Abstract
The Symphytum genus has been mainly used in traditional medicine, containing its anti-inflammatory activity. Symphytum spp.’s active components, such as allantoin, polyphenols, flavonoids, and alkaloids, can act on several intentions in the signaling pathway, constrain pro-inflammatory enzymes, reducing the construction of inflammatory chemokine’s and cytokines, and decreasing oxidative stress, which afterward suppresses inflammation procedures. Preclinical and clinical trials have reported the prevailing anti-inflammatory effect of several Symphytum species. This review presents an overview of the anti-inflammatory activities of different products and bioactive constituents in this genus. The papers with the English language were gathered from 2000 to 2021. This review may provide a scientific base for establishing innovative and alternative techniques for isolating a single individual from this genus to attenuate inflammatory disorders. The Symphytum genus is waiting for researchers to develop safe and effective anti-inflammatory agents for additional investigation of other different mechanisms of action.
Keywords: Inflammation, Boraginacea, Comfrey, Wound Healing, Arthritis, Rheumatoid
1. Context
Inflammation is a local, protecting reaction to injury or microbial invasion. It must be controlled precisely since insufficiencies or excesses of the inflammatory response could cause morbidity, shorten lifetime and decrease quality of life (1). Inflammation is generally characterized by redness, edema, fever, and pain, which might bring about loss of organ function dealing with the involved tissue (2). Inflammation could be classified into two groups, acute and chronic inflammation, and each possesses certain types of treatments. Drugs such as steroidal anti-inflammatory drugs (SAID) and non-steroidal anti-inflammatory drugs (NSAID) are usually utilized for the treatment of acute inflammatory complaints (3, 4). These medications have not been fully efficient for treating chronic inflammatory complaints; they temporarily suppress the diseases. In addition, patients suffering from adverse effects of the mentioned drugs, such as gastrointestinal disorders, ulcers, and liver disease, will increase, consequently (5). Therefore, there is a need for different and safe anti-inflammatory medicines, and one of the current and essential research candidates is herbal products (6). Unlike the drugs (SAID and NSAID), which pose an impact by a single component, medicinal plants might act by multitudes of different active compounds. The synergistic effect may occur and impress the targeted elements of the molecular pathways (7). Most developed or developing countries have herbal pharmacopeia separately; in addition to the usage of herbal products in the treatment procedure by the health care team, there is the more widespread usage of unofficial herbal treatments among the people in the society. Their use is grounded either in traditional medicine or modern medicine (8). There is a prevalent tendency to use medicinal herbs for the treatment of disorders (9). Since ere could be some potential side effects, contraindications, and severe drug-herb interactions through the use of herbal medicines, it is necessary to control and monitor the safety and effectiveness of those medicinal herbs (10, 11).
In this review, among the numerous herbal products used in traditional medicines to manage disorders, plants of Symphytum L. genus were selected to focus on anti-inflammatory effects. Symphytum L. spp. has centuries-old diverse usages in traditional medicine. Interestingly, the origin of the Latin name of Symphytum L. is divided into two syllables; symphis refers to the growth and strengthening of the bones, and phyton means the plants that were believed to help wounds healing, referring to its usage in the old days. Several randomized controlled studies have demonstrated the effectiveness of products made from different species of Symphytum L. for the oral and topical treatment of inflammatory disorders such as wound healing, swelling of joints and muscles in rheumatoid arthritis, acute myalgia in the back, sprains, and strains after sports accidents (12). The roots of S. officinale L. have a high reputation as a natural remedy in traditional medicine, especially in Europe, since millenary years ago (13). S. officinale L. has been well accepted in Northern America, so Native Americans admired the healing effects and included S. officinale L. roots in their main therapeutic armamentarium (14). There are internal and external applications for roots and leaves of Symphytum L. spp. External dosages forms are as an ointment, compress or alcoholic digestion, and internal dosages forms are as herbal tea or tincture (15).
In this review, we tried to assess the reports to determine the Symphytum L., genus’s prominence in the treatment of the inflammation process, elucidate the existing mechanisms of action, and compare the anti-inflammatory effects of Symphytum L. genus with Anchusa L., Borago L., Lithospermum L., Nonea L., Pulmonaria L. other genera belong to the Boraginaceae family. There are several genera of the mentioned family with valuables anti-inflammatory properties.
2. Search Strategy
We gathered about 200 detailed, evidence-based data through scientific papers published online from inception 2000 to 2021, and the number of studies included was 133. The search terms were, “comfrey AND clinical therapeutic”, “Boraginaceae AND inflammation,” “ inflammatory diseases”, “Boraginaceae”, “Anchusa L. ”, “Borago L. ”, “Lithospermum L. ”, “Nonea L. ”, and, “Pulmonaria L. ” in the title and keywords and the language was English. A wide range of databases, including Science Direct, PubMed/Medline, and Scopus search engines, were searched to identify relevant reports.
3. Inflammation
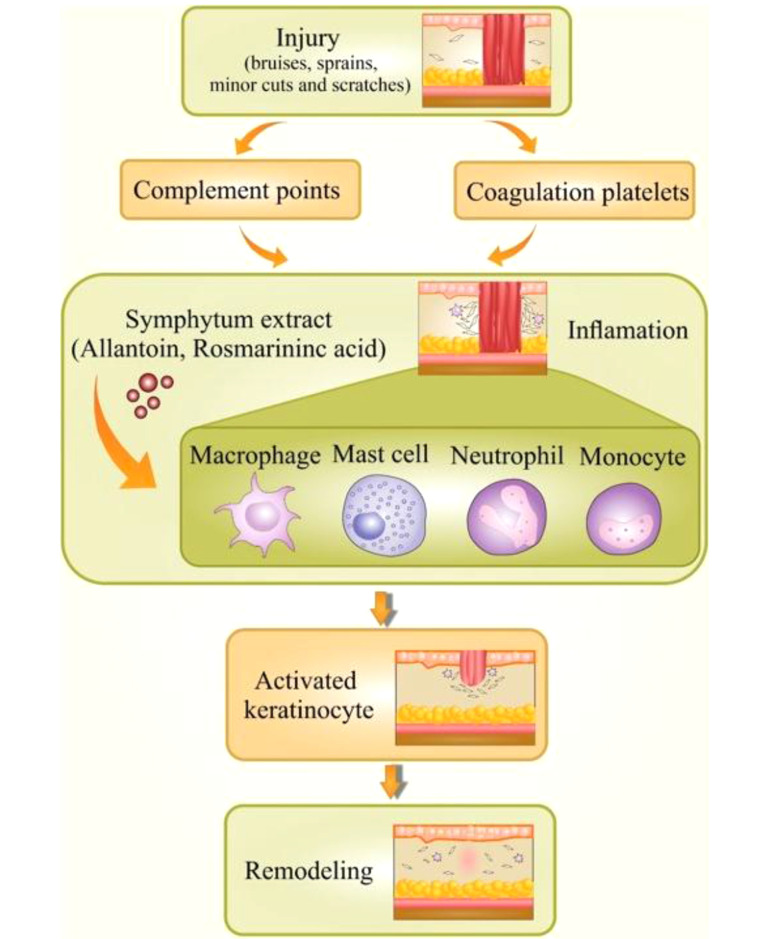
Not surprisingly, inflammation is responsible for contributing to half of the world’s global burden of disorders. Chronic inflammatory diseases are one of the most significant causes of death globally (16, 17). It is an incredibly dynamic process that responds to a various stimuli containing chemical, physical or microbial injury. Inflammation is a valuable reaction in body defense, signifying the first response to injury (18). A controlled inflammatory response is an excellent complex process that acts over a mechanism bringing about the clearance of injuries (19). As far as we know, the initiation of the inflammation process is mediated via signals and controlled through individual mediators. Different types of mediators and cells are implicated in the process of inflammation, which could regulate cell migration, chemotaxis, and proliferation in a significantly coordinated way (20, 21) Commonly, an incidence causes damaged cells to release chemicals, including histamine, bradykinin, and prostaglandins. These chemicals trigger inflammation and activate the endothelial cells in blood vessels close to the site of trauma. This action lets adhesion and transmigration of leucocytes from the blood circulation inside the hurt tissue. Acquisition of this ability, endothelial cells convey a wide-ranging variety of pro-inflammatory genes such as cell adhesion molecules (P-selectin, E-selectin, VCAM-1), cytokines, and chemokines [interleukin IL-1, IL-6, IL-8], enzymes (iNOS, COX-2, SOD), etc. (Figure 1) (22).
Figure 1. Inflammation and wound healing process.
Inflammation may turn into a chronic condition that leads to the damage of the body structures and tissues due to the generation of potent biochemical mediators; chronic inflammation is extended for several months or even years. Frequently, the effects of chronic inflammation could be different from the impacts of acute inflammation of the hurt tissue, and the body's ability to heal the damage of chronic and acute inflammation is different (18). Additional responses of the body to the inflammation mediators might be related to chronic inflammation (23).
