Abstract
As one of the most common malignancies, gastric cancer (GC) is the third leading cause of cancer-related deaths in China. GC is asymptomatic in early stages, and the majority of GC mortality is due to delayed symptoms. It is an urgent task to find reliable biomarkers for the identification of GC in order to improve outcomes. A combination of dried blood spot sampling and direct infusion mass spectrometry (MS) technology was used to measure blood metabolic profiles for 166 patients with GC and 183 healthy individuals, and 93 metabolites including amino acids, carnitine/acylcarnitines and their derivatives, and related ratios were quantified. Multiple algorithms were used to characterize the changes of metabolic profiles in patients with GC compared to healthy individuals. A biomarker panel was identified in training set, and assessed by tenfold cross-validation and external test data set. After systematic selection of 93 metabolites, a biomarker panel consisting of Ala, Arg, Gly, Orn, Tyr/Cit, Val/Phe, C4-OH, C5/C3, C10:2 shows the potential to distinguish patients with GC from healthy individuals in tenfold cross-validation model (sensitivity: 0.8750, specificity: 0.9006) and test set (sensitivity: 0.9545, specificity: 0.8636). This metabolomic analysis makes contribution to the identification of disease-associated biomarkers and to the development of new diagnostic tools for patients with GC.
Subject terms: Cancer, Biomarkers, Diseases, Oncology
Introduction
As one of the most common malignancies, gastric cancer (GC) is the third cause of cancer-related deaths in China1. According to GLOBOCAN 2018 data, around 1,034,000 new cases and more than 782,000 deaths occurred for GC in 20182. GC is a multifactorial and multistep process, beginning with active chronic gastritis caused by Helicobacter pylori infection3. It is often described as a stepwise progression from non-active gastritis via chronic active gastritis into precursor lesions of GC and finally GC4,5. Most GC is adenocarcinoma, which derives from glandular epithelium of gastric mucosa4. However, GC is asymptomatic in early stages, and the majority of GC mortality is due to the delayed symptoms. It has been found that 5-year overall survival rate for patients with GC diagnosed at advanced stages is reduced down to 20%6. Among screening methods for early detection of GC, endoscopy as a sensitive method is most commonly used7, nevertheless, the risk of complications and patient discomfort limit its wide use. Furthermore, despite traditional circulating biomarkers of cancer were achieved, the diagnostic efficacy was not satisfactory for patients with GC due to their low sensitivity8. Because of that, finding reliable biomarkers for disease identification is of highest interest to improve outcomes for patients with GC.
Investigating the quantity or type of molecules in organisms via metabolomics can provide better understanding as to the biochemical status in a system or indicate the changes that have occurred within the metabolome9,10. Metabolomics technology can aid in cancer discovery and in building cancer diagnostic tools, and can provide opportunity to understand the molecular mechanism. Metabolic changes in blood are the key events in the development of carcinoma, which could be characterized by mapping global metabolic profiles, and this analytic technique has been used to interpret possible mechanisms and to identify novel metabolic biomarkers for GC11,12. Liquid chromatography mass spectrometry (LC–MS), one of the most commonly used platforms in metabolomic studies, can be applied to detect biomolecules for its peak resolution, high sensitivity, and sufficient reproducibility13. It has been found that the levels of 16 metabolites detected by LC–MS were altered in patients with GC compared to healthy control group, involving in Gly, Ala, Pro and hexadecanoic acid, which showed potential for developing biomarkers and therapeutic interventions for GC14. Another study showed that the ratio of kynurenine/tryptophan was associated with observed metabolic changes in patients with GC, and the monitoring of tryptophan metabolites could be used to identify potential biomarkers for GC11. However, both of them were relatively small-sized studies, and further clinical sample analysis is still needed for patients with GC. Besides, few reports were available in characterizing metabolic profiles of amino acids and carnitine/acylcarnitines for patients with GC. Dried blood spot (DBS) sampling is a microvolume sampling technique involving the collection of blood samples by heel or finger puncture. It as compared to conventional whole blood sampling has relatively high stability, requires a smaller blood volume, offers a simpler storage and easier transfer, reduces infection risk by infectious pathogens, and can be as an alternative method to metabolomics study15,16. The combination of DBS and MS can provide a high-throughput, reliable and stable determination for a broad array of analytes, which can be satisfactorily used to select high specificity and sensitivity biomarkers to some kinds of diseases17,18. In the present study, a combination of DBS sampling and MS was utilized to detect biomarkers based on altered levels of amino acids and carnitine/acylcarnitines in patients with GC compared to normal individuals. Nine parameters including 4 amino acids, 2 acylcarnitines and 3 related ratios were detected as potential biomarkers for GC in training set, which were further used to build prediction model for distinguishing patients with GC from healthy individuals. Hence, the changes in some amino acid and carnitine/acylcarnitine levels might indicate the existence of GC invasion, and the study’s findings suggest new insights into GC detection.
Materials and methods
Study design and participants
In this study, a total of 349 participants, including 183 healthy controls (HC) and 166 patients with GC, were enrolled between 2015 and 2019 from the First Affiliated Hospital of Jinzhou Medical University. Among all participants, 161 healthy individuals and 144 patients with GC were defined as training set. Other participants (22 healthy individuals and 22 patients with GC) were used as test set. Potential biomarker selection and prediction model building were performed in the training set. The external test set was used to evaluate biomarker candidates. The clinicopathologic characteristics of the whole participants were shown in Table 1. As shown in Table 1, the stages for all patients with GC were determined according to TNM (tumor-node-metastasis): stage I, 87 patients; stage II, 45 patients; stage III, 25 patients; stage IV, 9 patients. There were no significant differences identified in gender, age, BMI, blood pressure between HC and GC groups in training set and test set. The individuals with diabetes, hypertension, cardiovascular disease, tumor, infection or other diseases that can influence biological indicators or gastric function were excluded in HC group. The exclusion criteria for GC group were: (1) metabolic diseases or other digestive diseases; (2) severe liver, kidney, heart, lung, and nervous and mental diseases; (3) other types of malignant diseases and acute diseases. All the subjects with missing data were excluded. The project was approved by Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University. Written informed consent was obtained from each participants. This study was conducted in accordance with tenets of the Declaration of Helsinki, and followed relevant Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University guidelines and regulations.
Table 1.
