Abstract
In recent years, extracellular vesicles (EVs), specifically exosomes, have emerged as a promising strategy for treating a wide spectrum of pathologies, such as cancer and COVID-19, as well as promoting tissue regeneration in various conditions, including cardiomyopathies and spinal cord injuries. Despite the great potential of EV-based therapies, poor yield and unscalable production of EVs remain big challenges to overcome to translate these types of treatment to clinical practices. Here, we review different strategies for enhancing EV yield by physical, biological or chemical means. Some of these novel approaches can lead to about 100-fold increase in EV production yield, thus bringing closer the clinical translation with regard to scalability and efficiency.
Keywords: Extracellular vesicles, Exosomes, Large-scale, Drug delivery
1. Introduction
Extracellular vesicles (EVs), including exosomes, are nanoscale lipid bilayer membrane capsules secreted by cells. They contain proteins and genetic materials for local or systemic cell communications (Kalluri and LeBleu, 2020; van Niel et al., 2018). Multiple EV-based therapies have been developed in recent years as a novel and alternative strategy to cell therapies to treat various medical conditions, such as cancer (Kamerkar et al., 2017; Koh et al., 2017), cardiovascular (Gao et al., 2020; Liu et al., 2018a; Yadid et al., 2020), neurological (Guo et al., 2019; Perets et al., 2018) and orthopedic diseases (Fan et al., 2020). The usage of EV-based therapies holds inherent advantages as compared to cell-based therapies. These include lower immunogenicity, better safety and regulatory aspects, and the potential to serve as off-the-shelf products. Yet, to reach industrial-scale production, the poor yield and scalability issues need to be addressed. The traditional EV production process is based on a costly 2D cell cultivation protocol that requires large quantities of cell culture plastics, media, and space, highlighting the need for more scalable methods.
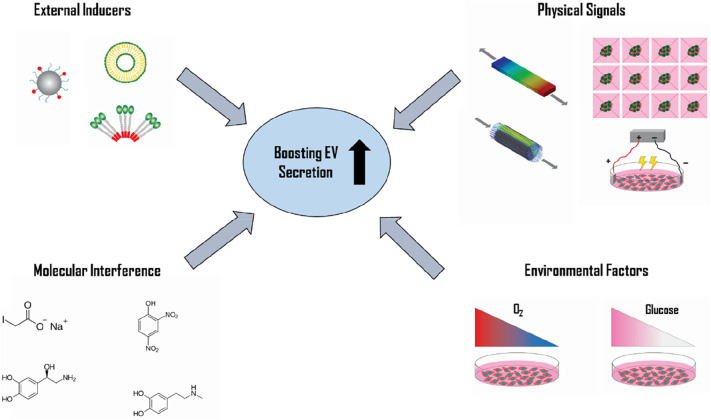
Recently, various factors have been discovered to stimulate exosome release. As a result, numerous approaches have been developed to increase EV secretion rates and improve production yield. In this review, we discuss novel methods to stimulate EV production and consider their strengths and limitations. We explore physical methods, such as mechanical loading, geometry, acoustic and electrical stimulation, as well as several non-physical protocols, including molecular interference, environmental factors and external inducers.
2. Stimulating EV production by physical signals
2.1. Mechanical loading
Mechanosensing is a fundamental pathway in cell-cell and cell-microenvironment communication. It includes a cell's reaction to a mechanical load, which can result in EV signaling secretion for near and far communication (Paolicelli et al., 2019; Turturici et al., 2014). Thus, mechanical stimulus may interfere with cell EV secretion mechanism and initiate enhanced production under certain loading conditions. Several studies have tested the influence of mechanical shear stress on cell behavior and EV secretion rate under direct flow or turbulence induction (Gazeau et al., 2021; Guo et al., 2021; Patel et al., 2019; Piffoux et al., 2017). Patel et al. demonstrated that the application of low shear rate (1.5 × 10−2 dyn/cm2) on human dermal microvascular endothelial cells (HDMECs) cultivated on a scaffold consisting of 3D pillars array resulted in a significant increase in EV secretion, at least 100-fold higher than that of the hydrostatic control (Patel et al., 2019). Another study revealed the application of moderate shear stress level (in the range of 0.5 to 5 dyn/cm2) on 3D engineered tissues made of DPSC constructs led to an increase of over 150-fold in EV production (Guo et al., 2021). Furthermore, the addition of the mechanosensing YAP-inhibitor resulted in a dramatic decrease in EV secretion, proving the active mechanosensitivity-mediated secretion process (Guo et al., 2021).
The application of cyclic tension load is another mechanical stimulus cells can experience in various tissues, such as skeletal and cardiac muscle. While exposed to cyclic stretch, hSkMCs were shown to increase EV secretion by an order of magnitude as compared to static control (Guo et al., 2021). Similarly, in the work of Wang et al., which studied the effect of mechanical environment on periodontal immune/inflammatory homeostasis, the authors reported a 30-fold increase in exosome secretion by periodontal ligament cells (PDL cells) under cyclic stretch application for 24 h, as compared to static cultivation (Wang et al., 2019).
The immediate advantages of such mechanical stimulations to increase EV secretion are clear and can be beneficial to the transfer to industrial mass production protocols in a scalable manner with relatively simple bioreactor systems (Fig. 1 ).
