Abstract
Identification and characterization of mycobacterial adhesins and complementary host receptors required for colonization and dissemination of mycobacteria in host tissues are needed for a more complete understanding of the pathogenesis of diseases caused by these bacteria and for the development of effective vaccines. Previous investigations have demonstrated that a 28-kDa heparin-binding mycobacterial surface protein, HBHA, can agglutinate erythrocytes and promote mycobacterial aggregation in vitro. In this study, further molecular and biochemical analysis of HBHA demonstrates that it has three functional domains: a transmembrane domain of 18 amino acids residing near the N terminus, a large domain of 81 amino acids consistent with an α-helical coiled-coil region, and a Lys-Pro-Ala-rich C-terminal domain that mediates binding to proteoglycans. Using His-tagged recombinant HBHA proteins and nickel chromatography we demonstrate that HBHA polypeptides which contain the coiled-coil region form multimers. This tendency to oligomerize may be responsible for the induction of mycobacterial aggregation since a truncated N-terminal HBHA fragment containing the coiled-coil domain promotes mycobacterial aggregation. Conversely, a truncated C-terminal HBHA fragment which contains Lys-Pro-Ala-rich repeats binds to the proteoglycan decorin. These results indicate that HBHA contains at least three distinct domains which facilitate intercalation into surface membranes, promote bacterium-bacterium interactions, and mediate the attachment to sulfated glycoconjugates found in host tissues.
Mycobacteria are among the most prominent pathogenic microorganisms, causing disease in both humans and animals, while tuberculosis is one of the world's leading causes of death. Although Mycobacterium bovis BCG vaccine is used in many parts of the world in immunization programs against tuberculosis, it has met with only limited success (5, 10). It is apparent that further research on the various steps associated with the pathogenesis of mycobacterial diseases is needed before the development of new vaccines or therapeutic approaches against these diseases can be achieved (6).
One of the initial and crucial events in bacterial pathogenesis is the adherence of the microorganism to its target tissue. The identification of adhesins involved in the first steps of colonization may suggest new approaches to block the infection, either by the development of novel drugs that interrupt this host-pathogen interaction or through new vaccine regimens. Mycobacteria have a tropism for the lung, and interactions of the tubercle bacillus with complement and mannose receptors on the alveolar macrophages have been implicated in the uptake of mycobacteria by these phagocytes (32, 33). Since these microorganisms are easily transmitted by aerosol, the first host structures they encounter will be those of the respiratory epithelium. As a result, interactions of the organism with respiratory epithelial cells or with the extracellular matrix during the initial and subsequent steps of infection may also be important in pathogenesis. Recent findings suggest that Mycobacterium tuberculosis may gain access to the lymphatic and circulatory systems by direct adherence and penetration of alveolar epithelial cells (23). Also, infection of pigs with Mycobacterium avium involves essential interactions with epithelial cells for which the requirement of specific receptors has been postulated but has yet to be characterized (4). Finally, binding to host receptors found on epithelial cells (35) or on interstitial matrix components may help the dissemination of the microorganism.
Many pathogens, including respiratory pathogens, have been shown to express a variety of structures on their surfaces which function as bacterial adhesins (2). Microbial adhesins, such as fimbriae (18) and the Bordetella pertussis filamentous hemagglutinin (8, 19, 22), have lectin-like properties, can agglutinate erythrocytes (RBC), and also bind to complementary glycoconjugates expressed on the surfaces of host cells. In addition, adhesins, such as filamentous hemagglutinin (19, 25) and the Yersinia enterocolitica YadA (1), appear to act as multifunctional adhesins, capable of mediating bacteria-host cell interactions, as well as promoting bacterial autoaggregation. Recently, the putative mycobacterial adhesin HBHA, which has been shown to agglutinate RBC and aggregate mycobacteria, has been identified (24, 26). The major goal of the work reported here was, by using molecular and biochemical strategies, to further characterize functional sites on the mycobacterial hemagglutinin.