Clinicopathologic characteristics of the whole participants.
| Characteristics | Training set | Test set | ||||
|---|---|---|---|---|---|---|
| HC | GC | p-value | HC | GC | p-value | |
| Total number | 161 | 144 | 22 | 22 | ||
| Male | 104 | 99 | 0.4427 | 12 | 12 | 1.0000 |
| Female | 57 | 45 | 10 | 10 | ||
| Age (mean, sd) | 55.9627 ± 11.1147 | 58.1389 ± 9.7798 | 0.1360 | 55.9091 ± 8.6845 | 56.0909 ± 8.0647 | 0.9430 |
| Weight (mean, sd) | 64.5404 ± 8.4609 | 63.7986 ± 7.9029 | 0.3216 | 64.9545 ± 7.5181 | 62.6818 ± 9.9350 | 0.3971 |
| Height (mean, sd) | 168.5963 ± 8.6822 | 169.3264 ± 8.3656 | 0.4193 | 167.9091 ± 8.1761 | 167.2273 ± 8.4173 | 0.7865 |
| BMI (mean, sd) | 22.6560 ± 2.0273 | 22.2415 ± 2.1597 | 0.0920 | 23.0368 ± 2.0542 | 22.4045 ± 3.0318 | 0.5416 |
| Systolic | 124.7019 ± 11.0152 | 125.7778 ± 8.0056 | 0.6186 | 122.8636 ± 8.5149 | 124.5909 ± 10.9225 | 0.5617 |
| Diastolic | 75.6770 ± 9.4846 | 74.4375 ± 8.1599 | 0.1732 | 72.8636 ± 9.6525 | 72.1364 ± 12.1275 | 0.4704 |
| I | 75 | 12 | ||||
| II | 39 | 6 | ||||
| III | 22 | 3 | ||||
| IV | 8 | 1 | ||||
HC, healthy control; GC, gastric cancer; BMI: body-mass index.
Blood sample collection and pretreatment
Labeled amino acid and relevant carnitine/acylcarnitine internal standards were mixed with pure methanol, individually. Stock solutions were prepared by the mixture of these dissolved isotope standards, and stored at 4 °C. The 100-fold dilution of stock solution was used as working solution. In quality control (QC) process, a pooled QC sample was obtained by the mixture of equal volumes (10 μL) from all collected samples.
Blood samples were collected after an overnight fasting for each participants in order to eliminate the disturbance of diet. DBS samples were collected by fingertip puncture. After wiping off the first drop of blood, 3–5 drops were collected onto a DBS card. A disc of 3 mm diameter was punched from a DBS card. The collected discs were put into Millipore MultiScreen HV 96-well plate (Millipore, Billerica, MA, USA) aimed at extracting metabolites. A working solution of 100 μL was added into a well containing a DBS disc. After 20 min gentle shaking, the plate was centrifuged at 1500 rpm for 2 min and, afterwards, the filtrate was collected into a new flat-bottom 96-well plate. In order to monitor the stability of MS analysis, 2 low-level and 2 high-level QC sample solutions were randomly put into 4 blank wells. The filtrate and QC solution were dried in pure nitrogen gas flow at 50 °C, and then these samples were derivatized with 60 μL mixture of acetyl chloride/1-butanol (10:90, v/v) at 65 °C for 20 min. After derivatized solution dried again, 100 μL mobile phase solution was mixed with each dried sample for the following metabolomics analysis.
Metabolomics analysis
The direct injection MS was used for quantitative metabolomic analysis on an AB Sciex 4000 QTrap system (AB Sciex, Framingham, MA) coupled with an electrospray ionization source, and the MS analysis was conducted under positive mode. A sample volume of 20 μL was injected into the system. The 80% acetonitrile aqueous was used as mobile phase. An initial flow rate was set to be 0.2 mL/min. Flow rate was decreased to 0.01 mL/min within 0.08 min, and remained stable until 1.5 min. Subsequently, the flow rate reverted back to 0.2 mL/min within 0.01 min, and maintained for 0.5 min. MS parameters were set as follows: ion spray voltage 4.5 kV, curtain gas pressure 20 psi, auxiliary gas temperature 350 °C. Sheath and auxiliary gas pressure was set at 35 psi. The scan modes and scan parameters were referred to previous report19. Analyst 1.6.0 software (AB Sciex) was applied to control system, align spectrum, and collect MS data. ChemoView 2.0.2 (AB Sciex) was used for absolute quantification purposes.
Data analysis
A multivariate analysis for metabolomics data was performed using SIMCA-P 12.0 software (Umetrics AB, Umea, Sweden). A principal component analysis (PCA) was used to supervise holistic metabolome alterations between patients with GC and healthy individuals and to inspect the stability of this study. In addition, a partial least squares discriminant analysis (PLS-DA) was applied to differentiate patients with GC from healthy individuals and to determine the important variables contributing to this classification based on variable importance in projection (VIP) values. Subsequently, a permutation test was used to evaluate the risk of over-fitting for PLS-DA model. T-test statistical analysis was used to identify the differential metabolites between HC and GC groups for parametric variables. Wilcoxon–Mann–Whitney test was performed for nonparametric variables. Benjamini–Hochberg false discovery rate (FDR) was used to adjust p-values for multiple hypothesis testing. Volcano plots were generated to screen important variables (VIP > 1, fold change (FC) > 1.2 or < − 1.2, adjusted p-value < 0.05) in GC group compared to HC group. In order to further investigate metabolite changes in GC group compared to HC group, significance analysis of microarrays (SAM) method was performed. Ultimately, potential biomarkers were selected by a stepwise selection method. These selected potential biomarkers were included to build a binary logistic regression model for distinguishing patients with GC from healthy individuals. The performance of this model was assessed by tenfold cross validation and external test set. Receiver-operating characteristic (ROC) curve was created to measure the ability of potential biomarkers to discriminate between patients with GC and healthy individuals. Statistical analysis was performed using SAS software. Online software MetaboAnalyst 5.0 was used for pathway analysis based on differential metabolites between HC and GC groups.
Results
Demographics of study samples
The workflow for this study was shown in Fig. 1. A total of 305 participants, including 161 healthy individuals (mean age 55.96 ± 11.11, range 27–82 years) and 144 patients with GC (mean age 58.13 ± 9.78, range 28–85 years), were recruited as training set to define biomarker candidates. In the training set, 104 (64.60%) males and 57 (35.40%) females for healthy individuals, and 99 (68.75%) males and 45 (31.25%) females for patients with GC, were included. In order to evaluate biomarker candidates, 44 blood samples (22 healthy individuals and 22 patients with GC) were collected as test set.
Figure 1.
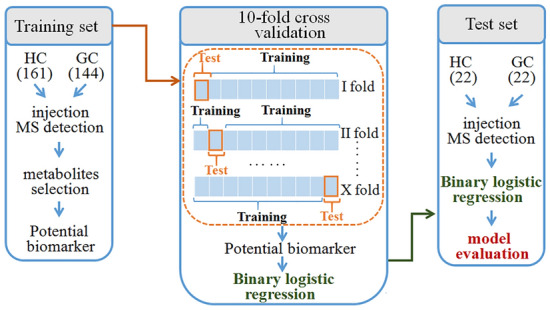
Design of the study. GC, gastric cancer; HC, healthy control.