Fig. 1.
Novel strategies for boosting extracellular vesicle secretion. EV secretion rate can be significantly enhanced up to two orders of magnitude using various triggers including physical signals, molecular interference, environmental factors, and external inducers.
2.2. Geometry
The traditional EV production protocols are based on 2D flask cultivation, lacking efficiency in the aspects of space, materials, and yield. Switching to 3D geometries provides more physiological cultivation conditions and results in a higher yield of EV secretion. Kim et al. compared the MSC EV secretion values of conventional 2D monolayer cell culture to 3D hanging droplet spheroids and 3D aggregates formed by poly(2-hydroxyethyl methacrylate) coating method (Kim et al., 2018). EV mass quantification revealed a 3-fold increase in EV secretion in favor of the 3D cultivation methods as compared to 2D monolayer. Additionally, lowering spheroid diameter led to an up to 6-fold increase in EV secretion, possibly due to the increase of total effective surface area. In another work, dynamic cultivation of 3D hMSC spheroids (200 μm microwell array cultured at 30 rpm on orbital shaker) resulted in a significant increase in EV secretion (Cha et al., 2018). Furthermore, the EVs contained a variety of therapeutic cytokines related to immunomodulation and angiogenesis and showed superior results in angiogenic and neurogenic assays in vitro. Lastly, a recently published paper proved the superiority of 3D cultivation in the form of engineered tissues as compared to the conventional 2D monolayer (Guo et al., 2021). Hydrostatic cultivation of 3D engineered tissues seeded with DPSCs or MSCs resulted in a 5-to-10-fold increase in EV secretion rate as compared to traditional 2D monolayer cultivation again, showing the beneficial effect of geometry, specifically 3D cultivation assays, in enhancing EV secretion rates.
2.3. Acoustic stimulation
Recently, another physical approach to stimulate EV production using sound waves was reported (Ambattu et al., 2020). By using high-frequency ultrasound (US) irradiation (4 W, 10 MHz), EV production was increased 8–10-fold. The stimulation profile included repeated cycles of 10 min US stimulation followed by 30 min of cell incubation. Stimulation was evoked by a piezoelectric actuator that induced surface-reflected bulk waves (SRBWs) with high cell viability rates (over 95%). The study further revealed that the reported enhanced EV secretion phenomena was regulated by calcium-dependent mechanisms. By exposing the cells to US stimulation, the authors showed an elevation in Ca2+ intracellular levels due to an increased permeability, which triggered the Endosomal Sorting Complexes Required for Transport Machinery (ESCRT) pathway and elevated CD63 and ALIX levels. This, in turn, led to increased EV production and secretion rates.
2.4. Electrical stimulation
Low level electric treatment (ET) has been shown to activate intracellular signaling including Rho-GTPase and endocytosis, which are known to be involved in exosome formation mechanism (Hasan et al., 2019). Based on this, Fukuta et al. tested the influence of low level electric treatment on EV secretion (Fukuta et al., 2020). The application of 0.34 mA/cm2 electrical field on murine melanoma and murine fibroblast cells for 60 min resulted in a significant increase in the EV secretion rate (1.7-fold) while maintaining cell viability and particle distribution. Moreover, the addition of Rhosin hydrochloride, a Rho-GTPase inhibitor, significantly decreased EV particle number, suggesting the involvement of Rho-GTPase activation in ET-mediated EV secretion. Finally, the quantification of cellular uptake of EVs collected with or without ET maintained similar uptake values suggested comparable EV functionality with or without ET stimulation.
3. Stimulating EV production by molecular interference
3.1. Inhibition of Glycolysis & oxidative phosphorylation
In general, cells under stress are known to increase exosome secretion (Ludwig et al., 2020). The combination of sodium iodoacetate (IAA) and 2,4-dinitrophenol (DNP) could induce a potent reduction of cellular energy charge by simultaneously blocking oxidative phosphorylation and glycolysis (Jackson et al., 2014). Therefore, Jackson et al. hypothesized that blocking cellular pathways of energy production using IAA and DNP would induce cellular stress and thus effectively boost exosome production (Ludwig et al., 2020). Indeed, simultaneous inhibition of glycolysis and oxidative phosphorylation by the combination of IAA/DNP led to energy depletion in the cells (decreased ATP levels, elevated AMP levels), stimulating cells to release adenosine, which activated the A2B receptor system and finally resulted in a 3-to-16-fold increase in exosome production. Those exosomes did not influence the cell migration when compared with exosomes derived without IAA/DNP stimulation. Despite these, other differences such as the heterogeneity, composition, and functional effects in other potency assays might change and should be examined.
3.2. Endolysosomal trafficking
The biogenesis of exosomes occurs during the process of endosomal maturation. Interestingly, it has been described that inhibition of lysosomal function prevents endosome maturation, leading to an accumulation of intraluminal vesicles in multivesicular bodies (Vanlandingham and Ceresa, 2009). Ortega et al. set out to evaluate whether interfering with endolysosomal trafficking through inhibition of endosomal maturation and/or reduction of lysosomal function would stimulate exosome release, and whether these exosomes retain their bioactive properties (Ortega et al., 2019). By using RNA interference technology, two known players in endosomal trafficking, NDRG1 and Rab7, were targeted. The reduction of NDRG1, but not Rab7, expression led to a two-fold increment in the release of exosomes. Two other well-known lysosomotropic agents, chloroquine and NH4Cl, were also tested. Similarly, they enhanced exosome release but did not alter the size, cargo and bioactivity of exosomes. Overall, the usage of these chemical compounds offers a straightforward approach to boost exosome production through inhibition of endolysosomal trafficking.
