Abstract
Background
Air pollution is speculated to increase the risks of COVID-19 spread, severity, and mortality.
Objectives
We systematically reviewed studies investigating the relationship between air pollution and COVID-19 cases, non-fatal severity, and mortality in North America and Europe.
Methods
We searched PubMed, Web of Science, and Scopus for studies investigating the effects of harmful pollutants, including particulate matter with diameter ≤2.5 or 10 μm ( or ), ozone (), nitrogen dioxide (), sulfur dioxide () and carbon monoxide (CO), on COVID-19 cases, severity, and deaths in Europe and North America through to June 19, 2021. Articles were included if they quantitatively measured the relationship between exposure to air pollution and COVID-19 health outcomes.
Results
From 2,482 articles screened, we included 116 studies reporting 355 separate pollutant-COVID-19 estimates. Approximately half of all evaluations on incidence were positive and significant associations (52.7%); for mortality the corresponding figure was similar (48.1%), while for non-fatal severity this figure was lower (41.2%). Longer-term exposure to pollutants appeared more likely to be positively associated with COVID-19 incidence (63.8%). , , , , and were most strongly positively associated with COVID-19 incidence, while and with COVID-19 deaths. All studies were observational and most exhibited high risk of confounding and outcome measurement bias.
Discussion
Air pollution may be associated with worse COVID-19 outcomes. Future research is needed to better test the air pollution-COVID-19 hypothesis, particularly using more robust study designs and COVID-19 measures that are less prone to measurement error and by considering co-pollutant interactions.
Keywords: Air pollution, Particulate matter, Nitrogen dioxide, Ozone, Pollutants, COVID-19, Pandemic, Respiratory health
1. Introduction
Air pollution is the greatest environmental health risk factor worldwide (Cohen et al., 2017). But has it also contributed to worse COVID-19 outcomes? Evidence that air pollution has systemic effects on cardiopulmonary and respiratory systems has led several researchers to speculate that it could increase COVID-19 incidence, severity, and mortality (Pozzer et al., 2020; Villeneuve and Goldberg, 2020).
Air pollution could exacerbate COVID-19 prognosis in multiple ways. Both acute and long-term exposure to air pollution increase susceptibility to and severity of respiratory and cardiovascular diseases by increasing oxidative stress and inflammation (Ciencewicki and Jaspers, 2007; Mehta et al., 2013). Particulate matter can reach the alveolar sacs in the lungs and travel further into the bloodstream, causing an inflammatory response that triggers and exacerbates respiratory diseases, including COVID-19 (Lai et al., 2021). Furthermore, other pollutants such as , and CO create oxidative stress, lung damage, and endothelial dysfunction (Lai et al., 2021). In addition to weakening the respiratory and immune system, air pollution has been hypothesized to aggravate COVID-19 infection severity through the overexpression of the angiotensin converting enzyme 2 (ACE-2), a coronavirus receptor, on surfaces of the respiratory tract (Paital and Agrawal, 2020).
In Europe and North America, air pollution is particularly harmful to aging and urban populations and continues to harm vulnerable groups. In 2016, more than 400,000 and 70,000 European deaths were attributable to and , respectively, and the urban population of the EU-27 and the UK continue to be exposed to pollution levels exceeding WHO thresholds (Khomenko et al., 2021; Sicard et al., 2021). Likewise, in Canada and in the U.S., three out of 10 people live in areas where ambient air quality standards are not met (United Nations, 2017). Furthermore, around 26% of the population in Europe and 23% of the U.S. and Canada population is 60 years or older (United Nations, 2019). For the elderly, long-term exposure to even low levels of air pollution can increase the risk of respiratory conditions (Danesh Yazdi et al., 2021).
It is currently unclear to what extent air pollution is exacerbating significant COVID-19 risks. Since 2020 hundreds of studies have investigated the association between exposure to air pollution and COVID-19. Many of the earlier studies use limited datasets and are prone to several confounding factors, which warrants caution in interpreting their results (Contini and Costabile, 2020). While existing prior systematic reviews suggest that air pollution may be linked to COVID-19 outcomes (see Marquès and Domingo (2022) for a recent review), most studies are from the very early stages of the pandemic and/or include limited evidence from North America and Europe, places where both air pollution and COVID-19 outcomes are likely to be less prone to measurement error (Maleki et al., 2021; Copat et al., 2020; Katoto et al., 2021). In this short time, the air pollution-COVID literature has increased exponentially and now covers additional pollutants, making it possible to ascertain potential heterogeneity of effects across PM, nitrous oxides, ozone, carbon monoxide and other pollutants known to be harmful to human health.
Here we perform a systematic review on studies investigating the relationship between air pollution and COVID-19 outcomes in Europe and North America, disaggregating the evidence by air pollutant type and length of exposure, as well as COVID-19 incidence, non-fatal severity, and mortality. Our focus on these regions is motivated by the similar pollution exposures, COVID-19 responses, and institutional and healthcare capacity relative to the rest of the world. Furthermore, the evidence for these settings is likely to be more reliable given the better air quality monitoring and comparable COVID-19 testing rates and reporting of outcomes (Hasell et al., 2020). Our final sample includes 69 studies covering populations in Europe, 46 in North America, and one which spans both.
2. Methods
2.1. Databases and search strategy
We searched PubMed, Web of Science, and Scopus for studies investigating the relationship between air pollutants and COVID-19 outcomes. We included published studies written in English with no restriction on the database inclusion start date through to June 19, 2021. The keywords used in the database search are composed of the concepts of 1) air pollution and 2) COVID-19. We included the most commonly measured air pollutants: particulate matter less than 10, 2.5, and 1 μm in diameter (, and , respectively), nitrogen oxides (), nitrogen dioxide (), ozone (), sulfur dioxide (), and carbon monoxide ().
For example, the search strategy on PubMed consisted of the following sets of keywords:
-
1)
((air pollutant) OR (air pollution) OR (air pollut*) OR (air contam*) OR (particulate matter) OR () OR () OR (PM1) OR (nitrogen oxides) OR () OR (nitrogen dioxide) OR () OR (ozone) OR () OR (sulfur dioxide) OR () OR (carbon monoxide))
-
2)
AND ((COVID-19) OR (SARS-COV-2))
The full search strategy is available in the Supplementary Materials.
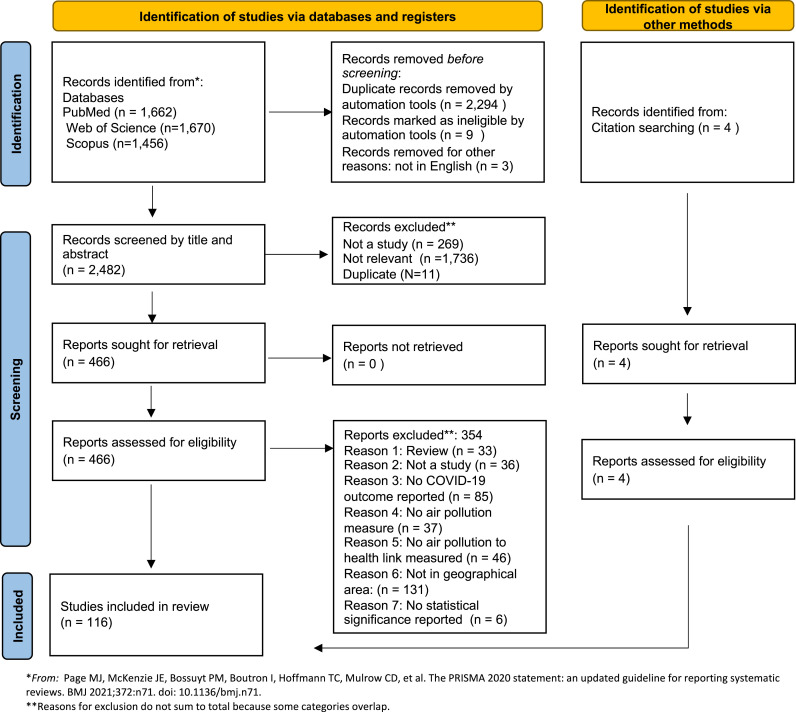
Fig. 1 reports the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Page et al., 2021) flow diagram that led to the inclusion of the studies. The initial search resulted in 1,662 articles from PubMed, 1,670 from Web of Science, and 1,456 from Scopus. After removing duplicates and ineligible items such as books and videos, two authors independently screened the title and abstract of 2,482 articles. Of these, we retrieved and analyzed 466 full-text reports for eligibility, including those where there was uncertainty based on title and abstract screening. We identified four additional texts from citations of included studies. Disagreements about inclusion were discussed between two authors and resolved by a third author. A total of 116 studies met the inclusion and geographical criteria and are included in the review.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) flow diagram of study selection.
2.2. Selection of articles and extraction of data
We included studies examining the relationship between ambient air pollutants, such as particulates (, ) or gases (, , , , ), and COVID-19 spread (e.g., transmission, incidence, prevalence), non-fatal severity (e.g., hospitalization), deaths (e.g., mortality, morbidity, excess deaths) and related outcomes in North America and Europe. We included quantitative analytical studies, such as ecological, cross-sectional, cohort, time-series, case-control, and quasi-experimental studies. We excluded qualitative or purely descriptive studies, reviews, letters to the editor, commentaries, responses, hypotheses, and editorials. We omitted animal studies and studies where the relevant gases (e.g., ozone) were administered in a clinical setting as part of a therapy. This review was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42021275236).
Two authors independently extracted the following: authors, title, journal, aim or the study, location, unit of analysis, study period, air pollution type, exposure length, data sources, details on the COVID-19 health outcome, study design, method for data analysis, covariates, and main results. Summary tables of the main features and results for each of the 116 studies are found in the Supplementary Materials Tables A1- A3.