Metabolic differences between LC and HC groups
A total of 93 variables including 23 amino acids, 26 carnitine/acylcarnitines, and 44 derived parameters and related ratios19 were detected from healthy participants and patients with GC for subsequent univariate and multivariate analyses. All detected variables were provided in Supplementary Table S1. Unsupervised PCA was executed for metabolomics data from blood samples of HC and GC groups in order to investigate the altered metabolites. There was a trend that GC group was separated from HC group based on 93 parameters in the training set (Fig. 2A). Furthermore, supervised PLS-DA was performed to determine the separations between HC and GC groups by all 93 variables. The PLS-DA score plot (Fig. 2B) showed the apparent separations between patients with GC and healthy individuals without over-fitting (Fig. 2C) in the training set.
Figure 2.
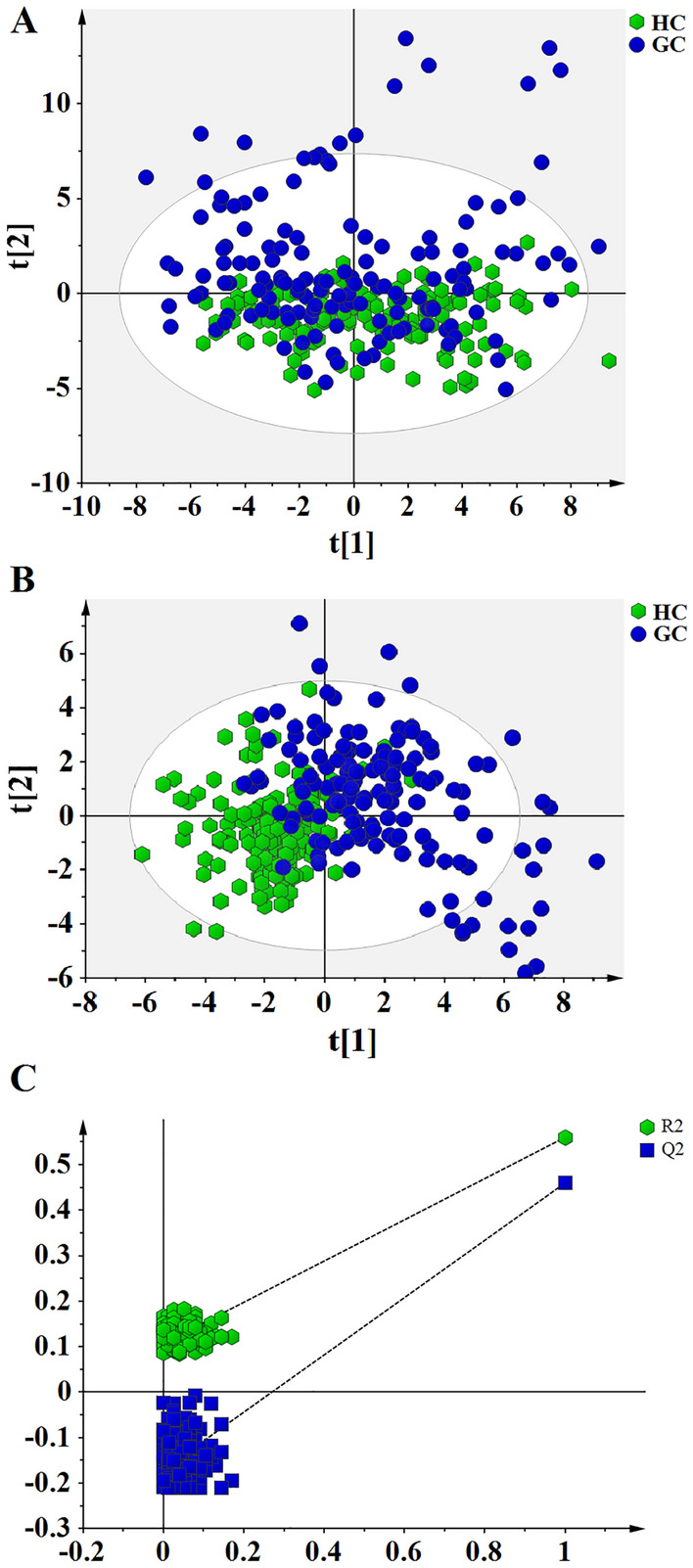
Score plots of PCA and PLS-DA analyses based on 93 metabolites for patients with GC and healthy individuals in the training set. (A) Score plot of PCA analysis, suggesting separating trend between GC and HC groups. The colors and shapes display the participants from different groups (healthy individuals and patients with gastric cancer). (B) Score plot of PLS-DA analysis, showing differential metabolic profiles in patients with GC compared to healthy individuals. (C) 200-times permutation test for evaluating the performance of PLS-DA model. The y-axis intercepts in test plot were R2 = (0.0, 0.109), Q2 = (0.0, -0.176).
The screening of significantly differential metabolites
Systematic screening of important metabolites was executed by multiple approaches. Firstly, a total of 29 metabolites were selected with VIP > 1, which can contribute to the classification between HC and GC groups according to PLS-DA score plot (Fig. 3A). Secondly, significant differences for all metabolites were evaluated with Wilcoxon–Mann–Whitney test or t-test, and FDR was controlled in order to adjust significance levels for hypothesis testing. A total of 45 parameters were retained with adjusted p-value < 0.05 (Fig. 3B). Thirdly, FC was calculated, and 30 features with FC > 1.2 or < − 1.2 were significantly altered in GC group compared to HC group. Together, 25 of these metabolites with VIP > 1, adjusted p-value < 0.05, and FC > 1.2 or < − 1.2 exhibited significant alterations in patients with GC compared to healthy individuals in the training set (Fig. 3C). All detected variables and their adjusted p-value, VIP, and FC values were shown in Supplementary Table S1.
Figure 3.
