Abstract
The COVID-19 pandemic created an explosion in the use of telehealth. However, telehealth consists of much more than a video discussion between doctor and patient. Since the onset of the COVID-19 pandemic, allergists have demonstrated a high level of synchronous telemedicine adoption with existing patients but have not taken full advantage of other virtual care modalities that have the potential to facilitate the efficient delivery of allergy care to the broader population. This is partially due to a lack of awareness about the various remote care services and how to implement and bill for them appropriately. This rostrum describes the spectrum of telehealth services, reviews existing literature on the use of telehealth in allergy, and provides suggestions about how allergists and immunologists can optimize the use of telehealth to optimize patient access and outcomes as well as receive appropriate compensation for specialty clinical services provided by themselves and their staff.
Key words: Telemedicine, Telehealth, Virtual care, Remote care, Remote physiologic monitoring, Remote therapeutic monitoring, Principal care management, Interprofessional consultation
INFORMATION FOR CATEGORY 1 CME CREDIT.
Credit can now be obtained, free for a limited time, by reading the review articles in this issue. Please note the following instructions.
Method of Physician Participation in Learning Process: The core material for these activities can be read in this issue of the Journal or online at the JACI: In Practice Web site: www.jaci-inpractice.org/. The accompanying tests may only be submitted online at www.jaci-inpractice.org/. Fax or other copies will not be accepted.
Date of Original Release: October 1, 2022. Credit may be obtained for these courses until September 30, 2023.
Copyright Statement: Copyright © 2022-2024. All rights reserved.
Overall Purpose/Goal: To provide excellent reviews on key aspects of allergic disease to those who research, treat, or manage allergic disease.
Target Audience: Physicians and researchers within the field of allergic disease.
Accreditation/Provider Statements and Credit Designation: The American Academy of Allergy, Asthma & Immunology (AAAAI) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The AAAAI designates this journal-based CME activity for 1.00 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
List of Design Committee Members: Sakina S. Bajowala, MD, Jennifer Shih, MD, Pooja Varshney, MD, and Tania Elliott, MD (authors); Michael Schatz, MD, MS (editor)
Learning objectives:
-
1.
To describe the various modalities of telehealth services that can be utilized to provide care for allergy/immunology patients.
-
2.
To identify barriers to the implementation of telehealth into allergy practice and strategies to overcome these challenges.
-
3.
To discuss how to effectively integrate telehealth and in-person care delivery for common allergic conditions.
Recognition of CommercialSupport: This CME has not received external commercial support.
Disclosure of Relevant Financial Relationships with Commercial Interests: Dr Bajowala is the owner of Wise Prince, LLC. Dr Elliott is a speaker for Teva. All other authors and reviewers reported no relevant financial relationships.
Introduction
Telemedicine (TM) is defined by the Centers for Medicare and Medicaid Services (CMS) as “the exchange of medical information from one site to another through electronic communication to improve a patient’s health.”1 Telehealth is an umbrella term incorporating a wide range of technologies such as video conferencing, telephones, facsimile machines, electronic mail systems, online patient portals, and remote patient monitoring (RPM) devices used to collect and transmit patient data for interpretation and treatment.1 , 2 All forms of telehealth overcome common barriers of time and transportation.3 Though gaining traction for years, adoption of telehealth grew exponentially during the COVID-19 global pandemic and facilitated the delivery of medical care during a time of unprecedented uncertainty and isolation.
Telemedicine visits provide health care services through synchronous, real-time audio and video communication1 and are known as virtual visits. Synchronous video visits require the patient and clinician to be online at the same time and offer the opportunity for face-to-face communication and a virtual physical examination. A facilitated video visit requires a patient to travel to a facility where a telefacilitator and digital examination equipment are available to perform a physical examination.3 Facilitated visits allow for the collection of more objective data as well as ancillary testing such as pulmonary function tests and skin tests. In an unfacilitated visit, patients use their own equipment to connect with the clinician.3 Although a complete physical examination is impossible in this type of visit, patients can be coached to measure basic vital signs (ie, weight, temperature, blood pressure) and useful physical findings can be observed by the examiner (ie, general appearance, pallor, oropharynx, respiratory pattern, neurologic status). Virtual physical examination can be enhanced with the use of peripheral devices, such as camera-enabled otoscopes and digital stethoscopes. A number of these devices are commercially available directly to patients for on-demand use. For certain visit types that do not require a physical examination, audio-only visits also allow for real-time communication between the clinician and patient.
Electronic consultations are initiated by referring clinicians, who send a brief description of the patient with specific questions to the consultant, who then reviews the case and issues written recommendations. Electronic consults may result in diagnosis and treatment recommendations, a request for in-person consultation, or an alternative referral.3 Electronic visits are patient-initiated, asynchronous, written communications through a patient portal or other online mechanism; virtual check-ins are also patient-initiated phone or secure digital communications with a physician or qualified health provider (QHP), generally to determine whether additional evaluation and management is needed.1
A subset of telehealth is RPM, which refers to the collection, transmission, and analysis of electronic data for disease treatment or management. With the advent of devices to collect these data, the term is often used synonymously with remote physiologic monitoring, which refers specifically to the collection of near real-time data from a US Food and Drug Administration (FDA)-cleared device. The CMS defines billable RPM as monitoring that occurs for 16 days or more in a 30-day period through an FDA-cleared device such as a biosensor, heart rate monitor, skin temperature patch, blood pressure cuff, glucometer, or pulse oximeter. Remote therapeutic monitoring (RTM) refers to the collection, transmission, and analysis of nonphysiologic data through a software platform or device for specific conditions, such as musculoskeletal disease, respiratory disease, or, more broadly, medication adherence. The software or device must be considered software as a medical device.4 This can facilitate early detection of asthma exacerbations as well as adherence with daily controllers.
