Summary
Background
During early stages of COVID-19 pandemic, antimicrobials were commonly prescribed.
Aim
To describe clinical, microbiological and antimicrobial use changes in bloodstream infections (BSI) of ICU patients during the first wave of COVID-19 pandemic compared to pre-COVID-19 era.
Methods
Observational cohort study of patients admitted to ICU of Bellvitge University Hospital was conducted during the COVID-19 pandemic (March-June 2020) and before COVID-19 pandemic (March-June 2019). Differences in clinical characteristics, antimicrobial consumption and incidence and aetiology of BSI were measured.
Findings
COVID-19 patients had significantly less comorbidities with obesity the only risk factor that increased in frequency. COVID-19 patients more frequently required invasive supportive care measures, had longer median ICU stay and higher mortality rates. The incidence of BSIs was higher in COVID-19 period (RR 3.2 [95%CI 2.2–4.7]), occurred in patients who showed prolonged median ICU stay (21days) and was associated with high mortality rate (47%). The highest increases in the aetiological agents were observed for AmpC-producing bacteria (RR 11.1 [95%CI 2.6–47.9]) and non-fermenting rods (RR 7.0 [95%CI 1.5–31.4]). The emergence of bacteraemia caused by Gram-negative rods resistant to amoxicillin-clavulanate, which was used as empirical therapy during early stages of the pandemic, led to an escalation towards broader-spectrum antimicrobials such as meropenem and colistin which was also associated with the emergence of resistant isolates.
Conclusions
The epidemiological shift towards resistant phenotypes in critically ill COVID-19 patients was associated with the selective use of antimicrobials. Our study provides evidence of the impact of empirical therapy on the selection of bacteria and their consequences on BSI over the subsequent months.
Keywords: COVID-19, ICU, Bloodstream infections, Broad-spectrum antimicrobials, Antimicrobial resistance, SARS-CoV-2
Introduction
COVID-19 pandemic remains a serious health threat worldwide. In Spain, SARS-CoV-2 virus was detected for the first time at the end of January 2020. From that time onwards there was an exponential increase in the number of cases to 248,960 cases and 28,346 deaths at the end of June 2020 [1,2]. Despite most infected patients being asymptomatic or having a mild clinical course, some may develop critical illness characterised by severe lung dysfunction, septic shock and/or extrapulmonary organ failure with high mortality rates (30–70%) [3,4].
While admission to the ICU is necessary for patients requiring life support, this also poses them at risk of acquiring nosocomial infections such as bloodstream infections (BSIs). Thus, management of nosocomial complications associated with COVID-19 in ICU patients may require the use of broad-spectrum antimicrobials. Furthermore, during the initial stages of COVID-19 pandemic, antimicrobials were commonly prescribed as empirical treatment in moderate or severe COVID-19 infections [5]. Empirical therapy was based on the management of other viral respiratory tract infections such as Influenza virus, where bacterial co-infections caused by Staphylococcus aureus or Streptococcus pneumoniae are frequently reported [[6], [7], [8]]. However, the incidence of concomitant bacterial infections in patients with COVID-19 appear to be lower than those reported for Influenza virus [[9], [10], [11], [12]]. In this regard, the WHO guide for Clinical Management of COVID-19 recommends empirical antimicrobial therapy in severe disease with clinical suspicion of bacterial infection, where treatment should be based on clinical judgment, patient’s host factors and local epidemiology [13].
The objectives of this study were to compare the patterns of antimicrobial use and the clinical and microbiological characteristics of BSI in ICU patients admitted to a Spanish tertiary referral hospital during pre-COVID-19 and COVID-19 periods.
Material and methods
Study design and clinical data
Bellvitge University Hospital (HUB) is a tertiary care centre with 700 beds and three mixed (medical-surgical) ICUs that account for 34 beds. As of March 2020, these units were converted to exclusively treat COVID-19 patients. Also, due to the high number of patients admitted (three times higher than the usual activity of the ICUs), other hospital units were converted to provide intensive care to COVID-19 and non-COVID-19 patients. We conducted an observational cohort study of all patients admitted to the three original ICUs during the first stages of COVID-19 pandemic (COVID-19 group, March to June 2020) and before COVID-19 pandemic (pre-COVID-19 group, March to June 2019). All COVID-19 patients had the SARS-CoV-2 diagnosis confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR). Clinical data were collected retrospectively. Data included age, gender, SOFA (Sequential Organ Failure Assessment) score at ICU admission, comorbidities and length and outcome of ICU stay. Only patients who stayed in ICU for more than 48 hours were considered for the analysis.
