Abstract
Glial cell activation is a hallmark of several neurodegenerative and neuroinflammatory diseases. During HIV infection, neuroinflammation is associated with cognitive impairment, even during sustained long-term suppressive antiretroviral therapy. However, the cellular subsets contributing to neuronal damage in the CNS during HIV infection remain unclear.
Using post-mortem brain samples from eight HIV patients and eight non-neurological disease controls, we identify a subset of CNS phagocytes highly enriched in LGALS3, CTSB, GPNMB and HLA-DR, a signature identified in the context of ageing and neurodegeneration.
In HIV patients, the presence of this phagocyte phenotype was associated with synaptic stripping, suggesting an involvement in the pathogenesis of HIV-associated neurocognitive disorder.
Taken together, our findings elucidate some of the molecular signatures adopted by CNS phagocytes in HIV-positive patients and contribute to the understanding of how HIV might pave the way to other forms of cognitive decline in ageing HIV patient populations.
Keywords: HIV, phagocytes, neuroinflammation, neurodegeneration, synapses
By comparing post-mortem brain samples of patients with HIV infection and non-neurological disease controls, Di Liberto et al. show that exacerbated synaptic elimination in HIV results from aberrant activated phagocytes. These abnormalities may drive the cognitive changes described in HIV-associated neurocognitive disorder.
Introduction
Neuronal injury and disruption of synaptic connectivity are common features of numerous neuro-inflammatory and neurodegenerative conditions often associated with cognitive decline.1 During viral infections of the CNS, both virus-induced cytopathic alterations and immune pathology contribute to neuronal damage.
In HIV infection, the virus can access the brain very early during primary infection2,3 via infected circulating monocytes that can cross the blood–brain barrier in response to chemotactic signals.4 Subsequently, the virus is further seeded to resident microglia and macrophages, collectively referred to as CNS phagocytes.5 Given their slow turnover rate (months to years) and self-renewal capacity,6 microglia can serve as viral reservoir and produce infectious virions,7 leading to a compartmentalized brain inflammation.8 Activated CD68+ phagocytes can frequently form glial nodules or even fuse giving rise to multinucleated giant cells that serve as neuropathological correlate of dementia9 especially in advanced form of disease.10 Perivascular and infiltrating CD8+ T cells actively monitor the CNS compartment and contribute to HIV control and clearance in the CNS; however, patients usually fail to control HIV infection because of virus mutational escape or T-cell exhaustion.11
In addition, viral replication in the CNS and HIV-RNA in the CSF can be detected even in non-viremic patients treated with combined antiretroviral therapy, a condition that can be associated with neurological impairment12,13 such as cognitive decline. While, the incidence of HIV-associated neurocognitive disorders (HAND) decreased in the era of combined antiretroviral therapy, the prevalence of patients with minor to moderate HAND remains high, even in well-treated patients.14,15 Indeed, virally suppressed HIV patients can show elevated levels of neopterin in the CSF, which is associated with phagocyte activation and cognitive decline,16 suggesting an involvement of CNS phagocytes in neuronal damage and degeneration.17
Synaptic degeneration represents an important histopathological hallmark of HAND.18–20 While persistent chronic inflammation is thought to contribute to cognitive decline, very little is known about the mechanistic underpinnings of CNS immune activation in the context of HAND. Given the ageing population of HIV-positive patients,21 deciphering the mechanisms that underpin such synaptic alterations in HIV is therefore critical for identifying new therapeutic targets to curb cognitive decline in HAND and other diseases.
During development and homeostasis, microglia play a crucial role in synaptic pruning by phagocytosis of aberrant synaptic connections, thereby shaping neural circuits.1 In contrast, aberrant phagocyte-mediated synaptic loss is associated with cognitive decline in both inflammatory disorders such as West Nile Virus infection,22 multiple sclerosis23 and neurodegenerative conditions such as Alzheimer’s disease24,25 and frontotemporal lobar degeneration.26
Transcriptional studies of neurodegenerative diseases conducted in murine models23,27,28 and human samples25,29–31 have started to elucidate the complex molecular landscape of an aberrant microglial response associated with neurodegeneration.
A variety of neurodegenerative diseases induce a common microglial signature characterized by upregulation of genes encoding for molecules involved in lysosomal function (CTSB, CTSD, CST7), surface receptors (TREM2, CLEC7A, ITGAX, AXL, GPNMB), secreted factors [secreted phosphoprotein 1 (SPP1), IL-1B, CCL2, C1Q] and lipid-metabolism (APOE, APOC1, LPL, CH25H) among others.27,28 This transcriptional programme eventually results in increased phagocytosis of endogenous materials (such as apoptotic cells, myelin, protein aggregates and synapses), enhanced immune vigilance and recruitment of other immune cells including peripheral monocytes to the CNS.
According to their initial description, these reactive phagocytes have been referred to as microglia in neurodegeneration (MGnD)28 or disease-associated microglia (DAM)27 and their response is mainly orchestrated by the upregulation of apolipoprotein E (APOE)/Triggering receptor expressed on myeloid cells 2 (TREM2) pathway and suppression of immune checkpoints restraining microglia to a homeostatic phenotype such as of TGF-β signalling. Despite a substantially conserved transcriptional signature across different neurodegenerative diseases, DAM and MgND phagocytes are, however, not considered as a panreactive phenotype. Instead, they display an arsenal of surface markers, cytokines and signalling pathways that are distinct from those expressed in neuroinflammatory conditions.32
Additionally, neurodegenerative phagocytes can give rise to a multitude of heterogeneous phenotypes that can be either protective or harmful depending on the specific disease context.33–36
These observations highlight the importance of deconvoluting the heterogeneity of microglia phenotypes to unravel their specific role in the pathogenesis of neurodegenerative diseases.
Indeed, complex diseases such as Neuro-HIV pose additional challenges given the coexistence of neurodegeneration, white matter alterations and chronic neuroinflammation.37
The switch from homeostatic to neurodegenerative microglia associated with the APOE/TREM2 signalling pathway has been proposed as a possible mechanism for neuronal toxicity during HIV infection.38 Indeed, elevated levels of soluble TREM2 in the CSF are a common feature of untreated HIV-1 infection. Those levels increase with CD4+ T-cell decline and peak in HIV-associated dementia.39 Beyond phagocytes, a reactive transcriptional program40 in disease-associated astrocytes (DAA) was described in murine models41 and human samples31 of Alzheimer’s disease, in which these cells can participate in neurodegeneration due to the expression of inflammatory and neurotoxic molecules.42
The role of neurodegenerative phagocytes in HIV-associated neuropathology has not been investigated so far.43 Here, we took advantage of brain samples from HIV-positive patients to investigate the presence and distribution of neurodegenerative phagocytes and their association with synaptic loss.
In HIV-positive patients, we observed that CNS phagocytes highly express molecules associated with neurodegeneration27,44 and are topographically distributed in inflammatory foci enriched in reactive astrocytes. In these brain samples, we noted that neurodegenerative phagocytes appose neurons and engulf synaptic material. This indicates that aberrant phagocyte activation may drive cognitive changes described in HAND. Finally, our findings suggest that HIV neuropathology and Alzheimer’s disease share a similar neurodegenerative signature associated with microglia activation and synaptic loss that could be explored for future therapeutic interventions.
Materials and methods
Human samples
All tissue samples were examined by at least two independent board-certified specialists in neuropathology who confirmed the diagnosis. Brain autopsies from HIV-positive patients (Neuro-HIV) [n = 8, four males and four females (41.4 ± 19.0 years, mean ± SD)], non-neurological disease (NND) [n = 8, four males and four females (40.4 ± 18.9 years, mean ± SD)] and systemic inflammation/sepsis [n = 4, two males and two females (72.0 ± 11.1 years, mean ± SD)] were obtained from the collection of the Department of Neuropathology of the University of Bern, and the Geneva University Hospitals. Their use for scientific purposes was in accordance with institutional ethical guidelines and was approved by the local ethics committee.