During the early months of the COVID-19 pandemic, telehealth enabled health care professionals to continue to care for patients remotely in a climate of physical distancing and fear of contagion. Allergists and immunologists were no exception to the explosion in telehealth adoption. Surveys revealed an increase in TM use among US allergists from 1% of visits before COVID to 54% of visits in April 2020, only 1 month after the institution of lockdowns across the United States.5 With the foreseeable transition of COVID-19 from pandemic to endemic, patients and clinicians alike have once again resumed in-person care. In fact, as early as August 2020, the share of TM visits by allergists had decreased to 23%.5 Nevertheless, telehealth’s benefits in terms of access to care, convenience, and efficiency have made this method of health care delivery integral to modern practice and unlikely to be abandoned despite the resumption of in-person care.
Moving forward, the ways in which telehealth services are administered cannot remain unchanged. Whereas the initial implementation of virtual care delivery was rushed by necessity and partially dependent on regulatory relaxation and payment expansion, a tightening of these laxities is now under way.6 This will require allergists to stay up-to-date with a rapidly evolving regulatory and payment landscape to ensure care is being provided in a compliant manner. In addition, much of the telehealth implemented by allergists during the COVID-19 pandemic has been limited to synchronous audio with or without video. A thoughtful approach to integrating the full spectrum of telehealth services into the post-COVID health care delivery landscape will be required if allergists are to optimize the provision of virtual care into the clinical tool kit.
History Of Telehealth
The idea of telehealth and RPM is not new. In 1924, Radio News magazine suggested the “radio doctor.” In the 1960s, the National Aeronautics and Space Administration first monitored the health of astronauts in space.
Before the COVID-19 pandemic, telehealth use had been increasing in the United States. More than 15 million Americans had received some form of remote medical care in 2015.7 Multiple benefits of telehealth in allergy and immunology have been reported, such as expanded access to underserved areas, reduced travel time and cost for patients,7 and equivalent or even improved asthma outcomes,8 , 9 including in school-based programs.10 Nevertheless, there have been obstacles to adoption, with unique challenges to allergy and immunology practice such as ensuring the continued delivery of in-person pulmonary function testing, skin testing, medication and food issues, allergen immunotherapy, and biologic injections.11 Despite these earlier considerations, growth and adoption of telehealth were slow before the COVID-19 pandemic because of these challenges and inconsistent payer coverage.
Early in the COVID-19 pandemic, many of these barriers were lifted, and TM use grew exponentially as patients and clinicians sought ways to continue to access and deliver health care safely. According to the May 2020 McKinsey report,12 overall TM use for office visits was 78 times higher in April 2020 than in February 2020 (prepandemic). This surge resulted from necessity and was spurred by increased patient and physician or QHP willingness to use telehealth. Regulatory changes that enabled greater access and reimbursement to perform telehealth also contributed to the telehealth surge. With this surge comes the opportunity to study and improve health care access, outcomes, and costs.
Telehealth Satisfaction And Outcomes
Telehealth has been used to manage disease processes in every specialty such as cardiology; burns; diabetes; obesity; emergency medicine; speech and hearing loss; ear, nose, and throat; psychology and psychiatry; radiology; oncology; home health care; asthma; genetics; and dentistry. One study looked at what type of visits were most common in tele-allergy. Food-related reactions (50.4%), urticaria and angioedema (23.2%), and rhinitis (18.1%) were the most common reasons for new referrals to TM during the COVID-19 pandemic. Of these new patient referrals, 29% did not require further allergy care; the overall experience was rated as very good or good for most patients (85%).13
In fact, patient and physician or QHP satisfaction with widespread adoption of TM during the pandemic has been positive overall. Patients consistently report a 95% to 100% satisfaction rate with TM compared with in-person appointments. They tend to cite the convenience of decreased travel times and costs as the main drivers for satisfaction with TM. Physicians and QHPs tend to be satisfied with TM when they have input into its development, there is administrative support, the technology is reliable and easy to use, and there is adequate reimbursement for its use.14
In a patient satisfaction study specific to an allergy clinic, 88% of patients rated their comfort level seeing a doctor via TM at 10 (the most positive rating). Moreover, 93% of respondents stated that their doctor explained their condition in an easily understood manner, 79% strongly agreed that connecting to their TM appointments was easy, and 77% would strongly recommend TM services to others. However, only 46% indicated a preference for future TM visits after resolution of the pandemic. There were various reasons stated by responders who would not preferentially elect for telehealth again. Some were disappointed with the limitations of the physical examination and access to ancillary testing such as laboratories and pulmonary function tests. Others expressed discomfort with video conferencing technology, whereas a minority expressed a compromise in the level of rapport with the doctor. Those who highly preferred TM visits expressed satisfaction with convenience, decreased wait times, and savings in money and transportation time.15 In another study that focused on allergy clinics, nearly 97% of patients were satisfied with the TM encounter, and 77.4% believed it was as satisfactory as an in-person encounter.16
There are several publications regarding the benefits of telehealth and RPM in allergy and immunology, but studies are lacking on health care outcomes managed by telehealth versus management by in-person visits. Systematic reviews in tele-pediatrics show that studies have not proven the clinical effectiveness of TM and have suggested further studies to assess the clinical outcomes of services provided through TM technologies.17 There is also modest empirical evidence for the effectiveness of electronic consults on important outcomes. Most studies are observational and within a single health care system, and comprehensive assessments are lacking. For the outcomes that were reported, findings are generally positive, with mixed results for clinician experience.18