2.3. Synthesis of studies
We synthesized the studies by COVID-19 outcome, pollutant type, length of air pollution exposure measured (short-term vs long-term exposure), and country. We classified the measure of air pollution exposure as long-term if exposure is one year or longer. Due to large heterogeneity in the measures of COVID-19 outcomes and air pollution exposures, we did not perform a meta-analysis. Furthermore, given that many of the studies are at risk of bias, a meta-analysis may be misleading since it is likely to compound the errors. In general, it is difficult to compare result magnitudes among all studies, given the different outcome measures and since many do not interpret their coefficients beyond their value and statistical significance. However, to facilitate the identification of general patterns across the different sets of outcomes, we classified the estimates reported into positive associations, negative associations, non-significant associations, and mixed associations (that is, within the same study, the direction of the relationship is dependent on e.g., the model specification and/or sample). Only estimates that are statistically significant at the 5% level are reported as positive or negative and are otherwise classified as non-significant. Six studies were omitted because they did not indicate p-values or whether their results were statistically significantly different than zero (Agapito et al., 2020; Carteni et al., 2021; Fronza et al., 2020; Razzaq et al., 2020; Sasidharan et al., 2020 ; Zoran et al., 2020). We created summary figures graphing the direction of the association found by outcome, pollutant, length of exposure, and country.
2.4. Risk of bias assessment
We used the World Health Organization's Risk of Bias (ROB) Assessment Instrument for Systematic Reviews Informing Global Air Quality Guidelines (World Health Organization, 2020) developed for the assessment of studies examining the relationship between air quality and health. We assessed the studies using the following six domains 1) confounding, 2) selection bias, 3) exposure assessment, 4) outcome measurement, 5) missing data, and 6) selective reporting. Each of these domains was scored either as “low”, “moderate”, or “high” risk of bias based on the specific guidelines provided by the WHO evaluation instrument. Potential confounders were identified based on the ROB instrument suggestions and further adapted to consider confounders that are particularly relevant to COVID-19 outcomes. For long-term studies, critical confounders include age, sex, individual or area-level socioeconomic status, and body mass index or co-morbidities. For short-term exposure studies, critical confounders include temperature, seasonality, long-term trends, and other pandemic-specific variables such as population levels of interactions and mobility, and lock-down measures. A separate evaluation was made for each COVID-19 outcome that a study investigates and independently assessed by two authors. A detailed explanation of the ROB assessment is included in the Supplementary Materials Risk of Bias Assessment section.
3. Results
3.1. Overview of studies
The 116 included studies vary in research design, unit of observation, study period, and of length of air pollution exposure measured. Most studies use either an ecological or time-series design, comparing air pollution and COVID-19 outcomes across regions or within a region across time. Three studies implement quasi-experimental designs (Cole et al., 2020; Isphording and Pestel, 2021; Persico and Johnson, 2021). The unit of analysis varies from individual-level (n = 8) to sub-regional level (e.g., city, county, province, state). Most studies analyze COVID-19 outcomes from the first half of 2020. 64 studies focus on long-term pollution exposure, 47 study short-term exposure and three investigate both (two do not specify the length). The studies provide evidence from eight European countries including Italy (n = 35), the U.K. (n = 11), Spain (n = 6), Germany (n = 3), France (n = 3), Austria (n = 2), Poland (n = 2) and the Netherlands (n = 1), and from North America including Canada (n = 3), the U.S. (n = 39), and Mexico (n = 4). Seven studies analyze multiple countries together: six study European countries, and one reports results for both Europe and North America at the continent-level (Pozzer et al., 2020).
We first describe the COVID-19 outcomes used to measure spread/cases, followed by non-fatal severity, and deaths. 80 studies focus on how various air pollutants relate to the spread of COVID-19, resulting in 184 evaluations. These studies employ measures such as incidence, prevalence, total cases, and other transmission dynamics based on officially reported cases. The papers studying incidence (n = 43) mostly use incidence case counts or crude incidence rates – one uses a standardized incidence ratio (Huang and Brown, 2021), and another uses the daily number of new cases divided by the number of existing cases (Moshammer et al., 2021). The incidence measurement period varies across studies from daily to weekly to all new cases from the beginning of the pandemic up to a specified date. Instead, four studies define their COVID-19 outcome as prevalence (Bilal et al., 2020; Filippini et al., 2020; Hendryx and Luo, 2020). However, several studies did not differentiate between incidence and prevalence and use the total cumulative number of cases as their measure of COVID-19 (n = 24). A few studies use other COVID-19 measures such as positivity rates (Ingram et al., 2021; Isaia et al., 2021), age-standardized positivity ratios (Cazzolla Gatti et al., 2020), standardized morbidity ratios (Sahu and Böhning, 2021), seeding and doubling time (Collivignarelli et al., 2021), the reproductive numbers R0 and Rt (Chakrabarty et al., 2021; To et al., 2021), and another measure of the outbreak rate (Messner and Payson, 2021). We refer to these spread-related outcomes collectively as spread/cases.
11 studies measure COVID-19 non-fatal severity or hospitalizations, for a total of 17 pollutant-outcome evaluations. The studies on non-fatal severity mostly employ hospitalization-related measures, which include number of hospitalizations, hospitalization rates, odds of hospitalization, cases in intensive care units (ICU), rate of urgent hospitalizations, and daily ICU admissions; one study measures non-fatal severity as the probability of developing COVID-19 pneumonia (Pegoraro et al., 2021). Finally, 70 studies investigate the relationship between air pollution and COVID-19 mortality-related outcomes, for a total of 154 pollutant-outcome evaluations. These measures include total counts of COVID-19 deaths (daily new deaths or cumulative up to a given date), mortality rates, case fatality rates, and excess mortality.
The studies analyze how COVID-19 outcomes relate to ambient exposure to the following air pollutants: (n = 82), (n = 42), (n = 34), (n = 41), (n = 4), (n = 6), (n = 8) and a more general multi-pollutant air quality index, AQI (n = 4). Nine studies include other pollutant measures such as ammonia (), benzene, benzidine, acetaldehyde sulfate, aerosol optical depth (AOD), and other generic non-methane volatile organic compounds (NMVOC). Most studies evaluate more than one pollutant.
3.2. Risk of bias (ROB) assessment
For each study, we assessed each COVID-19 outcome separately, resulting in 171 evaluations. The ROB varies widely across the six evaluated categories. There was high risk of confounding in most evaluations (n = 115), moderate risk in eight evaluations and low risk in 48 of the evaluations. Most of the studies classified as high risk of confounding fail to adjust for critical variables, such as age, sex, socioeconomic status, and body-mass index. Time series studies fail to account for confounders such as mobility, lockdown measures, and seasonality. Selection-bias risk was low in most evaluations (n = 134), moderate in 24, and at high risk in 13. Air pollution exposure assessment risk was high in 11 assessments, moderate in six evaluations, and low in 154, as studies mostly employ high quality air pollution data that is measured by government or environmental agencies. COVID-19 outcome measurement risk was high in most of the evaluations (n = 148), moderate in 14, and low in only nine, where studies use excess deaths or explicitly account for testing capacity. The main concerns with COVID-19 measurements are potential errors in the measurement of officially reported COVID-19 outcomes that are systematically related to air pollution exposure, particularly COVID-19 testing, healthcare capacity, socioeconomic status, and comorbidities. We classified missing data risk of bias as unclear in 150 of the evaluations since most studies do not report whether and how much data was missing or whether it was imputed. Selective reporting is mostly at low risk of bias, as in 156 of the evaluations all effect estimates are presented for all hypotheses that the studies aim to test. An overview graph of the results and a detailed description of the risk of bias assessment is found in the Supplementary Materials ROB Assessment and Fig. A5.
3.3. Direction of relationship across COVID-19 spread/cases, hospitalizations, and deaths
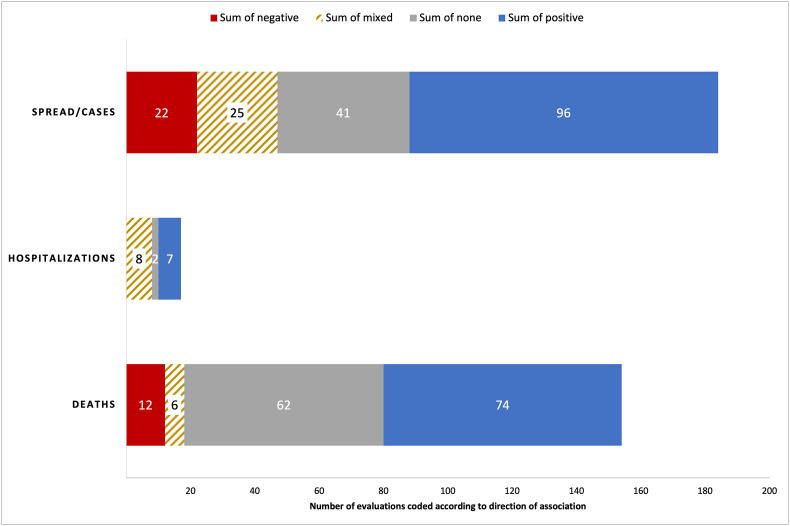
Many studies examine more than one COVID-19 outcome and air pollutant, and we report on each pollutant-outcome pair as a separate evaluation. Fig. 2 summarizes the distribution of the 355 total evaluations across COVID-19 spread/cases, non-fatal severity measured as hospitalizations (but including one study that measures severity as risk of developing COVID-19 pneumonia), and deaths, coded by the direction of association found with air pollution. Across all evaluations and outcomes, close to half report positive associations with air pollution (n = 177; 49.9%). Non-significant associations are found in 105 evaluations (29.6%), while negative and mixed associations are found in 34 (9.6%) and 39 (11.0%) of the evaluations, respectively. We next disaggregate the results by the COVID-19 outcomes shown in Fig. 2.
Fig. 2.
Direction of the relationship between air pollution and COVID-19 cases/spread, hospitalizations, and deaths. The relationship was coded separately for each pollutant and COVID-19 outcome pair for each study. Includes all countries and pollutants. None is defined as having found no statistically significant association.
3.3.1. Spread/cases
The category “spread/cases” includes all outcomes related to spread as previously defined. 96 out of 184 evaluations (52.2%) find that COVID-19 spread/cases are positively associated with higher air pollution. Furthermore, 41 evaluations (22.3%) find no statistically significant results, 25 find mixed results, and 22 find negative associations. The analyses on spread/cases are at high risk of confounding bias in 76.7% of the outcome evaluations and low risk in 19.8% of the cases. Only 16 papers studying COVID-19 spread have low confounding bias, for a total of 31 pollutant-COVID-19 evaluations. Among these evaluations, 15 (48.4%) find positive air pollution-spread associations, 14 (45.2%) find no significant associations, one finds mixed results, and one finds a negative association between air pollution and COVID-19 spread. Compared to all evaluations, those with low risk of confounding bias find a higher share with no significant associations (45.2% vs 22.3% in full sample) and almost none find mixed or negative associations. A slightly lower percent finds positive associations (48.4% vs 52.2% in full sample). This suggests that accounting for confounding bias is likely to decrease the number of studies that find negative or mixed associations and increase the number of evaluations that fail to find a significant relationship.