3.3. Adiponectin
Adiponectin (APN) is a high-molecular-weight (HMW) multimer secreted exclusively by adipocytes. HMW multidimer adiponectin accumulates in a variety of tissues, such as the heart and skeletal muscles, and interacts with T-cadherin, a unique glycosylphosphatidylinositol-anchored (GPI-anchored) cadherin (Fukuda et al., 2017). Recent studies have demonstrated that adiponectin isolated from serum can enter through the endosomal route via binding to T-cadherin, The adiponectin/T-cadherin system subsequently enhances exosome biogenesis and secretion (Fukuda et al., 2017; Kita et al., 2019; Obata et al., 2018). Based on these observations, Nakamura et al. stimulated exosome biogenesis and secretion via adiponectin binding to T-cadherin on human adipose-derived MSCs (hMSCs), resulting in an about 3-fold increase in exosome production compared to those MSCs without APN stimulation. The exosome production mediated by APN was dose-dependent within the range of physiological plasma APN concentrations (Nakamura et al., 2020). The group further evaluated the effect of circulating APN on exosome production in vivo and reported a dose dependent effect of APN on stimulating exosome release to enhance hMSC-driven therapy of heart failure in mice.
3.4. Small molecule modulators
Recent study optimized a quantitative high throughput screen (qHTS) assay to identify compounds that modulate exosome biogenesis and/or release by aggressive prostate cancer cells (Datta et al., 2018). Based on this finding, Wang et al. set out to examine the effects of selected small molecule modulators on exosome production from MSCs (Wang et al., 2020). Four FDA-approved drugs (fenoterol, norepinephrine, N-methyldopamine, and mephenesin), as well as another nutritional supplement, (forskolin) were selected. The treatment of MSCs with a combination of N-methyldopamine and norepinephrine led to 3-fold increase in exosome production by enhancing the expression of genes related to the ESCRT-independent pathway, such as nSMase2. Importantly, the intrinsic regenerative effects of MSC exosomes were not altered, such as inducing angiogenesis, polarizing macrophages, or downregulating collagen expression. Therefore, these compounds could be employed to boost exosome secretion from MSCs for practical application.
4. Stimulating EV production by environmental factors
4.1. Hypoxia
Hypoxia, the condition of insufficient oxygen, is an environmental stress factor that stimulates exosome release. It has been extensively studied in a context of tumor biology. Most tumors have an increased oxygen demand from proliferating cancer cells and low oxygen supply due to poor vascularization. In response to hypoxia, cancer cells release exosomes containing proangiogenic micro-RNAs and growth factors (Shao et al., 2018). Hypoxia can also be utilized for therapeutic exosome production (Bister et al., 2020). Exposure of cancer cells to severe hypoxic conditions for 24 h resulted in 2- to 3-fold increase in exosome release (King Hamish et al., 2012; Tse et al., 2020). Prolonged moderate hypoxia (1% oxygen for 48 h) was less effective for lung cancer cells, and caused only 1.3-fold exosomal elevation (Hamish W King et al., 2012). Other cell lines, such as pancreatic cancer cells and Ewic sarcoma cells, responded with a 2- to 3-fold EV increase even under moderate hypoxia (1% oxygen for 24-72 h) (Zeng et al., 2021). The activation of the hypoxia inducible factor (HIF) signaling pathway seems to be largely responsible for this effect (King Hamish et al., 2012; Simko et al., 2017). Further research confirms that the overexpression of HIF without hypoxic stimulation had a similar effect on exosome levels (Gonzalez-King et al., 2017). A number of studies demonstrated slightly enhanced EV secretion upon moderate hypoxic treatment (0.5–1% oxygen) of MSCs (Gonzalez-King et al., 2017; Liu et al., 2020; Zhu et al., 2017). When hypoxic stimulation was performed at 5%, no elevation in exosome release was observed, however the properties of the exosomes were still affected (Almeria et al., 2019; Han et al., 2019). One study demonstrated decreased EV release upon exposure of MSCs to hypoxic conditions. The opposite effect may be explained by prolonged exposure (72 h at 1% oxygen) (Liu et al., 2018b).
Overall, hypoxic stress has the potential to increase exosome production. It has been shown that brief, severe exposures to hypoxia seem to show a greater influence on exosomes secretion when compared to their exposure to a prolonged, moderate state. So far, the investigation of hypoxia's influence on EVs focused on the change in exosome properties rather its effect on numbers, therefore the optimal conditions for increased release may not have been identified. Yet, according to currently available data, hypoxic stimulation seems to be far less effective compared to most of the above-mentioned strategies.