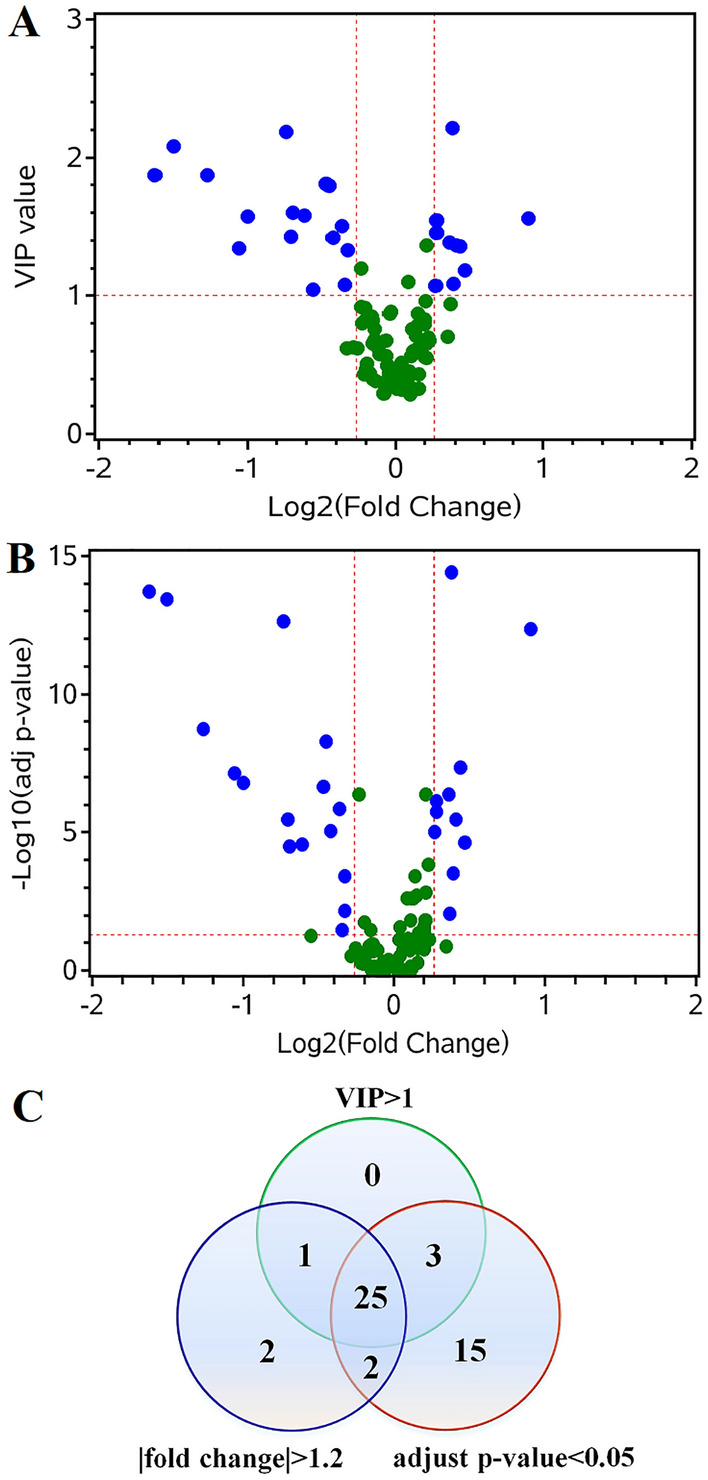
Identification of potential biomarkers for distinguishing patients with GC from healthy individuals in training set. (A) The plot of VIP value versus fold change (FC). The differential metabolites were defined with VIP > 1 and FC > 1.2 or < -1.2 between patients with GC and healthy individuals. The selected metabolites were colored in blue. (B) The plot of adjusted p-value versus FC. The changed metabolites were displayed with adjusted p-value < 0.05 and FC > 1.2 or < -1.2 in patients with GC compared to healthy individuals. (C) Venn diagram demonstrates differential metabolites in GC group compared with healthy group. Twenty-five differential metabolites were selected with VIP > 1 and adjusted p-value < 0.05 and FC > 1.2 or < − 1.2.
SAM was used to further supervise and define the significant metabolite changes in patients with GC compared to healthy individuals (Fig. 4). Finally, 23 metabolites contribute to the discrepancy between the two groups (Table 2). Among these metabolites, the levels of 15 features were significantly increased and, conversely, the levels of 8 features were distinctively decreased in patients with GC compared to healthy individuals.
Figure 4.
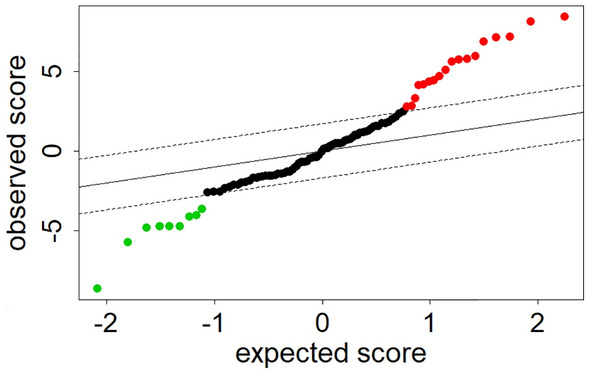
Significance analysis of microarrays for a comparison of patients with GC and healthy individuals in training set at false discovery rate of zero. The levels of 9 metabolites were significantly decreased, and 18 metabolites were significantly increased in patients with GC compared to healthy individuals.
Table 2.
The differential parameters between patients with GC and healthy individuals.