Focusing on the 84 evaluations that measure spread/cases as incidence, 48 (57.1%) find that increasing levels of air pollution exposure are positively associated with higher incidence, 12 evaluations find non-statistically significant results, 10 find negative, and 14 find mixed associations. For example, Fang et al. (2021) find that in the U.S., each 1 μg/m3 (microgram per cubic meter) increase in the annual average concentration of exposure is associated with a 7.56% (95% CI: 3.76%, 11.49%) increase in COVID-19 incidence risk, while in Italy, De Angelis et al. (2021) find that a 1 μg/m3 increase in annual concentrations of is associated with a 5.8% higher incidence rate. Similarly, among 68 evaluations that measure spread as the cumulative total number of cases, 36 (52.9%) find positive associations, 14 find non-significant associations, 10 find negative, and eight find mixed results. In terms of magnitude, for example, Travaglio et al. (2021) find that in the United Kingdom, increases of 1 μg/m3 in the long-term average of and are associated with a 12% and 8% increase in COVID-19 cases, respectively.
14 evaluations from four studies use prevalence to measure COVID-19 spread (Bilal et al., 2020; Filippini et al., 2020; Hendryx and Luo, 2020; Dragone et al., 2021) with varying results according to pollutant. Five evaluations (35.7%) find a positive relationship between air pollution and higher prevalence, eight evaluations (57.1%) from one single study find no significant associations (Dragone et al., 2021), and one evaluation finds a negative association. Bilal et al. (2020) find that in Germany, daily , , and are positively associated with active cases but that is negatively associated. For Northern Italy, Filippini et al. (2020) find a positive association between short-term satellite-measured tropospheric levels and SARS-CoV-2 prevalence at levels beyond 130 μmol/m2, suggesting there may be non-linearities in the relationships. Finally, Hendryx and Luo (2020) find that long-term exposure to is positively associated with prevalence in the U.S., but they fail to find a significant association for . The mixed results within studies indicate potential interactions among pollutants.
3.3.2. Hospitalizations/non-fatal severity
Among 17 evaluations from 11 studies on hospitalizations/non-fatal severity, eight evaluations (47.1%) find mixed results. In the U.S., Mendy et al. (2021) find that is positively associated with hospitalizations among COVID-19 patients with pre-existing respiratory diseases but negatively associated among those without. Instead, both Lolli et al. (2020) and Linares et al. (2021) find that the results depend on location, as they find significant positive relationships in only some cities/regions in their samples. Furthermore, Diaz et al. (2021) find that the results are either positive or null depending on whether air pollution is measured daily or averaged over 14 days. Seven evaluations find positive associations between pollutants and hospitalizations: five of these study (Cole et al., 2020; Berg et al., 2021; Bowe et al., 2021; Cascetta et al., 2021; Frontera et al., 2020) and one (Deguen and Kihal-Talantikite, 2021). Additionally, one study finds a positive association between exposure to and developing COVID-19 pneumonia (Pegoraro et al., 2021). Finally, two evaluations from one study find non-significant relationships between , , and hospitalizations (Cole et al., 2020). The analyses on hospitalizations are at high risk of confounding bias in 63.6% of the outcome evaluations, and at low risk in 36.4% of the cases. Only four papers studying hospitalizations and non-fatal severity had low risk of confounding bias, for a total of six evaluations. Of these, half (n = 3) find positive associations, two non-significant associations, and one finds mixed associations.
3.3.3. Deaths
Among 154 evaluations on COVID-19 mortality, about half (n = 74; 48.1%) find positive associations with pollutants, 62 (40.3%) evaluations find non-significant associations, 12 (7.8%) find negative associations, and six find mixed results. The analyses on mortality are at high risk of confounding bias in 56.8% of the outcome evaluations and at low risk in 36.5% of the cases. From the 23 papers with low confounding bias, there are a total of 62 evaluations. Of these, 27 (43.5%) evaluations find positive associations between air pollution and COVID-19 deaths, 32 (51.6%) find no significant effects, two (3.2%) find negative results, and one (1.6%) finds no significant results. Relative to all evaluations on COVID-19 deaths, those with low confounding bias have a higher percentage of nonsignificant results, and a lower percentage of positive, negative, and mixed results. We next stratify the results by type of mortality measures used.
44 out of 154 evaluations on mortality use measures of mortality rates or ratio. Among these, about half (n = 23) find positive associations with air pollutants and 19 find no significant associations. The magnitude of the relationship for those finding positive results varies across countries, pollutants, and models. For instance, in one of the most cited studies, Wu et al. (2020) use a negative binomial model and find that in the U.S., an increase of 1 μg/m3 in the 2000–2016 average (8.4 μg/m3; SD 2.5 μg/m3) is associated with an 11% (95% CI: 6%, 17%) increase in a county's COVID-19 mortality rate. Also in the U.S. but focusing on , Lipsitt et al. (2021) find that an interquartile range increase of 8.7 ppb in the long-term mean annual leads to an increase in COVID-19 mortality of 35% (95% CI: 23%, 48%), 44% (95% CI: 11%, 86%), and 60% (CrI: 37%, 88%), using Poisson, negative binomial, and spatial random effect models, respectively, highlighting the sensitivity of using different models. Instead in Italy, Borro et al. (2020) find that when short-term average concentration levels increase from 10 to 25 μg/m3 the mortality rate doubles from 4.5 to 9%.
Other evaluations focus on total cumulative death counts (n = 41): 21 find positive associations with pollutants, 10 find no significant associations, and nine find negative associations. Instead, focusing on case fatality rates or ratios, six out of 19 evaluations find positive relationships with pollutants, 10 find no significant associations, and three find mixed results. For example, in a U.S. study that uses multi-pollutant models, an interquartile range increase (4.6 ppb) in was associated with a COVID-19 case-fatality rate increase of 11.3% (95% CI: 4.9%, 18.2%), while in contrast, and were not significantly associated with deaths (Liang et al., 2020).
3.4. Results by pollutant
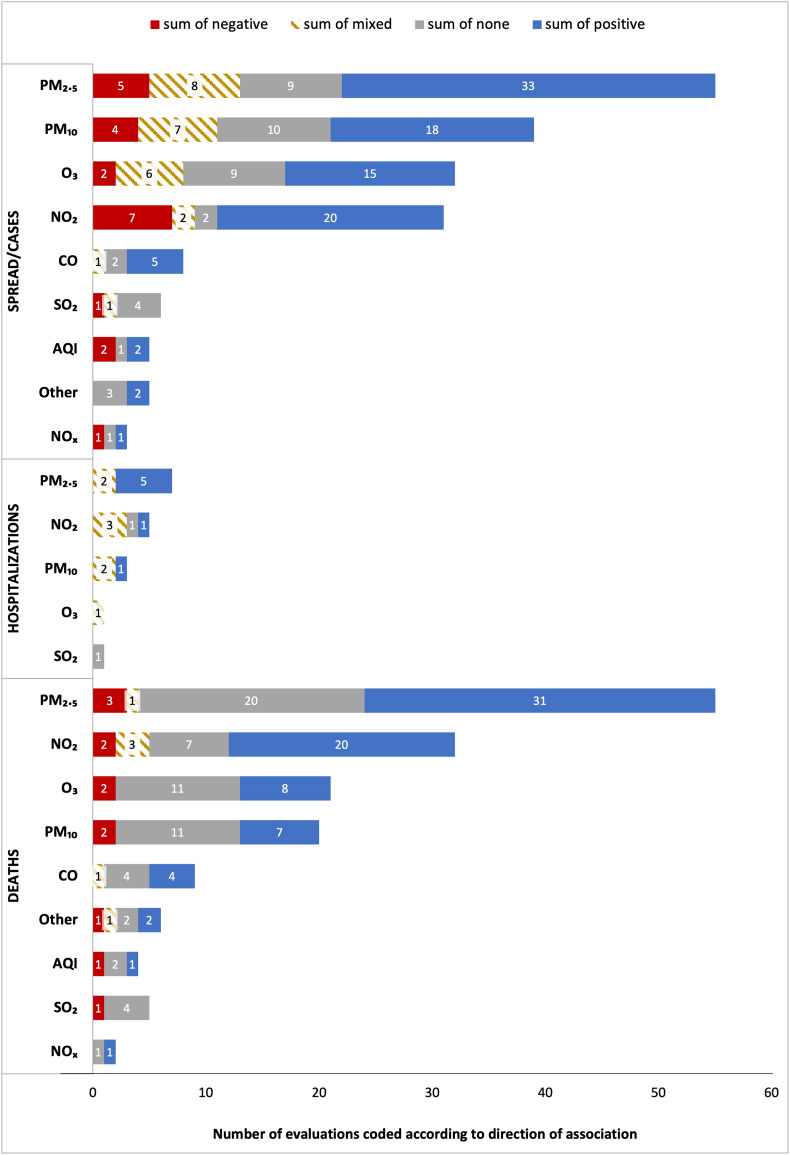
Fig. 3 graphs the direction of the relationships found among specific pollutants and COVID-19 spread/cases, hospitalizations, and deaths. Each pollutant-outcome estimate is treated as a different evaluation. Overall, among the top five pollutants measured ( , , , , and CO) studies most frequently find that COVID-19 spread/cases rise as these pollutants increase. and are also most frequently associated with more deaths. Instead, for and the most frequent result is no statistically significant relationship with COVID-19 deaths.
Fig. 3.
Direction of the relationship between air pollution and COVID-19 cases/spread, hospitalizations, and deaths split by pollutant. The relationship was coded separately for each pollutant and COVID-19 outcome pair for each study. Pollutants included: particulate matter less than 10 and 2.5 μm in diameter ( and respectively); ozone (); nitrogen dioxide (), nitrogen oxides , sulfur dioxide (), carbon monoxide () and a more general multiple-pollutant air quality index, AQI. The category “other” includes other measures of air pollution such as ammonia (), benzene, benzidine, acetaldehyde SO4, aerosol optical depth (AOD), and other non-methane volatile organic compounds (NMVOC).
is the most-studied pollutant, and among 55 evaluations, more than half (n = 33; 60.0%) find that higher levels are associated with increasingly higher COVID-19 spread; instead, nine evaluations find non-significant results, seven find mixed results, and five find negative relationships. For hospitalizations, five out of seven evaluations (71.4%) find positive associations, and for deaths 31 out of 55 (56.4%) find that higher levels are related to higher COVID-19 mortality.