Most outcome studies relating to the field of allergy and telehealth focus on asthma. A few studies before the pandemic showed that children with asthma seen by TM or through in-person visits can achieve comparable degrees of asthma control.8 A virtual group of pediatric patients achieved excellent asthma therapeutic and disease control outcomes after 1 year compared with those who received standardized office-based care. They were more adherent to diary submission and had better inhaler scores at 52 weeks, but there were no other differences in therapeutic or disease control outcome measures.19 In a systematic review, outcomes were examined for school-age children with asthma involving asthma-based telemedical education. Clinical outcomes showed mixed support for improvements in airway inflammation, medication use, symptom burden and symptom-free days, and spirometry.20 In adult patients, combined TM involving tele-case management or tele-consultation appeared to be an effective telehealth intervention to improve asthma control and quality of life.9
Outcome studies involving remote monitoring of patient data in allergy are also scarce. A study looking at electronic health interventions, especially mobile health interventions, showed that they are effective and acceptable in improving patient adherence to inhaled corticosteroids (ICS) in asthma.21 Another randomized behavioral controlled trial demonstrated that patient self-monitoring via an electronic medication monitor and smartphone app plus remote clinician feedback on inhaler use helped maintain baseline adherence to ICS-containing controller medications and decrease the percentage of days with short-acting β-agonist use compared with the control group. These results are encouraging because ICS adherence typically declines over time. In addition to overreliance on short-acting β-agonists, it is consistently associated with increased asthma morbidity, mortality, and costs in asthma care.22
During the COVID-19 pandemic, it has been extremely important to monitor respiratory vital signs, but many people needed this monitoring to occur remotely owing to self-isolation at home. In addition, even robust health care systems were facing a shortage of health care professionals, personal protective equipment, beds, and mechanical ventilators in intensive care units.23 This highlights the need for alternative medical solutions, including RPM.24
Technological solutions grew for the remote monitoring of respiratory rate (RR) in COVID-19 patients, spurred by new FDA policies25 during the COVID-19 pandemic. Respiratory rate monitoring by RPM can be achieved employing the built-in camera of a smart device that can be used to record RR from respiratory-induced chest wall movements or superficial changes in the facial perfusion of a seated patient. The built-in microphone of a smartphone can be used to record RR from the breathing sounds of the patient. A mattress can be adapted for continuous RR monitoring by registering the breathing-related chest wall movements of the patient. Radio wave or Wi-Fi signal sources can be used to register RR values by modulating the transmitted signals by respiratory-related thoracic movements. A smart garment can be worn to record RR continuously from the respiratory-related periodic changes in chest wall circumference.26
A few studies have demonstrated that RPM can facilitate a safe discharge home after a hospitalization for COVID-19 and can assist patients in tapering home oxygen therapy. However, the assumed benefits of RPM (shorter hospitalizations, fewer readmissions, and safe tapering of home oxygen therapy) have yet to be confirmed in randomized controlled trials.27 Extrapolating these findings, RPM of respiratory status may be useful in the future for asthma treatment and other respiratory needs in the field of allergy. As use of telehealth and RPM become standard of care, there will need to be more outcome studies to use these modalities fully.
Recommended Clinical Approach
Similar to bedside manner, web-side manner refers to the core communication competencies that clinicians should practice when delivering virtual visits. In preparing for a visit, carefully consider attire, room setup, and background. Clinicians are advised to dress as if they are in the office seeing patients face-to-face: a white coat, business casual attire, or scrubs are all appropriate options. In setting up their virtual office, clinicians should seek a well-lit environment (facing a window often works well) and a simple background. Care should be taken to avoid facing open doors, people walking behind the clinician, and unkempt spaces, all of which all contribute to an unprofessional visit. Simple and professional virtual backgrounds (solid color, clinic logo, or office image) can be used when the existing live background is not ideal.
Owing to new technology, remote physical examinations are more accessible than ever before. Digital stethoscopes and otoscopes are commercially available, but this may not be applicable for all patients and it might be reserved for select high-risk populations (ie, immunodeficiency patients, poorly controlled asthmatic individuals). Similarly, a simple, inexpensive peak flow may be effective for establishing both a baseline and understanding breathing status for patients. Digital inhalers can evaluate both the quality of inhalation and inhaler use. High-resolution photography via smartphone cameras can allow for an accurate assessment of a skin examination without requiring additional purchases on the part of the patient. Patients should be properly educated on how to take a high-resolution photo. Telemedicine platforms and/or patient portals should allow for photo uploads and zoom in to allow closer examination. The ability to perform a virtual exam notwithstanding, it is reasonable to defer a physical examination if it does not contribute to the goals of the visit, such as during a TM encounter to review laboratory results.
A key benefit of TM is the ability to meet the patient outside the sterile, clinical setting. For allergists, this presents unique opportunities. Through video, a physician or QHP can essentially join a patient for a home visit and actively participate in care in ways not possible in a strictly clinical setting. For example, for patients with asthma or allergic rhinitis, a video visit can uncover previously overlooked sources of exposure such as tapestries, rugs, or other decorations. Medication reconciliation can also be facilitated virtually with patients. Of course, care must be taken to ensure patients are comfortable with such visits. Setting expectations remains paramount in this situation.