| No | Parameters | HC (mean ± SD) | GC (mean ± SD) | Statusa | Adjusted p-value |
|---|---|---|---|---|---|
| 1 | Asp | 28.9299 ± 12.3803 | 44.2080 ± 31.3087 | ↑ | < 0.0001 |
| 2 | Arg | 6.9661 ± 4.5362 | 19.7555 ± 19.3990 | ↑ | < 0.0001 |
| 3 | Gly | 187.0274 ± 54.2258 | 255.2199 ± 112.4842 | ↑ | < 0.0001 |
| 4 | Ser | 52.8482 ± 15.5849 | 67.8629 ± 29.7788 | ↑ | < 0.0001 |
| 5 | Orn | 17.3904 ± 7.5393 | 53.694 ± 64.3894 | ↑ | < 0.0001 |
| 6 | C3DC | 0.0681 ± 0.0462 | 0.1107 ± 0.0920 | ↑ | < 0.0001 |
| 7 | C4-OH | 0.0620 ± 0.0338 | 0.1293 ± 0.1688 | ↑ | < 0.0001 |
| 8 | C18:1 | 0.5388 ± 0.2005 | 0.7210 ± 0.3814 | ↑ | < 0.0001 |
| 9 | Gly/Ala | 1.1504 ± 0.5417 | 1.9130 ± 0.9737 | ↑ | < 0.0001 |
| 10 | Orn/Cit | 0.7861 ± 0.4212 | 1.8922 ± 1.8845 | ↑ | < 0.0001 |
| 11 | C2/C0 | 0.3841 ± 0.1422 | 0.5307 ± 0.2511 | ↑ | < 0.0001 |
| 12 | C3DC/C10 | 0.5130 ± 0.4537 | 1.0268 ± 0.9963 | ↑ | < 0.0001 |
| 13 | C5/C3 | 0.0765 ± 0.0339 | 0.1235 ± 0.1010 | ↑ | < 0.0001 |
| 14 | C2 | 12.3469 ± 3.5780 | 15.4624 ± 8.7437 | ↑ | 0.0069 |
| 15 | C5 | 0.1167 ± 0.0464 | 0.1480 ± 0.0884 | ↑ | 0.0354 |
| 16 | Ala | 182.8131 ± 58.0390 | 150.6334 ± 60.2686 | ↓ | < 0.0001 |
| 17 | Pro | 492.3674 ± 166.7648 | 408.097 ± 231.8517 | ↓ | < 0.0001 |
| 18 | Cit/Arg | 5.3826 ± 4.3734 | 2.8778 ± 2.9849 | ↓ | < 0.0001 |
| 19 | Met/Phe | 0.4766 ± 0.1452 | 0.3931 ± 0.1275 | ↓ | < 0.0001 |
| 20 | Tyr/Cit | 1.4338 ± 0.6711 | 1.0788 ± 0.5934 | ↓ | < 0.0001 |
| 21 | Val/Phe | 3.7288 ± 0.9200 | 2.8610 ± 0.7869 | ↓ | < 0.0001 |
| 22 | C3/C2 | 0.1397 ± 0.0522 | 0.1088 ± 0.0529 | ↓ | < 0.0001 |
| 23 | C10:2 | 0.7606 ± 0.4541 | 0.5502 ± 0.3974 | ↓ | < 0.0001 |
HC, healthy control; GC, gastric cancer; Asp, aspartic acid; Arg, arginine; Gly, glycine; Ser, serine; Orn, ornithine; C3DC, malonylcarnitine; C4-OH, hydroxybutyrylcarnitine; C18:1, octadecenoylcarnitine; Ala, alanine; Cit, citrulline; C2, acetylcarnitine; C0, free carnitine; C10, decanoylcarnitine; C5, isovalerylcarnitine; C3, propionylcarnitine; Pro, proline; Met, methionine; Phe, phenylalanine; Tyr, tyrosine; Val, valine; C10:2, decadienoylcarnitine.
aDefined as the increased (upward arrow) or decreased (downward arrow) levels of metabolites in patients with GC compared to healthy individuals.
To further clarify the metabolic pathways which may be affected by GC, the differential metabolites between healthy individuals and patients with GC (Table 2) were imported into MetaboAnalyst 5.0 for pathway analysis. As shown in Fig. 5, 8 metabolic pathways were highlighted focusing on amino acid metabolism, urea cycle, malate-aspartate shuttle, lipid metabolism, and so on.
Figure 5.
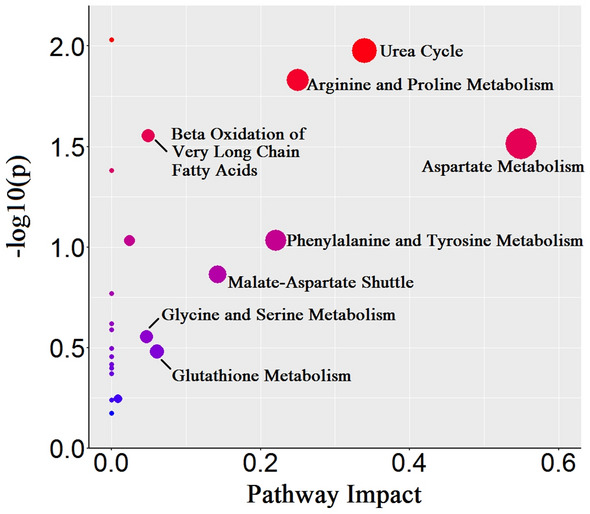
A pathway impact analysis based on differential metabolites between GC and HC groups in training set. Eight perturbed metabolic pathways were indicated for patients with GC.
Building prediction model
A stepwise logistic regression was conducted towards 23 selected metabolites (Table 2) in the training set. Finally, 9 features were identified including Ala, Arg, Gly, Orn, Tyr/Cit, Val/Phe, C4-OH, C5/C3, C10:2 (Fig. 6). A logistic regression model was developed as follows: Logit probability = 2.05 − 1.35 × Ala + 5.68 × Orn + 1.80 × Arg + 2.39 × C4-OH − 0.90 × Tyr/Cit − 0.62 × Val/Phe + 1.20 × C5/C3 − 1.35 × C10:2 + 0.76 × Gly. The diagnostic performance of this metabolic panel was evaluated by tenfold cross validation and external test set (Table 3). Furthermore, ROC curve was drawn to assess the potential of metabolic panel to distinguish between patients with GC and healthy individuals (Fig. 7). The area under ROC curve (AUC) is 0.9586 (95%CI 0.9384–0.9788) in the training set. The sensitivity and specificity were 0.8611 and 0.9565 in the training set, respectively. During the process of tenfold cross validation, all samples in the training set were randomly divided into 10 partitions in order to cross-validate the predicted model. Additionally, 44 blood samples including 22 patients with GC and 22 healthy individuals were used as test set to further assess the diagnostic potential of 9 selected metabolic biomarkers. As shown in Table 3, the AUC of 0.9438 (95%CI 0.9163–0.9714) and 0.9318 (95%CI 0.8525–1.0000) was determined in tenfold cross validation and test set, respectively. Additionally, both of sensitivity and specificity were also satisfactory in tenfold cross validation (sensitivity: 0.8750; specificity: 0.9006) and test set (sensitivity: 0.9545; specificity: 0.8636).
Figure 6.
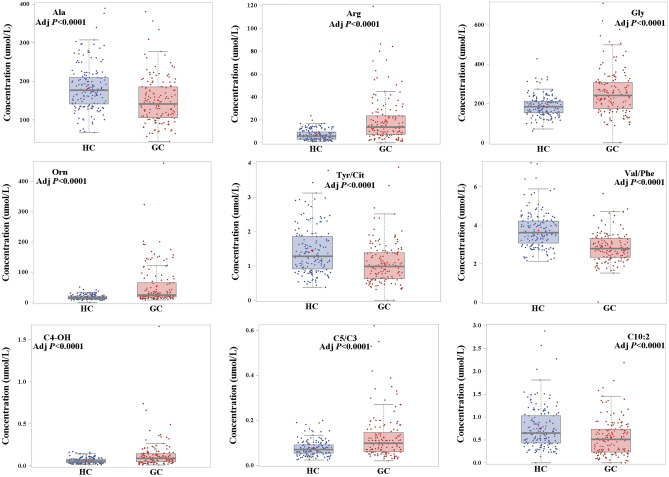
Blood concentrations for potential metabolic biomarkers contributing to the building of prediction model in training set.
Table 3.
