |
| Speech impairment | Profound | Profound |
| Behaviour | No concerns | No concerns |
| Cerebral MRI | Hypoplastic corpus callosum, enlarged inner and outer CSF spaces at age 7 months; progressive frontotemporal and mesial temporal atrophy at 4 years 10 months | Generalized volume loss, borderline thinning of the corpus callosum at 11 months; progression of generalized volume loss at 26 months |
| Hearing | Normal, not tested | Normal |
| Eyes | Cortical visual impairment, nystagmus | Cortical visual impairment, intermittent disconjugate gaze |
| Seizures | ||
| Age of onset | 9 months: absences; 19 months: generalized seizures | 11 months: right face/arm twitching, abnormal EEG |
| Initial seizure type | Absences, generalized | Focal with impaired awareness (hemi-clonic), atypical absence |
| Current seizure type | Absences | Focal with impaired awareness (hemi-clonic), 3 years 9 months: ESES |
| Response to treatment | Well (generalized seizures), poor (absences) | Well controlled on Depakene |
| EEG | Normal at age 7 months | 11 months: diffusely slow, no anterior posterior frequency amplitude gradient, slow spike and wave in sleep, multifocal spikes. Focal motor seizure captured, some irregular generalized spikes with possible eye blinking and unresponsive. 3 years 9 months: mild diffuse slowing, occasional multifocal sharps and 2–3 Hz spike and wave during wakefulness, near continuous slow spike and wave during sleep meeting criteria for ESES |
| Feeding | ||
| Feeding difficulties | G tube placement at 4 years | Since birth, s/p G tube placement at 13 months |
| Cardiac features | ||
| Echocardiography | Normal | Limited study with tiny patent foramen ovale, normal function |
| Electrocardiography | Normal | Normal, including 24 h patch recording |
| Other findings | ||
| Facial dysmorphism | Bitemporal narrowing, large ears, flared medial eyebrows, open mouth with tented upper lip | Microcephalic with posterior plagiocephaly, mild bitemporal narrowing, ears are low set and appear larger relative to head size, mild ptosis bilaterally, mouth held hanging open, high arched palate, teeth are widely spaced and blunted due to frequent bruxism, small hands and feet |
| Sleep disturbance | Yes | Obstructive sleep apnoea |
| Insensibility to pain | Yes | Yes |
ESES = electrical status epilepticus in sleep; s/p = status post.
Figure 1.
MRI scans of patients with biallelic variants in CACNA2D1 and determination of CACNA2D1 mRNA and α2δ-1 protein levels in patient-derived fibroblasts. (A) In Patient 1, T2-weighted sagittal MRI shows hypoplastic corpus callosum and T1-weighted axial image shows enlarged ventricles and frontotemporal CSF spaces at the age of 7 months. At the age of 4 years and 10 months, progressive frontotemporal and mesial temporal atrophy was noted. (B) At the age of 11 months, T1-weighted sagittal and T2-weighted axial MRI images of Patient 2 show non-specific findings of delayed myelination within the frontal and parietal white matter for age and prominent perivascular spaces. At the age of 26 months, T1-weighted sagittal MRI shows interval generalized volume loss with extra-axial spaces and thinning of the corpus callosum. T2-weighted axial images show an increase in ventricular size in addition to increased intra-axial spaces. Permission to publish MRI scans was provided for the two patients shown here. (C) Relative quantification of CACNA2D1 transcripts by reverse transcription-qPCR (RT-qPCR) using two CACNA2D1-specific primer pairs generating amplicons for exons 6–7 and 10–11. RNA was obtained from fibroblasts of Patients 1 and 2 and three healthy individuals (Controls 1–3). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control; and the amount of target mRNA relative to GAPDH mRNA is presented. Mean ± standard error of the mean (SEM) of three independent experiments, each performed in triplicate, is shown. One-way ANOVA with Bonferroni post hoc test for multiple comparisons was used for statistical analysis: ns, P > 0.05; ***P = 0.0001; ****P < 0.0001. Datasets of independent RT-qPCR experiments with technical triplicates are shown in Supplementary Fig. 2. (D) Representative immunoblot of whole-cell lysates obtained from fibroblasts of Patients 1 and 2 and three controls. The amount of α2δ-1 was monitored with an anti-α2δ-1 antibody. An anti-tubulin antibody was used to demonstrate equal loading. A band corresponding to α2δ-1, which shows a low expression in fibroblasts, was observed in all control cells. Uncropped blots are shown in Supplementary Fig. 3. (E) Band intensities were quantified using the ChemiDoc imaging system. Mean ± SEM with individual data-points of three independent experiments is shown. One-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons was used for statistical analysis: **P < 0.0065; ***P < 0.0005; ****P < 0.0001. Ctrl = control; ex = exon; ns = not significant; P = patient.