MATERIALS AND METHODS
All mycobacterial strains used in these studies were from either the mycobacterial collection of the Laboratory of Mycobacteria, Center for Biologics Evaluation and Research (CBER), Food and Drug Administration (FDA), or the Trudeau Institute (Saranac Lake, N.Y.) mycobacterial collection. The monoclonal antibodies D2 and E4 have been described previously (30), and the mouse anti-HBHA polyclonal antibody was prepared in the Laboratory of Mycobacteria, CBER, FDA, with a DNA vaccine construct (17).
Purification of native HBHA.
Mycobacteria were grown in 2 liters of Long's synthetic medium (Quality Biological Inc., Gaithersburg, Md.) until late log phase. The bacteria were then pelleted by centrifugation, washed once in Dulbecco's phosphate-buffered saline (DPBS) containing 0.05% Tween 80 (DPBS/Tw), resuspended in 100 ml of DPBS/Tw, and heated at 60°C for 30 min. The bacteria were centrifuged at 20,000 × g for 20 min, washed with DPBS/Tw, and resuspended in 25 ml of DPBS/Tw containing a 5 mM concentration of protease inhibitor 4-(2-aminomethyl)-benzenesulfonyl fluoride hydrochloride (AEBSF; ICN Biomedicals Inc., Aurora, Ohio). The mixture was sonicated intermittently for 25 min on ice and then centrifuged at 20,000 × g for 20 min. The sonication and centrifugation steps were repeated on the cell pellet, and the supernatants were pooled and centrifuged at 100,000 × g for 1 h. The pellet was discarded, and the final supernatant was resuspended to a final concentration of 2% Triton X-114 for separation of the material into hydrophilic and hydrophobic phases by methods described by Nair et al. (27). The aqueous phase, which contains most of the HBHA protein, was diluted 1:2 in DPBS and was passed through a heparin-Sepharose CL-6B (Pharmacia/LKB, Piscataway, N.J.) column (1 by 5 cm) equilibrated with DPBS. The column was then washed with 100 ml of DPBS, and the bound material was eluted by a 0 to 500 mM NaCl gradient in 100 ml of DPBS. Fractions eluting at a final NaCl concentration of 300 mM were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 12% gel followed by Coomassie brilliant blue R-250 staining and Western blotting with monoclonal antibodies directed against HBHA (26).
Molecular methods and recombinant protein purification.
The genes encoding the entire HBHA molecule and the N-terminal fragment were amplified from M. tuberculosis H37Rv chromosomal DNA with Vent polymerase (New England BioLabs, Beverly, Mass.) by using the common primer HB5Nd (5′-AAG CTT ATG GCT GAA AAC TCG AAC ATT-3′) found at the N terminus combined with HB3B (5′-GAA TTC GGA TCC CTA TGC GGT TTG CAT CCA A-3′) for the entire gene and with HB3B1 (5′-GAA TTC GGA TCC GAA GCT CTG CTG CTG GCT GCG-3′) for the N-terminal fragment. The amplified fragments were cloned in Zero Blunt cloning vector (Invitrogen, San Diego, Calif.) and then in pET15 (Novagen Inc., Madison, Wis.) expression vector by using the NdeI and BamHI sites. The C-terminal clone for HBHA was derived by cutting the complete gene with XhoI and BamHI and then inserting it in pET15b. Escherichia coli BL21(DE3)pLysS was transformed with the three plasmids, and the expressed recombinant histidine-tagged proteins were purified with the X-press system (Invitrogen) under native conditions. The N-terminal recombinant HBHA fragment (N-h-HBHA) extends from amino acid 1 to 116, and the C-terminal recombinant HBHA fragment (C-h-HBHA) extends from amino acid 87 to 199.
Nickel chromatography.
To investigate HBHA interactions, we used a qualitative procedure similar to that described by Rigal et al. for the TolB proteins of E. coli (29). Purified recombinant His-tagged HBHA proteins were immobilized on nickel-chelating resin columns (ProBond; Invitrogen) by methods suggested by the manufacturer for the X-press system and subsequently were washed with 200 mM imidazole. Under these conditions most of the E. coli proteins were washed off the column. Columns were then washed with phosphate buffer, pH 7.0, containing 150 mM NaCl. Aliquots (0.3 ml) of the nickel matrix containing similar amounts of immobilized full-length recombinant HBHA (h-HBHA), N-h-HBHA, and C-h-HBHA, as determined by SDS-PAGE, were transferred to a microcentrifuge tube, and identical samples of H37Ra cell lysate or purified native HBHA were added and mixed gently at 4°C for 1 h, in a total volume of 0.7 ml. The H37Ra cell lysates used were those preparations collected after ultracentrifugation as described above for the purification of native HBHA. All columns were then washed in 8 column volumes of phosphate buffer with increasing concentrations of 50, 100, 200, and 300 mM imidazole, followed by elution with 500 mM imidazole. Fractions were then concentrated by trichloroacetic acid precipitation and analyzed for protein content, followed by SDS-PAGE and Western blotting.