Performance of metabolite biomarker panel for distinguishing patients with GC from healthy individuals in the training set, tenfold cross validation, and test set.
| Training set | tenfold cross validation | Test set | |
|---|---|---|---|
| AUC (95%CI) | 0.9586 (0.9384–0.9788) | 0.9438 (0.9163–0.9714) | 0.9318 (0.8525–1.0000) |
| Sensitivity | 0.8611 | 0.8750 | 0.9545 |
| Specificity | 0.9565 | 0.9006 | 0.8636 |
GC, gastric cancer; AUC, area under receiver operating characteristic curve.
Figure 7.
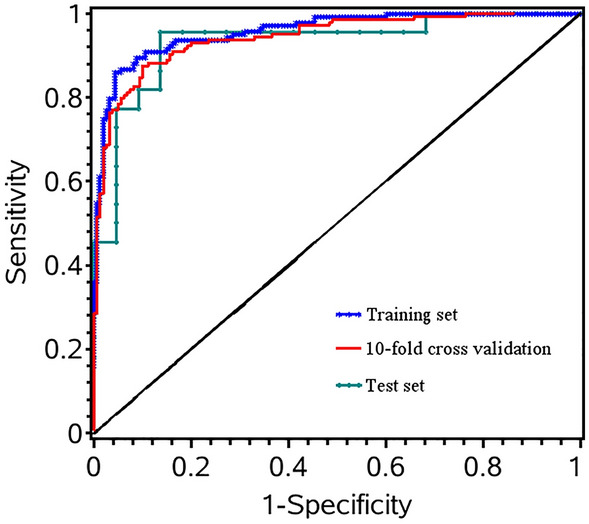
Receiver operating characteristic (ROC) curve was established to examine the performance of metabolite biomarker panel in distinguishing patients with GC from healthy individuals. ROC curve was marked with blue star for training set, red line for tenfold cross validation, and cyan dot for test set.
Discussion
Currently, identification of novel blood biomarkers remains a pivotal goal for GC, and the limitations of modern technology for the detection and treatment of the disease emphasize the necessity of finding novel potential biomarkers. However, few biomarker candidates can be translated into clinical applications due to limited diagnostic performance or study cohorts20. Specific physiological or pathological conditions are able to perturb blood metabolites, which can be used as potential biological indicators in normal and pathological biological processes. Thus, the detection of perturbed small molecular metabolites can provide a powerful tool for cancer diagnosis. In the present study, a total of 349 subjects were recruited, including 183 healthy individuals and 166 patients with GC, and were divided into training set of 305 subjects and test set of 44 subjects. A combination of DBS sampling and direct injection MS analysis was performed to detect metabolite biomarkers for GC. After systematic selection, there were significant differences in the levels of 23 metabolites between patients with GC and healthy individuals (Table 2). Furthermore, independent predictors was identified by a stepwise logistic regression analysis, and a biomarker panel consisting of Ala, Arg, Gly, Orn, Tyr/Cit, Val/Phe, C4-OH, C5/C3, C10:2 was used to construct prediction model for GC.
Metabolic reprogramming was regarded as a central hallmark of cancer. The amino acid and lipid metabolic pathways were disturbed in patients with GC as revealed by differential pathway analysis (Fig. 5). Identifying how metabolism shifts in patients with cancer can contribute to disease diagnosis and prediction. Amino acids were disturbed by the imbalance in protein metabolism due to the influences of host-tumor interactions and metabolic requirements of tumor cells to specific amino acids21, which exhibited potential usage in improving diagnosis and detection of early-stage cancer22. In the present study, 23 metabolites were significantly altered with VIP > 1, adjusted p-value < 0.05, and FC > 1.2 or < − 1.2 in patients with GC compared to healthy individuals, involving Asp, Arg, Gly, Ser, Orn, Ala, and Pro. Interestingly, the levels of Asp, Arg, Gly, Ser, and Orn were increased in patients with GC compared to healthy individuals (Table 2). As a non-essential amino acid, Asp is the basic substrate for the synthesis of pyrimidine and purine nucleosides. Furthermore, Ser is also involved in the synthesis of purine nucleotides via Gly, and it can influence cell growth and invasion of cancer cells23. The increased uptake rates of Asp and Ser imply that these amino acids are needed in fueling nucleoside biosynthesis for tumor proliferation24. As a semi-essential amino acid, Arg is a key component in the body and involved in cell division, immune system, and hormone biosynthesis, and contributes to immunosurveillance, tumor growth and metastasis25, which implies that Arg is required to fuel tumor cell metabolism. Its metabolism may be influenced by the overexpressed argininosuccinate synthase 1 (ASS1) in GC26. The high expression of ASS1 can lead to increased NO production, which promotes gluconeogenesis via S-nitrosylation of pyruvate carboxylase and phosphoenolpyruvate carboxykinase 2. The increased gluconeogenesis may further enhance the levels of Ser and Gly in nucleotide synthesis27. Increased level of Arg can lead to decreased ratio of Cit/Arg, and this ratio has been found to reflect NO production28. Orn acts both as a substrate of ornithine decarboxylase (ODC) to produce polyamines and as a substrate of ornithine aminotransferase (OAT) to produce Pro, and all these products are involved in the promotion of cancer progression29. Therefore, altered levels of Orn and Pro suggested the disorders of ODC and OAT metabolism and the requirements of these amino acids in GC progression. It has been found that ratio of Orn/Cit can reflect a shift in arginine metabolism30. This ratio is influenced by increased level of Orn, reconfirming the altered arginine metabolism in GC.
The upregulated glycolysis in cancer metabolism, also known as the Warburg effect, promotes compensatory pathways, especially oxidation of fatty acids31. Carnitine/acylcarnitines, intermediates of fatty acid oxidation, are essential for fatty acid oxidation and energy metabolism, and accumulated as a consequence of metabolic defect. Considering these adaptations, carnitine pool is uniquely positioned to supervise the perturbations of carnitine/acylcarnitine metabolism, and it is useful to discover the disturbed metabolic pathways during cancer development and progression32. Carnitine palmitoyl transferase 1 (CPT1) is associated with metabolism of acylcarnitines by catalyzing the conversion of acyl-CoA into acylcarnitine, and controls the entry of long-chain fatty acid into mitochondrial matrix for energy production via fatty acid β-oxidation. A recent study reported that CPT1A is upregulated in patients with GC, and is involved in GC progression33, which may account for the distinct accumulations of acylcarnitines in GC. In addition, the expression of carnitine acetyltransferase (CrAT) has been reported to be upregulated in cancer, which may also promote alterations in carnitine metabolism34. In this study, the levels of 4 short-chain acylcarnitines (C2, C3DC, C4-OH, C5) and one long-chain acylcarnitine (C18:1) were increased in patients with GC compared to healthy individuals (Table 2), and the accumulations of acylcarnitine metabolites may be due to the abnormal expression of these enzymes. Ratio C2/C0 was enhanced in patients with GC, which further indicated the increased fatty acyl mitochondrial transport and β-oxidation of fatty acids in GC35. Furthermore, it has been reported that short-chain carnitine-acylcarnitine translocase in mitochondria and short-chain acylcarnitine levels may be related to the metabolism of branched-chain amino acids (BCAA)36, and changed ratio of C3/C5 implicates altered flux through BCAA metabolic pathways37. The abnormal levels of short-chain acylcarnitines may further influence ratio Val/Phe. Several studies reported that free carnitine and short-, medium-, and long-chain acylcarnitines were disturbed in patients with cancer, and showed potential as candidate biomarkers for the development of certain cancers38,39. Taken together, these findings suggested that the detection of carnitine/acylcarnitine changes may provide a promising new strategy against GC.