Within samples of a study group (Neuro-HIV, NND, systemic inflammation), no statistical differences between sex were observed regarding the obtained results. Available information about pharmacological treatment is indicated in Supplementary Table 1. Neuro-HIV and NND cases were matched for brain region, age and, when possible, also for sex.
Histology
CNS tissue was fixed with 4% formalin and was embedded in paraffin as described previously.45
For immunofluorescence staining, after antigen retrieval (Pascal citrate, 10 mM, pH 6.0, 30 s, 125°C, 21 psi) and blocking of non-specific binding (foetal calf serum 10% in PBS), formalin-fixed sections were incubated with primary antibodies. Bound antibodies were visualized with appropriate species-specific Cy2-, Cy3- or Cy5-conjugated secondary antibodies or anti-rabbit tyramide signal amplification. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen).
Immunostained sections were scanned using a Pannoramic 250 FLASH II (3DHISTECH) Digital Slide Scanner with objective magnification of ×20 or ×40. Positive signals were quantified by a blinded experimenter using Pannoramic Viewer software (3DHISTECH) and ImageJ/FIJI (NIH Image analysis).
To analyse the distribution patterns of CD8, CD68, HLA-DR, galectin-3 (LGALS3), GPNMB, CTSB, P2RY12, glial fibrillary acidic protein (GFAP) and Vimentin in a representative Neuro-HIV sample, we used an image analysis ruleset based on the Definiens Cognition Network Language as previously described.45 2D-signal density maps were stacked and visualized as 3D-surface plots (FIJI plugin). Additionally, the immunostainings from adjacent tissues sections were digitally registered, then segmented in tiles (n = 792) and positive signals were detected. Average histological marker density within the tile was used as variable to compute the Pearson’s correlation coefficient r.
For representative images, white balance was adjusted and contrast was linearly enhanced using the tools levels, curves, brightness and contrast in Adobe Photoshop CC. Image processing was applied uniformly across all images within a given dataset.
Antibodies
Primary antibodies: mouse anti-Human Beta-Amyloid (Clone 6F/3D; 1:100, Dako, Cat. No. M0872), mouse IgG2b anti-Cathepsin B (CTSB) (clone H-5, 1:50, Santa Cruz Biotechnology, Cat. No. sc-365558), rat IgG2a anti-LGALS3 (clone M3/38, 1:200, BioLegend, Cat. No. 125401), rabbit anti-Iba1 (polyclonal, 1:50, WAKO Cat. No. 019-19741, directly labelled with anti-rabbit Alexa Fluor 647), mouse IgG2b anti-SPP1 (clone MAB-1433, 1:100, RD Systems, Cat. No. 190312), rabbit anti-P2Y12R (polyclonal, 1:1000, Sigma-Aldrich Cat. No. HPA014518), goat anti-GPNMB (polyclonal, 1:100, RD Systems, Cat. No. AF2550), mouse IgG1 anti-HLA-DR (clone CR3/43, 1:50, DAKO, Cat. No. M0775), mouse IgG3 anti-CD68 (clone PG-M1, 1:100, DAKO, Cat. No. M0876), mouse IgG1 anti-CD8a (clone C8/144B, 1:50, Abcam, Cat. No. ab75129), chicken anti-GFAP (polyclonal, 1:2000, Abcam, Cat. No. ab4674), rabbit anti-NeuN (clone EPR12763, 1:50, Abcam, Cat. No. ab190195, directly labelled with Alexa Fluor 488), rabbit anti-phosphorylated-Signal Transducer and Activator of Transcription 1 (p-STAT1) (clone 58D6, 1:1000, Cell Signalling Technology, Cat. No. 9167), mouse anti-human phosphorylated Tau (p-Tau) (Ser202, Thr205) (clone AT8, 1:100, ThermoFisher, Cat. No. MN1020), mouse IgG1 anti-synaptophysin (clone 27G12,1:50, Novocastra, Cat. No. NCL-L-SYNAP-299), mouse IgG1 anti-HIV-p24 (clone Kal-1, 1:100, DAKO, Cat. No. M0857) and mouse IgG1 anti-Vimentin (clone V9, 1:20, DAKO, Cat. No. M0725).
Secondary antibodies: anti-mouse IgG2b Alexa Fluor 488 (ThermoFisher, Cat. No. A21141), anti-goat Cy2 (Jackson Immuno Research Laboratories, Cat. No. 705-225-147), anti-rabbit Alexa Fluor 488 (Jackson ImmunoResearch Laboratories, Cat. No. 712-545-153), anti-mouse IgG1 Alexa Fluor 555 (ThermoFisher, Cat. No. A21127), anti-rat Cy3 (Jackson ImmunoResearch Laboratories, 712-165-153), anti-mouse IgG3 Atto 647 (LifeSpan Biosciences, LS-C209483), anti-chicken Alexa Fluor 647 (ThermoFisher, Cat. No. A21449), Alexa Fluor 647 Antibody Labelling Kit (ThermoFisher, Cat. No. A20186), anti-rabbit tyramide signal amplification Plus Cyanine 3 (Perkin Elmer, Cat. No. NEL744001KT, used to amplify the signals of anti-p24 and anti-pSTAT1). Secondary antibodies were used at 1:200 dilution. For immunohistochemistry, bound primary antibodies were visualized either by an Envision HRP with 3,30-diaminobenzidine (DAB) as chromogen (Dako Cat. No. K4001; hemalaun counterstaining of nuclei). p-Tau staining was carried out on a Ventana Discovery Ultra platform. Slides were post-treated with CC1 for 40 mins, non-specific binding and endogenous peroxidase were blocked using, respectively, 2% NGS solution and Discovery Inhibitor reagent according to the manufacturer’s protocol. Mouse anti-human p-Tau (clone AT8, 1:100, ThermoFisher, Cat. No. MN1020) was applied at a 1:3000 dilution and incubated for 60 min at 37°C, OMap anti-Ms HRP secondary antibody was applied and incubated for 24 min. A Discovery Purple kit was used as enzymatic substrate for 40 min, then slides were counterstained using Haematoxylin II.
Marker selection for neurodegenerative phagocytes
We extracted the top differentially expressed genes from previously identified neurodegeneration-associated microglial states: DAM27 (GSE98969); MGnD28 (GSE101689), activated response microglia44 (GSE127893), white matter-associated microglia46 (GSE166304) and human CSF-derived myeloid cells from HIV38 (GSE117397). Curated lists of upregulated genes (Supplementary Table 4) were used to generate a Venn diagram in which signatures overlapping in more than three datasets were highlighted, thus showing their annotated genes and considered as core signatures (Fig. 1C). Among these core signatures, four degeneration-associated microglia markers were selected because of their differential regulation operated by APOE or TREM2 and the availability of antibodies against those targets. In particular, we selected antibodies against LGALS3 and GPNMB that are both regulated in a TREM2- and APOE-dependent manner,28 as well as SPP1 and CTSB that are TREM2-dependent and -independent molecules, respectively.27
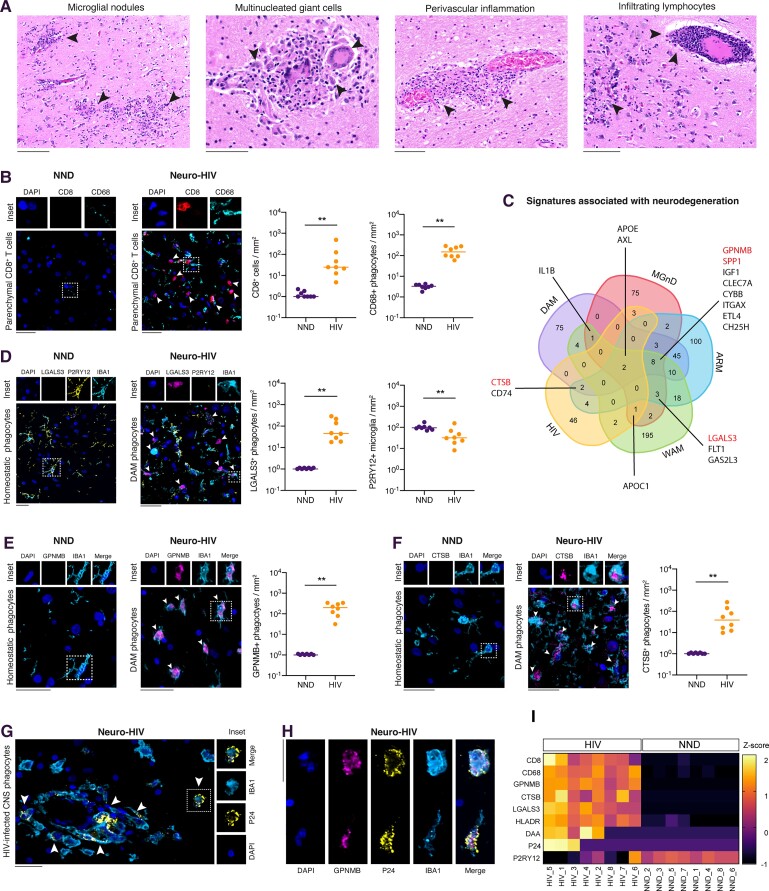
Figure 1.