3.1. Inflammatory Markers
The inflammation process and its molecular basis have led to identifying inflammatory markers. These markers might suggest newfound therapy targets in the management of inflammatory-based diseases (24). Conditions with a prominent initiation of the inflammatory markers are placed into three main groups: (1) infections; (2) some hematological malignancies; (3) and autoimmune diseases. Specific proteins are regularly released from the inflammation site in-vivo, circulate in the bloodstream and bring about the whole involvement organ in a specific part of the body. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), plasma viscosity (PV), ferritin, and fibrinogen are leading acute phase markers in the inflammation process (25). Detection and measurement of these inflammatory markers through various blood tests are frequently utilized to reveal the acute phase of inflammation that might be a sign of specific diseases and follow up the recovery process, seeing as the crucial role of these markers in treatment response. , The amount of these inflammatory markers can also be used as a universally accepted but non-specific test of severe diseases such as rheumatoid arthritis and systemic vasculitis (26). As far as we know, the marker “CRP” is signified to be principally beneficial in detecting bacterial infections. The marker “ESR” is usually revealed to the plasma viscosity, the test measures the rate of red blood cells and erythrocytes, and this marker is also independent of sex or age (27). Several reports in ethnopharmacological studies have been conducted about the effectiveness of herbal medicines in the inflammation process due to the interruption of inflammatory markers (28, 29).
4. Symphytum L.
Plants from Symphytum L. spp. are common inhabitants of humid meadows and edge of lake parts of Europe, Asia, and America, belonging to Boraginaceae. The genus Symphytum L. is also known worldwide as comfrey, knitbone, bruise wort, and slipprty wort (30, 31). Symphytum L. includes about 40 species as the most famous ones are: S. officinale L., S. x uplandicum Nyman L., S. asperrimum L., S. pregrinum ledab L., S. kurdicum L., S. caucasicum Bieb L., S. asperum Lepech L., and S. tuberosum L. (32, 33). S. officinale L.is the most renowned member of this genus that is repeatedly recorded in scientific reports with the common comfrey name and is well-known for its anti-inflammatory property (31, 34). Different therapeutic activities of Symphytum spp. have been reported to several phytochemicals, like; Allantoin, phenolic, glycopeptides, polysaccharides, and pyrrolizidine alkaloids (14). Except for Symphytum L. genus of Boraginaceae other genera from this family were reported for their anti-inflammatory effects (35). Other species of family-like, are cultivated worldwide and native to Europe, Asia. Anchusa strigosa L. was used as an antiulcer, wound healing, antirheumatic, and antiarthritis (36, 37). Anchusa L. genus contained phytochemicals such as pyrrolizidine alkaloids, tannins, triterpenes, and polyphenols (38). The genus Borage seeds oil is the substantial herbal resource of the gamma-linolenic acid (30% - 40%) that is prescribed as an anti-inflammatory agent (39). Borage seed extract is also used to treat diabetes, arthritis, and autoimmune disorders (40). The genus Lithospermum contained shikonin, shikalkin, pyrrolizidine alkaloids, flavonoids, and polyphenols (41). It was used as burns healing, antimicrobial and, anti-inflammatory agent (42). The genus Nonea L. has been traditionally used in the treatment process of diabetes respiratory diseases and was used as a wound-healing agent and anti-inflammatory agent (43). It has been established that the genus Nonea L. is the rich source of polyphenols, alkaloids, and fatty acids (44). The genus Pulmonoria L., an herbal medicine, was used against respiratory and urinary disorders (45, 46). It has been reported that Rosmarinic acid, flavnoides, and phenolic compounds were the active ingredients in this genus (47). The genus Echium L. is mainly used as a sedative, anti-inflammatory agent, and anxiolytic possession, treating disorders including fissures of the hands and snakebites (48). It has been established that the Echium genus contained phytochemicals like naphthoquinones, flavonoids, terpenoids, and polyphenols (49).
5. Phytochemicals and Anti-inflammatory Properties of Symphytum L. spp.
Chronic inflammatory systemic diseases like rheumatoid arthritis are considered whole lifetime debilitating disorders, increasing the mortality rate bringing about high costs in healthcare for the patients and public health organizations (50). Accordingly, pharmacotherapy in these inflammatory disorders is one of the vital healthcare issues. Herbal products hold a unique place among the public and researchers, revealing appealing natural sources for detection and proceeding with novel drug candidates. Regarding the natural products, different constitutes and preparations from S. officinale L. have been extensively used to treat inflammatory disorders such as painful muscle and joint illnesses bone and wound healing (18). In a study by Thibane et al., leaves of S. officinale L. were used to stimulate healing, reduce inflammation, resulting in the alleviation of joint and muscle disorders and more facilitated functional improvements (51). In a randomized, placebo-controlled, double-blind study by Koll et al., this genus was also renowned for its effectiveness in treating gonarthrosis, acute lower and upper back pain, and blunt injuries (52). S. officinale L. has oral and topical preparations (53), used for its analgesic and anti-inflammatory activities, validated by modern clinical studies; however, the molecular base of action is still elusive (18, 54). Some scientific shred of evidence is explaining the pathogenesis of inflammation and inflammatory diseases, underlining the role of the Symphytum L. genus in this process (5, 24). A reputable source, European Scientific Cooperative on Phytotherapy Monograph (ESCOP), introduced a monograph for S. officinale L. discussing some properties of this plant. Specifically, it has been indicated that the roots have beneficial diverse therapeutic indications such as discolorations and wound healing, strains recovery, osteoarthritis, tend vaginitis, epicondylitis, arthritis, knee joint injuries, skin inflammation, tendinitis syndrome, non-active gonarthrosis, mastitis, insect bites, and fractures, proved by numerous clinical trials (55, 56). Although S. officinale L. is the most acknowledged species in this genus, other species represent significant bioactivities like S. x uplandicum L. (Russian comfrey) with wound healing effect, and another plant is S. caucasicum L. with burn healing effects (57, 58). Diverse biological effects have been reported from the selected species of the Boraginaceae family, which especially have a reputation in the treatment process of inflammation (Table 1).
Table 1. Names, Active Compounds, Biological Activities, and Geographical Distribution in Mentioned Species of the Boraginaceae Family.
| Genus | Active Components | Biological Activity | Geographical Distribution | References |
|---|---|---|---|---|
| Symphytum | Allantoin, pyrrolizidine alkaloids, choline, tannins, rosmarinic acid, and triterpenoid saponins. | Wound healing effects, strains recovery, osteoarthritis, tend vaginitis, epicondylitis, arthritis, knee joint injuries, skin inflammation. | Europe, Asia, and America | (59) |
| Pulmonaria | Allantoin, Rosmarinic acid, flavenoides, and phenolic compounds. | Treating respiratory disorders and urinary disorders anti-lithiasis activities. Wound healing effects. | Europe and western Asia | (47) |
| Nonea | Phenolic compounds, pyrrolizidine alkaloids, fatty acids, flavonoid, and saponines. | Treating diabetes, respiratory disorders, and wound healing agent. | the Mediterranean districts | (44) |
| Anchusa | Allantoin, pyrrolizidine alkaloids, tannins, triterpenes, and phenolic compounds. | Antiulcer, wound healing, Diaphoretic, antipyretic, narcotic, antipyretic, antirheumatic, and antiarthritis. | tropical and Mediterranean districts | (38, 60) |
| Echium | Allantoin, naphthoquinones,flavonoids, terpenoids, and phenolic compounds. | sedative, and anxiolytic, treating disorders including fissures of the hands, general scratches, and snakebites. | Mediterranean, North Africa and Europe | (49) |
| Borage | Allantoin and gamma-linolenic acid | Treating multiple sclerosis, diabetes, arthritis, eczema, and autoimmune disorders. | Europe, North Africa and Asia | (39) |
| Lithospermum | Allantoin, pyrrolizidine alkaloids, shikonin, shikalkin, flavonoids, and phenolic compounds. | Wounds and burns healing, antimicrobial, and antiparasitic agent. | Native to Europe, Asia, Africa. | (61) |
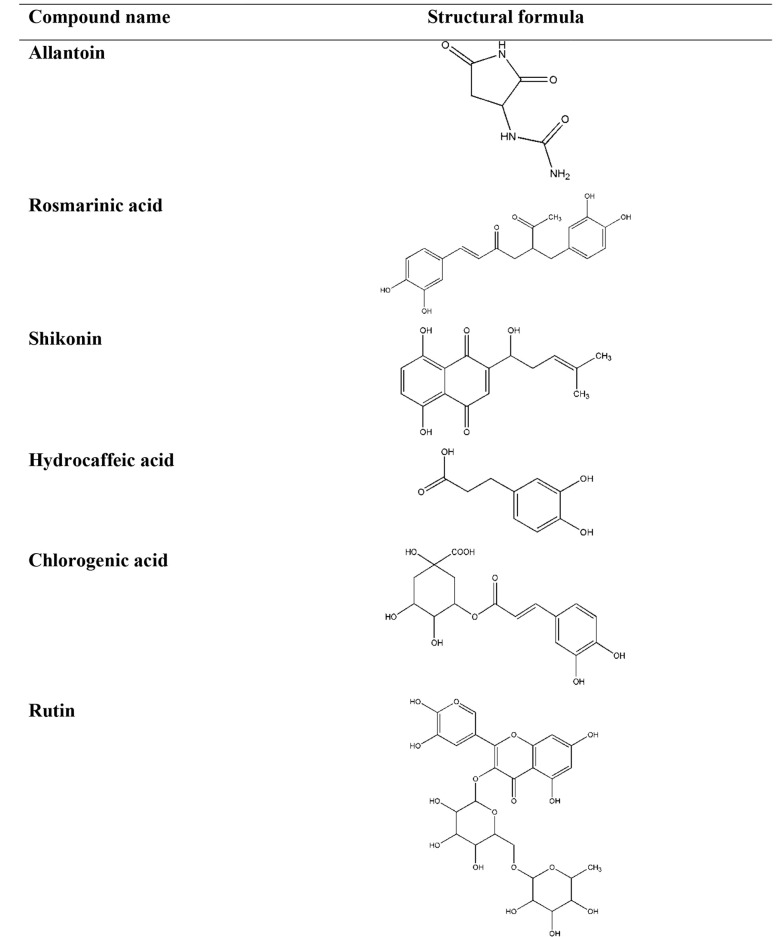
Diverse mechanisms of the anti-inflammatory preparations from the Symphytum and selected members of the Boraginaceae family L. spp. have been revealed to the constituents playing roles in these procedures (62). It has been confirmed that allantoin, choline, tannins, rosmarinic acid and its derivatives, poly[3-(3,4-dihydroxyphenyl) glyceric acid], shikonin, triterpenoid saponins, and essential oil were of the leadings phytopharmaceuticals present in these plants (63, 64). Considerably, these constituents vary depending on the plant species and special part of the herb. The chemical structures of phytochemicals, structure activity relationship (SAR), and the source of the phytochemicals have important effects on the anti-inflammatory activities of a plant (Table 2 and Figure 2) (65, 66).