The evidence for is less consistent than that of , particularly for deaths. A total of 18 out of 39 (46.2%) evaluations find that leads to more spread/cases, while 10 find non-significant associations. Instead, among 20 evaluations on COVID-19 deaths, 11 (55.0%) find no statistically significant results, while seven (35.0%) find that higher levels of lead to more deaths. Among three evaluations of and hospitalizations, one finds a positive association (Pegoraro et al., 2021) and two find mixed results (Linares et al., 2021; Diaz et al., 2021).
The results for are similar to those of . Out of the 32 evaluations between and COVID-19 spread/cases, 46.9% (n = 15) find positive associations, nine find non-significant associations, and only two find negative associations. For COVID-19 deaths, 11 out of 21 evaluations (52.4%) find no significant association and eight (38.1%) find that higher levels lead to more COVID-19 deaths. Only one evaluation analyzes hospitalizations, finding that is positively associated with the rate of urgent hospitalizations in some regions of Spain but not in others (Linares et al., 2021).
Across all pollutants, is the one with the highest percentage of evaluations finding a positive relationship with spread/cases and deaths: 20 out of 31 evaluations (64.5%) find that increasing levels of lead to higher spread/cases, while 20 out of 32 evaluations (62.5%) on deaths find a positive relationship. Instead, seven out of 31 evaluations (22.6%) find a negative relationship between and spread/cases, and for deaths this is only two out of 32 (6.3%).
Many studies evaluate more than one pollutant, but only some consider interactions among the pollutants in their models. Notably, Liang et al. (2020) use co-pollutant models and find that an increase of 4.6 parts per billion of is associated with case-fatality and mortality rate increases of 11.3% (95% CI 4.9%–18.2%) and 16.2% (95% CI 8.7%–24.0%), respectively; similarly an increase in of 2.6 mg/m3 is associated with a 14.9% (95% CI 0.0%–31.9%) increase in the COVID-19 mortality rate, adjusting for co-pollutants.
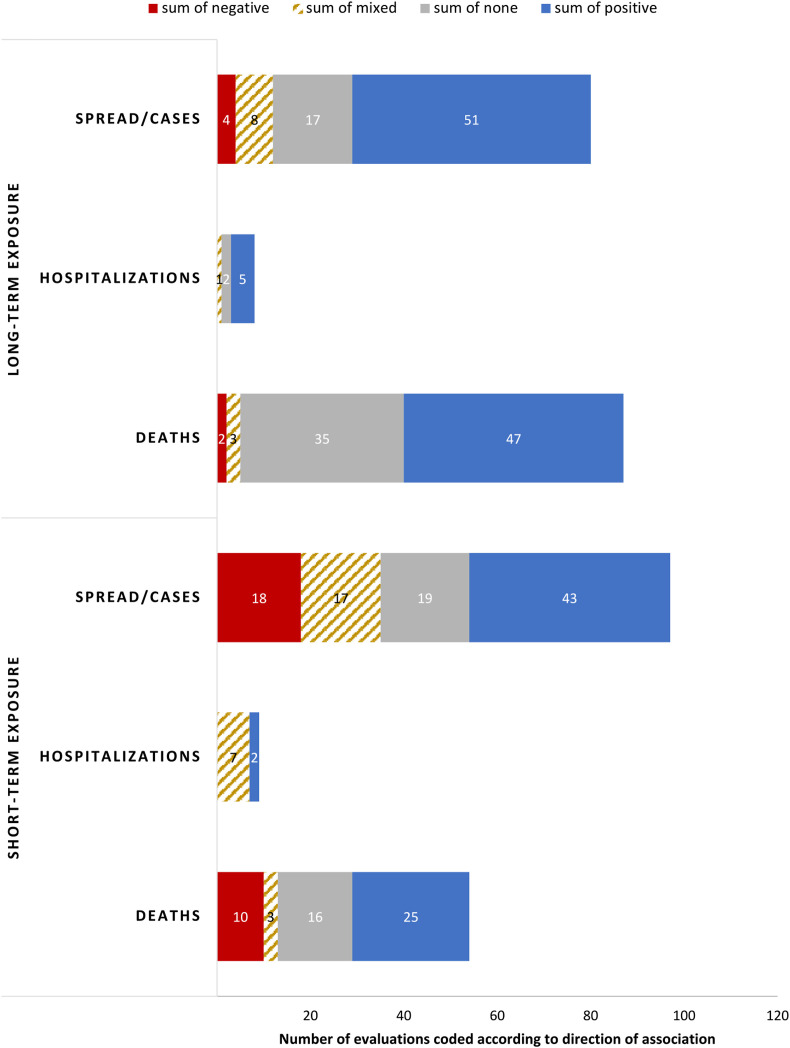
3.5. Results by length of exposure
Fig. 4 displays the direction of the association between pollutants and COVID-19 outcomes, stratified by length of exposure to air pollution. We classify the measure of air pollution exposure as long-term if is one year or longer. In this section, we omit two studies whose measure of the length of air pollution exposure was unclear. Additionally, three studies examine both long-term and short-term exposure and we code each relationship separately.
Fig. 4.
Direction of the relationship between air pollution and COVID-19 cases/spread, hospitalizations, and deaths split by length or air pollution exposure measured. The relationship was coded separately for each pollutant and COVID-19 outcome pair for each study. We classify the measure of air pollution exposure as long-term if is one year or longer. Two studies investigated both short-term and long-term exposure and their findings are reported separately for each short- and long-term evaluation. Two studies where the length of pollution measure is unclear (14 evaluations) are omitted.
Long-term exposure to air pollution is more frequently positively associated with increased COVID-19 cases and deaths compared to short-term exposure. Specifically, for COVID-19 spread/cases 63.8% (51 out of 80) of the evaluations studying long-term exposure to pollutants find positive associations, compared with 44.3% (43 out of 97) of the evaluations on short-term exposure. For deaths the gap is smaller, with 54.0% (47 out of 87) of the evaluations finding positive associations with long-term exposure to air pollution, compared to 46.3% (25 out of 54) of the evaluations studying short-term exposure. There are fewer evaluations on hospitalizations, and five out of eight find positive associations with air pollution in the long-term exposure studies, while two out of nine find positive associations in the short-term exposure studies.
Conversely, short-term exposure to air pollution is more frequently found to be negatively associated with COVID-19 spread/cases and deaths (in 18.6% and 18.5% of the evaluations, respectively) compared with the results for long-term exposure to air pollutants, in which only 5.0% and 2.3% of evaluations find that higher levels of pollutants are associated with less spread/cases and deaths, respectively.
3.6. Results of individual-level studies
A total of eight studies use individual-level data. Seven investigate the relationship between long-term exposure to air pollution and COVID-19 outcomes and one analyzes both long- and short-term air pollution exposure. Most of the studies come from the UK (n = 4) and these use UK Biobank data, while two studies come from the U.S, one from Mexico, and one from Italy. Nine evaluations from three studies focus on the relationship between pollutants and COVID-19 spread: among these, five find that pollution is positively associated with COVID-19 spread and four find no significant relationships. From three evaluations on hospitalizations, two find that pollution is positively associated with risk of hospitalization and one finds mixed results: a positive association for patients with pre-existing conditions and negative associations with patients without pre-existing conditions. Finally, for COVID-19 deaths, among 10 evaluations from three studies, six evaluations do not find evidence that air pollution is associated with COVID-19 deaths, three find positive associations, and one finds a negative association. Overall, for each of the main outcomes (COVID-19 spread, hospitalizations, and deaths) there are only three separate studies mostly coming from the UK and using the same datasets, calling for a need for more evidence using individual-level data and for studies investigating the consequences of short-term exposure to air pollution, since only one study focused on this.
3.7. Country-specific results
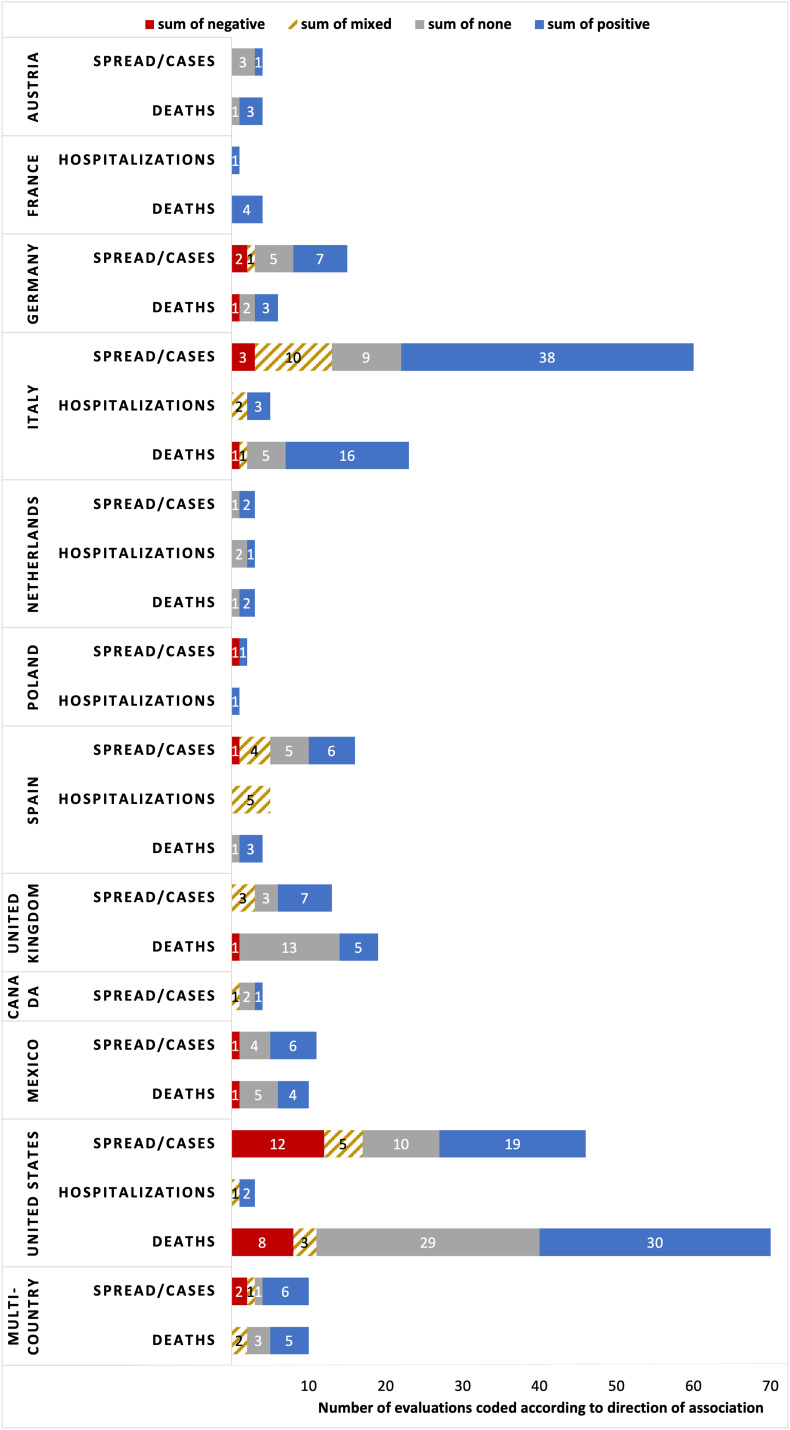
Fig. 5 shows the results of the direction of the relationships by country for cases/spread, hospitalizations, and deaths.