CNS phagocytes express a neurodegenerative signature in Neuro-HIV. (A) Haematoxylin and eosin staining of brain sections indicating neuropathological changes in Neuro-HIV indicated as arrowheads, including microglial nodules, multinucleated giant cells, perivascular giant cells and infiltrating lymphocytes. Scale bars = 150 μm. (B) Representative immunostainings and quantification for CD8, CD68 and nuclei (DAPI) in brain sections of NND and Neuro-HIV patients. (C) Venn diagram showing the overlap of upregulated genes in microglia from neurodegeneration models including DAM, MGnD and ARM as well as ageing white matter-associated microglia and human CSF-derived myeloid cells from HIV patients (HIV). Genes overlapping in at least three conditions are annotated in the diagram and considered as core signatures. Markers selected for histological analyses are indicated in red. (D) Representative immunostainings and quantification for LGALS3, P2RY12, IBA1 and nuclei (DAPI) in brain sections of NND and Neuro-HIV patients. Arrowheads indicate neurodegenerative phagocytes (DAM). (E) Representative immunostainings and quantification for GPNMB, IBA1 and nuclei (DAPI) in brain sections of NND and Neuro-HIV patients. Arrowheads indicate neurodegenerative phagocytes (DAM). (F) Representative immunostainings and quantification for CTSB, IBA1 and nuclei (DAPI) in brain sections of NND and Neuro-HIV patients. Arrowheads indicate neurodegenerative phagocytes (DAM). (G) Representative immunostaining and quantification for HIV p24 and IBA1 in brain sections of Neuro-HIV patients. Arrowheads indicated CNS phagocytes productively infected with HIV. (H) Representative immunostainings for GPNMB, HIV p24, IBA1 and nuclei (DAPI) from a brain section of a Neuro-HIV patient. (I) Heat map of normalized cellular densities for CD8, CD68, GPNMB, CTSB, LGALS3, HLA-DR, DAA, P24 and P2RY12 in NND and Neuro-HIV patients. The colour scale represents the z-score obtained with the following formula z = (x − )/S, where x is the raw value (positive cells/mm2) for each brain sample, is the mean of raw values of both groups (NND and HIV) and S is the standard deviation of raw values of both groups (NND and HIV). (B–F) Symbols represent individual samples (n = 8 per group). Data are shown on a base 10 logarithmic scale. Lines indicate the median. Scale bar = 30 μm. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05; ns = not significant by non-parametric paired Wilcoxon test.
Neuropathological evaluation
Immunohistochemistry for phospho-Tau was performed to stage the evaluated samples according to Braak methodological protocol.47 Amyloid-β detected by immunohistochemistry at autopsy was classified using CERAD (Consortium to Establish a Registry for Alzheimer’s Disease), a semi-quantitative scale to stage amyloid-β neuritic plaques and fibrillar amyloid aggregates.48
Confocal imaging and quantification of synaptic terminals
For each sample, single-plane confocal images were acquired (Zeiss LSM800), sampling 0.1 mm2 of brain tissue at ×63 magnification. FIJI software was used to calculate the perimeter of neuronal soma (µm) and enumerate perisomatic synaptophysin (SYP)-positive terminals (number of synapses/µm). Synapses in direct contact with neuronal soma or in its close vicinity (<2 µm) were considered perisomatic synapses. For each sample, 35 neurons were evaluated. This approach allowed us to stratify perisomatic synaptic density in relation to contact with phagocytes.
The total number of synaptic terminals in the tissue (synaptic punctae/µm2) was detected through a custom image analysis ruleset using Definiens Developer XD software as previously described.45
Quantification of phagocyte engulfing synaptic terminals
For each sample, single-plane confocal images were acquired, sampling 0.06 mm2 of tissue at ×63 magnification. Imaris software (Bitplane) was used to build a co-localization channel of SYP- and CD68-positive punctae and to perform a 3D reconstruction of the cell. IBA1+ cells showing co-localized SYP+ CD68+ punctae were counted as phagocytes engulfing synaptic terminals, expressed as the number of SYP+ CD68+ phagocytes/mm2. The rate of engulfed synapses was calculated in relation to the total number of SYP terminals in the imaged tissue.
Quantification and statistical analysis
Data are shown as individual values. Horizontal lines represent the median as reported in the figure legends. Normal distribution was confirmed using the D’Agostino–Pearson omnibus normality test where appropriate. To compare two groups, a paired non-parametric Wilcoxon test (two-tailed) was used as indicated in figure legends. Variance between samples was tested using the Brown–Forsythe test. To compare multiple groups with equal variance, a one-way ANOVA test was used while Kruskal–Wallis test was applied for groups with unequal variance. Post hoc tests for multiple comparisons are indicated in the figure legends. Linear regression and Pearson correlation coefficient r and R2 were calculated. Correlation matrices illustrate the correlation coefficients between two variables displaying the Pearson’s correlation coefficient r within each cell. Histological marker densities (positive cells/mm2) or synaptic densities (synaptic boutons/µm) are considered as variables and used as the input to calculate the correlation between two variables. Z-scores were computed according to the formula z = (x − ) / S, where x is the raw value (positive cells/mm2) for each brain sample, is the mean of raw values of both groups (NND and HIV) and S is the standard deviation of raw values of both groups (NND and HIV). In figures, asterisks denote statistical significance as *P < 0.05; **P < 0.01; ***P < 0.001. Statistical analysis was performed in GraphPad Prism9.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. All software is freely or commercially available.
Results
Phagocytes express a neurodegenerative signature in Neuro-HIV
To investigate whether CNS phagocytes express cellular and molecular hallmarks of neurodegeneration-associated microglia signatures,27,28 we analysed brain samples obtained from HIV-positive individuals (Neuro-HIV, Supplementary Table 1) and patients with NND (Supplementary Table 2 and Supplementary Fig. 1).
The neuropathological picture of Neuro-HIV was dominated by the presence of microglial nodules, multinucleated giant cells and lymphocytic infiltration (Fig. 1A), composed especially of CD8+ T cells that could be found in both perivascular space (Supplementary Fig. 1A) and parenchyma (Fig. 1B). In contrast to control brain sections of NND, CD68+ microglial nodules highlighting an activated response of microglia and macrophages were frequently found in the brains of HIV-positive individuals (Fig. 1B).