Table 2. The Classifications and Chemical Structures of the Important Phytochemicals Used in the Treatment of Inflammation.
| Group Compound | Compound Name | Source | Anti-inflammatory Effects | References |
|---|---|---|---|---|
| Imidazolidine-type alkaloid | Allantoin | Aerial parts and roots of Symphytum, Aerial parts of Borage, Aerial parts and roots of Lithospermum, Aerial parts and roots of Anchusa, Aerial parts and roots of Echium, Aerial parts and roots of Nonea, Aerial parts and roots of Pulmonaria | stimulating cell proliferation, improving regeneration of damaged tissues | (67, 68) |
| Phenolic acid | Rosmarinic acid | Aerial parts and roots of Symphytum, Aerial parts and roots of Borage, Aerial parts and roots of Lithospermum, Aerial parts and roots of Anchusa, Aerial parts and roots of Echium, Aerial parts and roots of Nonea, Aerial parts and roots of Pulmonaria | inhibits the formation of pro-inflammatory mediators, inhibits the formation of lipoxygenase, inhibits the formation of 5-HETE, inhibits cytokine release | (67, 69) |
| Naphthoquinone | Shikonin | Roots of Symphytum, Roots of Lithospermum, Roots of Echium | suppresses the transcriptional activation of the TNF-α promoter | (67, 70, 71) |
| Phenolic acid | Hydrocaffeic acid | Aerial parts and roots of Symphytum, Aerial parts and roots of Lithospermum, Aerial parts and roots of Echium, Aerial parts and roots of Nonea | inhibited the release of IL-1β | (67, 72) |
| Phenolic acid | Chlorogenic acid | Aerial parts of Symphytum, Aerial parts of Lithospermum, Aerial parts of Nonea, Aerial parts and roots of Pulmonaria | inhibits NO, inhibits pro-inflammatory cytokines | (67, 73-75) |
| Flavonoid | Rutin | Aerial parts of Symphytum, Aerial parts of Lithospermum, Aerial parts of Anchusa, Aerial parts of Echium | suppresses the production of TNF-α, suppresses interleukin 6, suppresses the activation of NF-κB | (68, 73, 76) |
Figure 2. The chemical structures of the important phytochemicals used in the treatment of inflammation.
Allantoin, 5-ureide-hydantoin, is a metabolic compound of uric acid oxidation stimulating cell proliferation and improving regeneration of damaged tissues, while the compound choline decreases capillary permeability and, as a result, acts as an anti-oedemateous (77, 78). It has been reported that the quantity of allantoin in the selected species was in the range of 0.6 - 11.8 mg.g-1 air-dried matter in the aerial parts and 0.1 - 34.9 mg.g-1 air-dry matter in the roots. The maximum amount of this compound was detected in Echium italicum L. aerial parts (9.59 ± 1.96 mg.g-1) and roots (34.89 ± 10.4 mg.g-1), whereas in S. officinale aerial parts, it was reported as 9.38 ± 2.72 and in roots, it was 25.77 ± 17.02 mg.g-1 (67). However, allantoin's molecular mechanism of action and its pharmacodynamic have remained unknown (79). Choline increases tissue perfusion through vasodilatation and supports the clearance of inflammations mediators from the involved tissue (78). Choline shows its anti-inflammatory effect by triggering alpha-7 nicotinic receptors and reducing cytokine production in macrophages (80). Rosmarinic acid, a phenolic compound in Symphytum L. spp., inhibits the formation of pro-inflammatory mediators, lipoxygenase, and 5-HETE also expresses antiphlogistic activity with no relative activity upon prostaglandin synthesis (81, 82). Moreover, rosmarinic acid binds to T-cells and blocks signaling pathways to the nucleus resulting in cytokine release inhibition like IL-1 that would be used in treating autoimmune diseases (83). The rosmarinic acid quantity in the selected plant species was in the range of 1.2 - 36.6 mg.g-1 air-dry matter in the aerial parts and 1.3 - 27.0 mg.g-1 air-dried matter in the roots. The maximum amount of this compound was detected in Pulmonaria mollis aerial parts (36.6 ± 1.2 mg.g-1) and roots of Anchusa undulata (27.0 ± 5.65 mg.g-1). In aerial parts of S. officinale, rosmarinic acid quantity was detected as 4.5 ± 1.5, and in roots, it was 7.1 ± 2.63 mg.g-1. Compared to S. officinale, the amount of rosmarinic acid was much more in the other species, S. cordatum, in aerial parts (12.4 ± 1.3) and less in the amount in the roots (7.19 ± 0.75) (67). Allantoin, rosmarinic acid, and choline were believed to be the most responsible components for the anti-inflammatory and wound-healing properties in the mentioned plants (84). Phenolic compounds of S. officinale L. have been ascertained to be used as an anti-inflammatory agent in experimentations both in-vitro and in-vivo (85). A polysaccharide; Poly[3-(3,4-dihydroxyphenyl)glyceric acid], showed antioxidant and anti-complement effects putting a stop to tissue damage that would be beneficial in several pathological disorders (86).
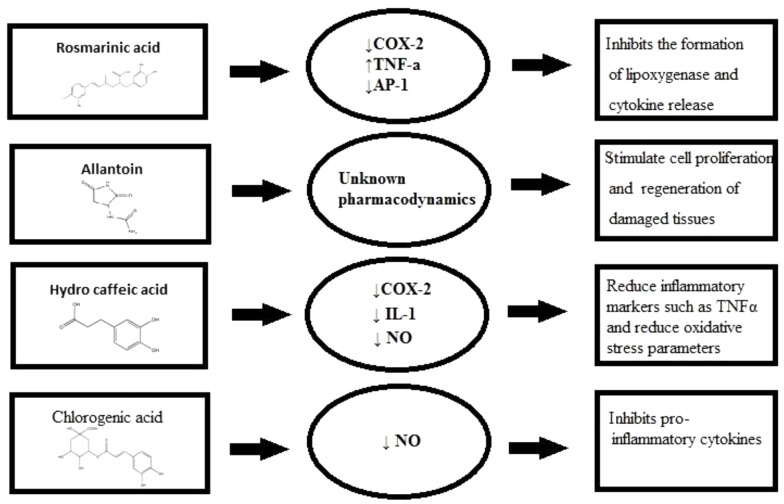
S. officinale L. has anti-inflammatory properties that could be attributed to inhibition of IL-1β release, significantly (87). Shikonin suppresses the transcriptional activation of the TNF-α promoter concluded inhibiting the binding of Transcription factor II D (a complex of proteins that binds to a TATA series on the DNA) complex to TATA box (a sequence of DNA) within the basal transcription machinery and so the consequent expression of the TNF-α protein. It is well accepted that shikonin possesses valuable therapeutic profits for skin-related inflammatory diseases and conceivably in inflammatory disorders attendant with increased TNF-α mediators (88). There are various molecular mechanisms accredited in the treatment of inflammations commended to describe their functioning mechanism, containing the targeting of various intracellular signaling pathways provoking through Nrf2, MAPK, NF-κB, PPAR, and AP1 (Figure 3) (20).
Figure 3. Anti-inflammatory components pharmacodynamics figure.
NF-κB signaling regulated several innate and adjustable immune functions. It is believed that NF-κB is a transcription factor that adjusts pro-inflammatory cytokine gene transcription. In the study, the roots of S. officinale L. were extracted by hydroalcoholic; solution. The mucilage depleted fraction impaired the interleukin-1 (IL-1) through induction of pro-inflammatory markers, an expression containing E-selectin, VCAM1, ICAM1, and COX-2. Data afforded evidence that S. officinale L. obstructed NF-κB signaling at two different stages: at first, it reduced the activation of IKK1/2, and later degradation of IκBα, and secondly, it inhibited the NFκB p65 nucleo-cytoplasmatic transporting and transactivation (18, 26). There is another established mechanism for diverse anti-inflammatory effects of Symphytum L. spp. through the two cyclooxygenase isoforms in charge of several signs of inflammation such as vascular disorders and pain. Logically, COX-1 and COX-2 are the main enzymes in the arachidonic acid metabolism pathway resulting in the synthesis of prostaglandins. The inhibitory action of the Symphytum L. spp. was determined to be specifically on COX-2, with no effect on COX-1 enzymatic activity. Of note, no direct inhibitory activity on the enzymatic activity was detected, but the expression of COX-2 itself was powerfully blocked (18, 89). Various genera of the Boraginacea family inhibits numerous inflammatory factors (Table 3).