Trio exome sequencing in Patient 1 and parents revealed in the proband the homozygous CACNA2D1 (NM_000722.3) frameshift variant c.818_821dupGAAC/p.(Ser275Asnfs*13). The variant is absent from public databases including gnomAD (v.2.1.1 and 3.1.1). The 4-bp duplication was validated in Patient 1’s DNA and fibroblast-derived complementary DNA. His healthy parents were heterozygous carriers (Tables 1 and 2 and Supplementary Fig. 1). In Patient 2, trio exome sequencing demonstrated compound heterozygosity for the CACNA2D1 variants c.13_23dupTGCCTGCTGGC [p.(Leu9Alafs*5)] and c.626G>A [p.(Gly209Asp)] (Tables 1 and 2 and Supplementary Fig. 1). His healthy mother was heterozygous for the c.13_23dupTGCCTGCTGGC variant and his healthy father for the c.626G>A variant. The 11-bp duplication is probably a loss-of-function variant; it has a worldwide minor allele frequency of 0.003% (gnomAD v.2.1.1), while the c.626G>A variant is absent in gnomAD (v.2.1.1 and 3.1.1). The missense variant c.626G>A/p.(Gly209Asp) in exon 7 is predicted to be damaging by in silico tools detailed in the Supplementary material.
In fibroblasts of Patient 1, CACNA2D1 mRNA level was reduced to 6–9% compared with control fibroblasts, while it was similar in Patient 2 and control fibroblasts (Fig. 1C and Supplementary Fig. 2). We next determined α2δ-1 levels in whole-cell lysates from cultured primary fibroblasts of Patients 1 and 2. Qualitatively, we detected little full-length α2δ-1 in Patient 1 fibroblasts, while it was present in Patient 2 and control cells (Fig. 1D and Supplementary Fig. 3). Quantification of α2δ-1 indicated 10–12% in Patient 1 and 31–38% in Patient 2 compared to controls (Fig. 1E). Together, these data indicate that Patient 1 carries biallelic CACNA2D1 loss-of-function alleles and Patient 2 harbours at least one CACNA2D1 loss-of-function variant.
Next, we investigated mRNA levels of the other CACNA2D genes in patient fibroblasts to identify possible compensatory effects. While mRNA levels of CACNA2D2 and CACNA2D4 were too low to be quantified, we detected 3- to 7-fold higher CACNA2D3 mRNA levels in Patient 2 fibroblasts compared to Patient 1 and control cells (Supplementary Fig. 4).
Glycine 209 is invariant in CACNA2D1
The α2δ-1 protein (also denoted as CACNA2D1) contains a von Willebrand factor-A domain and four Ca2+ channel and chemotaxis receptor (Cache) domains,15 organized into two double-Cache (dCache) domains.16 The p.(Gly209Asp) (G209D) amino-acid substitution in the CACNA2D1 gene product of Patient 2 is within the gabapentin and amino-acid binding pocket of its dCache_1 domain.16 This Gly residue is important for maintaining a three-strand beta-sheet stability and simultaneously providing a critical turn in the structure. G209 is absolutely invariant in both CACNA2D1 and CACNA2D2 orthologues in all vertebrates and paralogues and predecessors from low invertebrates (Supplementary Fig. 5).