To characterize the phenotype of CNS phagocytes in Neuro-HIV, we took advantage of signature molecules shared by multiple neurodegenerative disease models (DAM,27 MGnD28 and activated response microglia44), ageing white matter models (white matter-associated microglia46) and human CSF-derived myeloid cells from HIV patients38 (Fig. 1C), thus selecting LGALS3, glycoprotein NMB (GPNMB), SPP1 and CTSB as markers indicating a degeneration-associated microglia/macrophage response.49 Moreover, these markers are differentially regulated by either APOE or TREM2; namely LGALS3 and GPNMB are induced by both APOE and TREM2,28 while SPP1 and CTSB are TREM2 dependent and independent molecules, respectively.27
In Neuro-HIV, CNS phagocytes downregulated homeostatic signature molecules such as P2Y12R (Fig. 1D) and acquired a neurodegenerative phenotype characterized by the expression of DAM signature molecules such as the immunomodulator LGALS3, CTSB and GPNMB (Fig. 1E and F). These inflammatory phagocytes frequently harboured productive HIV infection (Fig. 1G and H); however, also uninfected or potentially latently infected phagocytes contributed to neuroinflammation.
Additionally, Neuro-HIV brain sections were populated by numerous CNS phagocytes expressing high levels of HLA-DR (Supplementary Fig. 1B), another marker of DAM response along with other molecules involved in major histocompatibility complex class II presentation.44,50 On the other hand, in HIV-positive individuals, we did not observe a consistent upregulation in SPP1 (Supplementary Fig. 1C), a molecule involved in tissue repair and remyelination.44 Despite reactive microglia with occasional microglial nodules are described in severe respiratory diseases with systemic inflammation,51–53 patients who died from sepsis/systemic inflammation (Supplementary Table 3) did not show phagocytes expressing the neurodegenerative signature markers LGALS3 and GPNMB (Supplementary Fig. 1F and G). However, white matter-associated phagocytes displayed higher levels of HLA-DR and CD68 and lower levels of P2RY12 compared with control samples as previously described,54 suggesting an activated phenotype (Supplementary Fig. 1B, D and E).
Parenchymal hotspots of reactive phagocytes were enriched in microglial cells expressing both the microglia-specific marker P2RY12 and LGALS3 (72.1 ± 24.0%, mean ± SD), compatible with transitional DAM microglia. However, the accumulation of IBA1+ LGALS3+ cells lacking P2RY12 indicated the presence of resident microglia, which further downregulated this homeostatic marker in their reactive response and also suggested the acquisition of this signature from monocyte-derived macrophages that are devoid of this receptor and are responsible for the introduction of HIV into the brain,4,55 thus creating a mingling of onthogenically different DAM phagocyte populations and participating in the establishment of a viral reservoir within the CNS (Supplementary Fig. 1H).
Since the microglial response can shift astrocyte signalling from physiological to pathological,56 we investigated the co-expression of molecules enriched in DAAs in murine models of Alzheimer’s disease and ageing41 by staining brain sections for GFAP, Vimentin and CTSB (Supplementary Fig. 1I).
Here, we observed that a substantial proportion of brains (62.5%) were populated by DAAs in Neuro-HIV (Fig. 1I), which highly correlated with the presence of CNS phagocytes showing a DAM response signature (Pearson’s correlation r = 0.80 for GPBMB and 0.74 for LGALS3, P < 0.001) (Supplementary Fig. 1J). Additionally, CNS phagocytes showing increased expression of DAM signature molecules could be identified in all Neuro-HIV cases, even in the absence of productive HIV infection or low CD8+ T-cell infiltration (Fig. 1I).
Neurodegenerative phagocytes occupy hotspots of brain inflammation
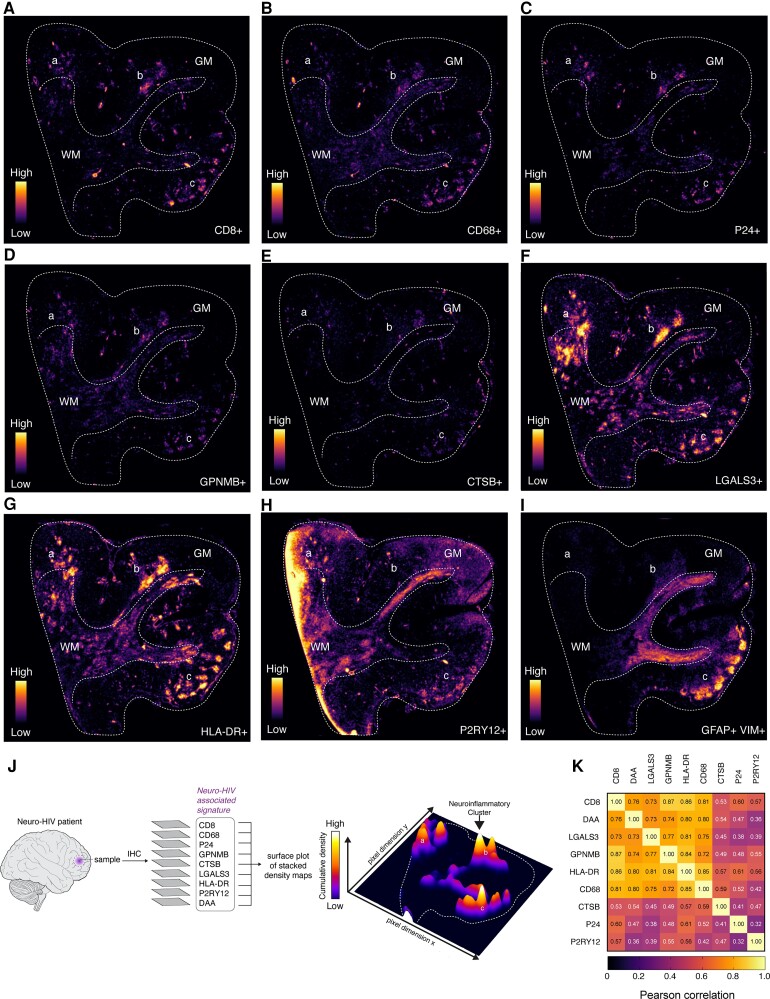
Since DAM were predominantly detected in the diseased CNS regions,27,28,57 we speculated whether neurodegenerative glial cells could occupy hotspots of brain inflammation in Neuro-HIV.
To address this question, we stained adjacent tissue sections from a representative Neuro-HIV sample for DAM markers (LGALS3, CTSB, GPNMB, HLA-DR), DAA markers (GFAP, Vimentin), homeostatic microglia markers (P2RY12), as well as CD68, CD8 and p24 for productive HIV infection. In order to visualize the expression in the spatial context, we generated a tissue density map for each marker (Fig. 2A–I). Thereby, we noted that focal areas of brain inflammation were predominantly localized in the grey matter in Neuro-HIV. We subsequently superimposed all stained sections to generate a bidimensional density map in which overlapping signals (peaks) were visualized in form of a 3D surface plot. This analysis revealed that high density clusters of DAM phagocytes, DAA astrocytes, CD8+ T cells and p24+ cells were frequently co-localized (Fig. 2J), and their topographic distribution highly correlated with each other (Fig. 2K). In contrast, this topographic distribution of reactive glial cells was not found in control samples (Supplementary Fig. 2A–J).
Figure 2.
Neurodegenerative glial cells are localized to hotspots of brain inflammation. (A–I) Bidimensional tissue density maps obtained from immunofluorescence of adjacent sections of a representative Neuro-HIV case for the following markers: CD8 (A), CD68 (B), p24 positive phagocytes (C), DAM signature markers including GPNMB (D), CTSB (E), LGALS3 (F), HLA-DR (G), homeostatic microglial marker P2RY12 (H) and DAA markers (I). (J) Individual bidimensional tissue maps were stacked and visualized as a 3D surface plot. White peaks correspond to regions enriched in all markers. Dashed lines indicate the transition between grey matter (GM) and white matter (WM). Cortical neuroinflammatory clusters are indicated (a–c). (K) The correlation matrix illustrates the Pearson’s correlation coefficients (r) between two variables within each cell of the matrix, indicating the degree of similar topographic distribution in the tissue. Average histological marker densities obtained from the whole tissue area (as indicated in A) are considered as variables and used as input to calculate the correlation between two variables. A colour-code scale is used to indicate the Pearson’s correlation coefficient.