4.2. Acidity
Low PH is also known to stimulate exosome release. Tumor-associated acidity, alongside hypoxia, are major determinants in inducing increased exosome release by human cancer cells (Logozzi et al., 2019; Logozzi et al., 2017; Parolini et al., 2009). The exosomal production seems to depend on the acidity in a direct ratio (Ban et al., 2015). HEK 293 T cells secreted 5-fold fewer exosomes in high pH (11.0) and 6-fold more exosomes in low (4.0) pH compared to normal pH condition. A gradual moderate decrease in the pH from 7.4 to 6.5 resulted in a profound, up to 69-fold, increased exosome release by the tumor cells. The magnitude of this effect was largely dependent on the cell line used (Logozzi et al., 2018). So far, moderately reduced pH seems to be a promising environmental factor to stimulate the exosome production; it is easy to apply and only mildly affects cell viability. However, whether acidity is efficient in therapeutically relevant cell types, such as MSCs, has yet to be investigated.
4.3. Starvation or hyperglycemia
There is limited evidence that insufficient or excess glucose can stimulate exosome release. Under conditions of glucose deprivation, immortalized H9C2 cardiomyocytes exhibited around 4-fold increased secretion of exosomes. Here, unlike in the case of hypoxia, prolonged starvation resulted in higher exosome release (Garcia et al., 2015). The combination of moderate hypoxia and hypoglycemia have also resulted in 3- to 4-fold increase in exosome production, as indicated for trophoblast cells (Rice et al., 2015). Systematic studies of starvation and hypoglycemia in the context of exosome release are still lacking to conclude on their effectiveness.
5. Stimulating EV production by external inducers
5.1. Liposomes
Another strategy to stimulate exosome secretion using liposomes was tested recently (Emam et al., 2018). Liposomes are known to interact with cell membranes and stimulate cell activity by endocytosis. Thus, liposomes might interfere with exosome formation and secretion process (Cui et al., 2005; Elsabahy and Wooley, 2013; Miller et al., 1998). In this recent study, several liposome variations were added to four types of cultivated tumor cell lines (Emam et al., 2018). The addition of neutral or cationic-bare liposomes resulted in a significant increment of exosome secretion (up to 3-fold higher as compared to the control). Interestingly, PEGylation of bare liposomes diminished exosome secretion. This might be a result of lipid-cell collision interaction, which needs further investigation. Additionally, exosomes harvested under fluidic cationic conditions showed significant increase in cellular uptake rates. The results of this study reflect a new possibility to regulate exosome secretion rate with customized liposomes.
5.2. Nanoparticles
The regulation and stimulation of exosome secretion by interfering with the endocytosis process were also tested by surface modified nanoparticles (Park et al., 2020). In the study of Park et al., iron-oxide magnetic nanoparticles (NPs) were coated with poly(lactic-co-glycolic acid) or PLGA and polyethyleneimine PEI. These 100 nm NPs entered MSCs and stimulated exosome secretion by 5-fold without magnetic field application and by 20-fold under magnetic field application. The upregulated endocytosis processes involve stimulation of the autophagy factor, Rab7, thereby promoting exosome secretion.
6. Conclusions
As discussed here, various stimuli, including physical, chemical and biological, hold great potential in significantly increasing EV secretion rate, and bring closer the ability to translate EV-based therapies into the clinic (as summarized in Table 1 ). Nevertheless, multiple aspects need to be addressed to ensure the safety, efficiency, and robustness of such technologies. The application of physical stimulus shows significant elevation in the secretion yield, especially by mechanical load, which can lead to ~150-fold higher secretion values compared to the traditional 2D cultivation. Yet, the loading intensity must be carefully chosen to meet the necessary threshold for secretion stimulation while avoiding excessive cell stress. Additionally, scaling up such technologies entails other concerns which need to be addressed during the design process. This includes the ability to maintain uniform loading field for all cultivated cells in a large volume stimulated by external means, such as flow, stretch, induced electric field or ultrasound induction. Alternatively, dense cell constructs with proper media exchange that prevent cell ischemia can be fabricated. It is worth mentioning that EVs secreted by cells exposed to mechanical strains showed improved functionality with regard to periodontal tissue homeostasis, bone homeostasis, or neurotrophic effects, when compared to their non-stimulated counterparts (Guo et al., 2021; Lv et al., 2020; Xiao et al., 2021). This is likely due to the altered cargos after mechanical stimulations, as Guo S and Debbi L et al. reported.
Table 1.
Stimulation methods for increasing EV secretion.