Aggregation assay.
Dispersed M. tuberculosis H37Ra cells were used in the aggregation test as previously described (26) to compare the abilities of native and recombinant HBHA constructs to promote the autoagglutination of the mycobacteria. Native HBHA was purified on heparin-Sepharose as described above, while His-tagged recombinant HBHA proteins were purified by Ni column chromatography, and the His-tagged recombinant protein from E. coli expressing the mycobacterial gene mpt64 (28) was used as a control. Titration experiments were performed starting at 50 μg per ml, and the results shown are the averages of three individual experiments.
Binding of HBHA to decorin.
Binding of HBHA to the proteoglycan decorin (37) (provided by David Mann, Holland Laboratories, American Red Cross) was assessed by incubating purified native or recombinant HBHA with 25 μg of decorin per ml immobilized to plastic in 96-well microtiter plates (Immunolon I; Nunclon). Wells were incubated with 1% casein for 1 h before adding various concentrations of purified HBHA diluted in 0.05 M Tris-buffered saline containing 0.002% Tween 20 (binding buffer). Following a 2-h incubation at room temperature wells were washed four times with binding buffer and anti-HBHA mouse serum (1:2,000) was added for 2 h in binding buffer. Wells were washed four times as described above and incubated with the second antibody (anti-mouse immunoglobulin G; alkaline phosphatase conjugated; 1:2,000; Sigma, Inc.). Wells were washed as described above, and, after a 30-min incubation with phosphatase substrate (pNPP; Kirkegaard & Perry Laboratories, Gaithersburg, Md.), plates were read for absorbance at 405 nm. Nonspecific binding was determined by measuring the binding of HBHA to wells coated with 1% casein. In some experiments, a final concentration of 0.5 mg of chondroitin sulfate A (Sigma, Inc., St. Louis, Mo.) per ml was added to HBHA prior to the addition to decorin-coated wells. Experiments were performed a minimum of two times with three wells for each data point.
Analytical procedures.
SDS-PAGE was performed as described originally by Laemmli (16), and immunoblot analyses were performed by standard procedures as described by Harlow and Lane (13). Protein concentrations were determined by the method of Bradford (7) with bovine serum albumin as a standard.
Software analysis.
Computer-assisted analysis available on the World Wide Web through the Expasy molecular biology server was used in predicting the secondary structure of the protein. DAS (dense alignment surface) (11) and TMpred (14) were used to analyze the transmembrane region of the protein. Coil (21), Parcoil (3), and Multicoil algorithms were used to analyze the coiled-coil region.
RESULTS
Several mycobacterial species express HBHA.
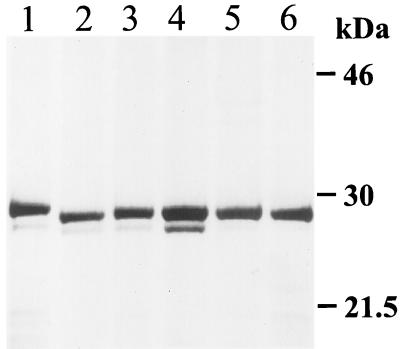
Recently, we have shown that a mycobacterial protein which agglutinates RBC (HBHA) and autoagglutinates mycobacteria can be purified from cell extracts and the growth fluid of M. tuberculosis and M. bovis BCG strains (24, 26). To determine if other mycobacteria can express an HBHA-like protein, cell extracts from various mycobacterial species were prepared and chromatographed on heparin-Sepharose. Figure 1 shows fractions from the purification analyzed by SDS-PAGE and stained with Coomassie blue. The M. tuberculosis and M. bovis strains tested as well as BCG have a band that migrates at 28 kDa, while HBHA proteins from M. avium, Mycobacterium intracellulare, and Mycobacterium smegmatis (data not shown) migrate slightly slower on SDS-polyacrylamide gels. All of these heparin-binding protein bands were recognized by antibodies reactive with the HBHA purified from M. tuberculosis (data not shown). This suggests that mycobacteria other than those species belonging to the M. tuberculosis complex can express an HBHA-like protein.