Table 3. Mechanisms of Anti-inflammatory Action of the Medicinal Plants are Mentioned in this Review Article.
The aqueous extract of S. officinale L. aerial parts stimulated peroxisome proliferator-activated receptor (PPARs) and down-regulated E-selectin (expressed on endothelial cells after activation by IL-1 or TNFα and played an essential role in inflammation) mRNA and IL-8 (15, 99). The wound healing activity of S. officinale L. leaves was evaluated using three preparations via carbomer gel, glycero-alcoholic solution, and emulsion/soft lotion upon open wound rat model, using allantoin as the positive control. The results showed that the emulsion induced healing of the tissue injury and proliferation in collagen deposition from 40% to 240% and reduced cellular inflammatory infiltration by 3 - 46% (15, 99, 100). Polysaccharides like poly[3-3,4-dihydroxyphenyl) glyceric acid] from S. officinale showed the ability to deactivate the formation of active oxygen species (AOS) that are molded by activated polymorphonuclear neutrophils (PMN), performing a significant role in the protection of the body from invasive microorganisms (101). The tissue was threatened when PMN initiated extra AOS production (102). When AOS rises, the enzyme xanthine oxidase (XO) catalyzes the oxygen transformation into a superoxide anion, causing tissue damage. It was concluded that the binding of superoxide anion formed by activation of PMN and by XO was essential for healing wounds and treating inflammations (103). Another study assessed the effects of hydroalcoholic extract on healing osteoarthritis pain on 200 patients divided into two groups. One group used S. officinale L. extract cream, and the other applied placebo cream. The results showed that the patients who applied S. officinale L. cream rated their pain 16 points lower than the other group in a short time (104, 105). Elsewhere, the effectiveness of the ointment of S. officinale L. extracts compared to diclofenac gel was evaluated in an observer-blinded study reporting the curve (AUC), the tenderness, and pain assessment at rest, movement by the patient, swelling, and ankle movement. According to the statistical data, the difference between the two groups was significant, and the benefit of the herbal ointment in excess of the diclofenac gel in the treatment process of distortions was observed. The S. officinale L. products were suggested as a safe and effective alternative to the standard topical treatments (Table 4) (5, 106).
Table 4. The Symphytum Genus Biological Activities and Active Compounds Related to Anti-Inflammatory Activities.
| Species | Anti-inflammatory Activity | Active Components | References |
|---|---|---|---|
| Symphytum officinale L. | Wound healing effects, Treating swelling of muscles, Treating arthritis, Treating sprains, contusions and strains after accidents | Allantoin, Rosmarinic acid, Hydro caffeic acid, Chlorogenic acid | (107-109) |
| Symphytum asperum Lepech. | Treating fractures and strains, Treating thrombophlebitis, Treating rheumatism , Treating gout, Wound healing effects | Poly [3-(3, 4-dihydroxyphenyl) glyceric acid], Caffeic acid, Rosmarinic acid, Chlorogenic acid, Salvianolic acid | (110) |
| Symphytum caucasicum | Burning healing effects, Wound healing effects | Poly [3-(3, 4-dihydroxyphenyl) glyceric acid], Allantoin | (63, 111) |
| Symphytum cordatum | Wound healing effects | Allantoin, Rosmarinic acid, Hydrocaffeic acid, P-hydroxybenzoic acid | (14, 67) |
| Symphytum × uplandicum Nyman | Wound healing effects, Treating swelling of muscles and myalgia, Treating arthritis, Treating sprains, contusions and strains after accidents, Treating joint distortion | Allantoin | (58, 112) |
| Symphytum anatolicum | Wound healing effects, Treating sprains and bruises | Caffeic acid, Allantoin, Chlorogenic acid, Rosmarinic acid, Isoquercitrin, Rutin, Hyperoside, Salvianolic acid C | (72, 113) |
6. Safety of Symphytum L. spp.
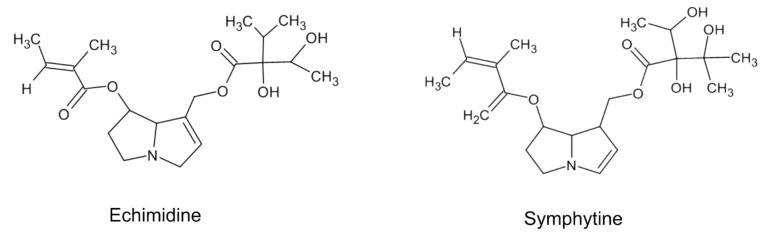
Toxicity is a crucial concern in deciding to choose a treatment procedure for a certain kind of disease. The safety of an herbal medication depends on the roots of administration, such as internal or external forms (114). It is deemed that preparation of Symphytum L. spp. Moreover, other plants of the Boraginaceae family are not safe; however, it is not supported through studies that administrated high doses of different crude compounds or prepared preparations to rodents or other animals (54). There exist not much epidemiological or clinical trial studies about the products to review them and clarify the actual toxicity in the utilized human doses (115). There is a shred of evidence that this genus, among the diversity of secondary metabolites, possesses pyrrolizidine alkaloids (PA) that are accepted as hepatotoxic phytochemicals (116). PAs are naturally inactive components and safe. They are metabolically triggered in the body by hepatic enzymes, specially CYP3A and CYP2B isoforms (117). Studies showed that toxicity varies according to the chemical structure of an individual PA in-vitro (118-120). Also, additional animals or rodents do not have the same response to PAs toxicity (121). Hence, a necessary factor to consider in determining the toxicity of a plant containing PAs is that all PAs with different chemical structures are not in the same toxicity; and various plant species have other content of PAs with different quantities (122). Different PAs are found in aerial parts and roots of Symphytum L. spp. with varying degrees of toxicity like echimidine, symlandine, symphytine, intermedine, lasiocarpine, lycopsamine, symviridine, and asperumine (Figure 4) (123-126).
Figure 4. Chemical structure of two important compounds of Pas.
The reported content of PAs in certain a plant depends not only on the extracted plant part but also on the extraction method. Since the chemical structure of the PAs are semi-polar, the concentration of PAs is shallow in extraction via a universal way of steeping parts of the plant in warm water (a polar solvent) as herbal tea (127, 128). A study investigated the PAs biosynthesis in this genus, and results revealed that there were no reported prominent adverse effects when the products were used externally. However, pharmacokinetics have reported low drug absorption via cutaneous roots (129). The safety and efficacy of S. officinale L. root extract topical preparation have been validated by numerous non-interventional studies and randomized clinical trials (52).
6.1. Liver Toxicity
Symphytum officinale roots consist of about 0.2% - 0.4% PAs (116). Internal use of comfrey root extracts is traditionally used for treating; its effectiveness and safety have never been assessed so far in controlled clinical trials however internally used preparations have not been recommended and were even restricted in the USA and Canada (120). The main liver injury caused by Symphytum and PAs is a veno-occlusive disease (VOD), a non-thrombotic obliteration of small hepatic veins leading to cirrhosis and ultimately liver failure. It may occur with either acute or chronic clinical signs with portal hypertension, hepatomegaly, and abdominal pain as the main features (122). To sum up, the risk of hepatotoxicity with the medicinal herbs preparations selected in this review article during the treatment procedure is influenced by the duration of the process, the source of the plant, the quantity of consumed, and the health crises of the patient (115, 130).
It has been accepted that the products with internal usages such as tablets and capsules in Europe and America should be labeled with the warning; “products contained a relatively low concentration of PAs” (31). It is established that the toxicity of Symphytum L. spp. is because of PAs, and the anti-inflammatory effects of this genus are primarily because of allantoin, rosmarinic acid, and other constituents. We might conclude that the natural products prepared from Symphytum L. spp. that might withdraw the toxic components would be utilized with more confidence and efficiency (131). There is a part in the Committee on Herbal Medicinal Products that dealt with the safety of Symphytum L. spp. Usage during pregnancy and lactation, in which there were no sufficient data.
In conclusion, its safety during pregnancy and lactation has not been established yet. So, due to the PAs toxicity, the use of Symphytum L. spp. Products during pregnancy and lactation are not recommended (132). Plus, no drug interactions have been reported till now (133). As a final point, all the mentioned medicinal plants belong to the Boraginaceae family, which are a source of PAs with different chemical structures; according to the quantity of PAs and non-availability of data on their safety of usage, we might consider their herbal preparations safe or dangerous to use (134, 135).
7. Conclusions
There is a strong shred of evidence, scientific reports, and clinical trials, as mentioned above, presenting an insight into the importance of Symphytum L. spp. Different pharmacological mechanisms for the effectiveness of plants of this genus in inflammation have been proposed to treat inflammatory diseases. Major Phytochemicals from Symphytum L. spp. contributed to propounding the outcome mentioned above, including allantoin, rosmarinic acid, and choline. PAs are known as hepatotoxic compounds. Several reports were reviewed, and it was established that different PAs with various chemical structures showed different toxicity levels, mainly depending on the administration route and usage duration. Nevertheless, it sounds logical and safer to utilize special extraction techniques for removing PAs from the Symphytum L. spp. herbal preparations. Overall, Symphytum L. genus, has valuable constituents for inflammation treatment, such as allantoin, various polyphenolic acids, and flavonoids; and there remains a great extent of unknowns, to seek about effectiveness of this genus with other different mechanisms of action.