Microbiological methods
To assess the impact of antimicrobial therapy on invasive infections, episodes of BSI during ICU stay were analysed. Data on respiratory bacterial isolation was also collected as a representative of the samples that could indicate patient’s colonization. Microbiological data were collected from the laboratory information system. Respiratory tract samples (RTS) included good quality sputum or tracheal aspirate, bronchial aspirate and bronchoalveolar lavage. Bloodstream infections (BSI) were defined as growth of bacteria or fungus from one or more blood cultures (BCs). True bacteraemia caused by common skin colonisers were considered in patients with clinical signs of infection and two or more positive BCs drawn from different venepuncture sites. Only one episode per microorganism, sample type and patient were included. Incidence data are reported as episodes per 100 patients.
RTS and BCs were processed using conventional procedures. All isolates were routinely identified by MALDI-TOF® MS (Bruker Daltonik) and tested for antimicrobial susceptibility by microdilution (Microscan®, Beckman Coulter) following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations and criteria [14].
For analysis purposes, BSI-related microorganisms were classified as follows: 1) Enterobacterales Group 1–2 2) Enterobacterales Group 3; 3) Non-fermenting Gram-negative rods 4) Staphylococcus aureus/Enterococcus spp. 5) Coagulase-negative staphylococci (CoNS) and 6) Candida spp. Enterobacterales classification was made according to their wild-type phenotypic pattern of drug susceptibility (Group 1: Escherichia coli, Proteus mirabilis; Group 2: Klebsiella pneumoniae, Klebsiella oxytoca, Citrobacter koseri and Citrobacter amalonaticus; Group 3: Enterobacter spp., Klebsiella aerogenes, Citrobacter freundii, Serratia marcescens, Morganella morganii, Providencia spp., Proteus vulgaris and Proteus penneri [AmpC-producing bacteria]). Due to their common susceptibility to amoxicillin-clavulanate, groups 1 and 2 were analysed together.
Microorganisms isolated from RTS were divided in five categories: 1) Potentially pathogenic respiratory tract bacteria (RTB) including Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumoniae 2) Enterobacterales Group 1–2; 3) Enterobacterales Group 3 4) Non-fermenting rods and 5) Staphylococcus aureus/Enterococcus spp.
Antimicrobial consumption
Monthly antimicrobial consumption was calculated using defined daily dose (DDD) per 100 patient-days of bed occupancy as defined elsewhere [15]. Data were obtained from the hospital electronic dispensing system. Antimicrobials were grouped according to their spectrum of activity: Broad-spectrum antimicrobials (piperacillin-tazobactam, cefepime, imipenem, meropenem and ertapenem), antimicrobials against meticillin-resistant Staphylococcus aureus (MRSA) and Enterococcus faecium (teicoplanin, daptomycin, linezolid), and systemic antifungals (azoles, echinocandins).
Statistical analysis
Differences between pre-COVID-19 and COVID-19 periods were assessed through SPSS® software package (SPSS, version 23) using T-test or Mann-Whitney U test for continuous variables and Chi-square or Fisher’s exact test for categorical variables, when appropriate. Differences in the incidence between periods were assessed by risk ratio (RR) and 95% confidence intervals were calculated. Statistical significance was set at P< 0.05 (two-sided).
Results
Clinical characteristics
During the study period, 424 (pre-COVID-19) and 263 patients (COVID-19) were admitted to ICU. Among them, 306 (72%) and 220 (84%) stayed in ICU for more than 48 hours and were selected for the analysis. Demographics and clinical data by group are shown in Table I. Both groups were similar regarding age and SOFA score at ICU admission. COVID-19 patients were more frequently males, had less comorbidities and had a significantly higher prevalence of obesity (13% vs 25%). A higher proportion of COVID-19 patients required mechanical ventilation (MV) (77% vs 87%), extracorporeal membrane oxygenation (ECMO) (2% vs 10%) and renal replacement therapy (12% vs 18%). Patients in COVID-19 group also showed longer median ICU stay (7 vs 10 days) and longer duration of mechanical ventilation (3 vs 12 days). ICU-mortality rate was also significantly higher for the COVID-19 group (21% vs 31%). When comparing patients who developed BSI during ICU stay, baseline characteristics were similar (Supplementary Table S1). Obesity and MV requirement remained in a significantly higher proportion in the COVID-19 group (13% vs 30% and 77% vs 98%, respectively).