The p.(Gly209Asp) variant disrupts plasma membrane α2δ-1 expression
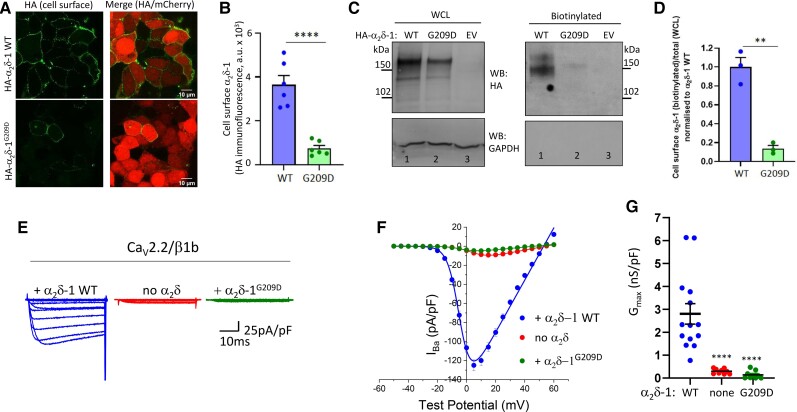
We then investigated the in vitro effect of the p.(Gly209Asp) variant on α2δ-1 as a calcium channel subunit. First, we compared cell surface expression of wild-type HA-α2δ-1 (HA-α2δ-1 WT) and HA-α2δ-1G20n9D in non-permeabilized cells.5 As shown by the haemagglutinin (HA) signal, the expression of HA-α2δ-1G209D at the cell surface was reduced by ∼80% compared to HA-α2δ-1 WT (Fig. 2A and B). In agreement, cell surface biotinylated HA-α2δ-1G209D was decreased, by 86.2%, compared to HA-α2δ-1 WT (Fig. 2C and D).
Figure 2.
The p.(Gly209Asp) variant disrupts α2δ-1 expression at the cell surface, and does not promote CaV2 calcium currents. (A) Representative confocal images of tsA-201 cells transfected with either HA-α2δ-1 wild-type (WT) or HA-α2δ-1G209D with mCherry. HA staining was performed in non-permeabilized conditions and shown in the left panels. The right panels represent the merged images with the 10 μm scale bar. (B) Bar charts (mean ± SEM with individual data-points, each representing mean of at least 60 cells) showing the expression at the cell surface (HA signal) of WT α2δ-1-HA (blue bar) and HA-α2δ-1G209D (green bar). Data obtained from six coverslips in two independent experiments, ****P < 0.0001, Student’s t-test. (C) Western blot experiments for HA-α2δ-1 (anti-HA Ab, top; molecular weight ∼140–170 kDa for the glycosylated uncleaved and proteolytically cleaved α2δ-1 proteins) and GAPDH (used as a control, bottom). Left panels show whole-cell lysate (WCL) input and right panels show cell surface biotinylated samples from tsA-201 cells transfected with HA-α2δ-1 WT (lane 1) or HA-α2δ-1G209D (lane 2) or empty vector (EV, lane 3). Western blots were performed under reducing conditions, such that the disulphide bonds between α2 and δ were broken. Uncropped blots are in Supplementary Fig. 10A. (D) Mean ± SEM and individual data-points of cell surface HA-α2δ-1 WT (blue bar) or HA-α2δ-1G209D (green bar) measured as a proportion of biotinylated over total protein normalized to HA-α2δ-1 WT. **P = 0.0012, Student’s t-test. (E) Example of whole-cell patch-clamp recordings for CaV2.2-HA co-expressed with β1b and either α2δ-1 WT (blue, left), empty vector (no α2δ, red, centre) or α2δ-1G209D (green, right). Holding potential −80 mV, steps between −50 and +60 mV for 50 ms (applies to all traces). (F) Mean (± SEM) IV relationships for the conditions shown in E. CaV2.2-HA co-expressed with α2δ-1 WT (n = 14, blue fileld circles), empty vector (no α2δ, n = 10, red filled circles) or α2δ-1G209D (n = 10, green filled circles). The individual and mean data were fit with a modified Boltzmann equation (see ‘Materials and methods’ section). (G) Gmax [nanosiemens (nS)/picofarad (pF)] from the IV relationships shown in F. Individual data (same symbols as in F) and mean ± SEM are plotted. ****P < 0.0001 versus wild-type (one-way ANOVA and Sidak’s post hoc test correcting for multiple comparisons).
The p.(Gly209Asp) variant abolishes ability of α2δ-1 to promote CaV2.2 currents
CaV2.2 currents were then measured in tsA-201 cells transfected with HA-tagged CaV2.2 with β1b and α2δ-1 wild-type, α2δ-1G209D or no α2δ. While α2δ-1 wild-type increased CaV2.2 currents by ∼13-fold, α2δ-1G209D produced no increase compared to without α2δ (Fig. 2E–G). Expression of all subunits was confirmed by western blotting and immunocytochemistry (Supplementary Fig. 6). This effect was not specific to CaV2.2, as α2δ-1G209D also did not increase CaV2.1 currents (Supplementary Fig. 7A–C).