Fig. 5.
Direction of the relationship between air pollution and COVID-19 cases/spread, hospitalizations, and deaths split by country. The relationship was coded separately for each pollutant and COVID-19 outcome pair for each study.
3.7.1. Europe
Austria (n = 2).
In two studies from Vienna, results vary by the length of air pollution exposure and pollutant. Hutter et al. (2020) report that levels of average 2019 and above the upper quartile (30 and 20 μg/m3, respectively) are significantly associated with higher risk of a COVID-19 diagnosis (hazard ratios of 1.44 and 1.16), while only is found to increase deaths. Instead, Moshammer et al. (2021) find that a short-term increase in 1 μg/m3 of leads to a 3.2% incidence increase, but find no significant association between and cases, nor for either pollutant and COVID-19 deaths.
France (n = 3).
Three studies in France find positive associations between pollution, hospitalizations, and deaths. Two related studies on the cities of Lyon, Marseille, and Paris during March and April of 2020 use Artificial Neural Networks (ANNs) experiments and machine learning techniques and find that short-term exposure to , , and beyond certain thresholds is positively associated with COVID-19 deaths (Magazzino et al., 2020; Mele et al., 2021). Furthermore, Deguen and Kihal-Talantikite (2021) use nation-wide data and find that 2014–2018 average is positively associated with hospitalizations, cases in intensive care units, and deaths, particularly for those regions in the highest tercile of housing overcrowding.
Germany (n = 3).
10 out of 21 evaluations in Germany find that air pollutants are positively associated with higher COVID-19 spread/cases and deaths. In one of the few quasi-experimental studies, Isphording and Pestel (2021) find that significantly increases both COVID-19 deaths and cases, but find no significant effect of in a period with relatively low ozone levels. Instead, Bilal et al. (2020) use Spearman correlations to find that is the only pollutant that is consistently associated with higher COVID-19 spread/cases and deaths. Finally, Huang and Brown (2021) find that a 1 μg/m3 rise in long-term exposure to increases the COVID-19 incidence rate by 5.58% (95% CI: 3.35%, 7.86%), while is positively associated with incidence in one out of two models. However, they find no association for other pollutants (, , benzene, arsenic, cadmium and nickel).
Italy (n =35).
Italy is the most-studied European country and most evaluations find positive relationships between air pollutants and COVID-19 cases/spread (38 out of 60; 63.3%); instead 15.0% and 16.7% find null and mixed results, respectively. The results differ according to length of air pollution exposure. Among the evaluations on long-term exposure, 85.7% (24 out of 28) find positive associations with spread/cases, while 41.9% of short-term exposure studies find positive associations (13 out of 31). Fiasca et al. (2020) investigate both short- and long-term exposure to air pollution and find that a 1 μg/m3 increase in and rises incidence rates by 1.56 and 1.24 × 104 people, respectively, considering average 2016–2020 pollution, and 2.79 and 1.24 × 104 people when measuring pollution during March–May 2020.
Among the 23 evaluations on deaths, 16 (69.6%) find positive associations with pollutants, five find no associations, and one finds a negative association. Using excess mortality, one of the most accurate COVID-19 measures, Coker et al. (2020) find that a 1 μg/m3 increase in is associated with a 9% (95% confidence interval: 6–12%) increase in COVID-19 related mortality.
For hospitalizations, three out of five evaluations find positive associations. Frontera et al. (2020) and Cascetta et al. (2021) find positive associations between hospitalizations and short- and long-term exposure to , respectively. For , Pegoraro et al. (2021) use individual-level patient data and report that higher exposure the month before COVID-19 diagnosis increased the likelihood of developing COVID-19 pneumonia. Finally, Lolli et al. (2020) find mixed positive and null associations for and and hospitalizations, depending on the city studied.
All but one study are at high risk of confounding bias. The exception is Cazzolla Gatti et al. (2020) who consider critical confounders such as the number of smokers in each region, income levels, the regional levels of obesity, as well as the number of hospital beds and COVID-19 tests performed. They use a machine learning model to find that air quality and are among the most important predictors of COVID-19 positivity and mortality rates. Furthermore, the Italian evidence may be overrepresented by five similar papers from one author finding that and are positively associated with COVID-19 cases (Coccia, 2020a, 2020b, 2020c, 2021a, 2021b).
Netherlands (n = 1).
For the Netherlands, Cole et al. (2020) find that long-term exposure to leads to higher COVID-19 cases, hospitalizations, and deaths; similarly, increases in lead to more COVID-19 cases and deaths. They find no association between and COVID-19 outcomes.
Poland (n = 2).
One study in the city of Warsaw uses Spearman rank correlation tests and finds that daily ground-level is negatively associated with the number of COVID-19 cases during April to June 2020 (Wiśniewski et al., 2021). Instead, a nationwide study finds that average PM2.5 during 2019–2020 is positively associated with COVID-19 cases and excess deaths (Kowalski et al., 2021).
Spain (n = 6).
In Spain, six out of the 16 evaluations on spread/cases find positive associations, five find no associations and four find mixed results. The results vary widely across and within studies according to pollutants, region studied, and measures used. Only Zaldo-Aubanell et al. (2021) find a negative association between and COVID-19 incidence, but alongside a positive association with . Five evaluations on hospitalizations from two studies find mixed results according to region and model used (Linares et al., 2021; Diaz et al., 2021). For COVID-19 deaths, three out of four evaluations find that higher air pollution is associated with increasing COVID-19 mortality (Zaldo-Aubanell et al., 2021; Saez et al., 2020).
United Kingdom (n = 12).
In the UK, most evaluations find positive associations with COVID-19 spread and no associations with deaths. Seven out of 13 evaluations find that COVID-19 spread/cases are positively associated with air pollution. Instead, for COVID-19 deaths the results are mostly null: 13 out of 19 evaluations find no significant associations and five find positive associations. Only one evaluation finds a negative association between and COVID-19 deaths, but in this same study a positive association is found between and deaths (Travaglio et al., 2021).
3.7.2. North America
Canada (n = 3).
To et al. (2021) find that is positively associated with COVID-19 incidence but only in institutional settings (e.g., long-term care homes, hospitals, correctional facilities) and find no significant associations with the reproductive number Rt. Additionally, Stieb et al. (2020) find no significant associations between long-term exposure to nationally but find positive associations in the city of Toronto (Stieb et al., 2021).
Mexico (n = 4).
Six of the 11 evaluations on COVID-19 spread/cases find positive associations with air pollution while four find no significant results. For deaths, four out of 10 evaluations find positive relationships and five find no associations. In Mexico City, Kutralam-Muniasamy et al. (2020) find negative associations between COVID-19 cases and deaths and , positive associations with and , and nonsignificant associations with . Consistent with this, López-Feldman et al. (2021) use individual-level data in Mexico City and report that short-term exposure to is not significantly associated with COVID-19 deaths but find positive associations for long-term exposure. Instead, in the city of Victoria , , and have positive associations with COVID-19 cases (Tello-Leal and Macias-Hernandez, 2021). Finally, a study in the state of Nuevo Leon finds that is positively related to COVID-19 cases and deaths but that is not significantly associated with any outcome (Kutralam-Muniasamy et al., 2021).
United States (n =39).
For spread/cases, among a total of 46 evaluations, 19 find positive associations, 10 find no significant associations, and 12 find negative associations. Only three evaluations analyze hospitalizations. Instead, 30 out of 70 evaluations find positive associations between air pollution and COVID-19 deaths, another 29 find no significant associations, and eight find negative relationships.
The direction of the associations varies with the length of air pollution exposure, with no clear consensus for short-term exposure to air pollution, and more consistently positive and null associations for long-term exposure. Out of 26 evaluations for COVID-19 spread/cases and short-term exposure to air pollution, the outcomes are split across positive and negative associations: 10 find positive associations while 11 find negative associations. Instead, for long-term exposure to air pollution, eight out of 14 evaluations find a positive association with COVID-19 cases, four find no significant association and one study finds a negative association: Ingram et al. (2021) report that was negatively associated with the COVID-19 positivity rate but also find positive associations with and non-significant associations with .
A similar pattern as in COVID-19 spread/cases emerges when focusing on COVID-19 deaths. Of 22 evaluations on short-term exposure to air pollution and deaths, eight find positive correlations, seven find negative associations, and six find no significant associations. Instead, for evaluations on long-term exposure to pollution, 20 out of 36 (55.56%) find a positive relationship with COVID-19 deaths, while 13 find no statistically significant association. Only Hu et al. (2021) find a negative association between long-term exposure to benzidine and COVID-19 deaths, but a positive association between and deaths and no significant association with other pollutants (, , , , ). Regarding hospitalizations, only long-term exposure to air pollution is assessed, and the three existing evaluations find that air pollution is positively associated with increased hospitalizations (Mendy et al., 2021; Berg et al., 2021; Bowe et al., 2021).