FIG. 1.
Comparison by SDS-PAGE of heparin-binding proteins from different mycobacterial species stained by Coomassie blue. Lanes: 1, M. avium; 2, M. tuberculosis H37Ra; 3, M. tuberculosis Erdman; 4, M. tuberculosis 956 clinical isolate; 5, M. bovis BCG; 6, M. bovis.
Description of functional sites on HBHA.
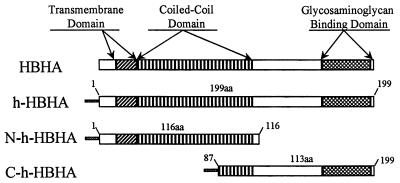
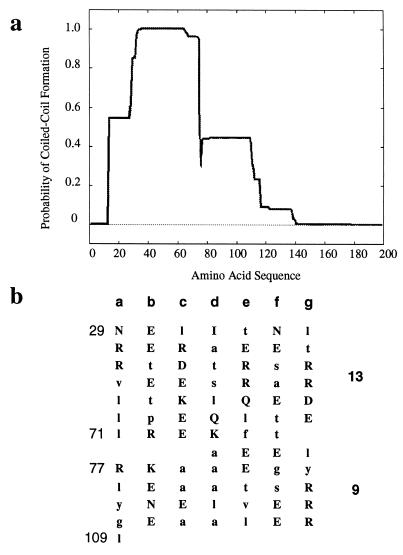
Comparative sequence analysis was performed on the HBHA amino acid sequence (24) to search for potentially important functional sites on the protein. A comparison of the sequences of HBHA genes obtained from both the M. tuberculosis H37Rv (Sanger Rv0475) and CDC1551 (Tigre no. 3721) genome banks demonstrates that the amino acid sequences of the hemagglutinin in both M. tuberculosis strains are identical. The DAS (dense alignment surface) method (11) and TMpred (14) were used to predict the presence of a transmembrane region at the N terminus of HBHA (amino acids 12 to 30) (Fig. 2). Analysis of the HBHA amino acid sequence using algorithms capable of recognizing putative coiled-coil regions indicates that such a region exists in HBHA. On the basis of the algorithm of Lupas (21) and the Parcoil and Multicoil analyses (3), a coiled-coil region in HBHA was predicted to extend over 81 amino acids (Fig. 3a). Figure 3b shows amino acid residues in the predicted region aligned as heptad repeats with the number of predicted α-helical turns occurring within the coiled-coil region. The particularly strong coiled-coil potential of amino acids 29 to 65 suggests that this region functions as a nucleator for a larger sequence, stabilizing the coiled-coil region perhaps up to amino acid residue 140. It is also likely that the 110 amino acid residues extending between amino acids 29 and 109 (Fig. 2) form a coiled-coil protein that could drive the formation of HBHA dimers.
FIG. 2.
Schematic showing the putative functional sites found in intact HBHA and the three His-tagged recombinant HBHA proteins used in this study. aa, amino acids.
FIG. 3.
(a) Schematic predicting a coiled-coil region in HBHA obtained by using the algorithm of Lupas (20, 21). (b) Predicted heptad repeats found in the coiled-coil domain of HBHA. Numbers on the left are the amino acid residues. The number of predicted α-helical turns within a given coiled-coil turn (3.6 residues per turn) is shown at the right. Hydrophilic amino acids are in uppercase, and hydrophobic amino acids are in lowercase.
Evidence for site-specific HBHA-HBHA interactions.