As telehealth continues to grow in popularity, hybrid visit schedules represent major opportunities for systems and clinicians to deliver high-quality care with efficient use of resources. In a post-COVID world, health care systems have struggled to hire various personnel, including key support ones such as medical assistants. Using telehealth, clinics have been able to share positions and maximize resource availability, and allow staff and physicians and QHPs to operate at the top of their licenses. Specific use cases lend themselves well to a hybrid approach within the allergy and immunology field:
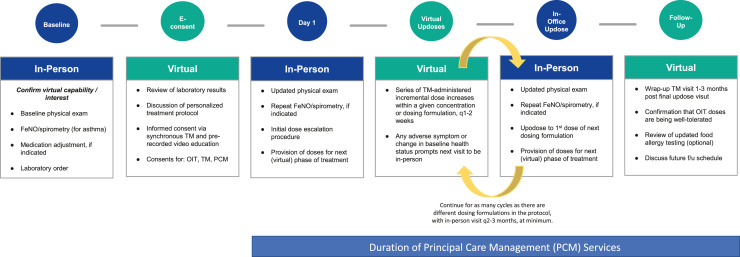
•Oral immunotherapy/food allergen desensitization: Physicians and QHPs may conduct in-person visits to introduce new concentration or dosing formulations and TM to raise the doses within a given concentration (Figure 1 ). Although standard recommendations for raising doses with FDA-approved peanut immunotherapy products call for all raises in doses to be performed in person, a retrospective review of 130 oral immunotherapy dose increases performed via TM reported only five adverse reactions, none of which required epinephrine.28
Figure 1.
Proposed schedule for hybrid in-person/telemedicine food allergen oral immunotherapy dose escalation protocol. E-consent, electronic consent; f/u, follow up; OIT, oral immunotherapy; q, every; TM, telemedicine.
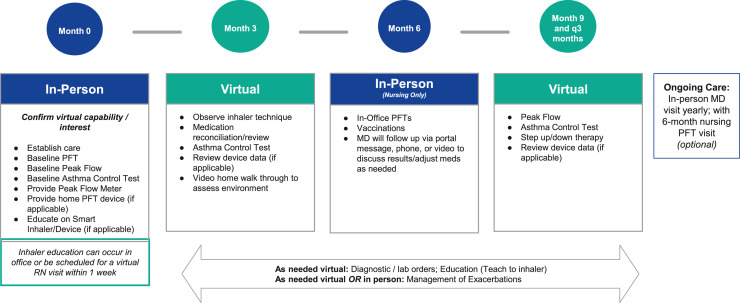
•Routine asthma management: Physicians and QHPs may use TM to observe inhaler technique, medication reconciliation, and video home walk-throughs (Figure 2 ).
Figure 2.
Sample hybrid in-person/telemedicine visit schedule for the management of persistent asthma. PFT, pulmonary function test; q, every.
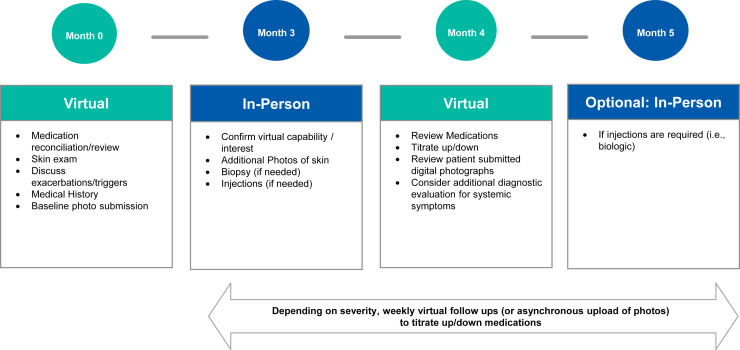
•Chronic urticaria: Telemedicine may be used for initial medication management, skin examinations, and baseline photo submissions (Figure 3 ).
Figure 3.
Sample hybrid in-person/telemedicine visit schedule for the management of chronic urticaria.
As the pandemic comes to a close, patients may continue to request TM visits, even as many physicians desire to return to normal. These hybrid care models offer an example of how to balance the convenience of virtual care with the importance of periodic in-person evaluation.
Like many of our colleagues in health care, allergists have been frustrated by declining reimbursements relative to the rapidly increasing costs of providing allergy care. The authors recommend that allergists and immunologists familiarize themselves with the billing codes for remote physiologic monitoring, remote therapeutic monitoring, and principal care management (PCM). These newer codes allow allergists to bill and receive compensation for time spent by clinicians and staff outside routine patient visits to manage complex patients, much of which until now has been provided without compensation. Because ongoing monitoring of patients with chronic or complex conditions is associated with improved outcomes, formalizing the process and billing for monitoring codes is advantageous for patients and allergists alike.29
Remote physiologic monitoring is a care management evaluation and management service involving the collection and analysis of physiologic data such as weight, heart rate, oxygen saturation, peak flow/FEV1, blood pressure, and glucose, which are used to develop and manage a treatment plan for established patients with an acute or chronic condition (Table I ). Such data must be collected and transmitted directly to the physician or QHP using a medical device, as defined by the Federal Food, Drug, and Cosmetics Act. In addition to physiologic monitoring itself, RPM includes codes for collecting and analyzing data by the physician or QHP. It also involves developing an updated care plan and interactively communicating the plan to the patient. This portion of the care management service can be performed by clinical staff under general supervision. Remote physiologic monitoring services can be managed directly by the allergist’s office. However, RPM services are often provided to patients in cooperation with a third-party vendor who supplies the monitoring devices and generates monthly reports in exchange for a share of the RPM revenue. Examples of RPM in allergy include home-based spirometry that transmits FEV1 data to the allergist’s electronic health record through 4G or Bluetooth, and pulse oximetry in patients recovering from COVID-19. The effective use of RPM allows allergists to care for complex or poorly controlled patients who may need active monitoring but do not have the resources to come into the clinic for frequent evaluation.