The only quasi-experimental study in North America and the U.S. comes from Persico and Johnson (2021), who use a difference in differences analysis to provide causal evidence. The authors use county-level data and variation in air pollution caused by rollbacks in environmental regulation enforcement and find that an 11.8% increase in pollution is associated with a 53% and 10% in increase in COVID-19 cases and deaths, respectively.
Multi-country (n = 7).
Among the 10 evaluations on COVID-19 spread/cases, six find a positive association with pollutants, two find a negative association, and one finds no significant association. Furthermore, Zerefos et al. (2020) find a positive association between and COVID-19 cases in March–April but not in May–June. For COVID-19 deaths, among 10 evaluations, five find positive associations with air pollution, three find non-significant results, and two find mixed results. At the continent level, Pozzer et al. (2020) find that in Europe 19% (CI: 8%, 41%) of COVID-19 mortality is due to anthropogenic emissions and that this figure is 17% (CI: 6%, 39%) in North America. Instead, focusing on 23 European countries, Rodríguez-Pose and Burlina (2021) report that is not significantly associated with COVID-19 excess mortality rates. Mixed results are found by Li et al. (2020) in Germany, Italy, and Spain: whether and AOD are statistically significant depends on the model, covariates and country under investigation.
4. Discussion
In this systematic review, we present the evidence on the relationship between air pollution and COVID-19 cases, deaths, and hospitalizations for Europe and North America. Most of the studies come from the U.S. and Italy. Studies focus on both short- and long-term exposure to air pollution of mostly , , , and . More than half of the evaluations (52.2%) find that COVID-19 spread/cases increase with higher air pollution, 22.3% find no statistically significant results and 12.0% find negative associations between air pollution and spread/cases. For COVID-19 deaths, 48.1% of the evaluations find positive associations, 40.3% find no associations, and 7.8% find negative associations. The evidence for hospitalizations points to mostly mixed (47.1%) and positive associations (41.2%), although there are relatively fewer evaluations on this outcome.
Among the top five pollutants studied, our analysis finds that , , , , and are most frequently positively associated with COVID-19 spread/cases. For deaths, the evidence is less consistent: and are most frequently associated with more deaths but for and the results are mostly null. Evaluations of find the same number of positive and non-significant relationships with deaths. We cannot make clear conclusions about the other pollutants such as and , since the number of evaluations is small. Many studies focus on , given that it is likely to penetrate deep into the respiratory and cardiovascular system, creating inflammation and leading to various health repercussions.
To summarize, we find that the largest share of evaluations reports a link between higher levels of air pollution and COVID-19 incidence/spread and mortality. There are relatively fewer studies on non-fatal COVID-19 hospitalizations/severity and the existing evidence on this is mostly mixed. The impact of short-term exposure to air pollution on COVID-19 remains the most inconclusive and risks of confounding bias and COVID-19 measurement bias is the main issue with most of the existing studies. Future research should focus on study designs that control for confounding factors, for example through quasi-experimental and natural experiments, and use measures of COVID-19 that are less likely to be measured with systematic errors, such as excess mortality.
Differences in results across studies depend mainly on the length of air pollution exposure measured, the COVID-19 outcome studied, and differences in adjustments for risk of bias. More studies find negative or mixed relationships when studying short-term exposure or COVID-19 spread. It is important to consider that the evidence on short-term air pollution exposure is in general at high risk of confounding bias. One of the biggest concerns in measuring the relationship between short-term air pollution and COVID-19 outcomes is reverse causality and adjustment for omitted variables, such as mobility, lockdowns, and weather patterns during the pandemic. Indeed, the timing of lockdowns may be one of the reasons why some studies find negative associations in time-series studies of the effect of short-term exposure to pollution (Gujral and Sinha, 2021). Furthermore, many studies report a positively significant relationship in some of their models, but after accounting for covariates this relationship disappears, suggesting there is confounding or omitted variable bias. When focusing only on the studies with low risk of confounding bias, we find that there are significantly fewer evaluations reporting negative or mixed associations, but also slightly fewer evaluations reporting positive evaluations, with more evaluations failing to find a significant relationship. However, the number of studies that are at low risk of confounding bias is relatively low to make more general conclusions and other biases such as COVID-19 outcome measurement error should also be considered when interpreting these results. Across countries, the main differences in results stem from the factors mentioned above and the number of available studies. Furthermore, there may be interactions among pollutants, as some studies find positive results for some pollutants, and negative or null results for other pollutants within the same sample.
Air pollution has been proposed to affect COVID-19 outcomes through several mechanisms. Some studies suggest that exposure to pollutants could lead to oxidative stress and a more stressed respiratory tract, which increases the likelihood of COVID-19 infection, hospitalization or death. The results from the studies in this review suggest that long-term exposure to air pollution weakens the respiratory system and increases susceptibility to COVID-19.
Another early proposed mechanism, particularly during the beginning of the pandemic, was through airborne transmission of COVID-19 via its attachment to pollution particles. However, this interpretation has not been supported by successive studies (Belosi et al., 2021; Ishmatov, 2022). Furthermore, in our review we find limited evidence for this interpretation, as found by the less clear relationship between short-term contemporaneous exposure to air pollutants and COVID-19. These results suggest that there is limited evidence that COVID-19 is carried by air pollution particles.
While the evidence for North America and Europe is important, a major limitation of this review is the limited regional scope and the fact that most of the countries are high-income countries (except for Mexico). In low- and middle-income countries, COVID-19 and the impact of air pollution are likely to be significantly more catastrophic, given fewer resources, less healthcare capacity, and higher exposure to outdoor air pollution. Nevertheless, the results for the relationship between air pollution and COVID-19 outcomes in North America and Europe can still inform the association and policy decisions in other parts of the world.
Another limitation of this review is that we do not carry out a meta-analysis due to the heterogeneity in the measurement of COVID-19 outcomes, even within spread/cases, hospitalizations, and deaths. Due to the large risk of confounding and other biases found in the studies, a meta-analysis may be misleading, as it will simply compound the errors. Furthermore, given that there is inconsistency in the direction of the effect for some of the outcomes, it may be misleading to quote an average value for the effect (Higgins et al., 2021). Nonetheless, an attempt was made to synthesize the studies according to the direction of the relationship between pollutants and COVID-19. Future analyses should seek to quantify and present the associations in a meta-analysis.
In this analysis, we are not able to analyze how lockdowns or quarantine measures and the subsequent changes in air pollution interacted with the COVID-19 outcomes to moderate the results. There were various levels of lockdown measures implemented during the pandemic, and these lockdown measures likely led to different air pollution changes and COVID-19 outcomes. The studies we include are carried out throughout various stages of the pandemic, and some studies account for differences in air pollution during lockdowns by focusing on measuring pollution only before the lockdown (Rovetta and Castaldo, 2020; Setti et al., 2020; Stufano et al., 2021). As discussed by Gujral and Sinha (2021), it is important to consider lockdown restrictions, as this could explain why some studies find a negative relationship between air pollution and COVID-19 spread (Bashir et al., 2020; Rui et al., 2021). Lockdowns cause large instantaneous drops in pollutants which may not be immediately followed by COVID-19 health outcomes due to the the dynamics of the virus. However, some studies find that PM is correlated with cases both before and after lockdowns (Delnevo et al., 2020; Kotsiou et al., 2021), and other studies find mixed results during the different stages of the lockdown period and by pollutant (Kutralam-Muniasamy et al., 2020). Other studies control for the lockdown stage but do not report different results, while some do not control for lockdown measures. We acknowledge that we cannot generalize beyond this and suggest that future research study how lockdown dynamics and quarantine measures affect the relationship between air pollution and COVID-19, as there may be potential non-linear relationships between the level of air pollution and COVID-19 outcomes.
Our study allows us to disaggregate the evidence of the effects on COVID-19 by outcome, pollutant, length of air pollution exposure, and country. This is important, as different institutional and contextual settings are likely to moderate the effects of pollutants on COVID-19 outcomes. We complement existing systematic reviews and focus on the evidence in Europe and North America, where there is more comparable air pollution exposure, institutional and healthcare capacity, and COVID-19 response relative to the rest of the world. Furthermore, the higher quality of data on both air pollution and COVID-19 outcomes in these regions relative to the rest of the world allows for more reliable results.
The included studies are subject to limitations. Many of the studies used correlation analyses such as Pearson's or Spearman's coefficients, which do not allow for the addition of covariates to account for confounding. Those using multivariate models are usually ecological. While the ecological study design is the most easily accessible given the data available, this study design is prone to ecological bias and has several limitations (Wu et al., 2020). Only three studies applied quasi-experimental designs, which aim to estimate causal relationships and are likely to account for confounding factors more robustly. In addition to employing more quasi-experimental designs, future evidence should include more individual-level data, and control for critical confounders.
Another challenge most studies face is that data on COVID-19 cases and deaths is prone to measurement error that is likely to vary across regions and time. Testing capacity and death classification are likely to be related to the timeframe of the pandemic, resources and air pollution exposure. Even confirmed COVID-19 death counts are likely underestimated as they depend on testing for classification. Excess mortality is likely to be a better measure of the pandemic burden and future studies should focus on this, although methods for this calculation differ (Barnard et al., 2021).
Studying country-specific results is important since there may be a need for specific policies that reduce air pollution. Indeed, rollbacks in environmental protections during COVID-19 have been causally found to be related to higher COVID-19 cases and mortality (Persico and Johnson, 2021). In Europe, most of the evidence focuses on Italy, highlighting regions that were chronically exposed to air pollution and hit particularly hard by COVID-19 in the early stages of the pandemic. However, there are very few Italian studies that are at a low risk of confounding bias and no studies that aim to estimate causal results. Furthermore, the evidence would benefit from more studies outside of Italy, and particularly from Eastern Europe, where daily limits of air pollution are most widely exceeded (Sicard et al., 2021).