These data indicate that neurodegenerative DAM phagocytes and DAA astrocytes closely interact within neuroinflammatory foci. These hotspots might be driven by locally secreted factors derived from CD8+ T-cell infiltrates and/or HIV-infected cells.
Phagocyte-mediated synaptic stripping in Neuro-HIV
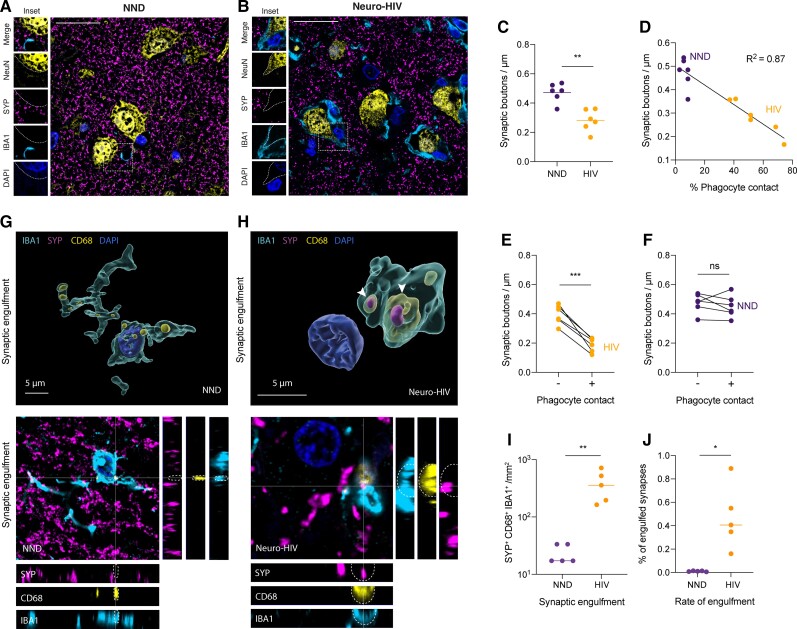
In pathological conditions associated with cognitive decline such as Alzheimer’s disease, frontotemporal lobar degeneration or West Nile Virus encephalitis, phagocytes have been shown to remove viable synapses by phagocytosis, leading to persistent memory impairment or other cognitive deficits.22,24,26 However, a specific role for DAM phagocytes in the process of synapse elimination and neuronal damage in Neuro-HIV has not yet been described.
Here, we noted that Neuro-HIV brains with prominent cortical inflammation were accompanied by a lower perisomatic synaptic density compared with age-matched control NNDs (Fig. 3A–C and Supplementary Fig. 3A), and in the absence of amyloid-β and p-Tau histopathology (Supplementary Fig. 3B–D). In Neuro-HIV, phagocytes were more frequently in contact with neuronal somata and this association correlated with reduced density of synaptic terminals (P < 0.0001, R2 = 0.87) (Fig. 3D and E). In contrast, no correlation between phagocyte contacts and synaptic density was observed in control NNDs (Fig. 3F).
Figure 3.
Phagocyte-mediated synaptic loss and neuronal p-STAT1 signalling in Neuro-HIV. (A and B) Confocal immunofluorescence images for synaptophysin (SYP), NeuN, IBA1 and nuclei (DAPI) from brain sections of Neuro-HIV patient (A) and NND (B). Scale bar = 25 μm. (C) Quantification of synaptic densities in Neuro-HIV and NND. Symbols represent individual samples (n = 6 per group; 35 neurons evaluated per patient). (D) Correlation of synaptic density with phagocyte apposition in Neuro-HIV and NND. Symbols represent individual samples (n = 6 per group; 35 neurons evaluated per patient). (E and F) Quantification of perisomatic bouton density in Neuro-HIV (E) and NND (F) matched for brain region and age (n = 35 neurons evaluated per patient) and stratified according to the presence (+) or absence (−) of contact with CD68+ cells. Symbols represent individual samples (n = 6 per group). (G and H) 3D cell reconstruction of confocal immunostainings of a homeostatic ramified phagocyte in NND without synaptic inclusions (G) and an amoeboid phagocyte in Neuro-HIV (H) exhibiting a SYP inclusion overlapping with a CD68+ phagosome. (I) Quantification of engulfed synaptic terminals (SYP) localized in the phagosomal compartment (CD68) of CNS phagocytes (IBA1) in brain sections of Neuro-HIV patients (n = 5) and NDD (n = 5). Number of SYP+ CD68+ phagocytes per mm2 are shown. (J) Proportion of engulfed synaptic terminals out of all detected SYP punctae in brain sections of Neuro-HIV patients (n = 5) and NDD (n = 5). (C–J) Lines indicate the median. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05; ns = not significant by paired Student’s t-test.
In addition, the co-localization of engulfed SYP presynaptic terminals with the CD68+ lysosome compartment inside IBA1+ cells of Neuro-HIV patients (Fig. 3G–I) indicated the active involvement of these phagocytes in synaptic elimination. The fraction of synapses found engulfed by phagocytes was <1% of the total synapses (0.47 ± 0.27%, mean and SD) in Neuro-HIV samples (Fig. 3J and Supplementary Fig. 3E), which was, however, at least 40 times higher compared to NND controls (0.01 ± 0.00%, mean and SD).
Moreover, synaptic density was inversely correlated with the expression of DAM signature elements in phagocytes or the presence of DAA signature elements in astrocytes (Pearson’s r = −0.70 for GPNMB, r = −0.62 for HLA-DR, r = −0.74 for DAAs, P < 0.05) (Supplementary Fig. 3F). Altogether, these findings point towards an important role of CNS phagocytes associated with neurodegeneration in synapse elimination.
Since the production of interferon gamma (IFN-γ) by CD8+ T cell does not correlate with HIV control,11 we speculated whether neuronal exposure to IFN-γ could drive STAT1 signalling and drive the recruitment of monocyte-derived macrophages, as we have recently shown in a model of viral encephalitis.58 Neuronal phosphorylated STAT1 (p-STAT1) was only identified in the Neuro-HIV case (Supplementary Fig. 3G) that showed the highest CD8+ T-cell infiltration among the samples evaluated. This suggests that neuronal exposure to IFN-γ could be considered collateral damage from a CD8+ T cell attacking HIV-infected phagocytes, thus contributing to synaptic loss.
Taken together, our findings indicate that phagocytes and astrocytes in Neuro-HIV acquire neurodegeneration-associated signatures and that CNS phagocytes perform synaptic stripping, thus participating in the immune-mediated cognitive decline in these patients.
Discussion
Synaptic loss is an early hallmark shared by several neurodegenerative diseases and represents a functional correlate of cognitive impairment. Recent studies have shown that microglia and astrocytes are actively involved in synapse elimination, thereby disrupting neuronal networks and contributing to neurodegeneration.59 Our study on HIV neuropathology reveals that CNS phagocytes acquire a neurodegenerative phenotype and are involved in synaptic stripping.