| Methods | Cell type | Treatment | Endosomal markers | Altered endogenous cargo | Increase in release | Scalability in GMP environment | Therapeutic use | Refs |
|---|---|---|---|---|---|---|---|---|
| Physical Signals | ||||||||
| Mechanical loading | HDMECs, DPSCs, hSkMCs, PDL | 0.5 ml/min flow induction for 48 h, 25% 1 Hz cyclic stretch for 48 h | CD9, CD63, CD81 | RhoG, ITGAV, CAPZA2, CKAP5, CDH13, ARPC2/4, MYH11, TUBB ↑ | 11 to 150 fold | +++ | drug delivery platform, neuronal regeneration, muscle regeneration | (Guo et al., 2021) |
| Geometry | MSCs, DPSCs | 3D aggregates, 3D constructs support by polymeric scaffold | CD9, CD63, CD81 | miR-210 ↑, miR-134, miR-137, miR-184↓ | 3 to 10 fold | +++ | pro-angiogenic properties | (Cha et al., 2018; Guo et al., 2021) |
| Acoustic stimulation | U87-MG human glioblastoma cells and A549 adenocarcinomic human alveolar basal epithelial cells | 7 repeated cycles of 10 min SRBWs stimulation (4 W, 10 MHz) followed by 30 min of cell incubation | CD63, Alix | syntenin-1 | 8 to 10 fold | + | drug delivery platform | (Ambattu et al., 2020) |
| Electric stimulation | murine melanoma and murine fibroblast | 0.34 mA/cm2 electrical field for 60 min | CD9, HSP70, and CD81 | − | ca. 1.7 fold | + | beneficial effects of transcranial direct current stimulation (tDCS) in the brain | (Fukuta et al., 2020) |
| Molecular Interference | ||||||||
| Glycolysis & oxidative phosphorylation inhibition | cancer cell lines: UMSCC47, PCI-13 and MEL526, | 10 μM IAA/DNP for over 48 h | TSG101 | − | 3 to 16-fold | ++ | − | (Ludwig et al., 2020) |
| Endolysosomal trafficking | SKOV-3 cells, cardiac progenitor cells | NDRG1 knocked down | Alix, TSG101, CD9, CD81 | − | up to 2 fold | ++ | Myocardial ischemia and ischemia/reperfusion injury. | (Ortega et al., 2019) |
| Adiponectin | MSCs | 20 mg/ml HMW-APN for 48 h | Syntenin, MFG-E8, Alix, CD63, TSG101 | ↑ miRNAs: let-7 family, miR-21, −100, −148a, −10, −26, and − 199, and others | ca. 3 fold | ++ | Pressure-overload heart failure | (Nakamura et al., 2020) |
| Small molecule modulators | MSCs | 100 μM NE/MeDA for 48 h | CD9, CD63, Hrs, TSG101, Stam1, Alix | ↑ proteins: COL15A1, COL11A1, LOXL2, AGRN, NID2, HSPG2, COM | ca. 3 fold | ++ | Ischemic or inflammatory diseases | (Wang et al., 2020) |
| Environmental Factors | ||||||||
| Hypoxia | Breast cancer cell lines | 1% for 48 h or 0.1% O2 for 24 h | CD63, TSG101, CD9 | miR-210 ↑ | up to 2 fold | +++ | − | (Hamish W. King et al., 2012) |
| Pancreatic cancer cell lines | 1% O2 for 48-72 h | CD63 | circZNF91↑, miR-23b-3p ↓ | up to 2 fold | +++ | − | (Zeng et al., 2021) | |
| MSCs | 1% O2 for 48 h | CD63, TSG101, CD9, CD81 | miR-126 ↑ | ca. 1.5 fold | +++ | bone fracture healing | (Liu et al., 2020) | |
| Acidity | metastatic prostate carcinoma LNCaP, metastatic melanoma Me30966, SaOS2 osteosarcoma, SKBR3 metastatic breast adenocarcinoma, and HCT116 colorectal carcinoma | pH 6.5 for 5 days | CD9, CD63, CD81, TSG101, Alix | − | sKBR3–6 fold, LNCaP - 9 fold, SaOS2–14 fold, HTC116–52 fold, Me30966–102 fold | +++ | − | (Logozzi et al., 2018) |
| metastatic melanoma cells | pH 6 for 4 days | Lamp-2, CD81, and Rab 5B | caveolin-1 ↑, membrane rigidity ↑, sphingomyelin/ganglioside GM3 (N-acetylneuraminylgalactosylglucosylceramide) content ↑, exosome fusion ↑ | up to 4 fold | +++ | delivery system for paracrine diffusion of tumor malignancy | (Parolini et al., 2009) | |
| HEK 293 | pH 4 for 3 days | CD9, CD63, HSP70 | − | up to 6 fold | +++ | − | (Ban et al., 2015) | |
| Starvation or hyperglycemia | immortalized H9C2 cardiomyocytes | glucose starved for 48 h | CD9, CD63, CD81 | 22 different miRs ↑ | ca. 3 fold | +++ | pro-angiogenic properties | (Garcia et al., 2015) |
| First-trimester trophoblast cells | 1% O2, hyperglycemia (25 mM glucose) for 48 h | CD63 | − | ca. 3 fold | +++ | induction if cytokine release | (Rice et al., 2015) | |
| External Inducers | ||||||||
| Liposomes | cancer cell lines:C26, B16BL6, MKN45, DLD-1 | 0.5-2 mM PEGylated/NL/CL1 for 48 h | TSG101, CD63 or CD81 | − | up to 3-fold | ++ | tumor metastasis | (Emam et al., 2018) |
| Nanoparticles | MSCs | 5-20 μg/ml PLGA-PEI PCS NPs (+) for 24 h | CD63, CD9, and CD81 | mmu-miR-2137, mmu-miR-3473b, mmu-miR-3473e, mmu-miR-3960, mmu-miR-5126, mmu-miR-5126, mmu-miR-455-3p ↑ | 5 to 20-fold | ++ | tissue regeneration or antioxidant efficacy | (Park et al., 2020) |
When it comes to boosting EV secretion via molecular interference, molecular compounds should meet FDA standards so that the EVs produced from cells stimulated by those compounds will be safe for further applications. To achieve this goal, the concentrations of stimulating compounds concentrations within the produced EVs should be quantified and analyzed to ensure that they would not pose any safety issue. In addition, it is important to note that the effective concentration of certain molecules works in specific cell lines may not work in other cell lines. Therefore, efforts are needed to optimize the dosages of molecules for each cell line for gaining the most abundant exosome production. Thirdly, while the functionality and cargos of EVs have been reported to be affected by mechanical stimulations (Guo et al., 2021), it remains to be determined whether the EVs primed after molecular interference will have altered cargos and what cargos have been changed to induce improved functionality.