Research investigating the impacts of long-term exposure to air pollution typically measures average air pollution exposure across one or more years and links it to later health outcomes. To directly link the relationship between past exposure and later outcomes, studies assume that the population studied remained in the same geographical area, or that people moved at random. Studies would benefit from addressing or making this limitation clear. Furthermore, the effect of acute exposure to air pollution is likely to depend on the cumulative or long-term exposure to air pollution; future studies should aim to study these interactions. Since air pollution is often a localized problem, measuring exposure across large geographical areas, such as a state, may lead to large measurement errors. Furthermore, many studies investigate several pollutants but these are often studied in separate models; incorporating multi-pollutant models, for example as in Liang et al. (2020), is a key priority for future research.
Reducing air pollution is a way to reduce harmful health effects responsible for millions of global premature deaths each year. Furthermore, we find that mitigating air pollution is likely to alleviate COVID-19 severity and deaths and to reduce the harms of any future potential respiratory pandemics. Immediate action is needed to implement policies that reduce air pollution, particularly for the most vulnerable populations that are more likely to be affected by air pollution and its deadly health consequences.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.114155.
Appendix A: Supplementary Materials
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Agapito G., Zucco C., Cannataro M. COVID-WAREHOUSE: a data warehouse of Italian COVID-19, pollution, and climate data. Int. J. Environ. Res. Publ. Health. 2020;17(15) doi: 10.3390/ijerph17155596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard S., Chiavenna C., Fox S., et al. Methods for modelling excess mortality across England during the COVID-19 pandemic. Stat Methods Med Res. Published online October. 2021;23 doi: 10.1177/09622802211046384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir M.F., Ma B.J., Bilal, et al. Correlation between environmental pollution indicators and COVID-19 pandemic: a brief study in Californian context. Environ. Res. 2020;187 doi: 10.1016/j.envres.2020.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosi F., Conte M., Gianelle V., Santachiara G., Contini D. On the concentration of SARS-CoV-2 in outdoor air and the interaction with pre-existing atmospheric particles. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K., Romer Present P., Richardson K. Long-term air pollution and other risk factors associated with COVID-19 at the census tract level in Colorado. Environ Pollut Barking Essex 1987. 2021;287 doi: 10.1016/j.envpol.2021.117584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal, Bashir MF, Benghoul M, et al. Environmental pollution and COVID-19 outbreak: insights from Germany. Air Qual Atmosphere Health. Published online August 3, 2020:1-10. doi:10.1007/s11869-020-00893-9. [DOI] [PMC free article] [PubMed]
- Borro M., Di Girolamo P., Gentile G., et al. Evidence-based considerations exploring relations between SARS-CoV-2 pandemic and air pollution: involvement of PM2.5-mediated up-regulation of the viral receptor ACE-2. Int. J. Environ. Res. Publ. Health. 2020;17(15) doi: 10.3390/ijerph17155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Gibson A.K., et al. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: cohort study. Environ. Int. 2021;154 doi: 10.1016/j.envint.2021.106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteni A., Cascetta F., Di Francesco L., Palermo F. Particulate matter short-term exposition, mobility trips and COVID-19 diffusion: a correlation analyses for the Italian case study at urban scale. Sustainability. 2021;13(8):4553. doi: 10.3390/su13084553. [DOI] [Google Scholar]
- Cascetta E., Henke I., Di Francesco L. The effects of air pollution, sea exposure and altitude on COVID-19 hospitalization rates in Italy. Int. J. Environ. Res. Publ. Health. 2021;18(2) doi: 10.3390/ijerph18020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzolla Gatti R., Velichevskaya A., Tateo A., Amoroso N., Monaco A. Machine learning reveals that prolonged exposure to air pollution is associated with SARS-CoV-2 mortality and infectivity in Italy. Environ Pollut Barking Essex 1987. 2020;267 doi: 10.1016/j.envpol.2020.115471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty R.K., Beeler P., Liu P., et al. Ambient PM2.5 exposure and rapid spread of COVID-19 in the United States. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciencewicki J., Jaspers I. Air pollution and respiratory viral infection. Inhal. Toxicol. 2007;19(14):1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- Coccia M. The effects of atmospheric stability with low wind speed and of air pollution on the accelerated transmission dynamics of COVID-19. Int. J. Environ. Stud. 2020;78(1):1–27. doi: 10.1080/00207233.2020.1802937. [DOI] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. How (Un)sustainable environments are related to the diffusion of COVID-19: the relation between coronavirus disease 2019, air pollution, wind resource and energy. Sustainability. 2020;12(22):9709. doi: 10.3390/su12229709. [DOI] [Google Scholar]
- Coccia M. How do low wind speeds and high levels of air pollution support the spread of COVID-19? Atmos. Pollut. Res. 2021;12(1):437–445. doi: 10.1016/j.apr.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Effects of the spread of COVID-19 on public health of polluted cities: results of the first wave for explaining the dejà vu in the second wave of COVID-19 pandemic and epidemics of future vital agents. Environ. Sci. Pollut. Res. Int. 2021;28(15):19147–19154. doi: 10.1007/s11356-020-11662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.J., Brauer M., Burnett R., et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet Lond Engl. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker ES, Cavalli L, Fabrizi E, et al. The effects of air pollution on COVID-19 related mortality in northern Italy. Environ. Resour. Econ. Published online August 4, 2020:1-24. doi:10.1007/s10640-020-00486-1. [DOI] [PMC free article] [PubMed]
- Cole M.A., Ozgen C., Strobl E. Air pollution exposure and covid-19 in Dutch municipalities. Environ. Resour. Econ. 2020:1–30. doi: 10.1007/s10640-020-00491-4. Published online August 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Abbà A., Caccamo F.M., et al. Can particulate matter be identified as the primary cause of the rapid spread of CoViD-19 in some areas of Northern Italy? Environ Sci Pollut Res Int. Published online February. 2021;26:1–13. doi: 10.1007/s11356-021-12735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D., Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere. 2020;11(4):377. doi: 10.3390/atmos11040377. [DOI] [Google Scholar]
- Copat C., Cristaldi A., Fiore M., et al. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: a systematic review. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh Yazdi M., Wang Y., Di Q., et al. Long-term association of air pollution and hospital admissions among medicare participants using a doubly robust additive model. Circulation. 2021;143(16):1584–1596. doi: 10.1161/CIRCULATIONAHA.120.050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis E., Renzetti S., Volta M., et al. COVID-19 incidence and mortality in Lombardy, Italy: an ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguen S., Kihal-Talantikite W. Geographical pattern of COVID-19-related outcomes over the pandemic period in France: a nationwide socio-environmental study. Int. J. Environ. Res. Publ. Health. 2021;18(4) doi: 10.3390/ijerph18041824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnevo G., Mirri S., Roccetti M. Particulate matter and COVID-19 disease diffusion in Emilia-Romagna (Italy). Already a cold case? Computation. 2020;8(2):59. doi: 10.3390/computation8020059. [DOI] [Google Scholar]
- Diaz J., Antonio-Lopez-Bueno J., Culqui D., Asensio C., Sanchez-Martinez G., Linares C. Does exposure to noise pollution influence the incidence and severity of COVID-19? Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragone R., Licciardi G., Grasso G., Del Gaudio C., Chanussot J. Analysis of the chemical and physical environmental aspects that promoted the spread of SARS-CoV-2 in the Lombard area. Int. J. Environ. Res. Publ. Health. 2021;18(3) doi: 10.3390/ijerph18031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Mu L., Zhu Y., Rao J., Heymann J., Zhang Z.F. Long-term exposure to PM2.5, facemask mandates, stay home orders and COVID-19 incidence in the United States. Int. J. Environ. Res. Publ. Health. 2021;18(12) doi: 10.3390/ijerph18126274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiasca F., Minelli M., Maio D., et al. Associations between COVID-19 incidence rates and the exposure to PM2.5 and NO2: a nationwide observational study in Italy. Int. J. Environ. Res. Publ. Health. 2020;17(24) doi: 10.3390/ijerph17249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T., Rothman K.J., Goffi A., et al. Satellite-detected tropospheric nitrogen dioxide and spread of SARS-CoV-2 infection in Northern Italy. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J. Infect. 2020;81(2):255–259. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronza R., Lusic M., Schmidt M., Lucic B. Spatial-Temporal variations in atmospheric factors contribute to SARS-CoV-2 outbreak. Viruses. 2020;12(6) doi: 10.3390/v12060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral H., Sinha A. Association between exposure to airborne pollutants and COVID-19 in Los Angeles, United States with ensemble-based dynamic emission model. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasell J., Mathieu E., Beltekian D., et al. A cross-country database of COVID-19 testing. Sci. Data. 2020;7(1):345. doi: 10.1038/s41597-020-00688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendryx M., Luo J. COVID-19 prevalence and fatality rates in association with air pollution emission concentrations and emission sources. Environ Pollut Barking Essex 1987. 2020;265(Pt A) doi: 10.1016/j.envpol.2020.115126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Chandler J., Cumpston M., Li T., Page M., Welch V. Cochrane handbook for systematic reviews of interventions. February 2021. https://training.cochrane.org/handbook/current/chapter-10#section-10-2
- Hu H., Zheng Y., Wen X., et al. An external exposome-wide association study of COVID-19 mortality in the United States. Sci. Total Environ. 2021;768 doi: 10.1016/j.scitotenv.2020.144832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Brown P.E. Population-weighted exposure to air pollution and COVID-19 incidence in Germany. Spat Stat. 2021;41 doi: 10.1016/j.spasta.2020.100480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter H.P., Poteser M., Moshammer H., et al. Air pollution is associated with COVID-19 incidence and mortality in Vienna, Austria. Int. J. Environ. Res. Publ. Health. 2020;17(24) doi: 10.3390/ijerph17249275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram C, Min E, Seto E, Cummings BJ, Farquhar S. Cumulative impacts and COVID-19: implications for low-income, minoritized, and health-compromised communities in king county, WA. J Racial Ethn Health Disparities. Published online June 14, 2021:1-15. doi:10.1007/s40615-021-01063-y. [DOI] [PMC free article] [PubMed]