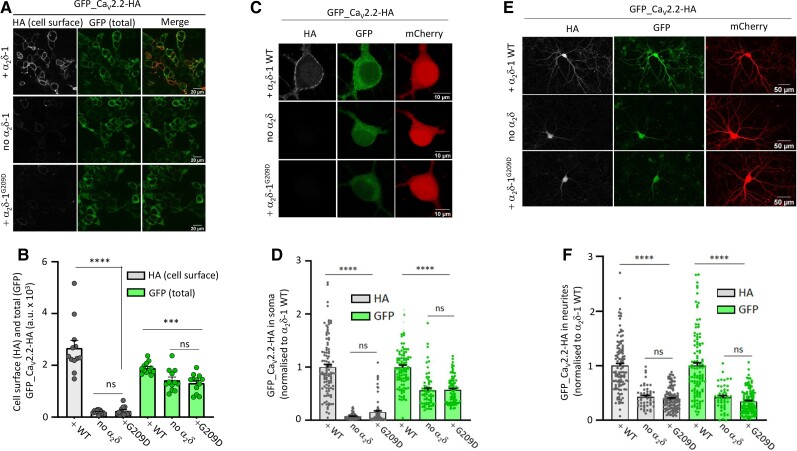
We next investigated expression of the calcium channel complex at the plasma membrane, using double-tagged GFP_CaV2.2-HA. When GFP_CaV2.2-HA was co-expressed with α2δ-1 wild-type, this resulted in an increase in its cell surface expression compared with no α2δ control (Fig. 3A and B). However, this effect was completely absent when CaV2.2 was co-expressed with α2δ-1G209D (Fig. 3A and B).
Figure 3.
α2δ-1G209D does not enhance CaV2.2 expression at the cell surface in tsA-201 cells and hippocampal neurons. (A) Representative confocal images of tsA-201 cells transfected with GFP_CaV2.2-HA with either α2δ-1 wild-type (WT) (without tag; top row), empty vector (no α2δ, middle row) or α2δ-1G209D (without tag; bottom row). HA staining was performed in non-permeabilized conditions (white, left), GFP signal shows total CaV2.2 (middle). The right panels represent the merged images (HA is shown in red). Scale bar = 20 μm. (B) Bar charts (mean ± SEM with individual data-points representing the mean of at least 50 cells from 12 coverslips) show CaV2.2 expressed at the cell surface (HA signal, grey bars) or total CaV2.2 (GFP, green bars) in the presence of α2δ-1 WT, empty vector (no α2δ-1) or α2δ-1G209D. Data obtained from four independent experiments; ns, P > 0.05; ***P = 0.0007, ****P < 0.0001, one-way ANOVA and Bonferroni post hoc test. (C) Representative confocal images of hippocampal somata imaged at ×63 objective and transfected with GFP_CaV2.2-HA and either α2δ-1 WT (top row), empty vector (no α2δ, middle row) or α2δ-1G209D (bottom row) together with β1b and mCherry. HA staining was performed in non-permeabilized conditions (grey, left), GFP signal (middle) and mCherry (transfection marker, right). Scale bar = 10 μm. (D) Bar charts (mean ± SEM with individual data-points) of HA (grey bars) and GFP (green bars) for α2δ-1 WT (n = 102), no α2δ (n = 63) and α2δ-1G209D (n = 82). Data obtained from three independent experiments; the values are normalized to α2δ-1 WT condition in each experiment. ns, P > 0.05; ****P < 0.0001, one-way ANOVA and Bonferroni post hoc test. (E) Representative confocal images of hippocampal neurons imaged at ×20 objective and transfected with GFP_CaV2.2-HA and either α2δ-1 WT (top row), empty vector (no α2δ, middle row) or α2δ-1G209D (bottom row) together with β1b and mCherry. HA staining was performed in non-permeabilized conditions (grey, left panels), GFP signal (middle) and mCherry (transfection marker, right). Scale bar = 50 μm. (F) Bar charts (mean ± SEM with individual data-points) of HA (grey bars) and GFP (green bars) for α2δ-1 WT (n = 114), no α2δ (n = 50) and α2δ-1G209D (n = 117). Data obtained from three independent experiments; the values are normalized to α2δ-1 WT condition in each experiment. ns, P > 0.05; ****P < 0.0001, one-way ANOVA and Bonferroni post hoc test. Note that for B, D and F, total CaV2.2 (GFP) was reduced in both the α2δ-1G209D and no α2δ conditions compared to α2δ-1 WT condition probably because of its increased degradation when CaV2.2 is poorly trafficked out of the endoplasmic reticulum.