Acknowledgments
The authors would like to thank the Research Vice-Chancellor of Tabriz University of Medical Sciences for financial support of this study. This article was written based on a data set of Ph.D. thesis, registered in Tabriz University of Medical Sciences (63073).
Footnotes
Authors' Contribution: Not declared by authors.
Conflict of Interests: The authors declare that they have no conflict of interest.
Funding/Support: Research Vice-Chancellor of Tabriz University of Medical Sciences.
Contributor Information
Elaheh Mahmoudzadeh, Email: chichest1991@gmail.com.
Hossein Nazemiyeh, Email: nazemiyehh@tbzmed.ac.ir.
Sanaz Hamedeyazdan, Email: yazdans@tbzmed.ac.ir.
References
- 1.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 2.Scotti F, Decani S, Sardella A, Iriti M, Varoni EM, Lodi G. Anti-inflammatory and wound healing effects of an essential oils-based bioadhesive gel after oral mucosa biopsies: preliminary results. Cell Mol Biol (Noisy-le-grand). 2018;64(8):78–83. [PubMed] [Google Scholar]
- 3.Pok LSL, Shabaruddin FH, Dahlui M, Sockalingam S, Mohamed Said MS, Rosman A, et al. Clinical and economic implications of upper gastrointestinal adverse events in Asian rheumatological patients on long-term non-steroidal anti-inflammatory drugs. Int J Rheum Dis. 2018;21(5):943–51. doi: 10.1111/1756-185X.13256. [DOI] [PubMed] [Google Scholar]
- 4.Hamedeyazdan S, Fathiazad F, Sharifi S, Nazemiyeh H. Antiproliferative activity of Marrubium persicum extract in the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev. 2012;13(11):5843–8. doi: 10.7314/apjcp.2012.13.11.5843. [DOI] [PubMed] [Google Scholar]
- 5.Smith DB, Jacobson BH. Effect of a blend of comfrey root extract (Symphytum officinale L.) and tannic acid creams in the treatment of osteoarthritis of the knee: randomized, placebo-controlled, double-blind, multiclinical trials. J Chiropr Med. 2011;10(3):147–56. doi: 10.1016/j.jcm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96(3):229–45. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 7.Vikrant A, Arya ML. A review on anti-inflammatory plant barks. Int J Pharmtech Res. 2011;3(2):899–908. [Google Scholar]
- 8.Sile I, Romane E, Reinsone S, Maurina B, Tirzite D, Dambrova M. Medicinal plants and their uses recorded in the Archives of Latvian Folklore from the 19th century. J Ethnopharmacol. 2020;249:112378. doi: 10.1016/j.jep.2019.112378. [DOI] [PubMed] [Google Scholar]
- 9.Sewell RD, Rafieian-Kopaei M. The history and ups and downs of herbal medicines usage. J HerbMed Pharmacol. 2014;3(1):1–3. [Google Scholar]
- 10.Boullata JI, Nace AM. Safety issues with herbal medicine. Pharmacotherapy. 2000;20(3):257–69. doi: 10.1592/phco.20.4.257.34886. [DOI] [PubMed] [Google Scholar]
- 11.Hamedeyazdan S, Sharifi S, Nazemiyeh H, Fathiazad F. Evaluating Antiproliferative and Antioxidant Activity of Marrubium crassidens. Adv Pharm Bull. 2014;4(Suppl 1):459–64. doi: 10.5681/apb.2014.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staiger C. Comfrey: A clinical overview. Phytother Res. 2012;26(10):1441–8. doi: 10.1002/ptr.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englert K, Mayer JG, Staiger C. Symphytum officinale L. - der Beinwell in der europäischen Pharmazie- und Medizingeschichte. Zeitschrift für Phytotherapie. 2005;26(4):158–68. doi: 10.1055/s-2005-915653. [DOI] [Google Scholar]
- 14.Salehi B, Sharopov F, Boyunegmez Tumer T, Ozleyen A, Rodriguez-Perez C, Ezzat SM, et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules. 2019;24(12) doi: 10.3390/molecules24122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogl S, Picker P, Mihaly-Bison J, Fakhrudin N, Atanasov AG, Heiss EH, et al. Ethnopharmacological in vitro studies on Austria's folk medicine--an unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J Ethnopharmacol. 2013;149(3):750–71. doi: 10.1016/j.jep.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehman S, Nabi B, Baboota S, Ali J. Natural anti-inflammatory agents for the management of osteoarthritis. In: Brahmachari G, editor. Discovery and Development of Anti-Inflammatory Agents from Natural Products. Amsterdam: Elsevier; 2019. [DOI] [Google Scholar]
- 17.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seigner J, Junker-Samek M, Plaza A, D'Urso G, Masullo M, Piacente S, et al. A Symphytum officinale Root Extract Exerts Anti-inflammatory Properties by Affecting Two Distinct Steps of NF-kappaB Signaling. Front Pharmacol. 2019;10:289. doi: 10.3389/fphar.2019.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tasneem S, Liu B, Li B, Choudhary MI, Wang W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol Res. 2019;139:126–40. doi: 10.1016/j.phrs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Ambriz-Perez DL, Leyva-Lopez N, Gutierrez-Grijalva EP, Heredia J, Yildiz F. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016;2(1) doi: 10.1080/23311932.2015.1131412. [DOI] [Google Scholar]
- 22.Mayer H, Bilban M, Kurtev V, Gruber F, Wagner O, Binder BR, et al. Deciphering regulatory patterns of inflammatory gene expression from interleukin-1-stimulated human endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24(7):1192–8. doi: 10.1161/01.ATV.0000131263.06296.77. [DOI] [PubMed] [Google Scholar]
- 23.Wiart C. Medicinal plants of Asia and the Pacific. 1st ed. Boca Raton, USA: CRC Press; 2006. [DOI] [Google Scholar]
- 24.Vostinaru O, Conea SIMONA, Mogosan CRISTINA, Toma C, Borza C, Vlase LAURIAN. Anti-inflammatory and antinociceptive effect of Symphytum officinale root. Rom Biotechnol Lett. 2018;23(6):14160–7. [Google Scholar]
- 25.Teymouri S, Rakhshandeh H, Baghdar HN, Yousefi M, Salari R. Analgesic Herbal Medicines in the Treatment of Knee Osteoarthritis: A Systematic Review. Curr Rheumatol Rev. 2019;15(4):290–303. doi: 10.2174/1573397115666190328150203. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Zhang L, Joo D, Sun SC. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson J, Round A, Hamilton W. Raised inflammatory markers. BMJ. 2012;344:e454. doi: 10.1136/bmj.e454. [DOI] [PubMed] [Google Scholar]
- 28.Dell'Agli M, Di Lorenzo C, Badea M, Sangiovanni E, Dima L, Bosisio E, et al. Plant food supplements with anti-inflammatory properties: a systematic review (I). Crit Rev Food Sci Nutr. 2013;53(4):403–13. doi: 10.1080/10408398.2012.682123. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A. Medicinal plants. India : Mittal Publications; 2010. [Google Scholar]
- 30.Oliveira PR, Santos FR, Duarte EF, Guimarães GS, Mattos NSC, Minafra CS. Symbiotics and Aloe vera and Symphytum officinale extracts in broiler feed. Semin Cienc Agrar. 2016;37(4):2677. doi: 10.5433/1679-0359.2016v37n4Supl1p2677. [DOI] [Google Scholar]
- 31.DerMarderosian A, Beutler JA. The review of natural products: the most complete source of natural product information. 3rd ed. St. Louis, USA: Facts and Comparisons; 2002. [Google Scholar]
- 32.Horinouchi CD, Otuki MF. Botanical briefs: comfrey (Symphytum officinale). Cutis. 2013;91(5):225–8. [PubMed] [Google Scholar]
- 33.Weigend M, Selvi F, Thomas DC, Hilger HH. Boraginaceae. In: Kadereit JW, Bittrich V, editors. Flowering Plants. Eudicots. 1st ed. Switzerland: Springer, Cham; 2016. pp. 41–102. [DOI] [Google Scholar]
- 34.Frost R, MacPherson H, O'Meara S. A critical scoping review of external uses of comfrey (Symphytum spp.). Complement Ther Med. 2013;21(6):724–45. doi: 10.1016/j.ctim.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopal PL, Dhilna KK, Kumar PS, John J. Herbs in inflammation-a review. Int J Ayurvedic Herb Med. 2013;3(4):1289–307. [Google Scholar]
- 36.Al-Quran S. Taxonomical and pharmacological survey of therapeutic plants in Jordan. J Nat Prod. 2008;1(1):10–26. [Google Scholar]
- 37.Yousefi K, Hamedeyazdan S, Hodaei D, Lotfipour F, Baradaran B, Orangi M, et al. An in vitro ethnopharmacological study on Prangos ferulacea: a wound healing agent. Bioimpacts. 2017;7(2):75–82. doi: 10.15171/bi.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Snafi AE. The pharmacology of Anchusa italica and Anchusa strigosa–A review. Int J Pharm Pharm Sci. 2014;6(4):7–10. [Google Scholar]