A high-performance biomarker panel consisting of Ala, Arg, Gly, Orn, Tyr/Cit, Val/Phe, C4-OH, C5/C3, C10:2 was identified and validated for separating patients with GC from healthy individuals, as displayed in Table 3. The tenfold cross-validation was performed to evaluate classifier performance by using the data in training set, which showed that the diagnostic performance of this biomarker panel was satisfactory (AUC: 0.9438). An independent test data set of 44 subjects, including 22 patients with GC and 22 healthy individuals, was applied to assess reliability of this metabolite biomarker panel. It showed that this biomarker panel can effectively discriminate patients with GC from healthy individuals (AUC: 0.9318). These results highlighted that the metabolite biomarker panel may act as a potential valuable tool to detect GC.
In this single-center case-control study, a combination of DBS sampling and MS was utilized for high-throughput detection of metabolites. A metabolite biomarker panel was identified with diagnostic potential for GC. Whereas, there were some limitations in this study. Firstly, since GC is considered as a stepwise progression from non-active gastritis4,5, we believe that this study will be more systematic when a reasonable amount of patients with gastritis can be recruited. Secondly, in this study, the detected metabolites were limited for the trade-off between coverage and cost, and more metabolites such as fatty acids will be detected in order to select more potential biomarkers. Thirdly, more patients with advanced GC will be recruited in order to perform metabolomics analysis based on the patients in different stages. Finally, a multi-institution study with a larger sample size is still required in order to further assess this study’s results.
Conclusion
In summary, a combination of DBS sampling and direct injection MS technology was used to detect metabolites for patients with GC and healthy individuals. Results obtained displayed significantly altered metabolomic profiles in patients with GC compared to healthy individuals. A metabolite biomarker panel of Ala, Arg, Gly, Orn, Tyr/Cit, Val/Phe, C4-OH, C5/C3, C10:2 was determined as an effective tool with satisfactory sensitivity and specificity for discriminating patients with GC from healthy individuals. Therefore, we believe that these selected metabolites have potential as novel biomarkers in the detection of GC.
Supplementary Information
Abbreviations
- GC
Gastric cancer
- HC
Healthy control
- MS
Mass spectrometry
- DBS
Dried blood spot
- QC
Quality control
- PCA
Principal component analysis
- PLS-DA
Partial least squared discriminant analysis
- VIP
Variable importance in projection
- FDR
False discovery rate
- FC
Fold change
- SAM
Significance analysis of microarrays
- ROC
Receiver operating characteristic
- AUC
Area under ROC curve
- Asp
Aspartic acid
- Arg
Arginine
- Gly
Glycine
- Ser
Serine
- Orn
Ornithine
- C3DC
Malonylcarnitine
- C4-OH
Hydroxybutyrylcarnitine
- C18:1
Octadecenoylcarnitine
- Ala
Alanine
- Cit
Citrulline
- C2
Acetylcarnitine
- C0
Free carnitine
- C10
Decanoylcarnitine
- C5
Isovalerylcarnitine
- C3
Propionylcarnitine
- Pro
Proline
- Met
Methionine
- Phe
Phenylalanine
- Tyr
Tyrosine
- Val
Valine
- C10:2
Decadienoylcarnitine
- ASS1
Argininosuccinate synthase 1
- ODC
Ornithine decarboxylase
- OAT
Ornithine aminotransferase
- CPT1
Carnitine palmitoyl transferase 1
Author contributions
X.W. wrote the main manuscript text. X.W. and H.A. did the statistical analysis. H.G. and Z.Z. provided supervision for data analysis and manuscript preparation. All authors reviewed the manuscript.
Funding
This study is supported by grants from Science and Technology Planning Project of Guiyang, Guizhou Province, China (Grant No. [2019]-9-4-28), research project in the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (Grant No. GZEYK[2020]6), Science and Technology Foundation of Health Commission of Guizhou Province (Grant No. gzwjkj2020-1-185), Science and Technology planning project of Guizhou Province(Grant No. ZK[2022] General 494).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xue Wu and Huaixuan Ao.
Contributor Information
Hui Gao, Email: 15224804@qq.com.
Zhitu Zhu, Email: zhituzhu12@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19061-3.