Table I.
Remote physiologic monitoring (RPM)
| Service summary | Current Procedural Terminology code | Description | 2022 work relative value units/practice expense relative value units (NF/F) | 2022 physician fee schedule (national average) |
|---|---|---|---|---|
| •Initiating visit •Medical device as defined under Federal Food, Drug, and Cosmetics Act •Device must transmit data electronically. Patient-reported data are not permitted •As evaluation and management code, permissible for clinical staff to perform care management services for Current Procedural Terminology 99457-8, under general supervision •Can be billed by only one provider per patient per month regardless of number of monitoring devices in use |
99453 | Remote monitoring of physiologic parameter(s) (eg, weight, blood pressure, pulse oximetry, respiratory flow rate), initial; setup and patient education on use of equipment) | 0.00/0.54/0.54 | NF: $19.03 F: $19.03 |
| 99454 | Remote monitoring of physiologic parameter(s) (eg, weight, blood pressure, pulse oximetry, respiratory flow rate), initial; device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 d) | 0.00/1.60/1.60 | NF: $55.72 F: $55.72 |
|
| 99091 | Collection and interpretation of physiologic data (eg, electrocardiogram, blood pressure, glucose monitoring) digitally stored and/or transmitted by patient and/or caregiver to physician or other qualified health care professional, qualified by education, training, licensure/regulation (when applicable) requiring a minimum of 30 min of time, each 30 d | 1.10/0.44/0.44 | NF: $56.41 F: $56.41 |
|
| 99457 | RPM treatment management services, clinical staff/physician/other qualified health care professional time in a calendar month requiring interactive communication with patient or caregiver during the month; first 20 min | 0.61/0.80/0.25 | NF: $50.18 F: $31.15 |
|
| 99458 | RPM treatment management services, clinical staff, physician, or other qualified health care professional time in a calendar month requiring interactive communication with patient or caregiver during the month; each additional 20 min | 0.61/0.53/0.25 | NF: $40.84 F: $31.15 |
F, facility; NF, non-facility.
New in 2022, RTM is designed to manage patients using medical devices that collect nonphysiologic data, such as therapy adherence and response (Table II ). Such data can be transmitted directly from the medical device to the clinician through integrated software, or they can be self-reported by the patient by manually inputting results into the device or approved software. Remote therapeutic monitoring is distinct from RPM in three key ways: (1) RTM involves the collection of nonphysiologic data rather than vital signs data; (2) RTM data can be self-reported by the patient; and (3) RTM codes are general medical codes rather than evaluation and management codes, so they cannot be billed by clinical staff under general supervision. This makes it difficult for RTM services to be provided effectively through a third-party service. An excellent example of how allergists can effectively employ RTM in practice is the use of digital asthma inhalers or inhaler attachments that time stamp inhaler actuations, measure the strength of inhalation, and generate reports for the patient and allergist within a mobile app. Such monitoring has been demonstrated to improve medication adherence in patients with severe asthma.30
Table II.
Remote therapeutic monitoring (RTM)
| Service summary | Current Procedural Terminology code | Description | 2022 work relative value units/practice expense relative value units (NF/F∗) | 2022 physician fee schedule (national average) |
|---|---|---|---|---|
| •Initiating visit •Medical device as defined under the Federal Food, Drug, and Cosmetics Act •Device must monitor at least 16 of each 30 d to bill 98976 •As general medical codes, RTM codes can be billed by physicians, nurse practitioners, physician assistants, physical therapists, occupational therapists, speech language pathologists, and clinical social workers •Because RTM is not an evaluation and management service, work performed by clinical staff under general supervision cannot be billed using Current Procedural Terminology 98980-1 |
98975 | RTM (eg, respiratory system status, musculoskeletal system status, therapy adherence, therapy response); initial setup and patient education on use of equipment | 0.00/0.54/0.54 | NF: $19.38 F: $19.38 |
| 98976 | RTM (eg, respiratory system status, musculoskeletal system status, therapy adherence, therapy response); device(s) supply with scheduled (eg, daily) recording(s) and/or programmed alert(s) transmission to monitor respiratory system, each 30 d | 0.00/1.60/1.60 | NF: $55.72 F: $55.72 |
|
| 98980 | RTM services, physician/ other qualified health care professional time in calendar month requiring at least one interactive communication with patient or caregiver during calendar month; first 20 min | 0.62/0.79/0.25 | NF: $50.18 F: $31.49 |
|
| 98981 | RTM treatment management services, physician or other qualified health care professional time in calendar month requiring at least one interactive communication with patient or caregiver during the calendar month; each additional 20 min (List separately in addition to code for primary procedure) | 0.61/0.52/0.25 | NF: $40.84 F: $31.49 |
F, facility; NF, non-facility.