Table I.
Clinical characteristics of patients admitted to ICU by period. Significant differences between periods are highlighted in bold
| Pre-COVID-19 group |
COVID-19 group |
P-value | |
|---|---|---|---|
| (n = 306) | (n = 220) | ||
| Characteristics | |||
| Age | 66 (56–74) | 64 (55–72) | 0.208 |
| Male sex | 205 (67%) | 169 (77%) | 0.014 |
| SOFA | 7 (5–9) | 6 (5–9) | 0.177 |
| Comorbidities | |||
| None | 19 (6%) | 35 (16%) | <0.001 |
| Two or more | 205 (67%) | 112 (51%) | <0.001 |
| Hypertension | 178 (58%) | 124 (56%) | 0.679 |
| Diabetes mellitus | 85 (28%) | 60 (27%) | 0.898 |
| Obesitya | 39 (13%) | 55 (25%) | <0.001 |
| Active Smoker | 81 (26%) | 28 (13%) | <0.001 |
| Cardiovascular disease | 150 (49%) | 87 (40%) | 0.031 |
| COPD | 41 (13%) | 12 (5%) | 0.001 |
| Chronic kidney disease | 62 (20%) | 30 (14%) | 0.049 |
| Transplant | 10 (3%) | 11 (5%) | 0.317 |
| Malignancies | 65 (21%) | 28 (13%) | 0.012 |
| During ICU stay | |||
| Vasopressor therapy | 222 (73%) | 163 (74%) | 0.694 |
| Renal replacement therapy | 37 (12%) | 40 (18%) | 0.051 |
| Bloodstream Infection | 31 (10%) | 57 (26%) | <0.001 |
| ECMO | 5 (2%) | 22 (10%) | <0.001 |
| Mechanical ventilation (MV) | 236 (77%) | 191 (87%) | 0.005 |
| Duration of MV (days) | 3 (1–11) | 12 (6–29) | <0.001 |
| Length of ICU stay (days) | 7 (4–14) | 10 (5–20) | <0.001 |
| UCI discharge | |||
| Death | 63 (21%) | 68 (31%) | 0.007 |
Data are median (Interquartile range) or n (%).
Obesity was defined according to the body mass index (BMI) categories which based on WHO classification. Obesity is considered by a BMI of >30 kg/m2. (WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Geneva: World Health Organization, 1995)
Microbiological analysis
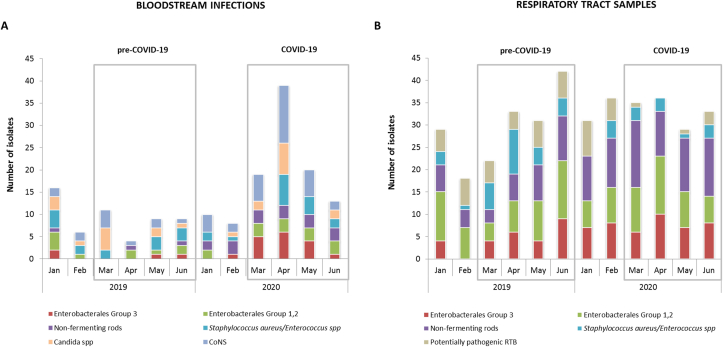
The monthly distribution of pathogens and their incidence by period are shown in Figure 1 and Table II, respectively. An overall increase in the incidence of BSI was detected in COVID-19 period (RR 3.2 [95% CI 2.2–4.7]). This was mostly related to an increase of pathogens belonging to Enterobacterales Group 3 (RR 11.1 [95%CI 2.6–47.9]), non-fermenting rods (RR 7.0 [95%CI 1.5–31.4]) and CoNS (RR 4.5 [95%CI 2.1–9.8]). There were not statistically significant differences in the number of RTS isolates between periods, but the incidence of pathogens of the RTB (RR 0.5 [95% CI 0.2–1.1]) and the S. aureus/Enterococcus spp. (RR 0.3 [95% CI 0.1–0.9]) groups were lower in COVID-19 patients. On the other hand, the incidence of Enterobacterales Group 3 (RR 1.8 [95% CI 1.1–3.0]) and non-fermentative rods increased (RR 2.4 [95% CI 1.5–3.7]). Interestingly, the occurrence of some epidemiological important multidrug resistant (MDR) isolates such as extended spectrum β-Lactamase (ESBL) or carbapenemase-producing Enterobacterales (CPE), MRSA and E. faecium was lower in the COVID-19 period. In contrast, the incidence of MDR Pseudomonas aeruginosa increased (RR 4.2 [95%CI 1.4–12.8]). This was unrelated to an increase of carbapenemase-producing isolates (only one strain producing VIM-type carbapenemase, detected in the pre-COVID-19 period). The genetic relatedness of MDR P. aeruginosa isolates was also studied through PFGE (pulsed-field gel electrophoresis), ruling out the existence of clonal dissemination (data not shown).