The environmental factors, such as hypoxia, pH and glucose content, have been mostly studied with regard to EVs property alteration, rather than EV secretion value. According to current data, pH is by far a more promising environmental factor, however it still must be tested in clinically relevant cell types, such as MSCs. The combination of environmental factors with physical stimulation may further improve the EV secretion value.
All the stimulants described here represent different stress factors. Yet, the downstream mechanism of EV secretion elevation has not been thoroughly investigated for each of the stimulants. Systematic comparison of most promising stimulants regarding downstream effects may lead to a common mechanism and potentially enable creation of genetically enhanced exosome secretion.
Notes
The authors declare no conflicts of interests.
Acknowledgements
This work is supported by the generous donation of Mr. Sunder Charnai (Recovery of chronic or delayed spinal cord injuries project) and the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG02C). We wish to thank Janette Zavin for her help with the illustrations and Adina Israel Fried for editorial assistance in preparing this manuscript.
References
- Almeria C., Weiss R., Roy M., Tripisciano C., Kasper C., Weber V., Egger D. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front. Bioeng. Biotechnol. 2019;0:292. doi: 10.3389/FBIOE.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambattu L.A., Ramesan S., Dekiwadia C., Hanssen E., Li H., Yeo L.Y. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism. Commun. Biol. 2020;3 doi: 10.1038/s42003-020-01277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban J.J., Lee M., Im W., Kim M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015;461:76–79. doi: 10.1016/J.BBRC.2015.03.172. [DOI] [PubMed] [Google Scholar]
- Bister N., Pistono C., Huremagic B., Jolkkonen J., Giugno R., Malm T. Hypoxia and extracellular vesicles: a review on methods, vesicular cargo and functions. J. Extracell. Vesicles. 2020;10 doi: 10.1002/JEV2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J.M., Shin E.K., Sung J.H., Moon G.J., Kim E.H., Cho Y.H., Park H.D., Bae H., Kim J., Bang O.Y. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018;8:1–16. doi: 10.1038/s41598-018-19211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Han S.J., Vangasseri D.P., Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol. Pharm. 2005;2:22–28. doi: 10.1021/MP049907K. [DOI] [PubMed] [Google Scholar]
- Datta A., Kim H., McGee L., Johnson A.E., Talwar S., Marugan J., Southall N., Hu X., Lal M., Mondal D., Ferrer M., Abdel-Mageed A.B. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci. Reports. 2018;81(8):1–13. doi: 10.1038/s41598-018-26411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabahy M., Wooley K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013;42:5552–5576. doi: 10.1039/C3CS60064E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emam S.E., Ando H., Lila A.S.A., Shimizu T., Ukawa M., Okuhira K., Ishima Y., Mahdy M.A., Ghazy F., Ishida T. A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biol. Pharm. Bull. 2018;41:733–742. doi: 10.1248/BPB.B17-00919. [DOI] [PubMed] [Google Scholar]
- Fan J., Lee C.-S., Kim S., Chen C., Aghaloo T., Lee M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano. 2020;14:11973–11984. doi: 10.1021/ACSNANO.0C05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Kita S., Obata Y., Fujishima Y., Nagao H., Masuda S., Tanaka Y., Nishizawa H., Funahashi T., Takagi J., Maeda N., Shimomura I. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J. Biol. Chem. 2017;292:7840. doi: 10.1074/JBC.M117.780734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta T., Nishikawa A., Kogure K. Low level electricity increases the secretion of extracellular vesicles from cultured cells. Biochem. Biophys. Reports. 2020;21 doi: 10.1016/j.bbrep.2019.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Wang L., Wei Y., Krishnamurthy P., Walcott G.P., Menasché P., Zhang J. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 2020;12:1318. doi: 10.1126/SCITRANSLMED.AAY1318. [DOI] [PubMed] [Google Scholar]
- Garcia N.A., Ontoria-Oviedo I., González-King H., Diez-Juan A., Sepúlveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One. 2015;10 doi: 10.1371/JOURNAL.PONE.0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazeau F., Pocard M., Pinto A., Marangon I., Méreaux J., Nicolás-Boluda A., Lavieu G., Wilhelm C., Sarda-Mantel L., Silva A.K.A. Immune reprogramming precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles. ACS Nano. 2021;15:3251–3263. doi: 10.1021/ACSNANO.0C09938/SUPPL_FILE/NN0C09938_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Gonzalez-King H., García N.A., Ontoria-Oviedo I., Ciria M., Montero J.A., Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35:1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- Guo S., Perets N., Betzer O., Ben-Shaul S., Sheinin A., Michaelevski I., Popovtzer R., Offen D., Levenberg S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and Tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. doi: 10.1021/ACSNANO.9B01892. [DOI] [PubMed] [Google Scholar]