Principal care management is intended to be a way to reimburse specialists for providing additional care to patients with a single chronic health condition that has resulted in recent hospitalization or puts the patient at significant risk for death, exacerbation or decompensation, or functional decline. This condition should require frequent adjustment of the medication or regimen, or the condition should be complicated by comorbidities. A number of requirements must be met to bill for PCM codes (Table III ). These include obtaining patient consent during an initiating visit, the use of a certified electronic health record, the development of a disease-specific care plan that is updated as clinically indicated and is available electronically to the patient, ongoing coordination of home- and community-based care, and provision of 24/7 on-call service for prompt evaluation of changes in the patient’s status. An example of the effective use of PCM in allergy is during the build-up phase of peanut desensitization, when a patient’s symptoms may require the frequent readjustment of doses or supportive medications outside scheduled office visits.
Table III.
Principal care management (PCM)
| Service summary | Current Procedural Terminology code | Description | 2022 work relative value units/practice expense relative value units (NF/F) | 2022 physician fee schedule (national average) |
|---|---|---|---|---|
| •Verbal consent •Initiating visit •Certified EHR •24/7 access “on call service” •Designated care team member •Disease-specific care management •Disease-specific electronic care plan •Management of care transitions/referrals •Home and community-based care coordination •Enhanced communication opportunities |
99424 | PCM services for single high-risk disease. First 30 min provided personally by physician or other qualified health care professional, per calendar month | 1.45/0.86/0.63 | NF: $83.40 F: $75.44 |
| 99425 | PCM services for single high-risk disease. Each additional 30 min provided personally by physician or other qualified health care professional, per calendar month | 1.00/0.66/0.44 | NF: $60.22 F: $52.60 |
|
| 99426 | PCM services for single high-risk disease. First 30 min of clinical staff time directed by physician or other qualified health care professional, per calendar month | 1.00/0.75/0.38 | NF: $63.33 F: $50.53 |
|
| 99427 | PCM services for single high-risk disease. Each additional 30 min of clinical staff time directed by physician or other qualified health care professional, per calendar month | 0.71/0.64/0.27 | NF: $48.45 F: $35.64 |
F, facility; NF, non-facility.
Requirements include one complex chronic condition lasting at least 3 mo, which is the focus of the care plan. The condition is of sufficient severity to place the patient at risk for hospitalization or to have been the cause of a recent hospitalization. The condition requires the development or revision of a disease-specific care plan. The condition also requires frequent adjustments in the medication regimen and/or management of the condition is unusually complex owing to comorbidities.
Awareness among allergists regarding available remote monitoring technologies and how best to apply them in practice is not widespread. Therefore, we recommend that specialty societies undertake concerted campaigns to train allergists about the appropriate use of RPM, RTM, and PCM.
Future Directions
Leveraging telehealth creatively can help future-proof the specialty of allergy and immunology against allergist shortages and the remote practice of allergy. Because of the failure of graduate medical education funding for fellowship positions to keep pace with medical school enrollment and health care use, a shortfall of approximately 480 allergists is estimated by 2025.31 , 32 Each US allergist provides specialty care for about 56 patient visits and approximately 14 new patients per week, so this will result in an unmet need of over 1.3 million visits and nearly 350,000 new patients per year.33 Noting this service gap, nonallergists (including direct-to-consumer TM companies) have aggressively marketed themselves to patients with allergic disease. Expanding our accessibility is important to ensure that patients with allergic and immunologic disease can receive timely care from fellowship-trained allergists and achieve the superior outcomes they deserve.
Telehealth and other remote services can be used as an effective tool to scale the allergist’s expertise across the broader population, especially in underserved areas. Although many allergists have already incorporated nonfacilitated synchronous TM into their practice, fewer have implemented facilitated visits. We believe this is an untapped opportunity with significant potential to offer comprehensive allergy diagnostics to patients who otherwise might go without a full workup. Facilitated visits are an excellent way for distant patients to undergo skin testing and pulmonary function testing with the benefit of real-time allergist interpretation and counseling. A hub-and-spoke model of care, with a central allergist and multiple distant sites with well-trained nurse facilitators to obtain vital signs, facilitate physical examinations, and administer testing, can expand a single allergist’s footprint into sparsely populated areas that might not otherwise have access to such highly specialized care. As part of the COVID-19 waivers put into place in 2020, CMS modified physician supervision requirements to allow physicians to provide direct supervision virtually, using real-time audio-video technology. In addition, CMS allowed physicians to enter into contractual arrangements with auxiliary personnel to provide care that would typically be provided incidental to physicians’ services. Under these new rules, an allergist could subcontract nursing services from a distant primary care office to allow for facilitated visits. Such arrangements would enable allergists to provide facilitated services in locations that might not have enough patient volume to support a dedicated full-time nurse. Many of the COVID-19 waivers adopted by CMS are set to expire once the public health emergency has officially ended. If allergists hope to have such regulatory relaxation made permanent, we need to take advantage of the flexibility to implement innovations in health care delivery and show evidence that doing so has contributed to cost savings and/or improved outcomes. We encourage our fellow allergists to advocate for funding for telehealth demonstration projects and to share the results of their efforts in expanding patient access via telehealth by submitting their findings for peer review.
Another route to improving access to allergy and immunology care remotely is a modification of the collaborative care model, currently in place for behavioral health.34 In this model, a psychiatrist remotely provides weekly caseload consultation to an embedded care manager for a defined panel of behavioral health patients who are tracked in a registry. The case manager coordinates with primary care physicians to care for the patients directly based on psychiatrist input. The collaborative care model expands access to specialist care for complex patients who may not otherwise be able to obtain this care outside the primary care physician’s office. In allergy and immunology, such a model would be useful to optimize the management of severe asthma, atopic dermatitis, or immune deficiency, and can increase patient access to specialty medications such as biologics, which are often restricted to prescription by specialists. Time-based interprofessional consultation codes can be effectively employed in these scenarios and can be used for synchronous telephone and internet consultation with the requesting clinician or asynchronous written communication within the electronic medical record (Table IV ).