Figure 1.
Overview of the pathogens' distribution showing the epidemiological change among microorganisms isolated from ICU patients at early stages of SARS-CoV-2 pandemic. A) Number of pathogens isolated from blood cultures by period (January-June 2019 and January-June 2020). B) Number of pathogens isolated from Respiratory Tract Samples by period (January-June 2019 and January-June 2020).
Table II.
Pathogens isolated from blood cultures and respiratory tract samples by period. Incidence is shown as number of episodes per 100 patients. Statistically significant differences between periods are highlighted in bold.
| Pre-COVID-19 |
COVID-19 |
RR (95% CI) | |||
|---|---|---|---|---|---|
| n | Incidence | n | Incidence | ||
| Bloodstream infections | |||||
| All episodes | 31 | 10.1 | 72 | 32.7 | 3.2 (2.2–4.7) |
| Polymicrobial episodes | 1 | 0.3 | 14 | 6.4 | 14.5 (2.6–147.0) |
| Enterobacterales Group 1-2 | 5 | 1.6 | 12 | 5.5 | 3.3 (1.2–9.3) |
| Enterobacterales Group 3 | 2 | 0.7 | 16 | 7.3 | 11.1 (2.6–47.9) |
| Non-fermentative rods | 2 | 0.7 | 10 | 4.5 | 7.0 (1.5–31.4) |
| Staphylococcus aureus/Enterococcus spp. | 7 | 2.3 | 12 | 5.5 | 2.4 (1.0–6.0) |
| CoNS | 8 | 2.6 | 26 | 11.8 | 4.5 (2.1–9.8) |
| Candida spp. | 8 | 2.6 | 11 | 5.0 | 1.9 (0.8–4.7) |
| Significative RTS isolation | |||||
| Enterobacterales Group 1-2 | 32 | 10.5 | 36 | 16.4 | 1.6 (1.0–2.4) |
| Enterobacterales Group 3 | 23 | 7.5 | 30 | 13.6 | 1.8 (1.1–3.0) |
| Non-fermentative rods | 27 | 8.8 | 46 | 20.9 | 2.4 (1.5–3.7) |
| Staphylococcus aureus/Enterococcus spp. | 23 | 7.5 | 8 | 3.6 | 0.3 (0.1–0.9) |
| Potentially pathogenic RTB | 18 | 5.9 | 4 | 1.8 | 0.5 (0.2–1.1) |
| MDR bacteriaa | |||||
| ESBL/CP-producing Enterobacterales | 10 | 3.3 | 4 | 1.8 | 0.6 (0.2–1.8) |
| MRSA/Enterococcus faecium | 10 | 3.3 | 5 | 2.3 | 0.5 (0.2–1.4) |
| MDR Pseudomonas aeruginosab | 4 | 1.3 | 12 | 5.5 | 4.2 (1.4–12.8) |
Includes all isolates collected from BSI and RTS.
MDR P. aeruginosa was defined according to Magiorakos et al. criteria [38].