- Guo S., Debbi L., Zohar B., Samuel R., Arzi R.S., Fried A.I., Carmon T., Shevach D., Redenski I., Schlachet I., Sosnik A., Levenberg S. Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett. 2021 doi: 10.1021/acs.nanolett.0c04834. [DOI] [PubMed] [Google Scholar]
- Han Yudi, Ren J., Bai Y., Pei X., Han Yan. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019;109:59–68. doi: 10.1016/J.BIOCEL.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Hasan M., Hama S., Kogure K. Low electric treatment activates rho GTPase via heat shock protein 90 and protein kinase C for intracellular delivery of siRNA. Sci. Reports. 2019;91(9):1–12. doi: 10.1038/s41598-019-40904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E.K., Cheng D., Mi Z., Gillespie D.G. Guanosine regulates adenosine levels in the kidney. Physiol. Rep. 2014;2 doi: 10.14814/PHY2.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science (80-) 2020:367. doi: 10.1126/SCIENCE.AAU6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. 5467659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Yun H.-W., Park D.Y., Choi B.H., Min B.-H. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng. Regen. Med. 2018;15:427–436. doi: 10.1007/s13770-018-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Hamish W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;121(12):1–10. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Hamish W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita S., Maeda N., Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Invest. 2019;129:4041–4049. doi: 10.1172/JCI129193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh E., Lee E.J., Nam G.H., Hong Y., Cho E., Yang Y., Kim I.S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/J.BIOMATERIALS.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Liu B., Lee B.W., Nakanishi K., Villasante A., Williamson R., Metz J., Kim J., Kanai M., Bi L., Brown K., Di Paolo G., Homma S., Sims P.A., Topkara V.K., Vunjak-Novakovic G. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018;25(2):293–303. doi: 10.1038/s41551-018-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zhang H., Mai J., Chen Z., Huang T., Wang S., Chen Y., Wang J. Distinct anti-fibrotic effects of exosomes derived from endothelial Colony-forming cells cultured under Normoxia and hypoxia. Med. Sci. Monit. 2018;24:6187–6199. doi: 10.12659/MSM.911306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li L., Rong Y., Qian D., Chen J., Zhou Zheng, Luo Y., Jiang D., Cheng L., Zhao S., Kong F., Wang J., Zhou Zhimin, Xu T., Gong F., Huang Y., Gu C., Zhao X., Bai J., Wang F., Zhao W., Zhang L., Li X., Yin G., Fan J., Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/J.ACTBIO.2019.12.020. [DOI] [PubMed] [Google Scholar]
- Logozzi M., Angelini D.F., Iessi E., Mizzoni D., Di Raimo R., Federici C., Lugini L., Borsellino G., Gentilucci A., Pierella F., Marzio V., Sciarra A., Battistini L., Fais S. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–329. doi: 10.1016/J.CANLET.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Logozzi M., Mizzoni D., Angelini D.F., Di Raimo R., Falchi M., Battistini L., Fais S. Microenvironmental pH and exosome levels interplay in human cancer cell lines of different Histotypes. Cancers. 2018;10 doi: 10.3390/CANCERS10100370. Page 370 10, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi M., Spugnini E., Mizzoni D., Di Raimo R., Fais S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019;381(38):93–101. doi: 10.1007/S10555-019-09783-8. [DOI] [PubMed] [Google Scholar]
- Ludwig N., Yerneni S.S., Menshikova E.V., Gillespie D.G., Jackson E.K., Whiteside T.L. Simultaneous inhibition of glycolysis and oxidative phosphorylation triggers a multi-fold increase in secretion of exosomes: possible role of 2′,3′-cAMP. Sci. Reports. 2020;101(10):1–12. doi: 10.1038/s41598-020-63658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv P., Gao P., Tian G., Yang Y., Mo F., Wang Z., Sun L., Kuang M., Wang Y. Osteocyte-derived exosomes induced by mechanical strain promote human periodontal ligament stem cell proliferation and osteogenic differentiation via the miR-181b-5p/PTEN/AKT signaling pathway. Stem Cell Res. Ther. 2020;111(11):1–15. doi: 10.1186/S13287-020-01815-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miller C.R., Bondurant B., McLean S.D., McGovern K.A., O’Brien D.F. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–12883. doi: 10.1021/BI980096Y. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Kita S., Tanaka Y., Fukuda S., Obata Y., Okita T., Nishida H., Takahashi Y., Kawachi Y., Tsugawa-Shimizu Y., Fujishima Y., Nishizawa H., Takakura Y., Miyagawa S., Sawa Y., Maeda N., Shimomura I. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol. Ther. 2020;28:2203. doi: 10.1016/J.YMTHE.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Kita S., Koyama Y., Fukuda S., Takeda H., Takahashi M., Fujishima Y., Nagao H., Masuda S., Tanaka Y., Nakamura Y., Nishizawa H., Funahashi T., Ranscht B., Izumi Y., Bamba T., Fukusaki E., Hanayama R., Shimada S., Maeda N., Shimomura I. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight. 2018;3 doi: 10.1172/JCI.INSIGHT.99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F.G., Roefs M.T., de Miguel Perez D., Kooijmans S.A., de Jong O.G., Sluijter J.P., Schiffelers R.M., Vader P. Interfering with endolysosomal trafficking enhances release of bioactive exosomes. Nanomedicine nanotechnology. Biol. Med. 2019;20 doi: 10.1016/J.NANO.2019.102014. [DOI] [PubMed] [Google Scholar]