- 39.Ramezani M, Amiri MS, Zibaee E, Boghrati Z, Ayati Z, Sahebkar A, et al. A Review on the Phytochemistry, Ethnobotanical Uses and Pharmacology of Borago Species. Curr Pharm Des. 2020;26(1):110–28. doi: 10.2174/1381612825666191216152733. [DOI] [PubMed] [Google Scholar]
- 40.Farhadi R, Balashahri MS, Tilebeni HG, Sadeghi M. Pharmacology of Borage (Borago officinalis L.) medicinal plant. Int J Plant Prod. 2012;3(2):73–7. [Google Scholar]
- 41.Boulos JC, Rahama M, Hegazy MF, Efferth T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019;459:248–67. doi: 10.1016/j.canlet.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Yazaki K. Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol (Tokyo). 2017;34(3):131–42. doi: 10.5511/plantbiotechnology.17.0823a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouffouk S, Mouffouk C, Bensouici C, Haba H. In vitro Cytotoxic Effect, Hemolytic, and Antioxidant Activities of the Algerian Species Nonea vesicaria Rchb. Curr Bioact Compd. 2020;16(8):1197–204. doi: 10.2174/1573407216666200109120431. [DOI] [Google Scholar]
- 44.Rehman SU, Faisal R, Shinwari ZK, Ahmad N, Ahmad IJAZ. Phytochemical screening and biological activities of Trigonella incisa and Nonea edgeworthii. Pak J Bot. 2017;49(3):1161–5. [Google Scholar]
- 45.Malinowska P. Effect of flavonoids content on antioxidant activity of commercial cosmetic plant extracts. Herba Pol. 2013;59(3):63–75. doi: 10.2478/hepo-2013-0017. [DOI] [Google Scholar]
- 46.Pielesz A, Paluch J. [Therapeutically active dressings--biomaterials in a study of collagen glycation]. Polim Med. 2012;42(2):115–20. [PubMed] [Google Scholar]
- 47.Krzyzanowska-Kowalczyk J, Pecio L, Moldoch J, Ludwiczuk A, Kowalczyk M. Novel Phenolic Constituents of Pulmonaria officinalis L. LC-MS/MS Comparison of Spring and Autumn Metabolite Profiles. Molecules. 2018;23(9) doi: 10.3390/molecules23092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosseini N, Abolhassani M. Immunomodulatory properties of borage (Echium amoenum) on BALB/c mice infected with Leishmania major. J Clin Immunol. 2011;31(3):465–71. doi: 10.1007/s10875-010-9502-6. [DOI] [PubMed] [Google Scholar]
- 49.Jin J, Boersch M, Nagarajan A, Davey AK, Zunk M. Antioxidant Properties and Reported Ethnomedicinal Use of the Genus Echium (Boraginaceae). Antioxidants (Basel). 2020;9(8) doi: 10.3390/antiox9080722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straub RH, Schradin C. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health. 2016;2016(1):37–51. doi: 10.1093/emph/eow001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thibane VS, Ndhlala AR, Finnie JF, Van Staden J. Modulation of the enzyme activity of secretory phospholipase A2, lipoxygenase and cyclooxygenase involved in inflammation and disease by extracts from some medicinal plants used for skincare and beauty. S Afr J Bot. 2019;120:198–203. doi: 10.1016/j.sajb.2018.06.001. [DOI] [Google Scholar]
- 52.Koll R, Buhr M, Dieter R, Pabst H, Predel HG, Petrowicz O, et al. Efficacy and tolerance of a comfrey root extract (Extr. Rad. Symphyti) in the treatment of ankle distorsions: results of a multicenter, randomized, placebo-controlled, double-blind study. Phytomedicine. 2004;11(6):470–7. doi: 10.1016/j.phymed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Avila C, Breakspear I, Hawrelak J, Salmond S, Evans S. A systematic review and quality assessment of case reports of adverse events for borage (Borago officinalis), coltsfoot (Tussilago farfara) and comfrey (Symphytum officinale). Fitoterapia. 2020;142:104519. doi: 10.1016/j.fitote.2020.104519. [DOI] [PubMed] [Google Scholar]
- 54.Brown AW, Stegelmeier BL, Colegate SM, Gardner DR, Panter KE, Knoppel EL, et al. The comparative toxicity of a reduced, crude comfrey (Symphytum officinale) alkaloid extract and the pure, comfrey-derived pyrrolizidine alkaloids, lycopsamine and intermedine in chicks (Gallus gallus domesticus). J Appl Toxicol. 2016;36(5):716–25. doi: 10.1002/jat.3205. [DOI] [PubMed] [Google Scholar]
- 55.ESCOP . ESCOP Monographs: the European Scientific Cooperative on Phytotherapy. UK: European Scientific Cooperative on Phytotherapy; 2002. [PubMed] [Google Scholar]
- 56.ESCOP . ESCOP Monographs: the scientific foundation for herbal medicinal products. UK: European Scientific Cooperative on Phytotherapy; 2003. [Google Scholar]
- 57.Mulkijanyan K, Barbakadze V, Novikova Z, Sulakvelidze M, Gogilashvili L, Amiranashvili L, et al. Burn healing compositions from Caucasian species of comfrey (Symphytum L.). Bull Georg Natl Acad Sci. 2009;3(3):114–7. [Google Scholar]
- 58.Barna M, Kucera A, Hladicova M, Kucera M. [Wound healing effects of a Symphytum herb extract cream (Symphytum x uplandicum NYMAN: ): results of a randomized, controlled double-blind study]. Wien Med Wochenschr. 2007;157(21-22):569–74. doi: 10.1007/s10354-007-0474-y. [DOI] [PubMed] [Google Scholar]
- 59.Ruzicka J, Berger-Büter K, Esslinger N, Novak J. Assessment of the diversity of comfrey (Symphytum officinale L. and S. × uplandicum Nyman). Genet Resour Crop Evol. 2021;68(7):2813–25. doi: 10.1007/s10722-021-01156-x. [DOI] [Google Scholar]
- 60.Hussain FHS, Ahamad J, Osw PS. A Comprehensive Review on Pharmacognostical and Pharmacological Characters of Anchusa Azurea. Advances in Medical, Dental and Health Sciences. 2019;2(3) [Google Scholar]
- 61.Cohen J. The Systematics of Lithospermum L.(Boraginaceae) and the Evolution of Heterostyly [dissertation]. New York, USA: Cornell University; 2010. [Google Scholar]
- 62.Chrubasik-Hausmann S. Phytomedicines for Inflammatory Conditions. New Jersey, USA: John Wiley & Sons; 2015. [DOI] [Google Scholar]
- 63.Barbakadze VV, Kemertelidze EP, Mulkijanyan KG, van den Berg AJJ, Beukelman CJ, van den Worm E, et al. Antioxidant and anticomplement activity of poly[3-(3,4-dihydroxyphenyl)glyceric acid] from Symphytum Asperum and Symphytum Caucasicum plants. Pharm Chem J. 2007;41(1):14–6. doi: 10.1007/s11094-007-0004-7. [DOI] [Google Scholar]
- 64.Neagu E, PĂun G, Radu LG. Phytochemical study of some Symphytum officinalis extracts concentrated by membranous procedures. Sci Bull Univ Politeh Buchacharest. 2011:3–7. [Google Scholar]
- 65.Ebada SS, Al-Jawabri NA, Youssef FS, El-Kashef DH, Knedel TO, Albohy A, et al. Anti-inflammatory, antiallergic and COVID-19 protease inhibitory activities of phytochemicals from the Jordanian hawksbeard: identification, structure–activity relationships, molecular modeling and impact on its folk medicinal uses. RSC Adv. 2020;10:38128–41. doi: 10.1039/D0RA04876C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taguchi R, Hatayama K, Takahashi T, Hayashi T, Sato Y, Sato D, et al. Structure-activity relations of rosmarinic acid derivatives for the amyloid beta aggregation inhibition and antioxidant properties. Eur J Med Chem. 2017;138:1066–75. doi: 10.1016/j.ejmech.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 67.Dresler S, Szymczak G, Wojcik M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm Biol. 2017;55(1):691–5. doi: 10.1080/13880209.2016.1265986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panchenko L, Muratova A, Biktasheva L, Galitskaya P, Golubev S, Dubrovskaya E, et al. Study of Boraginaceae plants for phytoremediation of oil-contaminated soil. Int J Phytoremediation. 2022;24(2):215–23. doi: 10.1080/15226514.2021.1932729. [DOI] [PubMed] [Google Scholar]
- 69.Pezeshki S, Petersen M. Rosmarinic Acid and Related Metabolites. In: Schwab W, Lange BM, Wüst M, editors. Biotechnology of Natural Products. Switzerland: Springer, Cham; 2018. pp. 25–60. [DOI] [Google Scholar]
- 70.Olennikov DN, Kruglov DS, Daironas ZV, Zilfikarov IN. Shikonin and its Esters from Buglossoides arvensis and Other Species of the Family Boraginaceae. Chem Nat Compd. 2020;56(4):713–5. doi: 10.1007/s10600-020-03127-7. [DOI] [Google Scholar]
- 71.Tufa T, Damianakos H, Graikou K, Chinou L. Comparative Study of Naphthoquinone Contents of Selected Greek Endemic Boraginaceae Plants - Antimicrobial Activities. Nat Prod Commun. 2017;12(2):179–80. [PubMed] [Google Scholar]