Table IV.
Interprofessional consultation codes for telephone, Internet, and electronic health record–based assessment and management service
| Current Procedural Terminology code | Reported by | Conclusion | Time required | Description | 2022 work relative value units/practice expense relative value units | 2022 physician fee schedule (national average) |
|---|---|---|---|---|---|---|
| 99446 | Consultant | Verbal and written | 5-10 min | Interprofessional telephone/Internet assessment and management service provided by consultative physician, including verbal and written report to patient's treating/requesting physician or other qualified health care professional | 0.35/0.15 | $18.69 |
| 99447 | Consultant | Verbal and written | 11-20 min | 0.70/0.29 | $36.68 | |
| 99448 | Consultant | Verbal and written | 21-30 min | 1.05/0.45 | $55.02 | |
| 99449 | Consultant | Verbal and written | ≥31 min | 1.40/0.62 | $73.71 | |
| 99451 | Consultant | Written (within electronic health record) | ≥5 min | Interprofessional telephone/Internet/electronic health record assessment and management service provided by consultative physician, including written report to patient's treating/requesting physician or other qualified health care professional | 0.70/0.30 | $36.34 |
| 99452 | Requesting/treating qualified health provider | Not applicable | ≥16 min | Interprofessional telephone/Internet/electronic health record referral service(s) provided by treating or requesting physician or other qualified health care professional | 0.70/0.30 | $37.03 |
Conclusion
In the face of a global pandemic, shifting patient expectations, physician shortages, and financial constraints, telehealth has offered allergists a versatile, reliable, and effective method of health care delivery with a high degree of patient satisfaction. It is clear that the provision of virtual care services was not merely a stopgap measure during the public health emergency, but that telehealth will be indispensable to modern health care delivery. Allergists and immunologists have readily adopted synchronous TM, and now we have an opportunity to embrace additional telehealth technologies and apply them to the challenges faced by our specialty. Rather than limiting ourselves to video chats in lieu of face-to-face patient visits, allergists should integrate a broad array of virtual care services into our repertoire. Implementation of best practices in TM web-side manner, environmental analysis, hybrid TM/in-person visit schedules, RPM, facilitated remote diagnostic testing, and collaborative care for complex patients are ways in which allergists can effectively use telehealth to improve patient access and outcomes. In doing so, we can improve our agility and insulate the specialty from an ever-changing health care landscape. Much of the regulatory laxity that has enabled the explosion of telehealth was forced by the COVID-19 pandemic, but it will require advocacy to make this flexibility permanent. Allergists should be supported in efforts to design and publish research demonstrating the cost-effectiveness and clinical utility of telehealth for our patients.
Footnotes
Conflicts of interest: S.S. Bajowala is the owner of Wise Prince, LLC. T. Elliott is a speaker for Teva. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Centers for Medicare and Medicaid Services Medicare Telemedicine Snapshot – December 2021 FAQ’s. https://www.cms.gov/files/document/medicare-telemedicine-snapshot-faqs.pdf
- 2.Centers for Medicare and Medicaid Services Telemedicine. https://www.medicaid.gov/medicaid/benefits/telemedicine/index.html
- 3.Portnoy J.M., Pandya A., Waller M., Elliott T. Telemedicine and emerging technologies for health care in allergy/immunology. J Allergy Clin Immunol. 2020;145:445–454. doi: 10.1016/j.jaci.2019.12.903. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services Telehealth and remote patient monitoring. https://telehealth.hhs.gov/providers/preparing-patients-for-telehealth/telehealth-and-remote-patient-monitoring/
- 5.American College of Allergy, Asthma and Immunology COVID Member Survey 7/30/20–8/16/20 and ACAAI COVID Member Survey 4/24/20–5/3/20. Used with permission from ACAAI. Copyright 2020. Data are protected by ACAAI copyright and are prohibited to be shared or published without ACAAI’s consent. Accessed August 27, 2022. www.college.acaai.org.
- 6.Bajowala S.S., Milosch J., Bansal C. Telemedicine pays: billing and coding update. Curr Allergy Asthma Rep. 2020;20:60. doi: 10.1007/s11882-020-00956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott T., Shih J., Dinakar C., Portnoy J., Fineman S. American College of Allergy, Asthma & Immunology position paper on the use of telemedicine for allergists. Ann Allergy Asthma Immunol. 2017;119:512–517. doi: 10.1016/j.anai.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 8.Portnoy J.M., Waller M., De Lurgio S., Dinakar C. Telemedicine is as effective as in-person visits for patients with asthma. Ann Allergy Asthma Immunol. 2016;117:241–245. doi: 10.1016/j.anai.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Chongmelaxme B., Lee S., Dhippayom T., Saokaew S., Chaiyakunapruk N., Dilokthornsakul P. The effects of telemedicine on asthma control and patients’ quality of life in adults: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2019;7:199–216. doi: 10.1016/j.jaip.2018.07.015. e11. [DOI] [PubMed] [Google Scholar]
- 10.Perry T.T., Turner J.H. School-based telemedicine for asthma management. J Allergy Clin Immunol Pract. 2019;7:2524–2532. doi: 10.1016/j.jaip.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Hare N., Bansal P., Bajowala S.S., Abramson S.L., Chervinskiy S., Corriel R., et al. Work Group Report: COVID-19: unmasking telemedicine. J Allergy Clin Immunol Pract. 2020;8:2461–2473. doi: 10.1016/j.jaip.2020.06.038. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bestsennyy O., Gilbert G., Harris A., Rost J. Telehealth: a quarter-trillion dollar post-COVID-19 reality? Healthcare Systems and Services. https://www.mckinsey.com/ Accessed August 27, 2022.