Antimicrobial consumption and emergence of resistant isolates
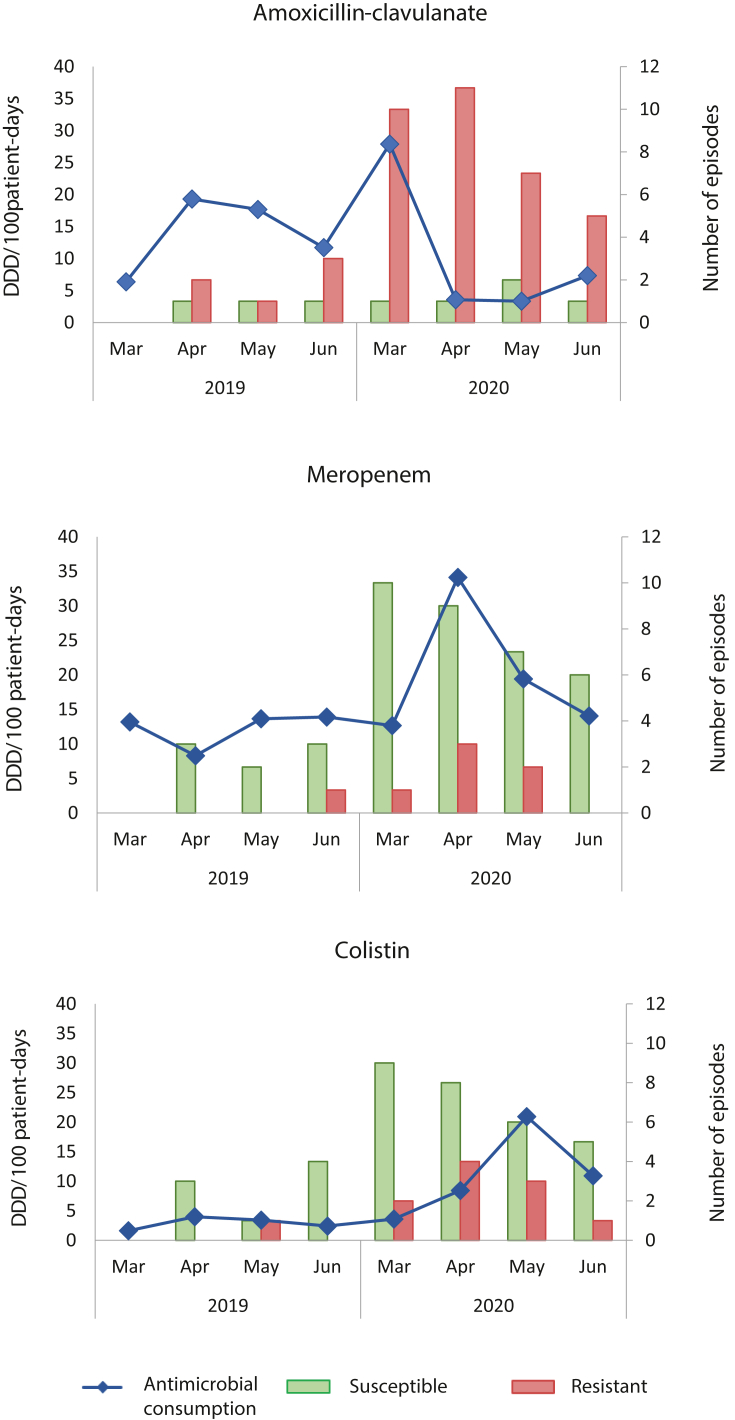
An increase in the consumption of most antimicrobials and antifungals was observed throughout the COVID-19 period (Supplementary Table S2). Data showed high amoxicillin-clavulanate usage during the first month of SARS-CoV-2 pandemic according to hospital recommendations regarding empirical antimicrobial treatment in patients with COVID-19. Amoxicillin-clavulanate usage increased from 6.4 DDD per 100 patients-day to 27.9 DDD per 100 patients-day in March 2020 compared to March 2019. As the pandemic progressed, we observed notable shifts in the use of antimicrobials. Amoxicillin-clavulanate consumption dropped in April 2020 (3.6 DDD per 100 patients-day) and the consumption of broad-spectrum antimicrobials such as meropenem increased (from 12.7 DDD per 100 patient-days to 34.1 DDD per 100 patient-days, from March to April). It is also remarkable the increased consumption of antimicrobials used for the treatment of infections caused by Gram-positive bacteria such as teicoplanin, linezolid or daptomycin (from 21.9 DDD per 100 patient-days in February 2020 to 61.9 DDD per 100 patient-days in April 2020). From May on a reduction in the use of most antimicrobials was observed except for colistin and ceftolozane-tazobactam that increased from 7.0 and 0.0 DDD per 100 patient-days in February 2020 to 20.9 and 12.2 DDD per 100 patient-days in May 2020, respectively.
We aimed to study whether these patterns of antimicrobial consumption were associated with the emergence of resistant isolates. Because the majority of BSI during the COVID-19 period were caused by Gram-negative bacilli, the analysis focused on these bacteria. As shown in Figure 2, the high rates of amoxicillin-clavulanate use in March 2020 were accompanied by the emergence of strains resistant to this antimicrobial. In contrast, the number of susceptible isolates remained stable. Likewise, the incidence of isolates susceptible to meropenem or colistin showed a decreasing trend from March, while that of resistant isolates reached its peak in April, coinciding with the increase in the use of these antimicrobials. The appearance of resistant isolates was mostly associated with bacterial species naturally resistant to these antimicrobials (AmpC-producing bacteria for amoxicillin-clavulanate and S. marcescens for colistin) or bacterial species that frequently develop antimicrobial resistance (P. aeruginosa for meropenem) (Supplementary Table S3).