α2δ-1G209D does not promote CaV2.2 cell surface expression or trafficking in hippocampal neurons
CaV2.2 is a neuronal calcium channel, and we therefore investigated the effect of α2δ-1G209D on CaV2.2 trafficking in neurons, as previously described.13 We first analysed cell surface expression of GFP_CaV2.2-HA in cultured hippocampal cell bodies. As expected, in the presence of α2δ-1 WT, GFP_CaV2.2-HA was strongly expressed at the cell surface (HA signal, Fig. 3C and D). In contrast, in the presence of α2δ-1G209D, GFP_CaV2.2-HA could not be detected at the cell surface, similar to no α2δ (Fig. 3C and D).
The neurites of these cells were then imaged. As expected, GFP_CaV2.2-HA showed strong expression when co-expressed with α2δ-1 WT; this was observed for both HA (cell surface) and green fluorescent protein (GFP) (total CaV2.2) (Fig. 3E and F). In contrast, α2δ-1G209D did not promote trafficking of CaV2.2 into hippocampal neurites (Fig. 3E and F). This is indicated by the finding that both HA (cell surface CaV2.2) and GFP (total CaV2.2) signals were reduced in parallel.
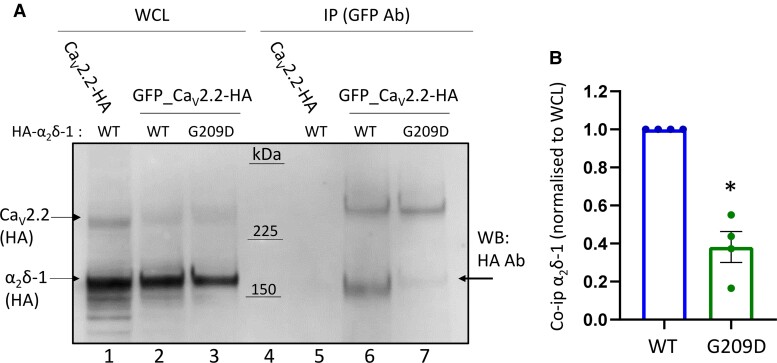
α2δ-1G209D shows reduced complex formation with CaV2.2 and limited proteolytic cleavage
To examine whether the lack of ability of α2δ-1G209D to promote calcium channel function was due to reduced interaction with CaV2.2, we performed co-immunoprecipitation experiments, using GFP_CaV2.2-HA (Fig. 4A). For α2δ-1 WT, robust interaction was shown by the presence of HA-α2δ-1 WT, co-immunoprecipitated by GFP_CaV2.2-HA, using anti-GFP antibody (Fig. 4A, lane 6). In contrast, very weak co-immunoprecipitation was observed for HA-α2δ-1G209D (Fig. 4A, lane 7), quantified in Fig. 4B. As a control, there was no co-immunoprecipitation of HA-α2δ-1 WT using CaV2.2-HA without a GFP tag (Fig. 4A, lane 5).
Figure 4.
α2δ-1G209D shows reduced interaction with CaV2.2. (A) GFP_CaV2.2-HA was co-expressed with β1b and either HA-α2δ-1 WT or HA-α2δ-1G209D. Both CaV2.2 and α2δ-1 proteins were detected in the same western blot using anti-HA antibodies. Immunoblots of whole-cell lysates (WCL) input (left) and immunoprecipitated (IP using GFP antibodies) samples (right) from tsA-201 cells transfected with CaV2.2-HA, β1b and HA-α2δ-1 WT (lanes 1 and 5) or GFP-CaV2.2-HA with either HA-α2δ-1 WT (lanes 2 and 6) or HA-α2δ-1G209D (lanes 3 and 7). Immunoblots with anti-HA antibody reveal CaV2.2-HA and GFP_CaV2.2-HA (top bands) and HA-α2δ-1 WT and HA-α2δ-1G209D (lower bands). Immunoprecipitation was performed with anti-GFP antibody to pull down GFP-CaV2.2-HA and the co-immunoprecipitated α2δ-1 is shown below this on the same blot. CaV2.2-HA lacking GFP tag, used as a negative control, shows a lack of co-immunoprecipitation (co-IP) with HA-α2δ-1 WT (lane 5). Arrow (lane 7) indicates increased molecular weight of α2δ-1G209D relative to α2δ-1 WT. Western blots were performed under reducing conditions, such that the disulphide bonds between α2 and δ were broken. Uncropped blots are in Supplementary Fig. 10B. (B) Mean ± SEM and individual data-points for co-immunoprecipitation of HA α2δ-1 WT (blue bar) or α2δ-1G209D (green bar) measured as a proportion of total protein (whole-cell lysates) and normalized to HA-α2δ-1 WT. *P = 0.0281 (ratio paired t-test on non-normalized data).