Prevention of cognitive decline60,61 in the ageing HIV-positive population62 represents an unmet clinical need.21 Indeed, although the introduction of combination antiretroviral therapy contributed to a decreased incidence in HIV-associated dementia, milder forms of cognitive disease remain a major element of morbidity.15,37,63,64 Even asymptomatic patients harbour a higher risk of developing symptomatic cognitive impairment,65,66 accelerated ageing67 or even signs of atrophy from neurodegeneration.68
In neurodegenerative and neuroinflammatory diseases, the defence housekeeping functions of microglia can be dysregulated, thus acquiring the capacity to damage neurons via chemokines, aberrant synaptic pruning and phagocytosis.17 In genome-wide association studies involving Alzheimer’s and Parkinson’s disease patients, numerous genes associated with an increased risk are expressed by CNS phagocytes69–72 and often represent their immune checkpoints governing the transition from their homeostatic sentinel state63 to an overreactive neurodegenerative response named DAM.27,28,44,73
Here, we hypothesized that this microglial transcriptional programme associated with neurodegeneration might also operate in other neurocognitive disorders including Neuro-HIV. Indeed, such a shared mechanism between Alzheimer’s disease and HAND has been suggested by a recent study on myeloid-like cells derived from the CSF of HIV-positive patients,38 despite obvious histopathological differences in terms of amyloid and Tau pathology. We observed that CNS phagocytes highly express LGALS3, GPNMB, CTSB and HLA-DR in brain sections of Neuro-HIV patients compared with NND controls. These changes are accompanied by a loss of the microglial homeostatic marker P2RY12, in accordance with the molecular switch operated by the activation of the APOE-TREM2 pathway, and probably because of brain infiltrating myeloid cells that lack this receptor and are involved in the dissemination of HIV within the CNS.4,74
In Alzheimer’s disease murine models, the transition from homeostatic microglia to DAM appears to be only partially TREM2 dependent.27,28 This phenotypic switch is initiated in a Trem2-independent fashion (DAM stage 1), which involves a reduction in the expression of homeostatic microglia checkpoint genes and upregulation of an intermediate DAM programme including Ctsb, Ctsd, Tyrobp and ApoE.27
Here, we show that neurodegenerative phagocytes express CTSB, which is a Trem2-independent molecule, but not SPP1, which is Trem2 dependent. This indicates that CNS phagocytes might have acquired a transcriptional programme that is tailored to the specific disease microenvironment induced by the HIV infection.
In this context, identifying crucial molecular targets to restore the neuroprotective function of phagocytes is particularly challenging. In particular, the complex landscape of responses adopted by these cells to face different neurodegenerative diseases is further modulated by environmental and genetic risk factors, as well as the divergence in this response between human diseases and mouse models.49 Indeed, the R47H and R62H loss-of-function variants of TREM2 are associated with fewer plaque-associated microglia, more neuritic dystrophy and increased risk of late-onset Alzheimer’s disease, suggesting a protective role of this receptor in disease development.75 In mice, TREM2-deficient microglia do not exhibit the typical increase in expression of HLA markers or DAM markers and are locked into a more homeostatic phenotype76 characterized by abundant autophagosomes, indicating metabolic stress.77 However, different studies conducted on murine models of Alzheimer’s disease found opposite outcomes of TREM2 ablation on neurodegeneration.78–83 This difference might emerge from the use of different models and TREM2 abrogation strategy, as well as the investigated disease stage. Therefore, TREM2 might be both protective and harmful, depending on the stage of disease progression and the role of microglial phenotype.
LGALS3 and GPNMB are also considered potential palatable targets for modulating the response of CNS phagocytes in neurodegeneration. Secreted LGALS3 can act as an opsonin84 binding to desialylated glycoproteins and glycolipids and activating the phagocytic receptor MerTK,85 thereby inducing the phagocytosis of opsonized structures. In addition, LGALS3 has also been shown to be an endogenous TREM2 ligand, thus modulating DAM phenotype.86 LGALS3-deficient mice exhibit a reduction in amyloid burden, inflammatory response and rescue of cognitive performance.86
Additionally, the extracellular domain of GPNMB can be cleaved by a disintegrin and metalloproteases 10 (ADAM10), thus allowing the interaction with multiple molecular targets in the extracellular spaces87 and therefore modulating vascular integrity,88 lysosomal function,89 cell adhesion and neuroinflammation.90 Although overexpression of GPNMB appears to be protective in murine models of ALS,91 its role in Neuro-HIV and other neurodegenerative diseases remains to be elucidated.
The plasticity displayed by CNS phagocytes demands further elucidation of the pathways governing their reactive phenotypes and their specific role in the disease stage to leverage this knowledge for the development of efficient therapeutics targeting microglia and infiltrating myeloid cells in neurodegenerative diseases.
DAM signature can be induced by various stimuli (protein aggregates, apoptotic cells, interferons) even in the absence of numerous other infiltrating immune cells.92 The latter is in accordance with our findings showing a DAM signature even in samples with a low density of CD8+ T cells. Altogether, this suggests that chronic brain inflammation contributes to neurodegeneration in HIV-positive individuals.
Some of these microglial changes could be associated with the presence of brain HIV reservoirs. Indeed, the upregulation of LGALS3 can be mediated by the HIV Tat protein through the binding of SP1 transcription factor93 and the amount of CTSB neuronal uptake is modulated by HIV replication levels.94 Of note, we observed that CNS phagocytes can display a DAM signature even without evidence of productive brain infection, thus indicating that the inflammatory milieu can induce changes even in uninfected phagocytes95 or latently infected cells. Indeed, latent HIV infection in the CNS is associated with increased levels of chromatin modifiers probably contributing to aberrant transcriptional changes, leading to inflammation and neurodegeneration.43
CNS phagocytes can indirectly damage neurons also by promoting reactive neurotoxic astrocyte functions in numerous neuroinflammatory and neurodegenerative conditions.96 Recently, a subset of reactive astrocytes, named DAA, has been described in murine models and human samples of Alzheimer’s disease31 and these are believed to become destructive with disease progression, due to expression of inflammatory and neurotoxic molecules.41 In our study, we show that astrocytes expressing markers of DAAs can be identified in a substantial proportion of HIV cases and their presence highly correlates with the density of phagocytes expressing markers of DAM. Indeed, this astrocyte response is probably induced by factors and a crosstalk between various cell types in the tissue microenvironment, including DAM phagocytes that are known to share a similar transcriptional landscape.41 This is further exemplified by the presence of both DAM and DAA at sites of focal brain inflammation, thus indicating that in neuroinflammatory hotspots different environmental cues might drive this neurodegenerative transcriptional programme in glial cells, thus participating in the pathogenesis of cognitive decline.
Neurodegeneration is accompanied by a progressive loss of neurons and synaptic terminals. This process can be fuelled by the neuroinflammatory microenvironment induced by HIV infection. These changes are probably accompanied by a neuronal increase in ‘eat-me signals’, decrease in ‘do not eat-me signals’ or by the binding of opsonins, therefore leading to an increased phagocytosis of stressed neurons or synapses.97 Indeed, even in absence of brain atrophy, a reduced synaptic density can be detected by SV2A PET imaging in virally suppressed HIV patients, thus suggesting a potentially reversible synaptic pathology for their neurocognitive dysfunction98 and a compartmentalized neuroinflammation despite antiretroviral therapy.
Although, the aberrant removal of damaged neurons or synapses during chronic inflammation can be harmful for cognitive function, phagocytosis of dead or dying neurons as well as neuronal debris per se is beneficial because it can curb inflammation.99,100 Therefore, it will be important to better understand whether, and under which circumstances, phagocytes remove still functioning but stressed neurons and synapses or rather neuronal corpses and debris.101,102 Thus, further studies are needed to address the beneficial and detrimental role, respectively, of CNS phagocytes in Neuro-HIV. Evidence is emerging for the pathogenic role of phagocytes in synaptic stripping in models of neuroinflammation22,103 and neurodegeneration24,26 in which interference with phagocytic functions results in improved outcomes.