- Paolicelli R.C., Bergamini G., Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2019;405:148–157. doi: 10.1016/J.NEUROSCIENCE.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Park D.J., Yun W.S., Kim W.C., Park J.E., Lee S.H., Ha S., Choi J.S., Key J., Seo Y.J. Improvement of stem cell-derived exosome release efficiency by surface-modified nanoparticles. J. Nanobiotechnology. 2020;18:1–17. doi: 10.1186/s12951-020-00739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., Colone M., Tatti M., Sargiacomo M., Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.B., Luthers C.R., Lerman M.J., Fisher J.P., Jay S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019;95:236–244. doi: 10.1016/J.ACTBIO.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets N., Hertz S., London M., Offen D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol. Autism. 2018;91(9):1–12. doi: 10.1186/S13229-018-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffoux M., Silva A.K.A., Lugagne J.B., Hersen P., Wilhelm C., Gazeau F. Extracellular vesicle production loaded with nanoparticles and drugs in a trade-off between loading, yield and purity: towards a personalized drug delivery system. Adv. Biosyst. 2017;1:1700044. doi: 10.1002/ADBI.201700044. [DOI] [PubMed] [Google Scholar]
- Rice G.E., Scholz-Romero K., Sweeney E., Peiris H., Kobayashi M., Duncombe G., Mitchell M.D., Salomon C. The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. J. Clin. Endocrinol. Metab. 2015;100:E1280–E1288. doi: 10.1210/JC.2015-2270. [DOI] [PubMed] [Google Scholar]
- Shao C., Yang F., Miao S., Liu W., Wang C., Shu Y., Shen H. Role of hypoxia-induced exosomes in tumor biology. Mol. Cancer. 2018;171(17):1–8. doi: 10.1186/S12943-018-0869-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko V., Iuliano F., Sevcikova A., Labudova M., Barathova M., Radvak P., Pastorekova S., Pastorek J., Csaderova L. Hypoxia induces cancer-associated cAMP/PKA signalling through HIF-mediated transcriptional control of adenylyl cyclases VI and VII. Sci. Rep. 2017;7 doi: 10.1038/S41598-017-09549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse S.W., Tan C.F., Park J.E., Gnanasekaran J., Gupta N., Low J.K., Yeoh K.W., Chng W.J., Tay C.Y., McCarthy N.E., Lim S.K., Sze S.K. Microenvironmental hypoxia induces dynamic changes in lung Cancer synthesis and secretion of extracellular vesicles. Cancers. 2020;12 doi: 10.3390/CANCERS12102917. page 2917 12, 2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturici G., Tinnirello R., Sconzo G., Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am. J. Physiol. - Cell Physiol. 2014;306 doi: 10.1152/AJPCELL.00228.2013/ASSET/IMAGES/MEDIUM/ZH00061474610001.JPEG. [DOI] [PubMed] [Google Scholar]
- van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;194(19):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Vanlandingham P.A., Ceresa B.P. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J. Biol. Chem. 2009;284:12110–12124. doi: 10.1074/JBC.M809277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Maruyama K., Sakisaka Y., Suzuki S., Tada H., Suto M., Saito M., Yamada S., Nemoto E. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 2019;0:1310. doi: 10.3389/FIMMU.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Bonacquisti E.E., Brown A.D., Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9 doi: 10.3390/CELLS9030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Zuo B., Tao B., Wang C., Li Y., Peng J., Shen C., Cui Y., Zhu J., Chen X. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann. Transl. Med. 2021;9:798. doi: 10.21037/ATM-21-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid M., Lind J.U., Ardoña H.A.M., Sheehy S.P., Dickinson L.E., Eweje F., Bastings M.M.C., Pope B., O’Connor B.B., Straubhaar J.R., Budnik B., Kleber A.G., Parker K.K. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci. Transl. Med. 2020;12:8005. doi: 10.1126/SCITRANSLMED.AAX8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Zhao Y., Chen Q., Zhu S., Niu Y., Ye Z., Hu P., Chen D., Xu P., Chen J., Hu C., Hu Y., Xu F., Tang J., Wang F., Han S., Huang M., Wang C., Zhao G. Hypoxic exosomal HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene. 2021;2021:1–13. doi: 10.1038/s41388-021-01960-w. [DOI] [PubMed] [Google Scholar]
- Zhu J., Lu K., Zhang N., Zhao Y., Ma Q., Shen J., Lin Y., Xiang P., Tang Y., Hu X., Chen J., Zhu W., Webster K.A., Wang J., Yu H. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells, Nanomedicine, Biotechnol. 2017;46:1–12. doi: 10.1080/21691401.2017.1388249. [DOI] [PMC free article] [PubMed] [Google Scholar]