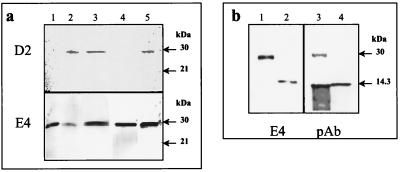
The presence of a potential coiled-coil domain in HBHA suggests that protein-protein interactions between HBHA proteins may occur. To investigate this, His-tagged recombinant proteins were constructed as shown in Fig. 2. h-HBHA, N-h-HBHA, and C-h-HBHA were cloned in pET15b, expressed in E. coli BL21(DE3)pLysS, and purified by nickel chromatography. We used the avidity of these His-tagged constructs for nickel (29) and our previous finding that the monoclonal antibody D2 recognizes native HBHA but not recombinant HBHA (Fig. 4a, lane 1) to investigate HBHA oligomerization. Previous investigations have suggested that D2 recognizes a specific sugar moiety found on mature native HBHA (24). An M. tuberculosis H37Ra cell lysate containing HBHA was incubated with a nickel column containing bound h-HBHA. The bound material eluted with 500 mM imidazole contained both native HBHA from the lysate and h-HBHA (Fig. 4a, lane 3), as indicated by detection with both D2, which recognizes native HBHA only, and E4, which reacts with both native and recombinant HBHA (24). Similar results were found when purified native HBHA was added to the nickel column conjugated with h-HBHA (Fig. 4a, lane 5). For comparison, lane 2 shows the flowthrough when the H37Ra lysate is chromatographed on nickel only and lane 4 shows the eluted h-HBHA from an h-HBHA nickel column after the addition of buffer only as a control. Together these results indicate that HBHA-HBHA interactions can occur.
FIG. 4.
Immunoblots showing HBHA-containing material analyzed by nickel chromatography and detected by the anti-HBHA monoclonal antibodies D2 and E4 and by polyclonal HBHA antisera. (a) Lane 1, purified recombinant His-tagged HBHA (h-HBHA); lane 2, flowthrough of H37Ra extract added to the Ni column only; lane 3, imidazole eluate from the h-HBHA Ni column after addition of H37Ra extract containing HBHA; lane 4, imidazole eluate from the h-HBHA Ni column after addition of buffer only; lane 5, imidazole eluate from the h-HBHA Ni column after addition of purified native HBHA. (b) Nickel column chromatography with purified recombinant His-tagged N-terminal (N-h-HBHA) or C-terminal (C-h-HBHA) fragments of HBHA; detection was with E4 and anti-HBHA polyclonal (pAb) antisera. Lanes 1 and 3, imidazole eluate following the addition of purified native HBHA to an N-h-HBHA Ni column; lanes 2 and 4, imidazole eluate following the addition of native HBHA to a C-h-HBHA Ni column. Arrows show the positions of the SDS-PAGE molecular mass standards.
The two recombinant HBHA fragments N-h-HBHA and C-h-HBHA, which migrate as 14- and 14.5-kDa proteins, respectively, were also bound to nickel columns and incubated with the purified native HBHA (Fig. 4b). When probed with E4, the imidazole eluate from the nickel column linked with N-h-HBHA was found to contain full-length native HBHA (Fig. 4b, lane 1), but full-length HBHA was not found in the material eluted from the nickel column containing the C-terminal fragment (lane 2). Similar results were found when an anti-HBHA polyclonal antiserum which recognizes the full-length HBHA and both of the recombinant fragments was used to detect the eluted fractions (Fig. 4b, lanes 3 and 4). These results indicate that HBHA can form multimers by using interactive sites found in the N-terminal fragment of HBHA, a region which contains the entire coiled-coil domain of HBHA.
Aggregation of M. tuberculosis is also promoted by the N-terminal domain of HBHA.
In an earlier report, we demonstrated that native purified HBHA promotes the aggregation of mycobacteria, a process that could be induced by contacts between HBHA molecules on the surfaces of mycobacteria (26). To determine if the region of HBHA containing the coiled-coil region can promote bacterial aggregation, the recombinant HBHA fragments were examined in an aggregation test using M. tuberculosis H37Ra cells (Table 1). h-HBHA aggregated M. tuberculosis, although more protein was required to produce a maximum effect compared to purified native HBHA. The N-terminal fragment containing the entire coiled-coil domain of HBHA also aggregated M. tuberculosis, giving maximum aggregation at a protein concentration of 12.5 μg per ml. No aggregation was observed with 50 μg of the C-terminal HBHA fragment per ml.