- Isaia G., Diemoz H., Maluta F., et al. Does solar ultraviolet radiation play a role in COVID-19 infection and deaths? An environmental ecological study in Italy. Sci. Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishmatov A. “SARS-CoV-2 is transmitted by particulate air pollution”: misinterpretations of statistical data, skewed citation practices, and misuse of specific terminology spreading the misconception. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isphording I.E., Pestel N. Pandemic meets pollution: poor air quality increases deaths by COVID-19. J. Environ. Econ. Manag. 2021;108 doi: 10.1016/j.jeem.2021.102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoto P.D.M.C., Brand A.S., Bakan B., et al. Acute and chronic exposure to air pollution in relation with incidence, prevalence, severity and mortality of COVID-19: a rapid systematic review. Environ. Health. 2021;20(1):41. doi: 10.1186/s12940-021-00714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomenko S., Cirach M., Pereira-Barboza E., et al. Premature mortality due to air pollution in European cities: a health impact assessment. Lancet Planet. Health. 2021;5(3):e121–e134. doi: 10.1016/S2542-5196(20)30272-2. [DOI] [PubMed] [Google Scholar]
- Kotsiou O.S., Kotsios V.S., Lampropoulos I., Zidros T., Zarogiannis S.G., Gourgoulianis K. PM2.5 pollution strongly predicted COVID-19 incidence in four high-polluted urbanized Italian cities during the pre-lockdown and lockdown periods. Int. J. Environ. Res. Publ. Health. 2021;18(10):5088. doi: 10.3390/ijerph18105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski P.A., Szwagrzyk M., Kielpinska J., Konior A., Kusy M. Numerical analysis of factors, pace and intensity of the corona virus (COVID-19) epidemic in Poland. Ecol. Inf. 2021;63 doi: 10.1016/j.ecoinf.2021.101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G., Pérez-Guevara F., Roy P.D., Elizalde-Martínez I., Shruti V.C. Impacts of the COVID-19 lockdown on air quality and its association with human mortality trends in megapolis Mexico City. Air Qual Atmosphere Health. 2020:1–10. doi: 10.1007/s11869-020-00960-1. Published online October 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G., Perez-Guevara F., Elizalde Martinez I., Chari S.V. Particulate matter concentrations and their association with COVID-19-related mortality in Mexico during June 2020 Saharan dust event. Environ Sci Pollut Res. Published online. 2021 doi: 10.1007/s11356-021-14168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A., Chang M.L., O'Donnell R.P., Zhou C., Sumner J.A., Hsiai T.K. Association of COVID-19 transmission with high levels of ambient pollutants: initiation and impact of the inflammatory response on cardiopulmonary disease. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Thomas R., El-Askary H., Piechota T., Struppa D., Abdel Ghaffar K.A. Investigating the significance of aerosols in determining the coronavirus fatality rate among three European countries. Earth Syst Environ. 2020;4(3):513–522. doi: 10.1007/s41748-020-00176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Shi L., Zhao J., et al. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. Innovat. Neurosurg. N. 2020;1(3) doi: 10.1016/j.xinn.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares C., Culqui D., Belda F., et al. Impact of environmental factors and Sahara dust intrusions on incidence and severity of COVID-19 disease in Spain. Effect in the first and second pandemic waves. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-14228-3. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitt J., Chan-Golston A.M., Liu J., Su J., Zhu Y., Jerrett M. Spatial analysis of COVID-19 and traffic-related air pollution in Los Angeles. Environ. Int. 2021;153 doi: 10.1016/j.envint.2021.106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolli S., Chen Y.C., Wang S.H., Vivone G. Impact of meteorological conditions and air pollution on COVID-19 pandemic transmission in Italy. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-73197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Feldman A., Heres D., Marquez-Padilla F. Air pollution exposure and COVID-19: a look at mortality in Mexico City using individual-level data. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazzino C., Mele M., Schneider N. The relationship between air pollution and COVID-19-related deaths: an application to three French cities. Appl. Energy. 2020;279 doi: 10.1016/j.apenergy.2020.115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki M., Anvari E., Hopke P.K., Noorimotlagh Z., Mirzaee S.A. An updated systematic review on the association between atmospheric particulate matter pollution and prevalence of SARS-CoV-2. Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquès M., Domingo J.L. Positive association between outdoor air pollution and the incidence and severity of COVID-19. A review of the recent scientific evidences. Environ. Res. 2022;203 doi: 10.1016/j.envres.2021.111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Shin H., Burnett R., North T., Cohen A.J. Ambient particulate air pollution and acute lower respiratory infections: a systematic review and implications for estimating the global burden of disease. Air Qual Atmosphere Health. 2013;6(1):69–83. doi: 10.1007/s11869-011-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele M., Magazzino C., Schneider N., Strezov V. NO2 levels as a contributing factor to COVID-19 deaths: the first empirical estimate of threshold values. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A., Wu X., Keller J.L., et al. Long-term exposure to fine particulate matter and hospitalization in COVID-19 patients. Respir. Med. 2021;178 doi: 10.1016/j.rmed.2021.106313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner W., Payson S.E. Contextual factors and the COVID-19 outbreak rate across U.S. counties in its initial phase. Health Sci Rep. 2021;4(1):e242. doi: 10.1002/hsr2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshammer H., Poteser M., Hutter H.P. Wien Klin Wochenschr; 2021. COVID-19 and Air Pollution in Vienna-a Time Series Approach. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paital B., Agrawal P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: a review. Environ Chem Lett. Published online September. 2020;18:1–18. doi: 10.1007/s10311-020-01091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro V., Heiman F., Levante A., Urbinati D., Peduto I. An Italian individual-level data study investigating on the association between air pollution exposure and Covid-19 severity in primary-care setting. BMC Publ. Health. 2021;21(1):902. doi: 10.1186/s12889-021-10949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico C.L., Johnson K.R. The effects of increased pollution on COVID-19 cases and deaths. J. Environ. Econ. Manag. 2021;107 doi: 10.1016/j.jeem.2021.102431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer A., Dominici F., Haines A., Witt C., Münzel T., Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020;116(14):2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq A., Sharif A., Aziz N., Irfan M., Jermsittiparsert K. Asymmetric link between environmental pollution and COVID-19 in the top ten affected states of US: a novel estimations from quantile-on-quantile approach. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Pose A., Burlina C. Institutions and the uneven geography of the first wave of the COVID-19 pandemic. J Reg Sci. Published online. 2021 doi: 10.1111/jors.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovetta A., Castaldo L. Relationships between demographic, geographic, and environmental statistics and the spread of novel coronavirus disease (COVID-19) in Italy. Cureus. 2020;12(11) doi: 10.7759/cureus.11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui R., Tian M., Tang M.L., Ho G.T.S., Wu C.H. Analysis of the spread of COVID-19 in the USA with a spatio-temporal multivariate time series model. Int. J. Environ. Res. Publ. Health. 2021;18(2) doi: 10.3390/ijerph18020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez M., Tobias A., Barceló M.A. Effects of long-term exposure to air pollutants on the spatial spread of COVID-19 in Catalonia, Spain. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu S.K., Böhning D. Bayesian spatio-temporal joint disease mapping of Covid-19 cases and deaths in local authorities of England. Spat Stat. Published online May. 2021;12 doi: 10.1016/j.spasta.2021.100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan M., Singh A., Torbaghan M.E., Parlikad A.K. A vulnerability-based approach to human-mobility reduction for countering COVID-19 transmission in London while considering local air quality. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., et al. Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2020-039338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard P., Agathokleous E., De Marco A., Paoletti E., Calatayud V. Urban population exposure to air pollution in Europe over the last decades. Environ. Sci. Eur. 2021;33(1):28. doi: 10.1186/s12302-020-00450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb D.M., Evans G.J., To T.M., Brook J.R., Burnett R.T. An ecological analysis of long-term exposure to PM2.5 and incidence of COVID-19 in Canadian health regions. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb, D.M., Evans, G.J., To, T.M., et al., Within-city variation in reactive oxygen species from fine particle air pollution and COVID-19. Am. J. Respir. Crit. Care Med. Published online April 2, 2021. 10.1164/rccm.202011-4142OC. [DOI] [PMC free article] [PubMed]
- Stufano A., Lisco S., Bartolomeo N., et al. COVID19 outbreak in Lombardy, Italy: an analysis on the short-term relationship between air pollution, climatic factors and the susceptibility to SARS-CoV-2 infection. Environ. Res. 2021;198 doi: 10.1016/j.envres.2021.111197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello-Leal E., Macias-Hernandez B.A. Association of environmental and meteorological factors on the spread of COVID-19 in Victoria, Mexico, and air quality during the lockdown. Environ. Res. 2021;196 doi: 10.1016/j.envres.2020.110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T., Zhang K., Maguire B., et al. UV, ozone, and COVID-19 transmission in Ontario, Canada using generalised linear models. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. Links between air pollution and COVID-19 in England. Environ Pollut Barking Essex 1987. 2021;268(Pt A) doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs: Population Division . World population prospects 2019. Volume II: Demographic Profiles (ST/ESA/SER.A/427) United Nations; 2019. https://population.un.org/wpp/Publications/Files/WPP2019_Volume-II-Demographic-Profiles.pdf [Google Scholar]
- United Nations Preventing pollution. UNEP - UN environment programme. 2017. http://www.unep.org/regions/north-america/regional-initiatives/preventing-pollution Published October 25. Accessed February 21, 2022.
- Villeneuve P.J., Goldberg M.S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Perspect. 2020;128(9) doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski Oskar, Kozak Wieslaw, Wisniewski Maciej. The ground-level ozone concentration is inversely correlated with the number of COVID-19 cases in Warsaw, Poland. Air Quality Atmosphere and Health. 2021 doi: 10.1007/s11869-021-01009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Risk Of Bias Assessment Instrument For Systematic Reviews Informing WHO Global Air Quality Guidelines. World Health Organization. Regional Office for Europe.https://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2020/risk-of-bias-assessment-instrument-for-systematic-reviews-informing-who-global-air-quality-guidelines-2020 [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6(45) doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldo-Aubanell Q., Lopez FC i, Bach A., et al. Community risk factors in the COVID-19 incidence and mortality in Catalonia (Spain). A population-based study. Int. J. Environ. Res. Publ. Health. 2021;18(7):3768. doi: 10.3390/ijerph18073768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerefos C.S., Solomos S., Kapsomenakis J., et al. Lessons learned and questions raised during and post-COVID-19 anthropopause period in relation to the environment and climate. Environ Dev Sustain. Published online November. 2020;19:1–23. doi: 10.1007/s10668-020-01075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.