- 13.Thomas I., Siew L.Q.C., Rutkowski K. Synchronous telemedicine in allergy: lessons learned and transformation of care during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2021;9:170–176.e1. doi: 10.1016/j.jaip.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen M., Waller M., Pandya A., Portnoy J. A review of patient and provider satisfaction with telemedicine. Curr Allergy Asthma Rep. 2020;20:72. doi: 10.1007/s11882-020-00969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanier K., Kuruvilla M., Shih J. Patient satisfaction and utilization of telemedicine services in allergy: an institutional survey. J Allergy Clin Immunol Pract. 2021;9:484–486. doi: 10.1016/j.jaip.2020.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafa S.S., Yang L., Mortezavi M., Vadamalai K., Ramsey A. Patient satisfaction with telemedicine encounters in an allergy and immunology practice during the coronavirus disease 2019 pandemic. Ann Allergy Asthma Immunol. 2020;125:478–479. doi: 10.1016/j.anai.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikhtaheri A., Kermani F. Telemedicine in diagnosis, treatment and management of diseases in children. Stud Health Technol Inform. 2018;248:148–155. [PubMed] [Google Scholar]
- 18.Vimalananda V.G., Orlander J.D., Afable M.K., Fincke B.G., Solch A.K., Rinne S.T., et al. Electronic consultations (E-consults) and their outcomes: a systematic review. J Am Med Inform Assoc. 2020;27:471–479. doi: 10.1093/jamia/ocz185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan D.S., Callahan C.W., Hatch-Pigott V.B., Lawless A., Proffitt H.L., Manning N.E., et al. Internet-based home monitoring and education of children with asthma is comparable to ideal office-based care: results of a 1-year asthma in-home monitoring trial. Pediatrics. 2007;119:569–578. doi: 10.1542/peds.2006-1884. [DOI] [PubMed] [Google Scholar]
- 20.Culmer N., Smith T., Stager C., Wright A., Burgess K., Johns S., et al. Telemedical asthma education and health care outcomes for school-age children: a systematic review. J Allergy Clin Immunol Pract. 2020;8:1908–1918. doi: 10.1016/j.jaip.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Jeminiwa R., Hohmann L., Qian J., Garza K., Hansen R., Fox B.I. Impact of eHealth on medication adherence among patients with asthma: a systematic review and meta-analysis. Respir Med. 2019;149:59–68. doi: 10.1016/j.rmed.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Mosnaim G.S., Stempel D.A., Gonzalez C., Adams B., BenIsrael-Olive N., Gondalia R., et al. The impact of patient self-monitoring via electronic medication monitor and mobile app plus remote clinician feedback on adherence to inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2021;9:1586–1594. doi: 10.1016/j.jaip.2020.10.064. [DOI] [PubMed] [Google Scholar]
- 23.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alwashmi M.F. The use of digital health in the detection and management of COVID-19. Int J Environ Res Public Health. 2020;17:2906. doi: 10.3390/ijerph17082906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration Enforcement policy for non-invasive remote monitoring devices used to support patient monitoring during the coronavirus disease-2019 (COVID-19) public health emergency. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-non-invasive-remote-monitoring-devices-used-support-patient-monitoring-during
- 26.Massaroni C., Nicolò A., Schena E., Sacchetti M. Remote respiratory monitoring in the time of COVID-19. Front Physiol. 2020;11:635. doi: 10.3389/fphys.2020.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mecklai K., Smith N., Stern A.D., Kramer D.B. Remote patient monitoring - overdue or overused? N Engl J Med. 2021;384:1384–1386. doi: 10.1056/NEJMp2033275. [DOI] [PubMed] [Google Scholar]
- 28.Schoonover A., Uyehara A., Goldman M. Continuing peanut oral immunotherapy via telemedicine during the COVID-19 pandemic. J Allergy Clin Immunol. 2021;147:AB109. [Google Scholar]
- 29.Merchant R.K., Inamdar R., Quade R.C. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract. 2016;4:455–463. doi: 10.1016/j.jaip.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Van Sickle D., Humblet O., Barrett M., Henderson K., Hogg C. 2016. Randomized, controlled study of the impact of a mobile health tool on asthma SABA use, control and adherence. Eur Respir J. 2016;48:PA1018. [Google Scholar]
- 31.Malick A., Meadows J.A. Allergy and Immunology Physician Workforce: where do we stand today? Ann Allergy Asthma Immunol. 2021;127:522–523. doi: 10.1016/j.anai.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce, National Center for Health Workforce Analysis. National and regional projections of supply and demand for internal medicine subspecialty practitioners: 2013-2025. Accessed March 26, 2021. https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/internalmedicine-subspecialty-report.pdf.
- 33.Center for Health Workforce Studies . 1999-2009/10. American Academy of Allergy Asthma and Immunology report on the allergy and immunology physician workforce.https://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/2012-AI-Physician-Workforce-Report.pdf [Google Scholar]
- 34.Jackson-Triche M.E., Unützer J., Wells K.B. Achieving mental health equity: collaborative care. Psychiatr Clin North Am. 2020;43:501–510. doi: 10.1016/j.psc.2020.05.008. [DOI] [PubMed] [Google Scholar]