Figure 2.
Evolution of the monthly number of BSI episodes caused by Gram-negative bacteria resistant to amoxicillin-clavulanate, meropenem and colistin and their consumption. Antimicrobial consumption is expressed as defined daily doses (DDD) per 100 patient-days.
Discussion
The early stages of SARS-Cov-2 pandemic were characterised by a lack of available evidence about the effective treatment of COVID-19 and consequently the management of critically ill patients was based on the accumulated experience in other pathogens. Recent studies have reported the frequency of co-infections and superinfections in moderate to severe COVID-19 patients [10,16] but data describing changes in local epidemiology during initial stages of COVID-19 pandemic are scarce. In this study, we found that the use of empirical therapy during early stages of SARS-CoV-2 pandemic was associated with the emergence of microorganisms with a characteristic resistance phenotype, which required a high use of broad-spectrum antimicrobials over the following months.
Compared to the pre-COVID period, patients with SARS-CoV-2 infection admitted to ICU required longer stay, more days on MV, more use of ECMO and had higher mortality rates. This is in concordance with reported data on critically ill patients with COVID-19 [17,18]. Our data also show that obesity may have a role on the development of severe SARS-CoV-2 infection, as observed by other authors [19,20]. In fact, critically ill COVID-19 patients showed lower frequencies of comorbidities related to disease severity such as malignancies, cardiovascular diseases, COPD or chronic renal failure. These data indicate that COVID-19 patients differed significantly from the usual population of critically ill patients. Longer stay and greater use of some invasive support such as MV or ECMO means greater exposure to nosocomial infections [21,22]. The uncontrolled systemic inflammation induced by SARS-CoV-2 may also facilitate the invasion of the bloodstream by bacterial pathogens [23]. Additionally, other factors such as the prone positioning, a high work overload and a reduced hand hygiene or skin-decolonisation could have contributed to the increased rate of BSIs [24,25].
In our setting, empirical antimicrobial therapy for hospitalised patients was recommended by local guidelines during early stages of COVID-19. The use of antimicrobials showed increased rates and a biphasic pattern, with amoxicillin-clavulanate and broad-spectrum antimicrobials being the most frequently prescribed drugs [26]. Increased antimicrobial consumption has already been reported in Spain and other countries [8,27]. The emergence and expansion of MDR bacteria has also been described which could be related to the high antimicrobial consumption [28,29]. Nevertheless, our data differ from a typical clonal MDR expansion. In the context of increased BSI frequency, the incidence of infections caused by particular groups of pathogens was significantly higher. For instance, the increase in Gram-negative bacteria resistant to amoxicillin-clavulanate was particularly high. This was related to an increase in several AmpC-producing species and non-fermenting rods suggesting that it was probably not part of an outbreak but rather caused by selection secondary to antimicrobial pressure. In fact, the frequency of BSI caused by some multidrug resistant isolates typically associated with nosocomial outbreaks such as ESBL/CPE-producing bacteria or MRSA/E. faecium was low. This could be associated with the fact that 1) these patients had less comorbidities and may have had less contact with the healthcare setting and 2) COVID-19 infection prevention measures could have helped reduce horizontal transmission and prevent nosocomial outbreaks. The use of amoxicillin-clavulanate could also justify the low frequency of isolation of pathogens associated with community-acquired pneumonia among respiratory samples that are frequently the cause of early ventilator-associated pneumonia. Data in this line have been recently reported in England indicating a reduction in the incidence of pneumococcal invasive disease because of the implementation of anti-COVID-19 transmission measures and also to the overuse of certain antimicrobials [30].
The initial selection of pathogens resistant to amoxicillin-clavulanate led to an antimicrobial escalation during the following months towards broader spectrum antimicrobials that in turn also led to an increase in the incidence of bacteria resistant to these antimicrobials. At this point the need to use empirical antimicrobials in patients with severe COVID-19 to prevent bacterial co-infection was not clear. The frequency of bacterial co-infection on admission appears to be infrequent in COVID-19 patients [10,31,32], but not the acquisition of secondary infections during hospitalisation, which has been frequently described [16,33]. It seems that the use of empirical antimicrobial therapy should be restricted to patients with a high clinical suspicion of bacterial infection, as recommended [13]. Additionally, our data highlights the extreme importance of antimicrobial stewardship programs that, in addition to reducing the inappropriate use of empirical antimicrobials, could also help shorten the duration of antimicrobial treatment. In fact, during the early phases of the pandemic, the antimicrobial stewardship program in the ICU had to be discontinued and this may have contributed to antimicrobial over-administration.