TABLE 1.
Aggregation of M. tuberculosis H37Ra in the presence of purified native and recombinant HBHAa
| HBHA protein | Amt of HBHA that induces maximum aggregation (μg/ml) |
|---|---|
| Native HBHA | 0.5 |
| h-HBHA | 2.5 |
| N-h-HBHA | 12.5 |
| C-h-HBHA | >50 |
| h-MPT64 | >50 |
Native HBHA and His-tagged recombinant HBHA proteins were purified as described in Materials and Methods. The His-tagged recombinant mycobacterial protein h-MPT64 (28) was purified in a similar fashion and used as a control.
The C-terminal domain of HBHA promotes binding of HBHA to the proteoglycan decorin.
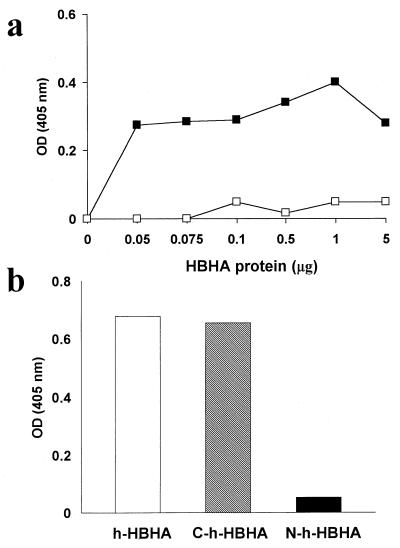
Previous evidence indicates that a heparin-binding region of HBHA is found in the C terminus of the protein (24). Since HBHA binds to heparin, it is likely that this mycobacterial protein also binds to proteoglycans. To test this hypothesis, we incubated various concentrations of partially purified HBHA with a purified preparation of the proteoglycan decorin, which was immobilized on plastic. Figure 5a shows that HBHA can bind to decorin (36, 37) in a dose-dependent fashion and that binding is inhibited in the presence of the glycosaminoglycan chondroitin sulfate. To localize the binding site on HBHA, we used the N- and C-terminal His-tagged HBHA recombinant proteins in the binding assay and found that only the C-terminal HBHA fragment bound to decorin (Fig. 5b). This extends observations, made previously (24), that the C-terminal region of HBHA containing Lys-Pro-Ala-rich repeats was implicated in binding to the glycosaminoglycan heparin.
FIG. 5.
Binding of native and recombinant HBHA to decorin. (a) Partially purified HBHA, extracted from M. tuberculosis H37Ra, from 0 to 5 μg of protein per ml, was incubated with decorin which had been immobilized on plastic. Bound HBHA (solid squares) was detected with polyclonal anti-HBHA sera and alkaline phosphatase-conjugated anti-mouse antibody in an enzyme-linked immunosorbent assay, as described in Materials and Methods. Open squares, binding of HBHA to decorin in the presence of 0.5 mg of chondroitin sulfate A/ml. (b) To measure the binding of recombinant His-tagged HBHA, purified recombinant HBHA (open bar), C-terminal recombinant HBHA (hatched bar), and N-terminal recombinant HBHA (solid bar) were incubated with decorin as described for panel a at a protein concentration of 5 μg per ml. OD (405 nm), optical density at 405 nm.
DISCUSSION
This investigation has provided evidence that the recently discovered mycobacterial hemagglutinin, HBHA, contains discrete functional domains that may facilitate its function as a bacterial adhesin. All M. tuberculosis and M. bovis strains, including BCG, that have been tested to date have a 28-kDa band visualized by SDS-PAGE and reactive with antibodies directed against HBHA. Other species of mycobacteria including M. avium, M. intracellulare, and M. smegmatis have a cross-reactive band that migrates at approximately 30 kDa. A leucine-rich putative transmembrane domain predicted by amino acid sequencing exists near the N terminus of HBHA (Fig. 2). It is likely that this domain serves to anchor HBHA in the outer lipid layer on the mycobacterial surface (9), as previously observed in immunoelectron microscopy studies (26). HBHA does not contain the consensus sequence LPXTGX, which has been found to anchor certain proteins at the surfaces of gram-positive organisms (34).