References
- 1.Wu C, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci. China Life Sci. 2019;62(5):640–647. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Amieva M, Peek RM. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol. Clin. North Am. 2013;42(2):261–284. doi: 10.1016/j.gtc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Lario S, et al. Plasma sample based analysis of gastric cancer progression using targeted metabolomics. Sci. Rep. 2017;7(1):17774. doi: 10.1038/s41598-017-17921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J. Cancer Prev. 2015;20:25–40. doi: 10.15430/JCP.2015.20.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohi O, et al. Diagnostic ability of magnifying endoscopy with blue laser imaging for early gastric cancer: A prospective study. Gastric Cancer. 2017;20(2):297–303. doi: 10.1007/s10120-016-0620-6. [DOI] [PubMed] [Google Scholar]
- 8.He CZ, et al. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87–91. doi: 10.1186/1471-230X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaushik AK, DeBerardinis RJ. Applications of metabolomics to study cancer metabolism. Biochim. Biophys. Acta Rev. Cancer. 2018;1870(1):2–14. doi: 10.1016/j.bbcan.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonian M, Mosallayi M, Mirzaei H. Circulating miR-21 as novel biomarker in gastric cancer: Diagnostic and prognostic biomarker. J. Cancer Res. Ther. 2018;14(2):475. doi: 10.4103/0973-1482.175428. [DOI] [PubMed] [Google Scholar]
- 11.Choi JM, Park WS, Song KY, Lee HJ, Jung BH. Development of simultaneous analysis of tryptophan metabolites in serum and gastric juice-an investigation towards establishing a biomarker test for gastric cancer diagnosis. Biomed. Chromatogr. 2016;30(12):1963–1974. doi: 10.1002/bmc.3773. [DOI] [PubMed] [Google Scholar]
- 12.Aa J, et al. Metabolic features of the tumor microenvironment of gastric cancer and the link to the systemic macroenvironment. Metabolomics. 2012;8:164–173. doi: 10.1007/s11306-011-0297-0. [DOI] [Google Scholar]
- 13.Huang Q, et al. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 2013;73(16):4992–5002. doi: 10.1158/0008-5472.CAN-13-0308. [DOI] [PubMed] [Google Scholar]
- 14.Liang Q, Wang C, Li B. Metabolomic analysis using liquid chromatography/mass spectrometry for gastric cancer. Appl. Biochem. Biotechnol. 2015;176(8):2170–2184. doi: 10.1007/s12010-015-1706-z. [DOI] [PubMed] [Google Scholar]
- 15.Lu WH, et al. Using matrix-induced ion suppression combined with LC-MS/MS for quantification of trimethylamine-N-oxide, choline, carnitine and acetylcarnitine in dried blood spot samples. Anal. Chim. Acta. 2021;1149:338214. doi: 10.1016/j.aca.2021.338214. [DOI] [PubMed] [Google Scholar]
- 16.Kirwan JA, et al. Preanalytical processing and biobanking procedures of biological samples for metabolomics research: A white paper, community perspective (for "precision medicine and pharmacometabolomics task group"-the metabolomics society initiative) Clin. Chem. 2018;64(8):1158–1182. doi: 10.1373/clinchem.2018.287045. [DOI] [PubMed] [Google Scholar]
- 17.Sriwi D, et al. Metabolomics profiling of cystic renal disease towards biomarker discovery. Biology. 2021;10(8):770. doi: 10.3390/biology10080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao G, et al. A metabolomic study for chronic heart failure patients based on a dried blood spot mass spectrometry approach. RSC Adv. 2020;10:19621–19628. doi: 10.1039/C9RA10684G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, et al. A dried blood spot mass spectrometry metabolomic approach for rapid breast cancer detection. Onco Targets Ther. 2016;9:1389–1398. doi: 10.2147/OTT.S95862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polanski M, Anderson NL. A list of candidate cancer biomarkers for targeted proteomics. Biomark. Insights. 2007;1:1–48. [PMC free article] [PubMed] [Google Scholar]
- 21.Elia I, et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 2017;8:15267. doi: 10.1038/ncomms15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eniu DT, et al. The decrease of some serum free amino acids can predict breast cancer diagnosis and progression. Scand. J. Clin. Lab. Investig. 2019;79(1–2):17–24. doi: 10.1080/00365513.2018.1542541. [DOI] [PubMed] [Google Scholar]
- 23.Li J, et al. Genetic profiles affect the biological effects of serine on gastric cancer cells. Front. Pharmacol. 2020;11:1183. doi: 10.3389/fphar.2020.01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LB, Shen JG, Zhang SZ, Ding KF, Zheng S. Amino acid uptake in arterio-venous serum of normal and cancerous colon tissues. World J. Gastroenterol. 2004;10(9):1297–1300. doi: 10.3748/wjg.v10.i9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Koussa H, El Mais N, Maalouf H, Abi-Habib R, El-Sibai M. Arginine deprivation: A potential therapeutic for cancer cell metastasis? A review. Cancer Cell Int. 2020;20:150. doi: 10.1186/s12935-020-01232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delage B, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer. 2010;126(12):2762–2772. doi: 10.1002/ijc.25202. [DOI] [PubMed] [Google Scholar]
- 27.Keshet R, et al. Targeting purine synthesis in ASS1-expressing tumors enhances the response to immune checkpoint inhibitors. Nat. Cancer. 2020;1:894–908. doi: 10.1038/s43018-020-0106-7. [DOI] [PubMed] [Google Scholar]
- 28.Benedetto C, et al. Increased l-citrulline/l-arginine plasma ratio in severe preeclampsia. Obstet. Gynecol. 2000;96(3):395–399. doi: 10.1016/s0029-7844(00)00939-x. [DOI] [PubMed] [Google Scholar]
- 29.Sivashanmugam M, Jaidev J, Umashankar V, Sulochana KN. Ornithine and its role in metabolic diseases: An appraisal. Biomed. Pharmacother. 2017;86:185–194. doi: 10.1016/j.biopha.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Kövamees O, Shemyakin A, Pernow J. Amino acid metabolism reflecting arginase activity is increased in patients with type 2 diabetes and associated with endothelial dysfunction. Diab. Vasc. Dis. Res. 2016;13(5):354–360. doi: 10.1177/1479164116643916. [DOI] [PubMed] [Google Scholar]
- 31.Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166(3):555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCann MR, George De la Rosa MV, Rosania GR, Stringer KA. l-Carnitine and acylcarnitines: Mitochondrial biomarkers for precision medicine. Metabolites. 2021;11(1):51. doi: 10.3390/metabo11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Li C, Song Y, Yan Z. Inhibition of carnitine palmitoyl transferase 1A-induced fatty acid oxidation suppresses cell progression in gastric cancer. Arch. Biochem. Biophys. 2020;696:108664. doi: 10.1016/j.abb.2020.108664. [DOI] [PubMed] [Google Scholar]
- 34.Sun C, Wang F, Zhang Y, Yu J, Wang X. Mass spectrometry imaging- based metabolomics to visualize the spatially resolved reprogramming of carnitine metabolism in breast cancer. Theranostics. 2020;10(16):7070–7082. doi: 10.7150/thno.45543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jourdan C, et al. Associations between thyroid hormones and serum metabolite profiles in an euthyroid population. Metabolomics. 2014;10(1):152–164. doi: 10.1007/s11306-013-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roe DS, Roe CR, Brivet M, Sweetman L. Evidence for a short-chain carnitine-acylcarnitine translocase in mitochondria specifically related to the metabolism of branched-chain amino acids. Mol. Genet. Metab. 2000;69(1):69–75. doi: 10.1006/mgme.1999.2950. [DOI] [PubMed] [Google Scholar]
- 37.Jachthuber Trub C, et al. Impact of lifestyle intervention on branched-chain amino acid catabolism and insulin sensitivity in adolescents with obesity. Endocrinol. Diabetes Metab. 2021;4(3):e00250. doi: 10.1002/edm2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enooku K, et al. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci. Rep. 2019;9(1):10663. doi: 10.1038/s41598-019-47216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Gao D, Jiang Y. Function, detection and alteration of acylcarnitine metabolism in hepatocellular carcinoma. Metabolites. 2019;9(2):36. doi: 10.3390/metabo9020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.