Our study adds potentially useful data on the impact of antimicrobial therapy on bacterial infection dynamics among COVID-19 patients. The study has some limitations of note. This is a single-centre experience, and our data may not be translated into other settings. Also, our study does not include patients less than 48 hours of ICU stay that could have led us to miss events which occurred in this population. However, the study design allowed us to homogenise the study population to reduce unknown confounding factors. Moreover, the systematic collection of clinical data performed by the same team minimises the existence of potential biases.
Conclusions
Our study shows a high frequency of BSI in severe COVID-19 patients which could contribute to the increased mortality seen in these patients. The use of empirical antimicrobial therapy during early stages of SARS-CoV-2 pandemic changed the epidemiology of BSIs towards microorganisms with a characteristic resistance phenotype, which in turn led to the use of broad-spectrum antimicrobials. This highlights the epidemiological and clinical impact of empirical antimicrobial therapy which should be thoroughly evaluated to avoid adverse effects.
Acknowledgments
We wish to thank the staff of the Microbiology Laboratory of Bellvitge University Hospital who contributed to this study on a daily routine. We must also thank the members of Intensive Unit Care of Bellvitge University Hospital who elaborated the clinical database.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.infpip.2022.100241.
CRediT author statement
Miriam Torrecillas: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Victor Daniel Gumucio: Conceptualization, Investigation, Formal analysis, Writing – review & editing. Ariadna Padullés: Investigation, Formal analysis, Writing – review & editing. Fe Tubau: Conceptualization, Methodology, Investigation, Writing – review & editing. Daniel Marco: Investigation, Writing – review & editing. Evelyn Shaw: Conceptualization, Writing – review & editing. Miguel Fernández-Huerta: Investigation, Writing – review & editing. Krystel Maisterra: Investigation, Writing – review & editing. Inmaculada Grau: Investigation, Writing – review & editing. Melanie Maria Petito: Investigation, Writing – review & editing. Dàmaris Berbel: Investigation, Writing – review & editing. Mireia Puig-Asensio: Investigation, Writing – review & editing. Xosé Luis Pérez: Investigation, Writing – review & editing. Ma Ángeles Domínguez: Supervision, Project administration, Funding acquisition, Writing – review & editing. Joan Sabater: Supervision, Writing – review & editing. Carmen Ardanuy: Supervision, Project administration, Funding acquisition, Writing – review & editing. Jordi Càmara: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Visualization.
Funding
This study was supported by Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias (CIBERES, CB06/06/0037) and de Enfermedades Infecciosas (CIBERinfec, CB21/13/00009) an initiative of the Instituto de Salud Carlos III, Madrid, Spain, co-funded by the European Regional Development Fund/European Social Fund (ERDF/ESF, “Investing in your future”). We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Conflict of interest statement
The authors declare no conflicts of interest.
Ethics approval and consent to participate
The Clinical Research Ethics Committee of Bellvitge University Hospital approved this research (PR076/21) and waived the requirement of written informed consent as this was a retrospective and non-interventional study with isolates obtained as part of the clinical routine. All confidential information was anonymized and protected according to national normative. All methods were performed in accordance with the relevant national guidelines and international regulations.
Consent for publication
Not applicable.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.ISCIII. Report on confirmed COVID-19 cases in Spain. COVID-19 report no 1. 2020.