- 72.Varvouni E, Zengin G, Graikou K, Ganos C, Mroczek T, Chinou I. Phytochemical analysis and biological evaluation of the aerial parts from Symphytum anatolicum Boiss. and Cynoglottis barrelieri (All.) Vural & Kit Tan (Boraginaceae). Biochem Syst Ecol. 2020;92:104128. doi: 10.1016/j.bse.2020.104128. [DOI] [Google Scholar]
- 73.Chaowuttikul C, Palanuvej C, Ruangrungsi N. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz J Pharm Sci. 2020;56:e17547. doi: 10.1590/s2175-97902019000317547. [DOI] [Google Scholar]
- 74.Neagu E, Radu GL, Albu C, Paun G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J Biol Sci. 2018;25(3):578–85. doi: 10.1016/j.sjbs.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Girsang E, Ginting CN, Lister INE, Gunawan KY, Widowati W. Anti-inflammatory and antiaging properties of chlorogenic acid on UV-induced fibroblast cell. PeerJ. 2021;9:e11419. doi: 10.7717/peerj.11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Negahdari R, Bohlouli S, Sharifi S, Maleki Dizaj S, Rahbar Saadat Y, Khezri K, et al. Therapeutic benefits of rutin and its nanoformulations. Phytother Res. 2021;35(4):1719–38. doi: 10.1002/ptr.6904. [DOI] [PubMed] [Google Scholar]
- 77.Savic V, Nikolic VD, Arsic IA, Stanojevic LP, Najman SJ, Stojanovic S, et al. Comparative Study of the Biological Activity of Allantoin and Aqueous Extract of the Comfrey Root. Phytother Res. 2015;29(8):1117–22. doi: 10.1002/ptr.5356. [DOI] [PubMed] [Google Scholar]
- 78.Kucera M, Kalal J, Polesna Z. Effects of Symphytum ointment on muscular symptoms and functional locomotor disturbances. Adv Ther. 2000;17(4):204–10. doi: 10.1007/BF02850297. [DOI] [PubMed] [Google Scholar]
- 79.Araujo LU, Grabe-Guimaraes A, Mosqueira VC, Carneiro CM, Silva-Barcellos NM. Profile of wound healing process induced by allantoin. Acta Cir Bras. 2010;25(5):460–6. doi: 10.1590/s0102-86502010000500014. [DOI] [PubMed] [Google Scholar]
- 80.Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105(2):201–7. doi: 10.1093/bja/aeq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alagawany M, Abd El-Hack ME, Farag MR, Gopi M, Karthik K, Malik YS, et al. Rosmarinic acid: modes of action, medicinal values and health benefits. Anim Health Res Rev. 2017;18(2):167–76. doi: 10.1017/S1466252317000081. [DOI] [PubMed] [Google Scholar]
- 82.Lee JH, Park KH, Lee MH, Kim HT, Seo WD, Kim JY, et al. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013;136(2):843–52. doi: 10.1016/j.foodchem.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 83.Stansbury J, Saunders P, Winston D, Zampieron ER. Rosmarinic Acid as a Novel Agent in the Treatment of Autoimmune Disease. J Restor Med. 2012;1(1):112–6. doi: 10.14200/jrm.2012.1.1013. [DOI] [Google Scholar]
- 84.Kucera M, Barna M, Horacek O, Kovarikova J, Kucera A. Efficacy and safety of topically applied Symphytum herb extract cream in the treatment of ankle distortion: results of a randomized controlled clinical double blind study. Wien Med Wochenschr. 2004;154(21-22):498–507. doi: 10.1007/s10354-004-0114-8. [DOI] [PubMed] [Google Scholar]
- 85.Chen L, Teng H, Xie Z, Cao H, Cheang WS, Skalicka-Woniak K, et al. Modifications of dietary flavonoids towards improved bioactivity: An update on structure-activity relationship. Crit Rev Food Sci Nutr. 2018;58(4):513–27. doi: 10.1080/10408398.2016.1196334. [DOI] [PubMed] [Google Scholar]
- 86.Barbakadze V, van den Berg AJJ, Beukelman CJ, Kemmink J, van Ufford H. Poly[3-(3,4-dihydroxyphenyl)glyceric acid] from Symphytum officinale roots and its biological activity. Chem Nat Compd. 2009;45(1):6–10. doi: 10.1007/s10600-009-9221-5. [DOI] [Google Scholar]
- 87.Trifan A, Skalicka-Wozniak K, Granica S, Czerwinska ME, Kruk A, Marcourt L, et al. Symphytum officinale L.: Liquid-liquid chromatography isolation of caffeic acid oligomers and evaluation of their influence on pro-inflammatory cytokine release in LPS-stimulated neutrophils. J Ethnopharmacol. 2020;262:113169. doi: 10.1016/j.jep.2020.113169. [DOI] [PubMed] [Google Scholar]
- 88.Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J Biol Chem. 2004;279(7):5877–85. doi: 10.1074/jbc.M309185200. [DOI] [PubMed] [Google Scholar]
- 89.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009;57(6):703–8. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 90.Krzyzanowska-Kowalczyk J, Kowalczyk M, Ponczek MB, Pecio L, Nowak P, Kolodziejczyk-Czepas J. Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors-In Vitro and In Silico Studies. Molecules. 2021;26(3) doi: 10.3390/molecules26030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curini M, Epifano F, Genovese S, Menghini A, Altinier G, Tubaro A, et al. Fatty acids profile and antiinflammatory activity of Nonea setosa R. et S. Phytother Res. 2006;20(5):422–3. doi: 10.1002/ptr.1886. [DOI] [PubMed] [Google Scholar]
- 92.Kuruuzum-Uz A, Suleyman H, Cadirci E, Guvenalp Z, Demirezer LO. Investigation on anti-inflammatory and antiulcer activities of Anchusa azurea extracts and their major constituent rosmarinic acid. Z Naturforsch C J Biosci. 2012;67(7-8):360–6. doi: 10.1515/znc-2012-7-802. [DOI] [PubMed] [Google Scholar]
- 93.Paun G, Neagu E, Albu C, Savin S, Radu GL. In Vitro Evaluation of Antidiabetic and Anti-Inflammatory Activities of Polyphenolic-Rich Extracts from Anchusa officinalis and Melilotus officinalis. ACS Omega. 2020;5(22):13014–22. doi: 10.1021/acsomega.0c00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moita E, Gil-Izquierdo A, Sousa C, Ferreres F, Silva LR, Valentao P, et al. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS One. 2013;8(3):e59131. doi: 10.1371/journal.pone.0059131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moreira R, Fernandes F, Valentao P, Pereira DM, Andrade PB. Echium plantagineum L. honey: Search of pyrrolizidine alkaloids and polyphenols, anti-inflammatory potential and cytotoxicity. Food Chem. 2020;328:127169. doi: 10.1016/j.foodchem.2020.127169. [DOI] [PubMed] [Google Scholar]
- 96.Ghasemian M, Owlia S, Owlia MB. Review of Anti-Inflammatory Herbal Medicines. Adv Pharmacol Sci. 2016;2016:9130979. doi: 10.1155/2016/9130979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sailaja AK. Herbal Medicine for the Treatment of Rheumatoid Arthritis. Journal of Physics & Optics Sciences. 2020;114:3. [Google Scholar]
- 98.Han KY, Kwon TH, Lee TH, Lee SJ, Kim SH, Kim J. Suppressive effects of Lithospermum erythrorhizon extracts on lipopolysaccharide-induced activation of AP-1 and NF-kappaB via mitogen-activated protein kinase pathways in mouse macrophage cells. BMB Rep. 2008;41(4):328–33. doi: 10.5483/bmbrep.2008.41.4.328. [DOI] [PubMed] [Google Scholar]
- 99.Araujo LU, Reis PG, Barbosa LC, Saude-Guimaraes DA, Grabe-Guimaraes A, Mosqueira VC, et al. In vivo wound healing effects of Symphytum officinale L. leaves extract in different topical formulations. Pharmazie. 2012;67(4):355–60. [PubMed] [Google Scholar]
- 100.Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, et al. E-selectin receptors on human leukocytes. Blood. 2008;112(9):3744–52. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barbakadze VV, Mulkidzhanyan KG, Merlani MI, Gogilashvili LM, Amiranashvili L, Shaburishvili EK. Extraction, composition and the antioxidant and anticomplement activities of high molecular weight fractions from the leaves of Symphytum asperum and S. caucasicum. Pharm Chem J. 2011;44(11):604–7. doi: 10.1007/s11094-011-0527-9. [DOI] [Google Scholar]
- 102.Marzano AV, Ortega-Loayza AG, Heath M, Morse D, Genovese G, Cugno M. Mechanisms of Inflammation in Neutrophil-Mediated Skin Diseases. Front Immunol. 2019;10:1059. doi: 10.3389/fimmu.2019.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jain P, Pandey R, Shukla SS. Natural Sources of Anti-inflammation. In: Jain P, Pandey R, Shukla SS, editors. Inflammation: Natural Resources and Its Applications. New Delhi, India: Springer; 2015. pp. 25–133. [DOI] [Google Scholar]