During viral infection, CNS phagocytes eliminate synapses in a complement C3 dependent22 or independent fashion.58 Here, we show that CNS phagocytes accumulate at neuronal somata and remove synaptic terminals in Neuro-HIV patients. Neuronal damage can also be induced by pro-inflammatory cytokines including IFN-γ produced by CD8+ T cells that can become unable to kill HIV-infected macrophages,11 following a prolonged antigenic stimulation leading to T-cell exhaustion.104 This process can be further expanded by a sustained upregulation of HLA-DR in CNS phagocytes throughout HIV infection.105,106
We previously demonstrated that neurons exposed to IFN-γ produced by CD8+ T cells can orchestrate a phagocyte-mediated synaptic loss via p-STAT1 signalling and downstream CCL2 in a mouse model of viral encephalitis.58 Here, we speculated whether a similar mechanism might take place in HIV-positive population. We found that the neuronal Janus Kinase-STAT1 signalling pathway is activated in Neuro-HIV cases with dense CD8+ T-cell infiltration, suggesting that even in the absence of viral infection in neurons, bystander exposure to IFN-γ may participate in neuronal damage and phagocyte-mediated synaptic loss. The absence of pSTAT1 in the other samples might reflect different disease stages, changes in viral load107 or complementary pathways leading to synaptic loss and cognitive decline.
A limitation of our cohort of HIV-positive patients is that these brain sections predominantly belong to advanced AIDS stages, therefore some of these neurodegenerative changes might be more pronounced compared with milder forms of the HAND spectrum. Nevertheless, it has been shown that signs of microglia or astrocytic activation can be detected in the CSF of HIV-infected patients with cognitive impairment, even when their HIV viral load is undetectable both in the serum and the CSF.38,108 Together with our findings, these data clearly point out that microglial response may be instrumental in the cognitive disorders of well-controlled HIV-infected patients.109
Taken together, our data indicate that neurodegenerative phagocytes can be detected in brain samples of HIV-positive patients and that CNS phagocyte-mediated synaptic stripping may represent a mechanistic substrate of cognitive decline in Neuro-HIV, which might be therapeutically exploited in the future.
Supplementary Material
Funding
The work in D.M.’s laboratory is supported by the Swiss National Science Foundation (number 310030_185321), and the European Research Council (grant agreement number 865026). R.D.P. is supported by the Swiss National Science Foundation (number 320030_179531).
Abbreviations
- DAA
disease-associated astrocytes
- DAM
disease-associated microglia
- DAPI
4′,6-diamidino-2-phenylindole
- HAND
HIV-associated neurocognitive disorders
- MGnD
microglia in neurodegeneration
- NND
non-neurological disease
Contributor Information
Giovanni Di Liberto, Department of Pathology and Immunology, University of Geneva, Geneva, Switzerland; Department of Clinical Neurosciences, Service of Neurology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Kristof Egervari, Department of Pathology and Immunology, University of Geneva, Geneva, Switzerland; Division of Clinical Pathology, Geneva University Hospital, Geneva, Switzerland.
Mario Kreutzfeldt, Department of Pathology and Immunology, University of Geneva, Geneva, Switzerland.
Christian M Schürch, Department of Pathology and Neuropathology, University Hospital and Comprehensive Cancer Center Tübingen, Tübingen, Germany.
Ekkehard Hewer, Institute of Pathology, University of Bern, Bern, Switzerland.
Ingrid Wagner, Department of Pathology and Immunology, University of Geneva, Geneva, Switzerland.
Renaud Du Pasquier, Department of Clinical Neurosciences, Service of Neurology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Doron Merkler, Department of Pathology and Immunology, University of Geneva, Geneva, Switzerland; Division of Clinical Pathology, Geneva University Hospital, Geneva, Switzerland.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 2018;19:622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11:e1004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: Central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. [DOI] [PubMed] [Google Scholar]
- 4. Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res. 2014;12:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. He J, Chen Y, Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. [DOI] [PubMed] [Google Scholar]
- 6. Huang Z, Tomitaka A, Raymond A, Nair M. Current application of CRISPR/Cas9 gene-editing technique to eradication of HIV/AIDS. Gene Ther. 2017;24:377–384. [DOI] [PubMed] [Google Scholar]
- 7. Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–641. [DOI] [PubMed] [Google Scholar]
- 8. Wallet C, De Rovere M, Van Assche J, et al. Microglial cells: The main HIV-1 reservoir in the brain. Front Cell Infect Microbiol. 2019;9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. [DOI] [PubMed] [Google Scholar]
- 10. Navia BA, Cho E-S, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. [DOI] [PubMed] [Google Scholar]
- 11. Collins DR, Gaiha GD, Walker BD. CD8+ T cells in HIV control, cure and prevention. Nat Rev Immunol. 2020;20:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fois AF, Brew BJ. The potential of the CNS as a reservoir for HIV-1 infection: Implications for HIV eradication. Curr HIV/AIDS Rep. 2015;12:299–303. [DOI] [PubMed] [Google Scholar]
- 13. Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–778. [DOI] [PubMed] [Google Scholar]
- 14. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simioni S, Cavassini M, Annoni J-M, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of Viremia. AIDS. 2010;24:1243–1250. [DOI] [PubMed] [Google Scholar]
- 16. Fleischman DA, Arfanakis K, Leurgans S, et al. Neopterin is associated with hippocampal subfield volumes and cognition in HIV. Neurol Neuroimmunol Neuroinflamm. 2018;5:e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hickman S, Izzy S, Sen P, Morsett L, Khoury J E. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levine AJ, Soontornniyomkij V, Achim CL, et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol. 2016;22:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Everall IP, Heaton RK, Marcotte TD, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: A quantitative Golgi study. Acta Neuropathol. 2004;107:97–110. [DOI] [PubMed] [Google Scholar]
- 21. Winston A, Spudich S. Cognitive disorders in people living with HIV. Lancet HIV. 2020;7:e504–e513. [DOI] [PubMed] [Google Scholar]
- 22. Vasek MJ, Garber C, Dorsey D, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jafari M, Schumacher A-M, Snaidero N, et al. Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat Neurosci. 2021;24:355–367. [DOI] [PubMed] [Google Scholar]
- 24. Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olah M, Menon V, Habib N, et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat Commun. 2020;11:6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lui H, Zhang J, Makinson SR, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290.e17. [DOI] [PubMed] [Google Scholar]
- 28. Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerrits E, Brouwer N, Kooistra SM, et al. Distinct amyloid-β and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol. 2021;141:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huttenrauch M, Ogorek I, Klafki H, et al. Glycoprotein NMB: A novel Alzheimer’s disease associated marker expressed in a subset of activated microglia. Acta Neuropathol Commun. 2018;6:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith AM, Davey K, Tsartsalis S, et al. Diverse human astrocyte and microglial transcriptional responses to Alzheimer’s pathology. Acta Neuropathol. 2022;143:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman BA, Srinivasan K, Ayalon G, et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep. 2018;22:832–847. [DOI] [PubMed] [Google Scholar]
- 33. Leyns CEG, Ulrich JD, Finn MB, et al. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci USA. 2017;114:11524–11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spiller KJ, Restrepo CR, Khan T, et al. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat Neurosci. 2018;21:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. d’Errico P, Ziegler-Waldkirch S, Aires V, et al. Microglia contribute to the propagation of Abeta into unaffected brain tissue. Nat Neurosci. 2022;25:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scheiblich H, Dansokho C, Mercan D, et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell. 2021;184:5089–5106.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saylor D, Dickens AM, Sacktor N, et al. Erratum: HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Farhadian SF, Mehta SS, Zografou C, et al. Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV. JCI Insight. 2018;3:e121718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gisslen M, Heslegrave A, Veleva E, et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol Neuroimmunol Neuroinflamm. 2019;6:e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Habib N, McCabe C, Medina S, et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci. 2020;23:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guttenplan KA, Weigel MK, Prakash P, et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature. 2021;599:102–107. [DOI] [PubMed] [Google Scholar]