Lupas (20) and others (38) have shown that a number of proteins contain coiled-coil domains, a feature that promotes interactions between homologous as well as heterologous proteins to form oligomeric structures. In coiled-coil proteins the interactions among the subunits are mediated by an amino acid sequence that follows specific rules outlined by Lupas (20). The HBHA sequence contains a region extending from amino acid 29 to 109 that is a putative coiled-coil domain as determined by the algorithm of Lupas and the Berger Parcoil and Multicoil analyses (3, 21). Our experiments using native HBHA, His-tagged recombinant HBHA proteins, specific anti-HBHA antibodies, and nickel chromatography provide direct evidence that HBHA proteins can interact with themselves to form multimers. Comparative experiments using truncated recombinant HBHA proteins indicate that multimer formation occurs within the expanse of HBHA containing the coiled-coil domain. Attempts at performing gel filtration chromatography to estimate the actual size of HBHA have failed to date, but this may be due to multimerization, the “stickiness” of the protein, or its avidity for plastic surfaces. Therefore, the exact nature of HBHA under physiological conditions remains to be determined.
We have also demonstrated that the recombinant HBHA protein and its N-terminal fragment containing the coiled-coil domain aggregate mycobacteria similarly to native HBHA. In contrast, the C-terminal HBHA fragment containing the heparin-binding domain does not induce aggregation. This is consistent with the finding that sulfated sugars do not inhibit HBHA-induced mycobacterial aggregation (8a). Thus, multimerization of HBHA may, in part, be responsible for the aggressive interactions between mycobacteria commonly visualized as “clumping” during in vitro growth. Since it has been shown that coiled-coil-containing proteins are capable of dynamic switching of monomer subunits (15), it is possible that HBHA may form reversible multimeric bridge-like structures connecting bacteria through the coiled-coil motif. In this model, the addition of purified HBHA would result in the induction of mycobacterial aggregation in vitro, as observed in our experiments. HBHA may function like other prokaryotic proteins, which have been shown to autoagglutinate bacterial protein, and it has been suggested that these factors are important for establishing infection and subsequent colonization (1, 25).
The C-terminal domain of HBHA contains an important site for interacting with sulfated glycoconjugates, and the avidity of HBHA for heparin has been used as a tool for purification of the mycobacterial protein (26). In contrast, HBHA does not bind to proteins such as bovine serum albumin, ovalbumin, and the extracellular glycoprotein fibronectin (8a). In these studies, we have demonstrated that HBHA can bind to the proteoglycan decorin, a macromolecule commonly found in interstitial tissues including the lung (31, 36, 37). Binding of the C-terminal HBHA recombinant protein but not the N-terminal fragment and inhibition of binding with chondroitin sulfate suggest that this interaction occurs between the sulfated glycosaminoglycan extending from the decorin core protein and the Lys-Pro-Ala repeats found at the C terminus of HBHA. Borrelia burgdorferi, the causative agent of Lyme disease, has been shown to express proteins that bind to decorin and mediate adherence of the organism to host tissues (12), probably by using a similar Lys-Ala-Pro-rich amino acid motif. In this manner, decorin or other proteoglycans may serve as receptors for HBHA and mediate attachment of mycobacteria to host tissues, as has been implied by earlier reports (24, 26). More-extensive investigations will be required to determine if there is more avidity of HBHA for certain types of proteoglycans than for others and to establish its physiological importance in bacteria-host interactions.
Although the precise function of the mycobacterial hemagglutinin, HBHA, still needs to be confirmed in vivo, the evidence presented here and in other reports (24, 26) strongly suggests that the HBHA molecule is well suited to function as a bacterial adhesin. The leucine-rich hydrophobic N-terminal domain may anchor HBHA into the cell walls of mycobacteria. The coiled-coil domain and the glycosaminoglycan-binding domain of HBHA may then be available to function at the surface of the bacterium to promote bacteria-bacteria interactions and attachment of the bacteria to host tissues.
ACKNOWLEDGMENTS
We thank Julie Rouse of CBER, FDA, for technical assistance on this project. We are also grateful to David Mann of the Holland Laboratories, American Red Cross, for the kind gift of purified decorin and to Zhongming Li, CBER, FDA, for providing the polyclonal anti-HBHA antisera. We also thank Alisdair Steven, NIAMS, NIH, for consultation on the coiled-coil domain of HBHA.