Interestingly, co-immunoprecipitated HA-α2δ-1G209D (Fig. 4A, lane 7, arrow) had a noticeably higher apparent molecular weight compared to HA-α2δ-1 WT (lane 6), and we found this was due to almost complete lack of proteolytic cleavage of HA-α2δ-1G209D into α2 and δ (Supplementary material and Supplementary Fig. 8).
In summary, these results show that α2δ-1G209D remains largely as the uncleaved immature form, indicating that it probably remains in the endoplasmic reticulum. In agreement with our previous results for uncleaved α2δ-1,17 it shows much less complex formation with CaV2.2. This result suggested that α2δ-1G209D would be unlikely to interfere with other α2δ proteins interacting with CaV2.2. In agreement with this, we found that α2δ-1G209D did not affect the ability of α2δ-3 to enhance CaV2.2 currents (Supplementary Fig. 9A–C). This result underscores that the p.(Gly209Asp) variant has a loss-of-function effect.
Discussion
In the current study, we show that biallelic loss-of-function variants in CACNA2D1 underlie DEE. In Patient 1 the homozygous frameshift variant p.(Ser275Asnfs*13) causes nonsense-mediated mRNA decay of mutated CACNA2D1 transcripts and absence of α2δ-1 in patient-derived fibroblasts. The variants p.(Leu9Alafs*5) and p.(Gly209Asp) in Patient 2 are a combination of a very early frameshift and a missense variant in trans, with the latter severely affecting CaV2 calcium channel function.
Our electrophysiological, biochemical and immunocytochemistry data show that α2δ-1G209D is completely non-functional, in that, unlike wild-type α2δ-1,5 it traffics extremely poorly to the cell surface, and does not enhance the function or trafficking of CaV2 channels in both non-neuronal cells and hippocampal neurons. Furthermore, α2δ-1G209D shows markedly reduced cleavage into α2 and δ, an enzymatic process that normally begins in the Golgi apparatus.13,17 This suggests that α2δ-1G209D does not traffic beyond the endoplasmic reticulum. Our previous finding that an uncleavable mutant α2δ-1 shows lower association with the CaV2.2 α1 subunit than the mature cleaved α2δ-1,17 indicates that the lack of proteolytic cleavage of α2δ-1G209D will probably contribute to the observed reduction in interaction of α2δ-1G209D with the CaV2.2 α1 subunit. This demonstrates the importance of a detailed understanding of α2δ-1 processing and function, in order to identify the basis for such deleterious variants.
Variants in CACNA2D1 have previously been associated with cardiac phenotypes in both humans and mice. Homozygous knockout of Cacna2d1 in mice resulted in a mild cardiac phenotype and reduced ventricular myocyte calcium current density.18 These mice also showed peripheral sensory deficits and delayed development of neuropathic pain-related responses.19 Relevant to this, both patients showed insensibility to pain (Table 2). Transgenic mice constitutively over-expressing α2δ-1 have no gross nervous system defects.20 However, they show spontaneous epileptiform EEG abnormalities and behavioural arrest,21 suggesting that not only the spatial and temporal expression but also the expression strength of α2δ-1 is critical for proper functioning of the mouse brain. Indeed, α2δ-1 is the major α2δ isoform in rodent cerebral cortex.22 Furthermore, an auto-antibody recognizing α2δ-1 is found in cases of autoimmune encephalitis23 and amyotrophic lateral sclerosis associated with type 2 diabetes.24
In humans, heterozygous variants in CACNA2D1 have previously been associated with inherited arrhythmogenic disease, including Brugada25 and short QT26 syndromes, as well as infantile spasms27 and intellectual disability and epilepsy.28 Re-evaluation of these monoallelic variants, together with genetic data presented here give rise to reasonable doubt about an association of these CACNA2D1 variants with disease (Supplementary material).