- 43. Desplats P, Dumaop W, Smith D, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frigerio C S, Wolfs L, Fattorelli N, et al. The major risk factors for Alzheimer’s disease: Age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 2019;27:1293–1306.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kreutzfeldt M, Bergthaler A, Fernandez M, et al. Neuroprotective intervention by interferon-γ blockade prevents CD8+ T cell–mediated dendrite and synapse loss. J Exp Med. 2013;210:2087–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Safaiyan S, Besson-Girard S, Kaya T, et al. White matter aging drives microglial diversity. Neuron. 2021;109:1100–1117.e10. [DOI] [PubMed] [Google Scholar]
- 47. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 49. Song WM, Colonna M. The identity and function of microglia in neurodegeneration. Nat Immunol. 2018;19:1048–1058. [DOI] [PubMed] [Google Scholar]
- 50. Lambert J-C, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matschke J, Lutgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020;19:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deigendesch N, Sironi L, Kutza M, et al. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020;140:583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144:2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zrzavy T, Hoftberger R, Berger T, et al. Pro-inflammatory activation of microglia in the brain of patients with sepsis. Neuropathol Appl Neurobiol. 2019;45:278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brown A. Understanding the MIND phenotype: Macrophage/microglia inflammation in neurocognitive disorders related to human immunodeficiency virus infection. Clin Transl Med. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Habbas S, Santello M, Becker D, et al. Neuroinflammatory TNFalpha impairs memory via astrocyte signaling. Cell. 2015;163:1730–1741. [DOI] [PubMed] [Google Scholar]
- 57. Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e6. [DOI] [PubMed] [Google Scholar]
- 58. Di Liberto G, Pantelyushin S, Kreutzfeldt M, et al. Neurons under T cell attack coordinate phagocyte-mediated synaptic stripping. Cell. 2018;175:458–471.e19. [DOI] [PubMed] [Google Scholar]
- 59. Henstridge CM, Tzioras M, Paolicelli RC. Glial contribution to excitatory and inhibitory synapse loss in neurodegeneration. Front Cell Neurosci. 2019;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chakradhar S. A tale of two diseases: Aging HIV patients inspire a closer look at Alzheimer’s disease. Nat Med. 2018;24:376–377. [DOI] [PubMed] [Google Scholar]
- 61. Turner RS, Chadwick M, Horton WA, Simon GL, Jiang X, Esposito G. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amst). 2016;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thakur KT, Boubour A, Saylor D, Das M, Bearden DR, Birbeck GL. Global HIV neurology: A comprehensive review. AIDS. 2019;33:163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. [DOI] [PubMed] [Google Scholar]
- 65. Grant I, Franklin DR Jr, Deutsch R, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82:2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: Is there a hidden epidemic? AIDS. 2010;24:1367–1370. [DOI] [PubMed] [Google Scholar]
- 67. Milanini B, Valcour V. Differentiating HIV-associated neurocognitive disorders from Alzheimer’s disease: An emerging issue in geriatric NeuroHIV. Curr HIV/AIDS Rep. 2017;14:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol. 2014;34:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 70. Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron. 1993;11:575–580. [DOI] [PubMed] [Google Scholar]
- 71. Henstridge CM, Hyman BT, Spires-Jones TL. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci. 2019;20:94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kia DA, Zhang D, Guelfi S, et al. Identification of candidate Parkinson disease genes by integrating genome-wide association study, expression, and epigenetic data sets. JAMA Neurol. 2021;78:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shemer A, Grozovski J, Tay TL, et al. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat Commun. 2018;9:5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R. The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol. 2018;17:721–730. [DOI] [PubMed] [Google Scholar]
- 76. McQuade A, Kang YJ, Hasselmann J, et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat Commun. 2020;11:5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ulland TK, Song WM, Huang SC, et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell. 2017;170:649–663.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang Y, Cella M, Mallinson K, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jay TR, Hirsch AM, Broihier ML, et al. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J Neurosci. 2017;37:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Ulland TK, Ulrich JD, et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med. 2016;213:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jay TR, Miller CM, Cheng PJ, et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J Exp Med. 2015;212:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schoch KM, Ezerskiy LA, Morhaus MM, et al. Acute Trem2 reduction triggers increased microglial phagocytosis, slowing amyloid deposition in mice. Proc Natl Acad Sci USA. 2021;118:e2100356118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Meilandt WJ, Ngu H, Gogineni A, et al. Trem2 deletion reduces late-stage amyloid plaque accumulation, elevates the Aβ42:Aβ40 ratio, and exacerbates axonal dystrophy and dendritic spine loss in the PS2APP Alzheimer’s mouse model. J Neurosci. 2020;40:1956–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nomura K, Vilalta A, Allendorf DH, Hornik TC, Brown GC. Activated microglia desialylate and phagocytose cells via neuraminidase, galectin-3, and Mer tyrosine kinase. J Immunol. 2017;198:4792–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Caberoy NB, Alvarado G, Bigcas J-L, Li W. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol. 2012;227:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boza-Serrano A, Ruiz R, Sanchez-Varo R, et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019;138:251–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hoashi T, Sato S, Yamaguchi Y, Passeron T, Tamaki K, Hearing VJ. Glycoprotein nonmetastatic melanoma protein B, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein. FASEB J. 2010;24:1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Winkler EA, Kim CN, Ross JM, et al. A single-cell atlas of the normal and malformed human brain vasculature. Science. 2022;375:eabi7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Robinet P, Ritchey B, Lorkowski SW, et al. Quantitative trait locus mapping identifies the Gpnmb gene as a modifier of mouse macrophage lysosome function. Sci Rep. 2021;11:10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Saade M, de Souza G A, Scavone C, Kinoshita PF. The role of GPNMB in inflammation. Front Immunol. 2021;12:674739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tanaka H, Shimazawa M, Kimura M, et al. The potential of GPNMB as novel neuroprotective factor in amyotrophic lateral sclerosis. Sci Rep. 2012;2:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Song WM, Colonna M. The microglial response to neurodegenerative disease. Adv Immunol. 2018;139:1–50. [DOI] [PubMed] [Google Scholar]
- 93. Fogel S, Guittaut M, Legrand A, Monsigny M, Hebert E. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology. 1999;9:383–387. [DOI] [PubMed] [Google Scholar]
- 94. Cantres-Rosario YM, Ortiz-Rodriguez SC, Santos-Figueroa AG, et al. HIV infection induces extracellular Cathepsin B uptake and damage to neurons. Sci Rep. 2019;9:8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. [DOI] [PubMed] [Google Scholar]
- 96. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. [DOI] [PubMed] [Google Scholar]
- 98. Weiss JJ, Calvi R, Naganawa M, et al. Preliminary in vivo evidence of reduced synaptic density in Human Immunodeficiency Virus (HIV) despite antiretroviral therapy. Clin Infect Dis. 2021;73:1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain. 2009;132:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: Beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98:813–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yang Y, Wang J-Z. Nature of tau-associated neurodegeneration and the molecular mechanisms. J Alzheimers Dis. 2018;62:1305–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Garber C, Soung A, Vollmer LL, et al. T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat Neurosci. 2019;22:1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kazer SW, Walker BD, Shalek AK. Evolution and diversity of immune responses during acute HIV infection. Immunity. 2020;53:908–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen P, Su B, Zhang T, et al. Perturbations of monocyte subsets and their association with T helper cell differentiation in acute and chronic HIV-1-infected patients. Front Immunol. 2017;8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liu L, Zhang Q, Chen P, et al. Foxp3+ Helios+ regulatory T cells are associated with monocyte subsets and their PD-1 expression during acute HIV-1 infection. BMC Immunol. 2019;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sanna PP, Fu Y, Masliah E, Lefebvre C, Repunte-Canonigo V. Central nervous system (CNS) transcriptomic correlates of human immunodeficiency virus (HIV) brain RNA load in HIV-infected individuals. Sci Rep. 2021;11:12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Du Pasquier RA, Jilek S, Kalubi M, et al. Marked increase of the astrocytic marker S100B in the cerebrospinal fluid of HIV-infected patients on LPV/r-monotherapy. AIDS. 2013;27:203–210. [DOI] [PubMed] [Google Scholar]
- 109. Rubin LH, Sacktor N, Creighton J, et al. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS. 2018;32:1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request. All software is freely or commercially available.