REFERENCES
- 1.Balligand G, Laroche Y, Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985;48:782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beachey E H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 3.Berger B, Wilson D B, Wolf E, Tonchev T, Milla M, Kim P S. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E, Young L S. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect Immun. 1994;62:2021–2026. doi: 10.1128/iai.62.5.2021-2026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 531–557. [Google Scholar]
- 6.Bloom B R, Murray C J. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Brennan M J, Shahin R D. Pertussis antigens that abrogate bacterial adherence and elicit immunity. Am J Respir Crit Care Med. 1996;154:S145–S149. doi: 10.1164/ajrccm/154.4_Pt_2.S145. [DOI] [PubMed] [Google Scholar]
- 8a.Brennan, M. J. Unpublished data.
- 9.Brennan P J, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 10.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 11.Cserzo M, Wallin E, Simon I, von Heijne G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- 12.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Antibodies, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 14.Hofmann K, Stoffel W. TMbase—a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 15.Hurme R, Berndt K D, Normark S J, Rhen M. A proteinaceous gene regulatory thermometer in Salmonella. Cell. 1997;90:55–64. doi: 10.1016/s0092-8674(00)80313-x. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun. 1999;67:4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg F, Lund B, Johansson L, Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987;328:84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- 19.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 20.Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 21.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 22.Makhov A M, Hannah J H, Brennan M J, Trus B L, Kocsis E, Conway J F, Wingfield P T, Simon M N, Steven A C. Filamentous hemagglutinin of Bordetella pertussis. A bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol. 1994;241:110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 23.McDonough K A, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menozzi F D, Bischoff R, Fort E, Brennan M J, Locht C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci USA. 1998;95:12625–12630. doi: 10.1073/pnas.95.21.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menozzi F D, Boucher P E, Riveau G, Gantiez C, Locht C. Surface-associated filamentous hemagglutinin induces autoagglutination of Bordetella pertussis. Infect Immun. 1994;62:4261–4269. doi: 10.1128/iai.62.10.4261-4269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menozzi F D, Rouse J H, Alavi M, Laude-Sharp M, Muller J, Bischoff R, Brennan M J, Locht C. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med. 1996;184:993–1001. doi: 10.1084/jem.184.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair J, Rouse D A, Morris S L. Nucleotide sequence analysis and serologic characterization of the Mycobacterium intracellulare homologue of the Mycobacterium tuberculosis 19 kDa antigen. Mol Microbiol. 1992;6:1431–1439. doi: 10.1111/j.1365-2958.1992.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 28.Oettinger T, Holm A, Mtoni I M, Andersen A B, Hasloov K. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect Immun. 1995;63:4613–4618. doi: 10.1128/iai.63.12.4613-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigal A, Bouveret E, Lloubes R, Lazdunski C, Benedetti H. The TolB protein interacts with the porins of Escherichia coli. J Bacteriol. 1997;179:7274–7279. doi: 10.1128/jb.179.23.7274-7279.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouse D A, Morris S L, Karpas A B, Mackall J C, Probst P G, Chaparas S D. Immunological characterization of recombinant antigens isolated from a Mycobacterium avium λgt11 expression library by using monoclonal antibody probes. Infect Immun. 1991;59:2595–2600. doi: 10.1128/iai.59.8.2595-2600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989;264:13369–13372. [PubMed] [Google Scholar]
- 32.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 33.Schlesinger L S, Horwitz M A. Phagocytosis of leprosy bacilli is mediated by complement receptors CR3 and CR3 on human monocytes and complement component C3 in serum. J Clin Investig. 1990;85:1304–1314. doi: 10.1172/JCI114568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneewind O, Model P, Fischetti V A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 35.Shepard C. Growth characteristics of tubercle bacilli and certain other mycobacteria in HeLa cells. J Exp Med. 1957;105:39–48. doi: 10.1084/jem.105.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westergren-Thorsson G, Hernnas J, Sarnstrand B, Oldberg A, Heinegard D, Malmstrom A. Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Investig. 1993;92:632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi Y, Mann D M, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 38.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]