Pathogenic variants in genes encoding several CaV channels have been associated with neurological diseases in humans, ranging from early-onset severe spinocerebellar ataxia with neurodevelopmental deficits to DEE (see Supplementary material). In CACNA2D2, rare biallelic loss-of-function variation has been reported in individuals with DEE, corpus callosum hypoplasia, cerebellar atrophy and ataxia.9,10 The two unrelated patients reported here show considerable clinical overlap with individuals carrying homozygous CACNA2D2 loss-of-function variants, such as global developmental delay and/or intellectual disability, epilepsy and hypoplasia of the corpus callosum. However, atrophy of the brain affects the cerebrum in the two affected individuals with CACNA2D1 variants, whereas cerebellar atrophy was consistently reported in subjects with CACNA2D2 variants.29 These data indicate that loss of α2δ-1 or α2δ-2 cannot be compensated by any of the other α2δ subunits during development. In agreement with this, important, non-overlapping roles for specific α2δ proteins in synapse formation in vitro have been identified recently, some of which may be calcium channel-independent.30
In conclusion, our data demonstrate that biallelic loss-of-function variants in CACNA2D1 underlie early-onset DEE characterized by microcephaly, profound developmental delay, seizures, visual impairment, truncal hypotonia, limb spasticity and movement disorder. These clinical features are similar to those in previously reported individuals with homozygous CACNA2D2 null alleles. Individuals with biallelic CACNA2D1 or CACNA2D2 variants all have corpus callosum hypoplasia, while patients with CACNA2D1 variants show progressive cerebral atrophy whereas subjects harbouring CACNA2D2 variants have cerebellar atrophy. The loss-of-function frameshift nature of two of the three identified CACNA2D1 variants, together with our functional studies demonstrating a loss-of-function effect for the amino-acid substitution p.(Gly209Asp), confirm disease causation of homozygous and compound heterozygous CACNA2D1 variants, while also calling into question a causative role of monoallelic CACNA2D1 variants in intellectual disability, epilepsy and/or inherited arrhythmogenic diseases.
Supplementary Material
Acknowledgements
We thank the patient families for participation in this study. We further thank Dennis Zorndt and Kanchan Chaggar for skillful technical assistance.
Abbreviations
- DEE
developmental and epileptic encephalopathy
- GFP
green fluorescent protein
Contributor Information
Shehrazade Dahimene, Department of Neuroscience Physiology and Pharmacology, University College London (UCL), London WC1E 6BT, UK.
Leonie von Elsner, Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Tess Holling, Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Lauren S Mattas, Neurology and Neurological Sciences, Pediatrics, Division of Medical Genetics, Stanford University and Lucile Packard Children's Hospital, Palo Alto, CA 94304, USA.
Jess Pickard, Department of Neuroscience Physiology and Pharmacology, University College London (UCL), London WC1E 6BT, UK.
Davor Lessel, Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Kjara S Pilch, Department of Neuroscience Physiology and Pharmacology, University College London (UCL), London WC1E 6BT, UK.
Ivan Kadurin, Department of Neuroscience Physiology and Pharmacology, University College London (UCL), London WC1E 6BT, UK.
Wendy S Pratt, Department of Neuroscience Physiology and Pharmacology, University College London (UCL), London WC1E 6BT, UK.
Igor B Zhulin, Department of Microbiology and Translational Data Analytics Institute, The Ohio State University, Columbus, OH, 43210, USA.
Hongzheng Dai, Department of Molecular and Human Genetics, Baylor College of Medicine/NGS-Molecular, Baylor Genetics, Houston, TX, USA.
Maja Hempel, Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Maura R Z Ruzhnikov, Neurology and Neurological Sciences, Pediatrics, Division of Medical Genetics, Stanford University and Lucile Packard Children's Hospital, Palo Alto, CA 94304, USA.
Kerstin Kutsche, Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Annette C Dolphin, Department of Neuroscience Physiology and Pharmacology, University College London (UCL), London WC1E 6BT, UK.
Funding
This study was supported by grants from Wellcome Trust (grant no. 206279\Z\17\Z to A.C.D.), National Institutes of Health (1R35GM131760 to I.B.Z.) and Deutsche Forschungsgemeinschaft (KU 1240/6-2 and KU 1240/10-1 to K.K.)