- 104.Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5):CD010538. doi: 10.1002/14651858.CD010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grube B, Grunwald J, Krug L, Staiger C. Efficacy of a comfrey root (Symphyti offic. radix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine. 2007;14(1):2–10. doi: 10.1016/j.phymed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 106.D'Anchise R, Bulitta M, Giannetti B. Comfrey extract ointment in comparison to diclofenac gel in the treatment of acute unilateral ankle sprains (distortions). Arzneimittelforschung. 2007;57(11):712–6. doi: 10.1055/s-0031-1296672. [DOI] [PubMed] [Google Scholar]
- 107.Trifan A, Zengin G, Sinan KI, Wolfram E, Skalicka-Wozniak K, Luca SV. LC-HRMS/MS phytochemical profiling of Symphytum officinale L. and Anchusa ochroleuca M. Bieb. (Boraginaceae): Unveiling their multi-biological potential via an integrated approach. J Pharm Biomed Anal. 2021;204:114283. doi: 10.1016/j.jpba.2021.114283. [DOI] [PubMed] [Google Scholar]
- 108.Le V, Dolganyuk V, Sukhikh A, Babich O, Ivanova S, Prosekov A, et al. Phytochemical Analysis of Symphytum officinale Root Culture Extract. Appl Sci. 2021;11(10):4478. doi: 10.3390/app11104478. [DOI] [Google Scholar]
- 109.Dey D, Jingar P, Agrawal S, Shrivastava V, Bhattacharya A, Manhas J, et al. Symphytum officinale augments osteogenesis in human bone marrow-derived mesenchymal stem cells in vitro as they differentiate into osteoblasts. J Ethnopharmacol. 2020;248:112329. doi: 10.1016/j.jep.2019.112329. [DOI] [PubMed] [Google Scholar]
- 110.Shaghayegh M. [Quantitative and qualitative study of pyrrolizidine alkaloids and biological activity of aerial organs of plants Arnebia decombens and Symphytum asperum by bioathography [dissertation]]. Mazandaran, Iran: Mazandaran University of Medical Sciences; 2020. [Google Scholar]
- 111.Barbakadze V, Kemertelidze E, Targamadze I, Mulkijanyan K, Shashkov AS, Usov AI. Poly[3-(3,4-dihydroxyphenyl)glyceric acid], a new biologically active polymer from Symphytum asperum Lepech. and S. caucasicum Bieb. (Boraginaceae). Molecules. 2005;10(9):1135–44. doi: 10.3390/10091135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barna M, Kucera A, Hladikova M, Kucera M. Randomized double-blind study: wound-healing effects of a Symphytum herb extract cream (Symphytumxuplandicum Nyman) in children. Arzneimittelforschung. 2012;62(6):285–9. doi: 10.1055/s-0032-1308981. [DOI] [PubMed] [Google Scholar]
- 113.Sarikurkcu C, Ozer MS, Tlili N. LC–ESI–MS/MS characterization of phytochemical and enzyme inhibitory effects of different solvent extract of Symphytum anatolicum. Ind Crops Prod. 2019;140:111666. doi: 10.1016/j.indcrop.2019.111666. [DOI] [Google Scholar]
- 114.Dasgupta A. Antiinflammatory Herbal Supplements. In: Actor JK, Smith KC, editors. Translational Inflammation. Massachusetts, USA: Academic Press; 2019. pp. 69–91. [DOI] [Google Scholar]
- 115.Rode D. Comfrey toxicity revisited. Trends Pharmacol Sci. 2002;23(11):497–9. doi: 10.1016/s0165-6147(02)02106-5. [DOI] [PubMed] [Google Scholar]
- 116.Stegemann T, Kruse LH, Brutt M, Ober D. Specific Distribution of Pyrrolizidine Alkaloids in Floral Parts of Comfrey (Symphytum officinale) and its Implications for Flower Ecology. J Chem Ecol. 2019;45(2):128–35. doi: 10.1007/s10886-018-0990-9. [DOI] [PubMed] [Google Scholar]
- 117.Peng C, Zhang X, Zhang F, Liu L, Shao Y, Xiang X, et al. Clinical efficacy and safety of anticoagulation therapy for Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: a retrospective multicenter cohort study. Eur J Gastroenterol Hepatol. 2020;32(9):1168–78. doi: 10.1097/MEG.0000000000001630. [DOI] [PubMed] [Google Scholar]
- 118.Neuman MG, Cohen L, Opris M, Nanau RM, Hyunjin J. Hepatotoxicity of Pyrrolizidine Alkaloids. J Pharm Pharm Sci. 2015;18(4):825–43. doi: 10.18433/j3bg7j. [DOI] [PubMed] [Google Scholar]
- 119.Seremet OC, Barbuceanu F, Ionica FE, Margina DM, GuTu CM, Olaru OT, et al. Oral toxicity study of certain plant extracts containing pyrrolizidine alkaloids. Rom J Morphol Embryol. 2016;57(3):1017–23. [PubMed] [Google Scholar]
- 120.Seremet OC, Olaru OT, Gutu CM, Nitulescu GM, Ilie M, Negres S, et al. Toxicity of plant extracts containing pyrrolizidine alkaloids using alternative invertebrate models. Mol Med Rep. 2018;17(6):7757–63. doi: 10.3892/mmr.2018.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moreira R, Pereira DM, Valentao P, Andrade PB. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int J Mol Sci. 2018;19(6) doi: 10.3390/ijms19061668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stickel F, Seitz HK. The efficacy and safety of comfrey. Public Health Nutr. 2000;3(4A):501–8. doi: 10.1017/s1368980000000586. [DOI] [PubMed] [Google Scholar]
- 123.Onduso SO. Determination of levels of pyrrolizidine alkaloids in Symphytum asperum Lepech growing in selected parts of Keniya [master's degree]. Nairobi, Kenia: Kenyatta University; 2014. [Google Scholar]
- 124.Mroczek T, Ndjoko-Ioset K, Głowniak K, Miętkiewicz-Capała A, Hostettmann K. Investigation of Symphytum cordatum alkaloids by liquid–liquid partitioning, thin-layer chromatography and liquid chromatography–ion-trap mass spectrometry. Analytica Chimica Acta. 2006;566(2):157–66. doi: 10.1016/j.aca.2006.03.016. [DOI] [Google Scholar]
- 125.Liu F, Wan SY, Jiang Z, Li SF, Ong ES, Osorio JC. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography-electrospray ionization mass spectrometry. Talanta. 2009;80(2):916–23. doi: 10.1016/j.talanta.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 126.Altamirano JC, Gratz SR, Wolnik KA. Investigation of pyrrolizidine alkaloids and their N-oxides in commercial comfrey-containing products and botanical materials by liquid chromatography electrospray ionization mass spectrometry. J AOAC Int. 2005;88(2):406–12. [PubMed] [Google Scholar]
- 127.Oberlies NH, Kim NC, Brine DR, Collins BJ, Handy RW, Sparacino CM, et al. Analysis of herbal teas made from the leaves of comfrey (Symphytum officinale): reduction of N-oxides results in order of magnitude increases in the measurable concentration of pyrrolizidine alkaloids. Public Health Nutr. 2004;7(7):919–24. doi: 10.1079/phn2004624. [DOI] [PubMed] [Google Scholar]
- 128.Savic V, Savic S, Nikolic V, Nikolic L, Najman S, Lazarevic J, et al. The identification and quantification of bioactive compounds from the aqueous extract of comfrey root by UHPLC-DAD-HESI-MS method and its microbial activity. Hem Ind. 2015;69(1):1–8. doi: 10.2298/hemind131202013s. [DOI] [Google Scholar]
- 129.Kruse LH, Stegemann T, Sievert C, Ober D. Identification of a Second Site of Pyrrolizidine Alkaloid Biosynthesis in Comfrey to Boost Plant Defense in Floral Stage. Plant Physiol. 2017;174(1):47–55. doi: 10.1104/pp.17.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schrenk D, Gao L, Lin G, Mahony C, Mulder PPJ, Peijnenburg A, et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment - A review. Food Chem Toxicol. 2020;136:111107. doi: 10.1016/j.fct.2019.111107. [DOI] [PubMed] [Google Scholar]
- 131.Ifeoma O, Oluwakanyinsola S. Screening of Herbal Medicines for Potential Toxicities. In: Gowder SJT, editor. New Insights into Toxicity and Drug Testing. London, UK: IntechOpen; 2013. [DOI] [Google Scholar]
- 132.Werner RDC, Merz ADB. Committee on Herbal Medicinal Products (HMPC). London, UK: European Medicines Agency ; 2007. [Google Scholar]
- 133.Gruenwald J, Brendler T, Jaenicke C. PDR for herbal medicines. Ontario, Canada: Thomson Reuters; 2007. [Google Scholar]
- 134.Ahmad L, He Y, Semotiuk A, Liu Q, Jan H. Pan-Himalaya Ethnomedicine safety: Lithospermeae (Boraginaceae) Herbal Remedies Containing Toxic Pyrrolizidine Alkaloids. J Complement Med Res. 2019;10(3):129. doi: 10.5455/jcmr.20190513073648. [DOI] [Google Scholar]
- 135.Knyazev S, Akhkubekova AA, Tamakhina AY, Loretts O, Kukhar V, Panfilova O, et al. Accumulation of alkaloids in plants of the family Boraginaceae depending on environmental conditions places of growth.; International Scientific and Practical Conference “Fundamental and Applied Research in Biology and Agriculture: Current Issues, Achievements and Innovations” (FARBA 2021).; Ored, Russian Federation. E3S Web of Conferences ; 2021. [Google Scholar]