- 2.World Health Organization . 2020. Coronavirus disease (COVID-2019) Situation report 162. [Google Scholar]
- 3.Working group for the surveillance and control of COVID-19 in Spain;Members of the Working group for the surveillance and control of COVID-19 in Spain. The first wave of the COVID-19 pandemic in Spain: characterisation of cases and risk factors for severe outcomes, as at 27 April 2020. Euro Surveill. 2020;25:1–13. doi: 10.2807/1560-7917.ES.2020.25.50.2001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas-Rüddel D., Winning J., Dickmann P., Ouart D., Kortgen A., Janssens U., et al. [Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020] Anaesthesist. 2020;69:225–235. doi: 10.1007/s00101-020-00758-x. [DOI] [PubMed] [Google Scholar]
- 5.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalil A.C., Thomas P.G. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care. 2019;23:258. doi: 10.1186/s13054-019-2539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttner B.D., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beović B., Doušak M., Ferreira-Coimbra J., Nadrah K., Rubulotta F., Belliato M., et al. Antibiotic use in patients with COVID-19: a ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75:3386–3390. doi: 10.1093/jac/dkaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend L., Hughes G., Kerr C., Kelly M., O’Connor R., Sweeney E., et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC-Antimicrobial Resist. 2020;2:dlaa071. doi: 10.1093/jacamr/dlaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothe K., Feihl S., Schneider J., Wallnöfer F., Wurst M., Lukas M., et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. 2021;40:859–869. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Amin A.K., Khanna P., Aali A., McGregor A., Bassett P., et al. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J Antimicrob Chemother. 2021;76:796–803. doi: 10.1093/jac/dkaa475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Clinical management of COVID-19: living guidance 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 [PubMed]
- 14.European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters. Version 110 2021. http://www.eucast.org.
- 15.WHO. Collaborating Centre for Drug Statistics Methodology, ATC classification index with DDDs, 2021. Oslo, Norway 2020: n.d.
- 16.Søgaard K.K., Baettig V., Osthoff M., Marsch S., Leuzinger K., Schweitzer M., et al. Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J Intensive Care. 2021;9:10. doi: 10.1186/s40560-021-00526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serafim R.B., Póvoa P., Souza-Dantas V., Kalil A.C., Salluh J.I.F. Clinical course and outcomes of critically ill patients with COVID-19 infection: a systematic review. Clin Microbiol Infect. 2021;27:47–54. doi: 10.1016/j.cmi.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalakis K., Ilias I. SARS-CoV-2 infection and obesity: Common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14:469–471. doi: 10.1016/j.dsx.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papazian L., Klompas M., Luyt C.-E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46:888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasselli G., Scaravilli V., Di Bella S., Biffi S., Bombino M., Patroniti N., et al. Nosocomial Infections During Extracorporeal Membrane Oxygenation: Incidence, Etiology, and Impact on Patients’ Outcome. Crit Care Med. 2017;45:1726–1733. doi: 10.1097/CCM.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 23.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 24.Buetti N., Ruckly S., de Montmollin E., Reignier J., Terzi N., Cohen Y., et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47:180–187. doi: 10.1007/s00134-021-06346-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker M.A., Sands K.E., Huang S.S., Kleinman K., Septimus E.J., Varma N., et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections. Clin Infect Dis. 2022;74:1748–1754. doi: 10.1093/cid/ciab688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abelenda-Alonso G., Padullés A., Rombauts A., Gudiol C., Pujol M., Alvarez-Pouso C., et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol. 2020;41:1371–1372. doi: 10.1017/ice.2020.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Zorn B. Antibiotic use in the COVID-19 crisis in Spain. Clin Microbiol Infect. 2021;27:646–647. doi: 10.1016/j.cmi.2020.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel A., Emerick M., Cabunoc M.K., Williams M.H., Preas M.A., Schrank G., et al. Rapid spread and control of multidrug-resistant gram-negative bacteria in COVID-19 patient care units. Emerg Infect Dis. 2021;27:1234–1237. doi: 10.3201/eid2704.204036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiri B., Sensi E., Marsiliani V., Cantarini M., Priante G., Vernelli C., et al. Antimicrobial stewardship program, COVID-19, and infection control: spread of carbapenem-resistant klebsiella pneumoniae colonization in ICU COVID-19 patients. what did not work? J Clin Med. 2020;9 doi: 10.3390/jcm9092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin-Chowdhury Z., Aiano F., Mensah A., Sheppard C.L., Litt D., Fry N.K., et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England. Clin Infect Dis. 2021;72 doi: 10.1093/cid/ciaa1728. e65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baskaran V., Lawrence H., Lansbury L.E., Webb K., Safavi S., Zainuddin N.I., et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol. 2021:70. doi: 10.1099/jmm.0.001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaba S.M., Jones G., Helsel T., Smith L.L., Avery R., Dzintars K., et al. Prevalence of co-infection at the time of hospital admission in COVID-19 patients, a multicenter study. Open Forum Infect Dis. 2021;8:ofaa578. doi: 10.1093/ofid/ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva D.L., Lima C.M., Magalhães V.C.R., Baltazar L.M., Peres N.T.A., Caligiorne R.B., et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J Hosp Infect. 2021;113:145–154. doi: 10.1016/j.jhin.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.