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Catterall WA, Lenaeus MJ, Gamal El-Din TM. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu Rev Pharmacol Toxicol. 2020;60:133–154. [DOI] [PubMed] [Google Scholar]
- 2. Dolphin AC, Lee A. Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat Rev Neurosci. 2020;21:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- 4. Davies A, Kadurin I, Alvarez-Laviada A, et al. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci U S A. 2010;107:1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cassidy JS, Ferron L, Kadurin I, Pratt WS, Dolphin AC. Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary α2δ-1 subunits. Proc Natl Acad Sci USA. 2014;111:8979–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ablinger C, Geisler SM, Stanika RI, Klein CT, Obermair GJ. Neuronal α2δ proteins and brain disorders. Pflugers Arch. 2020;472:845–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barclay J, Balaguero N, Mione M, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valence S, Cochet E, Rougeot C, et al. Exome sequencing in congenital ataxia identifies two new candidate genes and highlights a pathophysiological link between some congenital ataxias and early infantile epileptic encephalopathies. Genet Med. 2019;21:553–563. [DOI] [PubMed] [Google Scholar]
- 9. Pippucci T, Parmeggiani A, Palombo F, et al. A novel null homozygous mutation confirms CACNA2D2 as a gene mutated in epileptic encephalopathy. PLoS ONE. 2013;8:e82154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edvardson S, Oz S, Abulhijaa FA, et al. Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. J Med Genet. 2013;50:118–123. [DOI] [PubMed] [Google Scholar]
- 11. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneeberger PE, von Elsner L, Barker EL, et al. Bi-allelic pathogenic variants in HS2ST1 cause a syndrome characterized by developmental delay and corpus callosum, skeletal, and renal abnormalities. Am J Hum Genet. 2020;107:1044–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kadurin I, Ferron L, Rothwell SW, et al. Proteolytic maturation of α2δ represents a checkpoint for activation and neuronal trafficking of latent calcium channels. eLife. 2016;5:e21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: A matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu J, Yan Z, Li Z, et al. Structure of the voltage-gated calcium channel Cav1.1 at 3.6 A resolution. Nature. 2016;537:191–196. [DOI] [PubMed] [Google Scholar]
- 16. Gumerov VM, Andrianova EP, Matilla MA, et al. Amino acid sensor conserved from bacteria to humans. Proc Natl Acad Sci U S A. 2022;119(10):e2110415119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferron L, Kadurin I, Dolphin AC. Proteolytic maturation of α2δ controls the probability of synaptic vesicular release. eLife. 2018;7:e37507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuller-Bicer GA, Varadi G, Koch SE, et al. Targeted disruption of the voltage-dependent calcium channel α2/δ-1-subunit. Am J Physiol Heart Circ Physiol. 2009;297:H117–H124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel R, Bauer CS, Nieto-Rostro M, et al. α2δ−1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J Neurosci. 2013;33:16412–16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li CY, Zhang XL, Matthews EA, et al. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faria LC, Gu F, Parada I, Barres B, Luo ZD, Prince DA. Epileptiform activity and behavioral arrests in mice overexpressing the calcium channel subunit α2δ−1. Neurobiol Dis. 2017;102:70–80. [DOI] [PubMed] [Google Scholar]
- 22. Schlick B, Flucher BE, Obermair GJ. Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience. 2010;167:786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee ST, Lee BJ, Bae JY, et al. CaV α2δ autoimmune encephalitis: A novel antibody and its characteristics. Ann Neurol. 2021;89:740–752. [DOI] [PubMed] [Google Scholar]
- 24. Shi Y, Park KS, Kim SH, et al. IgGs from patients with amyotrophic lateral sclerosis and diabetes target CaVα2δ1 subunits impairing islet cell function and survival. Proc Natl Acad Sci USA. 2019;116:26816–26822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burashnikov E, Pfeiffer R, Barajas-Martinez H, et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Templin C, Ghadri JR, Rougier JS, et al. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur Heart J. 2011;32:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hino-Fukuyo N, Kikuchi A, Arai-Ichinoi N, et al. Genomic analysis identifies candidate pathogenic variants in 9 of 18 patients with unexplained West syndrome. Hum Genet. 2015;134:649–658. [DOI] [PubMed] [Google Scholar]
- 28. Valentino F, Bruno LP, Doddato G, et al. Exome sequencing in 200 intellectual disability/autistic patients: New candidates and atypical presentations. Brain Sci. 2021;11:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Punetha J, Karaca E, Gezdirici A, et al. Biallelic CACNA2D2 variants in epileptic encephalopathy and cerebellar atrophy. Ann Clin Transl Neurol. 2019;6:1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schöpf CL, Ablinger C, Geisler SM, et al. Presynaptic α2δ subunits are key organizers of glutamatergic synapses. Proc Natl Acad Sci USA. 2021;118:e1920827118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.